Abstract
Objectives:
to evaluate the accessibility, success rate, and attributable complications and to describe the maneuver for central line insertion via proximal basilic or axillary veins in neonates.
Methods:
This retrospective study included all infants admitted to the neonatal intensive care unit and had an axillary central line inserted or attempted. Success rate, complications, and outcomes were reviewed.
Results:
Axillary central line was attempted in 85 infants and was successful in 78 infants with a success rate of 91.7%. The median postnatal age of patients was 8 days (2 days–92 days), and the median weight of patients at the procedure was 2600 g (590 g–3900 g). The median corrected gestational age of patients at the procedure was 36 weeks (23 weeks–46 weeks). No serious complication was observed in any of the 85 infants.
Conclusion:
This study demonstrated a high success rate for insertion of proximal basilic and axillary veins central lines in neonates with difficult vascular access. This procedure was feasible in very low birth and extremely low birth preterm infants, especially in those who failed previous central line attempts.
Keywords: central lines, proximal basilic vein, axillary vein, neonates
Introduction
Central venous catheters have been used in the neonatal population for long-term medication infusion and total parenteral nutrition. They have been associated with fewer complications compared to umbilical venous catheterization and surgical central line and a high success insertion rate.1,2 Intravenous (IV) access may represent a unique challenge in many neonates as their veins are often poorly visualized. Repeated and failed venipunctures are a source of significant stress to hospitalized infants and their parents. 3 Healthcare providers may face frustrations having no options when managing an infant with difficult intravenous access that is desperately needed for a long duration. 4
Many studies addressed central lines’ indications, techniques, and complications in infants.5–7 Having a standardized maneuver for vein cannulation is shown to better contribute to the safety and success of the procedure compared to random cannulation trials. One of the veins whose cannulation is well described in the literature is the long saphenous vein in the lower limb. 8 Although it is safe and feasible, long saphenous central line cannulation has limitations due to its longest course between the medial malleolus in the ankle region and the inferior vena cava. In addition, lower limb veins have valves that may interfere with line threading. Other veins have also been used for central line insertions, such as veins in the dorsum of the hand, basilic and cephalic veins in the antecubital fossa and the popliteal veins. The axillary vein and proximal basilic veins are relatively large veins. The distance between the axilla and the superior vena cava is relatively short; therefore, threading a central line via axillary vein would be technically easier than threading a catheter for longer distance from the ankle, for example, to the inferior vena cava. These features make axillary cannulation suitable in the smallest size premature infants. However, they are rarely accessed in neonates. We are unaware of any report or description of the accessibility, maneuver, and safety for central line insertion in neonates’ basilic or axillary veins.
We hypothesized that central line insertion in the proximal basilic or axillary veins is feasible and safe when the proper cannulation technique is applied. This study aims to evaluate the accessibility, success rate, and attributable complications and to describe the maneuver for line insertion via proximal basilic or axillary veins in neonates.
Methods
Setting
This retrospective analysis included all infants admitted to the neonatal intensive care unit (NICU) at Cleveland Clinic Foundation over 4 years, from January 2018 through December 2021. The study was approved by the Institutional Review Board at Cleveland Clinic (study # 22-751) with a waiver of consent and waiver of HIPAA authorization. A preprint version of the study is available at https://www.researchsquare.com/article/rs-2518718/latest.pdf.
Definitions
According to the World Congress on Vascular Access Foundation, there are several types of central venous access devices: 9 Epicutaneo-caval catheters (ECC) are inserted under direct visualization in peripheral superficial veins with their tip in the superior or inferior vena cava. This type of catheters are mostly utilized in neonates; they have small bore (1–2.7 Fr), and they are not suitable for blood withdrawal, blood transfusions, or hemodynamic monitoring. Catheters that are inserted in deep veins (at least 7 mm deep from the skin) are utilized; they have wider bores (⩾3 Fr) and can be used for blood sampling and transfusions. These catheters are often referred to as Centrally Inserted Central Catheter, CICC (if venipuncture in the cervicothoracic area) or Femorally Inserted Central Catheters, FICC (venipuncture of common femoral vein).
Patients
Infants included in the study had the following criteria: (a) admitted to the NICU during the period January 1st, 2018–December 31st, 2021, (b) being born at term (⩾37 weeks) or preterm (<37 weeks) gestation, (c) had central line insertion attempted that was not successful, and (d) subsequently had an axillary central line inserted or attempted. Infants were not included in this study if: (a) they had a central line inserted successfully when utilizing the conventional technique in other peripheral veins (e.g., in the basilic or saphenous vein), or (b) they did not require central line insertion during their hospital stay.
Sample size calculation
To detect an effect size of 35% for the mean, using a two-tailed t-test, a sample size of 72 would be adequate with 90% power (α = 0.05, β = 0.9 and σ = 10%). With an estimated two axillary line attempts per month, a review of data of at least 36 months would be required. The study was planned to retrieve data on infants during the full 4 years in anticipation of any data attrition or if some months had an inadequate number of axillary lines.
Central line insertion practice
All infants with the anticipated need for intravenous access >4 days will have a central line inserted. This includes mainly very low birth weight infants (BW < 1500 g) and infants with congenital anomalies requiring surgical intervention. Umbilical venous catheters are inserted immediately after birth and can be used up to 5–7 days postnatally. If an infant continues to need central venous access, a central line is inserted before the removal of umbilical venous catheters. Lines are inserted by neonatal advanced practice providers and neonatal physicians. Straight and prominent veins are preferred for the central line placement. Distal veins are attempted for cannulation before proximal veins. Most of the time, a central line is placed near the antecubital region in the basilic or cephalic veins or close to the ankle region in the long saphenous vein anterior to the medial malleolus. If these veins are not accessible, proximal veins are attempted by the most experienced caregivers. In such a scenario, the proximal basilic and axillary veins are the preferred veins for cannulation.
Proximal basilic or axillary vein cannulation
Anatomic background
The basilic vein meets the brachial vein in the axilla to create the axillary vein, which travels medially to the brachial and axillary arteries. The axillary vein is not easily accessible to percutaneous cannulation in the intermediate or the proximal portion as it runs deep underneath the muscles in the armpit. The distal portion of the axillary vein runs relatively superficial at the lower border of the pectoralis major and the teres major muscles.
Technique
Apply chlorhexidine (2% chlorhexidine gluconate in 70% isopropyl alcohol) swabs to the entire extremity, including the axilla, the shoulder region, and a wide margin of the scapula, and allow it to dry. Insert the entire upper extremity into the hole of the sterile drape. Measure the distance between the planned insertion site and the superior vena cava junction with the right atrium approximately at the 3rd costo-sternal junction. Trim the central line accordingly. The line used in this experience was silicone-based without guide wire that comes with an introducer needle (BD-Canada, Ontario, Canada). Keep the arm position abducted between 100° and 130°, with the shoulder elevated with an appropriately sized neck roll. Compress the axillary vein using the “Thumb and Index Technique” with the index finger deep in the axilla pressing gently against the humerus bone head and the thumb at the mid of the arm stretching the tissue in the upper part of the arm with the other three fingers supporting the back of the arm (Figure 1). With this technique, the index finger will function as a tourniquet, and the proximal portion of the basilic vein and the distal portion of the axillary vein will become prominent. Alternatively, the primary provider can position the arm as described above, and an assistant can push with his/her index finger in the middle of the axilla against the humerus head. Before cannulation, the provider must palpate to ensure it is not the pulsatile axillary artery. Once the introducer is inserted and blood return is seen in the hub, the rest of the procedure will be similar to the cannulation of other veins as previously described.
Figure 1.

Thumb-and-index technique for cannulation of the axillary vein.
The index finger is positioned deep in the axilla, gently pressing against the head of the humerus bone while the thumb is positioned at the mid of the humerus bone stretching the skin of the upper part of the arm. The other three fingers are supporting the back of the arm.
If the vein was not visualized, raise the hand of the infant while the index finger is still pressing against the humerus head. This increases venous return and may help fill the veins and make them more visible. When the vein is not visible in bigger neonates, an alternative method is to palpate the axillary artery against the humerus head and then insert the introducer at a point medial to and parallel to the visualized or palpated axillary artery.10,11 Once inserted, test the functionality of the line by withdrawing and flushing with the heparinized normal saline (1 unit/1 ml). The portable chest radiograph is performed at the bedside, with the procedure field maintained sterile. Line is dressed using transparent film dressing (Tegaderm 3M, MN, USA) and secured with hypoallergenic adhesive strips (Steri-Strip, 3M, MN, USA) after confirming the position of the line tip radiographically.
Central line management
Fluid infusion is started immediately after confirmation of line position. Heparin is added to all fluids infusing via umbilical or central lines at a dose of 0.5 U/ml according to the internal procedure. Intravenous tubing is changed sterilely by two neonatal nurses as previously described.12,13 The medications are infused via a closed medication system. Central line dressing is changed every 7 days or when soiled.
Data collection
The institutional review board approved this retrospective descriptive study. All infants with axillary lines inserted or attempted were retrieved from the medical records starting January 2018 through December 2021. Demographic and clinical data were collected on infants with axillary lines inserted using this new technique. Data included the infant’s gestational age, postnatal age at the time of the procedure, birth weight, weight at the time of the procedure, catheter features, success rate, number of insertion attempts, the reason for removal, clinical indications, outcomes, and complications.
Statistical analysis
Means and standard deviations were used to describe continuous parametric variables, medians with ranges were used for continuous non-parametric variables, and percentages were used for categorical variables. We used Microsoft Excel 2020 for all the above calculations.
Results
Eighty-five infants were identified in the hospital records with attempts of brachial/axillary central line placement. Seventy-eight of them were successfully inserted using the insertion technique described above (78/85, success rate 91.7%), Figure 2. Characteristics of the study population are shown in Table 1. The central line was inserted on the first venipuncture attempt in 28 infants (28/78, 35.9%) and the second venipuncture attempt in 30 infants (30/78, 38.5%). Maximum attempts are three attempts; pain control is used if the patients are not on pain medications and need multiple attempts. The median postnatal age at the time of the procedure was 8 days (2 days–92 days), and the median weight of patients was 2600 g (590 g–3900 g). The median corrected gestational age of patients was 36 weeks (23 weeks–46 weeks). The duration of the line used was 21 days (3 days–92 days). ECC line size was 26 gauge in all cases except for one case where a 28-gauge catheter was used. Out of the seven unsuccessful attempts, three cases of lines were inserted inadvertently in the axillary artery and were discovered on a chest X-ray. All of them were immediately removed (Figure 3).
Figure 2.
Algorithm for infants included in the study.
Table 1.
Demographic and clinical characteristics of the study population.
| Characteristics | N = 85 |
|---|---|
| Patient’s weight at the time of the procedure (g) | 2600 (590–3900) |
| Postmenstrual age of patients at the time of the procedure (weeks) | 36 (23–46) |
| Postnatal age at the time of the procedure | 8 (2–92) |
| The duration of the line use (days) | 21 (3–92) |
| Primary Outcome (success) a | 78/85 (91.7%) |
| Axillary line inserted on the first attempt a | 28/78 (35.9%) |
| Axillary line inserted on the second attempt a | 30/78 (38.5%) |
| The length of the central line inserted inside the body (cm) | 10.5 (8–12) |
Data are expressed in median (range), except with adata are expressed in number (%).
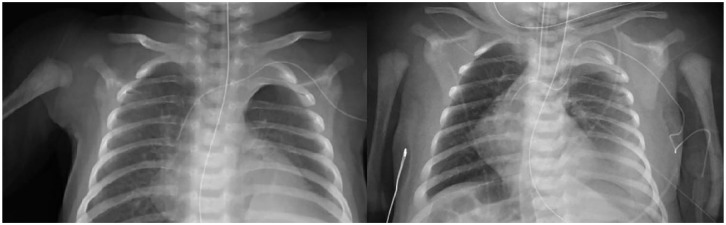
Figure 3.
Chest radiographs for axillary line position.
On the left, a chest X-ray demonstrating central line inserted in the right axillary vein with its tip at the superior vena cava-right atrial junction. On the right, a chest X-ray demonstrates the line accidentally inserted in the left axillary artery with its tip in the ascending thoracic aorta.
The side most inserted was the left (89.7 %). The length of the central line inserted and introduced inside the body was 10.5 cm (range 8 cm–12 cm). The tip was located in a central vein in all patients, which was confirmed by an x-ray. The duration of line use was 28 days (range: 14 days–35 days). Central lines were removed in three cases due to clotting/malfunctioning; otherwise, they were uneventfully removed in the rest of the cases.
Discussion
This study demonstrated a total of 78 central lines inserted successfully using the described insertion technique with a success rate of 91.7%. The median postnatal age of patients was 8 days (2 days–92 days), and the median weight of patients at the procedure was 2600 g (590 g–3900 g). The patients’ median corrected gestational age at the procedure was 36 weeks (23 weeks–46 weeks).
In newborns, central lines are safe and reliable vascular access. On the other hand, the insertion of the central line is an extremely delicate technique with a high failure rate.14–16 For infants who require venous access for more than a few days, central lines have become a more common modality of intervention; the optimal vein for catheter insertion should be chosen when the indication for line installation has been confirmed. Basilic or cephalic veins in the upper extremities, temporal or posterior auricular veins in the head, and saphenous veins in the lower extremities are all frequent superficial veins used for catheterization.17–22 However, in a NICU, ultrasound-guided central line placement may require fewer attempts and less time than the traditional approach. Additionally, it may increase overall success rates, reduce complications, and verify the tip position of the catheter to make the procedure safer and avoid or minimize ionizing radiation for the newborn.23–26 Polyurethane catheters could be the favored choice for newborns; it may significantly reduce risks such as infection, thrombosis, intraluminal occlusion, and mechanical complications in neonates. 24
This study has the strength of having a high success rate and fewer complications reported in the literature with 78 infants that have difficulties getting intravenous vascular access within 4 years. In addition, the study could help healthcare providers and provide solutions for infants with difficulties with intravenous vascular access. The study inherited some limitations, like a small sample size and short study duration. However, we think the sample size and duration of the study can provide important educational points about feasibility. The maneuver for inserting proximal basilic and axillary veins central lines in neonates was described.
Conclusion
This study demonstrated a high success rate for central line insertion when using the described axillary maneuver in 78 infants that had difficulties getting other intravenous vascular accesses. This procedure was feasible in very low birthweight and extremely low birthweight preterm infants, and the line was securely maintained for a long time. The study could help healthcare providers and provide solutions for infants with difficult central vascular access.
Acknowledgments
We thank Cleveland Clinic Foundation Marketing Department for drawing Figure 1 and providing permission for its copyright.
Footnotes
Author contributions: Hany Aly: Conceptualized and designed the study, interpreted the analysis, drafted the initial manuscript, and reviewed and revised the manuscript. Mohamed A Mohamed: Critically revised the manuscript and statistical analyses and approved the final draft of the manuscript. Mohsen A.A. Farghaly: Drafted the initial manuscript, and reviewed and revised the manuscript. Sehar Ejaz and Komail Malik: Drafted the initial manuscript, and reviewed and revised the manuscript. Ibrahim Qattea: Conceptualized and designed the study, conducted the analysis, drafted the initial manuscript, and reviewed and revised the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ibrahim Qattea  https://orcid.org/0000-0002-6318-6418
https://orcid.org/0000-0002-6318-6418
References
- 1. Durand M, Ramanathan R, Martinelli B, et al. Prospective evaluation of percutaneous central venous silastic catheters in newborn infants with birth weights of 510 to 3,920 grams. Pediatrics 1986; 78(2): 245–250. [PubMed] [Google Scholar]
- 2. Klein JF, Shahrivar F. Use of percutaneous silastic central venous catheters in neonates and the management of infectious complications. Am J Perinatol 1992; 9(4): 261–264. [DOI] [PubMed] [Google Scholar]
- 3. Humphrey GB, Boon CM, van Linden van den Heuvell GF, et al. The occurrence of high levels of acute behavioral distress in children and adolescents undergoing routine venipunctures. Pediatrics 1992; 90(1 Pt 1): 87–91. [PubMed] [Google Scholar]
- 4. Ozkiraz S, Gokmen Z, Ince DA, et al. Peripherally inserted central venous catheters in critically Ill premature neonates. J Vasc Access 2013; 14(4): 320–324. [DOI] [PubMed] [Google Scholar]
- 5. Westergaard B, Classen V, Walther-Larsen S. Peripherally inserted central catheters in infants and children—indications, techniques, complications and clinical recommendations. Acta Anaesthesiol Scand 2013; 57(3): 278–287. [DOI] [PubMed] [Google Scholar]
- 6. Pittiruti M, Celentano D, Barone G, et al. A GAVeCeLT bundle for central venous catheterization in neonates and children: a prospective clinical study on 729 cases. J Vasc Access. Epub ahead of print 9 May 2022. DOI: 10.1177/11297298221074472. [DOI] [PubMed] [Google Scholar]
- 7. Pittiruti M. Vascular access in paediatric patients: classification and indications. In: Biasucci DG, Disma NM, Pittiruti M. (eds.) Vascular access in neonates and children. Cham: Springer, 2022, pp. 3–24. [Google Scholar]
- 8. Uygun I, Okur MH, Otcu S, et al. Peripherally inserted central catheters in the neonatal period. Acta Cir Bras 2011; 26(5): 404–411. [DOI] [PubMed] [Google Scholar]
- 9. Barone G, Pittiruti M. Epicutaneo-caval catheters in neonates: new insights and new suggestions from the recent literature. J Vasc Access 2020; 21(6): 805–809. [DOI] [PubMed] [Google Scholar]
- 10. Oriot D, Defawe G. Percutaneous catheterization of the axillary vein in neonates. Crit Care Med 1988; 16(3): 285–286. [DOI] [PubMed] [Google Scholar]
- 11. Metz RI, Lucking SE, Chaten FC, et al. Percutaneous catheterization of the axillary vein in infants and children. Pediatrics 1990; 85(4): 531–533. [PubMed] [Google Scholar]
- 12. Timsit JF, Baleine J, Bernard L, et al. Expert consensus-based clinical practice guidelines management of intravascular catheters in the intensive care unit. Ann Intensive Care 2020; 10(1): 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Evidence-Based Medicine Group, Neonatologist Society, Chinese Medical Doctor Association. [Operation and management guidelines for peripherally inserted central catheter in neonates (2021)]. Zhongguo Dang Dai Er Ke Za Zhi 2021; 23(3): 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thiagarajan RR, Ramamoorthy C, Gettmann T, et al. Survey of the use of peripherally inserted central venous catheters in children. Pediatrics 1997; 99(2): E4. [DOI] [PubMed] [Google Scholar]
- 15. Janes M, Kalyn A, Pinelli J, et al. A randomized trial comparing peripherally inserted central venous catheters and peripheral intravenous catheters in infants with very low birth weight. J Pediatr Surg 2000; 35(7): 1040–1044. [DOI] [PubMed] [Google Scholar]
- 16. Bayley G. Technique for insertion of percutaneous central venous catheters in the newborn period. Arch Dis Child Fetal Neonatal Ed 2003; 88(3): F256–F257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chock VY. Therapeutic Techniques: peripherally Inserted Central Catheters in Neonates. NeoReviews 2004; 5(2): e60–e62. [Google Scholar]
- 18. Citak A, Karaböcüoğlu M, Uçsel R, et al. Central venous catheters in pediatric patients-subclavian venous approach as the first choice. Pediatr Int 2002; 44(1): 83–86. [DOI] [PubMed] [Google Scholar]
- 19. Finck C, Smith S, Jackson R, et al. Percutaneous subclavian central venous catheterization in children younger than one year of age. Am Surg 2002; 68(4): 401–404. [PubMed] [Google Scholar]
- 20. Rhondali O, Attof R, Combet S, et al. Ultrasound-guided subclavian vein cannulation in infants: supraclavicular approach. Paediatr Anaesth 2011; 21(11): 1136–1141. [DOI] [PubMed] [Google Scholar]
- 21. Lausten-Thomsen U, Merchaoui Z, Dubois C, et al. Ultrasound-guided subclavian vein cannulation in low birth weight neonates. Pediatr Crit Care Med 2017; 18(2): 172–175. [DOI] [PubMed] [Google Scholar]
- 22. Breschan C, Graf G, Arneitz C, et al. Retrospective evaluation of 599 brachiocephalic vein cannulations in neonates and preterm infants. Br J Anaesth 2022; 129(5): e138–e140. [DOI] [PubMed] [Google Scholar]
- 23. Al Sofyani K, Julia G, Abdulaziz B, et al. Ultrasound guidance for central vascular access in the neonatal and pediatric intensive care unit. Saudi J Anaesth 2012; 6(2): 120–124. Erratum in: Saudi J Anaesth. 2012 Oct-Dec;6(4):372. Khouloud, Al Sofyani [corrected to Al Sofyani, Khouloud]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seckold T, Walker S, Dwyer T. A comparison of silicone and polyurethane PICC lines and postinsertion complication rates: a systematic review. J Vasc Access 2015; 16(3): 167–177. [DOI] [PubMed] [Google Scholar]
- 25. Breschan C, Platzer M, Jost R, et al. Ultrasound-guided supraclavicular cannulation of the brachiocephalic vein in infants: a retrospective analysis of a case series. Paediatr Anaesth 2012; 22(11): 1062–1067. [DOI] [PubMed] [Google Scholar]
- 26. Barone G, Pittiruti M, Ancora G, et al. Centrally inserted central catheters in preterm neonates with weight below 1500 g by ultrasound-guided access to the brachio-cephalic vein. J Vasc Access 2021; 22(3): 344–352. [DOI] [PubMed] [Google Scholar]