Abstract
Tissue-resident memory T cells (Trm) are a sub-population of memory T cells that reside in skin tissue. Recent studies have revealed potential role of Trm in the reoccurrence of psoriasis, as these cells tend to be profusely infiltrated in the lesions observed during psoriasis relapse. Trm can be classified into CD8+ Trm cells that are distributed mainly in the epidermis and CD4+ Trm cells in the dermis. CD8+ Trm is derived from circulating memory T cells and CD49a−CD8+ Trm takes a crucial role in psoriasis relapse. In contrast, CD4+ Trm may originate from exTh17 cells and exTreg cells emerging from the inflammatory process. Since IL-23 can activate Trm, neutralizing antibodies against IL-23 are suggested to be more effective in clinical treatment. This review will focus on Trm cells in psoriasis relapsed lesions to reveal their mechanisms in the pathogenesis, relapse and transformation of psoriasis.
Keywords: Psoriasis, Trm, Relapse, Th17
Graphical abstract
Highlights
-
•
Trm are a sub-population of memory T cells that reside in skin tissue.
-
•
Trm is a potential factor for psoriasis relapse.
-
•
Biological agents for Trm can achieve better therapeutic results.
-
•
CD4+ Trm may originate from Th17 and Treg.
1. Introduction
Psoriasis is a T-cell mediated autoimmune disease with clinical manifestations characterized by erythema and scaling. Studies in immunology and genetics have confirmed the key role of TNF-α, IL-17 and IL-23 in the pathogenesis of psoriasis. Biologics that target TNF-α, IL-17 and IL-23 are now widely employed in clinical practice, and have achieved promising therapeutic results [Grolleau et al., 2023]. However, a significant concern arises as the majority of patients often experience relapse after discontinuing therapy upon clinical cure [Puig, L et al., 2022]. Of further concern is the observation that patients with moderate to severe psoriasis with greater improvements in skin lesions continue to experience varying degrees of secondary failures in the long-term maintenance treatment of psoriasis [Puig, L et al., 2022]. Both the relapse of psoriasis and the secondary failure of biologics can substantially affect the quality of patients' lives [Lin, L et al., 2022; Kearney and McKenna, 2022].
Recently, the role of tissue-resident memory T cells (Trm) in psoriasis relapse has attracted much attention [Ghaffarinia, A et al., 2023]. Post psoriasis inflammation, some memory T cells express skin-specific homing antigens, such as CD69, CD103, and cutaneous lymphocyte antigen (CLA), which are subsequently converted into Trm residing in the skin [Vu, T. T., Koguchi-Yoshioka, H., Watanabe, R. 2021]. Trm are known to have a surveillance function and reactivate upon reinfection with the same antigen [Rosato, P. C et al., 2023]. Among psoriasis patients, these Trm are reactivated and produce IL-17 and IL-22 in response to specific environmental triggers, thereby recruiting inflammatory cells and mediating psoriasis relapse [Vu, T. T., Koguchi-Yoshioka, H., Watanabe, R. 2021]. The mechanism of Trm involvement in psoriasis relapse has not yet been fully elucidated, and further discussion is needed on how Trm contribute to psoriasis relapse, and how they affect the therapeutic efficacy in treating psoriasis.
In this mini-review, we will focus on summarizing the progress of Trm research within psoriatic skin, discussing the above-mentioned problems, and presenting the current challenges in Trm research.
2. The source of Trm and surface markers
As early as 2015, the team of Thomas S Kupper and Rachael A Clark identified four main types of memory T cells present in human skin: central memory T cells (Tcm), migratory memory T cells (Tmm), and CD4 (+) and CD8 (+) CD103 (+) resident memory T cells (Trm)[Watanabe, R et al., 2015]. Among them, Trm represents a non-circulating tissue-resident subpopulation that plays an important role in immune surveillance and protective responses to reinfection. Within the skin, Trm can be classified into two subgroups, CD4+CD69+ Trm, located in the dermis, and CD8+CD69+CD103+ Trm in the epidermis [Szabo, P. A., Miron, M., Farber, D. L. 2019].
Presently, research on tissue-resident memory T cells has focused on CD8+ Trm, as they constitute the majority of Trm infiltration in psoriatic skin [10]. Previous studies have recorded approximately twice the number of CD103+CD8+ Trm in psoriatic lesional and non-lesional skin compared to controls.
During the phase of inflammation reduction, effector CD8+ T cells can differentiate into KLRG1highIL-7Rlow short-lived effector cells (SLEC) and KLRG1lowIL-7Rhigh memory precursor effector cells (MPEC), which together form a pool of effector cells [Behr, F. M et al., 2021]. Subsequently, the number of SLECs gradually diminishes, while MPECs progrssively differentiate into memory T cells capable of self-renewal, including Tcm, Tem and Trm cells [Behr, F. M et al., 2021]. Tcm exhibit high expression of CCR7 and CD62L. They preferentially reside in secondary lymphoid organs and have the ability to rapidly proliferate and differentiate into effector T cells upon antigen stimulation. In contrast Tem are mainly distributed in the peripheral tissues, where they can quickly and abundantly produce various inflammatory factors [Behr, F. M et al., 2021; Schenkel, J. M., Masopust, D. 2014].
The transformation of precursor memory cells into CD8+ Trm cells is accompanied by the upregulation of integral proteins such as CD103, CD69, and CD49a, which are responsible for the tissue residence of Trm cells [Reilly, E. C et al., 2020]. Specifically, CD103 is the alpha chain of integrin αEβ7 that enables CD8+ T cells to adhere to epithelial cells. It promotes T cell CD103 upregulation by enhancing the TGF-β signaling pathway [Ghaffarinia, A et al., 2023]. CD69 downregulates sphingosine phosphate receptor (S1PR1), transiently inhibiting T cell efflux through lymphatics and promotes T cell residency in tissues [Walsh, D. A et al., 2019]. CD49a, also known as very late antigen-1 (VLA-1), binds to collagen in the extracellular matrix, further contributing to Trm cell residency [Cheuk, S et al., 2017].
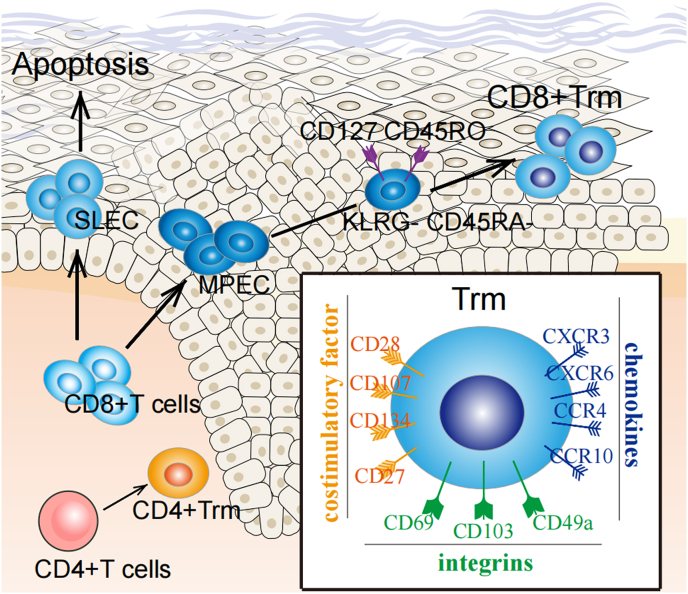
CD8+ Trm cells express numerous chemokine receptors, such as CXCR3, CXCR6, CCR4 and CCR10 [Ferreira, C et al., 2020; Wein, A. N et al., 2019;,van der Gracht et al., 2021]. Among them, CXCR3 drives precursor memory cells into the epidermis [Ferreira, C et al., 2020], while CXCR6 fosters Trm formation by binding to the CXC chemokine ligand CXCL16 [Wein, A. N et al., 2019]. CCR4 plays a role in T cell migration to the skin and acts as vital homing molecule for Trm formation [van der Gracht et al., 2021]. Trm express specific co-signaling factors, including the immunoglobulin superfamily (CD28 and ICOS) and the tumor necrosis factor receptor (TNFR) superfamily (e.g., 4-1BB (CD137), OX40 (CD134), CD27), which also play a key role in the regulation of immunity [Lin, R et al., 2020; Peng, C et al., 2022]. In addition to the above markers, there are other specific skin homing markers such as CLA (cutaneous lymphocyte-associated antigen) (Fig. 1).
Fig. 1.
(1) Surface markers of Trm: Integral proteins (CD69, CD103, CD49a), which are primarily responsible for the tissue residence of Trm. Chemokine receptors (CXCR3, CXCR6, CCR4, CCR10), capable of driving precursor memory cells into the skin. Costimulatory molecules (CD28, etc.) responsible for maintaining the stability of T cells. (2) Source of CD8+Trm:Circulating CD8+ T cells are able to differentiate into SLEC and MPEC. SLEC apoptosis after the inflammation subsides and MPEC continue to differentiate. One of the memory precursor cells with the phenotype KLRG1-CD127+CD45RO+CD45RA- eventually differentiates into CD8+Trm.SLEC: short-lived effector cells; MPEC: memory precursor effector cells.
CD4+ T cells exhibit limited proliferative capacity on their own, and the generation of CD4+ memory T cells post-antigen clearance is generally low [Nguyen, Q. P et al., 2019]. The availability of CD4+ Trm cells for research is restricted, leading to significant gaps in the understanding of these cells.
CD4+ Trm cells may be derived from circulating memory cells. First, similar to the precursor cells of CD8+ Trm, the memory CD4+ population in the circulatory system is able to express KLRG1 and CD127 [Nguyen, Q. P et al., 2019], but the role of these factors has not been elucidated. Second, CD4+ Trm demonstrate strong migratory capacity and a fraction of CD4+CLA+CD103+ T cells in the skin can re-enter the circulation and migrate to new skin sites, re-expressing CD69 and adopting a Trm phenotype [Klicznik, M. M et al., 2019]. The surface markers of CD4+ Trm share similarities with those of CD8+ Trm. Mediated resident integral proteins such as CD69 and CD49a are also abundantly expressed in CD4+ Trm [Szabo et al., 2019]. Unusually, CD103 is not commonly expressed in CD4+ Trm. When evaluated collectively, approximately 66% of the cell population exhibits CD4+CD103-[Watanabe, R et al., 2015]. A majority of CD4+ Trm express cutaneous lymphocyte antigen (CLA), which functions as a ligand for E selectin to promote cell residence in the skin [Ryan, G. E et al., 2021]. Chemokines (CCR4, CCR6 and CCR10, CXCR3) are also important markers of CD4+ Trm [Reschke, R et al., 2022].
The above markers are not simultaneously expressed In all Trm cells and a combination of several markers is typically utilized for identification. The commonly used markers for this purpose include CD69+, CD103+, CLA+, CD49a+, CXCR6+ or CCR7- [Ryan, G. E et al., 2021].
3. Functions of CD4+Trm and CD8+Trm in psoriasis
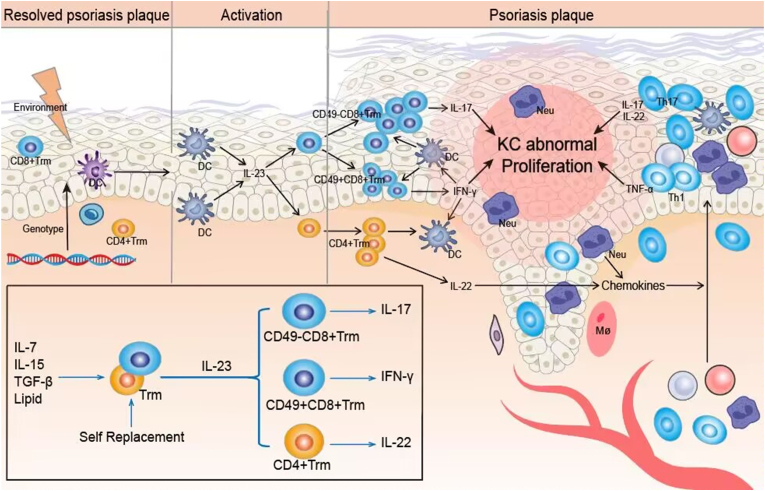
In psoriatic plaques that have remitted following treatment, CD8+ Trm are retained within the epidermis, and CD4+ Trm the dermis. When stimulated by external factors, dendritic cells, Langerhans cells secrete IL-23, which interacts with IL-23R on the surface of Trm. This activates the Trm, contributing to the relapse of psoriasis [Whitley, S. K et al., 2022]. During relapse, CD8+ Trm primarily produce IL-17, while CD4+ Trm is involved in the pathogenesis mainly by secreting cytokines such as IL-22 [Owczarczyk Saczonek, A et al., 2020](Fig. 2).
Fig. 2.
Self-proliferation in the presence of IL-7, IL-15, TGF-β and lipid. (ii) Activation phase: Trm is activated in response to stimulation by IL-23 secreted by DCs. (iii)Onset stage: CD49a−CD8+Trm secretion of IL-17 is involved in psoriasis relapse. IFN-γ secreted by CD49a−CD8+ Trm acts on DCs to form an inflammatory cycle while activating KC. IL-22 secreted by CD4+ Trm recruits inflammatory cells into the superficial dermis to participate in psoriasis relapse.
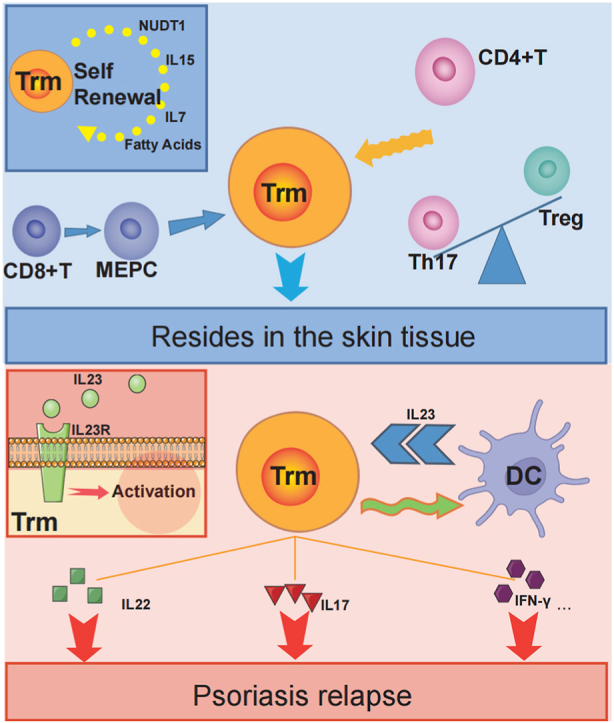
Cytokines (IL-15, IL-7), NUDT1, fatty acids and Trm turnover are all key factors in Trm residency [Mazzoni, A et al., 2020; Huang, B et al., 2022]. Specifically, IL-15 and IL-7 act as survival factors required for Trm residency [Mazzoni, A et al., 2020], and NUDT1 enhances CD8+ T cell tissue residence via the PARP1-TGFβR axis [Huang, B et al., 2022].
The maintenance of tissue memory T cells necessitates the continuous uptake of exogenous lipids. The expression levels of Fabp4 and Fabp5 in Trm are up-regulated, enhancing the lipid uptake capacity of Trm. In contrast, Fabp4/Fabp5-deficient mice exhibit significantly downregulated Trm levels and are more susceptible to viral infections [Grolleau et al., 2023]. The immune function of CD8+ Trm is equally dependent on the uptake of exogenous fatty acids [Pan, Y et al., 2017]. In addition, Richard A Flavell's team has shown through cell proliferation experiments that Trm is able to continuously turnover in the skin, which is an important reason why Trm is able to reside in the skin [Mazzoni, A et al., 2020].
The amount of CD8+ Trm in the epidermis correlates with the degree of inflammation at the time of relapse [Cheuk, S et al., 2017]. Cheuk S identified two different CD8+CD103+ Trm cell populations that can be recognized by CD49a. Among these, CD8+ Trm which do not express CD49a, are considered a major pathogenic factor in psoriasis, secreting IL-17 to mediate disease development [16]. In contrast CD49a+CD8+ Trm secretes mainly IFN-γ, and CD49a aids in maintaining CD8+ Trm persistence and responsiveness [Bromley, S. K et al., 2020]. IFN-γ can be pathogenic, leading to Th17-type inflammation, and is not inhibited by currently employed IL-17 monoclonal antibodies. IFN-γ may promote disease by activating DCs and stimulating keratinocytes to release adhesion molecules that drive T lymphocytes into inflammatory plaques [Srivastava, A et al., 2021].
CD4+ Trm play a central role in psoriasis relapse by secreting IL-22, which enhances the anti-apoptotic capacity of keratinocytes and leads to excessive proliferation and abnormal differentiation of keratinocytes [Owczarczyk Saczonek, A et al., 2020]. IL-22 induces the production of antimicrobial binding proteins (β-defensin 2 (BD2), BD3, S100A7, S100A8, S100A9, lipocalin 2 (LCN2)) and granulocyte chemokines (e.g. CXCL1, CXCL5 and CXCL8) that amplify psoriatic inflammation [Keir, M., Yi, Y., Lu, T., Ghilardi, N. 2020]. CD4+Trm increases the expression of Bcl-3, which is essential for Th1, Th2, and Th17 cells and can exacerbate psoriatic inflammation by promoting the proliferation of CD4+Trm and Th17 [Tohyama, M et al., 2018].
4. CD4+ Trm may originate from Th17 and Treg
Central memory cells enter and reside in the surrounding tissue to form CD8+ Trm cells [Schenkel, J. M., Masopust, D. 2014]. This process is associated with TCR stimulation affinity, the mTOR pathway, and the IL-15 signaling pathway [Zhou, A. C et al., 2019; You, Z et al., 2021]. CD4+Trm may originate from circulating memory cells [Nguyen, Q. P et al., 2019], though this assertion lacks conclusive evidence.
Th17 cells are central to the pathogenesis of psoriasis and act through the IL-23/Th17 cell axis [Griffiths, C. E. M et al., 2021]. IL-23, produced by dendritic cells and other antigen-presenting cells, contributes to the proliferation of Th17 cells. These Th17 cells secrete various pro-inflammatory cytokines, such as IL-17A, IL-17F, TNF-α, and IL-22, which recruit neutrophils and stimulate keratinocyte hyperproliferation. This leads to aberrant differentiation, mediating the development of psoriasis [Griffiths, C. E. M et al., 2021].
Th17 cells, which are derived from CD4+ T cells, exhibit some plasticity [Zhou, L., Chong, M. M., Littman, D. R. 2009]. For example, Th17 cells may convert into Th1 cells when stimulated by IFN-[Zhou et al., 2009, Zhou et al., 2009]. Moreover, Th17 and Treg cells are known to interconvert [Qin, Y., Gao, C., Luo, J. 2022; Li, X., Jiang, M., Chen, X., Sun, W. 2022]. Treg primarily inhibit inflammation in psoriasis by acting directly on antigen-presenting and inflammatory cells through pathways involving CTLA-4, TGF-β, and GITR, and by reducing the immune response through the secretion of IL-10, IL-4, and TGF-β [Qin et al., 2022]. Stability of Treg cell number and function is closely related to relapse after psoriasis treatment [Li, X., Jiang, M., Chen, X., Sun, W. 2022]. Current biologics targeting psoriasis (TNF-α, IL-17, IL-23) have been successful in achieving stable therapy by shifting the Th17/Treg balance towards Treg cell differentiation [Whitley, S. K et al., 2022; Mehta, H et al., 2021].
Chung-Gyu Park et al. concluded that the conversion of Th17 to Treg is not a one-step process. Rather, Th17 cells are first converted to exTh17 cells during the inflammatory process. Among the surface markers of exTh17, in addition to CD161, CCR6, which maintains Th17, CXCR3 is also expressed [Jung, S et al., 2023; Yadava, K et al., 2019]. Although these exTh17 cells no longer secrete IL-17, they still retain some inflammatory properties, such as the production of cytokines including IL-2, TNF, IFN-γ, and GM-CSF [Yadava, K et al., 2019]. In 2019, Amezcua Vesely, M. C used a model of Klebsiella pneumoniae to re-validate that some of these exTh17 cells transformed from Th17 cells were functionally and behaviorally consistent with Trm and were in a stable proliferative state [Amezcua Vesely, M. C et al., 2019]. This has led to the hypothesis that some exTh17 cells are able to exhibit a memory phenotype and reside in local tissues.
Treg can be categorized into two distinct types: thymus-derived natural Tregs (tTregs) and peripheral Tregs (pTregs)[Nussbaum, L., Chen, Y. L., Ogg, G. S. 2021]. Foxp3 is a major regulator of Tregs, and the loss of Foxp3 expression in certain inflammatory environments can lead to the formation of exTreg [Shi and Chi, 2019]. In patients with psoriasis, there is an increased expression of IL-6, IL-21 and IL-23 in skin lesions. These cytokines induce STAT3/RORγT signaling, resulting in a loss of Foxp3 expression and exTreg formation [Procaccini, C et al., 2010]. Such exTreg are capable of producing IL17 but still maintain some inhibitory function [Ayyoub, M et al., 2009; Beriou, G et al., 2009]. Jeffrey A Bluestone's team employed lineage-tracking mice to study such exTreg cells, finding that even when converted to metastasis, they still express pro-inflammatory cytokines (IFN-γ and IL-17), suggesting that they function as activated memory cells [Zhou, X et al., 2009].
In summary, Th17 cells transform into exTh17, and some exTh17 may acquire a memory phenotype and reside in the skin. Some Treg form exTreg, which function as memory cells in the inflammatory process. However, the questions of whether these memory cells are tissue-resident memory T cells, and their potential relationship with each other, have not been elucidated in psoriasis.
5. The use of biological agents and the challenges of future treatment
In recent years, biologics have significantly increased the effectiveness and safety of psoriasis treatment [Grolleau et al., 2023]. However, the problem of psoriasis recurrence remains unresolved [Puig, L et al., 2022]. The interval between recurrences may decrease as the number of recurrences increases in patients [Kasprowicz-Furmańczyk, M et al., 2021]. This is inextricably linked to Trm cells, which are substantially more prevalent in psoriatic lesions than in normal skin. These cells remain at elevated levels in recurrent lesions, and are pathogenic [Kasprowicz-Furmańczyk, M et al., 2021].
During the treatment of psoriasis, Th17 cells are transformed into exTh17, which evades drug treatment and Treg cell suppression and eventually transforms into Trm, which resides in the skin in an “inactive” state. APC cells such as Langerhans cells at already remitted sites express more IL-23 in response to external stimuli and activate Trm cells. Activated CD49a+Trm secrete IFN-γ to stimulate DCs to produce IL-6 and TNF-α, which positively feeds back to antigen-presenting cells such as Langerhans cells to form auto-inflammatory microcirculation. CD49a−CD103+CD8+ Trm cells secrete more IL-17 upon activation to contribute to psoriasis-like inflammation. Basal keratin-forming cells with stem cell properties present in skin lesions maintain memory capacity in inflammation by increasing Aim2 [Zhang, Y., Xu, X., Cheng, H., Zhou, F. 2023]. Together, these factors contribute to uncontrolled KC proliferation/differentiation and persistent inflammation in psoriasis [Bromley, S. K et al., 2020; Qin, Y., Gao, C., Luo, J. 2022].
Currently, inhibitors against IL-23 show better therapeutic efficacy, exhibiting better therapeutic efficacy and durability. Notable drugs include guselkumab (282 days), lixanizumab (295 days), and utekinumab (201 days) that are all able to maintain efficacy to 200 or even close to 300 days [Tian and Lai, 2022]. Kristian Reich concluded from a two-year-long phase III clinical trial that guselkizumab has a higher long-term treatment effect, with a much higher percentage of patients (84%) maintaining PASI90 at week 48 than in other treatment groups, where 84.1% of patients still maintain PASI90 at week 252. Anti-IL-23 biologics are mostly effective and safe for patients with refractory psoriasis, especially if other biologics have failed [Reich, K et al., 2021]. In 2022, Sarah K Whitley demonstrated the ability of IL-23 inhibitors to deplete Trm cells in the skin by constructing a mouse model [Whitley, S. K et al., 2022]. Mehta analyzed the changes in the number of Trm and Treg cells at psoriatic lesions and non-lesions after IL-23 inhibitor treatment and found that IL-23 inhibitors significantly reduced Trm cells and were able to maintain the number of Treg cells [Mehta, H et al., 2021]. This provides some evidence that treatment targeting Trm is more beneficial in delaying the time to relapse.
Although the mechanism has not been elucidated, Trm cells have a definite role in psoriasis relapse. However, there are a number of problems with the isolation and culture of Trm cells. First, due to the special characteristics of the skin tissue, which is rich in adhesive bands and bridging particles, the number of isolated live cells is low making the culture of these cell populations more difficult. Second, the maintenance mechanism of Trm cells has not been thoroughly investigated, thus culture and study isolated Trm cells in vitro remains understudied. Yet, despite these challenges, there have been many attempts that yielded successful isolations and cultures in some laboratories [Du, W et al., 2021]. There is a long way to go in the research of Trm culture.
6. Concluding remarks and future perspectives
Recently, with the popularization of biological agents, psoriasis treatment has achieved good results, however, almost 100% psoriasis patients relapse after stopping medication, making relapse the biggest challenge for psoriasis treatment. Tissue-resident memory (Trm) cells infiltrating the skin are closely associated with psoriasis relapse and are likely to be the next therapeutic target. In this review, we summarized the outcome of recent research on the origin, common markers and functions of Trm cells. The conjecture that Trm may originate from exTh17 cells and exTreg cells was proposed, and may provide a reference for future studies on skin Trm, psoriasis relapse.
CRediT authorship contribution statement
Canbin Dong: Investigation, Writing – original draft. Lanmei Lin: Investigation, Writing – original draft. Juan Du: Writing – review & editing.
Declaration of competing interest
The authors have no conflict of interest to declare.
Data availability
No data was used for the research described in the article.
References
- Amezcua Vesely M.C., Pallis P., Bielecki P., Low J.S., Zhao J., Harman C.C.D., et al. Effector TH17 cells give rise to long-lived TRM cells that are essential for an immediate response against bacterial infection. Cell. 2019;178(5):1176–1188. doi: 10.1016/j.cell.2019.07.032. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyoub M., Deknuydt F., Raimbaud I., Dousset C., Leveque L., Bioley G., Valmori D. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc. Natl. Acad. Sci. U.S.A. 2009;106(21):8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr F.M., Beumer-Chuwonpad A., Kragten N.A.M., Wesselink T.H., Stark R., van Gisbergen K.P.J.M. Circulating memory CD8+ T cells are limited in forming CD103+ tissue-resident memory T cells at mucosal sites after reinfection. Eur. J. Immunol. 2021;51(1):151–166. doi: 10.1002/eji.202048737. [DOI] [PubMed] [Google Scholar]
- Beriou G., Costantino C.M., Ashley C.W., Yang L., Kuchroo V.K., Baecher-Allan C., Hafler D.A. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113(18):4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley S.K., Akbaba H., Mani V., Mora-Buch R., Chasse A.Y., Sama A., Luster A.D. CD49a regulates cutaneous resident memory CD8+ T cell persistence and response. Cell Rep. 2020;32(9) doi: 10.1016/j.celrep.2020.108085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuk S., Schlums H., Gallais Sérézal I., Martini E., Chiang S.C., et al. CD49a expression defines tissue-resident CD8+ T cells poised for cytotoxic function in human skin. Immunity. 2017;46(2):287–300. doi: 10.1016/j.immuni.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Lenz D., Köhler R., Zhang E., Cendon C., Li J., et al. Rapid isolation of functional ex vivo human skin tissue-resident memory T lymphocytes. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.624013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira C., Barros L., Baptista M., Blankenhaus B., Barros A., et al. Type 1 Treg cells promote the generation of CD8+ tissue-resident memory T cells. Nat. Immunol. 2020;21(7):766–776. doi: 10.1038/s41590-020-0674-9. [DOI] [PubMed] [Google Scholar]
- Ghaffarinia A., Ayaydin F., Póliska S., Manczinger M., Bolla B.S., et al. Psoriatic resolved skin epidermal keratinocytes retain disease-residual transcriptomic and epigenomic profiles. Int. J. Mol. Sci. 2023;24(5):4556. doi: 10.3390/ijms24054556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths C.E.M., Armstrong A.W., Gudjonsson J.E., Barker J.N.W.N. Psoriasis. Lancet (London, England) 2021;397(10281):1301–1315. doi: 10.1016/S0140-6736(20)32549-6. [DOI] [PubMed] [Google Scholar]
- Grolleau C., Le Cleach L., Shourick J., Sbidian E., Afach S. Long-term clinical trials of biologics in plaque psoriasis demonstrate heterogeneous study designs. Br. J. Dermatol. 2023;188(5):677–678. doi: 10.1093/bjd/ljac142. [DOI] [PubMed] [Google Scholar]
- Huang B., Lyu Z., Qian Q., Chen Y., Zhang J., et al. NUDT1 promotes the accumulation and longevity of CD103+ TRM cells in primary biliary cholangitis. J. Hepatol. 2022;77(5):1311–1324. doi: 10.1016/j.jhep.2022.06.014. [DOI] [PubMed] [Google Scholar]
- Jung S., Lee S., Kim H.J., Kim S., Moon J.H., Chung H., et al. Mesenchymal stem cell-derived extracellular vesicles subvert Th17 cells by destabilizing RORγt through posttranslational modification. Exp. Mol. Med. 2023;55(3):665–679. doi: 10.1038/s12276-023-00949-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprowicz-Furmańczyk M., Czerwińska J., Placek W., Owczarczyk-Saczonek A. Assessment of the tissue resident memory cells in lesional skin of patients with psoriasis and in healthy skin of healthy volunteers. Int. J. Environ. Res. Publ. Health. 2021;18(21) doi: 10.3390/ijerph182111251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney N., McKenna K. Real world use of biologic drug levels and anti-drug antibodies in patients with psoriasis - does therapeutic drug monitoring have a place in routine clinical practice? J. Dermatol. Treat. 2022;33(3):1676–1681. doi: 10.1080/09546634.2021.1898526. [DOI] [PubMed] [Google Scholar]
- Keir M., Yi Y., Lu T., Ghilardi N. The role of IL-22 in intestinal health and disease. J. Exp. Med. 2020;217(3) doi: 10.1084/jem.20192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klicznik M.M., Morawski P.A., Höllbacher B., Varkhande S.R., Motley S.J., et al. Human CD4+CD103+ cutaneous resident memory T cells are found in the circulation of healthy individuals. Sci. Immunol. 2019;4(37) doi: 10.1126/sciimmunol.aav8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Jiang M., Chen X., Sun W. Etanercept alleviates psoriasis by reducing the Th17/Treg ratio and promoting M2 polarization of macrophages. Immun. Inflamm. Dis. 2022;10(12):e734. doi: 10.1002/iid3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Wang Y., Lu X., Wang T., Li Q., et al. The inflammatory factor SNP may serve as a promising biomarker for Acitretin to Alleviate secondary failure of response to TNF-a monoclonal antibodies in psoriasis. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.937490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Zhang H., Yuan Y., He Q., Zhou J., et al. Fatty acid oxidation controls CD8+ tissue-resident memory T-cell survival in gastric Adenocarcinoma. Cancer Immunol. Res. 2020;8(4):479–492. doi: 10.1158/2326-6066.CIR-19-0702. [DOI] [PubMed] [Google Scholar]
- Mazzoni A., Maggi L., Montaini G., Ramazzotti M., Capone M., et al. Human T cells interacting with HNSCC-derived mesenchymal stromal cells acquire tissue-resident memory like properties. Eur. J. Immunol. 2020;50(10):1571–1579. doi: 10.1002/eji.202048544. [DOI] [PubMed] [Google Scholar]
- Mehta H., Mashiko S., Angsana J., Rubio M., Hsieh Y.M., et al. Differential changes in inflammatory mononuclear phagocyte and T-cell profiles within psoriatic skin during treatment with Guselkumab vs. Secukinumab. J. Invest. Dermatol. 2021;141(7):1707–1718. doi: 10.1016/j.jid.2021.01.005. e9. [DOI] [PubMed] [Google Scholar]
- Nguyen Q.P., Deng T.Z., Witherden D.A., Goldrath A.W. Origins of CD4+ circulating and tissue-resident memory T-cells. Immunology. 2019;157(1):3–12. doi: 10.1111/imm.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum L., Chen Y.L., Ogg G.S. Role of regulatory T cells in psoriasis pathogenesis and treatment. Br. J. Dermatol. 2021;184(1):14–24. doi: 10.1111/bjd.19380. [DOI] [PubMed] [Google Scholar]
- Owczarczyk Saczonek A., Krajewska-Włodarczyk M., Kasprowicz-Furmańczyk M., Placek W. Immunological memory of psoriatic lesions. Int. J. Mol. Sci. 2020;21(2):625. doi: 10.3390/ijms21020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Tian T., Park C.O., Lofftus S.Y., Mei S., Liu X., et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature. 2017;543(7644):252–256. doi: 10.1038/nature21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C., Huggins M.A., Wanhainen K.M., Knutson T.P., Lu H., et al. Engagement of the costimulatory molecule ICOS in tissues promotes establishment of CD8+ tissue-resident memory T cells. Immunity. 2022;55(1):98–114. doi: 10.1016/j.immuni.2021.11.017. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procaccini C., De Rosa V., Galgani M., Abanni L., Calì G., Porcellini A., et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33(6):929–941. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig L., Costanzo A., Muñoz-Elías E.J., Jazra M., Wegner S., et al. The biological basis of disease recurrence in psoriasis: a historical perspective and current models. Br. J. Dermatol. 2022;186(5):773–781. doi: 10.1111/bjd.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Gao C., Luo J. Metabolism characteristics of Th17 and regulatory T cells in autoimmune diseases. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.828191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich K., Warren R.B., Lebwohl M., Gooderham M., Strober B., et al. Bimekizumab versus Secukinumab in plaque psoriasis. N. Engl. J. Med. 2021;385(2):142–152. doi: 10.1056/NEJMoa2102383. [DOI] [PubMed] [Google Scholar]
- Reilly E.C., Lambert Emo K., Buckley P.M., Reilly N.S., Smith I., et al. TRM integrins CD103 and CD49a differentially support adherence and motility after resolution of influenza virus infection. Proc. Natl. Acad. Sci. U.S.A. 2020;117(22):12306–12314. doi: 10.1073/pnas.1915681117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschke R., Shapiro J.W., Yu J., Rouhani S.J., Olson D.J., et al. Checkpoint blockade-induced dermatitis and colitis are dominated by tissue-resident memory T cells and Th1/Tc1 cytokines. Cancer Immunol. Res. 2022;10(10):1167–1174. doi: 10.1158/2326-6066.CIR-22-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G.E., Harris J.E., Richmond J.M. Resident memory T cells in autoimmune skin diseases. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.652191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel J.M., Masopust D. Tissue-resident memory T cells. Immunity. 2014;41(6):886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Chi H. Metabolic control of Treg cell stability, plasticity, and tissue-specific heterogeneity. Front. Immunol. 2019;10:2716. doi: 10.3389/fimmu.2019.02716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Luo L., Lohcharoenkal W., Meisgen F., Pasquali L., Pivarcsi A., Sonkoly E. Cross-talk between IFN-γ and TWEAK through miR-149 amplifies skin inflammation in psoriasis. J. Allergy Clin. Immunol. 2021;147(6):2225–2235. doi: 10.1016/j.jaci.2020.12.657. [DOI] [PubMed] [Google Scholar]
- Szabo P.A., Miron M., Farber D.L. Location, location, location: tissue resident memory T cells in mice and humans. Sci. Immunol. 2019;4(34) doi: 10.1126/sciimmunol.aas9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D., Lai Y. The relapse of psoriasis: mechanisms and mysteries. JID Innov. : Skin Sci. Mol. Popul. Health. 2022;2(3) doi: 10.1016/j.xjidi.2022.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohyama M., Shirakata Y., Hanakawa Y., Dai X., Shiraishi K., Murakami M., et al. Bcl-3 induced by IL-22 via STAT3 activation acts as a potentiator of psoriasis-related gene expression in epidermal keratinocytes. Eur. J. Immunol. 2018;48(1):168–179. doi: 10.1002/eji.201747017. [DOI] [PubMed] [Google Scholar]
- van der Gracht E.T.I., Behr F.M., Arens R. Functional heterogeneity and therapeutic targeting of tissue-resident memory T cells. Cells. 2021;10(1):164. doi: 10.3390/cells10010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T.T., Koguchi-Yoshioka H., Watanabe R. Skin-resident memory T cells: pathogenesis and implication for the treatment of psoriasis. J. Clin. Med. 2021;10(17):3822. doi: 10.3390/jcm10173822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D.A., Borges da Silva H., Beura L.K., Peng C., Hamilton S.E., et al. The functional requirement for CD69 in establishment of resident memory CD8+ T cells varies with tissue location. J. Immunol. 2019;203(4):946–955. doi: 10.4049/jimmunol.1900052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Gehad A., Yang C., Scott L.L., Teague J.E., et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 2015;7(279) doi: 10.1126/scitranslmed.3010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wein A.N., McMaster S.R., Takamura S., Dunbar P.R., Cartwright E.K., et al. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J. Exp. Med. 2019;216(12):2748–2762. doi: 10.1084/jem.20181308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley S.K., Li M., Kashem S.W., Hirai T., Igyártó B.Z., Knizner K., et al. Local IL-23 is required for proliferation and retention of skin-resident memory TH17 cells. Sci. Immunol. 2022;7(77) doi: 10.1126/sciimmunol.abq3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadava K., Medina C.O., Ishak H., Gurevich I., Kuipers H., Shamskhou E.A., et al. Natural Tr1-like cells do not confer long-term tolerogenic memory. Elife. 2019;8 doi: 10.7554/eLife.44821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z., Li Y., Wang Q., Zhao Z., Li Y., Qian Q., et al. The clinical significance of hepatic CD69+ CD103+ CD8+ resident-memory T cells in autoimmune hepatitis. Hepatology (Baltimore, Md.) 2021;74(2):847–863. doi: 10.1002/hep.31739. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xu X., Cheng H., Zhou F. AIM2 and psoriasis. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1085448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A.C., Batista N.V., Watts T.H. 4-1BB regulates effector CD8 T cell accumulation in the lung tissue through a TRAF1-, mTOR-, and antigen-dependent mechanism to enhance tissue-resident memory T cell formation during respiratory influenza infection. J. Immunol. 2019;202(8):2482–2492. doi: 10.4049/jimmunol.1800795. [DOI] [PubMed] [Google Scholar]
- Zhou L., Chong M.M., Littman D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Zhou X., Bailey-Bucktrout S.L., Jeker L.T., Penaranda C., Martínez-Llordella M., Ashby M., et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10(9):1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.