Pediatric cardiac arrest (CA) remains a significant cause of morbidity and mortality in the United States. Growth differentiation factor‐15 (GDF‐15) is a cardioprotective cytokine released after ischemia/reperfusion injury to cardiomyocytes that impacts myocardial recovery. 1 GDF‐15 deficiency increases mortality in animal models of myocardial ischemia. 1 Adult CA studies revealed that increased serum GDF‐15 postarrest is associated with unfavorable outcome and mortality. 2 , 3 We recently reported that serum GDF‐15 is increased after pediatric CA and that early elevations were associated with hospital mortality. 4 We now expand these findings in a secondary analysis of serum from patients enrolled in a multicenter study of pediatric CA and examine whether GDF‐15 is specific as a marker of cardiovascular dysfunction.
The parent study, POCCA (Personalizing Outcomes after Child Cardiac Arrest) (NCT02769026), enrolled children aged 48 hours to 17 years with in‐ or out‐of‐hospital CA and reported the association of blood‐based biomarkers at 3 timepoints (0–24, 24–48, and 48–72 hours postarrest) with neurobehavioral outcome at 1‐year postarrest. 5 This study included patients with serum from at least 2 timepoints and measured GDF‐15 by Luminex Assay (LXSAHM‐03, Lot: L145440; R&D Systems). A technician blinded to patient data performed measurements. Samples below the lower limit of detection of each standard curve were assigned the lower limit of detection of their plate. Statistical analysis (Kruskal–Wallis and post‐hoc Wilcoxon rank sum) was performed in StataIC 15 (StataCorp, College Station, TX). Both POCCA and this secondary analysis were approved by the relevant institutional review boards. Written informed consent from a parent or guardian was required for participation, and written patient assent was obtained when appropriate (based on local center guidelines). The data that support the findings of this study are available upon reasonable request and an appropriate data use agreement.
We included serum from 111 pediatric patients (59% male) after CA (age: 31.3 [6.87–129.9] months, median [interquartile range]) and 20 healthy controls (40% male, age: 37.7 [18.6–63.6] months). Consistent with our prior work, 0 to 24 hours postarrest, GDF‐15 levels were markedly elevated (median 3230 [1537–10 107] pg/mL) versus controls (313 [311–405] pg/mL, P <0.0001) (Figure [A]). For the 76 patients with outcome data available and serum at 0 to 24 hours, GDF‐15 levels were associated with unfavorable outcome (death or Vineland Adaptive Behavioral Score 3rd edition <70) at 1 year (8676 [2711–22 059] pg/mL versus 3490 [1522–9202] pg/mL, P<0.05) (Figure [B]).
Figure 1. Association of GDF‐15 with event cause and CV after pediatric CA.
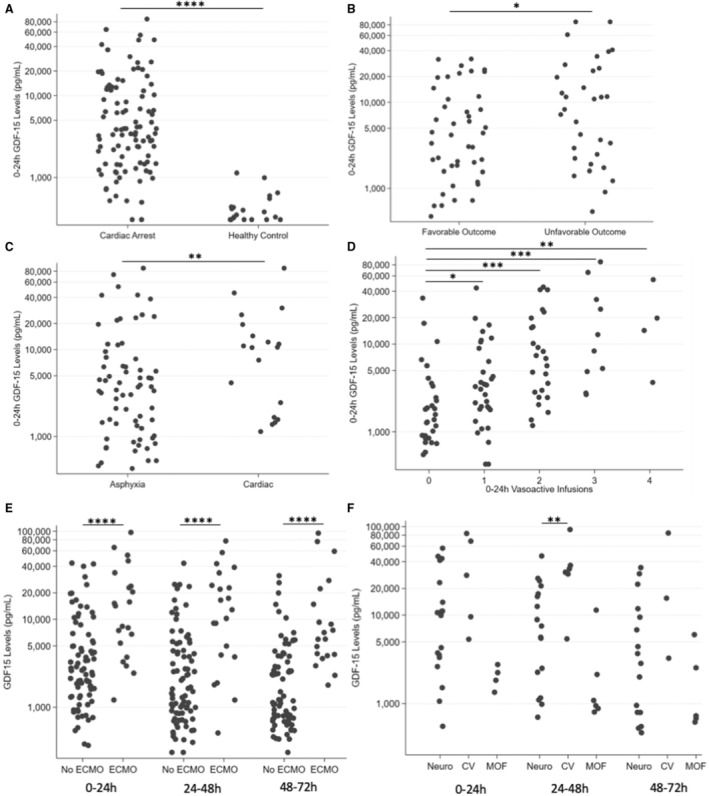
A, GDF‐15 levels are increased at 0 to 24 hours after CA (N=100) compared with healthy controls (N=20) (P <0.0001, Wilcoxon rank sum). B, Increased GDF‐15 levels at 0 to 24 hours postarrest are associated with unfavorable outcome at 1 year (N=44, favorable outcome, N=32 unfavorable outcome) (P <0.05, Wilcoxon rank sum). C, GDF‐15 levels are elevated at 0 to 24 hours in arrests of a cardiac cause (N=19) compared with arrests of a respiratory cause (N=68) (P <0.01, Wilcoxon rank sum). D, Increased GDF‐15 levels are associated with increasing vasoactive infusion use in the first 24 hours postarrest (N=29, 0 infusions; N=32, 1 infusion; N=25, 2 infusions; N=10, 3 infusions; N=4, 4 infusions) (P=0.0001, Kruskal–Wallis; post‐hoc Wilcoxon rank sum P <0.05, P <0.001, P <0.001, and P<0.01, respectively). E, GDF‐15 levels were markedly increased in patients with CA who required ECMO (N=21) compared with those who did not require ECMO (N=79) (P <0.0001, Wilcoxon rank sum comparison of 0‐ to 24‐hour levels). This finding persisted at later timepoints (P <0.0001 at 24–48 hours and 48–72 hours), total patients requiring ECMO (N=23) vs no ECMO (N=88). F, GDF‐15 levels differed in patients by mechanism of death. At 0 to 24 hours, there were serum samples from 19 patients who died from severe neurologic injury or brain death, 5 patients who died from cardiovascular failure, and 4 patients who died from multi‐organ failure (P <0.05, Kruskal–Wallis). At 24–48 hours, there were serum samples from 17 patients who died from neurologic injury or brain death, 6 patients who died from cardiovascular failure, and 6 patients who died from multi‐organ failure (P <0.01, Kruskal–Wallis). At 24–48 hours postarrest, GDF‐15 levels in patients who died from cardiovascular failure were significantly higher than in patients who died from neurologic injury or brain death (P <0.01, post‐hoc Wilcoxon rank sum). CA indicates cardiac arrest; CV, cardiovascular failure; ECMO, extracorporeal membrane oxygenation; GDF‐15, growth differentiation factor‐15; MOF, multi‐organ failure; and Neuro, neurologic injury or brain death. Each timepoint was evaluated independently (no repeated‐measures analysis was performed). All plots have the y‐axis on a log scale. *P <0.05, **P <0.01, ***P <0.001, ****P <0.0001.
When we examined GDF‐15 levels by arrest cause, the 19 patients with cardiac cause had >3‐fold increase in 0 to 24 hours GDF‐15 levels versus the 68 patients with a respiratory cause (9310 [2478–19 187] pg/mL versus 2987 [1181–6828] pg/mL, P<0.01) (Figure [C]). Because 0 to 24 hours GDF‐15 was associated with cardiac cause, we examined whether GDF‐15 was associated with vasoactive infusion requirement in the first 24 hours postresuscitation or the need for extracorporeal membrane oxygenation support. GDF‐15 differed significantly by the number of vasoactive infusions required and increased with each additional infusion (Kruskal–Wallis, P=0.0001) (Figure [D]). Patients requiring no infusions had lower GDF‐15 levels than those requiring 1, 2, 3, or 4 infusions (median 1474 [867–3017] pg/mL versus 2873, interquartile range: [1625–6677] pg/mL versus 7034 [2643–17 634] pg/mL versus 9336 [4623–38 232] pg/mL versus 16 204 [9059–37 480] pg/mL, respectively). GDF‐15 levels were ≈6‐fold greater at 0 to 24 hours in the 21 patients who required extracorporeal membrane oxygenation versus the 79 patients who did not (14 642 [6623–28 994] pg/mL versus 2539 [1342–5568] pg/mL, P <0.0001) (Figure [E]). This difference persisted at later timepoints (P <0.0001 at 24–48 and 48–72 hours). A sensitivity analysis, restricted to respiratory arrests, revealed similar associations between GDF‐15, extracorporeal membrane oxygenation, and vasoactive infusions (Wilcoxon rank sum, P <0.01, and Kruskal–Wallis, P=0.0006, respectively).
We then examined whether GDF‐15 differed by cause of death for patients with in‐hospital mortality. We divided causes of death into 3 categories: cardiovascular failure, multi‐organ failure, severe neurologic injury, or brain death. For the 31 patients with a reported cause of death, GDF‐15 differed significantly by cause of death at 0 to 24 and 24 to 48 hours (P <0.05 and P <0.01, respectively, Kruskal–Wallis) (Figure [F]). At 24 to 48 hours postarrest, GDF‐15 levels were >4‐fold higher in patients who died from cardiovascular failure versus severe neurologic injury or brain death (32 838 [30 269–36 489] pg/mL versus 7912 [2187–18 044] pg/mL, P <0.01, post‐hoc Wilcoxon rank sum).
In this secondary analysis of POCCA, we identified serum GDF‐15 levels as a biomarker of cardiovascular failure. Although prior adult studies emphasize the potential of GDF‐15 for neuro‐prognostication postarrest, most of these arrests had a cardiac cause or occurred in patients with pre‐existing cardiovascular disease. 2 , 3 In contrast, most arrests in our study were respiratory arrests, which allowed us to better distinguish cardiac and brain biomarkers. Here, we found that serum GDF‐15 is associated with both a cardiac cause of arrest and postresuscitation cardiovascular failure, suggesting possible release from injured myocardium.
Limitations of our study include a small sample size and short GDF‐15 time course. The overlapping ranges of GDF‐15 across groups may limit its utility as sole discriminatory biomarker for cardiovascular dysfunction. Luminex assessments can impact measurement accuracy, though data were consistent with our prior single‐center study using ELISA. 4
In conclusion, GDF‐15 is a biomarker with a previously unrecognized association with arrest of cardiac cause and cardiovascular failure in the pediatric postarrest population. Future studies should seek to validate this finding and explore the utility of GDF‐15 to predict and monitor postarrest cardiovascular failure in the pediatric intensive care unit.
Appendix
Collaborators—POCCA Investigators
Hülya Bayır, MD, Ashok Panigrahy, MD, and David Maloney, BS (UPMC Children's Hospital of Pittsburgh, Pittsburgh, PA).
Sue R. Beers, PhD and Amery Treble‐Barna, PhD (University of Pittsburgh School of Medicine, Pittsburgh, PA).
Tamara L. Haller, BS (University of Pittsburgh School of Public Health, Pittsburgh, PA).
Jose Pineda, MD, MSCI (UCLA Mattel Children's Hospital, University of California, Los Angeles, Los Angeles, CA).
Christopher Newth, MD (Children's Hospital Los Angeles, Los Angeles, CA).
Alexis A. Topjian, MD, MSCE (Children's Hospital of Philadelphia, University of Pennsylvania School of Medicine, Philadelphia, PA).
Craig A. Press, MD, PhD (Children's Hospital of Philadelphia, University of Pennsylvania School of Medicine, Philadelphia, PA).
Aline B. Maddux, MD, MSCS (University of Colorado School of Medicine and Children's Hospital Colorado, Aurora, CO).
Frederick Willyerd, MD (Phoenix Children's Hospital, Phoenix, AZ).
Elizabeth A. Hunt, MD, PhD and Ashley Siems, MD, MEd (Johns Hopkins Children's Center, Baltimore, MD).
Melissa G. Chung, MD (Nationwide Children's Hospital, Columbus, OH).
Lincoln Smith, MD (University of Washington School of Medicine, Seattle, WA).
J. Wesley Diddle, MD (Children's National Hospital, Washington, DC).
Jason Patregnani, MD (Barbara Bush Children's Hospital, Portland, ME).
Juan Piantino, MD (Oregon Health & Science University, Portland, OR).
Binod Balakrishnan, MD and Michael T. Meyer, MD, MS, FCCM (Children's Wisconsin, Medical College of Wisconsin, Milwaukee, WI).
Stuart Friess, MD (St. Louis Children's Hospital, St. Louis, MO).
Sources of Funding
This work was supported by National Institutes of Health (NIH) T32 HD040686 (J.R.H.), The Zoll Foundation (J.R.H.), The Laerdal Foundation (J.R.H.), NIH NS105721 (T.C.J.), NIH NS096714 (E.L.F.), and the Ake Grenvik Endowment (P.M.K.).
Disclosures
Dr Kochanek has multiple grants from federal agencies, including the National Institutes of Health and the US Army. Dr Kochanek has also served as an expert witness on several cases over the past 36 months, has received honoraria for numerous lectures at national meetings and/or as a guest professor at various institutions of higher education, and has received stipends for editing or authoring books and/or chapters. Drs Kochanek and Jackson have received multiple patents and have several submitted patent applications, but no money has been generated. Dr Jackson is also a co‐founder of the life science startup Constellation Neuroscience. The remaining authors have no disclosures to report.
Acknowledgments
This project used the UPCI Cancer Biomarkers Facility: Luminex Core Laboratory. We thank Chunyan Wang for assistance assembling clinical data for this secondary analysis.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT02769026.
This manuscript was sent to Kori S. Zachrison, MD, MSc, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 4.
Contributor Information
Patrick M. Kochanek, Email: kochanekpm@pitt.edu.
the POCCA Investigators:
Hülya Bayır, Ashok Panigrahy, David Maloney, Sue R. Beers, Tamara L. Haller, Amery Treble‐Barna, Jose Pineda, Christopher Newth, Alexis A. Topjian, Craig A. Press, Aline B. Maddux, Frederick Willyerd, Elizabeth A. Hunt, Ashley Siems, Melissa G. Chung, Lincoln Smith, J. Wesley Diddle, Jason Patregnani, Juan Piantino, Binod Balakrishnan, Michael T. Meyer, and Stuart Friess
References
- 1. Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini‐Vittori M, Korf‐Klingebiel M, Napp LC, Hansen B, et al. GDF‐15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17:581–588. doi: 10.1038/nm.2354 [DOI] [PubMed] [Google Scholar]
- 2. Richter B, Uray T, Krychtiuk KA, Schriefl C, Lenz M, Nurnberger A, Kastl SP, Wojta J, Heinz G, Schwameis M, et al. Growth differentiation factor‐15 predicts poor survival after cardiac arrest. Resuscitation. 2019;143:22–28. doi: 10.1016/j.resuscitation.2019.07.028 [DOI] [PubMed] [Google Scholar]
- 3. Rueda F, Cediel G, Garcia‐Garcia C, Aranyo J, Gonzalez‐Lopera M, Aranda Nevado MC, Serra Gregori J, Oliveras T, Labata C, Ferrer M, et al. Growth differentiation factor 15 and early prognosis after out‐of‐hospital cardiac arrest. Ann Intensive Care. 2019;9:119. doi: 10.1186/s13613-019-0593-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herrmann JR, Fink EL, Fabio A, Au AK, Berger RP, Janesko‐Feldman K, Clark RSB, Kochanek PM, Jackson TC. Serum levels of the cold stress hormones FGF21 and GDF‐15 after cardiac arrest in infants and children enrolled in single center therapeutic hypothermia clinical trials. Resuscitation. 2022;172:173–180. doi: 10.1016/j.resuscitation.2021.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fink EL, Kochanek PM, Panigrahy A, Beers SR, Berger RP, Bayir H, Pineda J, Newth C, Topjian AA, Press CA, et al. Association of blood‐based brain injury biomarker concentrations with outcomes after pediatric cardiac arrest. JAMA Netw Open. 2022;5:e2230518. doi: 10.1001/jamanetworkopen.2022.30518 [DOI] [PMC free article] [PubMed] [Google Scholar]


