Abstract
There is an increasing global burden of diabetes mellitus (DM) and chronic kidney disease (CKD), coupled with a high burden of people with HIV (PWH). Due to an increased lifespan on ART, PWH are now at risk of developing non-communicable diseases, including DM. Africa has the greatest burden of HIV infection and will experience the greatest increase in prevalence of DM over the next two decades. In addition, there is a rising number of people with CKD and progression to kidney failure. Therefore, there is an urgent need for the early identification and management of all 3 diseases to prevent disease progression and complications. This is particularly important in Africa for people with CKD where there is restricted or no access to dialysis and/or transplantation. This review focuses on the epidemiology and pathophysiology of the interaction between HIV infection and DM and the impact that these diseases have on the development and progression of CKD. Finally, it also aims to review the data on the management, which stems from the growing burden of all three diseases.
Keywords: chronic kidney disease, renal replacement therapy, antiretroviral therapy, Diabetes mellitus
Introduction
The global prevalence of human immunodeficiency virus (HIV) infection continues to place a significant burden on healthcare systems, especially in sub-Saharan Africa (SSA), with South Africa remaining the epicentre. In addition, the impact of the COVID-19 pandemic has seen an increase in annual HIV infections in many countries and a decline in targets for prevention and treatment.1 The introduction of antiretroviral therapy (ART) has resulted in a significant decline in the morbidity and mortality of people with HIV (PWH). With this increased life span, PWH are at risk of developing non-communicable chronic diseases, similar to those of the general population. The collision of the three pandemics, DM, HIV and kidney failure (KF) has significantly impacted on morbidity and mortality, as well as treatment costs. In areas where HIV infection is most prevalent, there is also restricted access to kidney replacement therapy (KRT). Therefore, this review seeks to address the epidemiology and pathophysiology of the interaction between HIV infection and DM and the impact that these diseases have on chronic kidney disease (CKD) progression. It also aims to discuss the implications for management, which stems from the growing burden of all three diseases.
Global Burden of Diabetes
Type 2 DM (T2DM) is one of the fastest growing global health emergencies of the 21st century. According to the International DM Federation (IDF), the global prevalence of DM in adults aged 20–79 was 10.5% in 2021, with an estimated 537 million adults living with DM. This is projected to increase to 783 million by 2045.2 Over the next 10–20 years the greatest increase in prevalence is expected to occur in Africa and, already, 80% of people with DM (PWD) are living in low- and middle-income countries (LMICs). DM is considered a leading cause of disability adjusted life years (DALYs), together with DM-related deaths estimated at 6.7 million worldwide in 2021. In LMICs, most of these deaths occur in people <60 years of age. The direct costs of managing DM are prohibitive for many economies. The global health expenditure due to DM has grown from USD 232 billion in 2007 to USD 966 billion in 2021 for adults aged 20–79 years.2 In many countries in Africa, especially South Africa, the financial burden of managing the morbidity from DM falls on a healthcare system already struggling with the burden of infectious diseases such as HIV and tuberculosis. A cost of illness study in the public sector in South Africa in 2018 showed the annual direct costs due to T2DM to be ZAR 2.7 billion if diagnosed and ZAR 21.8 billion if undiagnosed with an estimated increase in annual total costs to ZAR 35.1 billion by 2030.3 A major challenge in Africa is that over 1 in 2 (54%) PWD are undiagnosed on the continent.4 In addition, those who are diagnosed often do not receive adequate care due to poor access to healthcare services, lack of resources, and low awareness levels.
Global Burden of CKD
CKD contributes significantly to the annual global mortality. This is particularly concerning, given the lack of access to KRT in many LMICs.5 In 2017, there was an estimated 843.6 million people reported to have CKD worldwide.6 Between 1990 and 2017, CKD caused an increase in mortality of 41.5% globally, resulting in it becoming the 12th leading cause of death globally.7 A systematic review and meta-analysis (including 100 studies) revealed the global prevalence of CKD stages 1–5 to be 13.4% and 10.6% for CKD stages 3–5.8 In 2017, there were 35.8 million DALYs attributed to CKD, with almost 33% due to diabetic kidney disease (DKD). The CKD burden predominates in the three lowest quintiles of socio-demographic indices. Given their level of development, the burden of CKD was much higher than expected in Oceania, SSA, and Latin America.7 More effective and targeted preventative interventions to reduce the CKD burden particularly addressing risk factors including DM, are urgently needed.
Impact of DM on CKD
The global prevalence of T2DM is increasing due to the rapidly increased prevalence of obesity, metabolic syndrome, and westernization of lifestyle. DKD is a microvascular complication of both type 1 DM (T1DM) and T2DM. Approximately 40% of people with T2DM will develop DKD which is associated with a high mortality.9 Although CKD may be the most recognizable consequence of DKD, most patients actually die from cardiovascular diseases and infections before needing KRT.10 Early detection and adequate treatment of DM can slow DKD progression; however, it still accounts for approximately 50% of cases of KF in the developed world. There are limited epidemiological data on CKD in PWD living in low and middle-income countries (LMICs). A systematic review on studies from Africa showed the prevalence of CKD in people with T1DM and T2DM varied from 11% to 83.7%.11 Incident event rates were 34.7%, 94.9%, and 18.4% for KF at 5 years, proteinuria at 10 years and for mortality from nephropathy at 20 years of follow-up, respectively. These figures suggest a greater incidence of DKD in Africa. Common determinants of DKD were duration of DM, blood pressure (BP), increasing age, obesity and glucose control.11
Impact of HIV Infection on CKD
HIV was first deemed an epidemic in the 1980s and remains an important contributor to the burden of disease, particularly in Africa. In 2020, there were 37.7 million PWH globally with 1.5 million new cases during that year. East Africa and SSA had the highest disease burden.1 PWH have an increased risk of developing both acute kidney injury (AKI) and CKD.12 ART has significantly altered the spectrum of kidney disease seen in this population.13–15 There has been a steady decline in HIV-associated nephropathy (HIVAN) since the introduction of ART, without which there is a rapid decline to KF requiring KRT.16 A systematic review and meta-analysis showed the prevalence of CKD in PWH to be 6.4% globally but SSA had the highest prevalence at 7.9%.17
Impact of Both HIV Infection and DM on CKD
With the improved access to ART and increased life expectancy, PWH are now contributing to the global prevalence of noncommunicable diseases (NCDs), including DM. In a systematic review and meta-analysis by Ekrikpo et al, sociodemographic and clinical factors such as gender, age, co-infections with hepatitis B and hepatitis C did not significantly affect the CKD estimates. However, CKD prevalence was significantly increased with comorbid hypertension (MDRD: 20.7% [95% CI 14.3–27.8%] vs not hypertensive 5.4% [95% confidence interval (CI) 3.4–7.9%]; p < 0.001) and DM (MDRD: 19.4% [95% CI 13.5–26.0%] vs non-DM 8.4% [95% CI 5.5–11.8%]; p < 0.001).17 In addition, the combination of HIV and DM led to an increased risk of progression compared to either alone.18 In South Africa, the HIV Directorate and HIV Clinician’s Society have made concerted efforts to upskill clinicians at primary level care to better manage NCDs within HIV clinics. Primary care guidelines have been developed to assist with this process coupled with a HIV/TB hotline to allow real-time conversations to assist with care. These initiatives could be adopted for other high burden regions on the continent.
Pathophysiology of DKD
The combination of hyperglycaemia, haemodynamic changes and ischaemia results in the activation of the renin-angiotensin-aldosterone system (RAAS), oxidative stress and ultimately fibrosis.19 The hallmark structural abnormalities include mesangial expansion, fewer podocytes, progressive thickening of the glomerular basement membrane and development of Kimmelstiel-Wilson nodules.20
Incipient DKD results from afferent arteriolar vasodilatation and an increase in efferent arteriolar resistance, thereby raising intra-glomerular pressure resulting in hyper-filtration.21 Angiotensin II, endothelin-1 and urotensin II cause vasoconstriction of the efferent arterioles, resulting in the production and release of pro-inflammatory and pro-fibrotic mediators. These haemodynamic alterations are important for the development of glomerulosclerosis and proteinuria.22
In view of the high-filtered glucose load, both sodium chloride and glucose are reabsorbed in the proximal tubules, through up-regulation of the sodium glucose co-transporter 2 (SGLT2). As a result, there is decreased delivery of sodium to the macula densa, dilating the afferent arterioles. There is simultaneous vasoconstriction of the efferent arteriole, due to activation of RAAS and down-stream activation of angiotensin II, giving rise to glomerular hypertension.23
Both hyperglycaemia and hyperinsulinaemia are central to the development of endothelial dysfunction with a direct relationship between the extent of hyperglycaemia and tissue damage.24 A complex interplay between endothelial dysfunction, protein kinase C and the polyol pathway results in increased reactive oxygen species, stimulation of advanced glycation end products, pro-inflammatory cytokines and chemokines leading to an inflammatory cascade. This culminates in vasoconstriction and kidney ischaemia, oxidative stress, podocyte injury and apoptosis, and ultimately fibrosis.25–29
Activation of the RAAS is responsible for the progression of DKD. Angiotensin II and transforming growth factor β1 are intimately involved in kidney fibrosis and tubular dysfunction.30 Damage to the basement membrane in the glomerular wall of the kidney leads to abnormal excretion of albumin and it is also responsible for the deposition of extracellular matrix proteins, particularly type IV collagen.31
The Association Between HIV Infection and the Development of DM
Data showing a direct link between HIV infection and the development of DM are conflicting and dependent on the population studied.32,33 However, a systematic review and meta-analysis assessing the incidence and prevalence of T2DM with HIV infection in Africa showed no association between the prevalence of T2DM and HIV infection or ART.34 PWH are now living longer and are at risk of developing the metabolic sequelae associated with a westernised lifestyle and aging, similar to those without HIV infection. Since 2016, the World Health Organisation (WHO) have recommended the initiation of ART at diagnosis; therefore, it is now difficult to dissect the contribution of HIV infection itself to the development of DM in PWH. Since the prevalence of DM is increasing globally in all populations, it is likely that traditional risk factors such as high carbohydrate intake, obesity, aging and sedentary lifestyle will contribute more to the risk of developing DM than HIV infection itself.
The Association Between ART and the Development of DM
Glucose dysregulation is a well-documented consequence of the treatment of HIV infection when using the initial types of ART, with a number of cross-sectional studies of variable size documenting a high prevalence of insulin resistance, impaired glucose tolerance (IGT) and overt DM amongst patients receiving these types of ART.35–38 Although protease inhibitors (PIs) have often been the main culprits, there is evidence also implicating the use of nucleoside reverse transcriptase inhibitors (NRTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs).33,38 The Multicenter Aids Cohort Study reported a 4 times greater incidence of DM in men with HIV on ART than that of men without HIV.39 However, a recent meta-analysis of 9 studies (n = 13,742 PWH) failed to show an association between PIs and the development of DM but did show an association with the development of the metabolic syndrome [(RR: 2.11; 95% CI 1.28–3.48; p-value 0.003)].40 The mechanisms of glucose dysregulation caused by the initial types of ART have been well studied in vitro. PIs, as a class, have been shown to selectively inhibit the transport function of Glut4, a major glucose transport molecule.41 Both PIs and NRTIs increase pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 from adipocytes, thereby causing insulin resistance.42,43 In addition, there is some evidence that stavudine decreases adiponectin, a peptide hormone secreted by adipocytes which correlates directly with insulin sensitivity.44
Contemporary ART is considered to be more “metabolically friendly” than the initial types of ART. The integrase strand transfer inhibitors (INSTIs), the first-choice ART recommended by the WHO, have been associated with weight gain.45 In the ADVANCE Study, Venter et al showed a significant increase in weight over 48 weeks in men and women using a dolutegravir (DTG)-based regimen when compared to those using a standard non-DTG-based regimen.46 Meta-analyses and systematic reviews have not shown an increased risk of developing DM when using an INSTI, despite the weight gain associated with their use. In a meta-analysis and systematic review by Kajogoo et al, that included 10 studies (n = 62,400 participants), participants on INSTI-based regimens were shown to have a similar incidence of DM to those on other ART regimens.47 In fact, Mulindwa et al showed in their meta-analysis of 13 pooled studies (n = 72,404) that there was a lower risk of incident DM with exposure to an INSTI than to any other ART (RR 0.80, 95% CI 0.67 to 0.96, I2 = 29%).48 However, they also showed that there was an increased risk of DM in PWH of African origin (RR 2.99, 95% CI 2.53 to 3.54, I2 = 0%).
HIV and the Presentation of DKD
In two early kidney biopsy series from adults with HIV in the United States (US), the prevalence of DM was found to be 5.4% and 6.7%, respectively. These two series were small, with 152 biopsies (1995–2004) in the one study and 89 biopsies (1995–2001) in the other.49,50 A more recent biopsy series (n = 437) from the US (2010–2018), noted that comorbidities of NCDs were more common. In this cohort, 57% had hypertension,31% had DM, obesity was observed in 11%, and cardiovascular disease in 9%.15
However, in contrast, a review of two large recent kidney biopsy cohorts of PWH from South Africa reported that neither had an increase in DM. Diana et al evaluated 690 biopsies (from 1989 to 2014) and found no change in the proportion of patients diagnosed with DKD (p = 0.810) or hypertensive nephropathy (p = 0.33), pre and post ART.13 Similar findings were observed by Wearne et al where no change in the proportion of those with DM was seen pre and post ART roll-out in 671 patients undergoing a kidney biopsy.14 In contrast to the studies from the USA, the two large studies from South Africa showed the prevalence of DM to range between 3.6% and 4%, and the trend did not change between 2005 and 2020. However, the cohorts from the two countries are different in that the South African studies included patients with more advanced disease with associated co-morbidities, such as tuberculosis, and who were ART-naïve or not adherent to treatment.13,14
When compared to other ethnicities, African-Americans have been shown to have a higher rate of KF. African-Americans have around a 3-fold increased incidence of treated KF compared to Caucasians.51 Part of this increased risk is at least in part due to the inheritance of an apolipoprotein L1 (APOL1) gene variant. The protein apolipoprotein L1, encoded by this gene, has a historical role in conferring innate immunity against most strains of Trypanosoma brucei.52 Initially identified in individuals of African descent, two gain-of-function (APOL1) variants, G1 or G2 have been found to have a high prevalence particularly in West Africa.52,53 These coding variants have since spread widely throughout the African diaspora, with frequencies of 21% for G1 and 13% for G2 observed among African Americans.54
APOL1 risk variants have been associated with an increased risk and accelerated progression of focal segmental glomerulosclerosis (FSGS), HIVAN and hypertensive nephrosclerosis (OR 7.3).55,56 There have also been studies suggesting a role in progression of DKD.57
Both DM and APOL1-associated kidney diseases are common entities, as such, they are likely to occur synchronously. In addition, obesity, commonly associated with DM, on its own, can cause glomerular damage resulting in proteinuria and reduction in GFR. In Black individuals with DM who have no other complications and a marginally elevated HbA1c, kidney dysfunction should not be assumed to be due to DKD. In this setting, APOL1 genotyping may help the decision-making in whether a biopsy is required in selected individuals.58
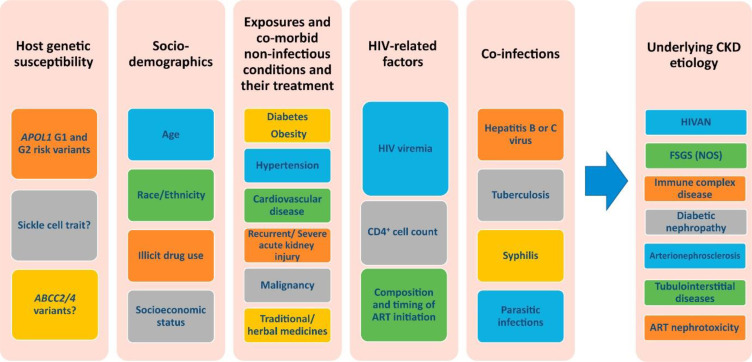
The data on the mechanisms of the progression of CKD in people with HIV and DM are sparse, however, a study by Osafo et al did show that kidney damage is accelerated when there is co-existing hypertension, HIV, genetic predisposition, and DM.59 This is demonstrated by the earlier onset of KF in African populations compared to those in developed countries (40–45 years versus 63 years).59 Figure 1 shows the HIV-related and traditional risk factors influencing the development and progression of CKD in PWH.12 Examples of risk factors increasing CKD progression include: co-infection with hepatitis B and C (2–3 fold increase) and episodes of AKI (3.8–20-fold increase).12 Similarities and differences of clinical presentation and investigations of CKD in HIV and DKD are demonstrated in Table 1 and 2, respectively.10,12,16,60–62 Patients with combined HIV and DM should have more frequent screening and follow-up, and more intensive management of existing CKD and its risk factors.
Figure 1.
HIV-related and traditional risk factors influencing the development and progression of CKD in PWH.
Notes: Reproduced with permission. Swanepoel CR, Atta MG, D’Agati et al Kidney disease in the setting of HIV infection: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney international. 2018;93(3):545–559. © 2017 International Society of Nephrology. Published by Elsevier Inc. Creative Commons CC-BY-NC-NDlicense.12
Abbreviations: APOL1, apolipoprotein L1; ABCC, ATP-binding cassette transporter proteins; ART, antiretroviral therapy; CKD, chronic kidney disease, FSGS (NOS) focal segmental glomerulosclerosis, not otherwise specified; GN, glomerulonephritis; HIVAN, HIV-associated nephropathy.
Table 1.
Similarities and Differences in the Clinical Presentation of CKD in HIV and DKD
| DM and CKD | HIV and CKD | |
|---|---|---|
| HPT | Often HPT before onset of CKD | HPT is usually not associated with HIVAN. Prior to CKD only HPT if known with pre-existing HPT |
| Phenotype | Frequently metabolic phenotype (T2DM) | BMI often not raised. |
| Oedema | Oedema often present | ± Oedema If CKD due to HIVAN – oedema unlikely Only oedematous if nephrotic syndrome or advanced CKD |
| Accompanying features | Other Target organ damage:
|
Often multiple pathologies on kidney biopsy
May have no specific features (HIVAN and ICGN can occur with any CD4 count) |
| Urine dipstick | Classical presentation: Usually albuminuria ± blood | Variable: Bland or Proteinuria ± blood (if ICGN, AIN or TB) |
| Natural history | Gradual onset of:
|
Presentation of kidney disease is varied depending on underlying cause. |
| Progression of disease | With poorly controlled HPT and DM eGFR can drop 10mL/min/year | HIVAN: without treatment can progress to ESKF in 3/12 Decline more rapid |
| Rapid decline in eGFR, what to consider? | AKI on CKD AIN (due to drug) PIGN or other GN |
Untreated HIVAN ICGN – can decline rapidly if crescentic AIN due to drug Intercurrent opportunistic infections: eg TB or IRIS |
Abbreviations: HPT, Hypertension; CKD, chronic kidney disease; DM, diabetes mellitus; T2DM, type 2 diabetes mellitus; BMI, body mass index, HIVAN, human immunodeficiency virus associated nephropathy; KF, kidney failure; IHD, ischaemic heart disease; CVA, cerebrovascular accident; PVD, peripheral vascular disease; GN, glomerulonephritis; ICGN, immune complex mediated GN; Hb, haemoglobin; eGFR, estimated glomerulofiltration rate; DKD, diabetic kidney disease; PIGN, post infectious glomerulonephritis, ESKF: end stage kidney failure, AIN: acute interstitial nephritis; AKI, acute kidney injury; TB, tuberculosis; IRIS, immune reconstitution syndrome.
Table 2.
Similarities and Differences in the Investigation of CKD in HIV and DKD
| DM and CKD | HIV and CKD | |
|---|---|---|
| Laboratory Investigations | ||
| Microscopy | Bland microscopy | Active urine on microscopy if ICGN |
| Urea | Urea – get symptomatic at a lower urea than normal population | Due to low muscle mass – Urea and creatinine may underestimate degree of KF. Cystatin C currently best measurement of eGFR. |
| Anaemia | Anaemia develops earlier in CKD | Anaemia likely multifactorial |
| Markers of MBD | MBD develops earlier in DKD | Tenofovir associated osteomalacia |
| UPCR | Absence of albuminuria does not exclude DKD
|
Variable- depending on cause:
|
| Special Investigations | ||
| Ultrasound | Increased kidney size despite reduced eGFR | Increased kidney size despite reduced eGFR in HIVAN |
| Renal biopsy | Classification of DKD histological features:
|
Multiple pathologies can be found:
|
| Indication for biopsy | Proteinuria with no retinopathy Onset of proteinuria rapid or onset of proteinuria <5 years in T1DM Macroscopic haematuria or active urine sediment Unexplained rapid decline in KF |
Spectrum of kidney disease is broad - Low threshold for biopsy Significant proteinuria (increasing trend or nephrotic range) Haematuria or active urine sediment Unexplained decline in kidney function |
Abbreviations: CKD, chronic kidney disease; DM, diabetes mellitus; MBD, mineral bone disease; HIVAN, human immunodeficiency virus associated nephropathy; KF, kidney failure; IHD, ischaemic heart disease; CVA, cerebrovascular accident; PVD, peripheral vascular disease; GN, glomerulonephritis; ICGN, immune complex mediated GN; Hb, haemoglobin; eGFR, estimated glomerulofiltration rate; DKD, diabetic kidney disease; EM, electron microscopy; PIGN, post infectious glomerulonephritis, ESKF: end stage kidney failure, AIN: acute interstitial nephritis; AKI, acute kidney injury; TB, tuberculosis; IRIS, immune reconstitution syndrome.
In a large cohort of 31,072 veterans with a baseline estimated GFR (eGFR) ≥45mL/min/1.73m2 in HIV-positive and matched HIV-negative individuals, there was a significant and graduated independent association between HIV and DM status and the risk of decline in eGFR. Co-existing HIV and DM had a greater effect on the relative risk of progression of CKD (HR 4.47, 95% CI 3.87–5,17), compared to either disease alone [HIV (2,8, 95% CI 2.50 −3,15) or DM (HR 2.48; 95% CI 2.19–2,80) only].18 Similar results were demonstrated in an earlier study by Choi et al who demonstrated a 4-fold increase in risk of KF in those with DM and a 7-fold increased risk in those with the combination of DM and HIV. However, a limitation of this study is that it only examined differences in ethnicity and did not adjust for other factors known to influence CKD progression.63 A single centre study reviewing PWH (n = 1494), comparing those with DM (n = 156) and those without, demonstrated a more frequent need for antihypertensives and lipid-lowering agents and a higher prevalence of kidney dysfunction (12.4% vs 7.1%, p = 0.030) in those with co-existing DM.64
A retrospective study of 653 PWD found that the majority of PWH on ART failed to achieve target glycaemic control, resulting in a greater incidence of neuropathy and nephropathy (when defined by overt proteinuria). Proteinuria was present in 25.7% of the HIV-positive patients and 15.4% of the HIV-negative patients. Obesity was also a concern; however, it was noted in both HIV negative and positive cohorts.65
Challenges in Calculating eGFR
Using the serum creatinine in the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation to estimate the GFR is the most practical and reliable way of determining the prevalence of CKD.66 This equation has recently been updated with the removal of the race- based correction factor for African Americans.67 To note, these equations were developed in the US and have not been validated against measured GFR (mGFR) in other regions. Whether this correction factor is applicable to patients of African descent living in Africa is controversial.
In a recent study by Fabian et al conducted in Uganda, Malawi and South Africa, 3025 subjects underwent determination of mGFR using the slope-intercept method for iohexol plasma clearance and this was compared to eGFR using a variety of creatinine and cystatin C-based equations (with or without the correction factor for African Americans). The principal findings of the study were that creatinine-based equations overestimated kidney function compared with mGFR, which was worsened by use of the inclusion of the race-based correction factor. The greatest bias occurred at low kidney function, such that the proportion with GFR < 60 mL/min/1·73 m² directly measured was more than double that estimated from creatinine. Cystatin C-based equations performed better than all creatinine-based equations. Using a model to impute kidney function based on mGFR, the estimated prevalence of impaired kidney function was more than two-times higher than creatinine-based estimates in populations across six countries in Africa.68 The authors speculate that the poor performance of creatinine-based eGFR equations is more likely due to non-GFR determinants of creatinine rather than ethnicity. These include lower muscle mass due to growth stunting, wasting or inflammation from chronic infection (eg tuberculosis and HIV), lower dietary protein ingestion, and undiagnosed liver disease.68
Over estimation of GFR with the resultant under estimation of CKD has major implications for health planning given the high costs of treating CKD and the high associated morbidity and mortality.
Medical Management of T2DM in PWH
There are only a few observational studies and no RCTs specifically assessing the use of any of the treatment options for managing T2DM in PWH. Han et al conducted a longitudinal cohort study in HIV-positive and HIV-negative veterans who were new users of oral DM medication (mostly metformin, sulphonylureas and thiazolidinediones). They found that the glycaemic response was independent of the initial class of medication and was not influenced by HIV infection.69 Interestingly, in their multivariable model, Black race and Hispanic ethnicity were associated with a poorer response to these DM medications. Since there are no robust data to guide treatment options for T2DM, most guidelines recommend that local guidelines for the pharmacologic management of T2DM are followed for PWH. However, there is an extra caution regarding the use of metformin. Metformin is often a good initial choice for the management of T2DM in PWH as insulin resistance is often one of the dominant pathophysiological mechanisms. No dosage adjustment is required when using metformin with non-nucleoside reverse transcriptase inhibitors, PIs or NRTIs. However, when metformin is used with INSTIs (eg dolutegravir), the area under the curve of metformin is increased, therefore, by consensus, the maximum daily dose of metformin when used with dolutegravir should be limited to 1000 mg daily.70 Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists have been shown to prevent progression of DKD in HIV-negative people with DM; however, this has not yet been studied in PWH. Tables 3–5 reflect the dose adjustments that are required for DM medications with CKD stage 3–5 and those on KRT.71–75
Table 3.
Adjustment of Diabetes Treatment in Chronic Kidney Disease
| Dose Adjustment for CKD 3–5 (*eGFR <30 ml/Min/1.73m2) | |
|---|---|
| Metformin | Contraindicated |
| Insulin | Insulin uptake, metabolism and elimination is reduced. Less insulin is required – titrate to need: eGFR 60–15: 25% reduction# eGFR <15: 50% reduction# |
| Sulphonylureas | |
| Gliclazide | eGFR < 30, use under specialist supervision |
| Glipizide | No dose adjustment based on eGFR [start conservatively (2,5 mg orally daily) and titrate up slowly in CKD]# |
| Glimepiride | eGFR <30: reduce dose to 1 mg orally daily eGFR < 15: not recommended |
| Glibenclamide | Use not recommended |
| SGLT2 inhibitors | |
| Canagliflozin | eGFR > 60: 100 mg daily, can titrate up to 300 mg orally daily eGFR 30–60: 100 mg orally daily eGFR < 30: Not recommended for initiation ● May continue for reducing risk of decline eGFR, ESKD, CV death and admission for HF |
| Dapagliflozin | eGFR > 45: no dose adjustment eGFR 25–45: 10 mg orally daily# eGFR < 25#: Not recommended for initiation ● May continue for reducing risk of decline eGFR, ESKD, CV death and admission for HF |
| Empagliflozin | eGFR > 30 no dose adjustment (A) CKD: eGFR > 20: 10 mg orally daily (may have a beneficial effect on CV health and reducing risk of decline eGFR) (B) For Glycaemic Control with No CVD or CVD Risk Factors eGFR < 30: Use not recommended (C) Heart Failure eGFR ≥ 20: No adjustment necessary eGFR < 20: Insufficient data to support a dosing recommendation in this population |
| Ertugliflozin | eGFR > 45: no dose adjustment eGFR < 45: use not recommended |
| Thiazolidinediones | |
| Pioglitazone | No dose adjustment required |
| Alpha-glucosidase inhibitors | |
| Acarbose | eGFR < 30: use not recommended |
| Miglitol | eGFR < 25: use not recommended |
Table 4.
Adjustment of Incretin Treatment in Chronic Kidney Disease
| Dose Adjustment for CKD 3–5 (*eGFR <30ml/min/1.73m2) | |
|---|---|
| GLP- 1 RA | |
| Dulaglutide | No dose adjustment |
| Exenatide | CrCl 30–50: caution with initiating or increasing the dose CrCl < 30: use not recommended If using a once weekly preparation: eGFR < 45: use not recommended |
| Liraglutide | No dose adjustment |
| Lixisenatide | eGFR 30–60: no adjustment necessary eGFR 15–29: monitoring of kidney function and gastrointestinal side effects is recommended eGFR < 15: use not recommended |
| Semaglutide | No dose adjustment |
| DPP-4 inhibitors | |
| Alogliptin | CrCl 30 −59: decrease to 12.5 mg orally daily CrCl < 30: decrease to 6.25 mg orally daily |
| Linagliptin | No dose adjustment required |
| Saxagliptin | eGFR < 45: decrease to 2.5 mg orally daily |
| Sitagliptin | eGFR 30–45: decrease to 50 mg orally daily eGFR < 30: decrease to 25 mg orally daily |
Notes: *eGFR, estimated glomerular filtration rate in ml/min/1.73m2.
Abbreviations: GLP-1 RA, glucagon-like peptide 1 receptor agonist; CrCl, creatinine clearance; DPP-4, dipeptidyl peptidase IV; mg, milligrams.
Table 5.
Adjustment of Diabetes Treatment for Kidney Failure-Dialysis Requiring
| Metformin | Contraindicated |
|---|---|
| Insulin | No specific dose adjustment is required. |
| Sulphonylureas | |
| Gliclazide | Discontinue and replace with insulin |
| Glipizide | Discontinue and replace with insulin |
| Glimepiride | Discontinue and replace with insulin |
| Glibenclamide | Discontinue and replace with insulin |
| SGLT2 inhibitors | |
| Canagliflozin | Contraindicated at present |
| Dapagliflozin | Contraindicated at present |
| Empagliflozin | Contraindicated at present |
| Ertugliflozin | Contraindicated at present |
| GLP- 1 RA | |
| Dulaglutide | Use with caution in ESKD, monitor kidney function closely in patient with gastrointestinal side effects |
| Exenatide | Contraindicated at present |
| Liraglutide | Use with caution in ESKD, when initiating or escalating doses in patients with renal impairment |
| Lixisenatide | Contraindicated at present |
| Semaglutide | Use with caution, monitor for side effects CrCl<15 requiring dialysis: Contraindicated** |
| DPP-4 inhibitors | |
| Alogliptin | 6.25 mg orally daily; administer without regard to timing of haemodialysis |
| Linagliptin | No dose adjustment required |
| Saxagliptin | 2,5 mg orally daily post dialysis |
| Sitagliptin | 25 mg orally daily without regard to timing of dialysis |
| Thiazolidinediones | |
| Pioglitazone | No dose adjustment required |
| Alpha-glucosidase inhibitors | |
| Acarbose | Contraindicated at present |
| Miglitol | Contraindicated at present |
Notes: **Advice from therapeutic index: South African Medical Formulary.
Abbreviations: SGLT2, sodium glucose co-transporter-2; GLP-1 RA, glucagon-like peptide 1 receptor agonist; DPP-4, dipeptidyl peptidase IV; CrCl, creatinine clearance; ESKD, end stage kidney disease; mg, milligrams.
In terms of DKD management, identification of additional risk factors for CKD is essential. In PWH, this includes a suppressed HIV viral load, early identification of hepatitis B and/or C virus coinfection, exclusion of nephrotoxins as well as managing episodes of AKI. All PWH should be on ART, including those with DKD. A baseline eGFR and assessment for microalbuminuria should be performed. However, a change in type of ART is required when the eGFR falls to <50 mL/min/1.73m2. Tenofovir, atazanavir and lopinavir should be avoided in those individuals with established CKD.76 Unfortunately, there are limited data concerning recommendations for the concurrent management of DKD in PWH. However, data from large RCTs in people without HIV-infection have shown the importance of DM and BP control in preventing and decreasing the progression of DKD.77,78 Angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) have an important role in slowing the GFR decline in DKD with albuminuria.79–81 In addition, ACE inhibitors and ARBs have been shown to prevent the progression of HIVAN.82
Additionally, a multi-faceted approach in the management of DKD in PWH should include intensive lipid management being careful with the concomitant use of simvastatin with PIs due to the increased risk of rhabdomyolysis. Comprehensive lifestyle interventions including a low protein and salt diet, moderate intensity physical activity, cessation of smoking, alcohol reduction and weight management.
Challenges in KRT with HIV and DM
The KDIGO controversies in HIV state that HIV status should not influence candidacy for KRT. The survival on KRT is similar for those patients on ART with viral suppression compared with HIV-negative patients.12,83,84 However, the survival of people with DM on KRT is significantly worse than those without DM.85–92 The outcome of PWH and DM on KRT is currently unknown.
The choice of dialysis for PWH and KF should be based on patient preference and resources.12,93 Both peritoneal dialysis (PD) and haemodialysis (HD) have disadvantages in the setting of DM. This includes vascular access complications due to accelerated atherosclerotic disease. In addition, the glucose load in PD may accelerate atherosclerosis and worsen DM control. Meta-analyses have demonstrated a superior survival for people with DM on HD compared to PD. This is thought to be due to the increased rate of infection, inadequate dialysis and volume control for patients on PD.94,95 In a cohort of 401 PD patients, followed up for 10 years, DM was shown to be an independent predictor for increased mortality and technique failure, but not for peritonitis-free survival.96
Both DM and HIV, cause marked vascular damage. “Inflamm-aging” describes the increased age-related co-morbidities which occur at a younger age in PWH.97 This accelerated aging may relate to chronic ART usage, immune activation and inflammation.98,99 This is evidenced by a high degree of vascular stiffening and “non-dipping” on ABPM.100 Similarly, endothelial dysfunction and inflammation is also demonstrated in DM.101
Numerous reports have reviewed the adverse effects of HIV and DM on arteriovenous fistula (AVF) creation, but as far as we are aware none have looked at the additive effect. In PWH, AVFs are preferable to dialysis catheters due to the increased risk of infection and stenosis.12,102 On review of 25,711 AVF creations, HIV did not increase the risk of reintervention, occlusion or mortality. In this cohort, 42% had a combination of DM and HIV, however a direct comparison between PWH with or without DM was not performed.103 PD catheter failure rates are similar to HIV negative patients. In addition, PD consumables must be discarded with appropriate infection control measures, as HIV persists in PD fluid.12
The combination of HIV and DM, or each as separate entities, poses an increased risk for cardiovascular disease which is a major cause of death in patients on KRT.104 Once again, there are no recommendations for which modality poses the lowest risk for those with a combination of HIV and DM. However, a meta-analysis and a comparative study comparing PD and HD have shown that PD had a lower incidence of cardiovascular and cerebrovascular events, than HD.105,106
Stock et al first described successful outcomes in HIV-positive transplant recipients from HIV-negative donors.107 Following these results, a crisis for dialysis slots in South Africa prompted clinicians to take a more liberal approach to donor selection. Consequently, a pivotal programme to use kidneys from HIV-positive donors for HIV-positive recipients was initiated in September 2008.108 In the setting of transplantation, HIV-positive recipients have excellent allograft survival at 1 and 3 years. In addition, the safety of transplantation in PWH is well-established.107–109 However, there is still significant bias toward transplantation access for PWH, with longer waiting times for referral, evaluation and waitlisting.110 In contrast, it is well known that DM can adversely affect kidney allograft and patient survival.111 Up to a two-fold higher mortality and graft loss has been described in transplant patients with pre-existing DM and those developing post-transplant DM.112,113
Little is known about the outcomes of PWH and DM post transplantation or the development of new onset DM post transplantation. Of the cohorts described, only Locke et al reported on prevalence of DM in the HIV transplant cohort, with DM being prevalent in 13.3% (57/113) of living and 11.7% (50/426) of deceased donor recipients, respectively. However, no comparison of the combination of HIV and DM was performed.109 There is an increased risk of rejection in PWH.107,108 The higher doses and type of immunosuppression needed to treat this increases the risk of developing DM post-transplant. In a study conducted by Barday et al in HIV-positive transplant recipients, there was an association between increased rate of rejection and use of PIs,12% of the cohort were on PIs at baseline.114 Furthermore, immunosuppression drug-level management is complex in transplanted PWH due to the drug interactions.115
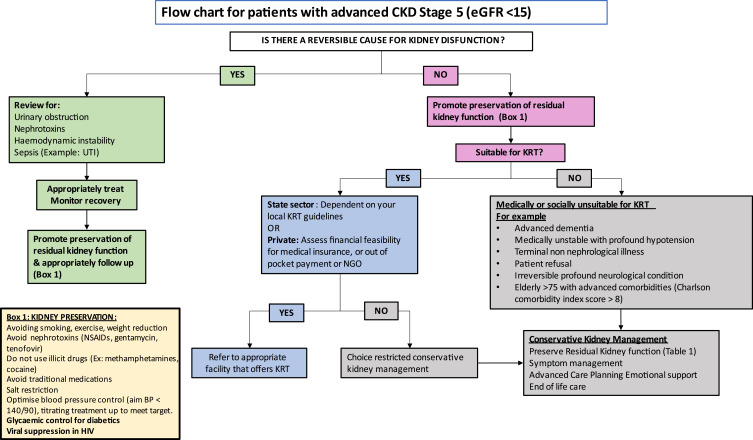
Although the prevalence of KF is increasing worldwide, major global inequalities exist with the largest treatment gaps occurring in LMICs.116,117 Access to lifesaving KRT remains limited or non-existent in many LMICs. It is reported that only 16% of patients requiring KRT on the African continent receive it.116 This means that chronic dialysis and transplantation are either not offered, are unaffordable or rationed resulting in the demise of many patients. The lack of access removes any element of “choice” for KRT (Figure 2). There has been considerable attention drawn to the importance of kidney supportive, conservative and palliative care by the International Society of Nephrology.118 However, in LMICs, there are limited resources to integrate palliative care into all levels of care despite it being demonstrated to be cost-effective.119 The cost of palliative care programs will vary by region and continent depending on access and infrastructure. Therefore, there needs to be greater emphasis on preventative measures to prevent CKD progression as well as improved palliative and conservative management for patients when KRT is limited or not available.120
Figure 2.
Choice restricted conservative kidney management.
Abbreviations: CKD, chronic kidney disease; UTI, urinary tract infection; eGFR, estimated glomerular filtration rate; KRT, kidney replacement therapy; NGO, non-government organisation; NSAIDs, non-steroidal anti-inflammatory drugs.
In a resource-limited setting, when dialysis is rationed, often PWD and PWH who are not virally suppressed, are excluded from KRT programmes.120 The concept of conservative kidney management is directly aligned with the Universal Health Coverage.118 It is crucial that in low resources settings there is early identification and management of kidney disease at all levels of care, this is particularly important in regions where KRT is limited.
Conclusion
Limited data exist on the burden of CKD, in patients with comorbid HIV and DM. Given the growing prevalence of HIV and DM, individually and in combination, this review highlights the need for a call to action to improve care as well as identify areas in which research is required. Future research should focus on the following: a better understanding of the molecular targets and genetic factors (APOL-1) that alter the trajectory of DKD, RCTs to show the efficacy of DM drugs in PWH, a better understanding of the long term metabolic sequelae of the use of INSTIs, especially in Black Africans where there is a possible early signal that they may be at increased risk of developing DM and, lastly, identify the impact of both DM and HIV on KRT. In Africa and other areas with limited access to KRT, strict blood pressure control, good diabetes management, viral suppression with ART and awareness of the multiple drug interactions/toxicity/dosing remain the foundation of therapy to prevent CKD. There is an urgent need to establish or upscale palliative care programs in areas with restricted access to KRT and high burden of disease as these have shown to be cost-effective in the long term.
Disclosure
The authors report no conflicts of interest related to this work.
References
- 1.UNAIDS. In Danger: UNAIDS global AIDS update 2022; 2022. Available from: https://www.unaids.org/en/resources/documents/2022/in-danger-global-aids-update. Accessed May 9, 2023.
- 2.International Diabetes Federation. IDF diabetes atlas 2021 - 10th edition; 2021. Available from: www.diabetesatlas.org. Accessed August 14, 2023. [PubMed]
- 3.Erzse A, Stacey N, Chola L, Tugendhaft A, Freeman M, Hofman K. The direct medical cost of type 2 diabetes mellitus in South Africa: a cost of illness study. Glob Health Action. 2019;12(1):1636611. doi: 10.1080/16549716.2019.1636611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bello AK, Levin A, Lunney M, et al. Status of care for end stage kidney disease in countries and regions worldwide: international cross sectional survey. BMJ. 2019;367. doi: 10.1136/bmj.l5873 [DOI] [PubMed] [Google Scholar]
- 6.Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C. A Single Number for Advocacy and communication—worldwide More Than 850 Million Individuals Have Kidney Diseases. Oxford University Press; 2019:1803–1805. [DOI] [PubMed] [Google Scholar]
- 7.Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765. doi: 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–308. doi: 10.1681/ASN.2012070718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. doi: 10.2215/CJN.11491116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noubiap JJN, Naidoo J, Kengne AP. Diabetic nephropathy in Africa: a systematic review. World J Diabetes. 2015;6(5):759. doi: 10.4239/wjd.v6.i5.759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanepoel CR, Atta MG, D’Agati VD, et al. Kidney disease in the setting of HIV infection: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2018;93(3):545–559. doi: 10.1016/j.kint.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diana NE, Davies M, Mosiane P, Vermeulen A, Naicker S. Clinicopathological correlation of kidney disease in HIV infection pre-and post-ART rollout. PLoS One. 2022;17(5):e0269260. doi: 10.1371/journal.pone.0269260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wearne N, Manning K, Price B, et al. The evolving spectrum of kidney histology in HIV-positive patients in South Africa. Kidney Int Rep. 2023;8:1087–1096. doi: 10.1016/j.ekir.2023.02.1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudose S, Santoriello D, Bomback AS, et al. The spectrum of kidney biopsy findings in HIV-infected patients in the modern era. Kidney Int. 2020;97:1006–1016. doi: 10.1016/j.kint.2020.01.018 [DOI] [PubMed] [Google Scholar]
- 16.Wearne N, Swanepoel CR, Boulle A, Duffield MS, Rayner BL. The spectrum of renal histologies seen in HIV with outcomes, prognostic indicators and clinical correlations. Nephrol Dial Transplant. 2012;27(11):4109–4118. doi: 10.1093/ndt/gfr702 [DOI] [PubMed] [Google Scholar]
- 17.Ekrikpo UE, Kengne AP, Bello AK, et al. Chronic kidney disease in the global adult HIV-infected population: a systematic review and meta-analysis. PLoS One. 2018;13(4):e0195443. doi: 10.1371/journal.pone.0195443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medapalli R, Parikh CR, Gordon K, et al. Comorbid diabetes and the risk of progressive chronic kidney disease in HIV-infected adults: data from the veterans aging cohort study. J Acquir Immune Defic Syndr. 2012;60(4):393. doi: 10.1097/QAI.0b013e31825b70d9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018;117(8):662–675. doi: 10.1016/j.jfma.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 20.Alsaad KO, Herzenberg AM. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: an update. J Clin Pathol. 2007;60(1):18–26. doi: 10.1136/jcp.2005.035592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dagar N, Das P, Bisht P, Taraphdar AK, Velayutham R, Arumugam S. Diabetic nephropathy: a twisted thread to unravel. Life Sci. 2021;278:119635. doi: 10.1016/j.lfs.2021.119635 [DOI] [PubMed] [Google Scholar]
- 22.Raptis AE, Viberti G. Pathogenesis of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S424–37. doi: 10.1055/s-2001-18600 [DOI] [PubMed] [Google Scholar]
- 23.Tuttle KR. Back to the future: glomerular hyperfiltration and the diabetic kidney. Diabetes. 2017;66(1):14–16. doi: 10.2337/dbi16-0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernhardt WM, Schmitt R, Rosenberger C, et al. Expression of hypoxia-inducible transcription factors in developing human and rat kidneys. Kidney Int. 2006;69(1):114–122. doi: 10.1038/sj.ki.5000062 [DOI] [PubMed] [Google Scholar]
- 25.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57(6):1446–1454. doi: 10.2337/db08-0057 [DOI] [PubMed] [Google Scholar]
- 26.Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev. 2008;4(1):39–45. doi: 10.2174/157339908783502370 [DOI] [PubMed] [Google Scholar]
- 27.Potenza MA, Gagliardi S, Nacci C, Carratu MR, Montagnani M. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr Med Chem. 2009;16(1):94–112. doi: 10.2174/092986709787002853 [DOI] [PubMed] [Google Scholar]
- 28.Flyvbjerg A. The role of the complement system in diabetic nephropathy. Nat Rev Nephrol. 2017;13(5):311–318. doi: 10.1038/nrneph.2017.31 [DOI] [PubMed] [Google Scholar]
- 29.Ramana KV. ALDOSE REDUCTASE: new insights for an old enzyme. Biomol Concepts. 2011;2(1–2):103–114. doi: 10.1515/BMC.2011.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritz E, Tomaschitz A. Aldosterone, a vasculotoxic agent--novel functions for an old hormone. Nephrol Dial Transplant. 2009;24(8):2302–2305. doi: 10.1093/ndt/gfp206 [DOI] [PubMed] [Google Scholar]
- 31.Suzuki D, Miyazaki M, Jinde K, et al. In situ hybridization studies of matrix metalloproteinase-3, tissue inhibitor of metalloproteinase-1 and type IV collagen in diabetic nephropathy. Kidney Int. 1997;52(1):111–119. doi: 10.1038/ki.1997.310 [DOI] [PubMed] [Google Scholar]
- 32.Sarkar S, Brown TT. Diabetes in people with HIV. Curr Diab Rep. 2021;21(5):13. doi: 10.1007/s11892-021-01382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dave JA, Lambert EV, Badri M, West S, Maartens G, Levitt NS. Effect of nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy on dysglycemia and insulin sensitivity in South African HIV-infected patients. J Acquir Immune Defic Syndr. 2011;57(4):284–289. doi: 10.1097/QAI.0b013e318221863f [DOI] [PubMed] [Google Scholar]
- 34.Prioreschi A, Munthali RJ, Soepnel L, et al. Incidence and prevalence of type 2 diabetes mellitus with HIV infection in Africa: a systematic review and meta-analysis. BMJ Open. 2017;7(3):e013953. doi: 10.1136/bmjopen-2016-013953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12(7):F51–8. doi: 10.1097/00002030-199807000-00003 [DOI] [PubMed] [Google Scholar]
- 36.Hadigan C, Corcoran C, Stanley T, Piecuch S, Klibanski A, Grinspoon S. Fasting hyperinsulinemia in human immunodeficiency virus-infected men: relationship to body composition, gonadal function, and protease inhibitor use. J Clin Endocrinol Metab. 2000;85(1):35–41. doi: 10.1210/jcem.85.1.6264 [DOI] [PubMed] [Google Scholar]
- 37.Hadigan C, Miller K, Corcoran C, Anderson E, Basgoz N, Grinspoon S. Fasting hyperinsulinemia and changes in regional body composition in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 1999;84(6):1932–1937. doi: 10.1210/jcem.84.6.5738 [DOI] [PubMed] [Google Scholar]
- 38.Levitt NS, Peer N, Steyn K, et al. Increased risk of dysglycaemia in South Africans with HIV; especially those on protease inhibitors. Diabetes Res Clin Pract. 2016;119:41–47. doi: 10.1016/j.diabres.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 39.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165(10):1179–1184. doi: 10.1001/archinte.165.10.1179 [DOI] [PubMed] [Google Scholar]
- 40.Echecopar-Sabogal J, D’Angelo-Piaggio L, Chanamé-Baca DM, Ugarte-Gil C. Association between the use of protease inhibitors in highly active antiretroviral therapy and incidence of diabetes mellitus and/or metabolic syndrome in HIV-infected patients: a systematic review and meta-analysis. Int J STD AIDS. 2018;29(5):443–452. doi: 10.1177/0956462417732226 [DOI] [PubMed] [Google Scholar]
- 41.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275(27):20251–20254. doi: 10.1074/jbc.C000228200 [DOI] [PubMed] [Google Scholar]
- 42.Limone P, Biglino A, Valle M, et al. Insulin resistance in HIV-infected patients: relationship with pro-inflammatory cytokines released by peripheral leukocytes. J Infect. 2003;47(1):52–58. doi: 10.1016/s0163-4453(03)00055-0 [DOI] [PubMed] [Google Scholar]
- 43.Lagathu C, Kim M, Maachi M, et al. HIV antiretroviral treatment alters adipokine expression and insulin sensitivity of adipose tissue in vitro and in vivo. Biochimie. 2005;87(1):65–71. doi: 10.1016/j.biochi.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 44.Lindegaard B, Keller P, Bruunsgaard H, Gerstoft J, Pedersen BK. Low plasma level of adiponectin is associated with stavudine treatment and lipodystrophy in HIV-infected patients. Clin Exp Immunol. 2004;135(2):273–279. doi: 10.1111/j.1365-2249.2004.02367.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanters S, Renaud F, Rangaraj A, et al. Evidence synthesis evaluating body weight gain among people treating HIV with antiretroviral therapy - a systematic literature review and network meta-analysis. EClinicalMedicine. 2022;48:101412. doi: 10.1016/j.eclinm.2022.101412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381(9):803–815. doi: 10.1056/NEJMoa1902824 [DOI] [PubMed] [Google Scholar]
- 47.Kajogoo VD, Amogne W, Medhin G. New onset type 2 diabetes mellitus risks with integrase strand transfer inhibitors-based regimens: a systematic review and meta-analysis. Metabol Open. 2023;17:100235. doi: 10.1016/j.metop.2023.100235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulindwa F, Kamal H, Castelnuovo B, et al. Association between integrase strand transfer inhibitor use with insulin resistance and incident diabetes mellitus in persons living with HIV: a systematic review and meta-analysis. BMJ Open Diabet Res Care. 2023;11(1):e003136. doi: 10.1136/bmjdrc-2022-003136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berliner AR, Fine DM, Lucas GM, et al. Observations on a cohort of HIV-infected patients undergoing native renal biopsy. Am J Nephrol. 2008;28(3):478–486. doi: 10.1159/000112851 [DOI] [PubMed] [Google Scholar]
- 50.Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66(3):1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x [DOI] [PubMed] [Google Scholar]
- 51.Laster M, Shen JI, Norris KC. Kidney disease among African Americans: a population perspective. Am J Kidney Dis. 2018;72(5):S3–S7. doi: 10.1053/j.ajkd.2018.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. doi: 10.1126/science.1193032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hung RK, Binns-Roemer E, Booth JW, et al. Genetic variants of APOL1 are major determinants of kidney failure in people of African ancestry with HIV. Kidney Int Rep. 2022;7(4):786–796. doi: 10.1016/j.ekir.2022.01.1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Limou S, Nelson GW, Kopp JB, Winkler CA. APOL1 kidney risk alleles: population genetics and disease associations. Adv Chronic Kidney Dis. 2014;21(5):426–433. doi: 10.1053/j.ackd.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kopp JB, Heymann J, Winkler CA. APOL1 Renal Risk Variants: Fertile Soil for HIV-Associated Nephropathy. Elsevier; 2017:514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akinbodewa AA, Adejumo AO, Koledoye OV, et al. Community screening for pre-hypertension, traditional risk factors and markers of chronic kidney disease in Ondo State, South-Western Nigeria. Niger Postgrad Med J. 2017;24(1):25–30. doi: 10.4103/npmj.npmj_161_16 [DOI] [PubMed] [Google Scholar]
- 57.Freedman BI, Murea M. Target organ damage in African American hypertension: role of APOL1. Curr Hypertens Rep. 2012;14:21–28. doi: 10.1007/s11906-011-0237-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedman DJ, Pollak MR. APOL1 nephropathy: from genetics to clinical applications. Clin J Am Soc Nephrol. 2021;16(2):294–303. doi: 10.2215/CJN.15161219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osafo C, Raji YR, Olanrewaju T, et al. Genomic approaches to the burden of kidney disease in sub-Saharan Africa: the human heredity and health in Africa (H3Africa) kidney disease research network. Kidney Int. 2016;90(1):2–5. doi: 10.1016/j.kint.2015.12.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, Group US. Risk factors for renal dysfunction in type 2 diabetes: U.K. prospective diabetes study 74. Diabetes. 2006;55(6):1832–1839. doi: 10.2337/db05-1620 [DOI] [PubMed] [Google Scholar]
- 61.Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63(1):225–232. doi: 10.1046/j.1523-1755.2003.00712.x [DOI] [PubMed] [Google Scholar]
- 62.Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA. 2016;316(6):602–610. doi: 10.1001/jama.2016.10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Volberding PA, O’Hare AM. Racial differences in end-stage renal disease rates in HIV infection versus diabetes. J Am Soc Nephrol. 2007;18(11):2968–2974. doi: 10.1681/ASN.2007040402 [DOI] [PubMed] [Google Scholar]
- 64.Kousignian I, Sautereau A, Vigouroux C, et al. Diagnosis, risk factors and management of diabetes mellitus in HIV-infected persons in France: a real-life setting study. PLoS One. 2021;16(5):e0250676. doi: 10.1371/journal.pone.0250676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pillay S, Mahomed F, Aldous C. A deadly combination-HIV and diabetes mellitus: where are we now? S Afr Med J. 2016;106(4):378–383. doi: 10.7196/SAMJ.2016.v106i4.9950 [DOI] [PubMed] [Google Scholar]
- 66.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inker LA, Eneanya ND, Coresh J, et al. New creatinine-and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fabian J, Kalyesubula R, Mkandawire J, et al. Measurement of kidney function in Malawi, South Africa, and Uganda: a multicentre cohort study. Lancet Glob Health. 2022;10(8):e1159–e1169. doi: 10.1016/S2214-109X(22)00239-X [DOI] [PubMed] [Google Scholar]
- 69.Han JH, Gordon K, Womack JA, et al. Comparative effectiveness of diabetic oral medications among HIV-infected and HIV-uninfected veterans. Diabet Care. 2017;40(2):218–225. doi: 10.2337/dc16-0718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song IH, Zong J, Borland J, et al. The effect of dolutegravir on the pharmacokinetics of metformin in healthy subjects. J Acquir Immune Defic Syndr. 2016;72(4):400–407. doi: 10.1097/QAI.0000000000000983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwenk MH. Seyffart’s directory of drug dosage in kidney disease. Kidney Int. 2012;81(10):931–932. doi: 10.1038/ki.2012.57 [DOI] [Google Scholar]
- 72.Merative Micromedex® DRUGDEX® (electronic version). Merative, Ann Arbor, Michigan, USA; 2023. Available from: https://www.micromedexsolutions.com/. Accessed May 19, 2023. [Google Scholar]
- 73.Alicic RZ, Neumiller JJ, Galindo RJ, Tuttle KR. Use of glucose-lowering agents in diabetes and CKD. Kidney Int Rep. 2022;7(12):2589–2607. doi: 10.1016/j.ekir.2022.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rossing P, Caramori ML, Chan JCN, et al. Executive summary of the KDIGO 2022 Clinical practice guideline for diabetes management in chronic kidney disease: an update based on rapidly emerging new evidence. Kidney Int. 2022;102(5):990–999. doi: 10.1016/j.kint.2022.06.013 [DOI] [PubMed] [Google Scholar]
- 75.South African Medicine Association. South African Medicine Formulary. South African Medicine Association; 2022. [Google Scholar]
- 76.Post FA. Managing chronic kidney disease in the older adults living with HIV. Curr Opin Infect Dis. 2017;30(1):4–11. doi: 10.1097/QCO.0000000000000333 [DOI] [PubMed] [Google Scholar]
- 77.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853. doi: 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 78.Lv J, Perkovic V, Foote CV, Craig ME, Craig JC, Strippoli GF. Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database Syst Rev. 2012;12:Cd004136. doi: 10.1002/14651858.CD004136.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.UK Prospective Diabetes Study (UKPDS) Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. doi: 10.1136/bmj.317.7160.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirst JA, Taylor KS, Stevens RJ, et al. The impact of renin-angiotensin-aldosterone system inhibitors on type 1 and type 2 diabetic patients with and without early diabetic nephropathy. Kidney Int. 2012;81(7):674–683. doi: 10.1038/ki.2011.413 [DOI] [PubMed] [Google Scholar]
- 81.Wang K, Hu J, Luo T, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers on all-cause mortality and renal outcomes in patients with diabetes and albuminuria: a systematic review and meta-analysis. Kidney Blood Press Res. 2018;43(3):768–779. doi: 10.1159/000489913 [DOI] [PubMed] [Google Scholar]
- 82.Yahaya I, Uthman OA, Uthman MM. Interventions for HIV-associated nephropathy. Cochrane Database Syst Rev. 2013;2013(1):CD007183. doi: 10.1002/14651858.CD007183.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahuja TS, Grady J, Khan S. Changing trends in the survival of dialysis patients with human immunodeficiency virus in the United States. J Am Soc Nephrol. 2002;13(7):1889–1893. doi: 10.1097/01.ASN.0000019773.43765.BF [DOI] [PubMed] [Google Scholar]
- 84.Jardine T, Wong E, Steenkamp R, Caskey FJ, Davids MR. Survival of South African patients on renal replacement therapy. Clin Kidney J. 2020;13(5):782–790. doi: 10.1093/ckj/sfaa012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ozener C, Arikan H, Karayaylali I, et al. The impact of diabetes mellitus on peritoneal dialysis: the Turkey Multicenter Clinic Study. Ren Fail. 2014;36(2):149–153. doi: 10.3109/0886022X.2013.843275 [DOI] [PubMed] [Google Scholar]
- 86.Locatelli F, Del Vecchio L, Cavalli A. How Can Prognosis for Diabetic ESRD Be Improved? Wiley Online Library; 2010:214–219. [DOI] [PubMed] [Google Scholar]
- 87.Passadakis PS, Oreopoulos DG. Diabetic Patients on Peritoneal Dialysis. Wiley Online Library; 2010:191–197. [DOI] [PubMed] [Google Scholar]
- 88.Yang X, Yi C, Liu X, et al. Clinical outcome and risk factors for mortality in Chinese patients with diabetes on peritoneal dialysis: a 5-year clinical cohort study. Diabetes Res Clin Pract. 2013;100(3):354–361. doi: 10.1016/j.diabres.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 89.Fang W, Qian J, Lin A, et al. Comparison of peritoneal dialysis practice patterns and outcomes between a Canadian and a Chinese centre. Nephrol Dial Transplant. 2008;23(12):4021–4028. doi: 10.1093/ndt/gfn372 [DOI] [PubMed] [Google Scholar]
- 90.Pk-t L, Szeto -C-C. Success of the Peritoneal Dialysis Programme in Hong Kong. Oxford University Press; 2008. [DOI] [PubMed] [Google Scholar]
- 91.Tien K-J, Lin -Z-Z, Chio -C-C, et al. Epidemiology and mortality of new-onset diabetes after dialysis: Taiwan national cohort study. Diabet Care. 2013;36(10):3027–3032. doi: 10.2337/dc12-2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sattar A, Argyropoulos C, Weissfeld L, et al. All-cause and cause-specific mortality associated with diabetes in prevalent hemodialysis patients. BMC Nephrol. 2012;13:1–9. doi: 10.1186/1471-2369-13-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Soleymanian T, Raman S, Shannaq FN, et al. Survival and morbidity of HIV patients on hemodialysis and peritoneal dialysis: one center’s experience and review of the literature. Int Urol Nephrol. 2006;38:331–338. doi: 10.1007/s11255-006-0080-8 [DOI] [PubMed] [Google Scholar]
- 94.Xue J, Li H, Zhou Q, Wen S, Zhou Q, Chen W. Comparison of peritoneal dialysis with hemodialysis on survival of diabetic patients with end-stage kidney disease: a meta-analysis of cohort studies. Ren Fail. 2019;41(1):521–531. doi: 10.1080/0886022X.2019.1625788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Han SS, Park JY, Kang S, et al. Dialysis modality and mortality in the elderly: a meta-analysis. Clin J Am Soc Nephrol. 2015;10(6):983–993. doi: 10.2215/CJN.05160514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang D, Yang Y, Li R, et al. Comparison of long-term outcomes between Chinese peritoneal dialysis patients with and without diabetes: a 10-year cohort study. J Diabetes Complications. 2021;35(5):107888. doi: 10.1016/j.jdiacomp.2021.107888 [DOI] [PubMed] [Google Scholar]
- 97.Sieg SF, Shive CL, Panigrahi S, Freeman ML. Probing the interface of HIV and inflammaging. Curr HIV. 2021;18:198–210. doi: 10.1007/s11904-021-00547-0 [DOI] [PubMed] [Google Scholar]
- 98.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manner IW, Baekken M, Oektedalen O, Os I. Hypertension and antihypertensive treatment in HIV-infected individuals. A longitudinal cohort study. Blood Press. 2012;21(5):311–319. doi: 10.3109/08037051.2012.680742 [DOI] [PubMed] [Google Scholar]
- 100.Borkum M, Heckmann J, Manning K, et al. High prevalence of “non-dipping” blood pressure and vascular stiffness in HIV-infected South Africans on antiretrovirals. PLoS One. 2017;12(9):e0185003. doi: 10.1371/journal.pone.0185003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Jager J, Dekker JM, Kooy A, et al. Endothelial dysfunction and low-grade inflammation explain much of the excess cardiovascular mortality in individuals with type 2 diabetes: the Hoorn Study. Arterioscler Thromb Vasc Biol. 2006;26(5):1086–1093. doi: 10.1161/01.ATV.0000215951.36219.a4 [DOI] [PubMed] [Google Scholar]
- 102.Mitchell D, Krishnasami Z, Young CJ, Allon M. Arteriovenous access outcomes in haemodialysis patients with HIV infection. Nephrol Dial Transplant. 2007;22(2):465–470. doi: 10.1093/ndt/gfl629 [DOI] [PubMed] [Google Scholar]
- 103.Dicken QG, Cheng TW, Farber A, et al. Patients with human immunodeficiency virus infection do not have inferior outcomes after dialysis access creation. J Vasc Surg. 2020;72(6):2113–2119. doi: 10.1016/j.jvs.2020.03.030 [DOI] [PubMed] [Google Scholar]
- 104.Johansen KL, Chertow GM, Gilbertson DT, et al. US renal data system 2021 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2022;79(4):A8–A12. doi: 10.1053/j.ajkd.2022.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zou M, Xie J, Lan L, et al. Safety and efficacy of hemodialysis and peritoneal dialysis in treating end-stage diabetic nephropathy: a meta-analysis of randomized controlled trials. Int Urol Nephrol. 2022;54(11):2901–2909. doi: 10.1007/s11255-022-03194-5 [DOI] [PubMed] [Google Scholar]
- 106.Sigrist M, Bungay P, Taal MW, McIntyre CW. Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant. 2006;21(3):707–714. doi: 10.1093/ndt/gfi236 [DOI] [PubMed] [Google Scholar]
- 107.Stock PG, Barin B, Murphy B, et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010;363(21):2004–2014. doi: 10.1056/NEJMoa1001197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Muller E, Barday Z, Mendelson M, Kahn D. HIV-positive–to–HIV-positive kidney transplantation—results at 3 to 5 years. N Engl J Med. 2015;372(7):613–620. doi: 10.1056/NEJMoa1408896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Locke JE, Gustafson S, Mehta S, et al. Survival benefit of kidney transplantation in HIV-infected patients. Ann Surg. 2017;265(3):604. doi: 10.1097/SLA.0000000000001761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Adekunle RO, Zhang R, Wang Z, Patzer RE, Mehta AK. Early steps to kidney transplantation among persons with HIV and end‐stage renal disease in ESRD network 6. Transpl Infect Dis. 2022;24(1):e13767. doi: 10.1111/tid.13767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lim WH, Wong G, Pilmore HL, McDonald SP, Chadban SJ. Long-term outcomes of kidney transplantation in people with type 2 diabetes: a population cohort study. Lancet Diabet Endocrinol. 2017;5(1):26–33. doi: 10.1016/S2213-8587(16)30317-5 [DOI] [PubMed] [Google Scholar]
- 112.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3(2):178–185. doi: 10.1034/j.1600-6143.2003.00010.x [DOI] [PubMed] [Google Scholar]
- 113.Kuo H-T, Sampaio MS, Vincenti F, Bunnapradist S. Associations of pretransplant diabetes mellitus, new-onset diabetes after transplant, and acute rejection with transplant outcomes: an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) database. Am J Kidney Dis. 2010;56(6):1127–1139. doi: 10.1053/j.ajkd.2010.06.027 [DOI] [PubMed] [Google Scholar]
- 114.Barday Z, Manning K, Freercks R, Bertels L, Wearne N, Muller E. Retrospective review of ART regimens in HIV-positive to HIV-positive kidney transplant recipients. Kidney Int Rep. 2022;7(9):2039–2046. doi: 10.1016/j.ekir.2022.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Muller E, Botha FC, Barday ZA, Manning K, Chin-Hong P, Stock P. Kidney transplantation in HIV-positive patients: current practice and management strategies. Transplantation. 2021;105(7):1492–1501. doi: 10.1097/TP.0000000000003485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975–1982. doi: 10.1016/S0140-6736(14)61601-9 [DOI] [PubMed] [Google Scholar]
- 117.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X [DOI] [PubMed] [Google Scholar]
- 118.Davison SN, Levin A, Moss AH, et al. Executive summary of the KDIGO controversies conference on supportive care in chronic kidney disease: developing a roadmap to improving quality care. Kidney Int. 2015;88(3):447–459. doi: 10.1038/ki.2015.110 [DOI] [PubMed] [Google Scholar]
- 119.Smith S, Brick A, O’Hara S, Normand C. Evidence on the cost and cost-effectiveness of palliative care: a literature review. Palliat Med. 2014;28(2):130–150. doi: 10.1177/0269216313493466 [DOI] [PubMed] [Google Scholar]
- 120.Wearne N, Davidson B, Mc Culloch M, Krause R, Krause R. Radically rethinking renal supportive and palliative Care in South Africa. Kidney Int Rep. 2021;6(3):568–573. doi: 10.1016/j.ekir.2020.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]