Abstract
Bacterial cells regularly confront simultaneous changes in environmental nutrient supply and osmolarity. Despite the importance of osmolarity and osmoregulation in bacterial physiology, the relationship between the cellular response to osmotic perturbations and other stresses has remained largely unexplored. Bacteria cultured in hyperosmotic conditions and bacteria experiencing nutrient stress exhibit similar physiological changes including metabolic shutdown, increased protein instability, dehydration, and condensation of chromosomal DNA. In this review, we highlight overlapping molecular players between osmotic and nutrient stresses. These connections between two seemingly disparate stress response pathways reinforce the importance of central carbon metabolism as a control point for diverse aspects of homeostatic regulation. We identify important open questions for future research, emphasizing the pressing need to develop and exploit new methods for probing how osmolarity affects phylogenetically diverse species.
Keywords: RpoS, stationary phase, crowding, intracellular density, periplasm, starvation, cytoplasmic shrinkage
Introduction
Osmolarity, the number of dissolved solute particles per liter of solution, is an important yet often ignored aspect of cellular physiology (Glossary) [1]. Single-celled bacteria typically cannot control their environment, and hence osmolarity broadly influences phenotypes from cell size [2] to antibiotic sensitivity [3]. Osmolarity dictates the water content of cells, impacting the activity of essential enzymes and modulating metabolic function [4,5]. The osmolarity differential between the inside and outside of the cell (turgor pressure, Glossary), which can be >10 atmospheres in some species [6,7], determines the mechanical force balance between the cell envelope and the environment [8], and maintaining positive turgor can be important for growth.
Under typical growth conditions, proteins, sugars, lipids, nucleic acids, and other small molecules within the cytoplasm collectively constitute a dense, highly crowded environment with concentrations as high as 400 mg/mL [9–11]. A substantial increase or decrease in density impacts molecular diffusion (Glossary) and interactions through the level of crowding [12,13]. Osmolarity is intrinsically related (although not identical) to intracellular density. Since osmolarity is the number of solute particles per liter, reducing intracellular osmolarity can be as simple as polymerizing hundreds of amino acids into a single protein or thousands of nucleotides into a molecule of DNA or RNA, as long as the subunits constitute a sizeable fraction of cellular mass. Thus, nutrient availability and metabolic rate have the potential to alter cytoplasmic osmolarity, and osmotic regulation may be required to maintain cellular homeostasis. Thus, the physical properties of the cytoplasm are intertwined with growth and directly impact intracellular organization and biochemistry.
The osmolarity of the bacterial cytoplasm is stringently regulated by molecular mechanisms that mediate accumulation or secretion of osmolytes. The importance of osmotic regulation is further underscored by its metabolic cost, involving the synthesis and breakdown of carbon-rich molecules. The osmoprotectants trehalose (a disaccharide of two glucose molecules), glycine betaine (amino acid derivative), and proline betaine (amino acid derivative) are manufactured or imported under hyperosmotic stress [1]. Thus, induction of osmoregulatory pathways requires repurposing nutrients from metabolic pathways that would otherwise provide cellular energy and synthesis capacity.
Just as many osmolytes are repurposed nutrients, nutrients can function as osmolytes, illustrating an intrinsic connection between osmolarity and nutrient availability such that a low-osmolarity environment may generically contain fewer molecules with nutritional potential, and vice versa. This coupling is particularly poignant for bacteria that face sudden shifts into drastically more dilute environments. Human gut commensals and pathogens face both osmotic shifts and feast-famine cycles as they transition between their host and the environment [14] and hence it would be natural to couple osmotic regulation to other aspects of physiology. Indeed, Leptospira interrogans utilizes the inevitable change in osmolarity when entering host tissues as a signal to upregulate virulence factors [15].
In this review, we discuss the impact of osmolarity on bacterial physiology and growth, emphasizing physiological and regulatory overlaps between the response to osmotic stress and to nutrient stress. Hyperosmotic or hypoosmotic stress result from a transition to an environment with higher or lower osmolarity, respectively. Although a low-osmolarity environment due to general dilution signifies a dearth of nutritional potential, under hypoosmotic conditions bacteria nevertheless use glucose to synthesize osmoregulated periplasmic glucans (OPGs, Glossary) [16]. This tension between two coincident stresses suggests that devoting precious resources to producing an osmoprotectant outweighs the benefits of using it for biosynthesis. Despite the intriguing nature of the OPGs and hypoosomotic stress as a whole, links with molecular crowding have made hyperosomotic stress the better studied of the two phenomena, and hence it will be the focus of this review.
Nutrient stress and hyperosmotic stress elicit similar changes in cytoplasmic density
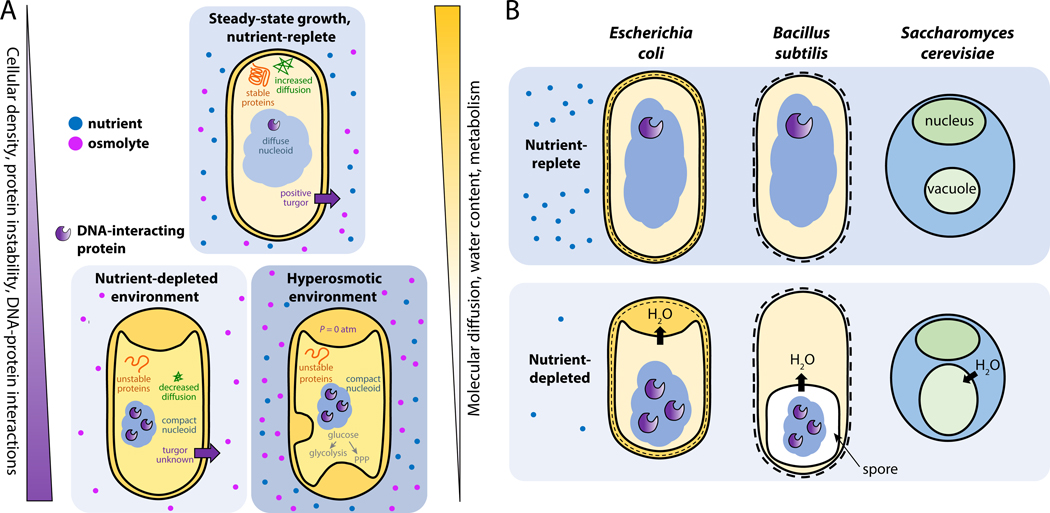
Plasmolysis—cytoplasmic dehydration and shrinkage coupled with enlargement of regions of the periplasm (Glossary)—is a visibly striking and well-known hallmark of the initial stages of hyperosmotic stress (Figure 1). Sudden or gradual nutrient depletion in Gram-negative bacteria similarly leads to plasmolysis, although the regions of periplasmic enlargement can differ from hyperosmotic shock (Figure 1A). During stationary phase (Glossary), a state of metabolic quiescence and cell shortening upon gradual nutrient depletion, the cytoplasm decreases in volume and becomes denser [11] and the periplasm increases in volume [17]. Likewise, during sudden depletion of carbon, nitrogen, or phosphorus, the inner membrane of E. coli [11], K. pneumoniae [11], and V. cholerae [18] retracts, resulting in a smaller, denser cytoplasm and enlarged periplasm. Budding yeast cells undergo a similar process under glucose starvation in which cell volume shrinks without any loss of cell mass, in this case due to vacuolar expansion [19] rather than the periplasmic expansion that occurs in bacteria [11]. The increased periplasmic volume at the expense of cytoplasmic volume during hyperosmotic shock and nutrient stress highlights the interplay between these compartments and raises the interesting possibility that one function of the bacterial periplasm may be to act as a vacuole-like organelle.
Figure 1: Nutrient and hyperosmotic stress have similar physiological effects on microbes.
A) In the model Gram-negative bacterium Escherichia coli, similar features distinguish cells under steady-state growth conditions from those under nutrient stress or hyperosmotic stress: protein instability, intracellular density, DNA-protein interactions, water content, and metabolic rate.
B) Escherichia coli (a model Gram-negative bacterium), Bacillus subtilis (a model Gram-positive bacterium), and Saccharomyces cerevisiae (a model unicellular eukaryote) all experience dehydration, cytoplasmic volume reduction, increased protein instability, and metabolic slowdown upon nutrient depletion. The crowded cytoplasm decreases molecular diffusion, and in bacteria this crowding increases the levels of DNA-protein interactions and nucleoid condensation.
Gram-positive bacteria lack a periplasm but still exhibit similar cytoplasmic changes as Gram-negative bacteria during starvation (Figure 1B). The model Firmicute Bacillus subtilis and other related species induce a programmed stress response pathway that culminates in the formation of a dormant, heat-resistant endospore [20]. Spore formation (Glossary) is characterized by nucleoid condensation [21] and dehydration, which contributes to heat tolerance [22]. The frequency of protein-DNA interactions also increases in spores, which provides protection against DNA damage [23]. Thus, a condensed, dehydrated state may generally increase resilience across the bacterial kingdom.
Notably, treatment of E. coli cells with DNP, a drug that uncouples the electron transport chain from ATP synthesis and leads first to an increase in metabolic activity and then to metabolic arrest, results in an expanded nucleoid rather than an expanded periplasm [24], highlighting the specificity of physiological responses to starvation. It remains unclear whether cytoplasmic volume fraction is intrinsically linked to growth rate and/or nutrient quality [17]; in E. coli, nutrient concentration during exponential growth non-monotonically impacts cytoplasmic volume fraction in stationary phase [11]. Altogether, comparison of cytoplasmic condensation between hyperosmotic shock and various modes of metabolic slowdown suggests that reduction in water content may be broadly beneficial but dependent on many aspects of growth history.
Physical mechanisms of enzymatic activity regulation during hyperosmotic and nutrient stress
The reduction in cytoplasmic volume elicited by nutrient stress and osmotic stress affects molecular crowding (Figure 1), a critical component of cellular physiology. Crowding inhibits many enzymatic reactions by physically slowing their kinetics, as diffusion coefficients decrease and reactant molecules encounter each other less frequently [4], or by inactivating enzymes through steric hindrance resulting from polymerization [25]. The increase in crowding during stationary phase in E. coli suggests that cells may face similar physiological challenges as under hyperosmotic stress [11,13], when density also increases, although the contribution of increased cytoplasmic crowding to stationary phase-dependent reductions in metabolic activity is unknown. In the budding yeast Saccharomyces cerevisiae (Glossary), the rates of cytoplasmic and nuclear diffusion decrease during glucose starvation, which has been suggested to be due to crowding [19], and mechanical stress-induced crowding inhibits translation and curtails cell growth [26]. Importantly, higher intracellular density is not necessarily associated with slow growth [27], and metabolic slowdown can inhibit molecular diffusion in the absence of obvious changes in cell volume or density [24]. Thus, the relationships among crowding, density, and growth may be more multi-faceted than previously appreciated.
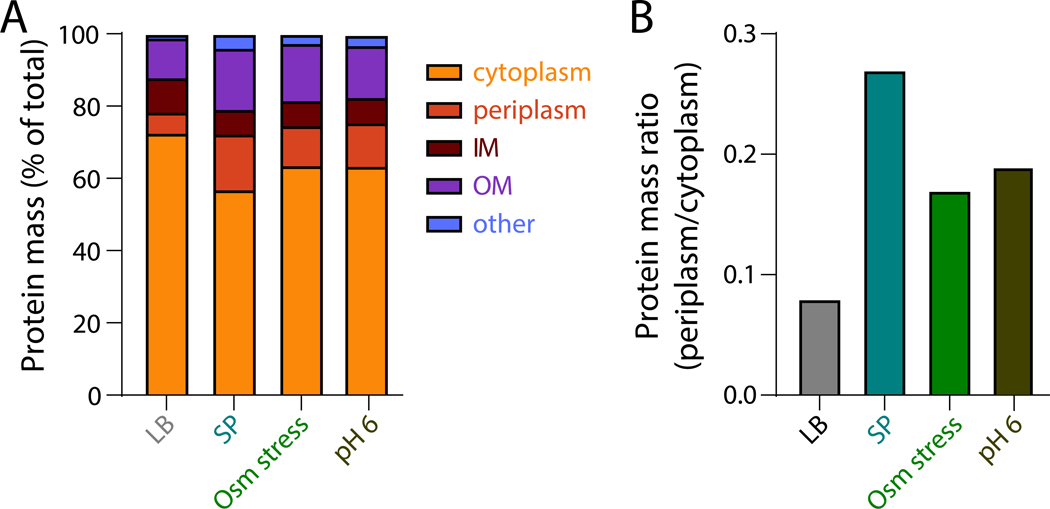
In addition to the physical effects of crowding, nutrient and hyperosmotic stress lead to changes in protein synthesis. During nutrient stress, bacteria downregulate protein synthesis through the stringent response and synthesis of the alarmone (p)ppGpp (Glossary) [28,29]. Hyperosmotic stress specifically inhibits translation elongation, leading to compensatory transcriptional upregulation of ribosomes in an attempt to maintain translational capacity [30]. Similarly, in budding yeast, translation and metabolic activity are initially inhibited during salt stress, which enables adaptation that avoids long-term defects in cell growth [26,31]. In addition to the shift in water content between the cytoplasm and periplasm that alters cytoplasmic volume fraction, nutrient and hyperosmotic stress also cause major redistribution of the proteome. Bacterial cells experiencing nutrient stress and cells experiencing hyperosmotic stress both have more biomass in the periplasm and outer membrane and a concomitant decrease in biomass in the cytoplasm and inner membrane compared to steady-state growth (Glossary) (Figure 2) [17]. Interestingly, similar changes are observed during growth at pH 6 as well (Figure 2), suggesting the potential for further overlap with the acid stress response pathway.
Figure 2: Proteome redistribution among cellular compartments and membranes upon stress.
A) During exponential growth in LB, the cytoplasm of E. coli cells contains 73% of the total protein mass, while the periplasm contains only 6%. During stationary phase (SP) or upon osmotic (Osm) stress in minimal medium supplemented with 50 mM NaCl, the cytoplasm accounts for only 57% and 64% of the proteome, respectively, and the expanded periplasm (Figure 1) accounts for 15% and 11% of the proteome, respectively.
B) The ratio of protein fraction between the periplasm and cytoplasm is greater during stationary phase, hyperosmotic stress, and pH stress than during exponential growth in LB.
Data plotted are from [17].
While an increase in cytoplasmic density is expected after a hyperosmotic shock due to the physical nature of the perturbation, why the cytoplasm shrinks during nutrient stress is unclear. It may be an active and beneficial response or an inevitable consequence of the physiological changes inherent in starvation. Regardless, the metabolic shutdown inherent in a dense, dehydrated state conserves energy [13,21] and boosts resistance to antibiotics [3], heat, and desiccation [10]. Nucleoid compaction driven by water efflux [21,29,32] also protects DNA from damage under stress [32–34]. Moreover, the reduction in size may itself be beneficial due to the increase in surface area-to-volume ratio, which decreases the distance molecules have to traverse from uptake to synthesis, utilization, and expulsion as waste [21]. Elucidating the genetic and/or physical mechanisms underlying these benefits will likely provide mechanistic insight into the links between nutrient and osmotic stress responses.
The role of ion trafficking in stress adaptation
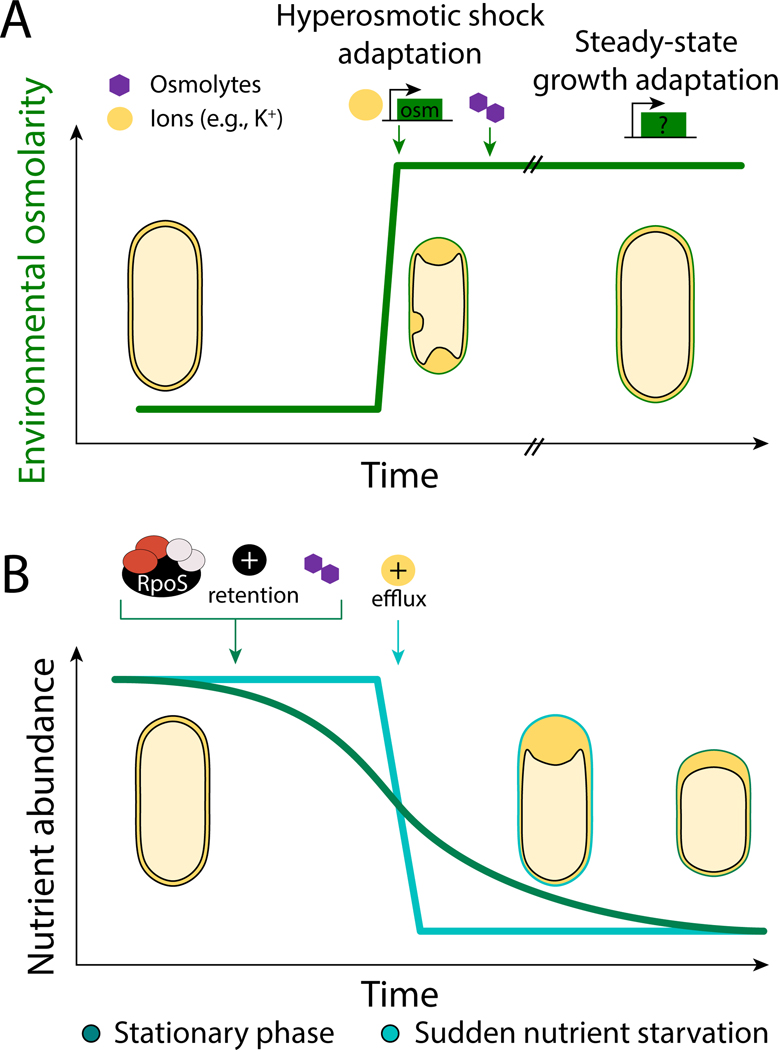
While the buildup of osmotic stress typically occurs on a short time scale (e.g., the transition of enteric bacteria from a host into a water supply), nutrient stress can increase quickly (e.g. a sudden shift to a rich or poor carbon source) or slowly (nutrient exhaustion and entry into stationary phase) (Figure 3). Adaptation to hyperosmotic stress is a two-step process initially involving quick and promiscuous import of any available ions and osmolytes to balance osmolarity and prevent loss of viability. This emergency response results in an increased concentration of ions including K+, which disrupts DNA-protein interactions and protein folding [1]. Ion trafficking is thus not a permanent solution, and as cells adapt to the new conditions, ions are effluxed as synthesis of compatible solutes such as glycine-betaine and trehalose restores water content [1]. Adaptation typically requires synthesis of proteins to generate and/or import osmolytes, costing energy and time.
Figure 3: The timelines of hyperosmotic and nutrient stress.
A) Hyperosmotic shock involves rapid onset of stress. Water efflux causes immediate plasmolysis, and cells initially respond with a rapid influx of ions, and later with synthesis or import of compatible solutes. The genes responsible for re-establishing osmotic homeostasis at steady state are not well understood.
B) Nutrient depletion can be sudden or gradual. In stationary phase, cells accumulate positively charged ions and trehalose in the cytoplasm and induce transcriptional changes. By contrast, cells that are starved by sudden removal of nutrients accumulate positively charged ions outside of the cytoplasm and the response does not rely on transcriptional regulation.
Nutrient stress also involves changes in ion flux (Figure 3B). Ions, particularly Na+ and H+, are essential for the transport of nutrients into and waste out of the cell as part of symporter and antiporter systems. In stationary phase, bacterial cells favor retention of K+, NH4+, and H+ [35], and accumulate the compatible solute trehalose [34]. In E. coli, the membrane depolarizes as cells transition from early to late exponential phase [36], but membrane potential is maintained during sudden starvation [11], suggesting differential regulation of ion flux. Cells suddenly starved of nutrients [11] maintain ATP levels for ~30 minutes, indicating that they possess the energy to adapt to dormancy and to synthesize proteins for the synthesis or transport of osmolytes. The importance of ion flux during both nutrient and hyperosmotic stress provides further evidence of the interconnectedness between these two stresses and the tension that arises as a result.
Overlap in transcriptional regulation between osmotic and nutrient stress responses
Several lines of evidence indicate common transcriptional regulation of the osmotic and nutrient stress responses in bacteria. In E. coli, the stationary-phase transcription factor RpoS (σs) induces hundreds of genes upon nutrient stress, including several osmoprotectant pathways [37]. Entry into stationary phase confers cross-protection against osmotic stress [38,39], which may be due to induction of one or more of these pathways but also could reflect the common physiological responses to these two stresses (Figure 1). Many genes under the control of RpoS are induced by hyperosmotic stress (Figure 3A), demonstrating an intrinsic link between osmoregulation and starvation. These genes include otsBA [40] and treA [41], which encode proteins that synthesize and breakdown trehalose, respectively; proP [40], which encodes a transporter for glycine betaine and other osmolytes; osmY [41], which encodes a periplasmic chaperone; and osmE [42–44] and osmB [41], which encode predicted lipoproteins. In addition to increasing cellular osmolarity, trehalose and glycine betaine also stabilize proteins [34,45], of notable benefit due to the increase in protein destabilization during both hyperosmotic stress and stationary phase. Interestingly, osmoregulated genes are highly induced by a small amount of RpoS, by contrast to the expression of canonical stationary phase genes that tend to require higher levels of the transcription factor [46].
The Rcs envelope stress response (Glossary) is also directly coupled to both nutrient and osmotic stress response pathways. The Rcs pathway is activated in response to changes in cell width and periplasmic dimensions [47], which are strongly affected by osmolarity and by nutrient stress (Figure 3) [48,49]. Rcs pathway activation also leads to expression of osmB and osmC [50], which are regulated by both osmotic stress and stationary phase [51] and encode enzymes that reduce organic hydroperoxides. Taken together, the co-regulation of stationary phase and osmotic genes may reflect a frequent need to adapt to both osmotic and nutrient stresses concurrently.
The Gram-negative cell envelope consists of an inner membrane, a thin layer of peptidoglycan cell wall, and an asymmetric outer membrane. The outer membrane plays a key role in mediating resistance to desiccation and antibiotics [14], and its capacity to bear substantial mechanical stress due to both its protein and lipopolysaccharide (Glossary) content was recently discovered [52]. Interestingly, the outer membrane of E. coli does not appear to bear mechanical stress during steady-state growth, but becomes critical during osmotic shock [53]. Production of the outer membrane lipoproteins OsmB and OsmE during osmotic stress may play a role in maintaining cell envelope integrity, although the mechanism is unknown. More generally, how transcriptional responses counteract the physical effects of osmotic and nutrient stress remains to be determined.
Prospects for future interrogation of the coupling between osmotic and nutrient stress responses
Progress in understanding the osmotic and nutrient properties of the cytoplasmic environment and their regulation has thus far largely relied on inferences. It is typically assumed that, like pH, there is an optimal cytoplasmic osmolarity. On the other hand, the turgor pressure of Gram-positive bacteria such as B. subtilis has been inferred to be an order of magnitude larger than that of E. coli [54,55], indicating a huge range of potential cytoplasmic osmolarity. Without the ability to measure cytoplasmic osmolarity, questions about its magnitude and that of turgor pressure will be difficult to address. Moreover, the functions of periplasmic contents remain largely mysterious, particularly during stress and across growth phases. Notably, for most organisms, no estimates of turgor or intracellular density have been made. Recent methodological and computational advances in quantitative phase imaging (QPI) have simplified the measurement of intracellular density [56,57], which should help to clarify the relationship between osmolarity and intracellular contents. Application of QPI and direct measurements of turgor through nanoscale probing [58] or other methods would expand our understanding of the relationship between osmolarity and physiology across growth conditions. Advances in metabolomics may also provide a window into osmolyte concentrations during perturbations (osmotic and otherwise); phenotypes of interest should generally be compared across media, which may have different osmolarities and/or contain different amounts of compatible solutes (trehalose, betaines, proline).
Beyond tools, we lack general principles regarding how osmolarity affects physiology, motivating a broad comparison across organisms to uncover both general and specific behaviors. The field has mainly focused on one organism (E. coli), resulting in narrow understanding of a challenge faced universally across the bacterial family tree.
Moreover, the lack of insight into how osmolarity homeostasis during steady-state growth is re-established long after adaptation to an initial shock means that well-studied genes may have unrecognized osmoregulatory roles in the absence of osmotic stress. It will also be fascinating to understand the intersection of osmotic and nutrient stress in microbes that inhabit environments highly distinct from the mammalian gut, from marine cyanobacteria to halophiles, to determine whether an organism’s behaviors are dictated by the osmotic variation in its natural environment. Application of oscillatory osmotic shocks has revealed qualitatively distinct phenomena linking osmolarity to growth behaviors in every organism studied thus far [2,27,59,60], indicating that there is likely much to be learned simply by looking. Addressing the crucial knowledge gaps discussed in this review would greatly enhance our understanding how nutrient and osmotic stresses are mechanistically coupled, and of cellular physiology more broadly.
Acknowledgements
The authors thank the Huang and Levin labs for helpful discussions. Funding was provided by a James McDonnell Postdoctoral Fellowship (to H.S.), NSF grant EF-2125383 (to K.C.H.), and NIH grants RM1-GM135102 (to K.C.H.) and R35-GM127331 (to. P.A.L.). K.C.H. is a Chan Zuckerberg Investigator.
Glossary:
- Physiology
processes important for bacterial viability such as metabolic and stress response pathways
- Turgor
the pressure exerted on the cell envelope as a result of higher intracellular osmolarity compared with the environment
- Osmoregulated periplasmic glucans (OPGs), formerly known as membrane derived oligosaccharides (MDOs)
polymers of D-glucose produced by Proteobacteria in hypoosmotic conditions with predicted roles in envelope and osmotic homeostasis
- Diffusion
the random movement of particles that serves to smooth variations in concentration
- Steady-state growth
a state with constant rate of cell expansion without shifts in the environment
- Stationary phase
a state of metabolic quiescence entered as cell density increases and nutrient levels decrease, characterized by cell shortening and regulation by the sigma factor RpoS
- Guanosine penta-phosphate and guanosine tetraphosphate ((p)ppGpp)
an alarmone that induces the stringent response under carbon or amino acid starvation
- Sporulation
a developmental strategy for adapting to unfavorable conditions through spore formation
- Budding yeast (Saccharomyces cerevisiae)
a unicellular fungus used as a model eukaryote
- Periplasm
The space between the inner and outer membrane of Gram-negative bacteria, which is less protected from the extracellular environment than the cytoplasm and contains a distinct enzymatic repertoire
- Rcs pathway
induces the expression of genes involved in processes such as capsule synthesis in response to envelope damage
- Lipopolysaccharide (LPS)
an anionic component of the outer leaflet of the outer membrane of Gram-negative bacteria, important for envelope stiffness and resistance to expansion by turgor
Footnotes
The authors declare no conflicts of interest.
Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wood JM: Bacterial osmoregulation: a paradigm for the study of cellular homeostasis. Annu Rev Microbiol 2011, 65:215–238. [DOI] [PubMed] [Google Scholar]
- 2.Rojas E, Theriot JA, Huang KC: Response of Escherichia coli growth rate to osmotic shock. Proc Natl Acad Sci U S A 2014, 111:7807–7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenwood D, O’Grady F: The effect of osmolality on the response of Escherichia coli and Proteus mirabilis to penicillins. Br J Exp Pathol 1972, 53:457–464. [PMC free article] [PubMed] [Google Scholar]
- 4.Minton AP: The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. Journal of biological chemistry 2001, 276:10577–10580. [DOI] [PubMed] [Google Scholar]
- 5.Rand RP, Parsegian VA, Rau DC: Intracellular osmotic action. Cell Mol Life Sci 2000, 57:1018–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch AL, Doyle RJ: The growth strategy of the Gram-positive rod. FEMS Microbiology Reviews 1986, 1:247–254. [Google Scholar]
- 7.Whatmore AM, Reed RH: Determination of turgor pressure in Bacillus subtilis: a possible role for K+ in turgor regulation. J Gen Microbiol 1990, 136:2521–2526. [DOI] [PubMed] [Google Scholar]
- 8.Rojas ER, Huang KC: Regulation of microbial growth by turgor pressure. Curr Opin Microbiol 2018, 42:62–70. [DOI] [PubMed] [Google Scholar]
- 9.Akabayov B, Akabayov SR, Lee SJ, Wagner G, Richardson CC: Impact of macromolecular crowding on DNA replication. Nat Commun 2013, 4:1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmerman SB, Trach SO: Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J Mol Biol 1991, 222:599–620. [DOI] [PubMed] [Google Scholar]
- 11. Shi H, Westfall CS, Kao J, Odermatt PD, Anderson SE, Cesar S, Sievert M, Moore J, Gonzalez CG, Zhang L, et al. : Starvation induces shrinkage of the bacterial cytoplasm. Proc Natl Acad Sci U S A 2021, 118. ** The authors characterize a phenotype of cytoplasmic shrinkage and periplasmic enlargement during complete carbon starvation, which has striking similarities to the hyperosmotic stress response. This study provides evidence for coupling among cytoplasmic size, water content, and envelope homeostasis during nutrient stress responses.
- 12. Delarue M, Brittingham GP, Pfeffer S, Surovtsev IV, Pinglay S, Kennedy KJ, Schaffer M, Gutierrez JI, Sang D, Poterewicz G, et al. : mTORC1 Controls Phase Separation and the Biophysical Properties of the Cytoplasm by Tuning Crowding. Cell 2018, 174:338–349 e320. ** The authors found that mTORC1 regulates molecular crowding and diffusion via ribosome concentration in budding yeast cells, linking nutrient availability to crowding.
- 13.Mourao MA, Hakim JB, Schnell S: Connecting the dots: the effects of macromolecular crowding on cell physiology. Biophys J 2014, 107:2761–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeidler S, Muller V: Coping with low water activities and osmotic stress in Acinetobacter baumannii: significance, current status and perspectives. Environ Microbiol 2019, 21:2212–2230. * This review discusses the survival strategies of bacteria including the pathogen Acinetobacter baumanii as they transition through environments that are osmotically challenging. It highlights the importance of water activity, which critically affects cellular physiology.
- 15.Matsunaga J, Lo M, Bulach DM, Zuerner RL, Adler B, Haake DA: Response of Leptospira interrogans to physiologic osmolarity: relevance in signaling the environment-to-host transition. Infect Immun 2007, 75:2864–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohin J-P: Osmoregulated periplasmic glucans in Proteobacteria. FEMS Microbiology Letters 2000, 186:11–19. [DOI] [PubMed] [Google Scholar]
- 17. Schmidt A, Kochanowski K, Vedelaar S, Ahrné E, Volkmer B, Callipo L, Knoops K, Bauer M, Aebersold R, Heinemann M: The quantitative and condition-dependent Escherichia coli proteome. Nature biotechnology 2016, 34:104–110. ** This study used quantitative mass spectrometry to generate proteomic data for E. coli cultured in nearly two dozen conditions, providing insight into adaptation in various nutritional, osmotic, and pH environments, particularly the distribution of proteins among each cellular compartment and membrane.
- 18.Brenzinger S, van der Aart LT, van Wezel GP, Lacroix JM, Glatter T, Briegel A: Structural and Proteomic Changes in Viable but Non-culturable Vibrio cholerae. Front Microbiol 2019, 10:793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joyner RP, Tang JH, Helenius J, Dultz E, Brune C, Holt LJ, Huet S, Muller DJ, Weis K: A glucose-starvation response regulates the diffusion of macromolecules. Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stragier P, Losick R: Molecular genetics of sporulation in Bacillus subtilis. Annual review of genetics 1996, 30:297–341. [DOI] [PubMed] [Google Scholar]
- 21.Koch AL: What size should a bacterium be? A question of scale. Annu Rev Microbiol 1996, 50:317–348. [DOI] [PubMed] [Google Scholar]
- 22.Piggot PJ, Hilbert DW: Sporulation of Bacillus subtilis. Curr Opin Microbiol 2004, 7:579–586. [DOI] [PubMed] [Google Scholar]
- 23.Setlow P: I will survive: DNA protection in bacterial spores. Trends in microbiology 2007, 15:172–180. [DOI] [PubMed] [Google Scholar]
- 24.Parry BR, Surovtsev IV, Cabeen MT, O’Hern CS, Dufresne ER, Jacobs-Wagner C: The bacterial cytoplasm has glass-like properties and is fluidized by metabolic activity. Cell 2014, 156:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barry RM, Bitbol AF, Lorestani A, Charles EJ, Habrian CH, Hansen JM, Li HJ, Baldwin EP, Wingreen NS, Kollman JM, et al. : Large-scale filament formation inhibits the activity of CTP synthetase. Elife 2014, 3:e03638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alric B, Formosa-Dague C, Dague E, Holt LJ, Delarue M: Macromolecular crowding limits growth under pressure. Nature Physics 2022, 18:411–416. ** The authors uses physical confinement, osmotic stress, and nanoparticle expression to demonstrate that molecular crowding and the accompanying reduction in diffusion reduces translational rate and growth rate in budding yeast. Since the physical principles of crowding are conserved, these findings likely apply to bacteria experiencing hyperosmotic or nutrient stress, which both increase cell density.
- 27. Knapp BD, Odermatt P, Rojas ER, Cheng W, He X, Huang KC, Chang F: Decoupling of Rates of Protein Synthesis from Cell Expansion Leads to Supergrowth. Cell Syst 2019, 9:434–445 e436. * The authors demonstrate in fission yeast that oscillatory osmotic shocks decouple synthesis of cytoplasmic molecules from cell expansion, leading to large increases in intracellular density. Halting the shocks triggered extremely fast growth, demonstrating that crowding need not inhibit synthesis or growth.
- 28.Diez S, Ryu J, Caban K, Gonzalez RL Jr., Dworkin J: The alarmones (p)ppGpp directly regulate translation initiation during entry into quiescence. Proc Natl Acad Sci U S A 2020, 117:15565–15572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navarro Llorens JM, Tormo A, Martínez-García E: Stationary phase in gram-negative bacteria. FEMS microbiology reviews 2010, 34:476–495. [DOI] [PubMed] [Google Scholar]
- 30. Dai X, Zhu M, Warren M, Balakrishnan R, Okano H, Williamson JR, Fredrick K, Hwa T: Slowdown of Translational Elongation in Escherichia coli under Hyperosmotic Stress. mBio 2018, 9. * This study shows that translational elongation slows dramatically upon hypersomotic stress in E. coli, even more than in nutrient-stressed cells, which the authors propose is the result of molecular crowding.
- 31.Pircher A, Bakowska-Zywicka K, Schneider L, Zywicki M, Polacek N: An mRNA-derived noncoding RNA targets and regulates the ribosome. Mol Cell 2014, 54:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Vries R: DNA condensation in bacteria: Interplay between macromolecular crowding and nucleoid proteins. Biochimie 2010, 92:1715–1721. * This study beautifully describes the mechanisms by which molecular crowding condenses DNA, including physical compaction and enhanced DNA-protein interactions that provide charge neutralization and induce DNA bending.
- 33.Murphy LD, Zimmerman SB: Condensation and cohesion of lambda DNA in cell extracts and other media: implications for the structure and function of DNA in prokaryotes. Biophys Chem 1995, 57:71–92. [DOI] [PubMed] [Google Scholar]
- 34.Moruno Algara M, Kuczyńska-Wiśnik D, Dębski J, Stojowska-Swędrzyńska K, Sominka H, Bukrejewska M, Laskowska E: Trehalose protects Escherichia coli against carbon stress manifested by protein acetylation and aggregation. Molecular Microbiology 2019, 112:866–880. [DOI] [PubMed] [Google Scholar]
- 35.Shabala L, Ross T, Newman I, McMeekin T, Shabala S: Measurements of net fluxes and extracellular changes of H+, Ca2+, K+, and NH4+ in Escherichia coli using ion-selective microelectrodes. Journal of Microbiological Methods 2001, 46:119–129. [DOI] [PubMed] [Google Scholar]
- 36.Bot CT, Prodan C: Quantifying the membrane potential during E. coli growth stages. Biophysical chemistry 2010, 146:133–137. [DOI] [PubMed] [Google Scholar]
- 37.Peterson CN, Mandel MJ, Silhavy TJ: Escherichia coli starvation diets: essential nutrients weigh in distinctly. J Bacteriol 2005, 187:7549–7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkins DE, Chaisson SA, Matin A: Starvation-induced cross protection against osmotic challenge in Escherichia coli. J Bacteriol 1990, 172:2779–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao NN, Kornberg A: Inorganic polyphosphate supports resistance and survival of stationary-phase Escherichia coli. J Bacteriol 1996, 178:1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kempf B, Bremer E: Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch Microbiol 1998, 170:319–330. [DOI] [PubMed] [Google Scholar]
- 41.Lange R, Barth M, Hengge-Aronis R: Complex transcriptional control of the sigma s-dependent stationary-phase-induced and osmotically regulated osmY (csi-5) gene suggests novel roles for Lrp, cyclic AMP (cAMP) receptor protein-cAMP complex, and integration host factor in the stationary-phase response of Escherichia coli. J Bacteriol 1993, 175:7910–7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bordes P, Repoila F, Kolb A, Gutierrez C: Involvement of differential efficiency of transcription by esigmas and esigma70 RNA polymerase holoenzymes in growth phase regulation of the Escherichia coli osmE promoter. Mol Microbiol 2000, 35:845–853. [DOI] [PubMed] [Google Scholar]
- 43.Conter A, Menchon C, Gutierrez C: Role of DNA supercoiling and rpoS sigma factor in the osmotic and growth phase-dependent induction of the gene osmE of Escherichia coli K12. J Mol Biol 1997, 273:75–83. [DOI] [PubMed] [Google Scholar]
- 44.Gutierrez C, Gordia S, Bonnassie S: Characterization of the osmotically inducible gene osmE of Escherichia coli K-12. Molecular microbiology 1995, 16:553–563. [DOI] [PubMed] [Google Scholar]
- 45.Stadmiller SS, Gorensek-Benitez AH, Guseman AJ, Pielak GJ: Osmotic Shock Induced Protein Destabilization in Living Cells and Its Reversal by Glycine Betaine. J Mol Biol 2017, 429:1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong GT, Bonocora RP, Schep AN, Beeler SM, Fong AJL, Shull LM, Batachari LE, Dillon M, Evans C, Becker CJ, et al. : Genome-Wide Transcriptional Response to Varying RpoS Levels in Escherichia coli K-12. Journal of Bacteriology 2017, 199:e00755–00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zietek M, Miguel A, Khusainov I, Shi H, Asmar AT, Ram S, Wartel M, Sueki A, Schorb M, Goulian M: Bacterial cell widening alters periplasmic size and activates envelope stress responses. bioRxiv 2022. [Google Scholar]
- 48.Pilizota T, Shaevitz JW: Plasmolysis and cell shape depend on solute outer-membrane permeability during hyperosmotic shock in E. coli. Biophys J 2013, 104:2733–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miguel A, Zietek M, Shi H, Sueki A, Maier L, Verheul J, den Blaauwen T, Van Valen DA, Typas A, Huang KC: Modulation of bacterial cell size and growth rate via activation of a cell envelope stress response. bioRxiv 2022. [Google Scholar]
- 50.Majdalani N, Gottesman S: The Rcs phosphorelay: a complex signal transduction system. Annu Rev Microbiol 2005, 59:379–405. [DOI] [PubMed] [Google Scholar]
- 51.Jung JU, Gutierrez C, Martin F, Ardourel M, Villarejo M: Transcription of osmB, a gene encoding an Escherichia coli lipoprotein, is regulated by dual signals. Osmotic stress and stationary phase. Journal of Biological Chemistry 1990, 265:10574–10581. [PubMed] [Google Scholar]
- 52. Sun J, Rutherford ST, Silhavy TJ, Huang KC: Physical properties of the bacterial outer membrane. Nat Rev Microbiol 2022, 20:236–248. ** This review discusses recent findings about the coupling between the structure/composition of the Gram-negative outer membrane and its mechanical properties.
- 53. Rojas ER, Billings G, Odermatt PD, Auer GK, Zhu L, Miguel A, Chang F, Weibel DB, Theriot JA, Huang KC: The outer membrane is an essential load-bearing element in Gram-negative bacteria. Nature 2018, 559:617–621. * The authors discover that the Gram-negative outer membrane is important for determining cell size during hyperosmotic chocks and for survival of oscillatory osmotic shocks, contradicting previous assumptions that the peptidoglycan cell wall is the sole contributor to mechanical integrity. They find that LPS and proteins contribute to outer membrane stiffness, which can be higher than that of the cell wall.
- 54.Cayley DS, Guttman HJ, Record MT Jr.: Biophysical characterization of changes in amounts and activity of Escherichia coli cell and compartment water and turgor pressure in response to osmotic stress. Biophys J 2000, 78:1748–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng Y, Sun M, Shaevitz JW: Direct measurement of cell wall stress stiffening and turgor pressure in live bacterial cells. Phys Rev Lett 2011, 107:158101. [DOI] [PubMed] [Google Scholar]
- 56. Oldewurtel ER, Kitahara Y, van Teeffelen S: Robust surface-to-mass coupling and turgor-dependent cell width determine bacterial dry-mass density. Proc Natl Acad Sci U S A 2021, 118. ** This study uses quantitative phase imaging to show that surface area-to-mass ratio is conserved in E. coli across a wide range of perturbations that affect cell shape.
- 57. Odermatt PD, Miettinen TP, Lemiere J, Kang JH, Bostan E, Manalis SR, Huang KC, Chang F: Variations of intracellular density during the cell cycle arise from tip-growth regulation in fission yeast. Elife 2021, 10. ** This study uses quantitative phase imaging to show that variations in fission yeast dry-mass density across the cell cycle reflect exponential biomass synthesis and non-exponential cell volume expansion. Differences in intracellular density across daughter cells were correlated with septal bending, supporting links between density and turgor.
- 58.Chen P, Xu L, Liu J, Hol FJ, Keymer JE, Taddei F, Han D, Lindner AB: Nanoscale probing the kinetics of oriented bacterial cell growth using atomic force microscopy. Small 2014, 10:3018–3025. [DOI] [PubMed] [Google Scholar]
- 59.Rojas ER, Huang KC, Theriot JA: Homeostatic Cell Growth Is Accomplished Mechanically through Membrane Tension Inhibition of Cell-Wall Synthesis. Cell Syst 2017, 5:578–590 e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou X, Halladin DK, Rojas ER, Koslover EF, Lee TK, Huang KC, Theriot JA: Bacterial division. Mechanical crack propagation drives millisecond daughter cell separation in Staphylococcus aureus. Science 2015, 348:574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]