Abstract
We studied the presence of tax and ltr genes from human T-cell lymphotropic virus type I (HTLV-I) provirus in the peripheral blood mononuclear cells from 15 seronegative patients with tropical spastic paraparesis or HTLV-I-associated myelopathy by PCR. Only a region of the tax gene from 10 patients was amplified. The nucleotide homologies of six Chilean isolates to the ATK-1 clone ranged between 98.7 and 99.4%.
Infection with human T-cell lymphotropic virus type I (HTLV-I) has been associated with the development of tropical spastic paraparesis or HTLV-I-associated myelopathy (TSP-HAM) (9, 17). Chilean studies have shown that almost 50% of patients with progressive spastic paraparesis (PSP) are HTLV-I seropositive (1). Diagnosis of this viral infection is done mainly by detection of specific antibodies (2, 7). The PCR assay has permitted detection of the provirus in peripheral blood mononuclear cells (PBMC) (5). Recently, some researchers have reported seronegative patients with PSP who are infected with HTLV-I (6, 16).
Seropositive and seronegative patients with PSP are clinically indistinguishable. A clinical study of these groups showed that seronegative patients had poor inflammatory response in their cerebrospinal fluid (CSF), absence of leukemoid lymphocytes in their peripheral blood, and less neurophysiologic involvement in the study of somatosensorial evoked potentials (1).
We studied 15 HTLV-I-seronegative patients with PSP (7 men and 8 women). They had an average age of 55.1 years (40 to 74 years) and an average paraparesis duration of 7.4 years (2 to 20 years). Other causes of PSP were excluded through clinical presentation according to cytochemical analysis of CSF and neurophysiological, radiological, immunological, and hematological analyses.
All patients had PSP with spasticity, hyperreflexia, and weakness of lower limbs; bilateral Babinski signs; and some sphincter disturbance. In addition to the spastic paraparesis, seven patients had brain involvement. Four of these developed pseudobulbar signs (dysartria, dysphagia, and affective lability). Three patients had basal ganglion involvement (two developed a Parkinsonian syndrome, and one showed diskinetic movements). Four patients had dacryosialadenitis, which was diagnosed by Schirmer’s test and by biopsies of minor salivary glands (3). Clinical data for each patient are presented in Table 1.
TABLE 1.
Clinical features of 15 HTLV-I-seronegative Chilean patients with PSP
| Patient no. | Sex | Age (yrs) | Evolution time (yrs) | Motor involvement | Sensitive symptom(s) | Spastic gait | Bladder involvement | Associated pathology | tax PCR |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 62 | 13 | Pseudobulbar signs and tetraparesis | Impairment in lower limbs and paresthesia | With support | Urgencya | None | Positive |
| 2 | M | 46 | 10 | Paraparesis | Vibratory sense impairment of lower limbs | Independent | Frequencyb | None | Positive |
| 3 | F | 61 | 20 | Paraparesis and Parkinsonian signs | Vibratory sense impairment of lower limbs and paresthesia | Independent | Frequency | Dacryosialadenitis | Positive |
| 4 | M | 74 | 5 | Paraparesis | None | With support | Frequency | None | Positive |
| 5 | F | 55 | 2 | Paraparesis and diskinesis | None | Independent | Frequency | Cutaneous lesions | Positive |
| 6 | M | 40 | 4 | Pseudobulbar signs and tetraparesis | None | With support | Urgency | Dacryosialadenitis | Positive |
| 7 | F | 48 | 3 | Pseudobulbar signs and tetraparesis | Tactile and vibratory sense impairment in lower limbs and paresthesia | Bedridden | Urgency and incontinencec | Dacryosialadenitis and hepatic cirrhosis | Positive |
| 8 | M | 73 | 4 | Paraparesis | None | Independent | Normal | Multiple neurofibramatosis | Positive |
| 9 | F | 42 | 3 | Paraparesis and Parkinsonian syndrome | Impairment in lower limbs and paresthesia | Independent | Frequency | Dacryosialadenitis and cutaneous lesions | Positive |
| 10 | M | 50 | 10 | Pseudobulbar signs and tetraparesis | None | Independent | Frequency | None | Positive |
| 11 | F | 70 | 6 | Paraparesis | None | Independent | Frequency | None | Negative |
| 12 | M | 52 | 12 | Paraparesis | Arm pain | With support | Normal | None | Negative |
| 13 | F | 30 | 1 | Paraparesis | None | Independent | Normal | None | Negative |
| 14 | M | 60 | 17 | Paraparesis | None | With support | Frequency | None | Negative |
| 15 | F | 56 | 4 | Paraparesis | Impairment in lower limbs and paresthesia | Independent | Normal | None | Negative |
Urinary urgency.
Urinary urgency and occasional urinary oncontinence.
Urinary frequency.
Determination of antibodies was accomplished by indirect immunofluorescence assay and Western blotting (WB) (8). DNA was extracted from purified PBMC according to a previously described method (5). By PCR, we amplified a region of 158 bp (primers SK43 to -44) of the tax gene and a region of 401 bp (primers LTR1 and LTR6) of the ltr gene (5). In six patients, amplified products of the tax gene were purified from agarose gels and cloned into the pGEM-T vector (Promega). Nucleotide sequence was determined by the dideoxy termination procedure with the Sequenase version 2.0 kit (U.S. Biochemicals). DNA sequences were aligned with the CLUSTAL V program (12).
All 15 patients were HTLV-I seronegative by indirect immunofluorescence and WB assays. Furthermore, all cases were negative for anti-p40 Tax antibodies by WB.
The tax gene was amplified from PBMC of 10 patients (5 men and 5 women), and the ltr gene was not detected in any of these patients. These results were confirmed through analysis of sequential samples from 6 patients (Table 1).
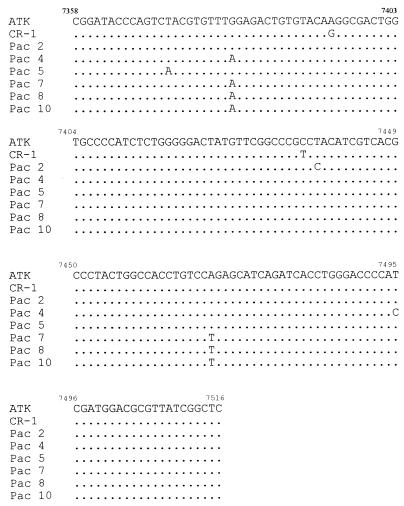
Sequences obtained from Chilean patients were compared with that of the HTLV-I prototype clone ATK-1 (Fig. 1). The tax sequences of patients 2, 4, 5, 7, 8, and 10 showed 99.4, 98.7, 99.4, 98.7, 98.7, and 98.7% homology, respectively, to the nucleotide sequence of the ATK-1 clone. Nucleotide homologies among samples in this region were 100% (patients 7, 8, and 10), 99.4% (patients 7, 8, and 10 compared to patient 4), 98.7% (patients 7, 8, and 10 compared to patients 2 and 5), 98.1% (patient 4 compared to patients 2 and 5), and 98.7% (patient 2 compared to patient 5). The nucleotide sequences of patients 4, 7, 8, and 10 had two nucleotide changes compared to the sequence of ATK-1; those of patients 2 and 5 had one nucleotide change. One of the nucleotide changes (nucleotide 7380) was common to four patients, and another (nucleotide 7469) was common to three patients (Table 2).
FIG. 1.
Nucleotide sequence of 158 bp of the tax gene from six Chilean HTLV-I-seronegative patients with PSP.
TABLE 2.
Nucleotide sequence homology of 158 bp of the tax gene between the ATK-1 clone and six HTLV-I-positive Chilean isolates
| Isolate | % Homology
|
||||||
|---|---|---|---|---|---|---|---|
| ATK-1 | Isolate 2 | Isolate 4 | Isolate 5 | Isolate 7 | Isolate 8 | Isolate 10 | |
| ATK-1 | 99.4 | 98.7 | 99.4 | 98.7 | 98.7 | 98.7 | |
| 2 | 99.4 | 98.1 | 98.7 | 98.7 | 98.7 | 98.7 | |
| 4 | 98.7 | 98.1 | 98.1 | 99.4 | 99.4 | 99.4 | |
| 5 | 99.4 | 98.7 | 98.1 | 98.7 | 98.7 | 98.7 | |
| 7 | 98.7 | 98.7 | 99.4 | 98.7 | 100 | 100 | |
| 8 | 98.7 | 98.7 | 99.4 | 98.7 | 100 | 100 | |
| 10 | 98.7 | 98.7 | 99.4 | 98.7 | 100 | 100 | |
We detected the tax gene in 10 of 15 HTLV-I-seronegative patients with PSP using PCR analysis. However, we did not detect the ltr gene in any of these patients. These results could not be explained by a different sensitivity in the genetic amplification method in different genomic regions of HTLV-I, because tax and ltr amplifications had similar sensitivities (6, 7).
Another hypothesis to explain our results is the presence of defective provirus in the PBMC from these patients. Yonaha-Nagato and Sumida found only the HTLV-I tax gene but not the gag, pol, or env gene in labial salivary gland samples from 29% of patients with Sjögren’s syndrome (23). Others researchers demonstrated the presence of a truncated HTLV-I genome from 72% of patients with Sezary’s syndrome (11). Our results showed the detection of tax but did not confirm ltr in 10 patients with TSP-HAM. These results support the hypothesis of an incomplete presence of the provirus in the PBMC of these seronegative TSP-HAM patients.
On the other hand, it is important to note that 40% of these seronegative, tax-positive TSP-HAM patients had developed chronic dacriosialoadenitis. This finding suggests that the development of some forms of Sjögren’s syndrome would be associated with the presence of HTLV-I provirus (3, 23).
Seropositive patients with TSP-HAM have high levels of antibodies against HTLV-I (1, 13, 15). However, our 10 tax-positive patients with TSP-HAM did not have antibodies against HTLV-I. This finding would discard an immune-inflammatory process as a pathogenic mechanism in these patients (20). Others researchers have suggested that antigens of HTLV or products encoding sequences homologous to the HTLV-I genes in PBMC from patients with TSP-HAM might be candidates for self-antigen and/or might lead to activation of autoreactive T lymphocytes by an immunoglobulin-mediated mechanism (19, 22). However, for our patients it seems more plausible to explain the pathogenesis by degenerative lesions of the central nervous system (21). We think that HTLV-I infection, in relation to genetic condition, has a direct effect on the development of spastic paraparesis.
HTLV-I is genetically very stable. A low degree of genetic variation (0.5 to 3%) has been described for HTLV-I strains from Japan, Africa, the Caribbean basin, and the Americas (4, 10, 14, 18). The tax fragment from our patients showed 98.7 to 99.4% homology with that of the ATK-1 genetic sequence. These findings showed that the amplified fragment is highly homologous to HTLV-I provirus sequence. In addition, the fact that some mutations in the tax gene were present in patients 3 and 4 suggests that the level of random mutation due to errors in Taq polymerase was not responsible for the overall differences observed. In summary, the results of this study suggest the presence of a defective HTLV-I provirus in these seronegative patients with TSP-HAM.
REFERENCES
- 1.Cartier L, Araya F, Castillo J L, Ruiz F, Gormaz A, Tajima K. Progressive spastic paraparesis associated with human T-cell leukemia virus type I (HTLV-I) Intern Med. 1992;31:1257–1261. doi: 10.2169/internalmedicine.31.1257. [DOI] [PubMed] [Google Scholar]
- 2.Cartier L, Araya F, Castillo J L, Verdugo R, Mora C, Gajdusek D C, Gibbs C. HTLV-I in Chile: a study of 140 neurological patients. Rev Med Chile. 1990;118:622–628. [PubMed] [Google Scholar]
- 3.Cartier L, Castillo J L, Cea J G, Villagra R. Chronic dacrosialadenitis in HTLV-I associated myelopathy. J Neurol Neurosurg Psychiatry. 1995;58:244–246. doi: 10.1136/jnnp.58.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De B K, Lairmore M D, Griffis K, Williams L J, Villinger F, Quinn T C, Brown C, Nzilambi N, Sugimoto M, Araki S, Folks T M. Comparative analysis of nucleotide sequences of the partial envelope gene (5′ domain) among human T lymphotropic virus type I (HTLV-I) isolates. Virology. 1991;182:413–419. doi: 10.1016/0042-6822(91)90692-5. [DOI] [PubMed] [Google Scholar]
- 5.Ehrlich G, Greenberg S, Abbott M. Detection of human T-cell lymphoma/leukemia viruses. In: Innis M, Gelfand D, Sninsky J, White T, editors. PCR protocols: a guide to methods and applications. San Diego, Calif: Academic Press, Inc.; 1990. pp. 325–336. [Google Scholar]
- 6.Galeno H, Ramirez E, Cartier L. HTLV-I provirus in seronegative chilean patients with tropical spastic paraparesis. Lancet. 1996;348:1170. doi: 10.1016/S0140-6736(05)65303-2. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 7.Galeno H, Ramirez E, Mora J, Ojeda M, Cartier L. Anti HTLV-I antibody titers in seropositive infected individuals. Rev Med Chile. 1994;122:1004–1007. [PubMed] [Google Scholar]
- 8.Gallo D, Penning L M, Hanson C V. Detection and differentiation of antibodies to human T-cell lymphotropic virus types I and II by immunofluorescence method. J Clin Microbiol. 1991;29:2345–2347. doi: 10.1128/jcm.29.10.2345-2347.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gessain A, Barin F, Vernant J C, Gout O, Calender A, de The G. Antibodies to human T lymphotropic virus type I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 10.Gessain A, Gallo R C, Franchini G. Low degree of human T-cell leukemia/lymphoma virus type I genetic drift in vivo as a means of monitoring viral transmission and movement of ancient human populations. J Virol. 1992;66:2288–2295. doi: 10.1128/jvi.66.4.2288-2295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh S K, Abrams J T, Terunuma H, Vonderheid E C, de Freitas E. Human T-cell leukemia type I tax/rex DNA and RNA in cutaneous T-cell lymphoma. Blood. 1994;84:2663–2671. [PubMed] [Google Scholar]
- 12.Higgins D G, Bleasby A J, Fusch R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992;8:189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson S, Gupta A, Mattson D, Mingioli E, McFarlin D. Immunological studies in tropical spastic paraparesis. Ann Neurol. 1990;27:149–156. doi: 10.1002/ana.410270209. [DOI] [PubMed] [Google Scholar]
- 14.Malik K T, Even J, Karpas A. Molecular cloning and complete nucleotide sequence of an adult T cell leukaemia virus/human T cell leukaemia virus type I (ATLV/HTLV-I) isolate of Caribbean origin: relationship to other members of the ATLV/HTLV-I subgroup. J Gen Virol. 1988;69:1695–1710. doi: 10.1099/0022-1317-69-7-1695. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa M, Izumo S, Ijichi S, Kubota H, Animura K, Kawabata M, Osame M. HTLV-I associated myelopathy: analysis of 213 patients based on clinical features and laboratory findings. J Neurovirol. 1995;1:50–61. doi: 10.3109/13550289509111010. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura M, Minglioli E, McFarlin D, Jacobson S. Demonstration of human T-cell lymphotropic virus type I (HTLV-I) from an HTLV-I seronegative South Indian patient with chronic, progressive spastic paraparesis. Ann Neurol. 1993;34:867–870. doi: 10.1002/ana.410340618. [DOI] [PubMed] [Google Scholar]
- 17.Osame M, Matsumoto M, Usuku K, Izumo S, Ijichi N, Amitani H, Tara M, Igata A. Chronic progressive myelopathy associated with elevated antibodies to human T lymphotropic virus type I and adult T-cell leukemia-like cells. Ann Neurol. 1987;21:117–122. doi: 10.1002/ana.410210203. [DOI] [PubMed] [Google Scholar]
- 18.Ratner L, Philpott T, Trowbridge D B. Nucleotide sequence analysis of isolates of human T-lymphotropic virus type I of diverse geographical origins. AIDS Res Hum Retroviruses. 1991;7:923–941. doi: 10.1089/aid.1991.7.923. [DOI] [PubMed] [Google Scholar]
- 19.Shoji H, Kuwasaki N, Kaji M, Miyamoto Y, Usuku K, Sonoda S, Osame M. HTLV-I-associated myelopathy and adult T-cell leukemia cases in a family. Eur Neurol. 1989;29:33–35. doi: 10.1159/000116373. [DOI] [PubMed] [Google Scholar]
- 20.Sonoda S, Yashiki S, Fujiyoshi T, Arima N, Tanaka H, Eiraku N, Izumo S, Osame M. Immunogenetic factors involved in the pathogenesis of adult T-cell leukemia and HTLV-I-associated myelopathy. Gann Monogr Cancer Res. 1992;39:81–93. [Google Scholar]
- 21.Usuku K, Nishisawa M, Matsuki K, Tokunaga K, Takahashi K, Eiraku N, Suehara M, Juji T, Osame M, Tabira T. Association of a particular amino acid sequence of the HLA-Dr beta-1 chain with HTLV-I-associated myelopathy. Eur J Immunol. 1990;20:1603–1606. doi: 10.1002/eji.1830200729. [DOI] [PubMed] [Google Scholar]
- 22.Usuku K, Sonoda S, Osame M, Yashiki S, Takahashi K, Matsumoto M, Sawada T, Tsuji K, Tara M, Igata A. HLA haplotype-linked high immune responsiveness against HTLV-I in HTLV-I-associated myelopathy: comparison with adult T-cell leukemia/lymphoma. Ann Neurol. 1988;23:S143–150. doi: 10.1002/ana.410230733. [DOI] [PubMed] [Google Scholar]
- 23.Yonaha-Nagato F, Sumida T. Expression of sequences homologous to HTLV-I pXIV gene in the labial salivary glands of Japanese patients with Sjögren’s syndrome and pathogenesis. Nippon Rinsho. 1995;53:2473–2478. [PubMed] [Google Scholar]