ABSTRACT
Cardiogenic shock is characterized by tissue hypoxia caused by circulatory failure arising from inadequate cardiac output. In addition to treating the pathologic process causing impaired cardiac function, prompt hemodynamic support is essential to reduce the risk of developing multiorgan dysfunction and to preserve cellular metabolism. Pharmacologic therapy with the use of vasopressors and inotropes is a key component of this treatment strategy, improving perfusion by increasing cardiac output, altering systemic vascular resistance, or both, while allowing time and hemodynamic stability to treat the underlying disease process implicated in the development of cardiogenic shock. Despite the use of mechanical circulatory support recently garnering significant interest, pharmacologic hemodynamic support remains a cornerstone of cardiogenic shock management, with over 90% of patients receiving at least 1 vasoactive agent. This review aims to describe the pharmacology and hemodynamic effects of current pharmacotherapies and provide a practical approach to their use, while highlighting important future research directions.
Keywords: cardiogenic, inotrope, mechanical circulatory support, shock, shock, vasopressor
Subject Categories: Myocardial Infarction, Cardiomyopathy, Cardiopulmonary Resuscitation and Emergency Cardiac Care
Nonstandard Abbreviations and Acronyms
- AR
adrenergic receptor
- CS
cardiogenic shock
- MCS
mechanical circulatory support
Cardiogenic shock (CS) is a clinical syndrome characterized by insufficient cardiac output to meet basal metabolic requirements, leading to life‐threatening end‐organ hypoperfusion. 1 The development of CS has downstream effects on the entire circulation, causing tissue hypoxia and injury, inflammation, and vasoplegia as part of a systemic inflammatory response syndrome in many cases. Physiologic compensatory mechanisms include endogenous sympathetic stimulation which augments cardiac output by increasing heart rate and myocardial contractility. 2 In addition to the direct cardiac effects produced by these endogenous compounds, peripheral vasoconstriction serves to increase systemic vascular resistance and mean arterial pressure (MAP). These compensatory responses occur at the expense of maladaptive increases in cardiac afterload, myocardial oxygen requirements, and filling pressures, with resulting reductions in coronary perfusion pressure. 2 Therefore, prompt hemodynamic support is essential to restore cellular metabolism and prevent worsening systemic and myocardial ischemia, which drives the “shock spiral” that in many cases leads to circulatory collapse and death. 1
The initial goals of therapy for patients with CS can broadly be defined by 2 overarching principles: first, to rapidly identify and treat the underlying cause of shock (for example, urgent revascularization in acute myocardial infarction related cardiogenic shock [AMI‐CS]) to enable cardiac recovery 3 ; second, to improve tissue perfusion and oxygenation through obtaining a minimum acceptable cardiac output and blood pressure, achieved through hemodynamic support strategies that may include the use of vasoactive medications and in select cases temporary mechanical circulatory support (MCS). The current review focuses on the pharmacologic hemodynamic supports that can be offered in this clinical context.
Epidemiology of Cardiogenic Shock 4
Defining CS has traditionally been challenging, owing to the various clinical, biochemical, and hemodynamic definitions used in the seminal outcome trials and societal guidelines. 5 The Society for Cardiovascular Angiography and Interventions has recently sought to harmonize these definitions through a pragmatic classification system that can be applied to a range of clinical setting and helps enable the diagnosis of CS across the full clinical spectrum of disease severity, ranging from “at risk” to fulminant circulatory collapse. 6 , 7 Importantly, the Society for Cardiovascular Angiography and Interventions diagnostic and staging system of CS has been validated across multiple clinical subgroups, including CS with and without AMI, patients admitted to intensive care, and those with CS complicating out‐of‐hospital cardiac arrest. 7 The use of a uniform definition for CS and its severity will play a crucial role for future epidemiologic and clinical trials, allowing direct comparison between outcomes associated with specific therapies, systems of care, and treatment protocols. 7
Despite the challenges with defining and diagnosing CS, it is a common problem in clinical practice with an estimated incidence of 408 per 100 000 hospitalizations based on the United States National Inpatient Sample. 8 Acute coronary syndromes are the most common cause of CS, accounting for 70% of cases. 9 , 10 The management of this cohort of patients is both costly and requires significant health care resource use. The average length of hospital stay ranges between 8.9 and 18.6 days with an associated cost of treating a patient with AMI‐CS in the United States of $41 774±$45252. 11 , 12 Despite significant improvements over the past 2 decades in contemporary revascularization techniques and supportive care, 1‐year mortality rates continue to range between 50% and 60%. 13
Although the epidemiology and outcomes of in‐hospital CS have been well described, there remains a paucity of data in relation to the incidence, treatment provision, and outcomes of CS in the prehospital environment. A recent population‐based cohort study of a large provincial emergency medical services registry demonstrated that the overall incidence of emergency medical services treated CS was 14.5 per 100 000 person‐years, with an overall 30‐day all‐cause mortality of 43.9%. 14 , 15 Despite large numbers of patients with CS receiving the initial phase of their treatment by emergency medical services, prehospital therapeutic interventions (eg, mechanical ventilation, and vasoactive medications), in addition to many in‐hospital interventions, are yet to be proven effective through high‐quality observational or randomized data. 16
Current Use of Vasoactive Medications in Clinical Practice
The use of vasoactive medications in CS is common. In the intensive care unit (ICU) setting, ≈25% of admitted patients receive at least 1 vasoactive medication, increasing to >90% in patients with CS. 17 , 18 Although comparative studies assessing these agents are limited, norepinephrine is increasingly administered for patients requiring hemodynamic support with CS. 18 , 19 The requirement for vasoactive medications is independently associated with short‐term mortality and a stepwise increase in risk of in‐hospital mortality has been observed with increasing number of vasoactive agents administered. 18 , 20 , 21 , 22 Furthermore, a dose‐dependent relationship with higher required peak doses to achieve hemodynamic stability is also associated with an increased risk of death. 18 Although the observed excess mortality risk associated with the use of this drug class is of concern, these findings are likely to be confounded by increased illness severity necessitating the use of multiple agents at high doses. Nonetheless, these data underscore the need for further evaluation of the utility of these commonly used medications, compared with alternate vasoactive sparing strategies such as MCS.
Cardiovascular Pharmacology and Pharmacodynamics of Vasoactive Medications
The study of the physiologic properties of vasoactive medications dates back to 1893, when the English physician George Oliver assessed the effects of various glandular extracts derived from sheep on radial artery vasoreactivity in his son. 23 Evolving from these early experiments, the contemporary conceptual framework for these agents was established and is broadly divided into 3 categories in accordance with their predominant hemodynamic effects: vasopressors, inotropes, and inodilators. Vasopressors improve perfusion to vital organs by increasing systemic vascular resistance and therefore MAP. 24 Inotropes augment cardiac output by increasing myocardial contractility and in many instances heart rate. Inodilators have the unique mixed effects of inotropy and arterial vasodilation. A summary of the commonly used vasoactive agents is presented in Table 1.
Table 1.
Common Vasoactive Medications, Indication for Clinical Use, Adverse Effects, and Receptor Affinity
| Agent | Indication | Side effects | β1 affinity | β2 affinity | α1 affinity |
|---|---|---|---|---|---|
| Catecholamines | |||||
| Epinephrine | CS with hypotension, vasoplegic shock, bradycardia, anaphylaxis | Hypertension, skin necrosis with extravasation, digital ischemia, tachycardia, myocardial ischemia, and arrhythmias | ++++ | +++ | +++ |
| Norepinephrine | CS with hypotension, vasoplegic shock | Hypertension, skin necrosis with extravasation, digital ischemia, arrhythmias | +++ | + | +++++ |
| Dobutamine | CS with preserved blood pressure (decompensated heart failure, low cardiac output state) | Tachycardia, myocardial ischemia, ventricular and atrial arrhythmias | ++++ | +++ | + |
| Metaraminol | Hypotension (vagally mediated), hypotension with severe aortic stenosis or obstructive hypertrophic cardiomyopathy | Reflex bradycardia, hypertension | Nil | Nil | +++++ |
| Phenylephrine | Hypotension (vagally mediated), hypotension with severe aortic stenosis or obstructive hypertrophic cardiomyopathy | Reflex bradycardia, hypertension | Nil | Nil | +++++ |
| Phosphodiesterase inhibitor | |||||
| Milrinone | CS with preserved blood pressure (decompensated heart failure, low cardiac output state), CS receiving chronic beta blocker therapy, and postcardiotomy shock | Hypotension, ventricular arrhythmia, drug accumulation in renal failure, myocardial ischemia | Inhibits phosphodiesterase 3‐mediated hydrolysis of cAMP, resulting in inotropy and vasodilation | ||
| Calcium sensitizer | |||||
| Levosimendan | CS with preserved blood pressure (decompensated heart failure, low cardiac output state) | Hypotension, tachycardia | Enhances calcium‐dependent troponin C binding and vascular smooth muscle potassium channel activation | ||
| Other | |||||
| Vasopressin | Refractory vasoplegic shock | Hypertension, myocardial ischemia (secondary to coronary spasm), skin ischemia, arrhythmias |
V1a receptor‐mediated vascular smooth muscle vasoconstriction V2 receptor‐mediated water retention |
||
| Methylene blue | Refractory vasoplegic shock | Hypertension, serotonin syndrome, hemolytic anemia (in those with glucose‐6‐phosphate dehydrogenase deficiency) | Inhibition of nitric oxide mediated of cGMP production, resulting in increased smooth muscle mediated vasocontriction | ||
+ through +++++, minimal to maximal relative receptor affinity; cAMP indicates cyclic adenosine monophosphate; and CS, cardiogenic shock.
Impact on Hemodynamic Parameters and Cardiac Performance
Conventional descriptions of the agonist‐receptor‐effector interactions promulgated in traditional pharmacology teaching is primarily based on small studies from the 1950s to 1960s, using variable drug doses and indirect surrogate measurements of in‐vivo receptor activity and downstream effects. 25 , 26 , 27 In these early mechanistic studies, the observed physiologic effects of catecholamine administration (in healthy subjects) on blood pressure, total peripheral resistance, and heart rate were used to extrapolate specific agonist‐receptor‐effector biology. However, it is becoming increasingly evident that there is a far more dynamic and nuanced receptor‐agonist interplay than previously appreciated. 28
This complex interaction has been assessed in pharmacodynamic studies in a sheep model where comparable doses of catecholamines (epinephrine, norepinphrine, and dopamine) were administered, and their doses titrated. Of note, all 3 drugs significantly and equally increased cardiac output, MAP, and right atrial pressure in a dose‐dependent fashion. 29 Similar observations have been made in clinical studies of CS, with the inotrope epinephrine being compared with the vasopressor noradrenaline and producing similar hemodynamic effects. 30 , 31 These findings suggest that the arbitrary vasopressor and inotrope definitions for catecholamines, in particular when the term “vasopressor” is used to describe predominantly peripheral arterial vasoconstrictive properties, do not necessarily reflect the true in vivo effects of these agents.
Myocardial Oxygen Consumption
Although the overarching purpose of vasopressors and inotropes in CS is to improve tissue oxygen delivery, these agents also increase myocardial oxygen demand, which in the setting of a cardiomyocyte oxygen supply–demand mismatch can cause further injury and contractile dysfunction. The energetic requirements of the myocardium is primarily met through oxidative metabolic pathways with <5% of ATP derived from glycolytic pathways. 32 Basal metabolic requirements account for 10% to 20% of the total myocardial oxygen requirements, with the remaining oxygen needs determined by variables that include heart rate, contractility, and systolic wall tension. 32 The relative contribution of these variables has been characterized through human and animal models, primarily using exercise to induce increased cardiac work. From these studies, heart rate has been shown to be the greatest contributor to myocardial oxygen consumption. Through augmentation with pacing, it was found that 30% to 40% of the heart's metabolic needs were secondary to heart rate variation. 32 However, this is likely an underestimate of the true impact of heart rate, due to pacing mediated reductions in end‐diastolic and stroke volumes, with some estimates of heart rate variation accounting for up to 50% to 70% of myocardial oxygen demand. 32 , 33 The contribution of contractility has been elegantly assessed through several animal and human studies, showing that increased myocardial contractility under physiological stress (exercise) accounts for 15% to 25% of the oxygen demand. 32 In addition to factors that influence myocardial oxygen requirements, supply is principally governed by the coronary perfusion pressure gradient, perfusion time (which occurs predominantly during diastole and is reduced with increased heart rate), and coronary artery vasomotor tone. 34 Therefore, the interplay between hemodynamic supports, cardiac filling pressures, heart rate variation, and coronary vasomotor tone has the potential to adversely influence myocardial oxygen supply–demand mismatch in the setting of CS.
Commonly Used Vasoactive Drug Classes: Pharmacology and Physiology
Catecholamines
The most commonly administered class of vasopressor and inotropic medications in the critical care setting are the sympathetic amines. 18 These agents produce their physiologic effects through the stimulation of the following receptors; alpha‐adrenergic (α1), beta‐adrenergic (β1 and β2), and dopamine (D1) receptors. 35 A comparison of the α‐ and β‐adrenergic receptor (AR) pharmacology on vascular smooth muscle and cardiac tissue is presented in Table 2 and the intracellular signaling effects of catecholamines, in addition to other vasoactive agents, are presented in Figures 1 and 2.
Table 2.
Comparative Receptor Pharmacology and Physiologic Effects of Stimulation of β‐ and α1‐Adrenergic Receptors
| β–adrenergic receptor | α1–adrenergic receptor | |
|---|---|---|
| Pharmacology | ||
| Agonists | Isoproterenol> dobutamine> epinephrine> norepinephrine> dopamine (relative receptor affinity) | Norepinephrine> epinephrine> dobutamine> dopamine (relative receptor affinity) |
| Antagonists | Propranolol (nonselective β1‐AR and β2‐AR), metoprolol (selective β1‐AR) | Prazosin |
| Mechanism of action | ||
| Receptor | GPCR | GPCR |
| Signaling system | ↑ cAMP and protein kinase A | ↑ Inositol 1, 4, 5‐triphosphate (IP3) and diacylglycerol (DAG) |
| Myocardial metabolism | +++ Oxygen consumption through β1‐AR stimulation | +/− Oxygen consumption |
| Cardiac effects | ||
| Electrophysiology | ++ Positive chronotropy (via β1‐AR stimulation) | +/− |
| Myocardial mechanics | ++ Contractility, lusitropy, stroke volume, and cardiac output (via β1‐AR stimulation) | +/− |
| Vascular smooth muscle | ||
| Coronary and peripheral arterioles | Dilatation (via β2‐AR stimulation) | Constriction |
GCPR indicates G‐protein coupled receptor; β1‐AR, beta 1 adrenergic receptor; β2‐AR, beta 2 adrenergic receptor; and cAMP, cyclic adenosine monophosphate.
Figure 1. Graphical representation of the intracellular signaling cascade following activation of the G‐protein coupled β1‐adrenergic receptor in cardiac tissue.
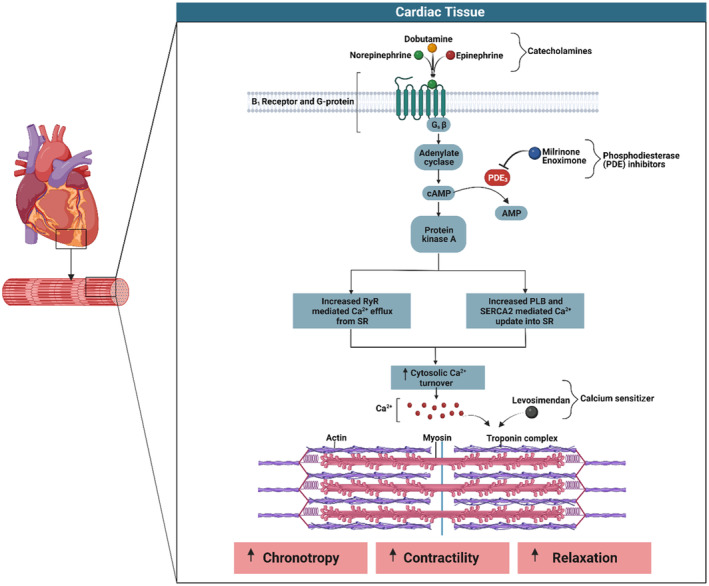
Mediated through the stimulatory (Gs) component of the β1‐adrenergic receptor, activation of the adenyl cyclase pathway occurs with a resulting increase in cAMP (cyclic adenosine monophosphate) and subsequent increased intracellular calcium cycling. The altered calcium handling results in a positive chronotropic response, increased myocardial contractility, and lusitropy. PLB indicates phospholamban; RyR, ryanodine receptor; SERCA, sarco/endoplasmic reticulum Ca2+ ATPase; and SR, sarcoplasmic reticulum.
Figure 2. Intracellular signaling within vascular smooth muscle following the activation of α1‐adrenergic, vasopressin‐1, β2‐adrenergic, and ATP‐sensitive potassium channel receptors.
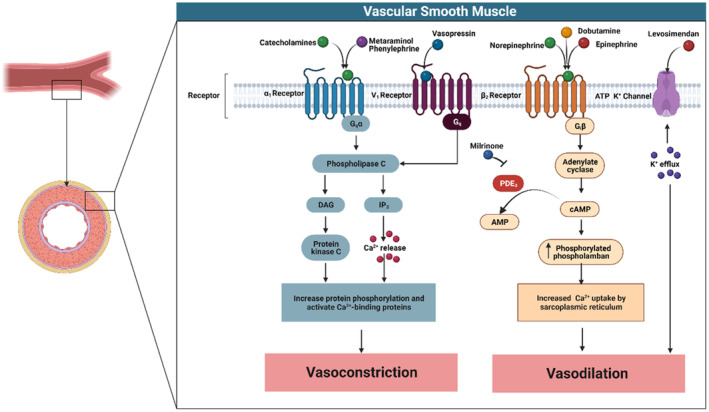
Stimulation of the α1‐adrenergic or vasopressin‐1 receptors causes activation of the Gq (G‐protein) subunit, resulting in downstream stimulation of the phospholipase C signaling pathway. Phospholipase C in turn activates inositol 1, 4, 5‐triphosphate (IP3) leading to Ca2+ release from the sarcoplasmic reticulum (SR), resulting in vasoconstriction. Conversely, β2‐receptor stimulation activates the inhibitory G‐protein (Gi) subunit causing vasodilatation through increased cAMP (cyclic adenosine monophosphase) activation and resulting phospholamban‐mediated Ca2+ uptake into the SR. Similarly, activation of the ATP‐sensitive potassium channel causes potassium influx that hyperpolarizes voltage‐dependent Ca2+ channels, reducing intracellular Ca2+ and vasomotor tone. AMP indicates adenosine monophosphate; DAG, diacylglycerol; PDE3, phosphodiesterase 3; and PLB, phospholamban.
The direct cardiac effects of catecholamines are mediated through the G‐protein–coupled β1‐AR, which comprise 80% of the β‐AR population within the left ventricle. 36 β1‐AR stimulation augments cardiac output through increased cytosolic Ca2+ cycling and phosphorylation of troponin I, leading to increased myocyte contractility, lusitropy (active relaxation), and positive chronotropy. 37 The second body system affected by catecholamines is vascular smooth muscle. The α1‐AR primarily modulates arteriolar smooth muscle tone. 38 Activation of the α1‐AR causes increased cytosolic calcium concentrations resulting in smooth muscle contraction and an accompanying increase in systemic vascular resistance and MAP. 38 Conversely, β2‐AR stimulation activates the inhibitory (Gi) signaling pathways in vascular smooth muscle, causing vasodilatation. 39
Norepinephrine, epinephrine, phenylephrine, and dopamine are potent vasopressors and have comparable effects in increasing MAP. 28 , 40 However, phenylephrine's exclusive α1‐AR agonist activity renders it unable to improve cardiac output and therefore should be avoided as a first‐line agent for hemodynamic support in CS. Unlike phenylephrine, norepinephrine, epinephrine, dobutamine, and dopamine (to a lesser extent) all augment cardiac output through stimulation of the myocyte β1‐AR. However, in contrast with dobutamine, dopamine, and epinephrine, norepinephrine has the capacity to improve cardiac output, without significantly increasing heart rate, and therefore may limit increases in myocardial oxygen consumption and potentially attenuate vasoactive medication related myocardial ischemia and injury. 31 , 41 , 42
Phosphodiesterase Inhibitors
PDE3 is an abundant enzyme in many tissues, including in cardiac myocytes and vascular smooth muscle. 43 PDE3's biological actions include the hydrolysis of cAMP. 43 Therefore, inhibition of PDE3 through agents such as milrinone leads to increased cytosolic cAMP levels. Within cardiac tissue, elevated cAMP levels lead to increased inotropy, while vasodilation occurs within blood vessels. These effects serve to augment cardiac output, reduce afterload, and reduce systemic and pulmonary vascular resistance. 44 The administration of milrinone, however, should be performed with caution in patients with severe renal impairment, as it is subject to renal elimination and runs the risk of toxic accumulation in this setting.
Calcium Sensitizers
Calcium sensitizers are a relatively recently developed novel class of inodilators. 45 , 46 Levosimendan, a routinely used calcium sensitizer (not routinely available in the United States), has both peripheral vasodilatory effects and enhances myocardial contractility, mediated through vascular smooth muscle potassium channel binding and cardiac myofilament calcium sensitization by calcium‐dependent troponin C binding, respectively. Levosimendan has similar hemodynamic effects to that of the PDE3 inhibitor milrinone and interestingly has been shown to have significant PDE3 inhibiting actions as well. 46 Furthermore, levosimendan has an active metabolite with a half‐life of >80 hours, allowing the hemodynamic effects to persist following completion of the initial infusion.
Arginine Vasopressin Antagonists
Arginine vasopressin (vasopressin), an endogenous nonapeptide hormone, exerts its cardiovascular effects through the activation of G‐protein coupled receptors V1a (in smooth muscle) and V2 (in the renal collecting tubules). Activation of the V1a receptor on vascular smooth muscle causes increased cytosolic Ca2+ and resulting vasoconstriction, and the V2 receptor activation leads to water retention via the distal convoluted tubule. 47
The use of vasopressin in the management of CS is principally mediated through a dose‐dependent increase in systemic vascular resistance. It has a limited impact on other hemodynamic parameters including cardiac output and pulmonary capillary wedge pressure. 48 Vasopressin may also have pleotropic effects that can ameliorate the underlying causes of the systemic inflammatory response syndrome mediated vasoplegia by reducing nitric oxide production and attenuate catecholamine resistance due to adrenergic receptor downregulation. 49 Furthermore, there are emerging data indicating that vasopressin, compared with catecholamines, may result in selective vasoconstriction in the systemic circulation and cause pulmonary arterial vasodilation, thereby reducing right ventricular afterload. 50 , 51
Guanylate Cyclase and Nitric Oxide Synthase Inhibitors
Methylene blue is a repurposed agent, previously used in the treatment of methemoglobinemia, but has recently generated interest for use in refractory vasoplegic shock. 52 In the setting of CS‐related systemic inflammatory response syndrome, methylene blue's therapeutic effects are exerted through the inhibition of nitric oxide mediated cGMP production, resulting in increased smooth muscle vasoconstriction. Methylene blue should be used with caution in patients treated with selective serotonin reuptake inhibitors as it may precipitate a serotonin syndrome and also in patients with known glucose‐6‐phosphate dehydrogenase deficiency due to the risk of developing hemolytic anemia.
Evidence for Use of Vasoactive Agents in Cardiogenic Shock
Current Societal Recommendations for Commencing Vasoactive Medications
Conducting randomized controlled trials in a population with CS has historically been challenging. Current societal recommendations have therefore been developed using data from small trials of variable quality, meta‐analyses, and consensus opinion, resulting in equipoise with respect to the recommended first‐line vasoactive agents to be used in the treatment of CS. 31 , 41 , 42 , 53 , 54 , 55 , 56 A summary of the available randomized trial data pertaining to the use of vasoactive medications are presented in Table 3. Despite limited high‐quality data, French, German, Austrian, and the European Society Cardiology guidelines addressing CS management endorse the use of norepinephrine or dobutamine as the first‐line vasoactive medications in CS. 16 , 19 , 57 , 58 In the United States, current guidelines remain less definitive. The American Heart Association's Heart Failure guidelines do not provide clear recommended first‐line vasoactive medication, suggesting clinicians use agents that are readily available, easy to administer, and they are familiar. 59 Furthermore, the American Heart Association's Scientific Statements on the contemporary management of CS recommends the use of dopamine or norepinephrine as first‐line treatments, whereas the recently published guideline for the invasive management of AMI‐CS suggests norepinephrine be used to support blood pressure in the initial stabilization period, unless further chronotropic support is required. 1 , 60 These recommendations are summarized in Figure 3.
Table 3.
Randomized Controlled Trials of Vasoactive Medications in Patients With Cardiogenic Shock
| Study | Setting | Population | Sample size | Agents assessed | Mortality | Adverse events |
|---|---|---|---|---|---|---|
| Milrinone as Compared With Dobutamine in the Treatment of Cardiogenic Shock (CAPITAL‐DOREMI), 2021 56 | ICU, single center | Cardiogenic shock | 192 | Dobutamine vs milrinone | No difference | Nil |
| Epinephrine Versus Norepinephrine for Cardiogenic Shock After Acute Myocardial Infarction (Optima CC), 2018 31 | ICU, multicenter | Post PCI AMI‐CS | 57 | Epinephrine vs norepinephrine | No difference |
Increased refractory shock in epinephrine Increased cardiac double product with epinephrine Increased serum lactate |
| Sepsis Occurrence in Acutely Ill Patients‐II (SOAP‐II), 2010 41 | ICU, multicenter | Undifferentiated shock | 1679 | Dopamine vs norepinephrine | No difference |
Increased arrhythmic events with dopamine Increased mortality in cardiogenic shock treated with dopamine. |
| A Comparison of Epinephrine and Norepinephrine in Critically Ill Patients (CAT), 2008 42 | ICU, multicenter | Undifferentiated shock | 280 | Epinephrine vs norepinephrine | No difference |
Epinephrine associated with metabolic acidosis requiring agent cessation No difference in mortality for cardiogenic shock subgroup |
| Effect of Tilarginine Acetate in Patients With Acute Myocardial Infarction and Cardiogenic Shock (TRIUMPH), 2007 53 | ICU, multicenter | Post PCI AMI‐CS | 398 | Tilarginine acetate vs placebo | No difference | Nil |
| Levosimendan vs Dobutamine for Patients With Acute Decompensated Heart Failure (SURVIVE), 2007 54 | In‐hospital, multicenter | Decompensated heart failure +/− low cardiac output | 1327 | Levosimendan vs dobutamine | No difference | Increased atrial fibrillation, hypokalemia, and headache with levosimendan |
| Efficacy and Safety of Intravenous Levosimendan Compared With Dobutamine in Severe Low‐Output Heart Failure (LIDO), 2002 55 | In‐hospital, multicenter | Low output heart failure (including decompensated heart failure and post cardiotomy) | 203 | Levosimendan vs dobutamine | No difference | Increased arrhythmia events and angina with dobutamine treatment |
AMI‐CS indicates acute myocardial infarction cardiogenic shock; ICU, intensive care unit; and PCI, percutaneous coronary intervention. Studies included were randomized controlled trials performed after the year 2000 that included patients with cardiogenic shock and included over 50 enrolled patients.
Figure 3. Summary of key cardiac societal guideline recommendations for the use of vasoactive medications in cardiogenic shock.
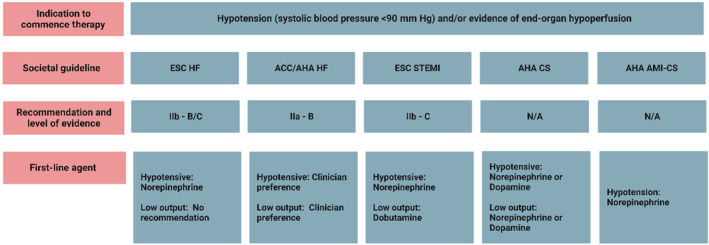
ESC HF, 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure 16 ; ACC/AHA HF, 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure 59 ; ESC STEMI, 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST‐Segment–Elevation 58 ; AHA CS, Contemporary Management of Cardiogenic Shock: A Scientific Statement from the American Heart Association 1 ; AHA AMI‐CS, Invasive Management of Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Scientific Statement from the American Heart Association. 60 ACC indicates American College of Cardiology; AHA, American Heart Association; AMI, acute myocardial infarction; CS, cardiogenic shock; ESC, European Society of Cardiology; HF, heart failure; HFSA, Heart Failure Society of America; and STEMI, ST‐segment–elevation myocardial infarction.
Treatment of Cardiogenic Shock With Associated Hypotension
The initial goal of therapy in patients with CS and associated hypotension should be focused on the restoration of perfusion to vital organs, achieved through augmenting MAP and cardiac output. 2 End‐organ blood flow is a direct correlate of MAP, and reduced systolic blood pressure in CS, in particular in the setting of AMI‐CS, is independently associated with an increased risk of mortality. 61
The safety and efficacy of dopamine in CS has been called into question by the SOAP‐II (Sepsis Occurrence in Acutely Ill Patients) trial. 41 This study compared dopamine and norepinephrine as first‐line therapies through a multicenter, ICU‐based randomized controlled trial of 1679 patients with shock of all causes. 41 Although there was no difference in the primary outcome of all‐cause 28‐day mortality in this cohort, dopamine was associated with increased arrhythmic events (n=207 [24.1%] versus n=102 [12.4%], P<0.001) and in a prespecified subgroup of 280 patients with CS there was an increased risk of 28‐day mortality (P=0.03). Although these findings are hypothesis generating, in the absence of alternate data supporting the use of dopamine, we advocate that noradrenaline should be used in preference to dopamine in the hemodynamic support in patients with CS and hypotension.
There is an emerging body of evidence to support the use of norepinephrine as the initial vasoactive agent for the management of CS with hypotension. OPTIMA CC (Epinephrine Versus Norepinephrine for Cardiogenic Shock After Acute Myocardial Infarction), a small prospective, double‐blinded, randomized controlled study, sought to compare the hemodynamic effects and tolerability of epinephrine with norepinephrine in AMI‐CS. 31 Despite recruiting a total of only 57 patients, there were several valuable insights gained from these data; (1) there were no differences observed in MAP, cardiac index, or stroke volume in patients treated with either epinephrine or norepinephrine; (2) comparable doses of both study drugs were required to achieve a target MAP of 70 mm Hg; (3) epinephrine treatment was associated with worse metabolic acidosis (P=0.0004) and increased lactate levels (P<0.0001); (4) those treated with epinephrine experienced significantly greater increases in heart rate (P<0.001) and a concomitant increase in the cardiac double product (P<0.001), an indirect surrogate of myocardial oxygen consumption, compared with norepinephrine treated patients; and (5) the study was terminated prematurely due to increased rates of refractory CS (odds ratio [OR], 8.24 [95% CI, 1.61–42.18], P=0.01) developing in the epinephrine‐treated arm. Additionally, a meta‐analysis that included 2583 patients with nonsurgical CS assessed the impact of epinephrine treatment on short‐term mortality outcomes. This study found that epinephrine treated patients had significantly greater adjusted risk of mortality (adjusted OR, 4.7 [95% CI, 3.4–6.4]). 62 Epinephrine's apparent lack of benefit relating to hemodynamic parameters, increased myocardial oxygen consumption, and the potential increased risk of developing refractory CS and death, when compared with norepinephrine, raise concerns about its use as a first‐line treatment in CS. However, further data are required to establish norepinephrine's superiority over epinephrine as the first‐line therapy in CS with hypotension.
Treatment Cardiogenic Shock With Preserved Blood Pressure and Low Cardiac Output State
To date there are no randomized studies comparing the safety and efficacy of inotropes with inodilators in CS. There are 3 commonly administered inodilators in clinical practice, each with a unique mechanism of action; β1‐ and β2‐AR agonist, dobutamine; PDE3 inhibitors such as milrinone; and the calcium sensitizer, levosimendan (not routinely available in the United States). Although these agents increase myocardial contractility and lusitropy like traditional inotropes, they also have the effect of reducing cardiac afterload through vasodilation. The use of milrinone and dobutamine has recently been compared through the CAPITAL DOREMI (Milrinone as Compared with Dobutamine in the Treatment of Cardiogenic Shock) study, a single‐center, double‐blinded randomized controlled trial. 56 This study included 192 patients (96 participants in each treatment arm) admitted to ICU with CS and randomized to receive either milrinone or dobutamine. There was no difference in the primary composite outcome of in‐hospital mortality, resuscitated cardiac arrest, cardiac transplantation or mechanical circulatory support, nonfatal myocardial infarction, stroke, or renal replacement therapy (relative risk [RR], 0.9 [95% CI, 0.69–1.19]). In a prespecified subgroup analysis, which included 65 patients with AMI‐CS, there was also no significant difference in the primary composite outcome between agents (hazard ratio [HR], 1.35 [95% CI, 0.73–2.47]). 63 However, the findings from this study should be interpreted with a degree of caution due to its small sample size potentially rendering the trial underpowered to detect the smaller than anticipated treatment effects in both the primary composite and secondary outcomes. Furthermore, its generalizability to other clinical settings may be limited as it was a single‐center study conducted in a quaternary level ICU. Nevertheless, considering these findings, patients who are normotensive and in a low cardiac output state, it is reasonable to consider the administration of either dobutamine or milrinone as a first‐line therapy, with the exception of severe renal impairment where dobutamine should be used in preference to milrinone.
Vasoactive Medications in Refractory Hypotension
The treatment of refractory hypotension in CS represents a significant challenge. In the setting of severe metabolic acidosis, which occurs due to tissue hypoxia and subsequent activation of anaerobic metabolic pathways, both in vivo and ex vivo experimental data has demonstrated reduced vascular and cardiac responsiveness to catecholamines. 64 The relatively preserved vasopressor effects in the setting of acidosis of vasopressin and methylene blue render these drugs a reasonable choice to trial as salvage therapy in cases of catecholamine‐refractory vasoplegia. 48 , 65
A Stepwise Approach to Vasoactive Therapy in Cardiogenic Shock
Presented in Figure 4 is a proposed stepwise approach for the use of vasoactive agents in CS. In the initial phase of therapy, we suggest that patients should be stratified into 2 phenotypes, those with hypotension (systolic blood pressure <90 mm Hg, MAP ≤65 mm Hg, or >30‐mm Hg reduction in MAP from baseline with evidence of hypoperfusion) or low cardiac output (determined clinically, biochemically, or through an invasive hemodynamic assessment) and preserved blood pressure. The algorithm advocates for the initial correction of hypotension, followed by the treatment of the low cardiac output state with the use of inodilator therapy. The timing and role for the use of MCS in this clinical situation remains less certain and is outside the scope of this review. However, persistent severe CS should prompt clinician consideration for MCS therapy at any stage of the proposed treatment pathway. This strategy leverages the emerging data supporting the use of norepinephrine as a first‐line therapy, while emphasizing the need for ongoing and repeated assessment throughout the treatment journey to tailor therapy based on the current prevailing hemodynamic status.
Figure 4. A stepwise approach to the use of vasoactive medications for the management of cardiogenic shock.
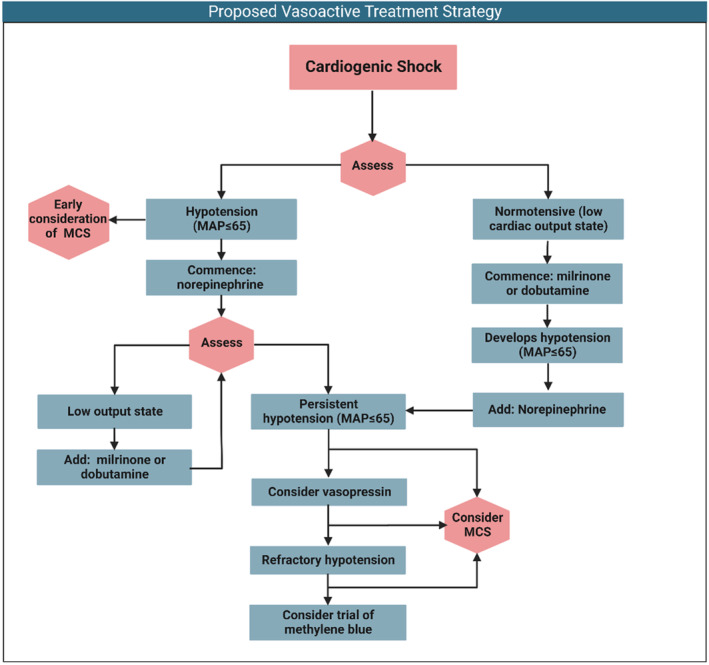
MAP indicates mean arterial pressure; and MCS, mechanical circulatory support.
Assessing Response to Therapy and Requirement for Titration of Hemodynamic Supports
Invasive Monitoring
Continuous blood pressure monitoring is essential to assess for progression of the underlying disease process and the therapeutic response to vasoactive therapy. The MAP, defined as the average blood pressure during a cardiac cycle, can be equated to the end‐organ “perfusion pressure.” 66 Accordingly, MAP is often used as treatment target for patients with CS and has been incorporated into the proposed treatment algorithm. The literature guiding specific MAP targets in a population with CS is limited and largely supported by observational data. 67 Nonetheless, current guidelines suggest a target MAP of ≥65 mm Hg. 60 , 68
The role of invasive hemodynamic assessment with a pulmonary artery catheter (PAC) is not clearly defined in CS. Although several randomized trials have assessed the use of the PAC in shock, it is important to note that these studies did not explicitly include patients with CS, nor did they assess the use of PAC‐derived data to guide a treatment algorithm relating to the use of vasoactive agents or MCS. 69 Nonetheless, the use of invasively derived measures of filling pressures and cardiac performance are of increasing clinical interest in the setting of CS. From a left ventricular perspective, in AMI‐CS the cardiac power output (defined as [(MAP–right atrial pressure) × CO]/451) is a well‐defined prognostic marker for short‐term outcomes, including mortality. 22 , 70 , 71 Furthermore, in a population with AMI‐CS, there is evidence suggesting that the cardiac power output can be used to assess the adequacy of hemodynamic support measures, with a cardiac power output >0.8 Watts associated with improved outcomes. 69 , 70 , 72 With respect to the right ventricle, there are several parameters including pulmonary artery pulsatility index (defined as pulmonary artery pulse pressure/right atrial pressure), right atrial pressure, and right ventricle stroke work index (defined as stroke volume index × [mean pulmonary artery pressures–mean right atrial pressure]), which have been shown to be predictive of in‐hospital mortality in AMI‐CS and decompensated heart failure. 73 , 74 In view of the paucity of randomized data supporting the use of PAC in CS, there is a considerable need for additional evidence to guide the use of PAC in this setting. At present, societal guidelines have insufficient evidence to recommend the routine use of PAC in this setting; however, upcoming trials including the ongoing PACCS trial (Pulmonary Artery Catheter in Cardiogenic Shock, NCT05485376) will greatly assist with addressing this knowledge gap.
Biochemical Assessment of Perfusion
Regular biochemical assessment can provide a “window” into the current perfusion status of the patient, in addition to classical clinical signs that may include mentation, skin quality, and urine output. In addition to direct measures of end‐organ function and injury, for example dynamic changes in serum creatinine when assessing for renal injury, serum lactate provides an important global measure of tissue perfusion. Absolute lactate levels have been shown to be a powerful prognostic biomarker in multiple shock states. 66 However, temporal changes in lactate levels may be more meaningful, owing to the highly dynamic nature of these measurements. In a cohort of the IABP‐SHOCK II (Intraaortic Balloon Pump in Cardiogenic Shock II) study, absolute lactate level and clearance at 8 hours was an independent predictor of mortality. 75 , 76 Furthermore, in the CAPITAL DOREMI study, the time taken for lactate normalization was the most powerful predictor of 30‐day mortality. 56 Serial measurements of biomarkers, in addition to routine clinical examination, can therefore provide clinicians with ongoing feedback about the effectiveness of the initial pharmacologic stabilization on perfusion (in particular for patients with low cardiac output state and preserved MAP) and indicate the need for further escalation of therapy with additional pharmacotherapy or potentially MCS.
Future Directions
Addressing a Lack of Randomized Data to Guide the Appropriate First‐Line Therapy in CS
The challenges of performing high‐quality randomized controlled trials in patients with CS have been well described. 77 Furthermore, the external validity and generalizability of the available trial data are a considerable issue as the majority of studies have exclusively recruited patients once they have undergone their initial prehospital and invasive cardiology management and are receiving supportive care within ICU at the time of randomization. Nonetheless, 2 seminal ICU trials are currently ongoing. First, the CAPITAL DOREMI‐II trial (NCT05267886) is recruiting patients with CS and assigning them to receive either milrinone, dobutamine, or placebo. This study will provide a crucial insight into the role of inodilators in the initial management of CS. Second, LevoHeartShock (NCT04020263) is a French prospective, double‐blind, multicenter randomized controlled trialof patients with CS already treated with noradrenaline or dobutamine and assigned to receive the addition of either levosimendan or placebo. This study will help inform clinicians about the utility of inodilator therapy, compared with traditional catecholamine agents.
Although these ICU‐based trials are likely to be instructive, given the high rates of prehospital treated CS, randomizing patients at first medical contact with emergency medical services may enhance the applicability of any findings to a variety of clinical settings. 14 , 78 As catecholamines are frequently used as a first‐line therapy in CS, a trial assessing the efficacy and safety of this drug class should be considered as an area of priority. Our group is currently seeking to address this clinical question through the PANDA trial (Paramedic Randomized Trial of Noradrenaline Versus Adrenaline in the Initial Management of Patients with Cardiogenic Shock, ACTRN12621000805875). This study will randomize patients with CS in the prehospital setting to receive either epinephrine or norepinephrine and will be sufficiently powered to detect a difference in 28‐day mortality and therefore guide first‐line catecholamine therapy.
Comparing Mechanical Circulatory Support With Medical Therapy, an Urgent Need for Randomized Data
MCS use in CS has increased dramatically over the past 15 years, despite the cost, associated risk of major complications, and limited evidence to support its use. 79 The rationale for the use of these devices is predicated on their ability to restore systemic perfusion, reduce cardiac filling pressures, limit myocardial oxygen consumption, and reduce vasoactive agent requirements. The Impella (Abiomed, Danvers) catheter‐based micro‐axial flow pump and venoarterial extracorporeal membrane oxygenation have been readily adopted to provide hemodynamic support and incorporated into a range of CS treatment algorithms. 9 , 72 , 79 This practice has occurred despite very limited randomized data supporting the safety and efficacy of these treatments, in addition to determining the appropriate timing and clinical indications for the commencement of these invasive therapies. 21 , 66 , 72 , 80 , 81 , 82 There are several randomized controlled trials ongoing that are seeking to assess the role MCS use with respect to the timing of initiation (NCT04184635, NCT03637205, NCT03813134) and their use compared with medical therapy alone (NCT01633502, NCT04184635, NCT03637205, NCT03813134). Although these studies will be crucial to inform the in‐hospital management of CS, MCS will not negate the need for pharmacologic support in both prehospital and in‐hospital environments during the treatment phase before patients are established on MCS and in settings where this technology is not readily available.
Conclusions
The use of vasoactive medications in CS is common and underpins the contemporary hemodynamic support strategy for these patients. We have sought to present the unique pharmacology of these medications and provide a pragmatic approach to their use. However, despite the ubiquitous use of these agents in critical care settings, there remains a lack of robust, outcomes‐based data, underscoring the need for further high‐quality trials to guide future practice.
Sources of Funding
D.S. is supported by a National Heart Foundation of Australia Fellowship. J.B. is supported by a National Health and Medical Research Council (NHMRC) and NHF Post Graduate Scholarships. D.K. is supported by an NHMRC Investigator Grant.
Disclosures
None.
Acknowledgments
Figures for this article were created using Biorender.com
This article was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 12.
References
- 1. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 2. Jentzer JC, Hollenberg SM. Vasopressor and inotrope therapy in cardiac critical care. J Intensive Care Med. 2021;36:843–856. doi: 10.1177/0885066620917630 [DOI] [PubMed] [Google Scholar]
- 3. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 4. Vincent J‐L, De Backer D. Circulatory shock. N Engl J Med. 2013;369:1726–1734. doi: 10.1056/NEJMra1208943 [DOI] [PubMed] [Google Scholar]
- 5. Vahdatpour C, Collins D, Goldberg S. Cardiogenic shock. J Am Heart Assoc. 2019;8:e011991. doi: 10.1161/JAHA.119.011991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O'Neill W, Ornato JP, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94:29–37. doi: 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
- 7. Naidu SS, Baran DA, Jentzer JC, Hollenberg SM, van Diepen S, Basir MB, Grines CL, Diercks DB, Hall S, Kapur NK, et al. SCAI SHOCK stage classification expert consensus update: a review and incorporation of validation studies. J Am Coll Cardiol. 2022;79:933–946. doi: 10.1016/j.jacc.2022.01.018 [DOI] [PubMed] [Google Scholar]
- 8. Osman M, Syed M, Patibandla S, Sulaiman S, Kheiri B, Shah MK, Bianco C, Balla S, Patel B. Fifteen‐year trends in incidence of cardiogenic shock hospitalization and in‐hospital mortality in the United States. J Am Heart Assoc. 2021;10:e021061. doi: 10.1161/JAHA.121.021061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Combes A, Price S, Slutsky AS, Brodie D. Temporary circulatory support for cardiogenic shock. Lancet. 2020;396:199–212. doi: 10.1016/S0140-6736(20)31047-3 [DOI] [PubMed] [Google Scholar]
- 10. Harjola V‐P, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, Parissis J, Banaszewski M, Silva‐Cardoso J, Carubelli V, et al. Clinical picture and risk prediction of short‐term mortality in cardiogenic shock: clinical picture and outcome of cardiogenic shock. Eur J Heart Fail. 2015;17:501–509. doi: 10.1002/ejhf.260 [DOI] [PubMed] [Google Scholar]
- 11. Lu DY, Adelsheimer A, Chan K, Yeo I, Krishnan U, Karas MG, Horn EM, Feldman DN, Sobol I, Goyal P, et al. Impact of hospital transfer to hubs on outcomes of cardiogenic shock in the real world. Eur J Heart Fail. 2021;23:1927–1937. doi: 10.1002/ejhf.2263 [DOI] [PubMed] [Google Scholar]
- 12. Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST‐elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3:e000590. doi: 10.1161/JAHA.113.000590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thiele H, Akin I, Sandri M, de Waha‐Thiele S, Meyer‐Saraei R, Fuernau G, Eitel I, Nordbeck P, Geisler T, Landmesser U, et al. One‐year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. 2018;379:1699–1710. doi: 10.1056/NEJMoa1808788 [DOI] [PubMed] [Google Scholar]
- 14. Bloom JE, Andrew E, Dawson LP, Nehme Z, Stephenson M, Anderson D, Fernando H, Noaman S, Cox S, Milne C, et al. Incidence and outcomes of nontraumatic shock in adults using emergency medical services in Victoria, Australia. JAMA Netw Open. 2022;5:e2145179. doi: 10.1001/jamanetworkopen.2021.45179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bloom JE, Andrew E, Nehme Z, Beale A, Dawson LP, Shi WY, Vriesendorp PA, Fernando H, Noaman S, Cox S, et al. Gender disparities in cardiogenic shock treatment and outcomes. Am J Cardiol. 2022;177:14–21. doi: 10.1016/j.amjcard.2022.04.047 [DOI] [PubMed] [Google Scholar]
- 16. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 17. Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird‐Zars VM, Barnett CF, Barsness GW, Burke JA, Cremer PC, et al. Epidemiology of shock in contemporary cardiac intensive care units: data from the Critical Care Cardiology Trials Network Registry. Circ Cardiovasc Qual Outcomes. 2019;12:e005618. doi: 10.1161/CIRCOUTCOMES.119.005618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jentzer JC, Wiley B, Bennett C, Murphree DH, Keegan MT, Kashani KB, Bell MR, Barsness GW. Temporal trends and clinical outcomes associated with vasopressor and inotrope use in the cardiac intensive care unit. Shock. 2020;53:452–459. doi: 10.1097/SHK.0000000000001390 [DOI] [PubMed] [Google Scholar]
- 19. Levy B, Bastien O, Bendjelid K, Cariou A, Chouihed T, Combes A, Mebazaa A, Megarbane B, Plaisance P, Ouattara A, et al. Experts' recommendations for the management of adult patients with cardiogenic shock. Ann Intensive Care. 2015;5:17. doi: 10.1186/s13613-015-0052-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kapur NK, Kanwar M, Sinha SS, Thayer KL, Garan AR, Hernandez‐Montfort J, Zhang Y, Li B, Baca P, Dieng F, et al. Criteria for defining stages of cardiogenic shock severity. J Am Coll Cardiol. 2022;80:185–198. doi: 10.1016/j.jacc.2022.04.049 [DOI] [PubMed] [Google Scholar]
- 21. Basir MB, Schreiber TL, Grines CL, Dixon SR, Moses JW, Maini BS, Khandelwal AK, Ohman EM, O'Neill WW. Effect of early initiation of mechanical circulatory support on survival in cardiogenic shock. Am J Cardiol. 2017;119:845–851. doi: 10.1016/j.amjcard.2016.11.037 [DOI] [PubMed] [Google Scholar]
- 22. Basir MB, Lemor A, Gorgis S, Taylor AM, Tehrani B, Truesdell AG, Bharadwaj A, Kolski B, Patel K, Gelormini J, et al. Vasopressors independently associated with mortality in acute myocardial infarction and cardiogenic shock. Catheter Cardiovasc Interv. 2022;99:650–657. doi: 10.1002/ccd.29895 [DOI] [PubMed] [Google Scholar]
- 23. Zarychanski R, Ariano RE, Paunovic B, Bell DD. Historical perspectives in critical care medicine: blood transfusion, intravenous fluids, inotropes/vasopressors, and antibiotics. Crit Care Clin. 2009;25:201–220. doi: 10.1016/j.ccc.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 24. Thooft A, Favory R, Salgado DR, Taccone FS, Donadello K, De Backer D, Creteur J, Vincent J‐L. Effects of changes in arterial pressure on organ perfusion during septic shock. Crit Care. 2011;15:R222. doi: 10.1186/cc10462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldenberg M. Pheochromocytoma and essential hypertensive vascular disease. Arch Intern Med. 1950;86:823. doi: 10.1001/archinte.1950.00230180028003 [DOI] [PubMed] [Google Scholar]
- 26. Allwood MJ, Cobbold AF, Ginsburg J. Peripheral vascular effects of noradrenaline, isopropylnoradrenaline and dopamine. Br Med Bull. 1963;19:132–136. doi: 10.1093/oxfordjournals.bmb.a070031 [DOI] [PubMed] [Google Scholar]
- 27. Duff RS. Effect of adrenaline and noradrenaline on blood vessels of the hand before and after sympathectomy. J Physiol. 1955;129:53–64. doi: 10.1113/jphysiol.1955.sp005338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Myburgh JA. An appraisal of selection and use of catecholamines in septic shock—old becomes new again. Crit Care Resusc. 2006;8:8. [PubMed] [Google Scholar]
- 29. Myburgh JA, Upton RN, Grant C, Martinez A. The cerebrovascular effects of adrenaline, noradrenaline and dopamine infusions under propofol and isoflurane anaesthesia in sheep. Anaesth Intensive Care. 2002;30:725–733. doi: 10.1177/0310057X0203000602 [DOI] [PubMed] [Google Scholar]
- 30. Levy B, Perez P, Perny J, Thivilier C, Gerard A. Comparison of norepinephrine‐dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Crit Care Med. 2011;39:450–455. doi: 10.1097/CCM.0b013e3181ffe0eb [DOI] [PubMed] [Google Scholar]
- 31. Levy B, Clere‐Jehl R, Legras A, Morichau‐Beauchant T, Leone M, Frederique G, Quenot J‐P, Kimmoun A, Cariou A, Lassus J, et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018;72:173–182. doi: 10.1016/j.jacc.2018.04.051 [DOI] [PubMed] [Google Scholar]
- 32. Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006 [DOI] [PubMed] [Google Scholar]
- 33. Sonnenblick EH, Braunwald E, Williams JF, Glick G. Effects of exercise on myocardial force‐velocity relations in intact unanesthetized man: relative roles of changes in heart rate, sympathetic activity, and ventricular dimensions. J Clin Invest. 1965;44:2051–2062. doi: 10.1172/JCI105312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson NP, Gould KL, De Bruyne B. Autoregulation of coronary blood supply in response to demand. J Am Coll Cardiol. 2021;77:2335–2345. doi: 10.1016/j.jacc.2021.03.293 [DOI] [PubMed] [Google Scholar]
- 35. Overgaard CB, Džavík V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation. 2008;118:1047–1056. doi: 10.1161/CIRCULATIONAHA.107.728840 [DOI] [PubMed] [Google Scholar]
- 36. Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S. Beta 1‐ and beta 2‐adrenergic‐receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1‐receptor down‐regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.RES.59.3.297 [DOI] [PubMed] [Google Scholar]
- 37. Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a [DOI] [PubMed] [Google Scholar]
- 38. Graham RM, Perez DM, Hwa J, Piascik MT. α1‐adrenergic receptor subtypes. Molecular structure, function, and signaling. Circ Res. 1996;78:737–749. doi: 10.1161/01.RES.78.5.737 [DOI] [PubMed] [Google Scholar]
- 39. Madamanchi A. Beta‐adrenergic receptor signaling in cardiac function and heart failure. Mcgill J Med. 2007;10:99–104. [PMC free article] [PubMed] [Google Scholar]
- 40. Levy B, Bollaert P‐E, Charpentier C, Nace L, Audibert G, Bauer P, Nabet P, Larcan A. Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock: a prospective, randomized study. Intensive Care Med. 1997;23:282–287. doi: 10.1007/s001340050329 [DOI] [PubMed] [Google Scholar]
- 41. De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent J‐L. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–789. doi: 10.1056/NEJMoa0907118 [DOI] [PubMed] [Google Scholar]
- 42. Myburgh JA, Higgins A, Jovanovska A, Lipman J, Ramakrishnan N, Santamaria J; CAT Study investigators . A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med. 2008;34:2226–2234. doi: 10.1007/s00134-008-1219-0 [DOI] [PubMed] [Google Scholar]
- 43. Young RA, Ward A. Milrinone: a preliminary review of its pharmacological properties and therapeutic use. Drugs. 1988;36:158–192. doi: 10.2165/00003495-198836020-00003 [DOI] [PubMed] [Google Scholar]
- 44. Nanayakkara S, Mak V, Crannitch K, Byrne M, Kaye DM. Extended release oral milrinone, CRD‐102, for advanced heart failure. Am J Cardiol. 2018;122:1017–1020. doi: 10.1016/j.amjcard.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 45. Packer M, Colucci W, Fisher L, Massie BM, Teerlink JR, Young J, Padley RJ, Thakkar R, Delgado‐Herrera L, Salon J, et al. Effect of levosimendan on the short‐term clinical course of patients with acutely decompensated heart failure. JACC: Heart Fail. 2013;1:103–111. doi: 10.1016/j.jchf.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 46. Hasenfuss G, Pieske B, Castell M, Kretschmann B, Maier LS, Just H. Influence of the novel inotropic agent levosimendan on isometric tension and calcium cycling in failing human myocardium. Circulation. 1998;98:2141–2147. doi: 10.1161/01.CIR.98.20.2141 [DOI] [PubMed] [Google Scholar]
- 47. Barrett LK, Singer M, Clapp LH. Vasopressin: mechanisms of action on the vasculature in health and in septic shock. Crit Care Med. 2007;35:33–40. doi: 10.1097/01.CCM.0000251127.45385.CD [DOI] [PubMed] [Google Scholar]
- 48. Jolly S, Newton G, Horlick E, Seidelin PH, Ross HJ, Husain M, Dzavik V. Effect of vasopressin on hemodynamics in patients with refractory cardiogenic shock complicating acute myocardial infarction. Am J Cardiol. 2005;96:1617–1620. doi: 10.1016/j.amjcard.2005.07.076 [DOI] [PubMed] [Google Scholar]
- 49. Kusano E, Tian S, Umino T, Tetsuka T, Ando Y, Asano Y. Arginine vasopressin inhibits interleukin‐1β‐stimulated nitric oxide and cyclic guanosine monophosphate production via the V1 receptor in cultured rat vascular smooth muscle cells. J Hypertens. 1997;15:627–632. doi: 10.1097/00004872-199715060-00009 [DOI] [PubMed] [Google Scholar]
- 50. Jeon Y, Ryu JH, Lim YJ, Kim CS, Bahk J‐H, Yoon SZ, Choi JY. Comparative hemodynamic effects of vasopressin and norepinephrine after milrinone‐induced hypotension in off‐pump coronary artery bypass surgical patients. Eur J Cardiothorac Surg. 2006;29:952–956. doi: 10.1016/j.ejcts.2006.02.032 [DOI] [PubMed] [Google Scholar]
- 51. Evora PRB. Broad spectrum vasopressors support sparing strategies in vasodilatory shock beyond the vascular receptors. Chest. 2020;157:471–472. doi: 10.1016/j.chest.2019.08.2211 [DOI] [PubMed] [Google Scholar]
- 52. Porizka M, Kopecky P, Dvorakova H, Kunstyr J, Lips M, Michalek P, Balik M. Methylene blue administration in patients with refractory distributive shock—a retrospective study. Sci Rep. 2020;10:1828. doi: 10.1038/s41598-020-58828-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. TRIUMPH Investigators ; Alexander JH, Reynolds HR, Stebbins AL, Dzavik V, Harrington RA, Van de Werf F, Hochman JS. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA. 2007;297:1657. doi: 10.1001/jama.297.15.joc70035 [DOI] [PubMed] [Google Scholar]
- 54. Mebazaa A, Nieminen MS, Packer M, Cohen‐Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Põder P, Kivikko M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA. 2007;297:1883–1891. doi: 10.1001/jama.297.17.1883 [DOI] [PubMed] [Google Scholar]
- 55. Follath F, Cleland J, Just H, Papp J, Scholz H, Peuhkurinen K, Harjola V, Mitrovic V, Abdalla M, Sandell E‐P, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low‐output heart failure (the LIDO study): a randomised double‐blind trial. Lancet. 2002;360:196–202. doi: 10.1016/S0140-6736(02)09455-2 [DOI] [PubMed] [Google Scholar]
- 56. Mathew R, Di Santo P, Jung RG, Marbach JA, Hutson J, Simard T, Ramirez FD, Harnett DT, Merdad A, Almufleh A, et al. Milrinone as compared with dobutamine in the treatment of cardiogenic shock. N Engl J Med. 2021;385:516–525. doi: 10.1056/NEJMoa2026845 [DOI] [PubMed] [Google Scholar]
- 57. Werdan K, Buerke M, Geppert A, Thiele H, Zwissler B, Rus M; guideline group* . Infarction‐related cardiogenic shock‐ diagnosis, monitoring and therapy. Dtsch Arztebl Int. 2021;118:88–95. doi: 10.3238/arztebl.m2021.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 59. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 60. Henry TD, Tomey MI, Tamis‐Holland JE, Thiele H, Rao SV, Menon V, Klein DG, Naka Y, Piña IL, Kapur NK, et al. Invasive management of acute myocardial infarction complicated by cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2021;143:e815–e829. doi: 10.1161/CIR.0000000000000959 [DOI] [PubMed] [Google Scholar]
- 61. Katz JN, Stebbins AL, Alexander JH, Reynolds HR, Pieper KS, Ruzyllo W, Werdan K, Geppert A, Dzavik V, Van de Werf F, et al. Predictors of 30‐day mortality in patients with refractory cardiogenic shock following acute myocardial infarction despite a patent infarct artery. Am Heart J. 2009;158:680–687. doi: 10.1016/j.ahj.2009.08.005 [DOI] [PubMed] [Google Scholar]
- 62. Leopold V, Gayat E, Pirracchio R, Spinar J, Parenica J, Tarvasmaki T, Lassus J, Harjola V‐P, Champion S, Zannad F, et al. Epinephrine and short‐term survival in cardiogenic shock: an individual data meta‐analysis of 2583 patients. Intensive Care Med. 2018;44:847–856. doi: 10.1007/s00134-018-5222-9 [DOI] [PubMed] [Google Scholar]
- 63. Jung RG, Di Santo P, Mathew R, Abdel‐Razek O, Parlow S, Simard T, Marbach JA, Gillmore T, Mao B, Bernick J, et al. Implications of myocardial infarction on management and outcome in cardiogenic shock. JAHA. 2021;10:e021570. doi: 10.1161/JAHA.121.021570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kimmoun A, Novy E, Auchet T, Ducrocq N, Levy B. Hemodynamic consequences of severe lactic acidosis in shock states: from bench to bedside. Crit Care. 2015;19:175. doi: 10.1186/s13054-015-0896-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leyh RG, Kofidis T, Strüber M, Fischer S, Knobloch K, Wachsmann B, Hagl C, Simon AR, Haverich A. Methylene blue: the drug of choice for catecholamine‐refractory vasoplegia after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2003;125:1426–1431. doi: 10.1016/S0022-5223(02)73284-4 [DOI] [PubMed] [Google Scholar]
- 66. Mathew R, Fernando SM, Hu K, Parlow S, Di Santo P, Brodie D, Hibbert B. Optimal perfusion targets in cardiogenic shock. JACC Adv. 2022;1:100034. doi: 10.1016/j.jacadv.2022.100034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Burstein B, Tabi M, Barsness GW, Bell MR, Kashani K, Jentzer JC. Association between mean arterial pressure during the first 24 hours and hospital mortality in patients with cardiogenic shock. Crit Care. 2020;24:513. doi: 10.1186/s13054-020-03217-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thiele H, Ohman EM, de Waha‐Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40:2671–2683. doi: 10.1093/eurheartj/ehz363 [DOI] [PubMed] [Google Scholar]
- 69. Saxena A, Garan AR, Kapur NK, O'Neill WW, Lindenfeld J, Pinney SP, Uriel N, Burkhoff D, Kern M. Value of hemodynamic monitoring in patients with cardiogenic shock undergoing mechanical circulatory support. Circulation. 2020;141:1184–1197. doi: 10.1161/CIRCULATIONAHA.119.043080 [DOI] [PubMed] [Google Scholar]
- 70. Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, LeJemtel TH, Cotter G; SHOCK Investigators . Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol. 2004;44:340–348. doi: 10.1016/j.jacc.2004.03.060 [DOI] [PubMed] [Google Scholar]
- 71. Lim HS. Cardiac power output revisited. Circ Heart Failure. 2020;13:e007393. doi: 10.1161/CIRCHEARTFAILURE.120.007393 [DOI] [PubMed] [Google Scholar]
- 72. Basir MB, Kapur NK, Patel K, Salam MA, Schreiber T, Kaki A, Hanson I, Almany S, Timmis S, Dixon S, et al. Improved outcomes associated with the use of shock protocols: updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2019;93:1173–1183. doi: 10.1002/ccd.28307 [DOI] [PubMed] [Google Scholar]
- 73. Jain P, Thayer KL, Abraham J, Everett KD, Pahuja M, Whitehead EH, Schwartz BP, Lala A, Sinha SS, Kanwar MK, et al. Right ventricular dysfunction is common and identifies patients at risk of dying in cardiogenic shock. J Card Fail. 2021;27:1061–1072. doi: 10.1016/j.cardfail.2021.07.013 [DOI] [PubMed] [Google Scholar]
- 74. Houston BA, Brittain EL, Tedford RJ. Right ventricular failure. N Engl J Med. 2023;388:1111–1125. doi: 10.1056/NEJMra2207410 [DOI] [PubMed] [Google Scholar]
- 75. Fuernau G, Desch S, de Waha‐Thiele S, Eitel I, Neumann F‐J, Hennersdorf M, Felix SB, Fach A, Böhm M, Pöss J, et al. Arterial lactate in cardiogenic shock. J Am Coll Cardiol Intv. 2020;13:2208–2216. doi: 10.1016/j.jcin.2020.06.037 [DOI] [PubMed] [Google Scholar]
- 76. Marbach JA, Stone S, Schwartz B, Pahuja M, Thayer KL, Faugno AJ, Chweich H, Rabinowitz JB, Kapur NK. Lactate clearance is associated with improved survival in cardiogenic shock: a systematic review and meta‐analysis of prognostic factor studies. J Card Fail. 2021;27:1082–1089. doi: 10.1016/j.cardfail.2021.08.012 [DOI] [PubMed] [Google Scholar]
- 77. Freund A, Desch S, Thiele H. Challenges in the conduct of randomised controlled trials in cardiogenic shock complicating acute myocardial infarction. J Geriatr Cardiol. 2022;19:125–129. doi: 10.11909/j.issn.1671-5411.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bloom JE, Nehme Z, Andrew E, Dawson LP, Fernando H, Noaman S, Stephenson M, Anderson D, Pellegrino V, Cox S, et al. Hospital characteristics are associated with clinical outcomes in patients with cardiogenic shock. Shock. 2022;58:204–210. doi: 10.1097/SHK.0000000000001974 [DOI] [PubMed] [Google Scholar]
- 79. Becher PM, Schrage B, Sinning CR, Schmack B, Fluschnik N, Schwarzl M, Waldeyer C, Lindner D, Seiffert M, Neumann JT, et al. Venoarterial extracorporeal membrane oxygenation for cardiopulmonary support: insights from a German registry. Circulation. 2018;138:2298–2300. doi: 10.1161/CIRCULATIONAHA.118.036691 [DOI] [PubMed] [Google Scholar]
- 80. Thiele H, Zeymer U, Neumann F‐J, Ferenc M, Olbrich H‐G, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 81. Batchelor RJ, Wheelahan A, Zheng WC, Stub D, Yang Y, Chan W. Impella versus venoarterial extracorporeal membrane oxygenation for acute myocardial infarction cardiogenic shock: a systematic review and meta‐analysis. J Clin Med. 2022;11:3955. doi: 10.3390/jcm11143955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ostadal P, Rokyta R, Karasek J, Kruger A, Vondrakova D, Janotka M, Naar J, Smalcova J, Hubatova M, Hromadka M, et al. Extracorporeal membrane oxygenation in the therapy of cardiogenic shock: results of the ECMO‐CS randomized clinical trial. Circulation. 2023;147:454–464. doi: 10.1161/CIRCULATIONAHA.122.062949 [DOI] [PubMed] [Google Scholar]


