Abstract
Background
Adult T-cell leukemia/lymphoma (ATL) is a lymphoid malignancy caused by HTLV-1 infection, with distinct geographical distribution. Despite advances in cancer treatment, the average survival rate of ATL is low. Conferone is a natural coumarin extracted from Ferula species with a wide range of pharmaceutical effects. In search for a novel chemotherapeutic agent, we investigated the cytotoxicity of conferone on ATL cells.
Methods
To obtain conferone, the methanolic extract of the roots of F. flabelliloba was subjected to silica gel column chromatography, followed by 1H- and 13C-NMR to confirm its structure. For cytotoxicity assay, MT-2 cells were treated with different concentrations of conferone (2.5, 5, 10, 20, and 40 µM) for 24, 48, and 72 h, and viability was evaluated by a colorimetric assay using alamarBlue. Cell cycle was analyzed by PI staining and flow cytometry, and qPCR was used to study the expression of candidate genes.
Results and Conclusion
Obtained findings indicated that conferone induced considerable cytotoxic effects on MT-2 cells in a time- and dose-dependent manner. In addition, accumulation of cells in the sub-G1 phase of the cell cycle was detected upon conferone administration. Moreover, conferone reduced the expression of CDK6, c-MYC, CFLIP L , and NF-κB (Rel-A) in MT-2 cells. Accordingly, conferone could be considered as a potent agent against ATL, although complementary investigations are required to define more precisely its mechanism of action.
Keywords: conferone, adult T-cell leukemia/lymphoma, natural coumarin, cytotoxicity, in vitro
Introduction
Adult T-cell leukemia/lymphoma (ATL) is a potentially aggressive neoplasm of mature T-lymphocytes associated with human T-lymphotropic virus type I (HTLV-I). 1 There are approximately 20 million HTLV-1-infected people across the world. 2 In tropical places such as South America, Central Africa, the Caribbean, South of Japan, and the Middle East, HTLV-I infection is endemic. ATL is common in individuals in their sixth and seventh decades.3,4 There are four clinical subtypes for ATL, from indolent, slowly progressive disease (smoldering and chronic) to aggressive and life-threatening disease (lymphoma and acute). Despite improvements in therapeutic strategies, the prognosis of ATL remains poor and the survival rate of patients is still low. 5 Cyclophosphamide, Adriamycin, vincristine, and prednisolone have been used as the standard first-line treatments for ATL. Although many patients do achieve either partial or complete remission, clinical outcomes of current modalities remain unfortunate. 6 Among all chemotherapeutic regimes, high response rates (ranging from 58% to 92%) were seen for acute ATL after co-administration of interferon (IFN-α) and antiviral agent zidovudine and also IFN-α and arsenic trioxide (ATO), but these combinatorial treatments failed to achieve significant impacts on survival. 7 Several mechanisms including overexpression of efflux pumps, TP53 mutations, and deregulation of oncogenes in leukemic cells are involved in disease chemoresistance and relapse. 8
Conferone (C24H28O4, Figure 1) is a natural coumarin derived from the fruits and roots of self-growing Ferula species. As an anticancer agent, conferone has antiangiogenic characteristics and suppresses p-glycoprotein-mediated drug efflux. 9 In addition, conferone possesses the potential to suppress cell proliferation, induce both apoptosis and necrosis, and generate free radicals in cancer cells. 10 To our knowledge, this is the first report studying the effects of conferone against ATL cells in vitro. In this regard, viability of MT-2 cells was assessed by alamarBlue assay, and changes induced on the cell cycle were detected by flow cytometry analysis upon propidium iodide (PI) staining. To ascertain the mechanism of conferone toxic action, quantitative polymerase chain reaction (qPCR) was used to investigate the expression of CDK6, c-MYC, cFLIPL, and NF-κB (Rel A).
Figure 1.
Chemical structure of conferone.
Materials and methods
Chemicals and reagents
alamarBlue and propidium iodide (PI) were purchased from Sigma-Aldrich (Germany). Dimethylsulfoxide (DMSO) and Triton X-100 were obtained from CinnaGen (Iran). RPMI-1640 was from Biosera (France), and fetal bovine serum (FBS), penicillin/streptomycin, and L-glutamine were from Gibco (Scotland). TriPure was produced by Roche (Germany), M-MuLV reverse transcriptase was from Thermo Scientific (USA), and SYBR green mix was from Takara (Japan).
Extraction of sesquiterpene coumarin conferone
Sesquiterpene coumarin conferone was extracted as previously described. 11 In summary, the roots of F. flabelliloba were collected, dried, powdered, and extracted with methanol by maceration method at ambient temperature. Then, the solvent was evaporated under vacuum pressure and a part of the extract was subjected to silica gel chromatography. Among obtained fractions, conferone (MW: 380.5 g/mol) was derived as white crystals form one fraction (18–21; PET: EtOAc [6:1]; 2.0 g; TLC [Hex: EtOAc 9 : 2.5]), and its structure was assigned by 1H- and 13C-nuclear magnetic resonance (NMR) spectra (Table 1). NMR experiments were run using Bruker DRX-500 and DRX-600 spectrometers at 300 K, and CDCl3 was used as the solvent (Carlo Erba, Italy). To note, the spectra were calibrated using the solvent signal as the internal standard (1H, d: 7.27 ppm; 13C, d: 77.0 ppm).
Table 1.
1H-NMR data (600 MHz, δ ppm)* and 13C-NMR data (125.7 MHz, δ ppm) obtained for conferone.
| Position of proton | Position of carbon | ||
|---|---|---|---|
| 2 | — | 2 | 161.5 |
| 3 | 6.28 d (9.6) | 3 | 113.4 |
| 4 | 7.66 d (9.6) | 4 | 143.7 |
| 5 | 7.39 d (8.4) | 5 | 129.2 |
| 6 | 6.86 dd (8.4, 2.3) | 6 | 113.6 |
| 7 | — | 7 | 162.2 |
| 8 | 6.85 d (2.3) | 8 | 101.7 |
| 9 | — | 9 | 156.3 |
| 10 | — | 10 | 113.0 |
| 1ʹ | 1.66 dd* β 2.30 m α |
1ʹ | 38.8 |
| 2ʹ | 2.31 m β 2.75 ddd (15.3, 6.6) α |
2ʹ | 34.8 |
| 3ʹ | — | 3ʹ | 216.4 |
| 4ʹ | — | 4ʹ | 47.9 |
| 5ʹ | 1.68 dd* | 5ʹ | 51.5 |
| 6ʹ | 2.00 like brd β 2.18 like brt α |
6ʹ | 24.3 |
| 7ʹ | 5.62 brs | 7ʹ | 124.0 |
| 8ʹ | — | 8ʹ | 132.7 |
| 9ʹ | 2.28* | 9ʹ | 53.4 |
| 10ʹ | — | 10ʹ | 36.2 |
| 11ʹ | a 4.10 dd (9.80, 5.20) b 4.21 dd (9.80, 4.80) |
11ʹ | 67.0 |
| 12ʹ | 1.75 s | 12ʹ | 25.6 |
| 13ʹ | 1.17 s | 13ʹ | 22.7 |
| 14ʹ | 1.13 s | 14ʹ | 25.6 |
| 15ʹ | 1.18 s | 15ʹ | 14.9 |
Overlapped with other signals.
Treatment of cells and viability assay
To assess the cytotoxic effects of natural coumarin conferone on human HTLV-1-infected T-cells, MT-2 cell line was purchased from Pasteur Institute (Tehran, Iran). For cell culture, RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 50 µl (W/V) penicillin/streptomycin, and 50 µl L-glutamine were used and cells were incubated at 37°C in 5% CO2.
To prepare different concentrations of conferone, at first a stock solution was prepared and stored using DMSO as the solvent, and then, serial dilutions were made by complete culture medium right before use. Accordingly, 0.4% DMSO was considered as the solvent control in all experiments. To determine viability, MT2 cells (50,000 cells per well in 96-well plate) were treated with increasing concentrations of conferone (5, 10, 20, 40, and 80 µM) for 24, 48, and 72 h. In addition, cells were treated with 4, 8, and 16 µM ATO (Sigma) as a standard chemical agent for ATL treatment. Then, alamarBlue (20 µl per well in the 96-well plate) was added at the end of each time point, and cells were incubated at 37°C. By using a microplate reader (Epoch), absorbance was measured at 600 nm, and cell viability (%) was determined according to the following equation: 100-((AT-AC)/(AB-AC) ×100), in which AT and AC were the absorbance of treated and untreated control cells, respectively, and AB was the absorbance of the blank control.
Detection of apoptosis
To further evaluate the effects of conferone on the cell cycle, PI staining followed by flow cytometry was applied. Briefly, MT-2 cells (50,000 cells per well in the 96-well plate) were incubated with conferone (40 µM) for 72 h. It should be noted that cells treated with 0.4% DMSO and untreated cells were considered as well. Briefly, MT-2 cells in each treatment were collected and centrifuged at 200 g for 5 min. Upon washing with cold phosphate-buffered saline (PBS) containing 5% FBS, cell pellets were resuspended in staining buffer containing 100 μg/ml PI, 0.1% Triton X-100, and 0.1% sodium citrate and incubated at 37°C in the dark for 30 min. Finally, flow cytometry (BD FACSCalibur) was carried out by FL2-H filter.
Gene expression analysis
To ascertain the molecular mechanism behind conferone cytotoxic action, the expression pattern of NF-κB (REL-A), CDK6, c-MYC, and cFLIP L was studied by qPCR. Briefly, the total cellular RNA was extracted from cells treated with 80 µM conferone and relevant controls using Triazole (Roche). Then, cDNAs were synthesized using random hexamer, dNTPs, and Revert Aid First Strand cDNA Synthesis kit (Thermo Scientific) according to the manufacturer’s protocol. qPCR was performed in Rotor-Gene 6000 detection system (Qiagen) with the SYBR green mix; primers are given in Table 2 for c-MYC, cFLIP L , and CDK6 genes. TaqMan probe and specific primers were employed for NF-κB (REL-A) . PCR cycling conditions were 94°C for 2 min, 94°C for 15 sec, 58°C for 30 sec, and 72°C for 45 sec. In all analyses, beta-2 microglobulin (B2M) transcripts were used as the internal control, and data were analyzed by standard curve relative method using the following formula: efficiency of the target gene^(ΔCt target)/efficiency of the reference gene^(ΔCt reference).
Table 2.
List of primers and probes used for qPCR analysis in the current study.
| Name of gene | Length (bp) | 5’→3’ |
|---|---|---|
| B 2 MG | 127 |
Forward: AATTGAAAAAGTGGAGCATTCAGA Reverse: GGCTGTGACAAAGTCACATGGTT |
| c-MYC | 159 |
Forward: ACTCTGAGGAGGAGGAACAAGAA Reverse: TGGAGACGTGGCACCTCTT |
| cFLIP L | 126 |
Forward: ATTGGCAATGAGACAGAGCTTC Reverse: CTCGGGCATACAGGCAAA |
| CDK6 |
Forward: GTTTCCAGATGGCTCTAACCTCAG Reverse: AAATATGCAGCCAACACTCCAGAG |
|
| Rel-A | 145 |
Forward: ACCCCTTCCAAGTTCCTATAGAAGAG Reverse: CGATTGTCAAAGATGGGATGAGAAAG Probe: ACTACGACCTGAATGCTGTGCGGCTCT |
| B2MG | 127 |
Forward: TTGTCTTTCAGCAAGGACTGG Reverse: CCACTTAACTATCTTGGGCTGTG Probe: TCACATGGTTCACACGGCAGGCAT |
Statistical analysis
The statistical significance for viability assay was analyzed by one-way ANOVA, Dunnet, and Sidak multiple comparison tests using GraphPad Prism. In addition, results of flow cytometry were analyzed by WinMDI software. qPCR data were analyzed using GraphPad Prism and Kolmogorov–Smirnov statistical test [12]. All data were reported as mean ± SD, and p-values less than 0.05, 0.01, 0.001, and 0.0001 were considered significant for all comparisons.
Results
Viability of MT-2 cells was reduced after conferone treatment
Conferone decreased the viability of MT-2 cells in a time- and concentration-dependent matter. As presented in Figure 2, upon 24, 48, and 72 h treatment with 20 µM conferone, viability was calculated as 85%, 75%, and 60%, respectively. Likewise, after treatment with 40 µM conferone during the same consecutive time periods, cell viability significantly (p < 0.0001) reduced and was determined as 77%, 60%, and 45%, respectively. To note, the assessment of ATO effects indicated that its highest concentration (16 µM) significantly (p < 0.0001) decreased cell viability down to 79% and 51% after 48 and 72 h, respectively.
Figure 2.
Viability assessment of MT-2 cells. Cells were treated with 2.5, 5, 10, 20, and 40 µM conferone and 4, 8 and 16 µM ATO and viability was assessed after 24 h (a), 48 h (b), and 72 h (c). alamarBlue assay was carried out for at least three times and results are presented as mean ± SD. (*p < .05, **p < .01, ***p < .001, and ****p < .0001).
Changes induced on the cell cycle were observed upon conferone treatment
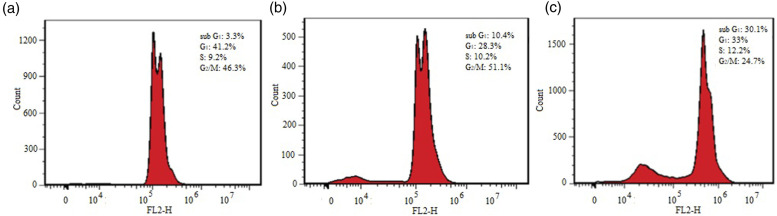
To determine whether conferone induced changes on the cell cycle, the DNA content of MT-2 cells was analyzed by flow cytometry. As presented in Figure 3, in untreated MT-2 cells, 3.3%, 41.2%, 9.2%, and 46.3% of cells were detected in the sub-G1, G1, S, and G2/M phases of the cell cycle, respectively. Upon treatment with DMSO solvent, 10.4%, 28.3%, 10.2%, and 51.1% of cells were detected in the sub-G1, G1, S, and G2/M phases of the cell cycle, respectively. Surprisingly, conferone treatment altered the distribution of cells in the cell cycle, as 30.1% of cells were detected in the sub-G1, 33% were detected in the G1, 12.2% of cells were detected in the S, and 24.7% of cells were detected in the G2/M phase of the cell cycle. This observation was in agreement with findings of the viability assay and confirmed cytotoxic effects of 40°µM conferone.
Figure 3.
MT2 cell cycle analysis by PI staining. Untreated cells (a), cells treated with 0.4% DMSO (b), and 40 µM conferone (c). Sub-G1 peak, as an indicative of dead cells, was specifically induced after conferone treatment.
Conferone downregulated the expression of NF-κB (REL-A), CDK6, c-MYC, and cFLIP L
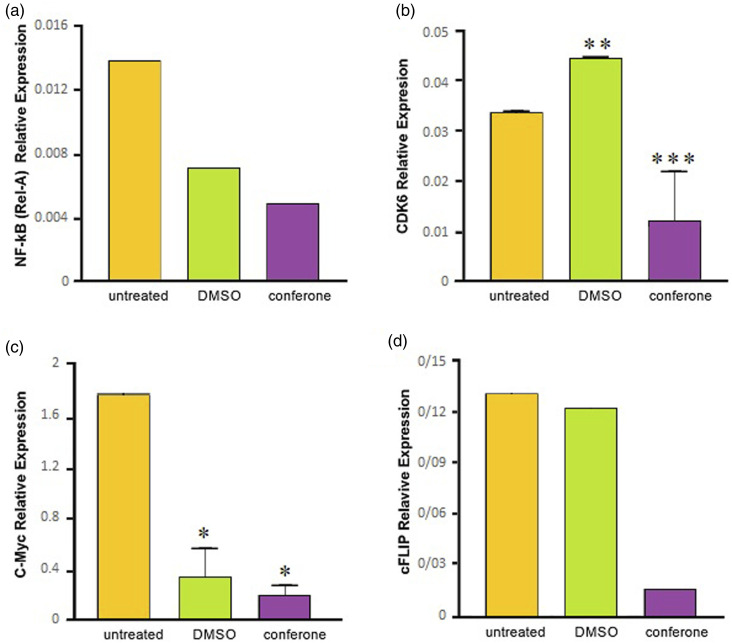
To ascertain molecular mechanisms underlying the effects of conferone, the expression patterns of NF-κB (REL-A), CDK6, c-MYC, and cFLIP l , all of which contributed to the proliferation and survival of ATL cells, were studied by qPCR. As shown in Figure 4, conferone decreased NF-κB (REL-A) expression and significantly (p < 0.001) reduced the expression of CDK6 to lower levels in comparison with DMSO control. Significant (p < 0.05) downregulation in c-MYC expression was also observed when conferone treatment was compared with its relevant control. Last but not least, conferone downregulated the expression of cFLIP L in comparison with other groups.
Figure 4.
qPCR analysis of NF-κB (REL-A) (a), CDK6 (b), c-MYC (c), and cFLIP L (d) expression 72 h after the treatment of MT2 cells with conferone. To note, relative expression was compared with relevant control. (*p < .05, **p < .01, and ***p < .001).
Discussion
ATL is known as a T-cell neoplasm associated with HTLV-1. Patients with the acute or lymphoma subtypes of ATL require therapeutic intervention, and although various chemotherapeutic regimens are currently available, clinical outcomes remain dismal. 12 To introduce a novel and more effective natural agent against ATL, the aim of present research was to investigate cytotoxic activity of conferone isolated from F. flabelliloba for the first time. Our findings revealed that conferone reduced the viability of MT-2 cells and attributed considerable accumulation of cells in the sub-G1 phase of the cell cycle. These findings were confirmed by molecular analysis, since significant downregulation of genes involved in the survival and proliferation of ATL cells was detected after conferone administration.
The NF-B (REL-A) transcription factor family regulates the expression of genetic networks critical for cell survival, proliferation, inflammation, and T-cell transformation.13–15 HTLV-1-Tax acts as a transcriptional activator via activation of nuclear factor-kB (NF-kB). Unlike normal T-cells, Tax-expressing T-cells constitutively express NF-kB. 16 Interestingly, our findings revealed that conferone was able to significantly reduce the expression of NF-B (REL-A) in MT-2 cells.
CDK6 is a family of cell cycle kinases that form complexes with D-type cyclins to help cells proceed through the early G1 phase of the cell cycle. Components of the CDK6-Cyclin D complexes are frequently altered in hematological malignancies.17–21 CDK6 inhibitors have various biological effects on cancer cells that can be exploited for therapeutic purposes, such as modulation of mitogenic kinase signaling, formation of a senescence-like phenotype, and increased immunogenicity of cancer cells. 22 In the present study, we demonstrated that conferone decreased the expression of CDK6 significantly.
c-MYC is a key transcription factor that promotes cell proliferation in the G1 phase by interacting with cyclin D and cyclin-dependent kinases-4/6.23,24 High level of c-MYC expression is required for carcinogenesis and maintenance of ATL.25–27 In addition, c-MYC protein and mRNA expression were substantially higher in lymphoma and acute types of ATL patients than in smoldering and chronic types. 28 Findings of the current study revealed significant reduction in c-MYC expression, implying that conferone has much potential as a negative regulator for this gene in ATL cells.
c-FLIPL is an anti-apoptotic protein with substantial expression in hematologic malignancies.29,30 c-FLIP L is highly expressed in bone marrow mononuclear cells obtained from ATL patients and is associated with prognosis of this malignancy. Moreover, it has been indicated that patients with peripheral T-cell lymphoma had elevated c-FLIP L expression. 31 Present results revealed that conferone significantly reduced the expression of c-FLIP L , that explains, to some extent, our observations regarding reduced viability and cell cycle changes.
The current research was carried out during COVID-19 pandemic, and due to global lockdown, laboratory work was profoundly restricted. Accordingly, there were limitations to develop this study on more ATL cell lines and/or carry out complementary analysis.
Conclusion
Findings of the present study indicated, for the first time, that conferone acts as a natural coumarin against ATL cells, and thus, could be considered as a potent pharmaceutical agent. Nevertheless, complementary studies on other cell lines are required to confirm observed effects of conferone in our study.
Ethical Statement
Ethical approval
This study was approved by the Research Council of Mashhad University of Medical Sciences (IR.MUMS.MEDICAL.REC. 970306).
Acknowledgments
The authors would like to thank Mr. Malaike and Mr. Feizy for their technical support.
Author Contributions: H. Rafatpanah supervised the project, M. Golizadeh and M. Mahdifar carried out the experiments, S. Mahdavi wrote the first draft, M. Iranshahi advised the project, and F.B. Rassouli designed and supervised the project and revised the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially supported by the Vice-Chancellor for Research and Technology, Mashhad University of Medical Sciences, Mashhad, Iran (Grant number: 970306).
ORCID iD
Fatemeh B Rassouli https://orcid.org/0000-0003-1889-0964
References
- 1.Rodríguez-Zúñiga MJM, Cortez-Franco F, Qujiano-Gomero E. (2018) Adult T-cell leukemia/lymphoma. Review of the literature. Actas Dermo-Sifiliográficas 109(5): 399–407. [DOI] [PubMed] [Google Scholar]
- 2.Ishitsuka K, Tamura K. (2014) Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol 15(11): e517–e526. [DOI] [PubMed] [Google Scholar]
- 3.Satake M, Yamada Y, Atogami S, et al. (2015) The incidence of adult T-cell leukemia/lymphoma among human T-lymphotropic virus type 1 carriers in Japan. Leuk Lymphoma 56(6): 1806–1812. [DOI] [PubMed] [Google Scholar]
- 4.Phillips AA, Shapira I, Willim RD, et al. (2010) A critical analysis of prognostic factors in North American patients with human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a multicenter clinicopathologic experience and new prognostic score. Cancer 116(14): 3438–3446. [DOI] [PubMed] [Google Scholar]
- 5.Shimoyama M. (1991) Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the lymphoma Sstudy group (1984-87). Br J Haematol 79(3): 428–437. [DOI] [PubMed] [Google Scholar]
- 6.Taylor GP, Matsuoka M. (2005) Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene 24(39): 6047–6057. [DOI] [PubMed] [Google Scholar]
- 7.Bazarbachi A, Plumelle Y, Ramos JC, et al. (2010) Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol 28(27): 4177–4183. [DOI] [PubMed] [Google Scholar]
- 8.Matutes E. (2007) Adult T-cell leukaemia/lymphoma. J Clin Pathol 60(12): 1373–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahmani A, Mousavi HZ, Salehi R, et al. (2020) Novel pH-sensitive and biodegradable micelles for the combined delivery of doxorubicin and conferone to induce apoptosis in MDA-MB-231 breast cancer cell line. RSC Advances 10(49): 29228–29246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheraghi O, Dehghan G, Mahdavi M, et al. (2016) Potent anti-angiogenic and cytotoxic effect of conferone on human colorectal adenocarcinoma HT-29 cells. Phytomedicine 23(4): 398–405. [DOI] [PubMed] [Google Scholar]
- 11.Iranshahi M, Kalategi F, Sahebkar A, et al. (2010) New sesquiterpene coumarins from the roots of Ferula flabelliloba. Pharm Biol 48(2): 217–220. [DOI] [PubMed] [Google Scholar]
- 12.Massey FJJ. (1951) The Kolmogorov-Smirnov test for goodness of fit. J Am Stat Assoc 46(253): 68–78. [Google Scholar]
- 13.Grilli M, Chiu J, Lenardo MJ. (1993) NF-kB and Rel: Participants in a multiform transcriptional regulatory system. Int Rev Cytol 143: 1–62. [DOI] [PubMed] [Google Scholar]
- 14.Baeuerle PA, Henkel T. (1994) Function and activation of NF-kB in the immune system. Annu Rev Immunol 12: 141–179. [DOI] [PubMed] [Google Scholar]
- 15.Baldwin AS. (1995) The NF-kB and IkB proteins: New discoveries and insights. Annu Rev Immunol 14: 649–681. [DOI] [PubMed] [Google Scholar]
- 16.Mori N, Fujii M, Ikeda S, et al. (1999) Constitutive activation of NF-kappaB in primary adult T-cell leukemia cells. Blood 93: 2360–2368. [PubMed] [Google Scholar]
- 17.Nebenfuehr S, Kollmann K, Sexl V. (2020) The role of CDK6 in cancer. Int J Cancer 147(11): 2988–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chilosi M, Doglioni C, Yan Z, et al. (1998) Differential expression of cyclindependent kinase 6 in cortical thymocytes and T-cell lymphoblastic lymphoma/leukemia. Am J Pathol 152: 209–217. [PMC free article] [PubMed] [Google Scholar]
- 19.Brito-Babapulle V, Gruszka-Westwood AM, Platt G, et al. (2002) Translocation t(2;7)(p12;q21-22) with dysregulation of the CDK6 gene mapping to 7q21-22 in a non-Hodgkin's lymphoma with leukemia. Haematologica 87: 357–362. [PubMed] [Google Scholar]
- 20.Chen D, Law ME, Theis JD, et al. (2009) Clinicopathologic features of CDK6 translocation-associated B-cell lymphoproliferative disorders. Am J Surg Pathol 33: 720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayette S, Tigaud I, Callet-Bauchu E, et al. (2003) B-cell chronic lymphocytic leukemias, 7q21 translocations lead to overexpression of the CDK6 gene. Blood 102: 1549–1550.In [DOI] [PubMed] [Google Scholar]
- 22.Goel S, DeCristo MJ, McAllister SS, et al. (2018) CDK4/6 inhibition in cancer: Beyond cell cycle arrest. Trends Cell Biol 28(11): 911–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mateyak MK, Obaya AJ, Sedivy JM. (1999) C-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points. Mol Cell Biol 19: 4672–4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dang CV, O’Donnell KA, Zeller KI, et al. (2006) The c-Myc target gene network. Semin Cancer Biol 16: 253–264. [DOI] [PubMed] [Google Scholar]
- 25.Mai S, Mushinski JF. (2003) c-Myc-induced genomic instability. J Environ Pathol Toxicol Oncol 22(3): 179–199. [DOI] [PubMed] [Google Scholar]
- 26.Palomero T, Lim WK, Odom DT, et al. (2006) NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci USA 103: 18261–18266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starza RL, Borga C, Barba G, et al. (2014) Genetic profile of T-cell acute lymphoblastic leukemias with MYC translocations. Blood 124: 3577–3582. [DOI] [PubMed] [Google Scholar]
- 28.Mihashi Y, Mizoguchi M, Takamatsu Y, et al. (2017) C-MYC and its main ubiquitin ligase, FBXW7, influence cell proliferation and prognosis in adult T-cell leukemia/lymphoma. Am J Surg Pathol 41(8): 1139–1149. [DOI] [PubMed] [Google Scholar]
- 29.He MX, He YW. (2013) CFLAR/c-FLIPL: A star in the autophagy, apoptosis and necroptosis alliance. Autophagy 9: 791–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sachanas S, Levidou G, Angelopoulou MK, et al. (2014) Apoptotic and proliferative characteristics of proliferation centers in lymph node sections of patients with chronic lymphocytic leukemia. Leuk Lymphoma 55: 571–582. [DOI] [PubMed] [Google Scholar]
- 31.Zheng Z, Cheng S, Wu W, et al. (2014) C-FLIP is involved in tumor progression of peripheral T-cell lymphoma and targeted by histone deacetylase inhibitors. J Hematol Oncol 7: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]