Abstract
Metabolic differences between colorectal cancer (CRC) and NI (NI) play an important role in early diagnoses and in-time treatments. We investigated the metabolic alterations between CRC patients and NI, and identified some potential biomarkers, and these biomarkers might be used as indicators for diagnosis of CRC. In this study, there were 79 NI, 50 CRC I patients, 52 CRC II patients, 56 CRC III patients, and 52 CRC IV patients. MS-MS was used to measure the metabolic alterations. Univariate and multivariate data analysis and metabolic pathway analysis were applied to analyze metabolic data and determine differential metabolites. These indicators revealed that amino acid and fatty acids could separate these groups. Several metabolites indicated an excellent variables capability in the separation of CRC patients and NI. Ornithine, arginine, octadecanoyl carnitine, palmitoyl carnitine, adipoyl carnitine, and butyryl carnitine/propanoyl carnitine were selected to distinguish the CRC patients and NI. And methionine and propanoyl carnitine, were directly linked to different stages of CRC. Receiver operating characteristics curves and variables importance in projection both represented an excellent performance of these metabolites. In conclusion, we assessed the difference between CRC patients and NI, which supports guidelines for an early diagnosis and effective treatment.
Keywords: metabolomics, colorectal cancer, biomarkers, MS/MS, statistical analysis
Introduction
Colorectal cancer (CRC), one of the most common cancers universally,(1) is closely related to mortality. Obesity and insulin resistance are two contributing factors for CRC.(2,3) Males are more vulnerable to death when they suffer from CRC than females, while females are more likely to develop advanced-stage CRC.(4) There has been an increasing number of CRC patients in China compared to the USA in the latest years, according to the paper published in 2022.(5) More than 10.0% of people died among patients diagnosed with colorectal cancer.(6) Obesity, alcohol consumption, diet, and other irregular lifestyles are potential risk factors for CRC.(7,8) Plant-based diets, coffee intake, and increasingly physical activities can prevent us from suffering CRC.(9) Abdominal pain and blood in the stool are the most common symptoms of CRC.(10) Several dietary fatty acids, amino acid, and nucleic acid metabolism are regarded as potential risk factors leading to CRC.(3) Most CRC have the risk of evolving from low-risk (LR) and high-risk (HR) adenomas to invasive cancer.(11) However, most early-staged CRC patients are asymptomatic and not easy to detect.(12) The accurate diagnosis of different stages of CRC will help to survive CRC, which offers opportunities for early detection and effective treatment.
The serodiagnosis of early-stage colorectal cancer has been extensively studied and some endogenous substances are reported as potential biomarkers for detecting CRC,(13) like miRNAs, amino acids, ctDNA,(14) and fatty acids.(15) Zhang et al.(13) found miR-451a (miRNAs) was dysregulated in early CRCs and could be used as a potential biomarker for the early clinical diagnosis. Peptides play increasingly important role in CRC diagnosis and CRC therapy.(16) Seyed Mostafa Parizadeh found peptide could be applied as vaccination in the treatment of CRC by inducing tumor-specific immune responses.(17) Bold et al.(18) found gut peptide could treat CRC patients by regulating the growth of gastrointestinal malignancies. Clinically, carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9), and cancer antigen 72-4 (CA 72-4) are classical tumor markers for CRCs. Overexpression of CEA or CA 19-9 both indicated higher tumor recurrence in patients, which was applied to diagnose and provide guidelines for CRC patients.(19) Kasanga et al.(20) reported the combination of CEA, CA 19-9, and Hsp90α together could help to distinguish early-staged CRC. They found the levels of Hsp90α were significantly higher in advanced CRCs (stage III and IV) compared to CRC I and CRC II because they could promote cell proliferation and cell apoptosis.(21) However, the detailed regulatory mechanism of these biomarkers in CRC patients are still opaque and the accuracy and sensitivity of individual biomarkers are compromised sometimes. Therefore, we intend to assess the difference between CRC patients and NI and try to give reference for an early diagnosis and effective treatment.
Current screening methods include one or more than one method combined: flexible sigmoidoscopy, fecal occult blood testing (FOBT), computed tomography colonography, air-contrast barium enema, nuclear magnetic resonance (NMR) based urine metabolomics, etc.(22,23) However, these methods fail to detect CRC in time, and they are unacceptable as golden rules by the medical profession, which prolongs or aggravates the recovery time. Interestingly, Monica Ghebrial found that patients could be diagnosed partly by gender. Males are more vulnerable to CRC when exposed to social stress and caffeine intake, while females are more likely diagnosed when they are suffering from household physical activity and hieratical problems.(2)
According to previous studies, the occurrence and development of CRC are closely related to the metabolic profiles of fecal fatty acids, purines, etc.(24) And there are also some related studies demonstrating that these metabolites can be used to diagnose colorectal cancer.(25)
Nucleic acid is also applied to process genetic information and regulate cellular processes,(26) which may help to recover from colorectal cancer.(27) It is reported that some fatty acids (PUFA) can reduce the risk of CRC occurrence, and free fatty acids are observed significantly lower in CRC than that in adjacent normal tissues.(28) Some amino acid can protect patients from CRC with the help of LC3B.(8) Up to now, there is still no recommended screening test to detect CRC by the International Agency for Research on Cancer.(29) The precise molecular mechanism of colorectal carcinogenesis has not been fully demonstrated yet.(8,30)
Metabolomics is used as a powerful tool to study diseases and discover potential biomarkers.(30,31) These biomarkers can reduce mortality by the detection of asymptomatic early-stage cancer and removal of premalignant precursor.(25) Liesenfeld et al.(30) found metabolic and transcriptomic differences in patients diagnosed with different stages of CRC. They used gas chromatography-tandem time-of-flight mass spectrometry and liquid chromatography-tandem quadrupole time-of-flight mass spectrometry to measure metabolites and they found that visceral adipose tissue (VAT) might be associated with CRC tumor stage. Tandem mass spectrometry is commonly used as multianalyte detection approach for its small sample value and high throughput capabilities,(32) such as newborn screening,(33) pinpointing the sites of attachment of drugs to DN, etc. We collected and analyzed data generated by tandem mass spectrometry based-metabolomics using plasma from the First Affiliated Hospital of Jinzhou Medical University. And we analyzed these data to investigate its metabolic changes and discover potential biomarkers for diagnosis.
Methods
Experiments design
This research was approved by the Ethics Committee for Clinical Research of the First Affiliated Hospital of Jinzhou Medical University and all candidates wrote the informed consent as the nature of the retrospective study asked. This study was consistence with the Helsinki Declaration of 1964 and its later amendments. 289 subjects (including 79 NI, 50 patients with stage I colon cancer, 52 patients with stage II colon cancer, 56 patients with stage III colon cancer, and 52 patients with stage IV colon cancer) were recruited randomly from the First Affiliated Hospital of Jinzhou Medical University from November 2019 to January 2021. Colonoscopy tests for all subjects were performed. The inclusion criteria for NI were: never detected with any other cancers and significant concomitant diseases (chronic heart failure/severe chronic liver/renal disease). These CRC patients were classified by clinical data and the rules of American Joint Committee.(30) The levels of the tumor markers CEA, CA19-9, and CA72-4 were measured for all patients. Other clinical backgrounds, like smoking history and BMI (body mass index), were all measured. Their ages ranged from 21 to 91 and we found no significant differences in the composition of gender and age among these different groups according to the t test (p>0.05). Among all these participants, 29.41% of females and 43.25% of males were diagnosed with different stages of CRC.
All subjects were divided into 4 cross-comparisons according to the stages of CRC. The candidates were selected by the following requirements: (1) colorectal cancer patients were diagnosed by histopathological diagnosis; (2) patients were diagnosed with CRC for the first time by the TNM staging method (2003) developed by the International Union Against Cancer (UICC) and the American Federation of Oncology (AJCC); (3) patients did not accept surgery, chemotherapy or radiotherapy; (4) patients with diabetes, inflammation, gastrointestinal disease, history of polyposis adenoma, cancer, and other serious diseases were excluded.
Data acquisition
In our study, we collected and analyzed blood samples from 5 groups (stage I–IV and NI) in the morning on an empty stomach and before surgery, they consisted of 289 patients admitted to the First Affiliated Hospital of Jinzhou Medical University. After extracting and derivatizing, the concentrations of fatty acids, nucleic acids and amino acid were detected by MS/MS.
A paper disc which was 3 mm equaled to 3.2 μl of whole blood was punched out from the dried blood sports filter paper. The paper disc was taken into a well in Millipore MultiScreen HV 96-well plate (Tullagreen, Ireland) containing 100 μl of working solution and subjected to extraction. The plate was centrifuged at 1,500 × g for 2 min after shaking gently (20 min, room temperature) and then the filtrate was put into a new flat bottom 96-well plate. The stability of MS analysis was monitored by four QC control samples as real samples, which contained randomly allocated two high-level and two low-level solutions. First, the QC solution was dried and filtrated under pure nitrogen at 50°C, and each sample was derived in a 60 μl mixed acetyl chloride and 1-butanol solution (10:90, v/v) at 65°C for 20 min. Then we dried derivative solutions under pure nitrogen gas at 50°C for another time. Finally, 100 μl mobile phase solution were was added into a well for further metabolic analysis.
The AB SCIEX 4000 Q Trap system (AB Sciex, Framingham, MA) was used for MS/MS analysis in a positive mode. The following conditions were used for operation: the injected volume of samples was 20 μl, and 80% ACN aqueous solution was the mobile phase. The initial elution flow rate was 0.2 ml/min, and then slowed down to 0.1 ml/min in 0.08 min and maintained for 1.5 min. Then, the flow rate reached 0.2 ml/min within 0.1 min and stayed for 0.5 min. The auxiliary gas was 350°C. Pressures of ion source gas I and gas 2 were set at 35 psi, and the curtain gas was set at 20 psi. Analyst v1.6.0 software (AB Sciex) was applied to process the raw data. Each metabolite was quantically analyzed based on Chemo View 2.0.2 software (AB Sciex) against different isotope standards.
Data processing
Data pre-processing
Metaboanalyst 5.0 platform was used to process all the data analyses, including performing metabolomic data analysis, visualization, and functional interpretation.
Univariate analysis
The p values were calculated using the false discovery ratio (FDR) of 92 metabolites, and they were considered statistically important when they were <0.05. Original and subsequent quantity was demonstrated by fold change. In this research, fold change was applied to describe the intensity and direction of metabolite changes. The logarithm of fold change (FC) at a base of 1.5 was applied to calculate and show the directions and extents of metabolism changes.
Multivariate data analysis
The principal component analysis (PCA) was used to demonstrate the distributed extent of raw data and orthogonal partial least squares discriminant analysis (OPLS-DA) was used to show the difference between two groups and their contributions to variables. P values and the variable importance in projection (VIP) were applied to find potential biomarkers. Then, we used (ROC) curve to assess these differential biomarker groups to diagnose. It could also be used as a technique to evaluate the predictive ability of the AUC model with the help of manually selected feature biomarkers.(34) ROC curve and AUC value were calculated and recorded for each model, and then models were constructed when their AUC values were higher than 0.650.(35)
Pathways analyses
The metabolic pathway analysis was evaluated with the enrichment analysis tool available on Metaboanalyst 5.0 platform. Biological pathways for each biomarker panel were plotted by this technique. All differential metabolites were altered within the metabolism of fatty acids and amino acid based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database.
Results
Baseline characteristics
The baseline characteristics of all experimental participants were shown in Table 1. A total of 289 participants (including 79 Nis, 50 patients with stage I colon cancer, 52 patients with stage II colon cancer, 56 patients with stage III colon cancer, and 52 patients with stage IV colon cancer) were enrolled. Men had significantly higher rates of colon cancer compared to females, there were separately 60.00%, 65.38%, 55.36%, and 56.86% of males were diagnosed with CRC I, CRC II, CRC III, and CRC IV, respectively. Moreover, the proportion of CRC patients in elder people was obviously higher compared to younger people. Approximately of 86 (29.76%) subjects had BMI ≥25 kg/m2 and 20 subjects had a positive history of smoking (Table 1). We found no significant difference in age or gender ratio in other groups.
Table 1.
Baseline characteristics of all experimental participants
| Characteristic | NI (n = 79) |
CRC I (n = 50) |
CRC II (n = 52) |
CRC III (n = 56) |
CRC IV (n = 52) |
p value | |
|---|---|---|---|---|---|---|---|
| Age (years) | 43.48 ± 11.08 | 66.08 ± 9.03 | 59.79 ± 12.76 | 66.08 ± 9.03 | 59.79 ± 12.76 | >0.05 | |
| Male sex (%) | 65.82 | 60 | 65.38 | 55.36 | 56.86 | >0.05 | |
| Positive history of smoking | 3 | 5 | 2 | 3 | 7 | >0.05 | |
| BMI (kg/m2) | <18.5 | 3 | 2 | 7 | 4 | 6 | >0.05 |
| 18.5–24.9 | 47 | 37 | 32 | 39 | 26 | >0.05 | |
| 25.0–29.9 | 26 | 10 | 12 | 11 | 17 | >0.05 | |
| 30.0–34.9 | 3 | 1 | 1 | 2 | 3 | >0.05 | |
Values are presented as mean ± SD.
Some serum protein and albumin levels among different groups of patients were assessed, such as TAP and AFP. And the results showed the levels of these factors were similar except for the increased contents of TAP and AFP in the fourth stage of CRC. The serum concentrations of CEA, CA19-9, and CA72-4 were detected in the same time, and the levels of CEA, CA72-4, and CA199 were shown in Fig. 1.
Fig. 1.
The blood laboratory results of different stages, the levels of carcinoembryonic antigen (CEA) (A), the levels of carbohydrate antigen 19-9 (CA19-9) (B), and the levels of cancer antigen 72-4 (CA72-4) (C). (*p<0.05, **p<0.01, ***p<0.001)
Univariate analysis
Metabolites were regarded as the most significant when their adjusted p value <0.05 and VIP >1.5 (Table 2). The heatmap was shown in Fig. 5 and 25 differential metabolites were selected to represent all detected comparisons.
Table 2.
Differential metabolites with VIP >1.5 and adjusted p values <0.05
| CRC I vs NI | VIP | p | FDR | FC | log2 (FC) |
|---|---|---|---|---|---|
| C18 | 1.816942 | 1.24E−37 | 2.2E−36 | 11.457 | 3.5182 |
| C16 | 1.801358 | 1.73E−37 | 2.3E−36 | 26.273 | 4.7155 |
| C18:2 | 1.581321 | 1.54E−22 | 9.53E−22 | 4.1326 | 2.047 |
| C4/C3 | 1.546064 | 1.17E−21 | 6.42E−21 | 10.187 | 3.3486 |
| CRC II vs NI | VIP | p | FDR | FC | log2 (FC) |
| Arg/Orn | 1.553826 | 1.39E−20 | 7.61E−20 | 11.674 | 3.5452 |
| Met/Phe | 1.536977 | 1.82E−18 | 8.89E−18 | 2.2877 | 1.1939 |
| C3/C16 | 1.512048 | 1.82E−18 | 8.89E−18 | 22.404 | 4.4857 |
| C4/C3 | 1.594945 | 1.53E−22 | 9.46E−22 | 10.506 | 3.3932 |
| C16 | 1.827176 | 1.84E−36 | 4.27E−35 | 17.096 | 4.0956 |
| C18 | 1.861812 | 1.32E−37 | 4.09E−36 | 10.451 | 3.3855 |
| CRC III vs NI | VIP | p | FDR | FC | log2 (FC) |
| C3/Met | 3.3663041 | 3.09E−33 | 9.59E−32 | 267.67 | 8.0643 |
| (C16 + C18)/C0 | 2.42154 | 1.81E−12 | 4.22E−11 | 2.2815 | 1.19 |
| Cit/Arg | 1.180143 | 0.001409 | 0.009356 | 1.7585 | 0.81434 |
| C10:2 | 1.091316 | 0.001572 | 0.009746 | 1.7133 | 0.77678 |
| C4/C8 | 1.263299 | 0.00056 | 0.004338 | 1.6854 | 0.75312 |
| CRC IV vs NI | VIP | p | FDR | FC | log2 (FC) |
| CO/(C16 + C18) | 2.52033 | 1.09E−22 | 5.07E−21 | 210.4 | 7.717 |
| C4-OH | 1.685166 | 1.53E−09 | 2.04E−08 | 4.6458 | 2.2159 |
| C6DC | 1.509 | 2.06E−06 | 0.0000147 | 2.7299 | 1.4489 |
| CRC III vs CRC I | VIP | p | FDR | FC | log2 (FC) |
| C3/Met | 1.958164 | 5.74E−37 | 5.28E−35 | 213.74 | 7.7397 |
| C0/(C16 + C18) | 1.826395 | 4.31E−30 | 2.35E−28 | 10.218 | 4.7635 |
| C16-OH | 1.879362 | 1.49E−27 | 3.42E−26 | 18.449 | 4.2055 |
| C5/C0 | 1.67155 | 1.54E−16 | 1.01E−15 | 16.821 | 4.0722 |
| C18-OH | 1.810133 | 2.67E−21 | 2.76E−20 | 16.315 | 4.0281 |
| C16-OH/C16 | 1.512287 | 1.62E−13 | 9.33E−13 | 6.853 | 2.7767 |
| Met/Leu | 1.640739 | 7.95E−17 | 5.63E−16 | 4.4459 | 2.1525 |
| C2/C0 | 1.930673 | 5.93E−30 | 2.36E−28 | 4.3378 | 2.117 |
| CRC IV vs CRC I | VIP | p | FDR | FC | log2 (FC) |
| C3/Met | 3.939144 | 7.8E−36 | 7.25E−34 | 208.7 | 7.7053 |
| C0/(C16 + C18) | 2.976943 | 6.21E−12 | 1.93E−10 | 1.9886 | 0.99173 |
| C5DC/C8 | 1.603277 | 0.001918 | 0.022296 | 4.1408 | 2.0499 |
| C2/C0 | 1.595155 | 0.005408 | 0.045719 | 1.2059 | 0.27013 |
| CRC IV vs CRC II | VIP | p | FDR | FC | log2 (FC) |
| C0/(C16 + C18) | 3.67983 | 7.43E−27 | 3.42E−25 | 209.21 | 7.7088 |
| C3/Met | 2.688987 | 9.89E−09 | 2.27E−07 | 2.0737 | 1.0522 |
| C10:2/C10 | 1.912797 | 0.0000603 | 0.000793 | 4.1103 | 2.0392 |
| CRC IV vs CRC III | VIP | p | FDR | FC | log2 (FC) |
| C0/(C16 + C18) | 4.02766 | 9.64E−24 | 4.43E−22 | 191.94 | 7.5845 |
| C3/Met | 2.807645 | 2.27E−08 | 5.22E−07 | 2.0732 | 1.0519 |
Fig. 5.
Differential metabolites were determined by five comparisons. The heatmap represented the level of 25 differential metabolites in five groups. NI, normal individual; 1, CRC I; 2, CRC II; 3, CRC III; 4, CRC IV.
Multivariate statistical analysis
The score plot of PCA (Fig. 2) and OPLS-DA (Fig. 3) generated from the original data set were applied to analyze these metabolites, which represented in the PCA plot of NI vs CRC stage I, the 1st PC and 2nd PC captured 24.3% and 16.2% of the total variates, respectively; in the PCA plot of NI vs CRC stage II, the 1st PC and 2nd PC captured 23.2% and 18.3% of the total variates, respectively; in the PCA plot of NI vs CRC stage III, the 1st PC and 2nd PC captured 6.3% and 10.8% of the total variates; in the PCA plot of NI vs CRC stage IV, the 1st PC captured 16.0% and 10.2%, respectively. We also built the PCA score plots of different CRC stages. In the PCA plot of CRC stage I vs CRC stage III, the 1st PC and 2nd PC captured 22.7% and 13.6% of the total variates. In the PCA plot of CRC stage I vs CRC stage IV, the 1st PC and 2nd PC captured 12.1% and 10.6% of the total variates. In the PCA plot of CRC stage II vs CRC stage IV, the 1st PC and 2nd PC captured 18.9% and 8.2% of the total variates, and in the PCA plot of CRC stage III vs CRC stage IV, the 1st PC and 2nd PC captured 13.4% and 11.6% of the total variates.
Fig. 2.
The score plot PCA of relative metabolite variations between CRC I, CRC II, CRC III, CRC IV with NI (A–D), CRC I vs CRC III, CRC I vs CRC IV, CRC II vs CRC IV, and CRC III vs CRC IV (E–H).
Fig. 3.
The score plot OPLS-DA of relative metabolite variations between CRC I, CRC II, CRC III, CRC IV with NI (A–D) and CRC stage I vs CRC stage III; CRC stage I vs CRC stage IV; CRC stage II vs CRC stage IV and CRC stage III vs CRC stage IV (E–H). The cumulative R2Y and Q2 were 0.961, 0.956; 0.953, 0.952; 0.961, 0.956; 0.802, 0.772; 0.913, 0.909; 0.735, 0.658; 0.756, 0.695; and 0.726, 0.635, separately.
The OPLS-DA models were used to obtain more clearer difference among all these comparisons, attention was paid to the following four models (Fig. 3): NI vs CRC stage I; CRC stage II; CRC stage III, and CRC stage IV, and their cumulative R2Y and Q2 were 0.961, 0.956; 0.953, 0.952; 0.961, 0.956; and 0.802, 0.772, separately. A clear difference was observed between the disease group from the non-disease group. Additionally, we also compare metabolites among different stages: CRC stage I vs CRC stage III; CRC stage I vs CRC stage IV; CRC stage II vs CRC stage IV and CRC stage III vs CRC stage IV, and their cumulative R2Y and Q2 were 0.913, 0.909; 0.735, 0.658; 0.756, 0.695; and 0.726, 0.635, separately.
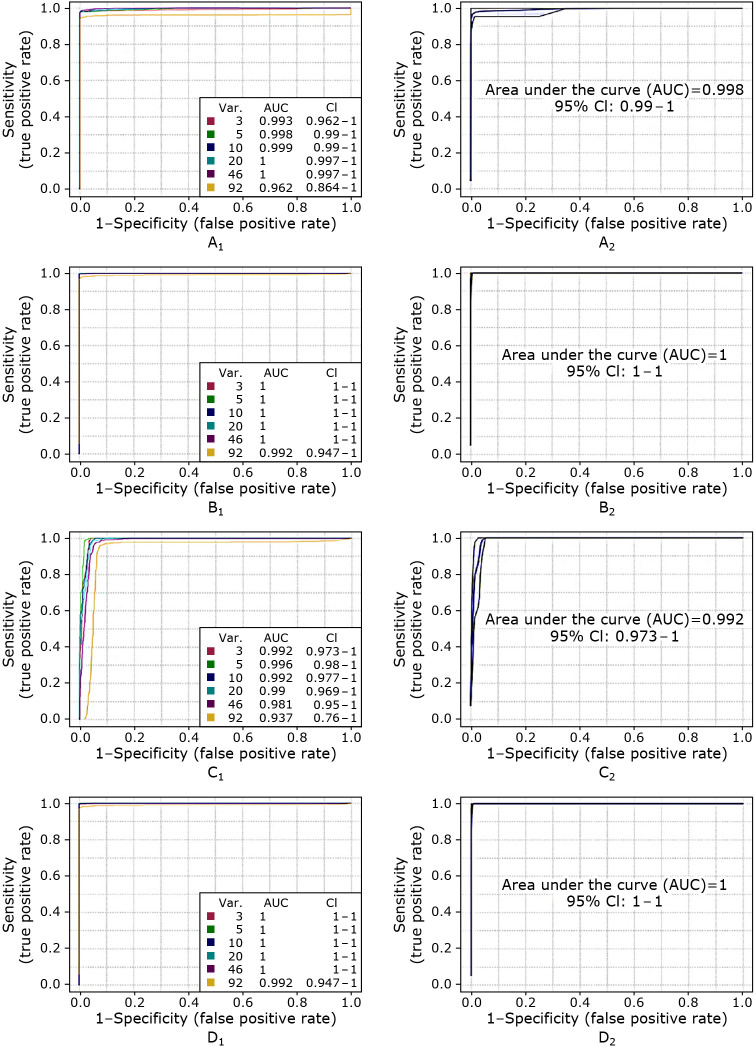
ROC curves and AUC values were used to validate each model and a new model would be constructed if its AUC value >0.650. The ROC curve among different stages was presented in Fig. 4. In (A1) it is shown that the ROC curve belonging to the model developed for CRC stage I vs CRC stage III feature when nine metabolites were selected as biomarkers, in (B1) for CRC stage I vs CRC stage IV, in (C1) for CRC stage II vs CRC stage IV, in (D1) for CRC stage III vs CRC stage IV and relevant ROC after the evaluation was present in Fig. 3 (A2), (B2), (C2), (D2), which were 0.998, 1.000, 0.992, and 1.000, separately.
Fig. 4.
The multivariate exploratory ROC of PLS-DA model combined biomarker (including CRC stage I vs CRC stage III (A1), CRC stage I vs CRC stage IV (B1), CRC stage II vs CRC stage IV (C1), CRC stage III vs CRC stage IV (D1) and their ROC after evaluations were shown in (CRC stage I vs CRC stage III (A2), CRC stage I vs CRC stage IV (B2), CRC stage II vs CRC stage IV (C2), CRC stage III vs CRC stage IV (D2).
Pathways analysis
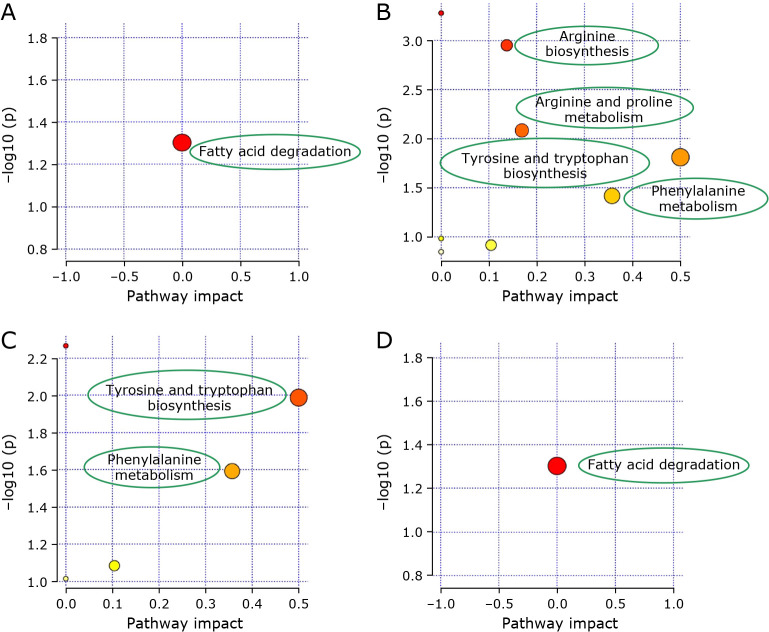
Pathway analysis of the systematic metabolome changes was built to distinguish different stages of CRC. The biological pathways, including metabolites, were constructed by enrichment analysis and shown in Fig. 6. They were built according to p values (y-axis) and pathway impact values (x-axis). The pathway was regarded as important when high impact values and pathway enrichment analysis were high. Metabolic changes involved arginine, and proline metabolism, phenylalanine, tyrosine and tryptophan biosynthesis for CRC stage II vs NI; phenylalanine, tyrosine, and tryptophan biosynthesis showed metabolic changes for CRC stage III vs NI; metabolic changes involved fatty acids degradation for both CRC stage I and IV vs NI. Therefore, these metabolic pathways were target pathways, which meant these selected biomarkers were closely related to colon cancer.
Fig. 6.
The bubble diagram of significantly changed metabolic pathways, including affected pathways of CRC I vs NI (A); CRC II vs NI (B); CRC III vs NI (C); and CRC IV vs NI (D) (small p value and big pathway impact factor indicate that the pathway is greatly influenced).
Discussion
Colorectal cancer (obesity and insulin resistance are the important contributing factors) is a really common disease related to death in the world. CRC can be cured by surgery and mortality can be reduced if the patients get an early diagnosis and appropriate treatment, which makes the screening strategy so critical for CRC. But most screening methods fail to satisfy convenience, accuracy, and affordability in the same time,(36) which results in patients’ high possibility of evolving to invasive cancer. Moreover, disease symptoms are not obvious in early-stages of CRC. In the same time, many people are not willing to date with CRC screening,(23) which sets the barrier for in-time diagnoses and effective treatment. The blood-based test would share the advantage in terms of convenience, accuracy, and no other requirement. Therefore, studying and assessing the metabolites of CRC patients in blood are important for further study.
Comparisons of NI with CRC stage I–IV and different stages of CRC
In our studies, compared with previous studies, men were not at greater age-specific risk for advanced-stage CRC than women (CRC stage I 60%, CRC stage II 65.38%, CRC stage III 55.36%, CRC stage IV 56.86%), maybe because the data were scarce.(37)
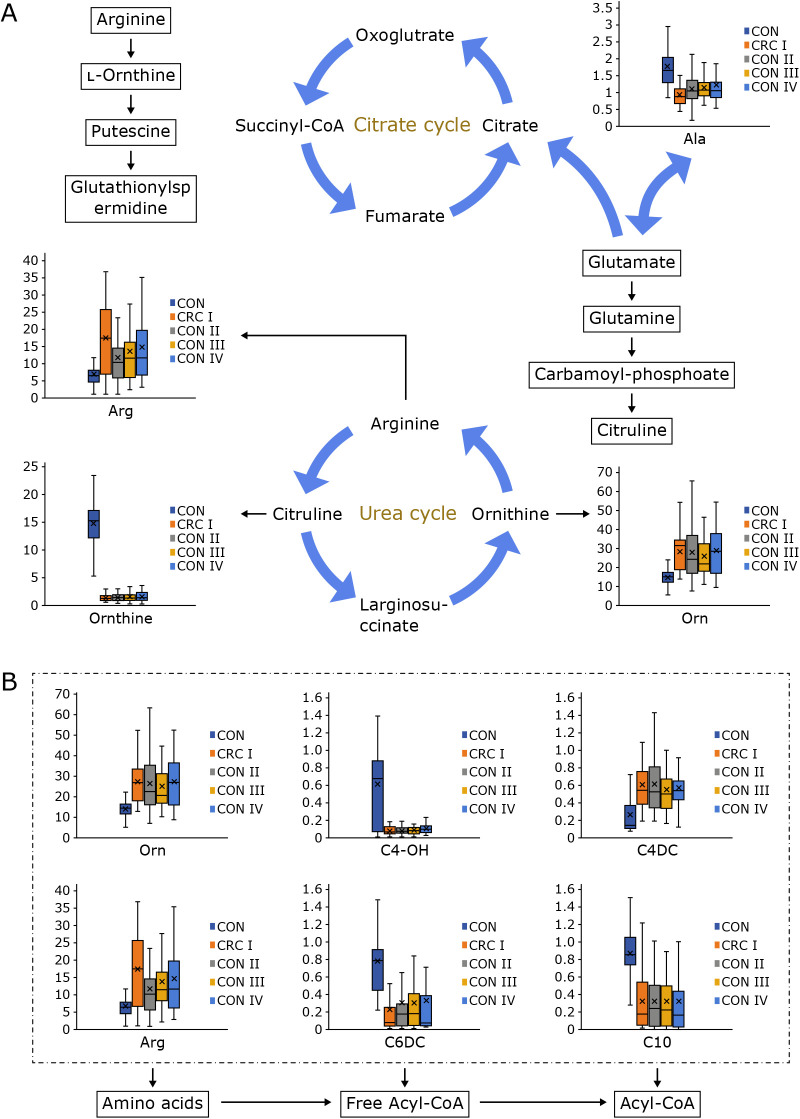
As shown in Fig. 7, for the discriminative metabolites, ornithine (Orn), arginine (Arg), octadecanoyl carnitine (C18), palmitoyl carnitine (C16) were down-regulated, while C0/(C16 + C18) and 3-hydroxy-butyryl carnitine (C4-OH) were up-regulated in CRC stage I–IV compared with Nis. What’s more, propanoyl carnitine/methionine were quite different among different stages, they were hugely down-regulated in CRC stage II–IV compared with CRC stage I.
Fig. 7.
The ROC curve with the AUC for predicting CRC among 289 cases follow-up including CRC I vs NI (A); CRC II vs NI (B); CRC III vs NI (C); and CRC IV vs NI (D) when the biomarkers are ornithine, arginine, octadecanoyl carnitine and palmitoyl carnitine adipoyl carnitine; and CRC I vs CRC III (E), CRC I vs CRC IV (F), CRC II vs CRC IV (G), CRC III vs CRC IV (H) when the biomarkers are propanoyl carnitine/methionine and acetyl carnitine/free carnitine, separately.
Ornithine, arginine, and methionine are essential amino acid, which are involved in insulin metabolism and therefore influence the development and progression of CRC.(38) Ornithine, which participated in the urea cycle, can aggravate tumor growth and contribute to disease progression by promoting pyrimidine synthesis.(39) Arginine is synthesized by urea cycle enzymes physiologically in normal cells. It’s extremely important to synthesize proteins and manage cellular activity in many aspects. But the significant difference between NI and CRC patients metabolically directly leads to the cells can’t sustain normal growth and survival, which means some kinds of tumors fail to depend on extracellular arginine.(40,41) This amino acid metabolism exists in almost each cell of the organism. What’s more, they are closely related to disease and health status.(40) They play multiple functions in supplying precursor materials and they are used as biomarkers for the detection and treatment of CRC.(42) Previous studies show amino acid which exists in CRC patients and varies among different stages.(43,44) Budhathoki et al.(45) found that branched-chain amino acid were inversely related to colorectal adenoma in males. Mackenzie et al.(46) found plasma free amino acid (PFAA) was slight in the early stage, whereas it varied during advanced-stage CRC, in which the progression of CRC could be predicted by this index. CRC-associated inflammation could induce gene mutations and make tumors grow steadily. As one of the essential amino acids, methionine can influence inflammation-induced colon cancer through carbon metabolism.(47) Some studies show methionine restriction help to prolong life span and inhibit the growth of tumor formation.(48)
Carnitine is described as a natural-origin amino acid and essential metabolite in the liver, kidneys, and placenta.(49) It is involved in lipid metabolism and beta-oxidation in human beings, which can transport long-chain fatty acids into the mitochondria for oxidation to produce energy (form acyl carnitine derivatives).(50) What’s more, the concentrations of carnitine and acyl carnitine are under the influence of dietary habits and abnormal fasting inhibits the TCA circle directly. Acetyl carnitines can decrease lipid peroxidation through their antioxidant capacity and anti-apoptotic effects.(51)
Carnitine can generate some necessary energy by transporting fatty acids and diagnose fatty acids oxidation disorders since it is involved in the beta-oxidation of long-chain fatty acids. Fatty acids, especially saturated fatty acids and monounsaturated fatty acids, are associated with CRC patients. Both males and females have released fatty acids into the bloodstream when they are fat, and sex differences contribute to a different flux of fatty acids.(42) C-Myc is a bHLH-zip pleiotropic transcription factor and regulates cancer metabolism by providing nucleic acids, proteins, and lipids for the development of cancer, which means its expression plays a vital role in the metabolic reprogramming of CRC. In summary, arginine biosynthesis, amino acid metabolisms, and fatty acids get involved in the occurrence and progression of CRC, which may help to the therapy of colorectal cancer.
Potential biomarkers for differential diagnosis
In this study, we got highly sensitive and accurate biomarkers between different stages of CRC with NIs. Six metabolites were screened for distinguishing CRC patients from NI and two metabolites for different stages of CRC. It showed that there were many similar metabolic pathways and metabolites affected in the metabolic process of CRC and NI. We found that ornithine, arginine, octadecanoyl carnitine, palmitoyl carnitine, adipoyl carnitine, and butyryl carnitine/propanoyl carnitine varied significantly between the NI and the CRC patients. Propanoyl carnitine and methionine were quite different among four stages of CRC. Then, ROC curves were constructed based on those mentioned metabolites as new biomarkers between different stages of CRC and NI and different stages of CRC. The AUC values were 0.901, 0.951, 0.972, 0.919 for different stages vs NI, separately. And the AUC values were 0.997 for CRC I vs III, 1.000 for CRC I vs IV, 0.960 for CRC II vs IV, and 0.975 for CRC III vs IV (Fig. 7). Therefore, ornithine, arginine, octadecanoyl carnitine, palmitoyl carnitine, adipoyl carnitine, butyryl carnitine/propanoyl carnitine, and methionine might be used as marker metabolites for the development of CRC.
In conclusion, we selected biomarkers and demonstrated the molecular mechanism of CRC with metabolomics. Six metabolites were selected as biomarkers to distinguish CRC patients from NI and two for different stages of CRC. They are involved in insulin metabolism, the urea cycle and pyrimidine synthesis and indicate the development and progression of CRC. However, further studies with large clinical data and reasonable experiments should be carried out to confirm their sensitivity and specificity in CRC.
Fig. 8.
The KEGG database was used to draw the metabolic pathways of differential biomarkers from four comparisons and plotted on (A), including arginine biosynthesis, arginine and proline metabolism, phenylalanine, tyrosine, and tryptophan biosynthesis, fatty acid degradation (B).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81501659) for financial support.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Nguyen S, Li H, Yu D, et al. Dietary fatty acids and colorectal cancer risk in men: a report from the Shanghai Men’s Health Study and a meta-analysis. Int J Cancer 2021; 148: 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghebrial M, Aktary ML, Wang Q, et al. Predictors of CRC stage at diagnosis among male and female adults participating in a prospective cohort study: findings from Alberta’s Tomorrow Project. Curr Oncol 2021; 28: 4938–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen SP, Bent S, Chen YH, Terdiman JP. Gender as a risk factor for advanced neoplasia and colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2009; 7: 676–681.e673. [DOI] [PubMed] [Google Scholar]
- 4.Hansen IO, Jess P. Possible better long-term survival in left versus right-sided colon cancer - a systematic review. Dan Med J 2012; 59: A4444. [PubMed] [Google Scholar]
- 5.Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022; 135: 584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 7.Younossi Z, Anstee Q, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15: 11–20. [DOI] [PubMed] [Google Scholar]
- 8.Tan J, Wang HL, Yang J, et al. JMJD2B-induced amino acid alterations enhance the survival of colorectal cancer cells under glucose-deprivation via autophagy. Theranostics 2020; 10: 5763–5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vargas AJ, Thompson PA. Diet and nutrient factors in colorectal cancer risk. Nutr Clin Pract 2012; 27: 613–623. [DOI] [PubMed] [Google Scholar]
- 10.Zanutto S, Ciniselli CM, Belfiore A, et al. Plasma miRNA-based signatures in CRC screening programs. Int J Cancer 2020; 146: 1164–1173. [DOI] [PubMed] [Google Scholar]
- 11.Gumpenberger T, Brezina S, Keski-Rahkonen P, et al. Untargeted metabolomics reveals major differences in the plasma metabolome between colorectal cancer and colorectal adenomas. Metabolites 2021; 11: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding Q, Sun Y, Zhang J, Yao Y, Huang D, Jiang Y. Utility and specificity of plasma heat shock protein 90 alpha, CEA, and CA199 as the diagnostic test in colorectal cancer liver metastasis. J Gastrointest Oncol 2022; 13: 2497–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Zhang D, Cui YP, Qiu Y, Miao C, Lu X. Identification of microRNA-451a as a novel circulating biomarker for colorectal cancer diagnosis. Biomed Res Int 2020; 2020: 5236236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang YC, Wang D, Jin L, et al. Circulating tumor DNA detectable in early- and late-stage colorectal cancer patients. Biosci Rep 2018; 38: BSR20180322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, He C, Qiu L, et al. Serum unsaturated free fatty acids: a potential biomarker panel for early-stage detection of colorectal cancer. J Cancer 2016; 7: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapira S, Fokra A, Arber N, Kraus S. Peptides for diagnosis and treatment of colorectal cancer. Curr Med Chem 2014; 21: 2410–2416. [DOI] [PubMed] [Google Scholar]
- 17.Parizadeh SM, Jafarzadeh-Esfehani R, Ghandehari M, et al. Personalized peptide-based vaccination for treatment of colorectal cancer: rational and progress. Curr Drug Targets 2019; 20: 1486–1495. [DOI] [PubMed] [Google Scholar]
- 18.Bold RJ, Ishizuka J, Townsend CM Jr. Progress toward hormonal therapy of gastrointestinal cancer. Ann Surg 1996; 223: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Zhang G, Shen J, Shen Y, Cai G. Elevated CEA and CA 19-9 levels within the normal ranges increase the likelihood of CRC recurrence in the Chinese Han population. Appl Bionics Biomech 2022; 2022: 8666724. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Kasanga M, Liu L, Xue L, Song X. Plasma heat shock protein 90-alpha have an advantage in diagnosis of colorectal cancer at early stage. Biomark Med 2018; 12: 881–890. [DOI] [PubMed] [Google Scholar]
- 21.Mamoori A, Wahab R, Vider J, Gopalan V, Lam AK. The tumour suppressor effects and regulation of cancer stem cells by macrophage migration inhibitory factor targeted miR-451 in colon cancer. Gene 2019; 697: 165–174. [DOI] [PubMed] [Google Scholar]
- 22.Pai RK. When to suspect and how to diagnose syndromic polyps and carcinomas of the gastrointestinal tract: focus on Lynch syndrome and colonic hamartomatous polyposis. Diagn Histopathol 2020; 26: 8–14. [Google Scholar]
- 23.Cui Y, Zaman A, Kaminsky AM, et al. Majority of individuals who have declined colonoscopy and stool test are willing to undergo blood test for CRC screening. Gastrointest Endosc 2021; 93: AB79. [Google Scholar]
- 24.Chen X, Shi BL, Qi RZ, Chang X, Zheng HG. Ultra-performance liquid chromatography/mass spectrometry-based metabolomics for discovering potential biomarkers and metabolic pathways of colorectal cancer in mouse model (ApcMin/+) and revealing the effect of Honokiol. Front Oncol 2021; 11: 671014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song EM, Byeon JS, Lee SM, et al. Fecal fatty acid profiling as a potential new screening biomarker in patients with colorectal cancer. Dig Dis Sci 2018; 63: 1229–1236. [DOI] [PubMed] [Google Scholar]
- 26.Yu Y, Wang J, Wang P, Wang Y. Quantification of azaserine-induced carboxymethylated and methylated DNA lesions in cells by nanoflow liquid chromatography-nanoelectrospray ionization tandem mass spectrometry coupled with the stable isotope-dilution method. Anal Chem 2016; 88: 8036–8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim SH, Becker TM, Chua W, et al. Circulating tumour cells and circulating free nucleic acid as prognostic and predictive biomarkers in colorectal cancer. Cancer Lett 2014; 346: 24–33. [DOI] [PubMed] [Google Scholar]
- 28.Hama K, Fujiwara Y, Hayama T, et al. Very long-chain fatty acids are accumulated in triacylglycerol and nonesterified forms in colorectal cancer tissues. Sci Rep 2021; 11: 6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinsky P, Rabeneck L, Lauby-Secretan B. The IARC perspective on colorectal cancer screening. N Engl J Med 2018; 379: 301–302. [DOI] [PubMed] [Google Scholar]
- 30.Liesenfeld DB, Grapov D, Fahrmann JF, et al. Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal cancer patients: the ColoCare study. Am J Clin Nutr 2015; 102: 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizota T, Hishiki T, Shinoda M, et al. The hypotaurine-taurine pathway as an antioxidative mechanism in patients with acute liver failure. J Clin Biochem Nutr 2022; 70: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silvestri C, Brodbelt JS. Tandem mass spectrometry for characterization of covalent adducts of DNA with anticancer therapeutics. Mass Spectrom Rev 2013; 32: 247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chace DH, Kalas TA. A biochemical perspective on the use of tandem mass spectrometry for newborn screening and clinical testing. Clin Biochem 2005; 38: 296–309. [DOI] [PubMed] [Google Scholar]
- 34.Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med 2013; 4: 627–635. [PMC free article] [PubMed] [Google Scholar]
- 35.Schieda N, Dilauro M, Moosavi B, et al. MRI evaluation of small (<4cm) solid renal masses: multivariate modeling improves diagnostic accuracy for angiomyolipoma without visible fat compared to univariate analysis. Eur Radiol 2016; 26: 2242–2251. [DOI] [PubMed] [Google Scholar]
- 36.Kahi CJ, Rex DK. Current and future trends in colorectal cancer screening. Cancer Metastasis Rev 2004; 23: 137–144. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen SP, Bent S, Chen YH, Terdiman JP. Gender as a risk factor for advanced neoplasia and colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2009; 7: 676–681.e671–673. [DOI] [PubMed] [Google Scholar]
- 38.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 2014; 10: 723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramos H, Calheiros J, Almeida J, et al. SLMP53-1 inhibits tumor cell growth through regulation of glucose metabolism and angiogenesis in a p53-dependent manner. Int J Mol Sci 2020; 21: 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Zhao X, Wei S, et al. Mechanism of paeoniflorin on ANIT-induced cholestatic liver injury using integrated metabolomics and network pharmacology. Front Pharmacol 2021; 12: 737630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raza MF, Wang Y, Cai Z, et al. Gut microbiota promotes host resistance to low-temperature stress by stimulating its arginine and proline metabolism pathway in adult Bactrocera dorsalis. PLoS Pathog 2020; 16: e1008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Li J, Wang H, Qi LW, Zhu Y, Lai M. Tyrosine and glutamine-leucine are metabolic markers of early-stage colorectal cancers. Gastroenterology 2019; 157: 257–259.e255. [DOI] [PubMed] [Google Scholar]
- 43.Aquilani R, Brugnatelli S, Dossena M, et al. Oxaliplatin-fluoropyrimidine combination (XELOX) therapy does not affect plasma amino acid levels and plasma markers of oxidative stress in colorectal cancer surgery patients: a pilot study. Nutrients 2019; 11: 2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto N, Miyagi Y, Chiba A, et al. Diagnostic modeling with differences in plasma amino acid profiles between non-cachectic colorectal/breast cancer patients and healthy individuals. Int J Med Med Sci 2008; 1: 1–8. [Google Scholar]
- 45.Budhathoki S, Iwasaki M, Yamaji T, Yamamoto H, Kato Y, Tsugane S. Association of plasma concentrations of branched-chain amino acids with risk of colorectal adenoma in a large Japanese population. Ann Oncol 2017; 28: 818–823. [DOI] [PubMed] [Google Scholar]
- 46.Mackenzie M, Baracos VE. Cancer-associated cachexia: altered metabolism of protein and amino acids. In: Cynober, ed. Amino Acid Metabolism and Therapy in Health and Disease, Boca Raton, FL: CRC Press, 2003; 339–354. [Google Scholar]
- 47.Zhou ZY, Wan XY, Cao JW. Dietary methionine intake and risk of incident colorectal cancer: a meta-analysis of 8 prospective studies involving 431,029 participants. PLoS One 2013; 8: e83588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp Gerontol 2003; 38: 47–52. [DOI] [PubMed] [Google Scholar]
- 49.Manta-Vogli PD, Schulpis KH, Dotsikas Y, Loukas YL. The significant role of carnitine and fatty acids during pregnancy, lactation and perinatal period. Nutritional support in specific groups of pregnant women. Clin Nutr 2020; 39: 2337–2346. [DOI] [PubMed] [Google Scholar]
- 50.Stroup BM, Nair N, Murali SG, et al. Metabolomic markers of essential fatty acids, carnitine, and cholesterol metabolism in adults and adolescents with phenylketonuria. J Nutr 2018; 148: 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou S, Wen H, Li H. Magnolol induces apoptosis in osteosarcoma cells via G0/G1 phase arrest and p53-mediated mitochondrial pathway. J Cell Biochem 2019; 120: 17067–17079. [DOI] [PubMed] [Google Scholar]