Abstract
Multiparametric magnetic resonance imaging (mpMRI) plays a vital role in prostate cancer diagnosis and management. With the increase in use of mpMRI, obtaining the best possible quality images has become a priority. The Prostate Imaging Reporting and Data System (PI-RADS) was introduced to standardize and optimize patient preparation, scanning techniques, and interpretation. However, the quality of the MRI sequences depends not only on the hardware/software and scanning parameters, but also on patient-related factors. Common patientrelated factors include bowel peristalsis, rectal distension, and patient motion. There is currently no consensus regarding the best approaches to address these issues and improve the quality of mpMRI. New evidence has been accrued since the release of PI-RADS, and this review aims to explore the key strategies which aim to improve prostate MRI quality, such as imaging techniques, patient preparation methods, the new Prostate Imaging Quality (PI-QUAL) criteria, and artificial intelligence on prostate MRI quality.
Keywords: Prostatic Neoplasms, Magnetic Resonance Imaging, Diagnostic Imaging
Introduction
Multiparametric magnetic resonance imaging (mpMRI) of the prostate has become an essential component in the diagnostic pathway of localized prostate cancer, enabling more precise detection and characterization of cancerous lesions. Prostate mpMRI has been shown to improve clinically significant prostate cancer (csPCa) diagnosis through targeted biopsies [1, 2]. It is also useful for ruling out csPCa, preventing unnecessary biopsies [3]. The quality of the MRI is crucial, given its major role in aiding cancer detection and guiding prostate biopsies. However, the widespread use of mpMRI has also resulted in high variability of scan quality for each sequence across different institutions and countries around the world [4].
As a response to the growth in the use of mpMRI, the Prostate Imaging Reporting and Data System (PI-RADS) guidelines, the most recent iteration being the PI-RADSv2.1, were introduced to standardize prostate mpMRI acquisition, interpretation, and reporting [5]. Importantly, PIRADSv2 established the minimum technical requirements for the acquisition of the individual MRI sequences with the goal of standardizing imaging protocols and reducing variability in image quality [6]. Nevertheless, adherence to these requirements is variable across both academic and non-academic institutions and does not necessarily translate into acquiring high-quality scans [7]. These issues are often unrecognized, and the diagnostic performance of mpMRI of the prostate, especially in non-academic centers, is assumed to be similar to published data when in practice this is most likely not the case as low quality images end up being excluded from the studies.
To improve the image acquisition quality control process, the Prostate Imaging Quality (PIQUAL) scoring system from the PRECISION study was introduced in 2020 [8]. PI-QUAL aims to assess prostate mpMRI quality and provide a standardized formal feedback mechanism for scan variability. However, even with a standardized quality scoring system for prostate MRI, there is still no clear consensus on the crucial factors that may impact MRI quality. The quality of mpMRI depends not only on the technical equipment-related factors (magnetic field strength of MRI, vendors, hardware/software) but also on image acquisition parameters, and patient-related factors, such as rectal distension, bowel peristalsis, and patient motion (Figure 1 and Table 1). The research initiatives undertaken to improve image quality in prostate MRI have been centered around optimizing image acquisition (e.g., use of endorectal coil [ERC]), employment of various patient preparation methods, and quality control methods (e.g., PI-QUAL). These issues were particularly amplified during the COVID-19 pandemic, as many centers across the world were forced to change their MRI protocols, such as discontinuing bowel preparations, due to disruptions in patient care [9].
Figure 1.

Factors that can impact prostate mpMRI quality.
Table 1.
Common terms related to MRI quality
| Terms | Definitions |
|---|---|
| Artifacts | Image features that are seen on a scan but not actually present; it can also refer to items outside the patient that may obscure or distort the image |
| Noise | Variability that is not part of a desired signal, appears as an irregular granular pattern and degrades image information |
| Motion artifact | Occurs with voluntary or involuntary patient or organ movement during image acquisition |
| Aliasing (wraparound) | Occurs when the field of view is smaller than the body part being imaged |
| Susceptibility artifact | Distortions or signal change due to local magnetic field inhomogeneities, often result from metallic object near homogeneous external magnetic field |
| Phase-encoded motion artifact | Occurs as a result of tissue/fluid movement during image acquisition; it manifests as ghosting or blurring in the direction of phase-encoding |
As prostate MRI has improved dramatically in recent years [10], this review outlines the studies aimed at improving prostate MRI quality that have been published in the last decade. This comprehensive review will focus on strategies which aim to improve prostate MRI quality, specifically the impact of imaging techniques, patient preparation methods, PI-QUAL, and artificial intelligence (AI).
Imaging Technique-Based Quality Improvement Strategies
Endorectal Coil vs. Surface Coil
Prostate gland is a walnut sized organ deeply located in the pelvis. Its relatively small size and deep location make it quite challenging to get a high-quality and stable signal for MRI. In order to get a high-quality signal from the prostate, use of a cavitary coil (also known as ERC), was proposed in 1989 by Schnall et al. [11]. This method has been reported to be useful in several studies for detection and staging of prostate cancer [12–14]. Despite documented benefits of ERC for prostate cancer diagnosis and staging, a variety of drawbacks regarding the use of ERC for MRI of the prostate also exist, including higher costs, invasiveness, and patient discomfort, all of which are associated with patient reluctance to undergo prostate MRI.
With increasing use of mpMRI in prostate cancer diagnosis and management, excellent quality images with high patient tolerance are needed. PI-RADSv2.1 guidelines acknowledge that the use of ERC enables a higher signal-to-noise ratio (SNR) [5]. When integrated with external phasedarray coil (PAC), ERC increases SNR in the prostate at any magnetic field strength. This is particularly true for older 1.5 T scanners; the use of combined ERC-PAC with 1.5 T scanners results in better SNR and therefore a better image quality compared to PAC only images [15, 16]. This may be particularly beneficial when high spatial resolution is preferred (i.e., cancer staging). ERC usage can also be helpful for intrinsically low-SNR sequences, such as diffusion-weighted imaging (DWI) and high temporal resolution dynamic contrast-enhanced (DCE) sequences. However, a higher magnetic field strength can be translated into a better SNR. With the introduction of more advanced hardware, such as 3 T MR scanners, the question arises: is ERC still necessary for prostate MRI? Studies investigating the impact of ERC on prostate MRI quality are summarized in Table 2.
Table 2.
Studies investigating endorectal coil
| Author & date | Country | Study design | Number of patients | Number of readers | MRI system | Comparison | Ground truth | Subjective image quality | Artifacts | SNR | Cancer detection |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dhatt et al. (2020) [17] | Canada | Prospective | 23 | 2 | 3T | ERC vs. NERC | Prostatectomy | Comparable | x | x | Comparable in detecting Gleason scores of 4+3 or greater, ERC was superior at detecting Gleason score of 3+4 |
| Ullrich et al. (2020) [18] | International | Retrospective | 150 | 6 | 3T | ERC vs. FSC | MR-TRUS fusion biopsy | Comparable | ERC had less motion artifacts on DWI but more motion artifacts on T2WI | ERC had a higher SNR on T2WI | x |
| Mirak et al. (2019) [28] | USA | Retrospective | 429 | 4 | 3T | ERC vs. NERC | Prostatectomy | x | x | x | Comparable, ERC had higher detection of posterior and peripheral cancers |
| O’Donohoe et al. (2018) [19] | USA | Prospective | 18 | 2 | 3T | ERC vs. WPC | None | Comparable | ERC had more artifacts on T2WI | ERC had higher SNR for both T2WI and DWI | x |
| Gawlitza et al. (2017) [20] | Germany | Retrospective | 41 | 2 | 3T | ERC vs. NERC | Prostatectomy | ERC had a better image quality score on both T2WI and DWI | Comparable | x | Comparable |
| Baur et al. (2016) [21] | Germany | Prospective | 45 | 2 | 3T | ERC vs. NERC | MR-TRUS fusion biopsy | ERC had a better image quality score on T2WI only | ERC had more artifacts on T2WI | x | Comparable |
| Barth et al. (2016) [22] | Switzerland | Prospective | 98 | 2 | 3T | ERC vs. NERC | None | ERC had a better image quality score on DWI by 1 reader | ERC had more artifacts on DWI | ERC had a lower SNR for DWI | x |
| Costa et al. (2016) [29] | USA | Prospective | 49 | 2 | 3T | ERC vs. NERC | Prostatectomy or combined biopsy | x | x | x | ERC had higher sensitivity for cancer detection |
| Shah et al. (2015) [23] | USA | Prospective | 186 | 2 | 1.5 and 3T | 1.5 T MRI with ERC vs. 3T MRI with NERC | Prostatectomy | 3T MRI without ERC had higher image quality score by 1 reader | 1.5T MRI with ERC had more artifacts | x | Comparable |
| Turkbey et al. (2014) [30] | USA | Prospective | 20 | 2 | 3T | ERC vs. NERC | Prostatectomy | x | x | x | ERC detected more cancer foci with higher sensitivity and PPV |
| Mazaheri et al. (2013) [26] | USA | Unspecified | 25 | 1 | 3T | ERC vs. NERC | Prostatectomy | x | x | ERC had higher SNR in both PZ and TZ for DWI | x |
| Kim et al. (2012) [27] | Korea | Retrospective | 30 | 2 | 3T | ERC vs. NERC | Prostatectomy | x | x | Comparable | Comparable |
Abbreviations: ERC=endorectal coil; NERC=non-endorectal coil; FSC=flexible surface coil; WPC=wearable pelvic coil; TRUS=transrectal ultrasound;
SNR=signal-to-noise ratio; PZ=peripheral zone; TZ=transition zone.
Regarding the effect of ERC on prostate MRI subjective image quality, prior studies have found mixed results. In most of the studies identified in this narrative review, the image quality rating protocols were similar [17–23]. Two to six radiologists evaluated image quality and assigned a rating based on a subjective 5-point Likert scale, rating on items such as distinction of zonal anatomy, presence of motion, and artifacts. It is important to note that these studies did not blind the readers concerning the coil setup, as ERC arrangements were difficult to conceal on scans. Some studies showed no significant differences in perceived image quality on both T2-weighted imaging (T2WI) and DWI for 3 T MRI [17–19], while others reported improved image quality with ERC [20–22]. Specifically, in a prospective single institution study with 45 patients, Baur et al. [21] described better overall T2WI quality ratings with ERC-PAC compared to PAC only (p = 0.004). However, they did not report a significant difference in quality for DWI (p = 0.39). This study only included patients without prostate cancer which were confirmed with at least one transrectal ultrasonography (TRUS)-guided systematic biopsy of the prostate. As a result, there could be a selection bias towards patients with prostate cancer in uncommon locations or benign conditions associated with persistently elevated prostate-specific antigen. In contrast, in a prospective two institutional study with 98 patients, Barth et al. [22] found no significant difference in overall image quality scores for T2WI between PAC-only and ERC-PAC groups (p = 0.56). For DWI quality, one reader reported similar assessment scores (3.03 ± 1.10 and 3.08 ± 0.80, respectively, p = 0.67), whereas another reader rated ERC setup higher (3.27 ± 0.81 and 3.66 ± 0.85, respectively, p < 0.05). In this study, however, not all scanning parameters were kept constant between ERC and non-ERC (NERC) MRIs. In summary, past studies provided variable results in subjective image quality comparisons between ERC and NERC. This variation likely arises from the limitations in blinding ERC cases within reader studies and variable center-specific adherence to image acquisition guidelines.
Another crucial aspect of quality assessment of MRI is the presence of artifacts, as they can affect visibility and influence image interpretation. The presence of imaging artifacts is often caused by motion or other sources, such as aliasing and susceptibility. Studies have consistently demonstrated that ERC use was associated with more artifacts on prostate MRI [19, 21, 22] (Figure 2). One study found that artifact severity score was greater in T2WI acquired with an ERC-PAC compared with just PAC (2.01 ± 0.42 vs. 1.39 ± 0.70, respectively, p = 0.003). Motion artifact emanating from the interface of the ERC and rectal wall was seen in 17 of 18 T2WI with ERC. However, no significant difference was reported for DWI [19]. Baur et al. demonstrated similar findings with more artifacts being noted in ERC group for T2WI [21]. In another study, Barth et al. [22] reported that susceptibility artifacts were more commonly observed in DWI while Ullrich et al. [18] reported ERC group had more motion artifacts in T2WI but less motion artifacts in DWI. One study reported no significant difference in artifacts for both ERC-PAC and PAC setup [20]. In the study, an anti-peristaltic agent (i.e., 1 mg of glucagon) was intravenously administrated twice during the examination. The use of anti-peristaltic agents could have reduced motion artifacts. Overall, studies suggested that ERC was associated with increased artifacts, particularly motion and susceptibility artifacts, for both T2WI and DWI. These findings may reflect that ERC are more prone to signal inhomogeneity due to the non-uniform reception profile and this effect could be further amplified by poor positioning of the patient and ERC. Susceptibility artifacts could be a result of the direct interface between soft tissue and air/liquids in the ERC. Moreover, incorrect positioning and rotation of the ERC may deform or displace the prostate and lead to artifacts [24] (Figure 3). It may even stimulate bowel peristalsis and thus induce more motion artifacts [18]. Artifacts may be reduced by completely deflating the ERC to remove air bubbles and reflating it before usage [22]. It must be also noted that if present, rectal air may mimic susceptibility artifacts seen with ERC usage.
Figure 2.

Axial T2W MRIs of a 63-year-old (serum PSA=9.5ng/ml) (A) and a 53-year-old (serum PSA=5ng/ml) (B) male obtained with endorectal coil. Use of endorectal coil resulted in motion related ghosting artifacts in both patients.
Figure 3.
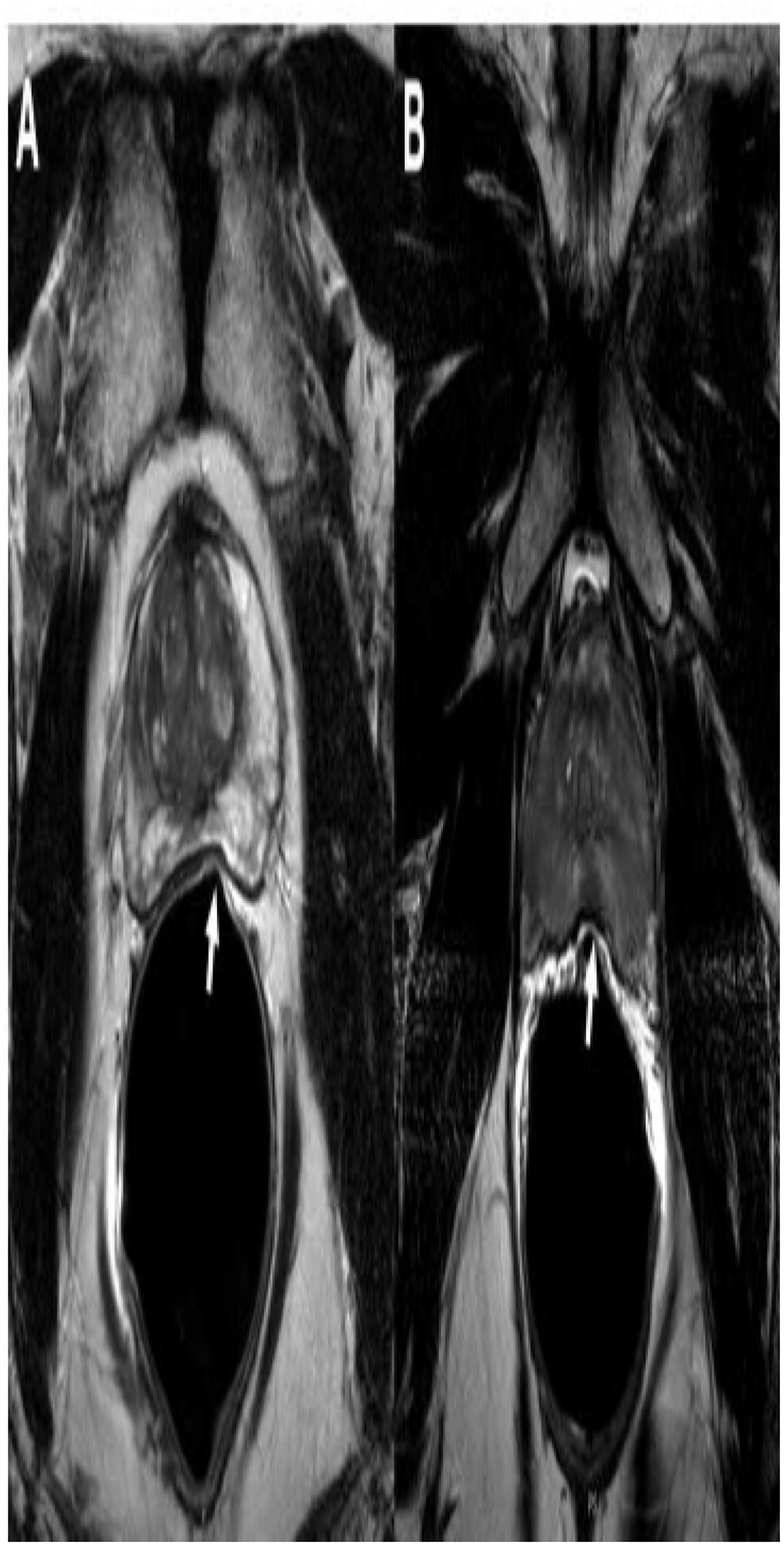
Axial T2W MRIs of a 60-year-old (serum PSA=4.8ng/ml) (A) and a 48-year-old (serum PSA=0.9ng/ml) (B) male obtained with endorectal coil. Use of endorectal coil resulted in deformations in the peripheral zone which is secondary to coil elements as a result of incorrect placement of the endorectal coils (arrows).
Apart from the subjective measures, MRI quality can be evaluated objectively, most often with quantifiable parameters such as SNR. SNR is typically measured within regions-of-interest, drawn on the whole prostate gland including the peripheral zone (PZ) and transitional zone (TZ). It is important to note that measurement of SNR for MRI scans with surface coils or various reconstruction techniques (i.e., parallel imaging) is difficult. These measurements are often estimations and may not reflect the true quantitative value [25]. Numerous studies have confirmed the use of ERC improves SNR for 3 T MRI on both T2WI and DWI [18, 19, 26]. One study measured a higher SNR for DWI with NERC group compared to ERC group [22]. However, there was no significant difference in SNR for T2WI acquired with NERC and ERC in the whole gland (22.7 ± 5.0 and 21.3 ± 6.6; p = 0.08). It is worth noting that not all parameters were kept constant between the sequences acquired with the ERC and NERC groups in this study. Specifically, number of excitations (SNR is generally proportional to the square root of the number of excitations) was higher for the NERC group, and it may have reduced the advantage of a higher resolution achieved by ERC. In addition, Kim et al. [27] reported no significant difference in T2WI SNR between the ERC and NERC groups, though the authors did note that SNR in the ERC group was higher without statistical significance (14.75 vs. 11.53, p = 0.08). Mazaheri et al. [26] found mean DWI SNR with ERC for PZ was 66.33 ± 27.07 and TZ was 32.69 ± 12.52 while mean SNR with NERC was 7.32 ± 2.30 and 6.13 ± 1.56, respectively (p < 0.0001 for both). Ullrich et al. [18] found T2WI SNRs with ERC for PZ was 76.74 and TZ was 52.49, while SNRs with NERC for PZ and TZ were 33.99 and 34.94, respectively (p <0.001 for both). In brief, ERC provided higher T2WI and DWI SNR in both PZ and TZ.
One of the most crucial questions is whether the use of ERC enhances (and ultimately improves) prostate cancer detection on MRI. Indeed, many studies have evaluated the diagnostic performance of ERC vs. surface coil in 3 T MRI. Diagnostic performance was most often investigated by parameters such as cancer detection rate, sensitivity, specificity, and positive predictive value. Most studies concluded that the diagnostic performance between ERC and surface coil were comparable [17, 20, 21, 27, 28]. However, in a prospective study with 49 patients comparing ERC and surface coil, Costa et al. [29] reported 78% and 43% sensitivity for overall lesion detection, and 92% and 47% sensitivity for index lesion (defined as the lesion with the highest Gleason score or the largest diameter for lesions with the same Gleason score) detection, respectively. In another prospective study with 20 patients, Turkbey et al. [30] reported 76% and 45% sensitivity for ERC and surface coil, respectively. Although these two studies were prospective studies, they were both single institutional studies and were limited by their relatively small sample size and by the fact that whole-mount histopathologic findings were not available for all patients in Costa et al [29]. Turkbey et al. used 6-channel cardiac coil during NERC MRI scans and 16-channel anterior cardiac coil for ERC scans. Because of this, it may not reflect a true one-to-one comparison of ERC and surface coil groups. Lastly, Mirak et al. [28] conducted a retrospective case-control study including 429 patients who underwent 3 T MRI with and without an ERC prior to prostatectomy. The study found ERC vs. surface coil had similar detection of overall and index prostate cancer. However, the ERC cohort had significantly higher detection of cancers located posteriorly and, in the PZ, but had lower detection rates of cancers located anteriorly and in the TZ. In the ERC and surface coil cohorts, 35.9% (66 of 184) and 48.4% (76 of 157) of anterior cancers (p = 0.02), 58% (200 of 345) and 48.1% (89 of 185) of posterior cancers (p = 0.03), 37.3% (41 of 110) and 54.4% (62 of 114) of TZ cancers (p = 0.01), and 53.7% (225 of 419) and 45.2% (103 of 228) of PZ cancers (p = 0.03) were detected, respectively. Restricting the study sample to surgery patients may result in selection bias, and findings may not be generalizable to overall population. Surgery patients will likely have more obvious and larger lesions on MRI, and ERC usage may not impact the diagnostic rates. However, without having a gold standard ground truth like whole-mount histopathology, it is difficult to evaluate the whole gland for cancer presence. Nevertheless, radiologists need to be aware of this mismatch between ERC and anterior/transition zone lesions especially as anterior and transition zones are often under-sampled on routine TRUS-guided biopsy [31].
1.5 Tesla vs. 3 Tesla
Another critical discussion on the use of hardware for prostate MRI scans is 1.5 T vs. 3 T magnets. Obviously 3 T magnets brings an advantage for the desired SNR, however there may be a few drawbacks. For patients with some models of medical instruments, such as aneurysm clips, vena cava filters, 3 T MRI may not be safe. Additionally, hip prosthesis can cause susceptibility artifacts and impact diagnostic performance of prostate MRIs. Metalwork can lead to signal drop out, making it difficult to distinguish between normal tissue and cancer suspicious lesions. The severity of the artifacts depends on the size, location, and composition of the prosthesis. Another challenge is the increased motion artifacts caused by the hip prosthesis. Patients with hip prostheses often have limited mobility and may experience pain or discomfort during the exam, which can lead to increased voluntary/involuntary motions. To overcome these issues, some proposed the utilization of 1.5 T MR scanners over 3 T for patients with hip prosthesis [32]. The rationale is that although 3 T MRI generates higher SNR compared to 1.5 T, it often suffers more from susceptibility and ghosting artifacts due to geometric distortion [33–35]. However, there is only one study so far that directly compares the image quality of 1.5 T and 3 T MRI for patients with hip prosthesis [36]. In a retrospective multicenter study with 140 patients with uni- or bilateral total hip replacement, Boschheidgen et al. [36] found no significant difference between subjective artifact severity in prostate mpMRI of patients with hip prosthesis between 1.5 T and 3 T MRI. This study is only a starting point and additional research is needed to establish a standardized imaging protocol for patients with hip prosthesis. Furthermore, deep learning reconstruction (DLR) may play an important role in 1.5 T MRI in the future as it has the potential to compensate for the relatively lower SNR.
In conclusion, the use of ERC provides significant improvement in SNR for both T2WI and DWI, but its effects on subjective image quality and cancer detection in 3 T MRI remains controversial, although most studies suggested ERC does not improve MRI diagnostic performance dramatically. The limited number of studies comparing 1.5 T MRI with ERC to 3 T MRI with surface coil have demonstrated no significant difference in image quality and cancer detection rates [23]. In addition, one should consider the drawbacks of ERC, such as motion and susceptibility artifacts, prostate distortion, cost of examination, and, importantly, patient discomfort. It’s important to note that evaluating the impact of ERC on DCE MRI, a component of mpMRI, has been difficult. It is impractical and unethical to administer intravenous contrast multiple times to each patient on the same day for research purposes. Therefore, there is minimal data regarding this issue. For patients with hip prothesis, there is also no clear consensus on whether 1.5 T or 3 T MRI provides better image quality. Lastly, as per PI-RADS v2.1 guidelines, an ERC should be employed to improve SNR on some older 1.5 T scanners or in patients with large intra-abdominal fat tissue, where SNR in the gland may be reduced with the use of PAC alone. Nonetheless, all MRI protocols should strive for the best and consistent quality possible with the scanners and tools available.
Patient Preparation-Based Quality Improvement Strategies
In addition to MRI acquisition methods, several patient-related factors can affect image quality of prostate mpMRI. Some of the common patient-related factors are rectal air/stool and intestinal peristalsis. These can induce artifacts that interfere with image interpretation and possibly mask cancer suspicious lesions (Figure 4). There is currently no consensus about the optimal patient preparation prior to prostate mpMRI according to PI-RADS v2.1 recommendations [5]. A variety of approaches have been explored to tackle these issues, such as having patients emptying their bowel prior to MRI or using prone imaging during MRI. More complex methods have also been employed, including the use of anti-spasmodic/anti-peristaltic agents hyoscine butylbromide (HBB), also known as scopolamine butylbromide, or glucagon to reduce rectal motion, or microenema to decrease rectal air and stool. Studies investigating the impact of patient preparation methods on prostate MRI quality are summarized in Table 3.
Figure 4.

mpMRI of a 72-year-old male with a serum PSA of 6.9 ng/mL. Axial (A), sagittal (B), coronal T2WI (C), high b-value DWI (D), ADC map (E), and DCE MRI (F). Patient has a focal lesion in right mid peripheral zone which is hypointense on axial (A) and coronal (C) T2WIs, and has a focal contrast uptake on DCE (F). However, due to large amount of rectal gas, visible on axial and sagittal T2WIs (asterisk), high b-value and ADC map images are distorted and lesion cannot be visualized (arrowheads). Targeted biopsy of the lesion revealed a Gleason grade 7 (3+4) prostate adenocarcinoma.
Table 3.
Studies investigating patient preparations
| Author & date | Study period | Country | Study design | Number of patients | Number of readers | MRI system | Comparison | Subjective image quality | Artifacts | Inter-observer agreement |
|---|---|---|---|---|---|---|---|---|---|---|
| Purysko et al. (2022) [50] | 2017–2018 | USA | Retrospective | 195 | 4 | 3 T | Enema + DR vs. enema vs. DR vs. no preparation | Enema + DR resulted in the highest overall image quality | No significant difference between groups | κ = 0.25–0.37 |
| Arnoldner et al. (2022) [48] | Unspecified | International | Prospective | 150 | 2 | 3 T | Enema + endorectal gel filling vs. no preparation | Enema + endorectal gel filling improved DWI and T2WI image quality and PI-QUAL scores | x | κ = 0.38–0.77 |
| Reischauer et al. (2021) [47] | 2017–2019 | Switzerland | Retrospective | 200 | 2 | 3 T | Enema vs. catheter | Enema resulted in the higher overall image quality | Enema had less susceptibility-related artifacts | κ = 0.920.95 |
| Schmidt et al. (2021) [49] | 2018–2020 | Switzerland | Retrospective | 180 | 2 | 3 T | HBB vs. enema vs. DR vs. combination of HBB, enema, and DR | Enema improved image quality of DWI and overall MRI | Enema reduced artifacts | ICC = 0.46 |
| Sathiadoss et al. (2021) [51] | 2019–2020 | Canada | Retrospective | 280 | 3 | 3 T | DR vs. enema vs. enema + DR vs. enema + DR + HBB vs. no preparation | Enema + DR resulted in the highest T2W and DWI image quality | Enema + DR resulted in the least DWI artifacts | κ = 0.15–0.78 |
| Coskun et al. (2020) [44] | 2016–2017 | USA | Retrospective | 117 | 2 | 3 T | Enema vs. no preparation | Enema reduced rectal gas but with only minor effects on overall image quality | No significant difference between groups | κ = 0.08–0.53 |
| Plodeck et al. (2020) [45] | 2017 | Germany | Retrospective | 114 | 2 | 3 T | Enema vs. no preparation | x | Enema had less artifacts on DWI | κ = 0.80 |
| Slough et al. (2018)[39] | 2015–2016 | UK | Prospective | 173 | 2 | 3 T | HBB vs. non-HBB | HBB improved image quality for T2WI | HBB reduced T2WI motion and blur | κ = 0.34–0.71 |
| Ullrich et al. (2017)[38] | Unspecified | Germany | Prospective | 103 | 2 | 3 T | HBB vs. non-HBB | HBB improved anatomic score on T2WI | HBB enhanced artifact score on T2WI | κ = 0.95–0.98 |
| Lim et al. (2014)[46] | 2013–2014 | Canada | Retrospective | 60 | 2 | 3 T | Enema vs. no preparation | No difference in image quality on T2W or ADC | No significant difference between groups | x |
| Roethke et al. (2013) [41] | 2010–2011 | Germany | Retrospective | 70 | 2 | 3 T | HBB vs. non-HBB | No significant difference between groups | No significant difference between groups | κ = 0.37–0.53 |
Abbreviations: DR=dietary restrictions; HBB=hyoscine butylbromide; ICC=intraclass correlation coefficient; κ=Cohen’s kappa coefficient.
Among anti-spasmodic agents, HBB has been the main focus of the majority of the studies on prostate MRI quality. The rationale behind the use of anti-spasmodic agents is to reduce motionrelated artifacts that deteriorate image quality. A prior study showed that the benefit of antiperistaltic agents on prostate MRI quality may be limited as the prostate is positioned away from the small bowel [37]. More recent studies on HBB, however, reported that its use is associated with improved subjective reader assessments, especially for T2WI. Most studies identified in this review focused on subjective image quality utilizing a 5-point Likert scale based on reader assessments. In a prospective study of 103 patients, Ullrich et al. [38] reported improvement in both visualization of anatomic structures (mean score 3.4 before administration vs. 4.4 after, p < 0.001) and motion-related artifacts (mean score 3.2 before vs. 4.2 after, p < 0.001) on T2WIs for about 68% of the patients. In addition, Slough et al. [39] showed better subjective T2WI quality after administration of HBB in 173 patients in a retrospective study (HBB score 3.63 vs. non-HBB 2.84, p < 0.001). The HBB group also had less blurring and motion artifacts compared to non-HBB group. No significant difference on DWI or apparent diffusion coefficient (ADC) image quality were reported in the aforementioned study. Although the results showed a trend towards improved quality of DCE, it did not reach statistical significance (HBB corrupt data points 2.47 vs. non-HBB 3.68, p = 0.052). This may be due to diminished effect of HBB over time after administration. Anti-peristaltic effect from HBB on average lasts around 20 minutes [40], suggesting that either a second bolus of HBB prior to DCE or a combination of HBB with longer-acting glucagon may be beneficial in the setting of longer imaging protocols. Lastly, it is worth noting that Roethke et al. [41] reported no consequential difference in subjective image quality or artifacts after administration of HBB. This may be partly due to the smaller sample size of 70 patients and moderate inter-observer agreement with Cohen’s kappa coefficients scores of 0.37–0.53. In summary, studies have demonstrated an improvement in image quality of T2WI after intravenous administration of HBB. Nonetheless, HBB administration increases MRI preparation time and cost. It is often associated with unwanted side effects, mostly related to its anti-cholinergic activity, such as dry mouth, blurred vision, and dizziness [42]. Therefore, the added costs (i.e., costs associated with administration of the medicine) and potential for adverse reactions should be taken into consideration when deciding on whether to use HBB for prostate MRI.
Rectal loading due to excessive air or stool induces DWI distortion and reduces image quality by causing susceptibility artifacts [43] (Figure 5). Susceptibility artifacts tend to occur in areas around the air–tissue interfaces, such as between the rectum and posterior part of the prostate, where most of prostate cancers are found. Removal of rectal air, or the use of prone imaging may overcome susceptibility artifacts. MRI technologists often need to identify these issues, but rescanning is often not possible due to scheduling constraints. Due to these reasons, several techniques to ensure minimal rectal loading before MRI have been evaluated, including enemas, rectal catheters, and dietary restrictions (DR). Self-administration of enema in a hospital setting before MRI may be stressful for many patients, leading to higher risk of unsuccessful bowel preparation. Administrating at home the day of the exam can be a suitable alternative to increase the success of the application and ensure enema can take effect before the study.
Figure 5.

mpMRI of a 55-year-old man with a serum PSA of 6.7 ng/mL. Axial (A), sagittal (B), coronal T2WI (C), high b-value DWI (D), and ADC map (E). Patient has a lesion in right apical-mid peripheral zone which is hypointense on axial (A) and coronal (C) T2WIs. Rectal gas, which can be seen on axial (A) and sagittal (B) T2WIs (asterisk), impacts the quality of high bvalue DWI (D) and ADC map (E) images which subsequently makes the intraprostatic lesion indistinguishable on these sequences (arrowheads). There is also an accompanying local distortion and shining in the posterolateral aspect of the left hemigland on high b-value DWI (D) (dashed arrow). Targeted biopsy of the lesion revealed a Gleason grade 7 (3+4) prostate adenocarcinoma.
Studies in the past have mostly examined enema administration vs. no patient preparation, and the results have been mixed [44–46]. There are also some concerns about the possibility of enemainduced peristalsis which may result in increased motion-related artifacts [5]. A study has suggested that enema administration was not associated with improvement in image quality nor a reduction in artifacts [46]. However, this study included a relatively small cohort of 60 patients, in which no patients had significant amount of rectal gas. Moreover, inter-reader agreement was not assessed. Coskun et al. [44] showed that enema may diminish rectal air but with minor effects on DWI distortion and overall image quality. In this study, there was a notable inter-reader variability regarding the assessment of image quality and artifacts, with Cohen’s kappa coefficients scores ranging from 0.08 to 0.53. Plodeck et al. [45] reported that application of enema significantly reduces both the incidence and severity of artifacts on DWI in a retrospective study of 114 patients. However, they did not directly analyze the effect of enema on overall image quality. In another study, Reischauer et al. [47] compared the use of enema to catheter preparation. The authors demonstrated that enema preparation was superior to catheter preparation regarding the image quality of DWI and reduced the occurrence of susceptibility artifacts. Direct comparison to nonenema group was not performed, therefore, no firm conclusion could be reached regarding enema vs. non-enema groups. Arnoldner et al. [48] incorporated endorectal gel-filling in addition to the conventional enema to create a novel rectal preparation protocol. In their prospective study, the authors concluded enema plus endorectal gel-filling improved overall image quality on DWI and lesion visibility on T2WI. They have also reported improved PI-QUAL scores with their bowel preparation technique. This study did not include an enema-only cohort either. Overall, studies have shown conflicting results on whether the use of enema improves image quality and reduces artifacts on prostate mpMRI. Further research is needed to validate the reports of these studies. More recent studies have investigated enema administration in combination with other patient preparation methods, such as HBB and DR [49–51]. In a retrospective study of 180 patients, Schmidt et al. [49] concluded that enema improved image quality while HBB and DR did not have any significant effects. Purysko et al. [50] found that enema and/or DE improved the overall image quality of prostate MRI by decreasing rectal distension and distortion of DWI. Similarly, in a study consist of 280 patients, Sathiadoss et al. [51] compared five different preparation strategies for prostate MRI: no preparation, DR, enema, enema plus DR, enema plus DR and HBB. The study reported enema plus DR before prostate MRI yielded the highest T2WI and DWI quality with the least artifacts on DWI. Rectal distension was reduced significantly with the administration of enema. Unlike enema and DR, HBB did not result in an improvement in image quality on T2WI or DWI or lessen DWI artifacts despite diminishing the motion artifacts on T2WI.
In brief, there was an existing heterogeneity in patient cohorts and methodologies in the aforementioned studies. Variation in dietary habits due to difference in locations and cultures can influence rectal distension and therefore affect image quality. In addition, inconsistency in study protocols, such as variability in timing of enema administration and dose/type of anti-peristaltic agents can impact image quality as well. All the studies identified in this review administered HBB intravenously [38, 39, 41, 49, 51]. HBB can be administered intramuscularly as well, and it has theoretical advantage of a more prolonged action [52]. Only one study compared intravenous HBB administration to intramuscular administration, and there were no significant difference in overall image quality between the two routes of administration [37]. More studies are needed to further investigate whether different administration routes of HBB impact prostate MRI quality. Overall, studies have shown that the use of anti-spasmodic agents such as HBB can reduce rectal motion, improve image quality, particularly T2WI. HBB should be given as late as possible to ensure that MRI takes place during the biological activity window of the anti-spasmolytics. Anti-spasmodic agents may be beneficial in some patients with hyper-motile intestine or flatulence. However, in many others it may not be necessary, and the additional cost and potential for adverse drug reactions should always be taken into consideration. On the other hand, past studies on the use of enema before MRI exams have contradicting results. Recent studies with larger patient cohorts seem to suggest that the use of a preparatory rectal enema alone or in conjunction with DR is superior to HBB in terms of improving mpMRI image quality with minimal side effects and additional cost.
Radiologist Evaluation-Based Standardized Quality Control Strategy
PI-RADS aims to standardize and improve the performance and interpretation of prostate mpMRI by setting minimum technical standards and providing suggestions to improve image quality. In real clinical practice, adherence to these minimum technical standards is highly variable, especially in non-academic institutions [53]. It must be noted that adherence to the PI-RADS technical recommendations does not necessarily translate into good-quality prostate mpMRIs [7]. With some MRIs, better results may be obtained with technical parameters outside the recommendations. While technical parameters are critical, several other factors such as patient motion, rectal air-related artifacts, and coil utilization can impact MRI quality. The available data published over the last decade are conflicting and draw mixed conclusions in terms of patient preparation and image acquisition. This is corroborated by the fact that studies often included a different subjective scoring system, mostly based on a 1–5 Likert scale. Even with the recently updated PI-RADS v2.1 recommendations, there is still vast inconsistency on how to produce images of the best quality. Furthermore, there is no consensus on what constitutes a good quality prostate MRI and no standardized criteria to assess image quality.
To address these problems and provide a more standardized assessment, Giganti et al. [8] introduced a novel scoring system, PI-QUAL, that evaluate each sequence against the PI-RADS standards and assigns a score to prostate MRI quality. The PI-QUAL score ranges from 1 to 5, where 1 indicates that none of the three MRI sequences (T2-WI, DWI and DCE) are of diagnostic quality, 3 indicates that at least two MRI sequences taken together are of acceptable diagnostic quality, and 5 means that each sequence is independently of optimal diagnostic quality with proper adherence to PI-RADS technical requirements (Figure 6).
Figure 6.

A diagram explaining how PI-QUAL scores are assigned and their implications.
As PI-QUAL was introduced only in 2020, a limited number of studies on PI-QUAL and its impact on improving prostate MRI quality have been published [36, 48, 54–58]. The full details are shown in Table 4. Studies have focused on evaluating the inter-reader agreement of PI-QUAL scores between two readers. Most found the reproducibility of PI-QUAL was moderate with Cohen’s kappa coefficients between 0.42–0.55 [48, 54–56]. Giganti et al. [58] reported substantial agreement between two experienced radiologists in the assessment of PI-QUAL (weighted kappa = 0.82, with 84% agreement), but it should be pointed out that the two readers were involved in the development of the PI-QUAL score [8], and presumably were more familiar with the scoring system than the readers from the other studies. They also had significantly more prostate MRI reading experience (both read >1,800 prostate MRI scans per year, one with 7 years of experience and the other with 20 years of experience) compared to average readers. Therefore, the true interreader agreement for PI-QUAL in clinical practice requires further validation.
Table 4.
Studies investigating PI-QUAL
| Author & date | Study period | Country | Study design | Number of patients | Number of readers | MRI system | PI-QUAL inter observer agreement | Objective | Findings |
|---|---|---|---|---|---|---|---|---|---|
| Karanasios et al. (2022) [55] | 2018 | UK | Retrospective | 247 | 3 | 3 T | κ = 0.47–0.51 | To assess the reproducibility and impact of PI-QUAL scores | Higher PI-QUAL scores are associated with decreased uncertainty in MRI and improved efficiency for cancer diagnosis |
| Potsch et al. (2022) [54] | 2018–2019 | Austria | Retrospective | 50 | 2 | 1.5 or 3 T | κ = 0.51 | To test the inter-reader agreement of PI-QUAL score for mpMRI and its impact on diagnostic performance in an MR-TRUS fusion biopsy population | PI-QUAL has a limited impact on PI-RADS diagnostic performance for MR-TRUS fusion biopsy; the prevalence of prostate cancer was significantly lower when the PI-QUAL score was < 3 |
| Girometti et al. (2022) [56] | 2020 | Italy | Retrospective | 66 | 2 | 1.5 T | κ = 0.51–0.55 | To investigate the interreader agreement of PI-QUAL for mpMRI | The reproducibility of PI-QUAL was moderate; higher PI-QUAL scores were associated with excellent inter-reader agreement for PI-RADSv2.1 categorization |
| Dinneen et al. (2022) [57] | Unspecified | UK | Retrospective | 121 | 3 | 1.5 or 3 T | x | To assess the influence of mpMRI scan quality on EPE diagnostic accuracy | EPE prediction was more reliable when the MRI scan had a higher PI-QUAL score |
| Arnoldner et al. (2022) [48] | Unspecified | International | Prospective | 150 | 2 | 3 T | κ = 0.42 | To investigate the effects of a rectal preparation regimen, that consisted of a rectal cleansing enema and an endorectal gel filling protocol, on PI-QUAL | Rectal preparation significantly improved PI-QUAL scores |
| Giganti et al. (2021) [58] | Unspecified | UK | Retrospective | 103 | 2 | 1.5 or 3 T | κ = 0.82–0.85 | To investigate the interreader reproducibility of the PI-QUAL score in patients enrolled in the NeuroSAFE PROOF trial | The reproducibility in the assessment of PI-QUAL between two radiologists with high expertise in prostate mpMRI was strong |
| Boschheidgen et al. (2021) [36] | 2016–2020 | Germany | Retrospective | 140 | 3 | 1.5 or 3 T | x | To evaluate image quality and diagnostic value of mpMRI in patients with total hip replacement at 1.5 and 3 T | PI-QUAL is applicable for prostate MRI in patients with total hip replacement |
Abbreviations: HBB=hyoscine butylbromide; κ=Cohen’s kappa coefficient; TRUS=transrectal ultrasound; EPE=extra-prostatic extension.
The standardized evaluation and reporting of image quality via PI-QUAL truly fulfills its purpose only if PI-QUAL scores have an objective impact on csPCa (defined as Gleason Grade Group ≥ 2) detection. The limited number of studies so far suggested that higher PI-QUAL score may improve the efficiency of diagnostic pathway of prostate cancer by reducing false-positive MRI calls and unnecessary biopsies. In a retrospective study with 50 patients undergoing MRI-TRUS fusion biopsy, Potsch et al. [54] reported that overall diagnostic performance did not differ between studies with optimal diagnostic quality compared to lower diagnostic quality (area under the receiver operating characteristics curve [AUC] for PI-QUAL > 3: 0.805 vs. for PI-QUAL ≤ 3: 0.839; p = 0.55;). However, the prevalence of csPCa was lower when the PI-QUAL score was ≤ 3 compared to > 3 (16.7% vs. 30.2%, p = 0.008). Scans with PI-QUAL > 3 also had higher positive predictive value (PI-QUAL > 3: 56% vs. PI-QUAL ≤ 3: 36%, p = 0.001). This suggested csPCa may be more apparent with higher quality scans, and the low-quality images may lead to falsepositive calls and subsequent unnecessary biopsies, lowering the biopsy yield of high PI-RADS scores. However, it is worth mentioning that this study used a modified PI-RADS scoring scheme where the prostate was divided into six regions and each region was assigned a PI-RADS score. In another retrospective study of 247 patients, Karanasios et al. [55] reported that when comparing PI-QUAL 5 to PI-QUAL 3 studies, the PI-RADS 1–2 calls increased from 50 to 87% and PI-RADS 3 calls decreased from 31.8 to 10.4%. More patients with PI-QUAL 1–3 underwent biopsy for PIRADS 1–2 (47%) and 3 (100%) MRIs compared to patients with PI-QUAL score 4–5 (30 and 75%, respectively). Dinneen et al. [57] specifically investigated the impact of PI-QUAL score on diagnostic accuracy of extra-prostatic extension on mpMRI. The authors reported the extra-prostatic extension prediction was more reliable with higher PI-QUAL scores with an AUC of 0.92 (0.88–0.96) for PI-QUAL 4–5 scans and AUC of 0.78 (95% CI: 0.72–0.84) for PI-QUAL 1–3 scans. In summary, studies suggest that better quality scans with higher PI-QUAL scores have limited effect on overall diagnostic performance of MRI-TRUS fusion biopsy, but they are associated with improved rates of extra-prostatic extension prediction and increased efficiency of prostate cancer diagnosis by reducing unnecessary biopsies.
Quality evaluation of MRI sequences is one of the key steps for successful prostate cancer diagnosis using MRI. The current version of PI-QUAL is only the starting point for the evaluation and standardization of prostate mpMRI quality and diagnostic reliability. Future prospective validations and refinements of PI-QUAL are under way. One study has shown that PI-QUAL score is strongly correlated with diagnostic value and artifact severity of mpMRI in patients with hip prosthesis at both 1.5 and 3 T [36]. This suggested that PI-QUAL can be applied to mpMRI in patients with hip prosthesis. Another factor to consider is that the current version of PI-QUAL only applies to mpMRI, therefore a biparametric MRI oriented PI-QUAL system may be needed in the future due to the rising interest towards prostate MRI without contrast [59]. Defining scan quality is highly subjective and experience dependent, and readers are likely to disagree on what constitutes optimal quality. The previous studies have shown that the reproducibility of PI-QUAL is likely moderate in clinical practice [48, 54–56]. Automated tools, including AI based on deep learning, may provide a more objective and consistent assessment of image quality and initial attempts to create semi-automated software have been made [60]. However, such algorithms are under development and not yet widely used in clinical practice. Until then, attempts on standardizing quality assessment with initiatives such as PI-QUAL holds a great value and initial results from dedicated teaching courses on image quality have been shown to be successful [61]. Its widespread adoption will be a crucial first step toward improving prostate MRI quality.
Artificial Intelligence-Based Quality Improvement Strategy
AI is an emerging technology that is more commonly used in radiology in the recent years. AI can improve the quality of prostate MRI through various approaches, such as image reconstruction and image quality assessment/classification. Although the use of AI is a relatively new topic for prostate MRI quality, there are few studies investigating the roles of AI in prostate MRI quality (Table 5). Image reconstruction is the process of converting raw k-space data acquired by an MR scanner into a final image. Traditional image reconstruction methods can be limited by various factors such as loss of SNR and introduction of artifacts [62–64]. On the other hand, DLR employs convolutional neural networks to learn the mapping from a large dataset of k-space data to the corresponding images. It can reduce noise and artifacts, therefore results in more accurate and detailed final images [65]. This approach has been shown to provide higher subjective overall image quality, SNR, and contrast-to-noise ratio (CNR) for both T2WI and DWI than images taken with the same parameters and processed using other approaches [66–73]. When correlated to pathology, one study showed diagnostic performance for the evaluation of extra-prostatic extension were similar between DLR and conventional protocol [69]. Furthermore, Wang et al. [72] found DLR with the ERC signal turned off had better subjective overall image quality and less artifacts compared to DLR with the ERC signal turned on and to the conventional reconstruction with ERC. This study suggested that scans acquired without ERC could potentially benefit from DLR to enhance image quality. Important to note, the groups with the ERC signal turned off still had the ERC in place. This removed confounding factors such as ERC positional differences and technical parameter variations, but it does not allow the direct assessment of the effect of removing the ERC on overall image quality. In addition to improving image quality, AIbased image reconstruction techniques have the potential to reduce scan time and provide more comfortable experience for the patients. mpMRI typically has a long examination time of more than 30 minutes; this can be especially difficult for patients who are elderly or claustrophobic. The duration of the MRI examination greatly influences its cost and accessibility. As the scan time increases, the images become more vulnerable to motion artifacts as well. Past studies have consistently shown that implementation of DLR can reduce MRI acquisition time (up to 76% reduction) while attaining similar or better image quality [66, 67, 69–71, 73–75]. This is achieved by reducing the number of excitations to accelerate acquisition time and subsequently improve SNR and CNR via DLR.
Table 5.
Studies investigating the role of artificial intelligence in prostate MRI quality
| Author & date | Study period | Country | Study design | Number of patients | Number of readers | MRI system | MRI sequence | Acceleration | Subjective image quality | SNR | CNR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Harder et al. (2022) [66] | Unspecified | Germany | Prospective | 33 | 4 | 3 T | T2WI | DLR reduced acquisition time by 58% | DLR had higher quality ratings compared to standard images | DLR resulted in higher SNR | DLR resulted in higher CNR |
| Tajima et al. (2022) [67] | 2021 | Japan | Prospective | 17 | 2 | 1.5 T | DWI | DLR reduced acquisition time by 64% | Comparable | x | DLR resulted in higher CNR for bone metastasis |
| Ueda et al. (2022) [68] | 2020 | Japan | Retrospective | 60 | 2 | 3 T | DWI | x | DLR had higher quality ratings compared to standard images | DLR resulted in higher SNR | DLR resulted in higher CNR |
| Johnson et al. (2022) [74] | Unspecified | USA | Retrospective | 113 | 4 | 3 T | T2WI and DWI | DLR reduced acquisition time by 73% | Comparable for both T2WI and DWI | x | x |
| Cipollari et al. (2022) [76] | 2020 | Italy | Retrospective | 312 | 3 | 3 T | T2WI, DWI, ADC, and DCE | x | x | x | x |
| Park et al. (2022) [69] | 2019–2020 | Korea | Retrospective | 109 | 3 | 3 T | T2WI | DLR reduced acquisition time by 38% | DLR had higher quality ratings compared to standard images | DLR resulted in higher SNR | DLR resulted in higher SNR |
| Gassenmaier et al. (2021) [70] | 2020 | Germany | Retrospective | 30 | 2 | 3 T | T2WI | DLR reduced acquisition time by 65% | DLR had higher quality ratings compared to standard images | x | x |
| Gassenmaier et al. (2021) [71] | 2020–2021 | Germany | Prospective | 60 | 2 | 3 T | T2WI | DLR reduced acquisition time by more than 60% | DLR had higher quality ratings compared to standard images | x | x |
| Kim et al. (2021) [75] | 2021 | Korea | Retrospective | 46 | 2 | 3 T | T2WI | DLR reduced acquisition time by 76% | Comparable | Comparable | Comparable |
| Wang et al. (2021) [72] | 2019 | USA | Retrospective | 31 | 3 | 1.5 or 3 T | T2WI | x | DLR with ERC signal turned off had best overall image quality score | x | x |
| Kaye et al. (2020) [73] | 2018–2019 | USA | Retrospective | 155 | 2 | 3 T | DWI | DLR reduced acquisition time by 70% | DLR had higher quality ratings compared to standard images | DLR resulted in higher SNR | x |
Abbreviations: DLR=deep learning reconstruction; SNR=signal-to-noise ratio; CNR=contrast-to-noise ratio; ERC=endorectal coil.
Additionally, AI can be used to assess the quality of prostate MRI scans and identify any artifacts or other issues that may be present. This ensures that images are of sufficient quality for accurate diagnosis and treatment planning. With AI, an MRI scan can be quickly evaluated for adequacy without relying on personal opinions of those involved. Finding out that image quality is unsatisfactory during MRI reading sessions, which may be hours/days after MRI acquisition is of limited value to the patients and physicians. Automated tools may warn the technical team during image acquisition and have them rescan the patient if necessary. In clinical practice, this would necessitate software that monitors image quality in real time during MRI exams and equipment with high computational powers. This can potentially improve MRI acquisition efficiency and reduce examination time and cost. So far, only one study on this topic has been published. Cipollari et al. [76] used a convolutional neural network-based image quality tool to retrospectively classify 326 mpMRI scans. The per-slice classification accuracy was 90.0% for T2WI, 80.0% for DWI, 76.6% for ADC, and 96.6% for DCE. The per-sequence accuracy was 100% for T2WI, DWI, and DCE, and 92.3% for ADC. While the results were promising, its impact on diagnostic performance was not evaluated. In addition, generalizability may be restricted as only a limited number of sequences (n = 869) generated from one MR scanner were used for training. The algorithm also employed a binary classification to assess image quality, a semiquantitative scale with multiple scores may be more applicable to address the complexity of MRI scans.
Overall, AI has the potential to improve the quality (SNR, CNR, and subjective image quality) of prostate MRI while significantly reducing image acquisition time, potentially leading to more accurate diagnosis and staging of prostate cancer. It is worth mentioning that these algorithms are still in the research phase and require further prospective investigation to fully prove their effectiveness and safety before clinical use. The use of AI-based techniques such as image reconstruction and image quality assessment have the potential to improve the accuracy and reliability of prostate mpMRI and ultimately improve clinical outcomes.
Conclusions
In conclusion, assurance of high-quality MRIs is of paramount importance for prostate mpMRI to contribute to clinical management of localized prostate cancer. Several strategies have been tried and reported to improve prostate MRI quality. Use of endorectal coil at 3T magnets has been documented to improve objective image quality by providing an increase in SNR. However, its effects on subjective overall image quality and cancer detection still remains controversial. Patient preparation-based strategies have been more studied in the last half decade. Among them, the use of anti-spasmodics such as HBB can reduce rectal motion and improve image quality, particularly for T2WI. On the other hand, studies on the use of enema before MRI exams have conflicting results but the current literature suggests that the use of a preparatory rectal enema in conjunction to DR is superior to HBB in terms of improving DWI image quality with minimal side effects and costs. There is compelling evidence to support the standardization of prostate MRI quality using objective and pre-defined criteria, and the PI-QUAL score is the first attempt to bridge this gap. With the limited number of studies to date, the reproducibility of the PI-QUAL has been shown to be moderate and higher PI-QUAL scores may improve efficiency of diagnostic pathway of prostate cancer by reducing false-positive MRI calls and unnecessary biopsies. Lastly, AI-based image reconstruction, which is a very new field, can significantly improve both objective and subjective image quality while reducing MRI acquisition time. For all of these quality improvement strategies, further prospective studies are needed to document their clinical impact and value.
Key Points:
Endorectal coil increases signal-to-noise ratio for T2WI and DWI sequences, but it results in more motion and susceptibility artifacts. It has no clear impact on subjective image quality and prostate cancer detection.
Hyoscine butylbromide (scopolamine butylbromide) reduces rectal motion and improves T2WI quality. Rectal enema alone or in conjunction to dietary restrictions is superior to hyoscine butylbromide alone in improving MRI quality.
Deep learning-based reconstruction can improve signal-to-noise ratio, contrast-to-noise ratio, and subjective image quality of prostate MRI while shortening image acquisition time.
Acknowledgments
Yue Lin: none
Enis C. Yilmaz: none
Mason J. Belue: none
Baris Turkbey: Cooperative research and development agreements with NVIDIA and Philips; Royalties from the National Institutes of Health; Patents in the field of artificial intelligence.
Abbreviations:
- ERC
Endorectal coil
- NERC
Non-endorectal coil
- SNR
Signal-to-noise ratio
- CNR
Contrast-to-noise ratio
- PAC
Phased-array coil
- HBB
Hyoscine butylbromide
- DR
Dietary restrictions
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Kasivisvanathan V, Rannikko AS, Borghi M et al. (2018) MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med 378:1767–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahdoot M, Wilbur AR, Reese SE et al. (2020) MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N Engl J Med 382:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed HU, El-Shater Bosaily A, Brown LC et al. (2017) Diagnostic accuracy of multiparametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389:815–822 [DOI] [PubMed] [Google Scholar]
- 4.Greer MD, Shih JH, Lay N et al. (2019) Interreader Variability of Prostate Imaging Reporting and Data System Version 2 in Detecting and Assessing Prostate Cancer Lesions at Prostate MRI. AJR Am J Roentgenol. 10.2214/AJR.18.20536:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turkbey B, Rosenkrantz AB, Haider MA et al. (2019) Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol 76:340–351 [DOI] [PubMed] [Google Scholar]
- 6.Weinreb JC, Barentsz JO, Choyke PL et al. (2016) PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 69:16–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sackett J, Shih JH, Reese SE et al. (2021) Quality of Prostate MRI: Is the PI-RADS Standard Sufficient? Acad Radiol 28:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giganti F, Allen C, Emberton M, Moore CM, Kasivisvanathan V, group Ps (2020) Prostate Imaging Quality (PI-QUAL): A New Quality Control Scoring System for Multiparametric Magnetic Resonance Imaging of the Prostate from the PRECISION trial. Eur Urol Oncol 3:615–619 [DOI] [PubMed] [Google Scholar]
- 9.Surasi DSS, Wang X, Bathala TK et al. (2021) The impact and collateral damage of COVID-19 on prostate MRI and guided biopsy operations: Society of Abdominal Radiology Prostate Cancer Disease-Focused Panel survey analysis. Abdom Radiol (NY) 46:4362–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giganti F, Rosenkrantz AB, Villeirs G et al. (2019) The Evolution of MRI of the Prostate: The Past, the Present, and the Future. AJR Am J Roentgenol 213:384–396 [DOI] [PubMed] [Google Scholar]
- 11.Schnall MD, Lenkinski RE, Pollack HM, Imai Y, Kressel HY (1989) Prostate: MR imaging with an endorectal surface coil. Radiology 172:570–574 [DOI] [PubMed] [Google Scholar]
- 12.Futterer JJ, Engelbrecht MR, Jager GJ et al. (2007) Prostate cancer: comparison of local staging accuracy of pelvic phased-array coil alone versus integrated endorectal-pelvic phased-array coils. Local staging accuracy of prostate cancer using endorectal coil MR imaging. Eur Radiol 17:1055–1065 [DOI] [PubMed] [Google Scholar]
- 13.Turkbey B, Pinto PA, Mani H et al. (2010) Prostate Cancer: Value of Multiparametric MR Imaging at 3 T for Detection-Histopathologic Correlation. Radiology 255:89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heijmink SWTPJ, Futterer JJ, Hambrock T et al. (2007) Prostate cancer: Body-array versus endorectal coil MR imaging at 3 T - Comparison of image quality, localization, and staging performance. Radiology 244:184–195 [DOI] [PubMed] [Google Scholar]
- 15.Hricak H, White S, Vigneron D et al. (1994) Carcinoma of the prostate gland: MR imaging with pelvic phased-array coils versus integrated endorectal--pelvic phased-array coils. Radiology 193:703–709 [DOI] [PubMed] [Google Scholar]
- 16.Futterer JJ, Scheenen TW, Huisman HJ et al. (2004) Initial experience of 3 tesla endorectal coil magnetic resonance imaging and 1H-spectroscopic imaging of the prostate. Invest Radiol 39:671–680 [DOI] [PubMed] [Google Scholar]
- 17.Dhatt R, Choy S, Co SJ et al. (2020) MRI of the Prostate With and Without Endorectal Coil at 3 T: Correlation With Whole-Mount Histopathologic Gleason Score. AJR Am J Roentgenol 215:133–141 [DOI] [PubMed] [Google Scholar]
- 18.Ullrich T, Kohli MD, Ohliger MA et al. (2020) Quality Comparison of 3 Tesla multiparametric MRI of the prostate using a flexible surface receiver coil versus conventional surface coil plus endorectal coil setup. Abdom Radiol (NY) 45:4260–4270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donohoe RL, Dunne RM, Kimbrell V, Tempany CM (2019) Prostate MRI using an external phased array wearable pelvic coil at 3T: comparison with an endorectal coil. Abdom Radiol (NY) 44:1062–1069 [DOI] [PubMed] [Google Scholar]
- 20.Gawlitza J, Reiss-Zimmermann M, Thormer G et al. (2017) Impact of the use of an endorectal coil for 3 T prostate MRI on image quality and cancer detection rate. Sci Rep 7:40640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baur AD, Daqqaq T, Wagner M et al. (2016) T2- and diffusion-weighted magnetic resonance imaging at 3T for the detection of prostate cancer with and without endorectal coil: An intraindividual comparison of image quality and diagnostic performance. Eur J Radiol 85:1075–1084 [DOI] [PubMed] [Google Scholar]
- 22.Barth BK, Cornelius A, Nanz D, Eberli D, Donati OF (2016) Comparison of image quality and patient discomfort in prostate MRI: pelvic phased array coil vs. endorectal coil. Abdom Radiol (NY) 41:2218–2226 [DOI] [PubMed] [Google Scholar]
- 23.Shah ZK, Elias SN, Abaza R et al. (2015) Performance comparison of 1.5-T endorectal coil MRI with 3.0-T nonendorectal coil MRI in patients with prostate cancer. Acad Radiol 22:467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatterjee A, Thomas S, Oto A (2020) Prostate MR: pitfalls and benign lesions. Abdom Radiol (NY) 45:2154–2164 [DOI] [PubMed] [Google Scholar]
- 25.Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO (2007) Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging 26:375–385 [DOI] [PubMed] [Google Scholar]
- 26.Mazaheri Y, Vargas HA, Nyman G, Shukla-Dave A, Akin O, Hricak H (2013) Diffusionweighted MRI of the prostate at 3.0 T: comparison of endorectal coil (ERC) MRI and phased-array coil (PAC) MRI-The impact of SNR on ADC measurement. Eur J Radiol 82:e515–520 [DOI] [PubMed] [Google Scholar]
- 27.Kim BS, Kim TH, Kwon TG, Yoo ES (2012) Comparison of pelvic phased-array versus endorectal coil magnetic resonance imaging at 3 Tesla for local staging of prostate cancer. Yonsei Med J 53:550–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirak SA, Shakeri S, Bajgiran AM et al. (2019) Three Tesla Multiparametric Magnetic Resonance Imaging: Comparison of Performance with and without Endorectal Coil for Prostate Cancer Detection, PI-RADS version 2 Category and Staging with Whole Mount Histopathology Correlation. J Urol 201:496–502 [DOI] [PubMed] [Google Scholar]
- 29.Costa DN, Yuan Q, Xi Y et al. (2016) Comparison of prostate cancer detection at 3-T MRI with and without an endorectal coil: A prospective, paired-patient study. Urol Oncol 34:255 e257–255 e213 [DOI] [PubMed] [Google Scholar]
- 30.Turkbey B, Merino MJ, Gallardo EC et al. (2014) Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: correlation with whole-mount histopathology. J Magn Reson Imaging 39:1443–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu X, Liu Z, Chang T et al. (2019) Transperineal Magnetic Resonance Imaging-Targeted Biopsy May Perform Better Than Transrectal Route in the Detection of Clinically Significant Prostate Cancer: Systematic Review and Meta-analysis. Clin Genitourin Cancer 17:e860–e870 [DOI] [PubMed] [Google Scholar]
- 32.Caglic I, Barrett T (2019) Optimising prostate mpMRI: prepare for success. Clin Radiol 74:831–840 [DOI] [PubMed] [Google Scholar]
- 33.Mazaheri Y, Vargas HA, Nyman G, Akin O, Hricak H (2013) Image artifacts on prostate diffusion-weighted magnetic resonance imaging: trade-offs at 1.5 Tesla and 3.0 Tesla. Acad Radiol 20:1041–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ullrich T, Quentin M, Oelers C et al. (2017) Magnetic resonance imaging of the prostate at 1.5 versus 3.0T: A prospective comparison study of image quality. Eur J Radiol 90:192–197 [DOI] [PubMed] [Google Scholar]
- 35.Soher BJ, Dale BM, Merkle EM (2007) A review of MR physics: 3T versus 1.5T. Magn Reson Imaging Clin N Am 15:277–290 [DOI] [PubMed] [Google Scholar]
- 36.Boschheidgen M, Ullrich T, Blondin D et al. (2021) Comparison and prediction of artefact severity due to total hip replacement in 1.5 T versus 3 T MRI of the prostate. Eur J Radiol 144:109949. [DOI] [PubMed] [Google Scholar]
- 37.Wagner M, Rief M, Busch J et al. (2010) Effect of butylscopolamine on image quality in MRI of the prostate. Clin Radiol 65:460–464 [DOI] [PubMed] [Google Scholar]
- 38.Ullrich T, Quentin M, Schmaltz AK et al. (2018) Hyoscine butylbromide significantly decreases motion artefacts and allows better delineation of anatomic structures in mp-MRI of the prostate. Eur Radiol 28:17–23 [DOI] [PubMed] [Google Scholar]
- 39.Slough RA, Caglic I, Hansen NL, Patterson AJ, Barrett T (2018) Effect of hyoscine butylbromide on prostate multiparametric MRI anatomical and functional image quality. Clin Radiol 73:216 e219–216 e214 [DOI] [PubMed] [Google Scholar]
- 40.Gutzeit A, Binkert CA, Koh DM et al. (2012) Evaluation of the anti-peristaltic effect of glucagon and hyoscine on the small bowel: comparison of intravenous and intramuscular drug administration. Eur Radiol 22:1186–1194 [DOI] [PubMed] [Google Scholar]
- 41.Roethke MC, Kuru TH, Radbruch A, Hadaschik B, Schlemmer HP (2013) Prostate magnetic resonance imaging at 3 Tesla: Is administration of hyoscine-N-butyl-bromide mandatory? World J Radiol 5:259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson W, Taylor MB, Carrington BM, Bonington SC, Swindell R (2007) The value of hyoscine butylbromide in pelvic MRI. Clin Radiol 62:1087–1093 [DOI] [PubMed] [Google Scholar]
- 43.Caglic I, Hansen NL, Slough RA, Patterson AJ, Barrett T (2017) Evaluating the effect of rectal distension on prostate multiparametric MRI image quality. Eur J Radiol 90:174–180 [DOI] [PubMed] [Google Scholar]
- 44.Coskun M, Mehralivand S, Shih JH et al. (2020) Impact of bowel preparation with Fleet’s enema on prostate MRI quality. Abdom Radiol (NY) 45:4252–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plodeck V, Radosa CG, Hubner HM et al. (2020) Rectal gas-induced susceptibility artefacts on prostate diffusion-weighted MRI with epi read-out at 3.0 T: does a preparatory micro-enema improve image quality? Abdom Radiol (NY) 45:4244–4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim C, Quon J, McInnes M, Shabana WM, El-Khodary M, Schieda N (2015) Does a cleansing enema improve image quality of 3T surface coil multiparametric prostate MRI? J Magn Reson Imaging 42:689–697 [DOI] [PubMed] [Google Scholar]
- 47.Reischauer C, Cancelli T, Malekzadeh S, Froehlich JM, Thoeny HC (2021) How to improve image quality of DWI of the prostate-enema or catheter preparation? Eur Radiol 31:6708–6716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arnoldner MA, Polanec SH, Lazar M et al. (2022) Rectal preparation significantly improves prostate imaging quality: Assessment of the PI-QUAL score with visual grading characteristics. Eur J Radiol 147:110145. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt C, Hotker AM, Muehlematter UJ, Burger IA, Donati OF, Barth BK (2021) Value of bowel preparation techniques for prostate MRI: a preliminary study. Abdom Radiol (NY) 46:4002–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Purysko AS, Mielke N, Bullen J et al. (2022) Influence of Enema and Dietary Restrictions on Prostate MR Image Quality: A Multireader Study. Acad Radiol 29:4–14 [DOI] [PubMed] [Google Scholar]
- 51.Sathiadoss P, Haroon M, Osman H, Ahmad F, Papadatos P, Schieda N (2022) Comparison of 5 Rectal Preparation Strategies for Prostate MRI and Impact on Image Quality. Can Assoc Radiol J 73:346–354 [DOI] [PubMed] [Google Scholar]
- 52.Tytgat GN (2008) Hyoscine butylbromide - a review on its parenteral use in acute abdominal spasm and as an aid in abdominal diagnostic and therapeutic procedures. Curr Med Res Opin 24:3159–3173 [DOI] [PubMed] [Google Scholar]
- 53.Cuocolo R, Stanzione A, Ponsiglione A et al. (2019) Prostate MRI technical parameters standardization: A systematic review on adherence to PI-RADSv2 acquisition protocol. Eur J Radiol 120:108662. [DOI] [PubMed] [Google Scholar]
- 54.Potsch N, Rainer E, Clauser P et al. (2022) Impact of PI-QUAL on PI-RADS and cancer yield in an MRI-TRUS fusion biopsy population. Eur J Radiol 154:110431. [DOI] [PubMed] [Google Scholar]
- 55.Karanasios E, Caglic I, Zawaideh JP, Barrett T (2022) Prostate MRI quality: clinical impact of the PI-QUAL score in prostate cancer diagnostic work-up. Br J Radiol 95:20211372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Girometti R, Blandino A, Zichichi C et al. (2022) Inter-reader agreement of the Prostate Imaging Quality (PI-QUAL) score: A bicentric study. Eur J Radiol 150:110267. [DOI] [PubMed] [Google Scholar]
- 57.Dinneen E, Allen C, Strange T et al. (2022) Negative mpMRI Rules Out Extra-Prostatic Extension in Prostate Cancer before Robot-Assisted Radical Prostatectomy. Diagnostics (Basel) 12:1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giganti F, Dinneen E, Kasivisvanathan V et al. (2022) Inter-reader agreement of the PIQUAL score for prostate MRI quality in the NeuroSAFE PROOF trial. Eur Radiol 32:879–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belue MJ, Yilmaz EC, Daryanani A, Turkbey B (2022) Current Status of Biparametric MRI in Prostate Cancer Diagnosis: Literature Analysis. Life (Basel) 12:804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giganti F, Lindner S, Piper JW et al. (2021) Multiparametric prostate MRI quality assessment using a semi-automated PI-QUAL software program. Eur Radiol Exp 5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giganti F, Cole AP, Fennessy FM et al. (2023) Promoting the use of the PI-QUAL score for prostate MRI quality: results from the ESOR Nicholas Gourtsoyiannis teaching fellowship. Eur Radiol 33:461–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang RK, Roth CG, Ward RJ, deJesus JO, Mitchell DG (2010) Optimizing Abdominal MR Imaging: Approaches to Common Problems. Radiographics 30:185–U210 [DOI] [PubMed] [Google Scholar]
- 63.Winkel DJ, Heye TJ, Benz MR et al. (2019) Compressed Sensing Radial Sampling MRI of Prostate Perfusion: Utility for Detection of Prostate Cancer. Radiology 290:702–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenkrantz AB, Geppert C, Grimm R et al. (2015) Dynamic contrast-enhanced MRI of the prostate with high spatiotemporal resolution using compressed sensing, parallel imaging, and continuous golden-angle radial sampling: preliminary experience. J Magn Reson Imaging 41:1365–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gassenmaier S, Kustner T, Nickel D et al. (2021) Deep Learning Applications in Magnetic Resonance Imaging: Has the Future Become Present? Diagnostics (Basel) 11:2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harder FN, Weiss K, Amiel T et al. (2022) Prospectively Accelerated T2-Weighted Imaging of the Prostate by Combining Compressed SENSE and Deep Learning in Patients with Histologically Proven Prostate Cancer. Cancers 14:5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tajima T, Akai H, Sugawara H et al. (2022) Feasibility of accelerated whole-body diffusion-weighted imaging using a deep learning-based noise-reduction technique in patients with prostate cancer. Magnetic Resonance Imaging 92:169–179 [DOI] [PubMed] [Google Scholar]
- 68.Ueda T, Ohno Y, Yamamoto K et al. (2022) Deep Learning Reconstruction of Diffusion-weighted MRI Improves Image Quality for Prostatic Imaging. Radiology 303:373–381 [DOI] [PubMed] [Google Scholar]
- 69.Park JC, Park KJ, Park MY, Kim MH, Kim JK (2022) Fast T2-Weighted Imaging With Deep Learning-Based Reconstruction: Evaluation of Image Quality and Diagnostic Performance in Patients Undergoing Radical Prostatectomy. J Magn Reson Imaging 55:1735–1744 [DOI] [PubMed] [Google Scholar]
- 70.Gassenmaier S, Afat S, Nickel D, Mostapha M, Herrmann J, Othman AE (2021) Deep learning-accelerated T2-weighted imaging of the prostate: Reduction of acquisition time and improvement of image quality. Eur J Radiol 137:109600. [DOI] [PubMed] [Google Scholar]
- 71.Gassenmaier S, Afat S, Nickel MD et al. (2021) Accelerated T2-Weighted TSE Imaging of the Prostate Using Deep Learning Image Reconstruction: A Prospective Comparison with Standard T2-Weighted TSE Imaging. Cancers (Basel) 13:3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X, Ma J, Bhosale P et al. (2021) Novel deep learning-based noise reduction technique for prostate magnetic resonance imaging. Abdom Radiol (NY) 46:3378–3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaye EA, Aherne EA, Duzgol C et al. (2020) Accelerating Prostate Diffusion-weighted MRI Using a Guided Denoising Convolutional Neural Network: Retrospective Feasibility Study. Radiol Artif Intell 2:e200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson PM, Tong A, Donthireddy A et al. (2022) Deep Learning Reconstruction Enables Highly Accelerated Biparametric MR Imaging of the Prostate. J Magn Reson Imaging 56:184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim EH, Choi MH, Lee YJ, Han D, Mostapha M, Nickel D (2021) Deep learningaccelerated T2-weighted imaging of the prostate: Impact of further acceleration with lower spatial resolution on image quality. Eur J Radiol 145:110012. [DOI] [PubMed] [Google Scholar]
- 76.Cipollari S, Guarrasi V, Pecoraro M et al. (2022) Convolutional Neural Networks for Automated Classification of Prostate Multiparametric Magnetic Resonance Imaging Based on Image Quality. J Magn Reson Imaging 55:480–490 [DOI] [PMC free article] [PubMed] [Google Scholar]


