Abstract
Background
Chronic respiratory failure and heart involvement may occur in Duchenne muscular dystrophy. We aimed to assess the prognostic value of the right ventricular (RV) systolic dysfunction in patients with Duchenne muscular dystrophy.
Methods and Results
We studied 90 genetically proven patients with Duchenne muscular dystrophy from 2010 to 2019, to obtain respiratory function and Doppler echocardiographic RV systolic function. Prognostic value was assessed in terms of death and cardiac events. The median age was 27.5 years, and median forced vital capacity was at 10% of the predicted value: 83 patients (92%) were on home mechanical ventilation. An RV systolic dysfunction was found in 46 patients (51%). In patients without RV dysfunction at inclusion, a left ventricular systolic dysfunction at inclusion was associated with a higher risk of developing RV dysfunction during follow‐up with an odds ratio of 4.5 (P=0.03). RV systolic dysfunction was significantly associated with cardiac events, mainly acute heart failure (62%) and cardiogenic shock (23%). In a multivariable Cox model, the adjusted hazard ratio was 4.96 (95% CI [1.09–22.6]; P=0.04). In terms of death, we found a significant difference between patients with RV dysfunction versus patients without RV dysfunction in the Kaplan–Meier curves (log‐rank P=0.045).
Conclusions
RV systolic dysfunction is frequently present in patients with Duchenne muscular dystrophy and is associated with increased risk of cardiac events, irrespective of left ventricular dysfunction and mechanical ventilation.
Registration
URL: https://www.clinicaltrials.org; unique identifier: NCT02501083.
Keywords: Duchenne muscular dystrophy, mortality, prognosis, right ventricle, ventilation
Subject Categories: Echocardiography, Prognosis
Nonstandard Abbreviations and Acronyms
- DMD
Duchenne muscular dystrophy
- TAPSE
tricuspid annular plane systolic displacement
Clinical Perspective.
What Is New?
Right ventricular systolic dysfunction is frequent in patients with Duchenne muscular dystrophy.
Right ventricular systolic dysfunction is associated with increased risk of cardiac events in patients with Duchenne muscular dystrophy.
A left ventricular systolic dysfunction is a risk factor for developing right ventricular dysfunction in Duchenne muscular dystrophy.
What Are the Clinical Implications?
It is essential to search systematically for a right ventricular systolic dysfunction in patients with Duchenne muscular dystrophy.
A closer ultrasound, right and left ventricular function ultrasound monitoring, is essential particularly in patients with left ventricular systolic dysfunction.
Duchenne muscular dystrophy (DMD) is an inherited myogenic disorder due to mutations in the dystrophin gene on chromosome Xp21.1. 1 Left ventricular (LV) systolic dysfunction is a usual complication in DMD, and abnormal myocardium may be present earlier in the pediatric population. 1 Cardiac drugs including angiotensin‐converting enzyme inhibitors and respiratory support using home mechanical ventilation have improved clinical outcomes in DMD. 2 , 3 However, the prognosis remains determined by respiratory failure and heart failure. 4 The use of noninvasive mechanical ventilation is often performed in DMD, but may affect the right ventricular (RV) systolic function. On the other hand, most cases of DMD exhibit simultaneously a restrictive respiratory failure. The right ventricle acts in a low‐pressure system and is sensitive to cardiac loading. 5 Hypoxemia and hypercapnic acidosis may affect RV performance by inducing increased pulmonary resistance. 6 , 7 In addition, hypercapnia and positive end‐expiratory pressure level may affect RV function. 8 In this context, the RV function may be affected in DMD, particularly in patients with restrictive pulmonary failure and on home mechanical ventilation. 9 In this study, we aimed to evaluate the prevalence of RV systolic dysfunction, to assess factors associated with RV dysfunction and to assess the impact of the RV dysfunction on death and cardiac events in patients presenting with DMD.
Methods
All data and supporting materials have been provided with the published article.
Subjects
We reviewed from our database all patients presenting with DMD and followed at the Home Mechanical Ventilation Unit of the Raymond Poincare University Hospital (tertiary neuromuscular center) between 2010 and 2019. We included 90 consecutive genetically proven DMD patients who benefited from an echocardiographic examination that included an RV systolic function assessment. Genetic data were extracted from the UMD‐DMD‐France database 10 maintained in the Genetics and Molecular biology lab, Cochin Hospital, Paris.
We excluded patients without RV function analysis (n=19), patients with other neuromuscular diseases (n=25), and patients without genetically proven DMD disease (n=10).
We systematically collected clinical and genetic characteristics, biological findings that included blood gas exchange, hepatic enzyme, creatinine level, albumin, hemoglobin, and lymphocytes. During follow‐up, death and cardiac events were systematically assessed. We also recorded the last available LV systolic function and RV systolic function available during follow‐up. The study was performed in compliance with the ethical principles formulated in the declaration of Helsinki and was approved by the comité de protection des personnes and the commission nationale de l'informatique et des libertés. Informed consent was obtained from patients. The UMD‐DMD‐France database was approved by the French ethical committees including Comité Consultatif sur le Traitement de l'Information en matière de Recherche dans le domaine de la Santé No. 08‐413/2008 and commission nationale de l'informatique et des libertés No. 1 326 937/2009. This study is registered as clinical trial NCT02501083.
Echocardiographic Doppler Analysis
All transthoracic echocardiographic examinations were performed using a Siemens CV70 device (Siemens Medical Solutions USA, Mountain View, CA). All measurements were performed according to the recommendations 11 and were averaged over 3 cardiac cycles. Several 2‐dimensional views were routinely recorded: parasternal long‐axis view; parasternal short‐axis view; and apical 2‐, 3‐, and 4‐chamber views. The following 2‐dimensional measurements were routinely assessed: (1) end‐diastolic measurements of the interventricular septum, posterior wall, and left ventricle; (2) left atrial diameter; (3) LV mass; and (4) LV ejection fraction using Simpson's method. The following Doppler parameters were routinely determined: (1) E and A waves of the mitral inflow; (2) aortic velocity; (3) systolic tricuspid regurgitation velocity, allowing to calculate the systolic pulmonary arterial pressure 12 , 13 , 14 ; and (4) peak systolic velocity from the septal insertion site of the mitral leaflet (septal s′), peak of early and late diastolic velocity from the septal insertion site of the mitral leaflet (septal e′ and septal a′). 13
The following RV systolic function was systematically assessed: (1) tricuspid annular plane systolic displacement (TAPSE) recorded from the apical 4‐chamber view using the M‐mode cursor positioned at the free wall angle of the tricuspid valve annulus 12 ; and (2) tricuspid RV s′ wave (RV s′ velocity) by tissue Doppler imaging from an apical 4‐chamber view. RV systolic dysfunction was defined as the presence of a TAPSE <17 mm or a peak RV s′<10 cm/s. 12
Genetic Analysis
DMD diagnosis was based on DMD gene analysis. Semiquantitative fluorescent multiplex polymerase chain reaction using genomic DNA was used to detect large exonic deletions and duplications in the DMD gene. Other types of mutations were detected by direct sequencing of the entire DMD gene exons or preceded by the analysis of muscle dystrophin mRNA by reverse transcription polymerase chain reaction. For each patient, we recorded the type of mutation and the exons of the gene involved; we also determined the most distal dystrophin domain theoretically involved by the DMD gene mutation and beyond which the protein is truncated if any dystrophin is produced. The dystrophin protein is composed of several functional domains, that is, the N‐terminal domain, rod domain (composed of 3 subdomains separated by 4 hinges [H1, H2, H3, and H4], which we respectively indicated as <H2, H2–H3, and >H3), cysteine‐rich domain, and C‐terminal domain.
Respiratory Function Analysis
Spirometry variables and lung volumes were measured routinely using a V max 229 SensorMedics System (Yorba Linda, CA) according to standard guidelines. The pulmonary function tests with spirometry were performed according to standard guidelines while the patient was sitting comfortably. 15 Vital capacity measurements during the slow volume measurement maneuver was recorded for each patient. We recorded diurnal pH, diurnal excess basis, diurnal pco 2, and nocturnal time with oxygen saturation <90% from nocturnal oximetry (performed with a Covidien Nellcor oximeter [Medtronic, Dublin, Ireland]).
Mechanical Ventilation Setting
Ventilator setting parameters were collected and included tidal volume (expressed both as raw value and indexed to ideal body weight), daily duration of mechanical ventilation, and positive end‐expiratory pressure. Bilevel ventilation was used in patients with nocturnal mechanical ventilation. Patients with permanent ventilation were ventilated using a volumetric mode. Invasive ventilation was defined by permanent ventilation using tracheostomy. Ventilation was considered efficient with no nocturnal hypoventilation according to Eagle et al criteria, 16 validated by our group in this population of patients. 17
Outcomes
Vital status was systematically recorded at the end of follow‐up. Death was defined as all‐cause death. Cardiac events were reported and included acute heart failure, cardiogenic shock, supraventricular arrhythmia, ventricular arrhythmia, and complete atrioventricular block.
Acute heart failure was defined as the rapid onset or rapid worsening of signs and symptoms of heart failure. 13 Cardiogenic shock was defined as the onset of acute heart failure associated with prolonged arterial hypotension <90 mm Hg or the need for vasopressors. 13
Statistical Analysis
Continuous variables were expressed as median (interquartile range) because of a nonnormal distribution. Categorical variables were expressed as number (percentage). We assessed the difference between groups using non‐parametric comparison when appropriate. Regarding continuous variables, normality was assessed using Shapiro–Wilk tests. Normally distributed variables were compared using t tests, while nonnormal variables were compared using Mann–Whitney tests. Regarding categorical variables, proportions were compared using Fischer's exact test. Cox regression survival models were constructed to assess the association between clinical events (cardiac events or death) and independent variables (after transformation because of linearity requirements). Because of the small number of events, the selected dependent variables were chosen on the basis of clinical relevance, and significant association in univariate comparison analysis. Analyses were adjusted on clinically relevant variables, known to be associated with the specified outcome (here, RV dysfunction). When possible, other variables were put into the multivariable model; these additional variables were retained on the basis of their association in the presented data set in regression analyses. Cardiac events were censored at 5 years of follow‐up. The time 0 in the Kaplan–Meier curves is the time of the initial Doppler echocardiography. A P value <0.05 was considered significant. Figures and statistical analyses were performed using SPSS version 25.0 (IBM, Armonk, NY).
Results
Study Population
Ninety adult patients with genetically proven DMD were included in this study (Table 1). The median age was 27.5 years, and 99% of patients were wheelchair‐bound. Dystrophin mutations were mainly exonic deletion (52%), followed by exonic duplication (12%), stop mutation (12%), small deletion (11%), intronic mutation (7.8%), small insertion (3.3%), and missense (1.1%). Patients had chronic restrictive lung disease with a median forced vital capacity at 10% of the predicted value. All the patients were ventilated at home or in a social health care institution independently to the duration of mechanical ventilation. Eighty‐three patients (92%) were under mechanical ventilation. Among them, 51 (57%) were ventilated 24 hours/24 hours. Fifteen (17%) of them remained under noninvasive ventilation by using a mask interface during the nighttime and a mouthpiece during the daytime, whereas 36 patients (40%) were tracheostomized and permanently ventilated. Ventilation was considered efficient with no nocturnal hypoventilation. The median diurnal arterial Paco 2) was 39.5 mm Hg.
Table 1.
Clinical, Biological, and Genetic Data of 90 Patients With DMD
| Parameters | 90 patients with DMD | |
|---|---|---|
| Median or N | IQR or % | |
| Age, y | 27.5 | 23 to 33 |
| Body mass index, kg/m2 | 17 | 14.5 to 22 |
| Body surface area, m2 | 1.4 | 1.24 to 1.64 |
| Wheelchair bound | 89 | 99 |
| Systolic blood pressure, mm Hg | 107 | 96 to 116 |
| Diastolic blood pressure, mm Hg | 64 | 59 to 75 |
| Heart rate, bpm | 84 | 75 to 97 |
| Angiotensin‐converting enzyme inhibitors | 84 | 93 |
| Beta blockers | 56 | 62 |
| Mineraloreceptor antagonists | 6 | 6.7 |
| Loop diuretics | 4 | 4.4 |
| Steroids | 8 | 9 |
| Left bundle branch block | 3 | 3.7 |
| LV ejection fraction <50% | 44 | 49 |
| Pulmonary vital capacity, % | 10 | 6 to 17 |
| Home mechanical ventilation | 83 | 92 |
| Home mechanical ventilation 24 h/24 h | 51 | 57 |
| Invasive ventilation | 36 | 40 |
| Tidal volume, mL | 550 | 468 to 650 |
| Tidal volume indexed to IBW, mL/kg | 9.25 | 7.79 to 10.9 |
| Positive end‐expiratory pressure, cmH2O | 2 | 0 to 4 |
| Diurnal Pco 2, mm Hg | 39.5 | 32.6 to 45 |
| pH | 7.41 | 7.38 to 7.46 |
| Excess basis | −0.4 | −2.2 to 2.25 |
| % Nocturnal oxygen saturation <90% | 0 | 0 to 0.2 |
| Biological markers | ||
| ALT, μ/L | 39 | 26 to 47 |
| AST, μ/L | 29 | 24 to 33.8 |
| GGT, μ/L | 27 | 17.2 to 48.8 |
| ALP, μ/L | 85 | 64 to 109 |
| Creatinine, μmol/L | 14 | 8 to 20 |
| Hemoglobin, g/dL | 13.6 | 12.8 to 14.4 |
| Lymphocytes/mm3 | 1810 | 1200 to 2080 |
| Albuminemia, g/L | 39.5 | 37 to 42 |
| Total bilirubin, μmol/L | 8 | 6 to 13 |
| Genetic results | ||
| Rod domain (H2–H3) | 43 | 49 |
| Rod domain (<H2) | 20 | 23 |
| Rod domain (>H3) | 13 | 15 |
| N terminal | 8 | 9 |
| Cysteine‐rich domain | 4 | 4.5 |
ALP indicates alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; DMD, Duchenne muscular dystrophy; GGT, gamma‐glutamyl transpeptidase; IBW, ideal body weight; IQR, interquartile range; and LV, left ventricular.
Table 2 represents the echocardiographic characteristics of the population.
Table 2.
Echocardiographic Data of 90 Patients With DMD
| Echocardiographic parameters | Median | IQR |
|---|---|---|
| LV end‐diastolic diameter, mm | 44 | 38–49 |
| Interventricular septal end‐diastolic thickness, mm | 7 | 6–7.3 |
| Posterior wall end‐diastolic thickness, mm | 7 | 7–8.7 |
| LV ejection fraction, % | 50 | 40–59.8 |
| Left atrial diameter, mm | 19.5 | 15.5–23 |
| Mitral E/A ratio | 1.68 | 1.4–2.16 |
| DTI septal e′ velocity, cm/s | 9 | 8–12 |
| DTI septal s′ velocity, cm/s | 9 | 6–10 |
| DTI septal a′ velocity, cm/s | 9 | 6–10 |
| DTI RV e′ velocity, cm/s | 12 | 10–15 |
| DTI RV a′ velocity, cm/s | 10 | 9–12 |
| TAPSE, mm | 15 | 13–18 |
| DTI RV s′ velocity, cm/s | 12 | 10–14 |
| Systolic pulmonary arterial pressure, mm Hg | 24 | 20–26.8 |
DMD indicates Duchenne muscular dystrophy; DTI, tissue Doppler imaging; LV, left ventricular; RV, right ventricular; and TAPSE, tricuspid annular plane systolic displacement.
RV Dysfunction and Associated Factors
An RV systolic dysfunction was found in 51% of patients (n=46). Tables 3 and 4 describe the characteristics of the study population according to RV systolic function. Patients with RV systolic dysfunction had reduced basal septal tissue Doppler imaging velocities. Liver enzyme levels and renal function (creatinine) were not associated with RV dysfunction. There was no association between genetic factors and RV dysfunction (chi‐squared test P=0.54).
Table 3.
Comparison Between Patients With and Without RV Systolic Dysfunction
| Parameters | Normal RV function | RV dysfunction | P value |
|---|---|---|---|
| (n=44) | (n=46) | ||
| Age, y | 27 (22 to 32.5) | 28.5 (23 to 35) | 0.83 |
| Body mass index, kg/m2 | 16.8 (14.8 to 21) | 17.5 (14.4 to 22.9) | 0.65 |
| Body surface area, m2 | 1.4 (1.3 to 1.6) | 1.4 (1.2 to 1.7) | 0.92 |
| Wheelchair bounded | 44 (100) | 45 (97.8) | 0.99 |
| Systolic blood pressure, mm Hg | 108 (99 to 118) | 106 (96 to 116) | 0.3 |
| Diastolic blood pressure, mm Hg | 65 (59 to 75) | 60 (59 to 75) | 0.99 |
| Heart rate, bpm | 87 (76 to 100) | 82 (70 to 94) | 0.37 |
| Angiotensin‐converting enzyme inhibitors | 41 (93.2) | 43 (93.5) | 0.99 |
| Beta blockers | 23 (52.3) | 33 (71.7) | 0.08 |
| Mineraloreceptor antagonists | 2 (4.5) | 4 (8.7) | 0.68 |
| Loop diuretics | 1 (2.3) | 3 (6.5) | 0.62 |
| Steroids | 2 (4.5) | 6 (13) | 0.27 |
| Left bundle branch block | 2 (4.9) | 1 (2.4) | 0.99 |
| Pulmonary forced vital capacity, % | 11 (6 to 20) | 9.5 (5 to 14) | 0.61 |
| Home mechanical ventilation 24 h/24 h | 24 (54.5) | 27 (60) | 0.67 |
| Tracheostomy | 18 (40.9) | 18 (39.1) | 0.99 |
| Diurnal Pco 2, mm Hg | 37.5 (29.5 to 43.5) | 42 (35.5 to 45.5) | 0.24 |
| pH | 7.42 (7.4 to 7.5) | 7.39 (7.4 to 7.4) | 0.13 |
| Excess basis, mmol/L | 0.35 (−2.3 to 2.5) | −0.5 (−2.2 to 2.2) | 0.66 |
| ALT, μ/L | 36.5 (26 to 45) | 42 (28 to 48) | 0.52 |
| AST, μ/L | 28 (23 to 33) | 30.5 (24 to 34.5) | 0.3 |
| GGT, μ/L | 25 (15 to 49) | 35 (20 to 48) | 0.79 |
| ALP, μ/L | 90 (64 to 115) | 85 (65 to 105.5) | 0.89 |
| Creatinine, μmol/L | 12.5 (7 to 20) | 14 (8 to 20) | 0.9 |
| Hemoglobin, g/dL | 13.25 (12.6 to 14.2) | 13.75 (13.1 to 14.5) | 0.29 |
| Lymphocytes/mm3 | 1780 (1190 to 2040) | 1845 (1405 to 2135) | 0.85 |
| Albuminemia, g/L | 39 (37 to 42) | 40 (37 to 42) | 0.99 |
| Total bilirubin, μmol/L | 8 (7 to 13) | 8 (6 to 14) | 0.82 |
Data expressed as median (IQR) or N (%). ALP indicates alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; GGT, gamma‐glutamyl transpeptidase; and RV, right ventricular.
Table 4.
Comparison of Echocardiographic Parameters Between Patients With and Without RV Systolic Dysfunction
| Parameters | Normal RV function (n=44) | RV dysfunction (n=46) | P value |
|---|---|---|---|
| LV ejection fraction, % | 50 (41.5–60) | 48.5 (40–55) | 0.69 |
| LVEDD, mm/m2 | 30.37 (24–35.8) | 30.07 (26.3–36.3) | 0.99 |
| IVS, mm | 7(6–7) | 7 (6–8) | 0.5 |
| PW, mm | 8 (6.5–9) | 7 (7–8) | 0.99 |
| Mitral E/A ratio | 1.9 (1.3–2.6) | 1.64 (1.4–2.1) | 0.69 |
| LA diameter, mm | 18.5 (16.5–21) | 20.5 (14–24) | 0.7 |
| Peak e′ RV, cm/s | 13 (10–15) | 11.5 (10–14) | 0.19 |
| Peak a′ RV, cm/s | 10 (9.5–13) | 9.5 (5–12) | 0.43 |
| Peak s′ septal, cm/s | 10 (8–11) | 8 (6–9) | 0.008 |
| Peak e′ septal, cm/s | 10 (9–12) | 9 (8–11) | 0.22 |
| Peak a′ septal, cm/s | 9.5 (6–10) | 8 (5.5–9.5) | 0.26 |
| TAPSE, mm | 20 (18–23) | 14 (12–15) | <0.001 |
| DTI RV s′ velocity, cm/s | 13 (11–15) | 10.5 (8.5–13) | 0.04 |
| Systolic pulmonary arterial pressure, mm Hg | 24.5 (20–28) | 24 (19–26) | 0.96 |
Data expressed as median (IQR). DTI indicates Doppler tissue imaging; IVS, interventricular septum end‐diastolic thickness; LA, left atrial; LVEDD, left ventricular end diastolic diameter; LVEDDi, indexed left ventricular end diastolic diameter; LV, left ventricular; PW, posterior wall end‐diastolic thickness; RV, right ventricular; and TAPSE, tricuspid annular plane systolic displacement.
Left and Right Ventricular Systolic Outcomes
We did not find any significant difference regarding the onset of new LV dysfunction in patients with RV dysfunction versus patients without RV dysfunction. In patients with previous RV dysfunction, the follow‐up LV ejection fraction was 45% (interquartile range, 35–56) versus 48% (interquartile range, 45–55) (P=0.26). However, in patients without RV dysfunction at inclusion (n=44), an LV ejection fraction <50% at inclusion was associated with more risk of developing RV dysfunction during follow‐up (12/20 [60.0%] versus 6/24 patients [25%]; odds ratio, 4.5 [95% CI, 1.28–14.4]; P=0.03).
Impact of RV Dysfunction on Clinical Events
Cardiac events occurred in 13 patients (14.4%). Cardiac events were distributed as follows: 8 cases of acute heart failure (62%), 3 cases of cardiogenic shock (23%), 2 cases of supraventricular arrhythmia (1 atrial fibrillation and 1 atrial flutter) (15%). Eleven events (23.9%) occurred in patients with RV systolic dysfunction versus 2 events (4.5%) in patients without RV dysfunction (P=0.008). Table 5 summarizes cardiac events in patients with RV dysfunction versus patients without RV systolic dysfunction. RV dysfunction was significantly associated with cardiac events (Figure 1). Three variables were put into the model due to clinical and statistical significance: LV ejection fraction, RV dysfunction and home mechanical ventilation 24 hours/24 hours. In the multivariable Cox model, for RV dysfunction, the adjusted hazard ratio (aHR) was 4.96 (95% CI, 1.09–22.6; P=0.04) and for LV ejection fraction, aHR for each percentage difference compared with reference, 0.93 (95% CI, 0.88–0.97; P=0.003).
Table 5.
Cardiac Events in Patients With RV Dysfunction Versus Patients Without RV Systolic Dysfunction
| Cardiac events | RVD group (n=46) | No RVD group (n=44) |
|---|---|---|
| AHF | 7 (15) | 1 (2.3) |
| Cardiogenic shock | 3 (6.5) | 0 (0) |
| Supraventricular arrythmia | 1 (2.2) | 1 (2.3) |
Data expressed as N (%). AHF indicates acute heart failure; RV, right ventricular; and RVD, right ventricular systolic dysfunction.
Figure 1. Long‐term cardiac events in patients with DMD with RVD or without RVD.
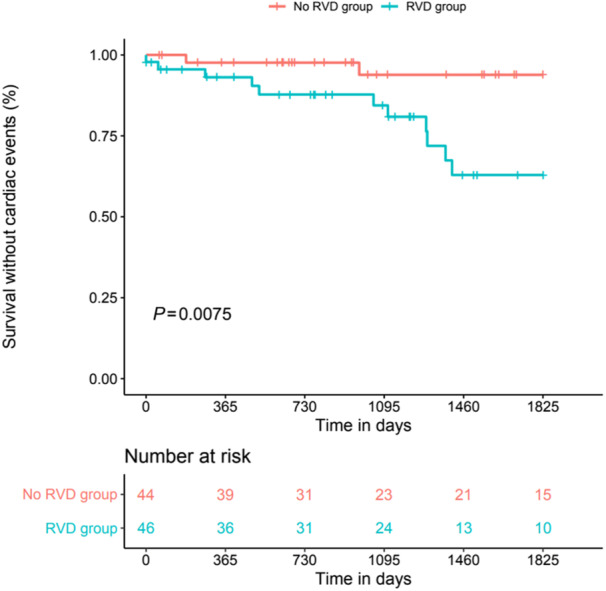
DMD indicates Duchenne muscular dystrophy; and RVD, right ventricular systolic dysfunction.
Death occurred in 7 patients (7.8%). Most of the patients died from cardiogenic shock and advanced heart failure (n=5). All the patients with DMD who experienced the onset of cardiogenic shock died during follow‐up. No ventricular arrhythmia was documented. Six patients died in the group with RV systolic dysfunction (13%) versus only 1 patient in the group without RV dysfunction (2.3%). There was a significant difference between the 2 groups in the Kaplan–Meier curves (log‐rank P=0.045; Figure 2); however, this difference did not translate in Cox survival analysis (P=0.08).
Figure 2. Long‐term survival in patients with DMD with RVD or without RVD.
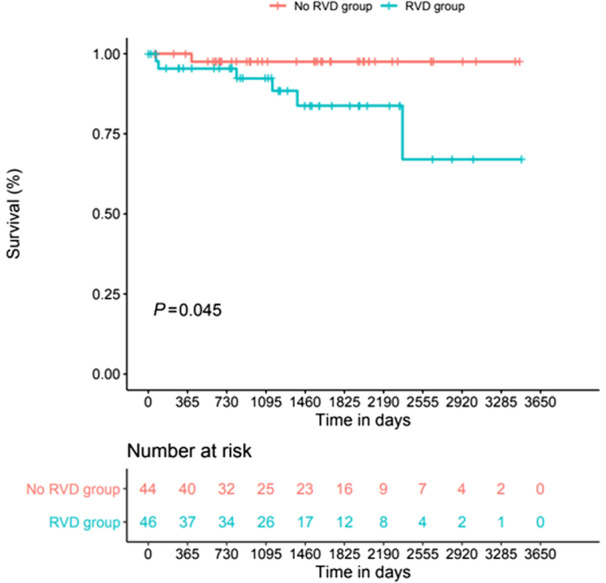
DMD indicates Duchenne muscular dystrophy; and RVD, right ventricular systolic dysfunction.
Discussion
This study highlighted 3 main findings. First, the prevalence of RV systolic dysfunction is high, reaching 51% in adults with DMD. Second, this RV systolic dysfunction is associated with increased risk of cardiac events independently of LV dysfunction. Third, patients with DMD with LV systolic dysfunction are at risk of developing RV dysfunction.
Our data are in agreement with the observations by Melacini et al, 18 reporting RV systolic impairment in 52% of patients with Becker muscular dystrophy. On the other hand, preserved RV systolic function has been described in pediatric DMD populations. 19 In a study that included patients with DMD aged 15.5 years and with nearly preserved pulmonary function, an association between RV ejection fraction and vital capacity was found. 9 In our study that included patients with DMD with severe respiratory dysfunction, we did not find this association, probably because of the respiratory severity of the patients and the fact that the majority of the patients were on home mechanical ventilation.
RV dysfunction in DMD may be due to multifactorial parameters. The right ventricle may be intrinsically affected, due to dystrophin deficiency, since the absence of dystrophin can cause myocardial damage via a disruption of the dytrophin–glycoprotein complex. 20 Pathophysiological aspects involved in the cardiomyopathy of patients with DMD are complex. Calcium dysregulation, due to excessive calcium influx through activation of plasma membrane channels is known to play a key role with a high activity of the ryanodine receptor 21 as well as inflammation. Recently, we found that a nicotinamide adenine dinucleotide defect is a major feature in this disease and that it is involved in the pathophysiology of cardiac disease in DMD. 22
Mechanical ventilation affects the RV because it causes a decrease in preload but may increase the RV afterload. 23 Conversely, a hypoventilated state can induce an increase in pulmonary vascular resistance, related to acidosis and hypercapnia. 23 The RV afterload may be increased because of hypoxemia, since hypoxemia can induce pulmonary vasoconstriction. 24 Similarly, RV afterload may be increased because of a higher positive end‐expiratory pressure, a situation known in critical care patients. 8 However, in our group of patients with DMD on home mechanical ventilation, positive end‐expiratory pressure level was not elevated, with a median at 2 cmH2O, and RV afterload was not high, attested by a median systolic artery pulmonary pressure at 24 mm Hg. Tidal volume is known to affect RV after loading in a cyclic manner in ventilated patients. 25 Even if we did not find any significant relationship between tidal volume and RV dysfunction in our study, we cannot exclude a long‐term deleterious impact of the cyclic increase of RV impedance with tidal volume in this group of patients with DMD. In addition, skeletal muscle status may affect RV loading because RV volume is increased in athletes, 26 , 27 whereas RV end‐diastolic volume is reduced in patients with DMD. 9 Finally, the splanchnic venous system affects venous return and hemodynamics, depending on the inspiratory descent of the diaphragm. 28 Since diaphragm dysfunction is frequent in patients with DMD, the impact of the vascular zone condition of the abdomen may contribute to RV preload.
The absence of elevated pulmonary pressure in these patients with a severe restrictive lung pattern is related to continuous mechanical ventilation that decreases hypercapnia and LV diastolic pressures. 29 Furthermore, in our study, patients were properly ventilated without nocturnal oxygen desaturation and without daytime hypercapnia.
RV function depends on preload, afterload, RV contractility, interaction with the left ventricle, pericardial state, and intrathoracic pressure. 30 RV dysfunction may be related to RV pressure overload, or RV volume overload. In our study, no patients disclosed significant tricuspid regurgitation that excluded volume overload. Conversely to spontaneous ventilation that decreases intrathoracic pressure, mechanical ventilation increases intrathoracic pressure and right atrial pressure. 31 This phenomenon affects venous return, which is a key determinant of cardiac output. Tidal volume ventilation is known to increase RV afterload. 32 In the context of cardiomyopathy related to absence of dystrophin, this phenomenon may worsen cardiac function and may predispose the patient with DMD to the onset of reduced cardiac output and cardiogenic shock in the long term. Our study highlights the importance of the LV function. In fact, the presence of previous LV dysfunction in DMD exposes the patient to the onset of RV dysfunction with an odds ratio of 4.5 (P=0.03). This finding emphasizes the interplay between left ventricle and right ventricle, since the 2 cavities share oblique fibers within the interventricular septum. 33 The LV systolic function is known to contribute to the RV systolic function via the systolic ventricular interaction. 33
Our results regarding the impact on death and prognosis in patients with DMD are similar to findings reported in other cardiomyopathies, 34 which emphasize the clinical importance of diagnosing RV systolic dysfunction in patients with DMD. In our study, death was mainly related to advanced heart failure and cardiogenic shock in patients with RV systolic dysfunction. This underscores the importance of RV echocardiography analysis in term of searching for RV systolic impairment in patients with DMD, particularly in patients with LV systolic dysfunction. In the case of RV systolic dysfunction onset, closer cardiac monitoring should be mandatory and optimal therapy addressed.
Limitations
The main limitation of our single‐center study is its limited number of patients and number of events. However, this disease is particularly uncommon, and patients were referred in our reference center with a definitive diagnosis of DMD. A second limitation is the echocardiographic evaluation of the RV function in these patients. In our echocardiography laboratory, for the RV function analysis, we analyzed at least 1 of the following parameters: TAPSE, RV peak systolic myocardial velocity, RV fractional area change, according to guidelines. 35 RV fractional area change may be difficult to obtain in patients with DMD because of wheelchair‐bound status and chest deformities. In contrast, measurement of RV peak systolic myocardial velocity is easy to perform with tissue Doppler imaging as well as the TAPSE. Since we mostly used simplified criteria for the diagnosis of RV systolic dysfunction such as TAPSE and tissue Doppler imaging RV s′ velocity. A cardiac magnetic resonance study focusing on the right ventricle could be of interest and more accurate, but a systematic cardiac investigation by cardiac magnetic resonance is difficult in patients with DMD and severe respiratory failure requiring permanent mechanical ventilation. Finally, inferior vena caval data are lacking in this study, and we did not have data regarding the venous pressure that may hamper the relevance of the pulmonary pressure.
Conclusions
The coexistence of LV systolic dysfunction and restrictive pulmonary failure is well described in patients with DMD. In the present study, we clearly demonstrated that RV systolic impairment is also present in half of patients with DMD and that this feature is associated with increased risk of cardiac events. DMD patients with LV systolic dysfunction are at risk of developing RV systolic dysfunction.
Sources of Funding
None.
Disclosures
None.
Acknowledgments
Study design and concept: Drs Fayssoil, Mansencal, Nardi, Yaou, Dubourg, Stojkovic, and Orlikowski; data collection: Drs Fayssoil, Nardi, and Yaou; imaging: Dr Fayssoil; statistical analysis: Dr Nguyen; drafting and revision of the manuscript: Drs Fayssoil, Mansencal, Nardi, Nguyen, Yaou, Lofaso, Leturcq, Amthor, Wahbi, Becane, Prigent, Bassez, Behin, Stojkovic, Fontaine, Duboc, Dubourg, Annane, Clair, Laforet, and Orlikowski.
This manuscript was sent to Mark W. Russell, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Cho MJ, Lee JW, Lee J, Shin YB. Evaluation of early left ventricular dysfunction in patients with Duchenne muscular dystrophy using two‐dimensional speckle tracking echocardiography and tissue Doppler imaging. Pediatr Cardiol. 2018;39:1614–1619. doi: 10.1007/s00246-018-1938-0 [DOI] [PubMed] [Google Scholar]
- 2. Nikhanj A, Yogasundaram H, Miskew Nichols B, Richman‐Eisenstat J, Phan C, Bakal JA, Siddiqi ZA, Oudit GY. Cardiac intervention improves heart disease and clinical outcomes in patients with muscular dystrophy in a multidisciplinary care setting. J Am Heart Assoc. 2020;9:e014004. doi: 10.1161/JAHA.119.014004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Porcher R, Desguerre I, Amthor H, Chabrol B, Audic F, Rivier F, Isapof A, Tiffreau V, Campana‐Salort E, Leturcq F, et al. Association between prophylactic angiotensin‐converting enzyme inhibitors and overall survival in Duchenne muscular dystrophy‐analysis of registry data. Eur Heart J. 2021;42:1976–1984. doi: 10.1093/eurheartj/ehab054 [DOI] [PubMed] [Google Scholar]
- 4. Landfeldt E, Thompson R, Sejersen T, McMillan HJ, Kirschner J, Lochmüller H. Life expectancy at birth in Duchenne muscular dystrophy: a systematic review and meta‐analysis. Eur J Epidemiol. 2020;35:643–653. doi: 10.1007/s10654-020-00613-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dandel M, Hetzer R. Echocardiographic assessment of the right ventricle: impact of the distinctly load dependency of its size, geometry and performance. Int J Cardiol. 2016;221:1132–1142. doi: 10.1016/j.ijcard.2016.07.014 [DOI] [PubMed] [Google Scholar]
- 6. Madden JA, Dawson CA, Harder DR. Hypoxia‐induced activation in small isolated pulmonary arteries from the cat. J Appl Physiol (1985). 1985;59:113–118. doi: 10.1152/jappl.1985.59.1.113 [DOI] [PubMed] [Google Scholar]
- 7. Hilde JM, Skjørten I, Grøtta OJ, Hansteen V, Melsom MN, Hisdal J, Humerfelt S, Steine K. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J Am Coll Cardiol. 2013;62:1103–1111. doi: 10.1016/j.jacc.2013.04.091 [DOI] [PubMed] [Google Scholar]
- 8. Mekontso Dessap A, Charron C, Devaquet J, Aboab J, Jardin F, Brochard L, Vieillard‐Baron A. Impact of acute hypercapnia and augmented positive end‐expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med. 2009;35:1850–1858. doi: 10.1007/s00134-009-1569-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehmood M, Ambach SA, Taylor MD, Jefferies JL, Raman SV, Taylor RJ, Sawani H, Mathew J, Mazur W, Hor KN, et al. Relationship of right ventricular size and function with respiratory status in Duchenne muscular dystrophy. Pediatr Cardiol. 2016;37:878–883. doi: 10.1007/s00246-016-1362-2 [DOI] [PubMed] [Google Scholar]
- 10. Tuffery‐Giraud S, Béroud C, Leturcq F, Yaou RB, Hamroun D, Michel‐Calemard L, Moizard MP, Bernard R, Cossée M, Boisseau P, et al. Genotype‐phenotype analysis in 2405 patients with a dystrophinopathy using the UMD‐DMD database: a model of nationwide knowledgebase. Hum Mutat. 2009;30:934–945. doi: 10.1002/humu.20976 [DOI] [PubMed] [Google Scholar]
- 11. Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (ACC/AHA/ASE Committee to update the 1997 guidelines for the clinical application of echocardiography). Circulation. 2003;108:1146–1162. doi: 10.1161/01.CIR.0000073597.57414.A9 [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 13. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4–131. doi: 10.1002/ejhf.2333 [DOI] [PubMed] [Google Scholar]
- 14. Tello K, Wan J, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, Roller F, Mohajerani E, Seeger W, Herberg U, et al. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular‐arterial coupling in severe pulmonary hypertension. Circ Cardiovasc Imaging. 2019;12:e009047. doi: 10.1161/CIRCIMAGING.119.009047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 16. Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12:926–929. doi: 10.1016/S0960-8966(02)00140-2 [DOI] [PubMed] [Google Scholar]
- 17. Ogna A, Nardi J, Prigent H, Quera Salva MA, Chaffaut C, Lamothe L, Chevret S, Annane D, Orlikowski D, Lofaso F. Prognostic value of initial assessment of residual hypoventilation using nocturnal capnography in mechanically ventilated neuromuscular patients: a 5‐year follow‐up study. Front Med (Lausanne). 2016;3:40. doi: 10.3389/fmed.2016.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Melacini P, Fanin M, Danieli GA, Fasoli G, Villanova C, Angelini C, Vitiello L, Miorelli M, Buja GF, Mostacciuolo ML, et al. Cardiac involvement in Becker muscular dystrophy. J Am Coll Cardiol. 1993;22:1927–1934. doi: 10.1016/0735-1097(93)90781-U [DOI] [PubMed] [Google Scholar]
- 19. Amedro P, Vincenti M, De La Villeon G, Lavastre K, Barrea C, Guillaumont S, Bredy C, Gamon L, Meli AC, Cazorla O, et al. Speckle‐tracking echocardiography in children with Duchenne muscular dystrophy: a prospective multicenter controlled cross‐sectional study. J Am Soc Echocardiogr. 2019;32:412–422. doi: 10.1016/j.echo.2018.10.017 [DOI] [PubMed] [Google Scholar]
- 20. Oreto L, Vita GL, Mandraffino G, Carerj S, Calabrò MP, Manganaro R, Cusmà‐Piccione M, Todaro MC, Sframeli M, Cinquegrani M, et al. Impaired myocardial strain in early stage of Duchenne muscular dystrophy: its relation with age and motor performance. Acta Myol. 2020;39:191–199. doi: 10.36185/2532-1900-022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fauconnier J, Thireau J, Reiken S, Cassan C, Richard S, Matecki S, Marks AR, Lacampagne A. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2010;107:1559–1564. doi: 10.1073/pnas.0908540107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Zélicourt A, Fayssoil A, Dakouane‐Giudicelli M, De Jesus I, Karoui A, Zarrouki F, Lefebvre F, Mansart A, Launay JM, Piquereau J, et al. CD38‐NADase is a new major contributor to Duchenne muscular dystrophic phenotype. EMBO Mol Med. 2022;14:e12860. doi: 10.15252/emmm.202012860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cecconi M, Johnston E, Rhodes A. What role does the right side of the heart play in circulation? Crit Care. 2006;10(Suppl 3):S5. doi: 10.1186/cc4832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marshall BE, Marshall C, Frasch F, Hanson CW. Role of hypoxic pulmonary vasoconstriction in pulmonary gas exchange and blood flow distribution. 1. Physiologic concepts. Intensive Care Med. 1994;20:291–297. doi: 10.1007/BF01708968 [DOI] [PubMed] [Google Scholar]
- 25. Vieillard‐Baron A, Loubieres Y, Schmitt JM, Page B, Dubourg O, Jardin F. Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol (1985). 1999;87:1644–1650. doi: 10.1152/jappl.1999.87.5.1644 [DOI] [PubMed] [Google Scholar]
- 26. Forsythe L, Somauroo J, George K, Papadakis M, Brown B, Qasem M, Oxborough D. The right heart of the elite senior rugby football league athlete. Echocardiography. 2019;36:888–896. doi: 10.1111/echo.14330 [DOI] [PubMed] [Google Scholar]
- 27. Bohm P, Schneider G, Linneweber L, Rentzsch A, Krämer N, Abdul‐Khaliq H, Kindermann W, Meyer T, Scharhag J. Right and left ventricular function and mass in male elite master athletes: a controlled contrast‐enhanced cardiovascular magnetic resonance study. Circulation. 2016;133:1927–1935. doi: 10.1161/CIRCULATIONAHA.115.020975 [DOI] [PubMed] [Google Scholar]
- 28. Takata M, Wise RA, Robotham JL. Effects of abdominal pressure on venous return: abdominal vascular zone conditions. J Appl Physiol (1985). 1990;69:1961–1972. doi: 10.1152/jappl.1990.69.6.1961 [DOI] [PubMed] [Google Scholar]
- 29. Shivkumar K, Ravi K, Henry JW, Eichenhorn M, Stein PD. Right ventricular dilatation, right ventricular wall thickening, and Doppler evidence of pulmonary hypertension in patients with a pure restrictive ventilatory impairment. Chest. 1994;106:1649–1653. doi: 10.1378/chest.106.6.1649 [DOI] [PubMed] [Google Scholar]
- 30. Harjola VP, Mebazaa A, Čelutkienė J, Bettex D, Bueno H, Chioncel O, Crespo‐Leiro MG, Falk V, Filippatos G, Gibbs S, et al. Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail. 2016;18:226–241. doi: 10.1002/ejhf.478 [DOI] [PubMed] [Google Scholar]
- 31. Pinsky MR. Cardiopulmonary interactions: physiologic basis and clinical applications. Ann Am Thorac Soc. 2018;15:S45–S48. doi: 10.1513/AnnalsATS.201704-339FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jardin F, Delorme G, Hardy A, Auvert B, Beauchet A, Bourdarias JP. Reevaluation of hemodynamic consequences of positive pressure ventilation: emphasis on cyclic right ventricular afterloading by mechanical lung inflation. Anesthesiology. 1990;72:966–970. doi: 10.1097/00000542-199006000-00003 [DOI] [PubMed] [Google Scholar]
- 33. Schwarz K, Singh S, Dawson D, Frenneaux MP. Right ventricular function in left ventricular disease: pathophysiology and implications. Heart Lung Circ. 2013;22:507–511. doi: 10.1016/j.hlc.2013.03.072 [DOI] [PubMed] [Google Scholar]
- 34. Becker MAJ, van der Lingen ACJ, Wubben M, van de Ven PM, van Rossum AC, Cornel JH, Allaart CP, Germans T. Characteristics and prognostic value of right ventricular (dys)function in patients with non‐ischaemic dilated cardiomyopathy assessed with cardiac magnetic resonance imaging. ESC Heart Fail. 2021;8:1055–1063. doi: 10.1002/ehf2.13072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713;quiz 786–8. doi: 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]


