Abstract
Herein, the dataset generated for Queeno et al. [1] is presented and described. Mammalian skeletal muscle slow (MyHC-I) fiber composition data was collated from 269 eligible studies identified via a systematic literature search and meta-analysis, following a structure similar to PRISMA [2]. Academic search systems were queried with terms relating to mammalian skeletal muscle fiber content and reference lists of selected articles were thoroughly investigated for additional studies. Eligible studies were those that provided skeletal muscle fiber composition data from mammalian species that were not subjected to experimental manipulations. Taxonomic information, sex, age, number of individuals sampled, average body mass (kg), average slow fiber content (%) of each skeletal muscle under investigation and fiber-typing methodology were collated from eligible studies when available. Muscle fiber composition data was collected from more than 200 skeletal muscles across 174 mammalian species, which will be of value to those interested in muscle physiology, interspecific muscle comparisons, and connections between muscle physiology, taxonomy, body mass, ecomorphology and locomotor strategy (among others).
Keywords: Muscle fiber composition, Myosin heavy chain I, MyHC I, Slow-twitch, Fiber typing, Interspecific muscle physiology comparison
Specifications Table
| Subject | Animal physiology |
| Specific subject area | Biology, muscle, anatomy, life sciences, meta-analysis, interspecific comparison, locomotion |
| Type of data | Tables Figures |
| How the data were acquired | A systematic literature search was conducted using academic search systems (Google Scholar, PubMed, and JSTOR) and library databases between June 1 2021 and November 30 2022 following a structure similar to PRISMA [2]. Reference lists of selected articles were also thoroughly investigated for additional studies. Data were extracted from the text, figures, tables, and supplementary materials of studies deemed eligible for the systematic review and meta-analysis (i.e. studies that provided skeletal muscle fiber composition data from mammalian species that were not subjected to experimental manipulations). |
| Data format | Secondary data Analyzed Filtered |
| Description of data collection | Taxonomic information, sex, age, number of individuals sampled, average body mass (kg), average slow fiber content (%) of each skeletal muscle under investigation and fiber-typing methodology were collated from eligible studies when available. If species body mass was not reported the mean was taken from published studies [3,4]. If muscle fiber content was reported from multiple sampling sites across a single muscle, the average across sampling sites was recorded. |
| Data source location | Eligible studies providing mammalian skeletal muscle fiber content are listed in Table 1. |
| Data accessibility | Repository name: Mendeley Data Data identification number: doi:10.17632/y47mj24ywy.3 Direct URL to data: https://data.mendeley.com/datasets/y47mj24ywy/3 |
| Related research article | S.R. Queeno, P.J. Reiser, C.M. Orr, T.D. Capellini, K.N. Sterner, M.C. O’Neill, Human and African ape myosin heavy chain content and the evolution of hominin skeletal muscle, Comp. Biochem. Physiol. A Mol. Integr. Physiol. 281 (2023) 111415. |
1. Value of the Data
-
•
This is the first meta-analysis of its kind, which compiles skeletal muscle fiber composition data across 174 mammalian species into a single, usable file.
-
•
These data will be of value to scientists interested in muscle physiology, interspecific muscle comparisons, and connections between muscle physiology, taxonomy, body mass, ecomorphology and locomotor strategy (among others).
-
•
These data highlight certain species, taxonomic orders, and muscles for which fiber composition data is lacking and needs investigation.
-
•
These data will spark interest in gathering muscle fiber composition data from currently unsampled (or underrepresented) species and muscles, generate interest in pursuing questions relating to muscle physiology and evolution, as well as analyses based on interspecific datasets.
2. Objective
Skeletal muscle slow fiber content varies across muscles and taxa and is one of the traits that distinguishes humans from other apes [1,5], yet no study to date has compiled these data into a single, usable format. The goal of this study was to compile mammalian skeletal muscle slow (MyHC-I) fiber composition data from published, peer-reviewed articles for interspecific comparison and analysis. This is the dataset referred to in Queeno et al. [1].
3. Data Description
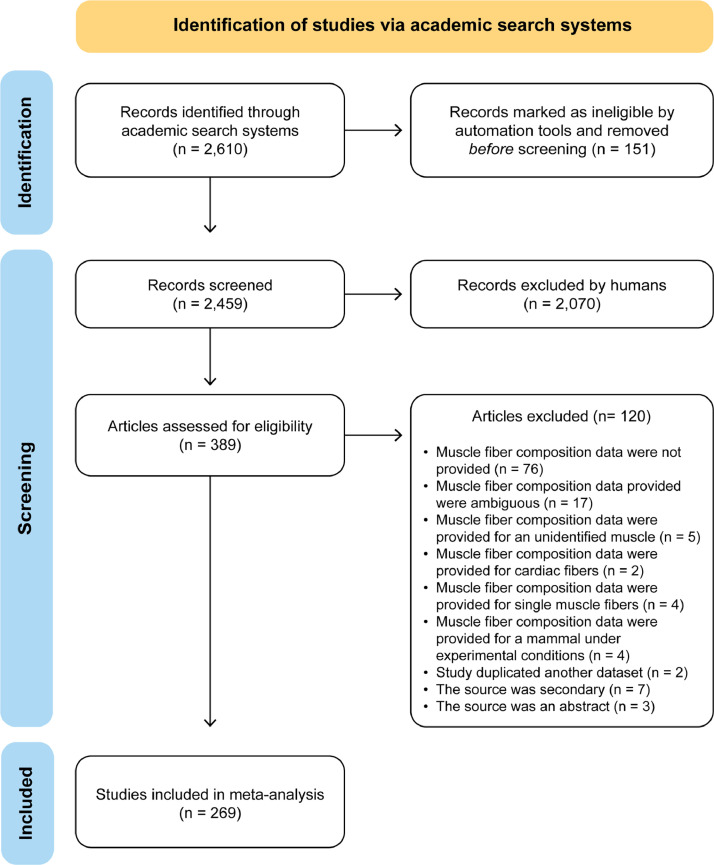
A total of 2610 studies were found from academic search systems (Google Scholar, PubMed, and JSTOR) and library databases according to the selection criteria (Fig. 1). Of these, 2459 studies were selected for screening. After reading the abstract, 389 articles were selected for full-text eligibility assessment. In total, 269 studies fully met the selection criteria and were selected for inclusion in the meta-analysis (Table 1).
Fig. 1.
PRISMA flow diagram adopted from Moher et al. [2] describing the systematic review and meta-analysis workflow.
Table 1.
Citations for the 269 studies that met the selection criteria and were included in the meta-analysis.
| Number | Citation |
|---|---|
| 1 | Acevedo, L.M., Rivero, J.L.L., 2006. New insights into skeletal muscle fibre types in the dog with particular focus towards hybrid myosin phenotypes. Cell Tissue Res 323, 283–303. https://doi.org/10.1007/s00441-005-0057-4 |
| 2 | Acosta, L., Roy, R.R., 1987. Fiber-type composition of selected hindlimb muscles of a primate (cynomolgus monkey). Anat Rec 218, 136–141. https://doi.org/10.1002/ar.1092180207 |
| 3 | Agostini, de Martino, L., Soltau, B., Hasselbach, W., 1991. The Modulation of the Calcium Transport by Skeletal Muscle Sarcoplasmic Reticulum in the Hibernating European Hamster. Zeitschrift für Naturforschung C 46, 1109–1126. https://doi.org/10.1515/znc-1991-11-1229 |
| 4 | Aigner, S., Gohlsch, B., Hämaläinen, N., Staron, R.S., Uber, A., Wehrle, U., Pette, D., 1993. Fast myosin heavy chain diversity in skeletal muscles of the rabbit: heavy chain IId, not IIb predominates. Eur J Biochem 211, 367–372. https://doi.org/10.1111/j.1432-1033.1993.tb19906.x |
| 5 | Almeida-Silveira, M.I., Pérot, C., Pousson, M., Goubel, F., 1994. Effects of stretch-shortening cycle training on mechanical properties and fibre type transition in the rat soleus muscle. Pflug Arch Eur J Physiol 427, 289–94. https://doi.org/10.1007/BF00374536 |
| 6 | Alnaqeeb, M.A., Al-Baker, E., 1994. Muscle fiber type, number and size in the EDL and soleus of Jaculus jaculus. J. Univ. Kuwait (Sci.) 21, 231–241. |
| 7 | Alvarez, G.I., Díaz, A.O., Longo, M. v., Becerra, F., Vassallo, A.I., 2012. Histochemical and Morphometric Analyses of the Musculature of the Forelimb of the Subterranean Rodent Ctenomys talarum (Octodontoidea). J Vet Med C: Anat Histol Embryol41, 317–325. https://doi.org/10.1111/j.1439-0264.2012.01137.x |
| 8 | Anapol, F.C., Jungers, W.L., 1986. Architectural and histochemical diversity within the quadriceps femoris of the brown lemur (Lemur fulvus). Am J Phys Anthropol 69, 355–375. https://doi.org/10.1002/ajpa.1330690308 |
| 9 | Ansved, T., 1995. Effects of immobilization on the rat soleus muscle in relation to age. Acta Physiol Scand 154, 291–302. https://doi.org/10.1111/j.1748-1716.1995.tb09913.x |
| 10 | Arbanas, J., Klasan, G.S., Nikolić, M., Cvijanović, O., Malnar, D., 2010. Immunohistochemical analysis of the human psoas major muscle with regards to the body side and aging. Coll Antropol 34 Suppl 2, 169–73. |
| 11 | Arbanas, J., Klasan, G.S., Nikolic, M., Jerkovic, R., Miljanovic, I., Malnar, D., 2009. Fibre type composition of the human psoas major muscle with regard to the level of its origin. J Anat 215, 636–41. https://doi.org/10.1111/j.1469-7580.2009.01155.x |
| 12 | Ariano, M.A., Edgerton, V.R., Armstrong, R.B., 1973. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem 21, 51–55. https://doi.org/10.1177/21.1.51 |
| 13 | Armstrong, R.B., Ianuzzo, C.D., Kunz, T.H., 1977. Histochemical and biochemical properties of flight muscle fibers in the little brown bat,Myotis lucifugus. J Comp Physiol B: Biochem Syst Environ Physiol 119, 141–154. https://doi.org/10.1007/BF00686562 |
| 14 | Armstrong, R.B., Phelps, R.O., 1984. Muscle fiber type composition of the rat hindlimb. Am J Anat 171, 259–272. https://doi.org/10.1002/aja.1001710303 |
| 15 | Armstrong, R.B., Saubert, C.W., Seeherman, H.J., Taylor, C.R., 1982. Distribution of fiber types in locomotory muscles of dogs. Am J Anat 163, 87–98. https://doi.org/10.1002/aja.1001630107 |
| 16 | Asmussen, G., Gaunitz, U., 1989. Temperature effects on isometric contractions of slow and fast twitch muscles of various rodents–dependence on fibre type composition: a comparative study. Biomed Biochim Acta 48, S536-41. |
| 17 | Augusto, V., Padovani, C.R., Rocha Campos, G.E., 2004. Skeletal muscle fiber types in C57Bl6J mice. Braz J Morphol Sci 21, 89–94. |
| 18 | Bao, T., Han, H., Li, B., Zhao, Y., Bou, G., Zhang, X., Du, M., Zhao, R., Mongke, T., Laxima, Ding, W., Jia, Z., Dugarjaviin, M., Bai, D., 2020. The distinct transcriptomes of fast-twitch and slow-twitch muscles in Mongolian horses. Comp Biochem Physiol Part D Genomics Proteomics 33, 100649. https://doi.org/10.1016/j.cbd.2019.100649 |
| 19 | Bär, A., Pette, D., 1988. Three fast myosin heavy chains in adult rat skeletal muscle. FEBS Lett 235, 153–155. https://doi.org/10.1016/0014-5793(88)81253-5 |
| 20 | Beecher, G.R., Cassens, R.G., Hoekstra, W.G., Briskey, E.J., 1965. Red and White Fiber Content and Associated Post-Mortem Properties of Seven Porcine Muscles. J Food Sci 30, 969–976. https://doi.org/10.1111/j.1365-2621.1965.tb01872.x |
| 21 | Bello, M.A., Roy, R.R., Martin, T.P., Goforth, H.W., Edgerton, V.R., 1985. Axial musculature in the dolphin (Tursiops truncatus): Some architectural and histochemical characteristics. Mar Mamm Sci 1, 324–336. https://doi.org/10.1111/j.1748-7692.1985.tb00019.x |
| 22 | Bloemberg, D., Quadrilatero, J., 2012. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7. https://doi.org/10.1371/journal.pone.0035273 |
| 23 | Bonington, A., Whitmore, I., Mahon, M., 1987. A histological and histochemical study of the cricopharyngeus muscle in the guinea-pig. J Anat 153, 151–61. |
| 24 | Boyd-Clark, L.C., Briggs, C.A., Galea, M.P., 2001. Comparative histochemical composition of muscle fibres in a pre- and a postvertebral muscle of the cervical spine. J Anat 199, 709–716. https://doi.org/10.1017/S0021878201008706 |
| 25 | Brandstetter, A.M., Picard, B., Geay, Y., 1998. Muscle fibre characteristics in four muscles of growing bulls I. Postnatal differentiation, Livest Prod Sci. |
| 26 | Brasseur, J.E., Curtis, R.L., Mellender, J.W., Rimm, A.A., Melvin, J.L., Sulaiman, A.R., 1987. Systematic distribution of muscle fiber types in the medical gastrocnemius of the laboratory mouse: A morphometric analysis. Anat Rec 218, 396–401. https://doi.org/10.1002/ar.1092180407 |
| 27 | Braund, K.G., Amling, K.A., Mehta, J.R., Steiss, J.E., Scholz, C., 1995. Histochemical and morphometric study of fiber types in ten skeletal muscles of healthy young adult cats. Am J Vet Res 56, 349–57. |
| 28 | Braund, K.G., Mcguire, J.A., Lincoln, C.E., 1982. Observations on Normal Skeletal Muscle of Mature Dogs: A Cytochemical, Histochemical, and Morphometric Study, Vet Pathol. |
| 29 | Brigham, R.M., Ianuzzo, C.D., Hamilton, N., Fenton, M.B., 1990. Histochemical and biochemical plasticity of muscle fibers in the little brown bat (Myotis lucifugus). J Comp Physiol B: Biochem Syst Environ Physiol 160, 183–186. https://doi.org/10.1007/BF00300951 |
| 30 | Burke, R.E., 1967. Motor unit types of cat triceps surae muscle. J Physiol 193, 141–160. https://doi.org/10.1113/jphysiol.1967.sp008348 |
| 31 | Burke, R.E., Levine, D.N., Salcman, M., Tsairis, P., 1974. Motor units in cat soleus muscle: physiological, histochemical and morphological characteristics. J Physiol 238, 503–514. https://doi.org/10.1113/jphysiol.1974.sp010540 |
| 32 | Burke, R.E., Levine, D.N., Tsairis, P., Zajac, F.E., 1973. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol 234, 723–748. https://doi.org/10.1113/jphysiol.1973.sp010369 |
| 33 | Burkholder, T.J., Fingado, B., Baron, S., Lieber, R.L., 1994. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol 221, 177–190. https://doi.org/10.1002/jmor.1052210207 |
| 34 | Carlson, H., 1978. Histochemical fiber composition of lumbar back muscles in the cat. Acta Physiol Scand 103, 198–209. https://doi.org/10.1111/j.1748-1716.1978.tb06207.x |
| 35 | Casinos, A., Milne, N., Jouffroy, F.K., Médina, M.F., 2016. Muscle fibre types in the reduced forelimb and enlarged hindlimb of the quokka (Setonix brachyurus, Macropodidae). Aust J Zool 64, 277–284. https://doi.org/10.1071/ZO15055 |
| 36 | Cebesoy, S., 2009. Morphology and histochemistry of primary flight muscles in Rhinolophus mehelyii. Afr J Biotechnol 8, 1160–1164. |
| 37 | Cebesoy, S., Ayvali, C., 2003. Morphology and histochemistry of primary flight muscles in Myotis myotis 16, 245–252. |
| 38 | Chanaud, C.M., Pratt, C.A., Loeb, G.E., 1991. Functionally complex muscles of the cat hindlimb. Exp Brain Res 85, 300–313. https://doi.org/10.1007/BF00229408 |
| 39 | Chang, H., Jiang, S., Ma, X., Peng, X., Zhang, J., Wang, Z., Xu, S., Wang, H., Gao, Y., 2018. Proteomic analysis reveals the distinct energy and protein metabolism characteristics involved in myofiber type conversion and resistance of atrophy in the extensor digitorum longus muscle of hibernating Daurian ground squirrels. Comp Biochem Physiol Part D Genomics Proteomics 26, 20–31. https://doi.org/10.1016/j.cbd.2018.02.002 |
| 40 | Choi, H., Selpides, P.-J.I., Nowell, M.M., Rourke, B.C., 2009. Functional overload in ground squirrel plantaris muscle fails to induce myosin isoform shifts. Am J Physiol Regul Integr Comp Physiol 297, R578-86. https://doi.org/10.1152/ajpregu.00236.2009 |
| 41 | Chopard, A., Pons, F., Marini, J.F., 2001. Cytoskeletal protein contents before and after hindlimb suspension in a fast and slow rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 280, R323-30. https://doi.org/10.1152/ajpregu.2001.280.2.R323 |
| 42 | Collatos, T.C., Edgerton, V.R., Smith, J.L., Botterman, B.R., 1977. Contractile properties and fiber type compositions of flexors and extensors of elbow joint in cat: implications for motor control. J Neurophysiol 40, 1292–1300. https://doi.org/10.1152/jn.1977.40.6.1292 |
| 43 | Cordonnier, C., Stevens, L., Picquet, F., Mounier, Y., 1995. Structure-function relationship of soleus muscle fibres from the rhesus monkey. Pflug Arch Eur J Physiol 430, 19–25. https://doi.org/10.1007/BF00373835 |
| 44 | Cornachione, A.S., Benedini-Elias, P.C.O., Polizello, J.C., Carvalho, L.C., Mattiello-Sverzut, A.C., 2011. Characterization of fiber types in different muscles of the hindlimb in female weanling and adult wistar rats. Acta Histochem Cytochem 44, 43–50. https://doi.org/10.1267/ahc.10031 |
| 45 | Cotton, C.J., Harlow, H.J., 2010. Avoidance of Skeletal Muscle Atrophy in Spontaneous and Facultative Hibernators. Physiol Biochem Zool 83, 551–560. https://doi.org/10.1086/650471 |
| 46 | Curry, J.W., Hohl, R., Noakes, T.D., Kohn, T.A., 2012. High oxidative capacity and type IIx fibre content in springbok and fallow deer skeletal muscle suggest fast sprinters with a resistance to fatigue. J Exp Biol 215, 3997–4005. https://doi.org/10.1242/jeb.073684 |
| 47 | Dada, S., Henning, F., Feldmann, D.C., Kohn, T.A., 2018. Baboon (Papio ursinus) single fibre contractile properties are similar to that of trained humans. J Muscle Res Cell Motil 39, 189–199. https://doi.org/10.1007/s10974-019-09509-x |
| 48 | de A. Braga, S., G. F. Padilha, F., M. R. Ferreira, A., 2016. Evaluation of Muscle Fiber Types in German Shepherd Dogs of Different Ages. Anat Rec 299, 1540–1547. https://doi.org/10.1002/ar.23464 |
| 49 | de Diego, M., Casado, A., Gómez, M., Martín, J., Pastor, J.F., Potau, J.M., 2020. Structural and molecular analysis of elbow flexor muscles in modern humans and common chimpanzees. Zoomorphology 139, 277–290. https://doi.org/10.1007/s00435-020-00482-5 |
| 50 | Delp, M.D., Duan, C., 1996. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80, 261–270. https://doi.org/10.1152/jappl.1996.80.1.261 |
| 51 | Dennington, S., Baldwin, J., 1988. Biochemical Correlates of Energy-Metabolism in Muscles Used to Power Hopping by Kangaroos. Aust J Zool 36, 229. https://doi.org/10.1071/ZO9880229 |
| 52 | Donselaar, Y., Eerbeek, O., Kernell, D., Verhey, B.A., 1987. Fibre sizes and histochemical staining characteristics in normal and chronically stimulated fast muscle of cat. J Physiol 382, 237–54. https://doi.org/10.1113/jphysiol.1987.sp016365 |
| 53 | Edgerton, V.R., Barnard, R.J., Peter, J.B., Maier, A., Simpson, D.R., 1975a. Properties of immobilized hind-limb muscles of the Galago senegalensis. Exp Neurol 46, 115–131. https://doi.org/10.1016/0014-4886(75)90036-9 |
| 54 | Edgerton, V.R., Smith, J.L., Simpson, D.R., 1975b. Muscle fibre type populations of human leg muscles. Histochem J 7, 259–266. https://doi.org/10.1007/BF01003594 |
| 55 | Edström, L., Lindquist, C., 1973. Histochemical fiber composition of some facial muscles in the cat in relation to their contraction properties. Acta Physiol Scand 89, 491–503. https://doi.org/10.1111/j.1748-1716.1973.tb05543.x |
| 56 | Edström, L., Nyström, B., 1969. Histochemical types and sizes of fibres in normal human muscles. A biopsy study. Acta Neurol Scand 45, 257–69. https://doi.org/10.1111/j.1600-0404.1969.tb01238.x |
| 57 | Egginton, S., 1990. Numerical and areal density estimates of fibre type composition in a skeletal muscle (rat extensor digitorum longus). J Anat 168, 73–80. |
| 58 | Eizema, K., van den Burg, M., Kiri, A., Dingboom, E.G., van Oudheusden, H., Goldspink, G., Weijs, W.A., 2003. Differential expression of equine myosin heavy-chain mRNA and protein isoforms in a limb muscle. J Histochem Cytochem 51, 1207–16. https://doi.org/10.1177/002215540305100911 |
| 59 | Eizema, K., van den Burg, M.M.M., de Jonge, H.W., Dingboom, E.G., Weijs, W.A., Everts, M.E., 2005. Myosin heavy chain isoforms in equine gluteus medius muscle: comparison of mRNA and protein expression profiles. J Histochem Cytochem 53, 1383–90. https://doi.org/10.1369/jhc.4A6609.2005 |
| 60 | Eng, C.M., Smallwood, L.H., Rainiero, M.P., Lahey, M., Ward, S.R., Lieber, R.L., 2008. Scaling of muscle architecture and fiber types in the rat hindlimb. J Exp Biol 211, 2336–2345. https://doi.org/10.1242/jeb.017640 |
| 61 | English, A.W., Letbetter, W.D., 1982. A histochemical analysis of identified compartments of cat lateral gastrocnemius muscle. Anat Rec 204, 123–30. https://doi.org/10.1002/ar.1092040205 |
| 62 | Enríquez, V., Granados, S., Arias, M.P., Calderón, J.C., 2015. Muscle Fiber Types of Gluteus Medius in the Colombian Creole Horse. J Equine Vet Sci 35, 524–530. https://doi.org/10.1016/j.jevs.2015.02.010 |
| 63 | Essén-Gustavsson, B., Fjelkner-Modig, S., 1985. Skeletal muscle characteristics in different breeds of pigs in relation to sensory properties of meat. Meat Sci 13, 33–47. https://doi.org/10.1016/S0309-1740(85)80003-6 |
| 64 | Essén-Gustavsson, B., Rehbinder, C., 1985. Skeletal muscle characteristics of reindeer (Rangifer tarandus L.). Comp Biochem Physiol A Physiol 82, 675–679. https://doi.org/10.1016/0300-9629(85)90450-5 |
| 65 | Feng, X., Zhang, T., Xu, Z., Choi, S.J., Qian, J., Furdui, C.M., Register, T.C., Delbono, O., 2012. Myosin heavy chain isoform expression in the Vastus Lateralis muscle of aging African green vervet monkeys. Exp Gerontol 47, 601–607. https://doi.org/10.1016/j.exger.2012.05.007 |
| 66 | Fitts, R.H., Bodine, S.C., Romatowski, J.G., Widrick, J.J., 1998. Velocity, force, power, and Ca2+ sensitivity of fast and slow monkey skeletal muscle fibers. J Appl Physiol 84, 1776–87. https://doi.org/10.1152/jappl.1998.84.5.1776 |
| 67 | Foehring, R.C., Hermanson, J.W., 1984. Morphology and Histochemistry of Flight Muscles in Free-Tailed Bats, Tadarida brasiliensis. J Mammal 65, 388–394. https://doi.org/10.2307/1381084 |
| 68 | Ford, D.M., Bagnall, K.M., McFadden, K.D., Reid, D.C., 1986. A Comparison of Muscle Fiber Characteristics at Different Levels of the Vertebral Column in the Rhesus Monkey. Cells Tissues Organs 126, 163–166. https://doi.org/10.1159/000146208 |
| 69 | Francisco, C.L., Jorge, A.M., Dal-Pai-Silva, M., Carani, F.R., Cabeço, L.C., Silva, S.R., 2011. Muscle fiber type characterization and myosin heavy chain (MyHC) isoform expression in Mediterranean buffaloes. Meat Sci 88, 535–541. https://doi.org/10.1016/j.meatsci.2011.02.007 |
| 70 | Fuentes, I., Cobos, A.R., Segade, L.A.G., 1998. Muscle fibre types and their distribution in the biceps and triceps brachii of the rat and rabbit. J Anat 192, 203–210. https://doi.org/10.1046/j.1469-7580.1998.19220203.x |
| 71 | Gao, Y.F., Wang, J., Wang, H.P., Feng, B., Dang, K., Wang, Q., Hinghofer-Szalkay, H.G., 2012. Skeletal muscle is protected from disuse in hibernating dauria ground squirrels.Comp Biochem Physiol A Mol Integr Physiol 161, 296–300. https://doi.org/10.1016/j.cbpa.2011.11.009 |
| 72 | Gellman, K.S., Bertram, J.E.A., Hermanson, J.W., 2002. Morphology, histochemistry, and function of epaxial cervical musculature in the horse (Equus caballus). J Morphol 251, 182–194. https://doi.org/10.1002/jmor.1082 |
| 73 | Gibson, M.C., Schultz, E., 1982. The distribution of satellite cells and their relationship to specific fiber types in soleus and extensor digitorum longus muscles. Anat Rec 202, 329–337. https://doi.org/10.1002/ar.1092020305 |
| 74 | Gillespie, M.J., Gordon, T., Murphy, P.R., 1987. Motor units and histochemistry in rat lateral gastrocnemius and soleus muscles: evidence for dissociation of physiological and histochemical properties after reinnervation. J Neurophysiol 57, 921–937. https://doi.org/10.1152/jn.1987.57.4.921 |
| 75 | Goldstein, B., 1971. Heterogeneity of Muscle Fibers in Some Burrowing Mammals. J Mammal 52, 515–527. https://doi.org/10.2307/1378586 |
| 76 | Gómez, M., Casado, A., de Diego, M., Pastor, J.F., Potau, J.M., 2022. Anatomical and molecular analyses of the deltoid muscle in chimpanzees (Pan troglodytes) and modern humans (Homo sapiens): Similarities and differences due to the uses of the upper extremity. Am J Primatol 84. https://doi.org/10.1002/ajp.23390 |
| 77 | Gonyea, W.J., Ericson, G.C., 1977. Morphological and histochemical organization of the flexor carpi radialis muscle in the cat. Am J Anat 148, 329–344. https://doi.org/10.1002/aja.1001480304 |
| 78 | Gorza, L., 1990. Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J Histochem Cytochem 38, 257–265. https://doi.org/10.1177/38.2.2137154 |
| 79 | Goto, M., Itamoto, K., Tani, Y., Miyata, H., Kihara, I., Mori, F., Tajima, T., Wada, N., 2013a. Distribution of Muscle Fibers in Skeletal Muscles of the African Elephant (Loxodonta africana africana). Mammal Study 38, 135. https://doi.org/10.3106/041.038.0210 |
| 80 | Goto, M., Kawai, M., Nakata, M., Itamoto, K., Miyata, H., Ikebe, Y., Tajima, T., Wada, N., 2013b. Distribution of muscle fibers in skeletal muscles of the cheetah (Acinonyx jubatus). Mamm Biol 78, 127–133. https://doi.org/10.1016/j.mambio.2012.07.001 |
| 81 | Gotoh, T., 2003. Histochemical properties of skeletal muscles in Japanese cattle and their meat production ability, Anim Sci J. |
| 82 | Gray, S.D., Renkin, E.M., 1978. Microvascular supply in relation to fiber metabolic type in mixed skeletal muscles of rabbits. Microvasc Res 16, 406–425. https://doi.org/10.1016/0026-2862(78)90073-0 |
| 83 | Graziotti, G.H., Chamizo, V.E., Ríos, C., Acevedo, L.M., Rodríguez-Menéndez, J.M., Victorica, C., Rivero, J.L.L., 2012. Adaptive functional specialisation of architectural design and fibre type characteristics in agonist shoulder flexor muscles of the llama, Lama glama. J Anat 221, 151–163. https://doi.org/10.1111/j.1469-7580.2012.01520.x |
| 84 | Graziotti, G.H., Palencia, P., Delhon, G., Rivero, J.L.L., 2004. Neuromuscular partitioning, architectural design, and myosin fiber types of the M. vastus lateralis of the llama (Lama glama). J Morphol 262, 667–681. https://doi.org/10.1002/jmor.10268 |
| 85 | Graziotti, G.H., Ríos, C.M., Rivero, J.-L.L., 2001. Evidence for Three Fast Myosin Heavy Chain Isoforms in Type II Skeletal Muscle Fibers in the Adult Llama ( Lama glama ), J Histochem Cytochem. |
| 86 | Green, H.J., Klug, G.A., Reichmann, H., Seedorf, U., Wiehrer, W., Pette, D., 1984. Exercise-induced fibre type transitions with regard to myosin, parvalbumin, and sarcoplasmic reticulum in muscles of the rat. Pflug Arch Eur J Physiol 400, 432–8. https://doi.org/10.1007/BF00587545 |
| 87 | Grotmol, S., Totland, G.K., Kryvi, H., Breistøl, A., Essén-Gustavsson, B., Lindholm, A., 2002. Spatial distribution of fiber types within skeletal muscle fascicles from standardbred horses. Anat Rec 268, 131–136. https://doi.org/10.1002/ar.10140 |
| 88 | Hämäläinen, N., Pette, D., 1993. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J Histochem Cytochem 41, 733–743. https://doi.org/10.1177/41.5.8468455 |
| 89 | Hansen, S., Cutts, J.H., Krause, W.J., Cutts, J.H., 1987. Distribution of fibre types in thirty-seven muscles of Didelphis virginiana. Anat Anz 164, 153–8. |
| 90 | Hazimihalis, P.J., Gorvet, M.A., Butcher, M.T., 2013. Myosin Isoform Fiber Type and Fiber Size in the Tail of the Virginia Opossum (Didelphis virginiana). Anat Rec 296, 96–107. https://doi.org/10.1002/ar.22614 |
| 91 | Hemingway, H.W., Burrows, A.M., Omstead, K.M., Zohdy, S., Pastor, J.F., Muchlinski, M.N., 2020. Vertical Clinging and Leaping Ahead: How Bamboo Has Shaped the Anatomy and Physiology of Hapalemur. Anat Rec 303, 295–307. https://doi.org/10.1002/ar.24183 |
| 92 | Hena, S., Sonfada, M., Shehu, S., Jibir, M., Bello, A., Omirinde, J., Gosomji, I., 2018. Determination of the Proportions of Muscle Fibre Types from Selected Muscles of the Forelimb: A Comparative Study of Cattle (Bos taurus indicus) and One-humped Camel (Camelus dromedaries). J Vet Anat 11, 39–52. https://doi.org/10.21608/jva.2018.45055 |
| 93 | Hermanson, J.W., Cobb, M.A., Schutt, W.A., Muradali, F., Ryan, J.M., 1993. Histochemical and myosin composition of vampire bat (Desmodus rotundus) pectoralis muscle targets a unique locomotory niche. J Morphol 217, 347–356. https://doi.org/10.1002/jmor.1052170309 |
| 94 | Hermanson, J.W., Foehring, R.C., 1988. Histochemistry of flight muscles in the jamaican fruit bat, Artibeus jamaicensis: Implications for motor control. J Morphol 196, 353–362. https://doi.org/10.1002/jmor.1051960308 |
| 95 | Hermanson, J.W., LaFramboise, W.A., Daood, M.J., 1991. Uniform myosin isoforms in the flight muscles of little brown bats,Myotis lucifugus. J Exp Zool 259, 174–180. https://doi.org/10.1002/jez.1402590205 |
| 96 | Hermanson, J.W., Ryan, J.M., Cobb, M.A., Bentley, J., Schutts, Jr., W.A., 1998. Histochemical and electrophoretic analysis of the primary flight muscle of several phyllostomid bats. Can J Zool 76, 1983–1992. https://doi.org/10.1139/z98-158 |
| 97 | Hershey, J.D., Robbins, C.T., Nelson, O.L., Lin, D.C., 2008. Minimal seasonal alterations in the skeletal muscle of captive brown bears. Physiol Biochem Zool 81, 138–147. https://doi.org/10.1086/524391 |
| 98 | Hesse, B., Fischer, M.S., Schilling, N., 2010. Distribution pattern of muscle fiber types in the perivertebral musculature of two different sized species of mice. Anat Rec 293, 446–63. https://doi.org/10.1002/ar.21090 |
| 99 | Hesse, B., Fröber, R., Fischer, M.S., Schilling, N., 2013. Functional differentiation of the human lumbar perivertebral musculature revisited by means of muscle fibre type composition. Ann Anat 195, 570–580. https://doi.org/10.1016/j.aanat.2013.07.003 |
| 100 | Hitomi, Y., Kizaki, T., Watanabe, S., Matsumura, G., Fujioka, Y., Haga, S., Izawa, T., Taniguchi, N., Ohno, H., 2005. Seven skeletal muscles rich in slow muscle fibers may function to sustain neutral position in the rodent hindlimb. Comp Biochem Physiol B Biochem Mol Biol 140, 45–50. https://doi.org/10.1016/j.cbpc.2004.09.021 |
| 101 | Ho, K.W., Heusner, W.W., van Huss, J., van Huss, W.D., 1983. Postnatal muscle fibre histochemistry in the rat. J Embryol Exp Morphol 76, 37–49. |
| 102 | Hochachka, P.W., Foreman III, R.A., 1993. Phocid and cetacean blueprints of muscle metabolism. Can J Zool 71, 2089–2098. https://doi.org/10.1139/z93-294 |
| 103 | Howells, K.F., Jordan, T.C., Howells, J.D., 1978. Myofibril content of histochemical fibre types in rat skeletal muscle. Acta Histochem 63, 177–182. https://doi.org/10.1016/S0065-1281(78)80023-3 |
| 104 | Huq, E., 2013. Physiological, Histological, and Mechanical Characteristics of Selected Epaxial Muscles in Primates. State University of New York at Stony Brook, Stony Brook. |
| 105 | Huq, E., Taylor, A.B., Su, Z., Wall, C.E., 2018. Fiber type composition of epaxial muscles is geared toward facilitating rapid spinal extension in the leaper Galago senegalensis. Am J Phys Anthropol 166, 95–106. https://doi.org/10.1002/ajpa.23405 |
| 106 | Hwang, K., Huan, F. and Kim, D.J., 2011. Muscle fiber types of human orbicularis oculi muscle. J Craniofac Surg 22, 1827-1830. https://doi.org/10.1097/scs.0b013e31822e8468 |
| 107 | Hyatt, J.P.K., Roy, R.R., Stuart, R., Talmadge, R.J., 2010. Myosin heavy chain composition of tiger (Panthera tigris) and cheetah (Acinonyx jubatus) hindlimb muscles. J Exp Zool A Ecol Genet Physiol 313, 45–57. https://doi.org/10.1002/jez.574 |
| 108 | Ichikawa, H., Matsuo, T., Higurashi, Y., Nagahisa, H., Miyata, H., Sugiura, T., Wada, N., 2019. Characteristics of Muscle Fiber‐Type Distribution in Moles. Anat Rec 302, 1010–1023. https://doi.org/10.1002/ar.24008 |
| 109 | Ito, J., 1998. Fiber Type Composition of Abdominal Muscles in Japanese Macaques(Macaca fuscata). Okajimas Folia Anat Jpn 74, 199–205. https://doi.org/10.2535/ofaj1936.74.6_199 |
| 110 | Johnson, M.A., Polgar, J., Weightman, D., Appleton, D., 1973. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18, 111–129. https://doi.org/10.1016/0022-510X(73)90023-3 |
| 111 | Jørgensen, K., Nicholaisen, T., Kato, M., 1993. Muscle fiber distribution, capillary density, and enzymatic activities in the lumbar paravertebral muscles of young men. Spine J 18, 1439–50. |
| 112 | Jouffroy, F.K., Médina, M.F., 1996. Developmental Changes in the Fibre Composition of Elbow, Knee, and Ankle Extensor Muscles in Cercopithecid Monkeys. Folia Primatologica 66, 55–67. https://doi.org/10.1159/000157185 |
| 113 | Jouffroy, F.K., Medina, M.F., Renous, S., Gasc, J.-P., 2003. Immunocytochemical characteristics of elbow, knee and ankle muscles of the five-toed jerboa (Allactaga elater). J Anat 202, 373–386. https://doi.org/10.1046/j.1469-7580.2003.00167.x |
| 114 | Jouffroy, F.K., Stern, J.T., Medina, M.F., Larson, S.G., 1999. Function and Cytochemical Characteristics of Postural Limb Muscles of the Rhesus Monkey: A Telemetered EMG and Immunofluorescence Study. Folia Primatol 70, 235-253. |
| 115 | Jürgens, K.D., 2002. Etruscan shrew muscle: the consequences of being small. J Exp Biol 205, 2161–2166. https://doi.org/10.1242/jeb.205.15.2161 |
| 116 | Kadim, I.T., Mahgoub, O., Al-Marzooqi, W., Khalaf, S.K., Mansour, M.H., Al-Sinani, S.S.H., Al-Amri, I.S., 2009. Effects of Electrical Stimulation on Histochemical Muscle Fiber Staining, Quality, and Composition of Camel and Cattle Longissimus thoracis Muscles. J Food Sci 74, S44–S52. https://doi.org/10.1111/j.1750-3841.2008.00992.x |
| 117 | Kanatous, S.B., Davis, R.W., Watson, R., Polasek, L., Williams, T.M., Mathieu-Costello, O., 2002. Aerobic capacities in the skeletal muscles of Weddell seals: key to longer dive durations? J Exp Biol 205, 3601–3608. https://doi.org/10.1242/jeb.205.23.3601 |
| 118 | Karlsson, A., Enfält, A.-C., Essén-Gustavsson, B., Lundström, K., Rydhmer, L., Stern, S., 1993. Muscle histochemical and biochemical properties in relation to meat quality during selection for increased lean tissue growth rate in pigs. J Anim Sci 71, 930–938. https://doi.org/10.2527/1993.714930x |
| 119 | Kawai, M., Minami, Y., Sayama, Y., Kuwano, A., Hiraga, A., Miyata, H., 2009. Muscle Fiber Population and Biochemical Properties of Whole Body Muscles in Thoroughbred Horses. Anat Rec 292, 1663–1669. https://doi.org/10.1002/ar.20961 |
| 120 | Kielhorn, C.E., Dillaman, R.M., Kinsey, S.T., McLellan, W.A., Mark Gay, D., Dearolf, J.L., Ann Pabst, D., 2013. Locomotor muscle profile of a deep (Kogia breviceps) versus shallow (Tursiops truncatus) diving cetacean. J Morphol 274, 663–675. https://doi.org/10.1002/jmor.20124 |
| 121 | Kiessling, K.-H., Kiessling, A., 1984. Fibre composition and enzyme activities in five different muscles from the svalbard reindeer. Comp Biochem Physiol A Physiol 77, 75–78. https://doi.org/10.1016/0300-9629(84)90014-8 |
| 122 | Kimura, T., 2002. Composition of psoas major muscle fibers compared among humans, orangutans, and monkeys. Z Morphol Anthropol 83, 305–14. |
| 123 | Kimura, T., 1994. Muscle Fiber Composition of the Anterior Tibial Muscle in Several Species of Prosimians. Primate Res 10, 25–31. https://doi.org/10.2354/psj.10.25 |
| 124 | Kimura, T., Kumakura, H., Inokuchi, S., Ishida, H., 1987. Composition of muscle fibers in the slow loris, using the m. biceps brachii as an example. Primates 28, 525–532. https://doi.org/10.1007/BF02380866 |
| 125 | Kohn, T.A., 2014. Insights into the skeletal muscle characteristics of three southern African antelope species. Biol Open 3, 1037–1044. https://doi.org/10.1242/bio.20149241 |
| 126 | Kohn, T.A., Burroughs, R., Hartman, M.J., Noakes, T.D., 2011a. Fiber type and metabolic characteristics of lion (Panthera leo), caracal (Caracal caracal) and human skeletal muscle. Comp Biochem Physiol A Mol Integr Physiol 159, 125–133. https://doi.org/10.1016/j.cbpa.2011.02.006 |
| 127 | Kohn, T.A., Curry, J.W., Noakes, T.D., 2011b. Black wildebeest skeletal muscle exhibits high oxidative capacity and a high proportion of type IIx fibres. J Exp Biol 214, 4041–4047. https://doi.org/10.1242/jeb.061572 |
| 128 | Kohn, T.A., Hoffman, L.C., Myburgh, K.H., 2007. Identification of myosin heavy chain isoforms in skeletal muscle of four Southern African wild ruminants.Comp Biochem Physiol A Mol Integr Physiol 148, 399–407. https://doi.org/10.1016/j.cbpa.2007.05.028 |
| 129 | Konno, T., Watanabe, K., 2012. Distribution of myofiber types in the crural musculature of sheep. Okajimas Folia Anat Jpn 89, 39–45. https://doi.org/10.2535/ofaj.89.39 |
| 130 | LaRosa, D.A., Cannata, D.J., Arnould, J.P.Y., O'Sullivan, L.A., Snow, R.J., West, J.M., 2012. Changes in muscle composition during the development of diving ability in the Australian fur seal. Aust J Zool 60, 81–90. https://doi.org/10.1071/ZO11072 |
| 131 | Larsson, L., Yu, F., 1997. Gender-related differences in the regulatory influence of thyroid hormone on the expression of myosin isoforms in young and old rats. Acta Physiol Scand 159, 81–9. https://doi.org/10.1046/j.1365-201X.1997.559328000.x |
| 132 | Lazareva, M. v., Trapeznikova, K.O., Vikhlyantsev, I.M., Bobylev, A.G., Klimov, A.A., Podlubnaya, Z.A., 2012. Seasonal changes in the isoform composition of the myosin heavy chains in skeletal muscles of hibernating ground squirrels Spermophilus undulatus. Biophys (Russ Fed) 57, 764–768. https://doi.org/10.1134/S0006350912060085 |
| 133 | Lefaucheur, L., Ecolan, P., Plantard, L., Gueguen, N., 2002. New Insights into Muscle Fiber Types in the Pig. J Histochem Cytochem 50, 719–730. https://doi.org/10.1177/002215540205000513 |
| 134 | Lefaucheur, L.L., Ecolan, P.P., 2005. Pattern of muscle fiber formation in Large White and Meishan pigs. Arch Tierz 48, 117–122. |
| 135 | Levine, M., Tjian, R., Tijan, R., 2003. Transcription regulation and animal diversity. Nature 424, 147–151. https://doi.org/10.1038/nature01763 |
| 136 | Lewis, D.M., Levi, A.J., Brooksby, P., Jones, J. v, 1994. A faster twitch contraction of soleus in the spontaneously hypertensive rat is partly due to changed fibre type composition. Exp Physiol 79, 377–86. https://doi.org/10.1113/expphysiol.1994.sp003772 |
| 137 | Lexell, J., Henriksson-larsen, K., Sjostrom, M., 1983. Distribution of different fibre types in human skeletal muscles 2. A study of cross-sections of whole m. vastus lateralis. Acta Physiol Scand 117, 115–122. |
| 138 | Lindholm, A., Piehl, K., 1974. Fibre Composition, Enzyme Activity and Concentrations of Metabolites and Electrolytes in Muscles of Standardbred Horses. Acta Vet Scand 15, 287–309. https://doi.org/10.1186/BF03547460 |
| 139 | Luziga, C., Miyata, H., Nagahisa, H., Oji, T., Wada, N., 2021. Muscle fibre distribution in forelimb, hindlimb and trunk muscles in three bat species: The little Japanese horseshoe, greater horseshoe and Egyptian fruit. Anat Histol Embryol 50, 685–693. https://doi.org/10.1111/ahe.12670 |
| 140 | Mabuchi, K., Szvetko, D., Pinter, K., Sreter, F.A., 1982. Type IIB to IIA fiber transformation in intermittently stimulated rabbit muscles. Am J Physiol Cell Physiol 242, C373–C381. https://doi.org/10.1152/ajpcell.1982.242.5.C373 |
| 141 | Malatesta, M., Perdoni, F., Battistelli, S., Muller, S., Zancanaro, C., 2009. The cell nuclei of skeletal muscle cells are transcriptionally active in hibernating edible dormice. BMC Cell Biol 10, 19. https://doi.org/10.1186/1471-2121-10-19 |
| 142 | Maréchal, G., Goffart, M., Reznik, M., Gerebtzoff, M.A., 1976. The striated muscles in a slow-mover, Perodicticus potto (prosimii, lorisidae, lorisinae). Comp Biochem Physiol A Physiol 54, 81–93. https://doi.org/10.1016/S0300-9629(76)80075-8 |
| 143 | Mashima, D., Oka, Y., Gotoh, T., Tomonaga, S., Sawano, S., Nakamura, M., Tatsumi, R., Mizunoya, W., 2019. Correlation between skeletal muscle fiber type and free amino acid levels in Japanese Black steers. Anim Sci J 90, 604–609. https://doi.org/10.1111/asj.13185 |
| 144 | Mattson, J.P., Miller, T.A., Poole, D.C., Delp, M.D., 2002. Fiber Composition and Oxidative Capacity of Hamster Skeletal Muscle. J Histochem Cytochem 50, 1685–1692. https://doi.org/10.1177/002215540205001214 |
| 145 | Maxwell, L.C., Barclay, J.K., Mohrman, D.E., Faulkner, J.A., 1977. Physiological characteristics of skeletal muscles of dogs and cats. Am J Physiol Cell Physiol 233, C14–C18. https://doi.org/10.1152/ajpcell.1977.233.1.C14 |
| 146 | McFadden, K.D., Bagnall, K.M., Mahon, M., Ford, D., 1984. Histochemical Fiber Composition of Lumbar Back Muscles in the Rabbit. Cells Tissues Organs 120, 146–150. https://doi.org/10.1159/000145909 |
| 147 | McIntosh, M., Ringqvist, J.S., Schmidt, E.M., 1986. Fiber Type Composition of Monkey Forearm Muscle. Anat Rec 211, 403–409. |
| 148 | Melichna, J., Macková, E. v, Semiginovský, B., Tolar, M., Stichová, J., Slavícek, A., Vanková, S., Bartůnĕk, Z., 1987. Effect of exercise on muscle fibre composition and enzyme activities of skeletal muscles in young rats. Physiol Bohemoslov 36, 321–8. |
| 149 | Meznaric, M., Čarni, A., 2020. Characterisation of flexor digitorum profundus, flexor digitorum superficialis and extensor digitorum communis by electrophoresis and immunohistochemical analysis of myosin heavy chain isoforms in older men. Ann Anat 227, 151412. https://doi.org/10.1016/j.aanat.2019.151412 |
| 150 | Moritz, S., Fischer, M.S., Schilling, N., 2007. Three-dimensional fibre-type distribution in the paravertebral muscles of the domestic ferret (Mustela putorius f. furo) with relation to functional demands during locomotion. Zoology 110, 197–211. https://doi.org/10.1016/j.zool.2007.01.004 |
| 151 | Mu, L., Sanders, I., 2002. Muscle Fiber-Type Distribution Pattern in the Human Cricopharyngeus Muscle. Dysphagia 17, 87–96. https://doi.org/10.1007/s00455-001-0108-2 |
| 152 | Muchlinski, M.N., Hemingway, H.W., Pastor, J., Omstead, K.M., Burrows, A.M., 2018. How the Brain May Have Shaped Muscle Anatomy and Physiology: A Preliminary Study. Anat Rec 301, 528–537. https://doi.org/10.1002/ar.23746 |
| 153 | Myatt, J.P., Schilling, N., Thorpe, S.K.S., 2011. Distribution patterns of fibre types in the triceps surae muscle group of chimpanzees and orangutans. J Anat 218, 402–412. https://doi.org/10.1111/j.1469-7580.2010.01338.x |
| 154 | Nemeth, P., Pette, D., 1981. Succinate dehydrogenase activity in fibres classified by myosin ATPase in three hind limb muscles of rat. J Physiol 320, 73–80. https://doi.org/10.1113/jphysiol.1981.sp013935 |
| 155 | Neufuss, J., Hesse, B., Thorpe, S.K.S., Vereecke, E.E., D'Aout, K., Fischer, M.S., Schilling, N., 2014. Fibre type composition in the lumbar perivertebral muscles of primates: implications for the evolution of orthogrady in hominoids. J Anat 224, 113–131. https://doi.org/10.1111/joa.12130 |
| 156 | Niederle, B., Mayr, R., 1978. Course of denervation atrophy in type I and type II fibres of rat extensor digitorum longus muscle. Anat Embryol (Berl) 153, 9–21. https://doi.org/10.1007/BF00569846 |
| 157 | North, M.K., Hoffman, L.C., 2017. Effect of Sex and Muscle on the Fiber-Type Composition and Cross-Sectional Area of Springbok (Antidorcas marsupialis) Muscle. Meat and Muscle Biology 1. https://doi.org/10.22175/mmb2017.01.0001 |
| 158 | North, M.K., Hoffman, L.C., 2015. The muscle fibre characteristics of springbok (Antidorcas marsupialis) longissimus thoracis et lumborum and biceps femoris muscle, in: 61st International Congress of Meat Science and Technology. Clermont-Ferrand. |
| 159 | Nowell, M.M., Choi, H., Rourke, B.C., 2011. Muscle plasticity in hibernating ground squirrels (Spermophilus lateralis) is induced by seasonal, but not low-temperature, mechanisms. J Comp Physiol B 181, 147–164. https://doi.org/10.1007/s00360-010-0505-7 |
| 160 | Olson, R.A., Womble, M.D., Thomas, D.R., Glenn, Z.D., Butcher, M.T., 2016. Functional Morphology of the Forelimb of the Nine-Banded Armadillo (Dasypus novemcinctus): Comparative Perspectives on the Myology of Dasypodidae. J Mamm Evol 23, 49–69. https://doi.org/10.1007/s00360-010-0505-7 |
| 161 | O'Neill, M.C., Umberger, B.R., Holowka, N.B., Larson, S.G., Reiser, P.J., 2017. Chimpanzee super strength and human skeletal muscle evolution. PNAS 114, 7343–7348. https://doi.org/10.1073/pnas.1619071114 |
| 162 | Ozaki, K., Matsuura, T., Narama, I., 2001. Histochemical and morphometrical analysis of skeletal muscle in spontaneous diabetic WBN/Kob rat. Acta Neuropathol 102, 264–70. https://doi.org/10.1007/s004010100363 |
| 163 | Peter, J.B., Barnard, R.J., Edgerton, V.R., Gillespie, C.A., Stempel, K.E., 1972. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry 11, 2627–33. https://doi.org/10.1021/bi00764a013 |
| 164 | Peters, S.E., Mulkey, R., Rasmussen, S.A., Goslow, G.E., 1984. Motor units of the primary ankle extensor muscles of the opossum (Didelphis virginiana): Functional properties and fiber types. J Morphol 181, 305–317. https://doi.org/10.1002/jmor.1051810305 |
| 165 | Peters, T., Kubis, H.P., Wetzel, P., Sender, S., Asmussen, G., Fons, R., Jurgens, K.D., 1999. Contraction parameters, myosin composition and metabolic enzymes of the skeletal muscles of the etruscan shrew Suncus etruscus and of the common European white-toothed shrew Crocidura russula (Insectivora: soricidae). J Exp Biol 202, 2461–2473. https://doi.org/10.1242/jeb.202.18.2461 |
| 166 | Petter, A., Jouffroy, F.K., 1993. Fiber type population in limb muscles of Microcebus murinus. Primates 34, 181–196. https://doi.org/10.1007/BF02381389 |
| 167 | Plaghki, L., Goffart, M., Beckers-Bleukx, G., Moureau-Lebbe, A., 1981. Some characteristics of the hind limb muscles in the leaping night monkey Aotus trivirgatus (primates, anthropoidae, cebidae). Comp Biochem Physiol A Physiol 70, 341–349. https://doi.org/10.1016/0300-9629(81)90188-2 |
| 168 | Plas, R.L.C., Degens, H., Meijer, J.P., de Wit, G.M.J., Philippens, I.H.C.H.M., Bobbert, M.F., Jaspers, R.T., 2015. Muscle contractile properties as an explanation of the higher mean power output in marmosets than humans during jumping. J Exp Biol 218, 2166–2173. https://doi.org/10.1242/jeb.117655 |
| 169 | Ponganis, P.J., Pierce, R.W., 1978. Muscle metabolic profiles and fiber-type composition in some marine mammals. Comp Biochem Physiol B Biochem Mol Biol 59, 99–102. https://doi.org/10.1016/0305-0491(78)90187-6 |
| 170 | Popova, S.S., Yurshenas, D.A., Mikhailova, G.Z., Bobyleva, L.G., Salmov, N.N., Tyapkina, O.V., Nurullin, L.F., Gazizova, G.R., Nigmetzyanov, I.R., Gusev, O.A., Zakharova, N.M., Vikhlyantsev, I.M., 2021. Stable Level of Giant Sarcomeric Cytoskeletal Proteins in Striated Muscles of the Edible Dormouse Glis glis during Hibernation. J Evol Biochem Physiol 57, 886–895. https://doi.org/10.1134/S0022093021040128 |
| 171 | Pösö, A.R., Nieminen, M., Raulio, J., Räsänen, L.A., Soveri, T., 1996. Skeletal muscle characteristics of racing reindeer (Rangifer tarandus). Comp Biochem Physiol A Physiol 114, 277–281. https://doi.org/10.1016/0300-9629(96)00014-X |
| 172 | Potau, J.M., Artells, R., Bello, G., Muñoz, C., Monzó, M., Pastor, J.F., de Paz, F., Barbosa, M., Diogo, R., Wood, B., 2011. Expression of Myosin Heavy Chain Isoforms in the Supraspinatus Muscle of Different Primate Species: Implications for the Study of the Adaptation of Primate Shoulder Muscles to Different Locomotor Modes. Int J Primatol 32, 931–944. https://doi.org/10.1007/s10764-011-9512-0 |
| 173 | Potau, J.M., Artells, R., Muñoz, C., Arias-Martorell, J., Pastor, J.F., de Paz, F.J., Barbosa, M., Bello-Hellegouarch, G., Pérez-Pérez, A., 2018. Quantification of Myosin Heavy Chain Isoform mRNA Transcripts in the Supraspinatus Muscle of Vertical Clinger Primates. Folia Primatol 88, 497–506. https://doi.org/10.1159/000485246 |
| 174 | Potau, J.M., Casado, A., de Diego, M., Ciurana, N., Arias‐Martorell, J., Bello‐Hellegouarch, G., Barbosa, M., de Paz, F.J., Pastor, J.F. and Pérez‐Pérez, A., 2018. Structural and molecular study of the supraspinatus muscle of modern humans (Homo sapiens) and common chimpanzees (Pan troglodytes). Am J Biol Anthropol 166, 934-940. |
| 175 | Pousson, M., Pérot, C., Goubel, F., 1991. Stiffness changes and fibre type transitions in rat soleus muscle produced by jumping training. Pflug Arch Eur J Physiol 419, 127–130. https://doi.org/10.1007/BF00372997 |
| 176 | Pullen, A.H., 1977. The distribution and relative sized of fibre types in the extensor digitorum longus and soleus muscles of the adult rat. J Anat 123, 467–86. |
| 177 | Queeno, S.R., Reiser, P.J., Orr, C.M., Capellini, T.D., Sterner, K.N., O'Neill, M.C., 2023. Human and African ape myosin heavy chain content and the evolution of hominin skeletal muscle. Comp Biochem Physiol A Mol Integr Physiol 281, 111415. |
| 178 | Rab, M., Neumayer, CH., Koller, R., Kamolz, L.-P., Haslik, W., Gassner, R., Giovanoli, P., Schaden, G., Frey, M., 2000. Histomorphology of rabbit thigh muscles: establishment of standard control values. J Anat 196, 203–209. https://doi.org/10.1046/j.1469-7580.2000.19620203.x |
| 179 | Rantanen, J., Rissanen, A., Kalimo, H., 1994. Lumbar muscle fiber size and type distribution in normal subjects. Eur Spine J 3, 331–5. https://doi.org/10.1007/BF02200146 |
| 180 | Raub, R.R., Bechtel, P.J., Lawrence, L.M., 1985. Variation in the distribution of muscle fiber types in equine skeletal muscles. J Equine Vet Sci 5, 34–37. https://doi.org/10.1016/S0737-0806(85)80084-8 |
| 181 | Reed, J.Z., Butler, P.J., Fedak, M.A., 1994. The metabolic characteristics of the locomotory muscles of grey seals (Halichoerus grypus), harbour seals (Phoca vitulina) and Antarctic fur seals (Arctocephalus gazella). J Exp Biol 194, 33–46. https://doi.org/10.1242/jeb.194.1.33 |
| 182 | Rehfeldt, C., Henning, M., Fiedler, I., 2008. Consequences of pig domestication for skeletal muscle growth and cellularity. Livest Sci 116, 30–41. https://doi.org/10.1016/j.livsci.2007.08.017 |
| 183 | Reid, W.D., Ng, A., Wilton, R., Milsom, W.K., 1995. Characteristics of diaphragm muscle fibre types in hibernating squirrels. Respir Physiol 101, 301–9. https://doi.org/10.1016/0034-5687(95)00036-d |
| 184 | Richmond, F.J., Abrahams, V.C., 1975. Morphology and enzyme histochemistry of dorsal muscles of the cat neck. J Neurophysiol 38, 1312–21. https://doi.org/10.1152/jn.1975.38.6.1312 |
| 185 | Richmond, F.J.R., Singh, K., Corneil, B.D., 2001. Neck Muscles in the Rhesus Monkey. I. Muscle Morphometry and Histochemistry. J Neurophysiol 86, 1717–1728. https://doi.org/10.1152/jn.2001.86.4.1717 |
| 186 | Riley, D.A., van Dyke, J.M., Vogel, V., Curry, B.D., Bain, J.L.W., Schuett, R., Costill, D.L., Trappe, T., Minchev, K., Trappe, S., 2018. Soleus muscle stability in wild hibernating black bears. Am J Physiol Regul Integr Comp Physiol 315, R369–R379. https://doi.org/10.1152/ajpregu.00060.2018 |
| 187 | Rivero, J.L., Serrano, A.L., Barrey, E., Valette, J.P., Jouglin, M., 1999. Analysis of myosin heavy chains at the protein level in horse skeletal muscle. J Muscle Res Cell Motil 20, 211–221. https://doi.org/10.1023/A:1005461214800 |
| 188 | Rivero, J.L., Talmadge, R.J., Edgerton, V.R., 1996. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in equine skeletal muscle and the influence of training. Anat Rec 246, 195–207. 10.1002/(SICI)1097-0185(199610)246:2<195::AID-AR6>3.0.CO;2-0 |
| 189 | Rivero, J.-L.L., 2018. Locomotor muscle fiber heterogeneity and metabolism in the fastest large-bodied rorqual: the fin whale (Balaenoptera physalus). J Exp Biol 221. https://doi.org/10.1242/jeb.177758 |
| 190 | Ronéus, M., Essén-Gustavsson, B., Lindholm, A., Persson, S.G., 1992. Skeletal muscle characteristics in young trained and untrained standardbred trotters. Equine Vet J 24, 292–4. https://doi.org/10.1111/j.2042-3306.1992.tb02838.x |
| 191 | Rourke, B.C., Cotton, C.J., Harlow, H.J., Caiozzo, V.J., 2006. Maintenance of slow type I myosin protein and mRNA expression in overwintering prairie dogs (Cynomys leucurus and ludovicianus) and black bears (Ursus americanus). J Comp Physiol B 176, 709–720. https://doi.org/10.1007/s00360-006-0093-8 |
| 192 | Rourke, B.C., Yokoyama, Y., Milsom, W.K., Caiozzo, V.J., 2004. Myosin isoform expression and MAFbx mRNA levels in hibernating golden-mantled ground squirrels (Spermophilus lateralis). Physiol Biochem Zool 77, 582–93. https://doi.org/10.1086/421753 |
| 193 | Rowlerson, A., Mascarello, F., Veggetti, A., Carpene, E., 1983. The arch fibre-type composition of the first branchial muscles in Carnivora and Primates. J Muscle Res Cell Motil 4, 443–472. |
| 194 | Roy, R.R., Bello, M.A., Powell, P.L., Simpson, D.R., 1984. Architectural design and fiber-type distribution of the major elbow flexors and extensors of the monkey (cynomolgus). Am J Anat 171, 285–293. https://doi.org/10.1002/aja.1001710305 |
| 195 | Roy, R.R., Bodine-Fowler, S.C., Kim, J., Haque, N., de Leon, D., Rudolph, W., Edgerton, V.R., 1991. Architectural and Fiber Type Distribution Properties of Selected Rhesus Leg Muscles: Feasibility of Multiple Independent Biopsies. Cells Tissues Organs 140, 350–356. https://doi.org/10.1159/000147081 |
| 196 | Roy, R.R., Graham, S., Peterson, J.A., 1988. Fiber type composition of the plantarflexors of giraffes (giraffa camelopardalis) at different postnatal stages of development. Comp Biochem Physiol A Physiol 91, 347–352. https://doi.org/10.1016/0300-9629(88)90429-X |
| 197 | Rupert, J.E., Rose, J.A., Organ, J.M., Butcher, M.T., 2015. Forelimb muscle architecture and myosin isoform composition in the groundhog (Marmota monax). J Exp Biol 218, 194–205. https://doi.org/10.1242/jeb.107128 |
| 198 | Rupert, J.E., Schmidt, E.C., Moreira-Soto, A., Herrera, B.R., Vandeberg, J.L., Butcher, M.T., 2014. Myosin isoform expression in the prehensile tails of didelphid marsupials: Functional differences between arboreal and terrestrial opossums. Anat Rec 297, 1364–1376. https://doi.org/10.1002/ar.22948 |
| 199 | Ryu, Y.C., Choi, Y.M., Lee, S.H., Shin, H.G., Choe, J.H., Kim, J.M., Hong, K.C., Kim, B.C., 2008. Comparing the histochemical characteristics and meat quality traits of different pig breeds. Meat Sci 80, 363–369. https://doi.org/10.1016/j.meatsci.2007.12.020 |
| 200 | Sahd, L., Doubell, N., Bennett, N.C., Kotzé, S.H., 2022. Hind foot drumming: Myosin heavy chain muscle fiber distribution in the hind limb muscles of three African mole‐rat species (Bathyergidae). Anat Rec 305, 170–183. https://doi.org/10.1002/ar.24712 |
| 201 | Salmov, N.N., Vikhlyantsev, I.M., Ulanova, A.D., Gritsyna, Yu. v., Bobylev, A.G., Saveljev, A.P., Makariushchenko, V. v., Maksudov, G.Yu., Podlubnaya, Z.A., 2015. Seasonal changes in isoform composition of giant proteins of thick and thin filaments and titin (connectin) phosphorylation level in striated muscles of bears (Ursidae, Mammalia). Biochem (Mosc) 80, 343–355. https://doi.org/10.1134/S0006297915030098 |
| 202 | Savolainen, J., Vornanen, M., 1995a. Myosin heavy chains in skeletal muscles of the common shrew (Sorex araneus): absence of a slow isoform and transitions of fast isoforms with ageing. Acta Physiol Scand 155, 233–239. https://doi.org/10.1111/j.1748-1716.1995.tb09968.x |
| 203 | Savolainen, J., Vornanen, M., 1995b. Fiber types and myosin heavy chain composition in muscles of common shrew (Sorex araneus). J Exp Zool 271, 27–35. https://doi.org/10.1002/jez.1402710104 |
| 204 | Sazili, A.Q., Parr, T., Sensky, P.L., Jones, S.W., Bardsley, R.G., Buttery, P.J., 2005. The relationship between slow and fast myosin heavy chain content, calpastatin and meat tenderness in different ovine skeletal muscles. Meat Sci 69, 17–25. https://doi.org/10.1016/j.meatsci.2004.06.021 |
| 205 | Schilling, N., 2005. Characteristics of paravertebral muscles - fibre type distribution pattern in the pika, Ochotona rufescens (Mammalia: Lagomorpha). J Zool Syst Evol Res 43, 38–48. https://doi.org/10.1111/j.1439-0469.2004.00295.x |
| 206 | Schmidt, M., Schilling, N., 2007. Fiber type distribution in the shoulder muscles of the tree shrew, the cotton-top tamarin, and the squirrel monkey related to shoulder movements and forelimb loading. J Hum Evol 52, 401–419. https://doi.org/10.1016/j.jhevol.2006.11.005 |
| 207 | Sickles, D.W., Pinkstaff, C.A., 1981. Comparative histochemical study of prosimian primate hindlimb muscles. II. Populations of fiber types. Am J Anat 160, 187–194. https://doi.org/10.1002/aja.1001600205 |
| 208 | Singh, K., Melis, E.H., Richmond, F.J.R., Scott, S.H., 2002. Morphometry of Macaca mulatta forelimb. II. Fiber-type composition in shoulder and elbow muscles. J Morphol 251, 323–332. https://doi.org/10.1002/jmor.1092 |
| 209 | Širca, A., Kostevc, V., 1985. The fibre type composition of thoracic and lumbar paravertebral muscles in man. J Anat 141, 131–7. |
| 210 | Skewes, O., Cádiz, P., Merino, V., Islas, A., Morales, R., 2014. Muscle fibre characteristics, enzyme activity and meat colour of wild boar (Sus scrofa s. L.) muscle with 2n=36 compared to those of phenotypically similar crossbreeds (2n=37 and 2n=38). Meat Sci 98, 272–278. https://doi.org/10.1016/j.meatsci.2014.06.001 |
| 211 | Smerdu, V., Ĉehovin, T., Ŝtrbenc, M., Fazarinc, G., 2009. Enzyme-and immunohistochemical aspects of skeletal muscle fibers in brown bear (Ursus arctos). J Morphol 270, 154–161. https://doi.org/10.1002/jmor.10673 |
| 212 | Snow, D.H., Guy, P.S., 1980. Muscle fibre type composition of a number of limb muscles in different types of horse. Res Vet Sci 28, 137–144. https://doi.org/10.1016/S0034-5288(18)32735-8 |
| 213 | Sokoloff, A.J., Yang, B., Li, H., Burkholder, T.J., 2007. Immunohistochemical characterization of slow and fast myosin heavy chain composition of muscle fibres in the styloglossus muscle of the human and macaque (Macaca rhesus). Arch Oral Biol 52, 533–543. https://doi.org/10.1016/j.archoralbio.2006.11.012 |
| 214 | Song, S., Ahn, C.H., Kim, G.D., 2020. Muscle fiber typing in bovine and porcine skeletal muscles using immunofluorescence with monoclonal antibodies specific to myosin heavy chain isoforms. Food Sci Anim Resour 40, 132–144. https://doi.org/10.5851/kosfa.2019.e97 |
| 215 | Soukup, T., Vydra, J., Černý, M., 1979. Changes in ATPase and SDH reactions of the rat extrafusal and intrafusal muscle fibres after preincubations at different pH. Histochemistry 60, 71–84. https://doi.org/10.1007/BF00495730 |
| 216 | Soukup, T., Zacharová, G., Smerdu, V., 2002. Fibre type composition of soleus and extensor digitorum longus muscles in normal female inbred Lewis rats. Acta Histochem 104, 399–405. https://doi.org/10.1078/0065-1281-00660 |
| 217 | Spainhower, K.B., Cliffe, R.N., Metz, A.K., Barkett, E.M., Kiraly, P.M., Thomas, D.R., Kennedy, S.J., Avey-Arroyo, J.A., Butcher, M.T., 2018. Cheap labor: myosin fiber type expression and enzyme activity in the forelimb musculature of sloths (Pilosa: Xenarthra). J Appl Physiol 125, 799–811. https://doi.org/10.1152/japplphysiol.01118.2017 |
| 218 | Spainhower, K.B., Metz, A.K., Yusuf, A.-R.S., Johnson, L.E., Avey-Arroyo, J.A., Butcher, M.T., 2021. Coming to grips with life upside down: how myosin fiber type and metabolic properties of sloth hindlimb muscles contribute to suspensory function. J Comp Physiol B: Biochem Syst Environ Physiol 191, 207–224. https://doi.org/10.1007/s00360-020-01325-x |
| 219 | Spiegel, N.B., Beaton, A.J.W., Mcgrath, J., Thompson, J.M., Wynn, P.C., Greenwood, P.L., 2002. Myofibre types in eight skeletal muscles from the eastern grey kangaroo (Macropus giganteus). Anim Prod Sci 24, 225–228. |
| 220 | Srinivasan, R.C., Lungren, M.P., Langenderfer, J.E., Hughes, R.E., 2007. Fiber type composition and maximum shortening velocity of muscles crossing the human shoulder. Clin Anat 20, 144–149. https://doi.org/10.1002/ca.20349 |
| 221 | Staron, R.S., Kraemer, W.J., Hikida, R.S., Fry, A.C., Murray, J.D., Campos, G.E.R., 1999. Fiber type composition of four hindlimb muscles of adult Fisher 344 rats. Histochem Cell Biol 111, 117–123. https://doi.org/10.1007/s004180050341 |
| 222 | Suzuki, A., 1995. Differences in distribution of myofiber types between the supraspinatus and infraspinatus muscles of sheep. Anat Rec 242, 483–490. https://doi.org/10.1002/ar.1092420406 |
| 223 | Suzuki, A., 1991. Composition of myofiber types in the pectoral girdle musculature of sheep. Anat Rec 230, 339–346. https://doi.org/10.1002/ar.1092300307 |
| 224 | Suzuki, A., 1990. Composition of myofiber types in limb muscles of the house shrew (Suncus murinus): Lack of type I myofibers. Anat Rec 228, 23–30. https://doi.org/10.1002/ar.1092280105 |
| 225 | Suzuki, A., 1972. Histochemical classification of individual skeletal muscle fibers in the sheep III. On the M. masseter. Anim Sci J 43, 161–166. |
| 226 | Suzuki, A., 1971a. Histochemical classification of individual skeletal muscle fibers in the sheep. I. On musculi semitendinosus, musculi longissimus dorsi, musculi psoas major, musculi latissimus dorsi and musculi gastrocnemus. Anim Sci Js 42, 39–54. |
| 227 | Suzuki, A., 1971b. Histochemical classification of individual skeletal muscle fibers in the sheep II. On M. serratus ventralis, M. supraspinatus, M. infraspinatus,M. semimembranosus, and M. triceps brachii (Caput longum). Anim Sci J 42, 463–473. |
| 228 | Suzuki, A., Hayama, S., 1994. Individual Variation in Myofiber Type Composition in the Triceps Surae and Flexor Digitorum Superficialis Muscles of Japanese Macaques. Anthropol Sci 102, 127–138. https://doi.org/10.1537/ase.102.Supplement_127 |
| 229 | Suzuki, A., Hayama, S., 1991. Histochemical classification of myofiber types in the triceps surae and flexor digitorum superficialis muscle of Japanese macaques. Acta Histochem Cytochem 24, 323–328. https://doi.org/10.1267/ahc.24.323 |
| 230 | Suzuki, A., Tamate, H., 1988. Distribution of myofiber types in the hip and thigh musculature of sheep. Anat Rec 221, 494–502. https://doi.org/10.1002/ar.1092210106 |
| 231 | Suzuki, A., Tamate, H., 1974. Histochemical classification of skeletal muscle fibers in the cattle. Acta Histochem Cytochem 7, 319–327. https://doi.org/10.1267/ahc.7.319 |
| 232 | Suzuki, A., Watanabe, K., Konno, T., Ohwada, S., 1999. Distribution of Myofiber Types in the Hip and Thigh Musculature of Pigs. Anim Sci J 70, 519–525. https://doi.org/10.2508/chikusan.70.519 |
| 233 | Talmadge, R.J., Grossman, E.J., Roy, R.R., 1996. Myosin heavy chain composition of adult feline (Felis catus) limb and diaphragm muscles. J Exp Zool 275, 413–20. 10.1002/(SICI)1097-010X(19960815)275:6<413::AID-JEZ3>3.0.CO;2-R |
| 234 | Thomason, D.B., Baldwin, K.M., Herrick, R.E., 1986. Myosin isozyme distribution in rodent hindlimb skeletal muscle. J Appl Physiol 60, 1923–1931. https://doi.org/10.1152/jappl.1986.60.6.1923 |
| 235 | Thorstensson, A., Carlson, H., 1987. Fibre types in human lumbar back muscles. Acta Physiol Scand 131, 195–202. https://doi.org/10.1111/j.1748-1716.1987.tb08226.x |
| 236 | Tinker, D.B., Harlow, H.J., Beck, T.D.I., 1998. Protein Use and Muscle‐Fiber Changes in Free‐Ranging, Hibernating Black Bears. Physiol Zool 71, 414–424. https://doi.org/10.1086/515429 |
| 237 | Tirrell, T.F., Cook, M.S., Carr, J.A., Lin, E., Ward, S.R., Lieber, R.L., 2012. Human skeletal muscle biochemical diversity. J Exp Biol 215, 2551–2559. https://doi.org/10.1242/jeb.069385 |
| 238 | Toniolo, L., Maccatrozzo, L., Patruno, M., Pavan, E., Caliaro, F., Rossi, R., Rinaldi, C., Canepari, M., Reggiani, C., Mascarello, F., 2007. Fiber types in canine muscles: Myosin isoform expression and functional characterization. Am J Physiol Cell Physiol 292. https://doi.org/10.1152/ajpcell.00601.2006 |
| 239 | Toole, J.F., Bullock, T.H., 1973. Neuromuscular responses of sloths. J Comp Neurol 149, 259–270. https://doi.org/10.1002/cne.901490209 |
| 240 | Tůmová, E., Chodová, D., Svobodová, J., Uhlířová, L., Volek, Z., 2015. Carcass composition and meat quality of czech genetic resources of nutrias (Myocastor coypus). Czech J Anim Sci 60, 479–486. https://doi.org/10.17221/8556-CJAS |
| 241 | Tůmová, E., Chodová, D., Uhlířová, L., Vlčková, J., Volek, Z., Skřivanová, V., 2016. Relationship between muscle fibre characteristics and meat sensory properties in three nutria (Myocastor coypus) colour types. Czech J Anim Sci 61, 217–222. https://doi.org/10.17221/59/2015-CJAS |
| 242 | Tůmová, E., Chodová, D., Vlčková, J., Němeček, T., Uhlířová, L., Skřivanová, V., 2017. Age-related changes in the carcass yield and meat quality of male and female nutrias (Myocastor coypus) under intensive production system. Meat Sci 133, 51–55. https://doi.org/10.1016/j.meatsci.2017.06.003 |
| 243 | Ustunel, I., Demir, R., 1997. A Histochemical, Morphometric and Ultrastructural Study of Gastrocnemius and Soleus Muscle Fiber Type Composition in Male and Female Rats. Cells Tissues Organs 158, 279–286. https://doi.org/10.1159/000147941 |
| 244 | van de Graaff, K.M., Frederick, E.C., Williamson, R.G., Goslow, G.E., 1977. Motor Units and Fiber Types of Primary Ankle Extensors of the Skunk (Mephitis mephitis). J Neurophysiol 40. |
| 245 | Vesely, M.J., Sanders, R., Green, C.J., Motterlini, R., 1999. Fibre type specificity of haem oxygenase-1 induction in rat skeletal muscle. FEBS Lett 458, 257–60. https://doi.org/10.1016/s0014-5793(99)01129-1 |
| 246 | von Mering, F., Fischer, M.S., 1999. Fibre type regionalization of forelimb muscles in two mammalian species, Galea musteloides (Rodentia, Caviidae) and Tupaia belangeri (Scandentia, Tupaiidae), with comments on postnatal myogenesis. Zoomorphology 119, 117–126. https://doi.org/10.1007/s004350050086 |
| 247 | Wada, N., Miyata, H., Tokuriki, M., 1994. Histochemical fiber composition of cat's tail muscles. Arch Ital Biol 132, 53–8. |
| 248 | Wall, C.E., Briggs, M.M., Huq, E., Hylander, W.L., Schachat, F., 2013. Regional variation in IIM myosin heavy chain expression in the temporalis muscle of female and male baboons (Papio anubis). Arch Oral Biol 58, 435–443. https://doi.org/10.1016/j.archoralbio.2012.09.008 |
| 249 | Watanabe, K., Suzuki, A., 1999. Distribution, Density, and Structure of Muscle Spindles in the Vastus Intermedius and the Peroneus Longus Muscles of Sheep. Okajimas Folia Anat Jpn 76, 203–219. https://doi.org/10.2535/ofaj1936.76.5_203 |
| 250 | Watson, R.R., Miller, T.A., Davis, R.W., 2003. Immunohistochemical fiber typing of harbor seal skeletal muscle. J Exp Biol 206, 4105–11. https://doi.org/10.1242/jeb.00652 |
| 251 | Wenfang, Yuye, F., Yang, B., Yang, H., Borjigin, G., Bao, H., Narenbatu, Demtu, 2019. Comparative studies on slaughter performance and skeletal muscle fibre type of alxa gobi camel and desert camel. J Camel Pract Res 26, 63. https://doi.org/10.5958/2277-8934.2019.00009.2 |
| 252 | Whiteman, J.P., Harlow, H.J., Durner, G.M., Regehr, E. v., Rourke, B.C., Robles, M., Amstrup, S.C., Ben-David, M., 2017. Polar bears experience skeletal muscle atrophy in response to food deprivation and reduced activity in winter and summer. Conserv Physiol 5. https://doi.org/10.1093/conphys/cox049 |
| 253 | Wigston, D.J., English, A.Wm., 1992. Fiber-type proportions in mammalian soleus muscle during postnatal development. J Neurobiol 23, 61–70. https://doi.org/10.1002/neu.480230107 |
| 254 | Williams, T.M., Dobson, G.P., Mathieu-Costello, O., Morsbach, D., Worley, M.B., Phillips, J.A., 1997. Skeletal muscle histology and biochemistry of an elite sprinter, the African cheetah. J Comp Physiol B 167, 527–535. https://doi.org/10.1007/s003600050105 |
| 255 | Williams, T.M., Noren, S.R., Glenn, M., 2011. Extreme physiological adaptations as predictors of climate-change sensitivity in the narwhal, Monodon monoceros. Mar Mamm Sci 27, 334–349. https://doi.org/10.1111/j.1748-7692.2010.00408.x |
| 256 | Williamson, R.G., Frederick, E.C., 1977. A functional analysis of ankle extension in the ricochetal rodent (Dipodomys merriami). Anat Histol Embryol 6, 157–66. https://doi.org/10.1111/j.1439-0264.1977.tb00430.x |
| 257 | Wojtysiak, D., Górska, M., Wojciechowska, J., 2016. Muscle Fibre Characteristics and Physico-Chemical Parameters of m. semimembranosus from Puławska, Polish Large White and Pietrain Pigs. Folia Biol (Praha) 64, 197–204. https://doi.org/10.3409/fb64_3.197 |
| 258 | Xu, R., Andres-Mateos, E., Mejias, R., MacDonald, E.M., Leinwand, L.A., Merriman, D.K., Fink, R.H.A., Cohn, R.D., 2013. Hibernating squirrel muscle activates the endurance exercise pathway despite prolonged immobilization. Exp Neurol 247, 392–401. https://doi.org/10.1016/j.expneurol.2013.01.005 |
| 259 | Zacharová, G., Knotková-Urbancová, H., Hník, P., Soukup, T., 1997. Nociceptive atrophy of the rat soleus muscle induced by bone fracture: a morphometric study. J Appl Physiol 82, 552–7. https://doi.org/10.1152/jappl.1997.82.2.552 |
| 260 | Zheng, A., Rahkila, P., Vuori, J., Rasi, S., Takala, T., Väänänen, H.K., 1992. Quantification of carbonic anhydrase III and myoglobin in different fiber types of human psoas muscle. Histochemistry 97, 77–81. https://doi.org/10.1007/BF00271284 |
| 261 | Zhong, W.W.H., Lucas, C.A., Hoh, J.F.Y., 2008. Myosin isoforms and fibre types in limb muscles of Australian marsupials: adaptations to hopping and non-hopping locomotion. J Comp Physiol B: Biochem Syst Environ Physiol 178, 47–55. https://doi.org/10.1007/s00360-007-0198-8 |
| 262 | Zhong, W.W.H., Withers, K.W., Hoh, J.F.Y., 2010. Effects of hypothyroidism on myosin heavy chain composition and fibre types of fast skeletal muscles in a small marsupial, Antechinus flavipes. J Comp Physiol B 180, 531–544. https://doi.org/10.1007/s00360-009-0431-8 |
| 263 | Żochowska, J., Lachowicz, K., Gajowiecki, L., Sobczak, M., Kotowicz, M., Zych, A., 2005. Effects of carcass weight and muscle on texture, structure and myofibre characteristics of wild boar meat. Meat Sci 71, 244–248. https://doi.org/10.1016/j.meatsci.2005.03.019 |
| 264 | Żochowska, J., Lachowicz, K., Gajowiecki, L., Sobczak, M., Kotowicz, M., Zych, A., 2006. Growth-related changes in muscle fibres, characteristics and rheological properties of wild boars meat. Med Weter 62, 47-50. |
| 265 | Żochowska-Kujawska, J., 2016. Effects of fibre type and structure of longissimus lumborum (Ll), biceps femoris (Bf) and semimembranosus (Sm) deer muscles salting with different Nacl addition on proteolysis index and texture of dry-cured meats. Meat Sci 121, 390–396. https://doi.org/10.1016/j.meatsci.2016.07.001 |
| 266 | Żochowska-Kujawska, J., Kotowicz, M., Sobczak, M., Lachowicz, K., Wójcik, J., 2019. Age-related changes in the carcass composition and meat quality of fallow deer (DAMA DAMA L.). Meat Sci 147, 37–43. https://doi.org/10.1016/j.meatsci.2018.08.014 |
| 267 | Żochowska-Kujawska, J., Lachowicz, K., Sobczak, M., 2012. Effects of fibre type and kefir, wine lemon, and pineapple marinades on texture and sensory properties of wild boar and deer longissimus muscle. Meat Sci 92, 675–680. https://doi.org/10.1016/j.meatsci.2012.06.020 |
| 268 | Żochowska-Kujawska, J., Lachowicz, K., Sobczak, M., Gajowiecki, L., Kotowicz, M., Zych, A., Medrala, D., 2007. Effects of massaging on hardness, rheological properties, and structure of four wild boar muscles of different fibre type content and age. Meat Sci 75, 595–602. https://doi.org/10.1016/j.meatsci.2006.09.018 |
| 269 | Żochowska-Kujawska, J., Sobczak, M., Lachowicz, K., 2009. Comparison of the texture, rheological properties and myofibre characteristics of SM (Semimembranosus) muscle of selected species of game animals. Pol J Food Nutr Sci 59, 243–246. |
Eligible studies providing mammalian skeletal muscle fiber content are listed alphabetically in Table 1.
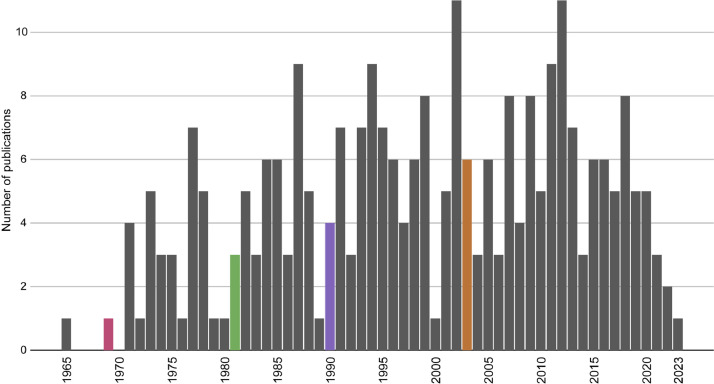
Fig. 2 The 269 eligible studies included in the meta-analysis were published between 1965 and 2023 (Fig. 2). Interest in, and ability to, determine the fiber composition of different skeletal muscles from various mammalian species increased in the 1970s. The earliest included study to use the histochemical assay for myofibrillar ATPase activity (mATPase) to determine fiber type across multiple skeletal muscles was published in 1969 [6]. Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was first used in 1981 [7,8], and immunohistochemistry with myosin antibodies (mABs) was first used in 1990 [9]. The first RNA-based method to determine skeletal muscle fiber composition, reverse transcription-polymerase chain reaction (RT-PCR), was first used in 2003 [10].
Fig. 2.
Number of mammalian skeletal fiber composition publications included in the meta-analysis per year. Colored bars denote the first appearance of each fiber-typing method: mATPase (raspberry), SDS-PAGE (green), mABs (purple), and RT-PCR (orange).
Table 2 is the dataset behind Fig. 3, Fig. 4.
Table 2.
Taxonomic composition, diversity, and representation of each of the 15 mammalian orders present in the 269 eligible studies.
| Order | No. of families | No. of genera | No. of species | No. of publications |
|---|---|---|---|---|
| Artiodactyla | 9 | 21 | 26 | 53 |
| Carnivora | 8 | 17 | 22 | 36 |
| Chiroptera | 6 | 12 | 16 | 8 |
| Cingulata | 1 | 1 | 1 | 1 |
| Dasyuromorphia | 1 | 1 | 1 | 1 |
| Didelphimorphia | 1 | 3 | 3 | 4 |
| Diprotodontia | 2 | 5 | 8 | 4 |
| Eulipotyphla | 2 | 7 | 10 | 6 |
| Lagomorpha | 2 | 2 | 2 | 7 |
| Perissodactyla | 1 | 1 | 3 | 13 |
| Pilosa | 2 | 2 | 2 | 3 |
| Primates | 11 | 35 | 50 | 64 |
| Proboscidea | 1 | 1 | 1 | 1 |
| Rodentia | 10 | 23 | 26 | 66 |
| Scandentia | 1 | 1 | 3 | 1 |
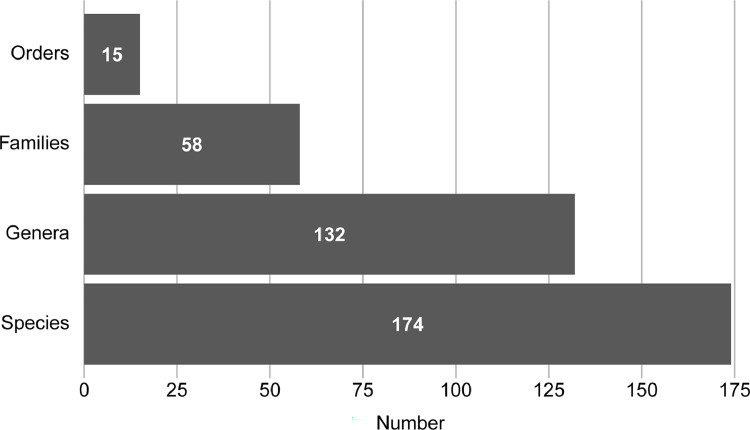
Fig. 3.
Taxonomic representation across the 269 eligible mammalian fiber composition studies included in the meta-analysis.
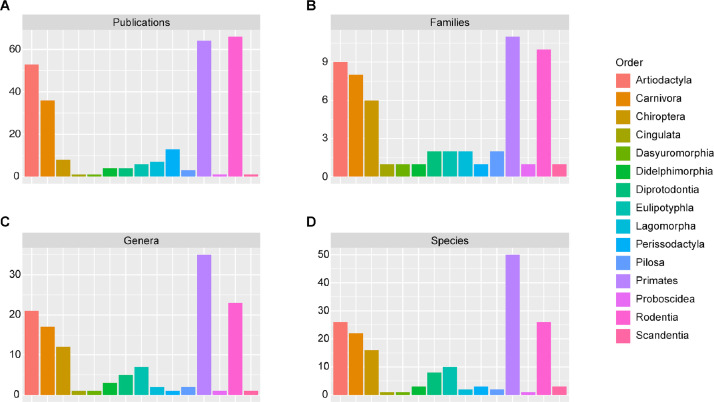
Fig. 4.
Study representation and taxonomic diversity across the 15 mammalian orders. (A) Number of eligible studies/publications per taxonomic order. (B) Number of taxonomic families per taxonomic order. (C) Number of genera per taxonomic order. (D) Number of species per taxonomic order.
From these 269 eligible studies, skeletal muscle fiber composition data was collated from 174 species belonging to class Mammalia (Fig. 3). These species represent 15 unique taxonomic orders, 58 families, and 132 genera.
Within class Mammalia, orders Rodentia, Primates, and Artiodactyla are the most highly represented in the literature, whereas orders Cingulata, Dasyuromorphia, Proboscidea, and Scandentia are the least represented (Fig. 4A). Similarly, orders Rodentia, Primates, and Artiodactyla have the most diverse representation in the literature in terms of number of unique families (Fig. 4B), genera (Fig. 4C), and species (Fig. 4D).
Table 3 quantifies the number of studies used in the meta-analysis that did not report the number of individuals from which skeletal muscle fiber composition data was recorded (n = 22) or the body mass of the individuals from which skeletal muscle fiber composition data was recorded (n = 136). Sixteen of the 269 eligible studies reported neither the number of individuals nor the body mass of individuals from which skeletal muscle fiber composition data was recorded.
Table 3.
Number of eligible studies missing relevant data for meta-analysis.
| Variable | No. of publications |
|---|---|
| Did report number of individuals sampled | 247 |
| Did not report number of individuals sampled | 22 |
| Did report body mass of sampled individuals | 133 |
| Did not report body mass of sampled individuals | 136 |
| Did not report either number of individuals sampled or body mass of sampled individuals | 16 |
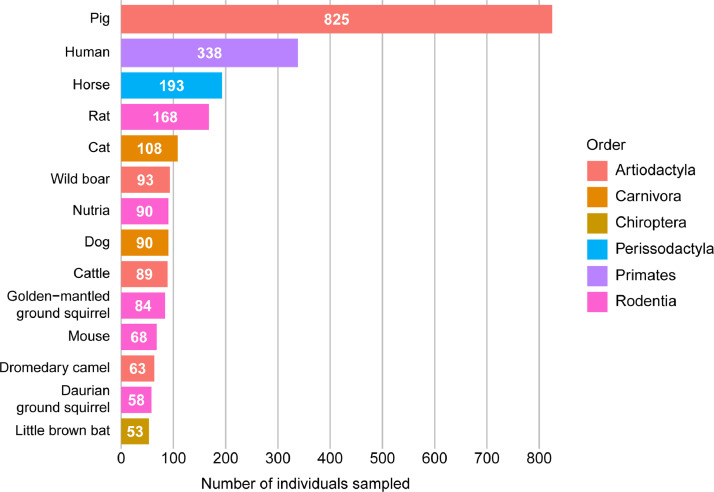
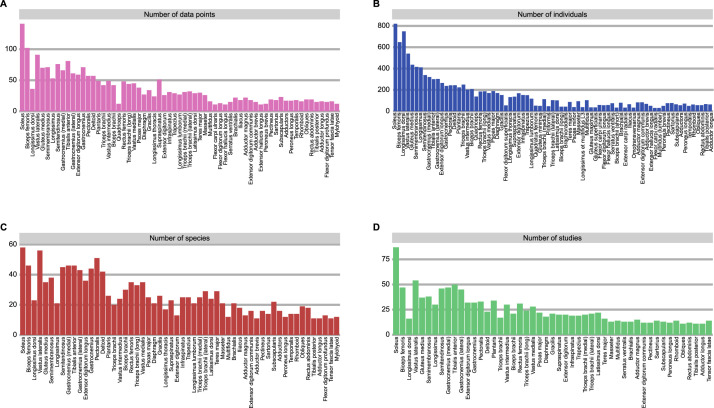
Fourteen species across 6 taxonomic orders had skeletal muscle fiber composition data from 50 or more individuals (Fig. 5). Five species had skeletal muscle fiber composition data from 100 or more individuals: pigs (n = 825), humans (n = 338), horses (n = 193), rats (n = 168), and cats (n = 108).
Fig. 5.
Species with greater than 50 sampled individuals.
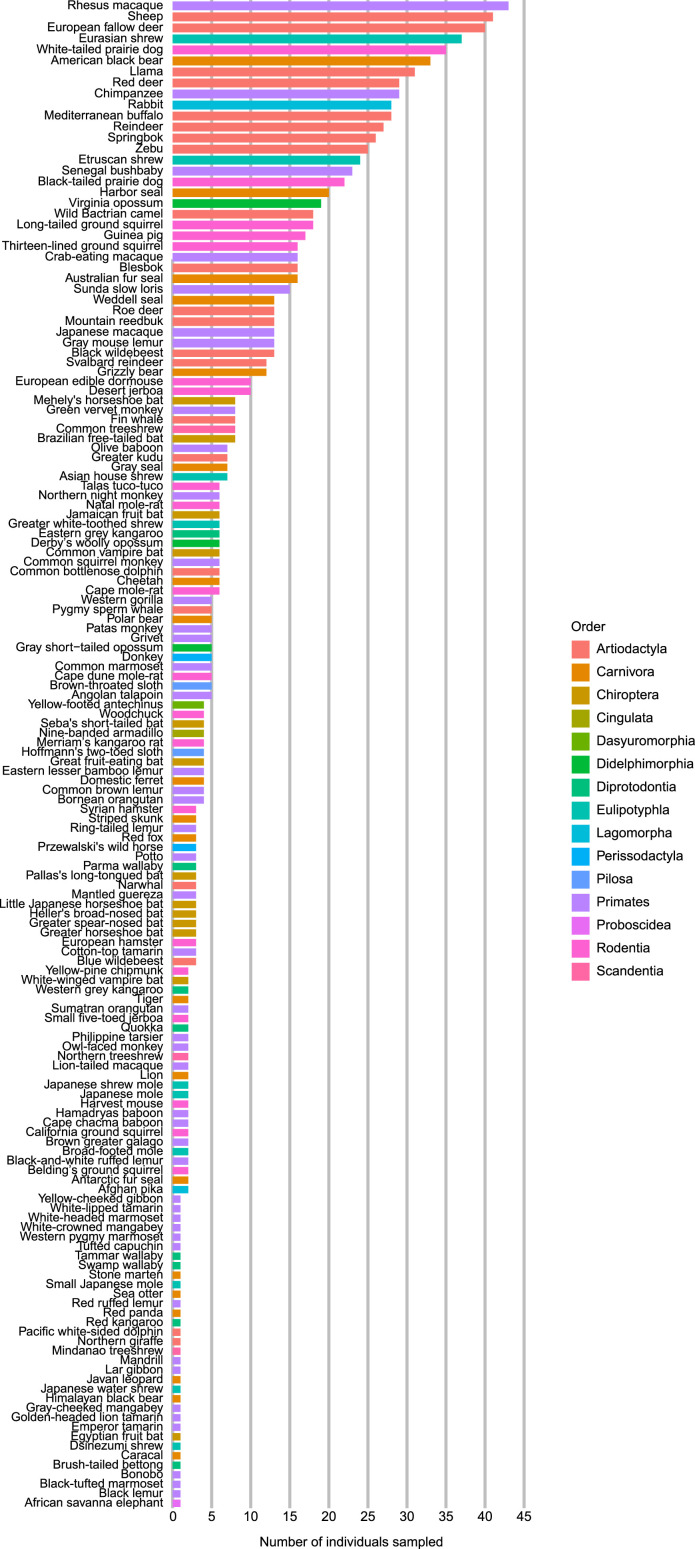
154 species had skeletal muscle fiber composition data from 50 or fewer individuals (Fig. 6). Of those, 33 species had skeletal muscle fiber composition data from only one individual: African savanna elephant, black lemur, black-tufted marmoset, bonobo, brush-tailed bettong, caracal, Dsinezumi shrew, Egyptian fruit bat, emperor tamarin, golden-headed lion tamarin, gray-cheeked mangabey, Himalayan black bear, Japanese water shrew, Lar gibbon, mandrill, Mindanao treeshrew, northern giraffe, Pacific white-sided dolphin, red kangaroo, red panda, red ruffed lemur, sea otter, small Japanese mole, stone marten, swamp wallaby, Tammar wallaby, tufted capuchin, western pygmy marmoset, white-crowned mangabey, white-headed marmoset, white-lipped tamarin, and yellow-cheeked gibbon.
Fig. 6.
Species with fewer than 50 sampled individuals.
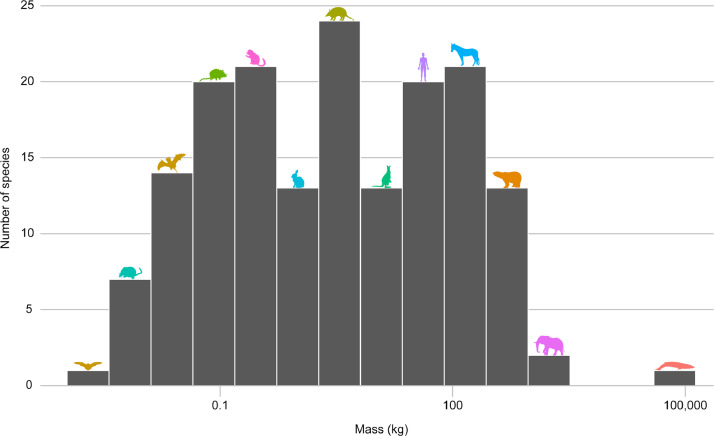
The 174 species included in the meta-analysis ranged in average body mass from 0.0019 kg (bumblebee bat, Craseonycteris thonglongyai) to 70,000 kg (fin whale, Balaenoptera physalus) (Fig. 7).
Fig. 7.
Body mass range of mammalian species included in the meta-analysis. Icons represent some of the species included within each mass bin and are colored by taxonomic order.
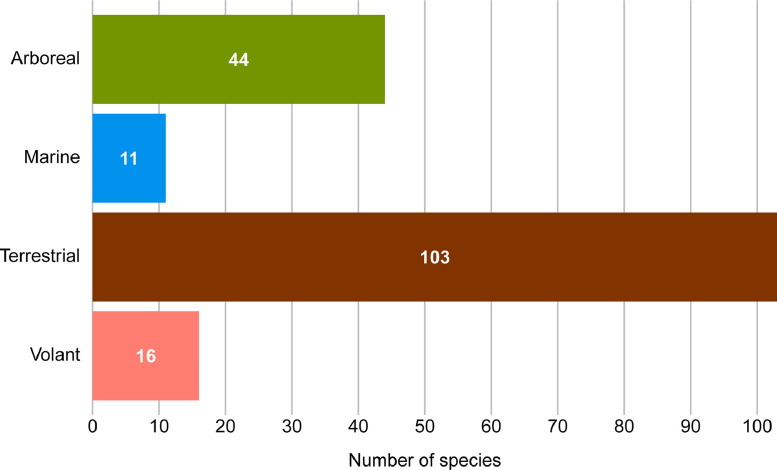
Most species providing skeletal muscle fiber composition data are terrestrial (n = 103), whereas marine (n = 11) species are the least represented (Fig. 8). Arboreal species are those that spend most of the time in the trees and locomote via arboreal quadrupedalism, vertical clinging and leaping, brachiation, suspension, and other scansorial activities. Marine species are those that spend most of the time in the ocean and locomote via swimming, diving, and other natatorial activities. Terrestrial species are those that spend most of the time on the ground and locomote via terrestrial quadrupedalism, bipedalism, or saltation, and may be fossorial, semi-fossorial, cursory, ambulatory, graviportal, generalized, amphibious, or scansorial. Volant species are those that primarily locomote via powered flight.
Fig. 8.
Broad locomotor strategy types employed by species included in the meta-analysis.
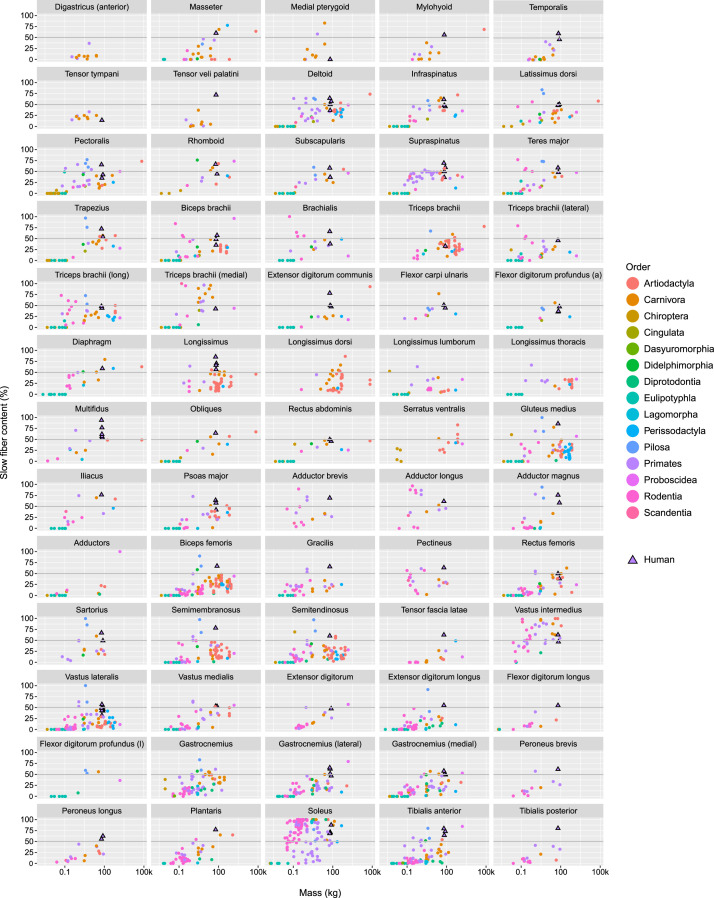
Skeletal muscle fiber composition data was provided for 238 muscles across 11 broad anatomical compartments: head, neck, shoulder, brachium (i.e. upper arm), antebrachium (i.e. lower arm), hand, trunk (i.e. back and abdominal musculature), pelvic/gluteal, thigh, leg and tail. Table 4 quantifies the number of unique skeletal muscle sampling terms (excluding differences in anatomical sampling location from the same muscle such as “superficial,” “deep,” “proximal,” “distal,” etc.) per anatomical compartment, as well as the number of data points per anatomical compartment. The anatomical compartment with the greatest number of unique skeletal muscle sampling terms is the trunk (n = 41). The most heavily sampled anatomical compartments are the leg (n = 684) and thigh (n = 661), followed by the shoulder (n = 340) and trunk (n = 331).
Table 4.
Number of unique skeletal muscle sampling terms and data points per anatomical compartment.
| Anatomical compartment | No. of unique skeletal muscle sampling terms | No. of data points |
|---|---|---|
| Head | 21 | 115 |
| Neck | 20 | 46 |
| Shoulder | 17 | 340 |
| Brachium | 12 | 238 |
| Antebrachium | 21 | 105 |
| Hand | 16 | 21 |
| Trunk | 41 | 331 |
| Pelvic/gluteal | 27 | 210 |
| Thigh | 27 | 661 |
| Leg | 23 | 684 |
| Tail | 13 | 27 |
| Total | 238 | 2,778 |
Sampling richness and taxonomic diversity across skeletal muscles from which fiber composition data was recorded (Table 5). Table 5 quantifies the number of data points, sampled individuals, sampled species, and studies per skeletal muscle, as well as the average (unweighted) slow fiber composition of each sampled skeletal muscle across species. Data from Table 5 is used for Fig. 9, Fig. 10.
Table 5.
Sampling characteristics and average (unweighted) slow fiber composition of each sampled skeletal muscle included in the meta-analysis. If the number of individuals sampled was not reported in the study, a value of “NA” is given in the “No. of individuals” column. Broad muscle terms marked as “undefined” are those from the literature that did not identify the specific muscle bellies included in the sample.
| Muscle | Anatomical compartment | No. of data points | No. of individuals | No. of species | No. of studies | Avg. slow fiber composition (%) |
|---|---|---|---|---|---|---|
| Buccinator | Head | 1 | 6 | 1 | 1 | 13.4 |
| Cheek pouch (undefined) | Head | 1 | 3 | 1 | 1 | 0.0 |
| Cheek pouch retractor | Head | 1 | 3 | 1 | 1 | 15.0 |
| Depressor conchae | Head | 1 | 10 | 1 | 1 | 30.0 |
| Digastricus (anterior) | Head | 10 | 18 | 10 | 1 | 9.0 |
| Digastricus (posterior) | Head | 1 | 7 | 1 | 1 | 2.3 |
| Extraocular | Head | 1 | NA | 1 | 1 | 0.0 |
| Frontalis | Head | 1 | 6 | 1 | 1 | 64.1 |
| Masseter | Head | 26 | 94 | 21 | 13 | 19.9 |
| Medial pterygoid | Head | 10 | 17 | 10 | 1 | 22.2 |
| Mylohyoid | Head | 12 | 21 | 12 | 3 | 20.5 |
| Nasorostralis superficialis | Head | 1 | 4 | 1 | 1 | 52.9 |
| Orbicularis oculi | Head | 3 | 21 | 2 | 3 | 12.5 |
| Orbicularis oris | Head | 1 | 10 | 1 | 1 | 15.0 |
| Orbicularis oris (marginal) | Head | 1 | 5 | 1 | 1 | 34.0 |
| Orbicularis oris (peripheral) | Head | 1 | 5 | 1 | 1 | 27.0 |
| Styloglossus | Head | 2 | 11 | 2 | 1 | 33.8 |
| Temporalis | Head | 18 | 50 | 14 | 7 | 16.9 |
| Tensor tympani | Head | 10 | 17 | 10 | 1 | 21.5 |
| Tensor veli palatini | Head | 10 | 17 | 10 | 1 | 15.0 |
| Tongue (undefined) | Head | 3 | NA | 2 | 2 | 0.0 |
| Biventer cervicis | Neck | 2 | 9 | 2 | 2 | 52.2 |
| Brachiocephalicus | Neck | 2 | 3 | 2 | 2 | 37.7 |
| Cleidocephalicus | Neck | 2 | 15 | 2 | 2 | 17.9 |
| Cleidocervicalis | Neck | 1 | 3 | 1 | 1 | 24.0 |
| Cleidomastoid | Neck | 1 | 3 | 1 | 1 | 23.0 |
| Cleidooccipital | Neck | 1 | 6 | 1 | 1 | 28.0 |
| Cricopharyngeus | Neck | 2 | 16 | 2 | 2 | 40.8 |
| Obliques capitis | Neck | 1 | 6 | 1 | 1 | 39.0 |
| Omotransversarius | Neck | 4 | 34 | 4 | 4 | 32.7 |
| Rectus capitis posterior major | Neck | 2 | 12 | 2 | 2 | 39.2 |
| Rectus capitis posterior minor | Neck | 1 | 6 | 1 | 1 | 23.0 |
| Rhomboideus capitis | Neck | 1 | 6 | 1 | 1 | 56.0 |
| Scalenus | Neck | 3 | 12 | 3 | 3 | 23.0 |
| Semispinalis capitis | Neck | 5 | 21 | 5 | 5 | 45.2 |
| Semispinalis cervicis | Neck | 1 | 6 | 1 | 1 | 38.0 |
| Splenius | Neck | 7 | 29 | 7 | 7 | 33.8 |
| Sternocephalicus | Neck | 3 | 12 | 3 | 3 | 29.2 |
| Sternohyoid | Neck | 3 | 13 | 3 | 3 | 21.9 |
| Sternomastoid | Neck | 3 | 19 | 3 | 3 | 28.0 |
| Thyroarytenoideus | Neck | 1 | 8 | 1 | 1 | 2.5 |
| Atlantoscapularis | Shoulder | 2 | 10 | 2 | 2 | 32.4 |
| Cleidobrachialis | Shoulder | 2 | 12 | 2 | 2 | 20.2 |
| Deltoid | Shoulder | 57 | 244 | 42 | 23 | 30.1 |
| Dorsoepitrochlearis | Shoulder | 1 | 3 | 1 | 1 | 28.0 |
| Infraspinatus | Shoulder | 31 | 152 | 25 | 19 | 27.7 |
| Latissimus dorsi | Shoulder | 29 | 102 | 24 | 22 | 30.7 |
| Pectoralis | Shoulder | 57 | 243 | 51 | 33 | 21.4 |
| Pectoralis abdominis lateralis | Shoulder | 1 | 4 | 1 | 1 | 38.3 |
| Rhomboid | Shoulder | 16 | 63 | 14 | 14 | 39.1 |
| Spinodeltoidius | Shoulder | 1 | 3 | 1 | 1 | 2.0 |
| Spinotrapezius | Shoulder | 2 | 7 | 2 | 2 | 42.3 |
| Subclavius | Shoulder | 1 | 6 | 1 | 1 | 23.3 |
| Subscapularis | Shoulder | 23 | 65 | 22 | 13 | 21.4 |
| Supraspinatus | Shoulder | 51 | 137 | 23 | 20 | 33.2 |
| Teres major | Shoulder | 30 | 87 | 29 | 16 | 27.3 |
| Teres minor | Shoulder | 7 | 26 | 6 | 6 | 44.3 |
| Trapezius | Shoulder | 29 | 151 | 25 | 19 | 30.1 |
| Biceps brachii | Brachium | 42 | 208 | 30 | 21 | 24.6 |
| Biceps brachii (long) | Brachium | 9 | 43 | 7 | 7 | 41.8 |
| Biceps brachii (short) | Brachium | 7 | 34 | 5 | 6 | 26.7 |
| Brachialis | Brachium | 22 | 80 | 21 | 13 | 30.4 |
| Coracobrachialis | Brachium | 5 | 17 | 4 | 5 | 41.3 |
| Tensor fascia antebrachii | Brachium | 2 | 10 | 2 | 2 | 32.1 |
| Triceps brachii | Brachium | 42 | 250 | 20 | 17 | 31.7 |
| Triceps brachii accessorius | Brachium | 1 | 3 | 1 | 1 | 90.0 |
| Triceps brachii (angular) | Brachium | 1 | 4 | 1 | 1 | 24.5 |
| Triceps brachii lateralis | Brachium | 32 | 104 | 29 | 21 | 19.7 |
| Triceps brachii longus | Brachium | 44 | 187 | 33 | 24 | 25.7 |
| Triceps brachii medialis | Brachium | 31 | 113 | 25 | 20 | 42.5 |
| Abductor pollicis longus | Antebrachium | 2 | 9 | 2 | 2 | 43.2 |
| Anconeus | Antebrachium | 3 | 18 | 3 | 3 | 87.6 |
| Brachioradialis | Antebrachium | 9 | 43 | 6 | 7 | 40.4 |
| Extensor carpi radialis | Antebrachium | 7 | 34 | 6 | 7 | 26.0 |
| Extensor carpi ulnaris | Antebrachium | 5 | 24 | 5 | 5 | 38.8 |
| Extensor digiti minimi (quinti) | Antebrachium | 2 | 7 | 2 | 2 | 59.6 |
| Extensor digitorum communis | Antebrachium | 18 | 84 | 16 | 12 | 24.0 |
| Extensor digitorum lateralis | Antebrachium | 2 | 5 | 2 | 2 | 16.7 |
| Extensor indicis proprius | Antebrachium | 1 | 6 | 1 | 1 | 57.7 |
| Extensor pollicis brevis | Antebrachium | 1 | 6 | 1 | 1 | 57.8 |
| Extensor pollicis longus | Antebrachium | 1 | 6 | 1 | 1 | 50.4 |
| Flexor carpi radialis | Antebrachium | 5 | 31 | 5 | 5 | 29.2 |
| Flexor carpi ulnaris | Antebrachium | 11 | 59 | 10 | 10 | 38.6 |
| Flexor digitorum communis | Antebrachium | 3 | 11 | 3 | 3 | 27.8 |
| Flexor digitorum profundus | Antebrachium | 15 | 73 | 13 | 9 | 21.5 |
| Flexor digitorum superficialis | Antebrachium | 8 | 55 | 7 | 8 | 40.2 |
| Flexor pollicis longus | Antebrachium | 1 | 6 | 1 | 1 | 44.1 |
| Palmaris longus | Antebrachium | 4 | 21 | 4 | 3 | 43.9 |
| Pronator quadratus | Antebrachium | 2 | 9 | 2 | 2 | 56.8 |
| Pronator teres | Antebrachium | 3 | 13 | 3 | 3 | 34.3 |
| Supinator | Antebrachium | 2 | 9 | 2 | 2 | 41.3 |
| Abductor digiti minimi | Hand | 2 | 12 | 1 | 2 | 56.5 |
| Abductor pollicis brevis | Hand | 2 | 12 | 1 | 2 | 66.0 |
| Adductor pollicis | Hand | 2 | 12 | 1 | 2 | 78.9 |
| Extensor digitorum brevis | Hand | 2 | 7 | 2 | 2 | 53.7 |
| Flexor digiti minimi brevis | Hand | 1 | 6 | 1 | 1 | 68.2 |
| Flexor digitorum brevis | Hand | 1 | 6 | 1 | 1 | 44.5 |
| Flexor pollicis brevis | Hand | 1 | 6 | 1 | 1 | 76.7 |
| Interosseous (Dorsal, 1st) | Hand | 2 | 12 | 1 | 2 | 62.5 |
| Interosseous (Dorsal, 4th) | Hand | 1 | 6 | 1 | 1 | 55.4 |
| Interosseous (Palmar, 1st) | Hand | 1 | 6 | 1 | 1 | 60.6 |
| Interosseous (Palmar, 3rd) | Hand | 1 | 6 | 1 | 1 | 46.9 |
| Lumbrical (1st) | Hand | 1 | 6 | 1 | 1 | 82.7 |
| Lumbrical (4th) | Hand | 1 | 6 | 1 | 1 | 86.3 |
| Lumbricals (undefined) | Hand | 1 | 4 | 1 | 1 | 38.4 |
| Opponens digiti minimi | Hand | 1 | 6 | 1 | 1 | 82.9 |
| Opponens pollici | Hand | 1 | 6 | 1 | 1 | 74.7 |
| Diaphragm | Trunk | 27 | 171 | 21 | 18 | 25.5 |
| Erector spinae | Trunk | 2 | 7 | 2 | 2 | 49.9 |
| Extensor caudae lateralis | Trunk | 2 | 10 | 2 | 2 | 38.5 |
| Extensor caudae medialis | Trunk | 3 | 14 | 3 | 3 | 54.2 |
| Flexor caudae lateralis | Trunk | 1 | 3 | 1 | 1 | 53.0 |
| Flexor caudae medialis | Trunk | 1 | 3 | 1 | 1 | 50.0 |
| Hypaxialis lumborum | Trunk | 2 | 7 | 2 | 2 | 52.5 |
| Iliocostalis | Trunk | 6 | 28 | 6 | 4 | 38.8 |
| Iliocostalis (lumbar) | Trunk | 6 | 23 | 6 | 3 | 46.4 |
| Iliocostalis (thoracic) | Trunk | 6 | 20 | 6 | 3 | 40.9 |
| Intercostals | Trunk | 4 | 14 | 4 | 3 | 51.8 |
| Intermammillares mammilloaccessorii L4 | Trunk | 1 | 3 | 1 | 1 | 67.5 |
| Intermammillares mammilloaccessorii L6 | Trunk | 1 | 3 | 1 | 1 | 81.2 |
| Interpinales | Trunk | 5 | 17 | 5 | 4 | 29.5 |
| Intertransversarii | Trunk | 5 | 22 | 5 | 5 | 52.8 |
| Longissimus | Trunk | 53 | 411 | 21 | 30 | 29.5 |
| Longissimus capitis | Trunk | 1 | 6 | 1 | 1 | 33.0 |
| Longissimus dorsi | Trunk | 36 | 749 | 23 | 16 | 29.7 |
| Longissimus (lumbar) | Trunk | 27 | 118 | 21 | 10 | 14.8 |
| Longissimus (thoracic) | Trunk | 24 | 133 | 17 | 10 | 21.4 |
| Longissimus (thoracic and lumbar) | Trunk | 4 | 17 | 2 | 3 | 11.6 |
| Longissimus et multifidus (L3) | Trunk | 2 | 38 | 1 | 1 | 21.4 |
| Longissimus et multifidus (Th9) | Trunk | 1 | 21 | 1 | 1 | 73.7 |
| Multifidus | Trunk | 16 | 104 | 12 | 14 | 42.1 |
| Multifidus (lumbar) | Trunk | 4 | 31 | 4 | 2 | 48.3 |
| Multifidus (thoracic) | Trunk | 3 | 10 | 3 | 1 | 42.0 |
| Obliques | Trunk | 19 | 57 | 19 | 11 | 23.7 |
| Panniculus carnosus | Trunk | 1 | 4 | 1 | 1 | 66.2 |
| Quadratus lumborum | Trunk | 4 | 12 | 4 | 3 | 31.4 |
| Rectus abdominis | Trunk | 19 | 57 | 18 | 12 | 22.0 |
| Rotatores | Trunk | 3 | 6 | 3 | 2 | 11.3 |
| Sacrospinalis | Trunk | 5 | 15 | 5 | 4 | 5.6 |
| Semispinalis and longissimus | Trunk | 1 | 3 | 1 | 1 | 46.3 |
| Serratus abdominis | Trunk | 9 | 16 | 9 | 2 | 0.0 |
| Serratus anterior | Trunk | 2 | 7 | 2 | 2 | 52.0 |
| Serratus cervicis | Trunk | 1 | 3 | 1 | 1 | 60.6 |
| Serratus dorsalis | Trunk | 1 | 3 | 1 | 1 | 57.0 |
| Serratus ventralis | Trunk | 15 | 75 | 10 | 13 | 36.6 |
| Spinalis transversospinalis (lumbar) | Trunk | 1 | 3 | 1 | 1 | 30.4 |
| Spinalis transversospinalis (thoracic) | Trunk | 2 | 9 | 2 | 2 | 69.4 |
| Transversus abdominis | Trunk | 5 | 17 | 5 | 5 | 41.8 |
| Capsularis | Pelvic/gluteal | 1 | 3 | 1 | 1 | 99.0 |
| Coccygeus | Pelvic/gluteal | 1 | 1 | 1 | 1 | 3.0 |
| Cremaster | Pelvic/gluteal | 1 | 3 | 1 | 1 | 33.0 |
| Gemelli (undefined) | Pelvic/gluteal | 1 | 3 | 1 | 1 | 69.0 |
| Gemellus inferior | Pelvic/gluteal | 3 | 10 | 3 | 3 | 34.1 |
| Gemellus superior | Pelvic/gluteal | 3 | 10 | 3 | 3 | 51.3 |
| Gluteofemoralis | Pelvic/gluteal | 4 | 19 | 4 | 2 | 7.6 |
| Gluteals (undefined) | Pelvic/gluteal | 4 | 21 | 3 | 3 | 29.2 |
| Gluteobiceps | Pelvic/gluteal | 1 | 7 | 1 | 1 | 19.8 |
| Gluteus accessorius | Pelvic/gluteal | 2 | 8 | 2 | 2 | 61.2 |
| Gluteus maximus | Pelvic/gluteal | 6 | 36 | 5 | 4 | 34.1 |
| Gluteus maximus ischiofemoralis | Pelvic/gluteal | 2 | 4 | 2 | 2 | 41.1 |
| Gluteus maximus proprius | Pelvic/gluteal | 2 | 4 | 2 | 2 | 19.2 |
| Gluteus medius | Pelvic/gluteal | 70 | 436 | 35 | 37 | 24.7 |
| Gluteus minimus | Pelvic/gluteal | 8 | 50 | 8 | 5 | 40.0 |
| Gluteus profundus | Pelvic/gluteal | 2 | 12 | 2 | 2 | 69.8 |
| Gluteus superficialis | Pelvic/gluteal | 8 | 36 | 7 | 6 | 15.7 |
| Iliacus | Pelvic/gluteal | 18 | 66 | 18 | 9 | 27.6 |
| Iliopsoas | Pelvic/gluteal | 2 | 9 | 2 | 2 | 46.7 |
| Levator ani | Pelvic/gluteal | 1 | NA | 1 | 1 | 0.0 |
| Obturator externus | Pelvic/gluteal | 5 | 16 | 5 | 5 | 37.7 |
| Obturator internus | Pelvic/gluteal | 4 | 13 | 4 | 4 | 38.9 |
| Piriformis | Pelvic/gluteal | 7 | 47 | 7 | 5 | 43.5 |
| Psoas major | Pelvic/gluteal | 38 | 189 | 25 | 22 | 23.8 |
| Psoas minor | Pelvic/gluteal | 4 | 39 | 3 | 4 | 33.2 |
| Psoas (major et minor) | Pelvic/gluteal | 3 | 6 | 3 | 2 | 1.0 |
| Quadratus femoris | Pelvic/gluteal | 9 | 52 | 8 | 8 | 77.0 |
| Abductor | Thigh | 1 | 3 | 1 | 1 | 20.0 |
| Adductor brevis | Thigh | 15 | 69 | 11 | 9 | 38.4 |
| Adductor longus | Thigh | 16 | 64 | 11 | 11 | 51.7 |
| Adductor magnus | Thigh | 22 | 83 | 13 | 15 | 22.3 |
| Adductor magnus ischiocondylaris | Thigh | 2 | 4 | 2 | 2 | 48.3 |
| Adductor magnus pubofemoralis | Thigh | 2 | 4 | 2 | 2 | 39.1 |
| Adductor tertius | Thigh | 1 | 6 | 1 | 1 | 1.2 |
| Adductors (undefined) | Thigh | 17 | 56 | 16 | 10 | 10.3 |
| Biceps femoris | Thigh | 102 | 648 | 46 | 47 | 21.0 |
| Biceps femoris (long) | Thigh | 3 | 10 | 3 | 3 | 55.0 |
| Biceps femoris (short) | Thigh | 3 | 10 | 3 | 3 | 40.9 |
| Cruralis | Thigh | 1 | 1 | 1 | 1 | 53.0 |
| Femorococcygeus | Thigh | 3 | 18 | 3 | 1 | 16.5 |
| Gracilis | Thigh | 34 | 152 | 26 | 21 | 17.6 |
| Hamstring (undefined) | Thigh | 1 | NA | 1 | 1 | 62.0 |
| Pectineus | Thigh | 19 | 76 | 16 | 12 | 33.2 |
| Quadriceps (undefined) | Thigh | 12 | 138 | 6 | 8 | 18.3 |
| Rectus femoris | Thigh | 48 | 185 | 35 | 31 | 17.7 |
| Sartorius | Thigh | 18 | 77 | 14 | 14 | 37.8 |
| Semimembranosus | Thigh | 71 | 416 | 38 | 38 | 21.2 |
| Semimembranosus accessorius | Thigh | 1 | 1 | 1 | 1 | 99.0 |
| Semitendinosus | Thigh | 76 | 339 | 45 | 46 | 20.4 |
| Tensor fascia latae | Thigh | 16 | 51 | 11 | 14 | 13.1 |
| Tenuissimus | Thigh | 1 | 1 | 1 | 1 | 20.0 |
| Vastus intermedius | Thigh | 49 | 203 | 24 | 30 | 60.8 |
| Vastus lateralis | Thigh | 91 | 540 | 56 | 54 | 16.4 |
| Vastus medialis | Thigh | 45 | 171 | 35 | 28 | 19.7 |
| Extensor digitorum | Leg | 26 | 169 | 13 | 20 | 15.1 |
| Extensor digitorum lateralis | Leg | 3 | 11 | 3 | 3 | 18.1 |
| Extensor digitorum longus | Leg | 59 | 265 | 36 | 32 | 10.9 |
| Extensor hallucis longus | Leg | 11 | 51 | 8 | 8 | 22.1 |
| Flexor digitorum | Leg | 2 | 10 | 2 | 2 | 31.4 |
| Flexor digitorum fibularis | Leg | 6 | 24 | 6 | 4 | 26.9 |
| Flexor digitorum longus | Leg | 13 | 56 | 10 | 8 | 13.7 |
| Flexor digitorum profundus | Leg | 11 | 22 | 11 | 5 | 19.3 |
| Flexor digitorum superficialis | Leg | 10 | 61 | 7 | 9 | 42.2 |
| Flexor digitorum tibialis | Leg | 5 | 22 | 5 | 3 | 18.2 |
| Flexor hallucis longus | Leg | 11 | 58 | 9 | 9 | 22.1 |
| Gastrocnemius | Leg | 71 | 237 | 44 | 32 | 25.4 |
| Gastrocnemius lateralis | Leg | 61 | 303 | 43 | 45 | 20.1 |
| Gastrocnemius medialis | Leg | 66 | 321 | 46 | 47 | 19.9 |
| Peroneals (undefined) | Leg | 2 | 9 | 1 | 1 | 12.5 |
| Peroneus brevis | Leg | 12 | 50 | 10 | 7 | 21.9 |
| Peroneus longus | Leg | 17 | 72 | 12 | 12 | 23.1 |
| Peroneus tertius | Leg | 4 | 20 | 4 | 3 | 10.0 |
| Plantaris | Leg | 49 | 224 | 26 | 34 | 17.7 |
| Popliteus | Leg | 7 | 21 | 7 | 5 | 31.3 |
| Soleus | Leg | 141 | 820 | 58 | 87 | 69.4 |
| Tibialis anterior | Leg | 81 | 301 | 46 | 50 | 16.8 |
| Tibialis posterior | Leg | 15 | 67 | 11 | 11 | 18.5 |
| Abductor caudae externus | Tail | 1 | 7 | 1 | 1 | 19.8 |
| Abductor caudae internus | Tail | 1 | 7 | 1 | 1 | 16.8 |
| Caudofemoralis | Tail | 5 | 7 | 4 | 3 | 1.4 |
| Flexor caudae brevis | Tail | 1 | 7 | 1 | 1 | 21.6 |
| Flexor caudae longus | Tail | 1 | 7 | 1 | 1 | 27.4 |
| Sacrocaudalis | Tail | 2 | 13 | 2 | 2 | 10.6 |
| Tail | Tail | 2 | 12 | 1 | 2 | 24.5 |
| Tail (distal) | Tail | 4 | 23 | 3 | 3 | 24.9 |
| Tail (mid dorsal) | Tail | 1 | 7 | 1 | 1 | 41.6 |
| Tail (mid ventral) | Tail | 1 | 7 | 1 | 1 | 40.0 |
| Tail (proximal) | Tail | 4 | 23 | 3 | 3 | 11.5 |
| Tail (tip) | Tail | 1 | 7 | 1 | 1 | 5.0 |
| Tail (transitional) | Tail | 3 | 16 | 3 | 2 | 20.3 |
Fig. 9.
The most represented skeletal muscles in the literature by (A) number of data points, (B) number of individuals sampled across taxa, (C) number of species, and (D) number of studies.
Fig. 10.
The relationship between skeletal muscle slow fiber content (% fibers) and body mass (kg) for muscles that are represented in 10 or more unique species (n = 65 muscles). Gray line represents a slow fiber composition of 50%, above which denotes muscles that are slow fiber dominant. Species (data points) are colored by taxonomic order. Humans within the order Primates are distinguished by outlined triangles.
Certain skeletal muscles are more represented than others in the literature, with the m. soleus being the most represented (Fig. 9). The mm. soleus, biceps femoris, vastus lateralis, tibialis anterior, semitendinosus, semimembranosus, gastrocnemius, gluteus medius and extensor digitorum longus are the most heavily sampled muscles in terms number of data points (Fig. 9A). The mm. soleus, longissimus dorsi, biceps femoris, vastus lateralis, gluteus medius, semimembranosus, longissimus, semitendinosus, gastrocnemius, tibialis anterior, and extensor digitorum longus are the most heavily sampled muscles in terms number of individuals (Fig. 9B). The mm. soleus, vastus lateralis, pectoralis, biceps femoris, gastrocnemius, tibialis anterior, semitendinosus, deltoid, semimembranosus, and extensor digitorum longus are the most heavily sampled muscles in terms number of unique species (Fig. 9C). The mm. soleus, vastus lateralis, tibialis anterior, biceps femoris, gastrocnemius, semitendinosus, semimembranosus, gluteus medius, plantaris, and pectoralis are the most heavily sampled muscles in terms number of studies (Fig. 9D).
Sixty-five skeletal muscles are represented in 10 or more unique species. For these skeletal muscles, the relationship between skeletal muscle slow fiber content (% fibers) and average species body mass (kg) is depicted in Fig. 10.
4. Experimental Design, Materials and Methods
4.1. Eligibility Criteria and Study Selection
Published, peer-reviewed data were compiled for meta-analysis between June 1 2021 and November 30 2022 following a structure similar to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [2], facilitating transparency and ease of reproducibility. Terms relating to mammalian skeletal muscle fiber composition (fiber type slow fast myofiber OR myosin OR “heavy chain” “skeletal muscle” -Xenopus -avian -denervated -transition -develop -developing -stimulated -training -switch -aging -athlete -cardiomyopathy -spaceflight -Duchenne -immobilization -suspension) were queried using academic search systems (Google Scholar, PubMed, and JSTOR) and library databases for relevant primary articles. Reference lists in relevant articles were thoroughly investigated for eligible studies. No restrictions were set on the date of publication.
Articles identified via academic search systems were screened for relevancy by reviewing published abstracts and data tables. When relevant articles were identified, the article DOI and/or citation were recorded and the article PDF and associated supplemental materials were downloaded and renamed under this format: “PublicationDate_FirstAuthorLastName_Species.pdf.” If multiple studies from the same first author from the same year exist, the second and third authors' last names were added to the file name.
Relevant articles were then assessed for eligibility. Eligible studies were published in the English language and provided muscle fiber composition data from at least one skeletal muscle from a species belonging to class Mammalia. Studies were not considered eligible for the meta-analysis for the following reasons: (i) muscle fiber composition data were not provided, (ii) muscle fiber composition data provided were ambiguous, (iii) muscle fiber composition data were provided for an unidentified muscle, (iv) muscle fiber composition data were provided for cardiac fibers, (v) muscle fiber composition data were provided for single muscle fibers, (vi) muscle fiber composition data were provided for a mammal under experimental manipulation (e.g. treadmill running, limb immobilization or dietary supplementation regime), (vii) the study duplicated another dataset, (viii) the source was secondary, and (ix) the source was an abstract (Fig. 1).
4.2. Data Extraction
The following data were recorded from the text, tables, figures, and supplementary materials of eligible studies when available: sampled species’ common name, scientific name, sex, age, breed/strain, method for classification of muscle fiber composition, slow muscle fiber terminology used (e.g. MyHC I, MHC I, Type I, beta, slow oxidative, red or slow twitch), number of individuals sampled, average body mass (kg), muscle(s) sampled and average percent slow fiber content (% fibers).
In most studies, body mass was reported; for those studies that did not report a body mass the species mean was taken from Clarke et al. [3] or Genoud et al. [4]. When muscle fiber content was recorded as the percentage of fast muscle fibers within a skeletal muscle, the proportion of slow muscle fibers was derived as 100 minus the total proportion of fast muscle fibers. If slow muscle fiber content was reported from multiple superficial and deep sampling sites across a single muscle, the average across the sampling sites was recorded as the percent slow muscle fiber content of that muscle. Skeletal muscle terms were then assessed for redundancy and updated when necessary to reflect modern anatomical terminology.
Given that most studies were published between 1965 and 1999, binomial and common names were updated when necessary to reflect the current understanding of phylogenetic relationships. Taxonomic data (i.e. class, infraclass, magnorder, superorder, order, suborder, infraorder, parvorder, clade, superfamily, family, subfamily, tribe, and subspecies) provided by NCBI Taxonomy [11] was recorded for each species when available. Species were then categorized into one of four locomotor types based on how the species navigates through its habitat matrix: arboreal, marine, terrestrial, and volant (Fig. 8).
Ethics Statements
This work meets all of the ethical requirements required for publication in Data in Brief. This work did not involve the use of animal or human subjects.
CRediT authorship contribution statement
Samantha R. Queeno: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization. Kirstin N. Sterner: Conceptualization, Supervision, Writing – review & editing. Matthew C. O'Neill: Conceptualization, Investigation, Data curation, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Thanks to P.J. Reiser, C.M. Orr and T.D. Capellini for their contributions to the corresponding article [1]. Thanks also to the Milwaukee Zoo and Smithsonian Institution for access to cadaveric material, and to R. Lieber for sharing his human MyHC dataset.
The study related to the corresponding article [1] was supported by the National Science Foundation (BCS 2018436 and BCS 1945809) and the Leakey Foundation.
Data Availability
References
- 1.Queeno S.R., Reiser P.J., Orr C.M., Capellini T.D., Sterner K.N., O'Neill M.C. Human and African ape myosin heavy chain content and the evolution of hominin skeletal muscle. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2023;281 doi: 10.1016/j.cbpa.2023.111415. [DOI] [PubMed] [Google Scholar]
- 2.Moher D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151:264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 3.Clarke A., Rothery P., Isaac N.J.B. Scaling of basal metabolic rate with body mass and temperature in mammals. J. Anim. Ecol. 2010;79:610–619. doi: 10.1111/j.1365-2656.2010.01672.x. [DOI] [PubMed] [Google Scholar]
- 4.Genoud M., Isler K., Martin R.D. Comparative analyses of basal rate of metabolism in mammals: data selection does matter. Biol. Rev. 2018;93:404–438. doi: 10.1111/brv.12350. [DOI] [PubMed] [Google Scholar]
- 5.O'Neill M.C., Umberger B.R., Holowka N.B., Larson S.G., Reiser P.J. Chimpanzee super strength and human skeletal muscle evolution. Proc. Natl. Acad. Sci. 2017;114:7343–7348. doi: 10.1073/pnas.1619071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edström L., Nyström B. Histochemical types and sizes of fibres in normal human muscles. a biopsy study. Acta Neurol. Scand. 1969;45:257–269. doi: 10.1111/j.1600-0404.1969.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 7.Plaghki L., Goffart M., Beckers-Bleukx G., Moureau-Lebbe A. Some characteristics of the hind limb muscles in the leaping night monkey Aotus trivirgatus (primates, anthropoidae, cebidae) Comp. Biochem. Physiol. A Physiol. 1981;70:341–349. doi: 10.1016/0300-9629(81)90188-2. [DOI] [Google Scholar]
- 8.Sickles D.W., Pinkstaff C.A. Comparative histochemical study of prosimian primate hindlimb muscles. II. populations of fiber types. Am. J. Anat. 1981;160:187–194. doi: 10.1002/aja.1001600205. [DOI] [PubMed] [Google Scholar]
- 9.Gorza L. Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J. Histochem. Cytochem. 1990;38:257–265. doi: 10.1177/38.2.2137154. [DOI] [PubMed] [Google Scholar]
- 10.Eizema K., van den Burg M., Kiri A., Dingboom E.G., van Oudheusden H., Goldspink G., Weijs W.A. Differential expression of equine myosin heavy-chain mRNA and protein isoforms in a limb muscle. J. Histochem. Cytochem. 2003;51:1207–1216. doi: 10.1177/002215540305100911. [DOI] [PubMed] [Google Scholar]
- 11.Schoch C.L., Ciufo S., Domrachev M., Hotton C.L., Kannan S., Khovanskaya R., Leipe D., Mcveigh R., O’Neill K., Robbertse B., Sharma S., Soussov V., Sullivan J.P., Sun L., Turner S., Karsch-Mizrachi I. NCBI taxonomy: a comprehensive update on curation, resources and tools. Database. 2020 doi: 10.1093/database/baaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.