Abstract
Objective
This study was performed to determine the effect of fasting on reproducibility of the glucose tolerance test. Due to individual variation in animal feeding behaviors, fasting animals prior to metabolic and behavioral experiments is widely held to reduce inter-subject variation in glucose and metabolic parameters of preclinical rodent models. Reducing variability is especially important for studies where initial metabolite levels can influence the magnitude of experimental interventions, but fasting also imposes stress that may distort the variables of interest. One such intervention is the glucose tolerance test (GTT) which measures the maximum response and recovery following a bolus of exogenous glucose. We sought to investigate how fasting affects the response of individual mice to a GTT.
Methods
Using simultaneous continuous glucose monitoring (CGM) and indirect calorimetry, we quantified blood glucose, physical activity, body temperature, metabolic rates, and food consumption levels on a minute-to-minute basis in adult male mice for 4 weeks. We tested the effects of a 4-h or 18-h fast on the GTT to examine the effect of food withdrawal in light or dark photoperiods. Studies were also performed with 4-h fasting in additional mice without implanted CGM probes.
Results
Contrary to our expectations, a 4-h fast during the light photoperiod promotes a paradoxical increase in inter-animal variation in metabolic rate, physical activity, body temperature, glycemia, and glucose tolerance. This hyperglycemic and hyper-metabolic phenotype promotes increased corticosterone levels and is consistent with a behavioral stress response to food deprivation, even in well-fed mice. We find that mice undergoing an 18-h fast entered torpor, a hibernation-like state. In addition to low body temperature and metabolic rate, torpor is also associated with glucose levels 56 mg/dl lower than those seen in mice with ad libitum access to food. Moreover, the time spent in torpor affects the response to a GTT.
Conclusion
Our results suggest fasting mice before glucose tolerance testing, and perhaps other experiments, can have the opposite of the intended effect where fasting can increase, rather than decrease, experimental variability.
Keywords: Standardization, Reproducibility, Method development, Glucose tolerance test, Indirect calorimetry, Continuous glucose monitoring
Highlights
-
•
Ad libitum mice maintain tight control of blood glucose levels.
-
•
Fasting increases the variation in glucose tolerance testing compared to mice with ad libitum access to food.
-
•
Fasting mice in the daytime increases glucose levels, metabolic rate, physical activity, and body temperature.
-
•
Prolonged fasting in the dark photoperiod drives mice into torpor.
1. Introduction
The glucose tolerance test (GTT), which measures circulating blood glucose levels following a bolus of exogenous glucose, is a fast, inexpensive, and widely used technique for comparing metabolic status amongst experimental animals. However, both the reliability and validity of GTTs are contingent upon the experimental protocols used. Multiple factors affect the sensitivity of GTT to measure glucose homeostasis including recent prandial history and environmental stressors. Standardized protocols for the GTT have yet to be broadly adopted [[1], [2], [3], [4], [5]].
To increase the interpretability of the responses, exogenous glucose should be administered to animals in the post-absorptive state. Additionally, by “resetting” the metabolism of experimental animals through fasting, it is believed that inter-subject glycemic variability will be reduced. The consequences of reduced variation are to increase the statistical power of the GTT, reduce the number of experimental animals required to detect a difference between groups and improve reproducibility of experiments utilizing GTTs. Thus, fasting has become a universal feature of efforts to standardize GTT protocols to harmonize glycemic states. Methodological recommendations have suggested that fasting for different durations may be optimal for reducing variation, including 2, 4, 6, or 16–18 h “overnight” fasting. It has been acknowledged that the metabolic impact of fasting during the light photoperiod, when mice graze to maintain levels of glycemia necessary to maintain euthermia, may differ from imposing fasting during the dark photoperiod when mice consume most of their nutrition [6,7]. However, the basic assumption that fasting reduces glycemic variability has not been formally tested in mice.
Modern technology now allows for continuous minute-to-minute glucose monitoring to measure glycemia without repeated blood draws in mice [[8], [9], [10]], allowing for the characterization of glycemic variability on time scales not permitted by intermittent sampling methods. Implantation of continuous glucose monitors in mice is an invasive procedure and does not obviate the utility of the classical glucose tolerance test. Ideally, insights gained from continuous glucose monitoring would allow for the optimization of the standard operating procedures of the GTT to maximize its power and physiologic validity. In this study, we sought to investigate whether and under what circumstances fasting could decrease the levels of experimental variation in the glucose tolerance test using continuous glucose monitoring (CGM) and indirect calorimetry.
2. Results
2.1. Circadian patterns in glycemia and metabolism
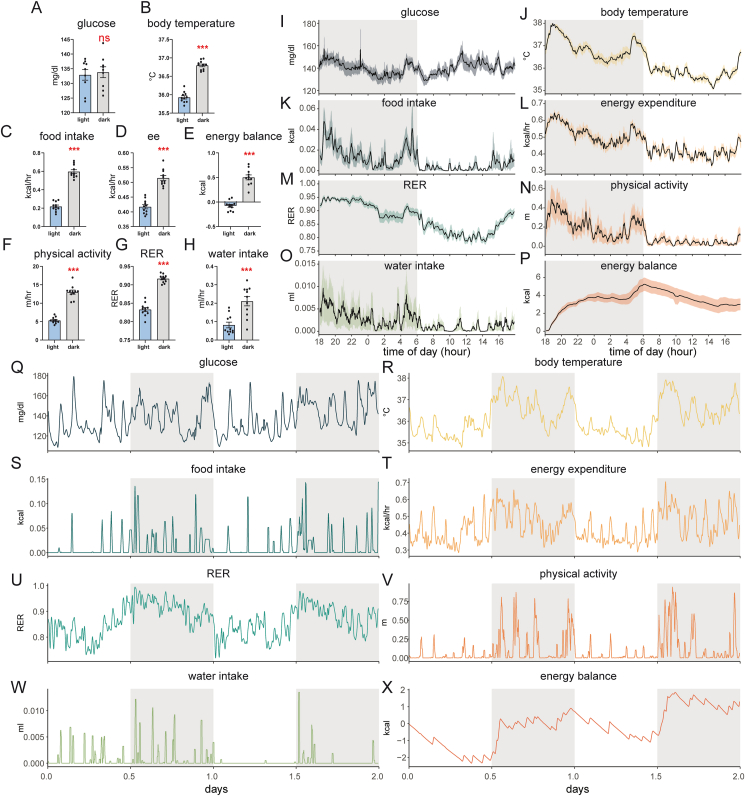
Humans and mice share many common aspects of metabolic regulation. CGM recordings of interstitial fluid in nondiabetic volunteers find similar levels of glucose in day and night monitoring periods [11]. We find that blood glucose levels in mice fed a standard chow diet are also similar between light and dark photoperiods, consistent with prior results using CGM in pregnant or pre-diabetic NOD mice [10,12]. In stark contrast, all other measured physiological parameters in mice exhibit substantial circadian variation (Figure 1). We find that dark photoperiods are associated with significant increases in body temperature, food intake, water intake, metabolic rate, and physical activity. These nocturnal behaviors promote positive energy balance (i.e., greater caloric intake than energy expenditure) and increased carbohydrate oxidation (denoted by elevated RER) during the dark photoperiod. Despite these large changes in other physiological parameters, glycemia remains surprisingly consistent through light and dark photoperiods. Given this glycemic stability, we sought to understand whether fasting could further lower the variability of metabolic parameters compared to mice with “ad libitum” unrestricted access to food. Due to the different physiological states of the mice, we examined the effect of fasting in both photoperiods.
Figure 1.
Circadian patterns in glycemia and metabolism. Representations of recordings with continuous glucose monitoring (CGM) and indirect calorimetry at 23 °C. A–H photoperiod average ± SEM n = 11 mice over 5 d. I–P average ± SEM recordings for n = 11 mice every 2 min for 1 d. Q-X recordings of a single mouse over 2 d. A, I, Q) blood glucose, B, J, R) body temperature, C, K, S) food intake, D, L, T) energy expenditure, E, P, X) energy balance (food intake minus energy expenditure) F, N, V) in-cage physical activity (beam break distance traveled), G, M, U) respiratory exchange ratio, H, O, W) water intake. Statistics on A–H by paired t-test; ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Wild-type C57Bl/6J male mice on a standard chow diet. Shaded areas represent the 12-h dark photoperiod.
2.2. Food restriction increases the variability of the glucose tolerance test
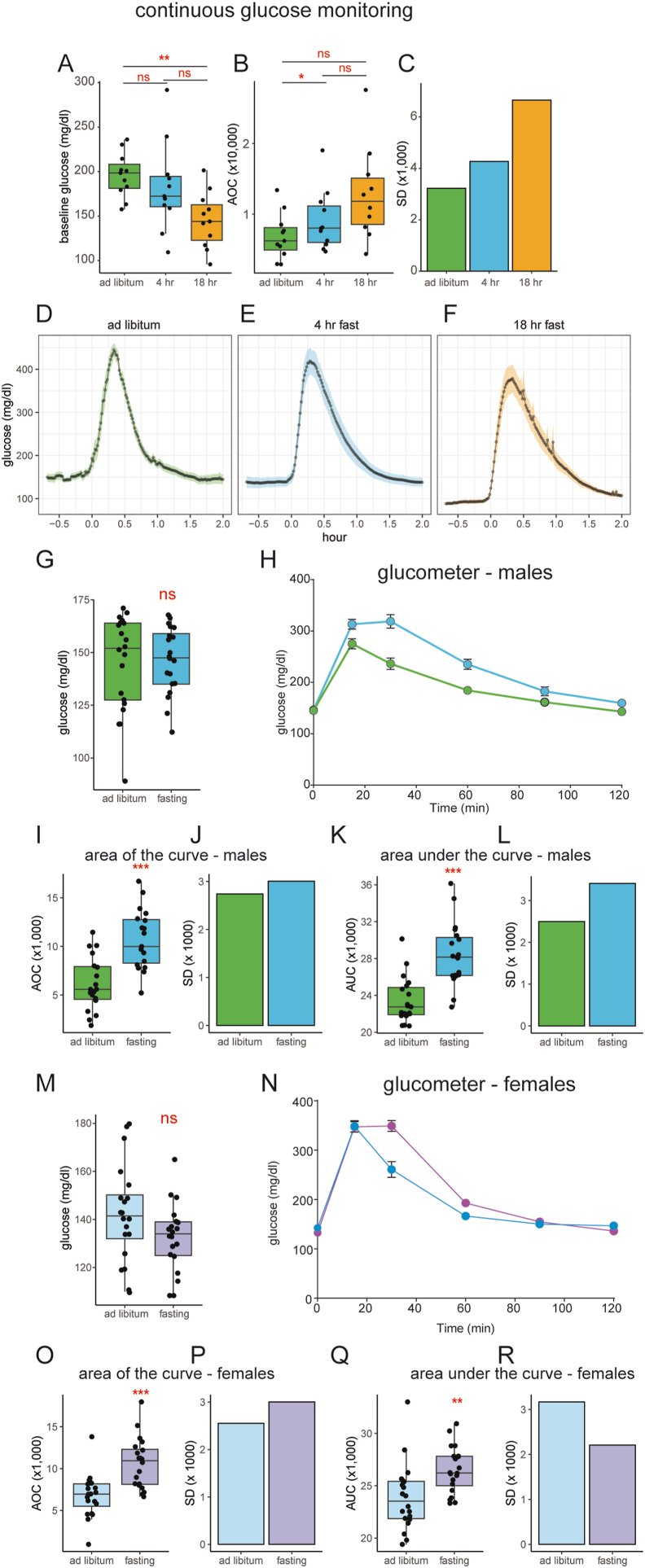
Our central question is whether fasting reduces the inter-subject glycemic variability of a GTT and in turn, increases its statistical power. To address this point, we performed continuous glucose monitoring in mice undergoing a GTT. Recordings were made under conditions of 1) unrestricted ad libitum access to food, 2) a 4-h light-photoperiod fast, or 3) an 18-h overnight fast. The GTT for all mice was performed at the same time of day, 1200 h, halfway through the light photoperiod. Mice with ad libitum access to food had higher average baseline glucose levels than mice following an 18-h fast but not a 4-h fast (Figure 2A). To account for differences in baseline glucose levels, we calculated the area of the curve (AOC), where baseline values for each mouse are subtracted from the area under the curve as described [4]. Despite receiving the same dose of glucose, the AOC was the lowest for ad libitum mice, followed by 4-h fast and 18-h fast respectively (Figure 2B). Importantly, we observe that ad libitum-fed mice have approximately half the variation in glucose excursion of overnight fasted mice as quantified by the standard deviation in the AOC (Figure 2C). The glucose excursion curve (GEC) shapes during the GTT were similar. However, the ad libitum mice had a sharper peak and more rapid drop in glucose levels when compared to 4-h or 18-h fasted mice (Figure 2D–F).
Figure 2.
Fasting-induced variation in glucose tolerance testing.A–F: Recordings with continuous glucose monitoring (CGM). A) baseline glucose levels: the mean of 30 min recordings prior to GTT glucose bolus. B) Area Of the Curve (AOC) of the glucose excursion curve GEC in (D–F). C) standard deviation (SD) of AOC in B. D–F) CGM GTT during ad libitum, 4-h or 18-h fasting for n = 11 mice. G–R) Recordings with a handheld glucometer for male (G–L) and female (M–R) mice G, M) baseline glucose levels. H, N) glucose tolerance tests under ad libitum and 4-h fasted conditions, I, O) AOC of the GTT, J, P) SD of AOC. K, Q) Area under the Curve (AUC) of the GTT. L, R) SD of AUC. n = 20 mice per group. ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
To exclude the possibility that these results are secondary to the implantation of telemetry probes, we sought to verify these results in an independent cohort of mice that have not undergone surgery. This independent cohort was examined under ad libitum food access or following a 4-h fast. There were no significant differences in baseline glucose when comparing ad libitum or 4-h fasted mice in either male or female cohorts (Figure 2G, M). In both sexes, we observe lower and smaller GEC in ad libitum fed mice during the GTT (Figure 2H, N). This experiment produces similar results to those seen with CGM recordings, with greater AOC values in fasted mice compared to ad libitum-fed mice (Figure 2I, O). Fasted male mice have a 10% higher variation in the AOC and a 36% greater variation in AUC. Fasted female mice have an 18% greater variation in AOC but a 30% lower variation when AUC is calculated. (Figure 2G–L). These results find larger variations in the AOC from GTT of 4-h fasted mice compared to non-fasted animals, similar to the CGM findings. In contrast, female mice exhibit trends dependent on the statistical analysis implemented (Figure 2M–R).
2.3. Physiologic variability under ad libitum and 4-h fasting conditions
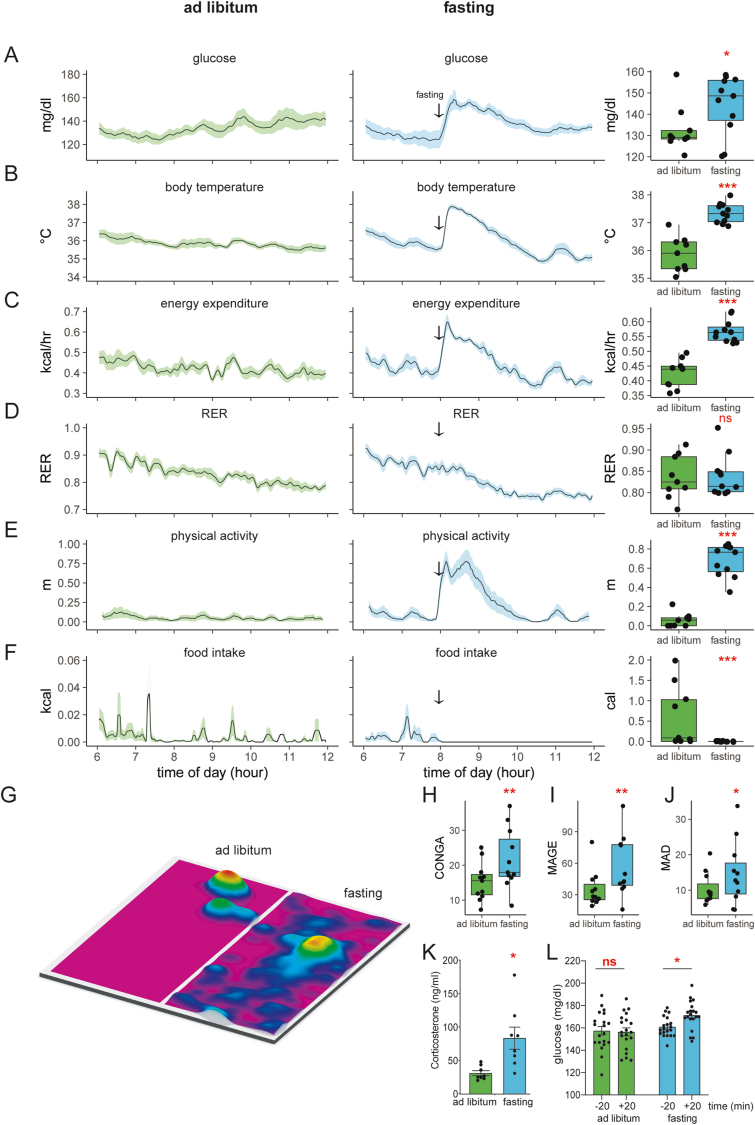
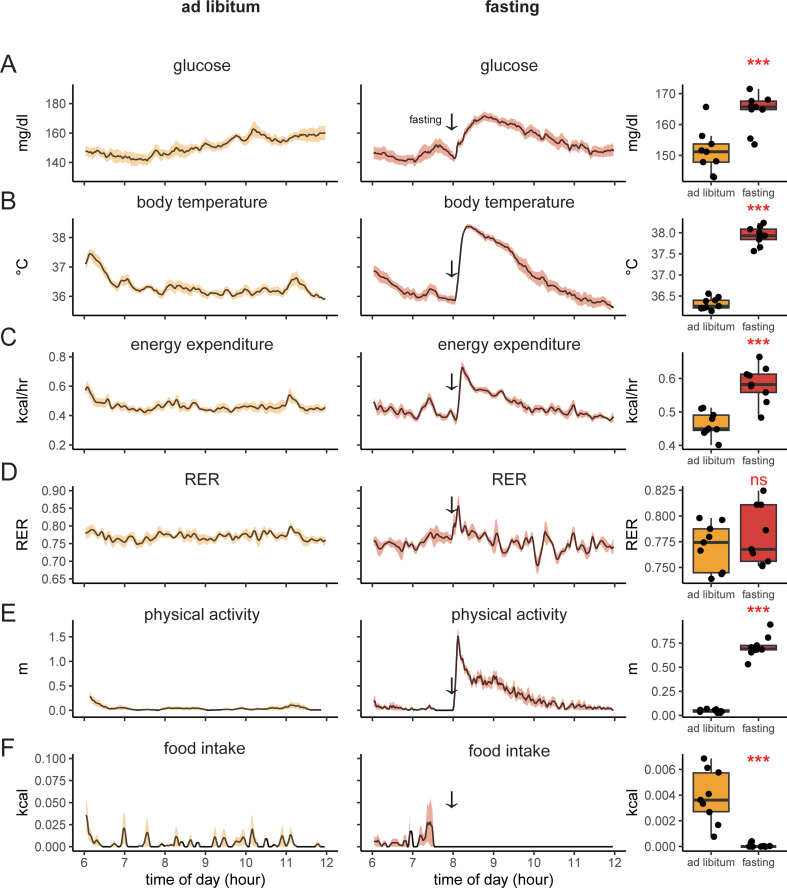
To understand the increased variability associated with a 4-h fast, we first examined the corresponding period under ad-libitum conditions. This interval includes the first 6 h of the light cycle as mice enter a phase of low physical activity and food intake. We monitored 11 young, healthy mice on a chow diet using CGM and indirect calorimetry. We observed tight blood glucose regulation: 136 ± 15 mg/dl (mean ± SD). Similar uniformity was seen in body temperature 36 ± 1 °C, and energy expenditure 0.44 ± 0.1 kcal/h. Despite food availability, the voluntarily low amount of food consumed corresponds to decreased respiratory exchange ratio (RER), representing the transition from metabolizing a high-carbohydrate diet to burning stored fat (Figure 3A–F). Physical activity levels are similarly low, with a positional heatmap consistent with sedentary behavior or sleep (Figure 3G). Overall, mice with ad libitum access to food have low variability in all parameters monitored. Moreover, the fatty acid oxidation indicated by a low RER value is consistent with a post-absorptive state.
Figure 3.
Fasting during the light photoperiod induces a hyper-glycemia and hyper-metabolic response.A–F) Recordings of chow fed and 4-h fasted mice. Left/green: ad libitum fed mice; center/blue: the same animals two days later fasted at 8 AM (arrow). Right: mean values for each animal between 0800 and 0900. A) blood glucose, B) body temperature, C) metabolic rate (energy expenditure), D) respiratory exchange ratio, E) in-cage physical activity (distance traveled) F) energy intake. The solid black line represents the mean value at each minute, the shaded area represents ± SEM, n = 11 male mice. G) A heatmap of position within the cage for one mouse from 0800 to 0900 under fasted or ad libitum conditions. The plotted area represents the physical location within the cage; height and color represent the accumulated time spent at each location. The food hopper is located in the top right corner. H–J) Estimates of glycemic variability including H) Continuous Overall Net Glycemic Action (CONGA), I) Mean Amplitude of Glycemic excursions (MAGE), J) Mean Absolute Deviation (MAD). K) Fasting induced corticosterone levels. L) Measurements of glucose before and after food removal with a hand-held glucometer (n = 20 male mice). ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
We next examined the 4-h fasting state by removing food from the mice at 0800 h, 2 h following the start of the light photoperiod. Cage change with food withdrawal during the light photoperiod provoked a strong hyper-metabolic physiological response. Within minutes of food withdrawal, we observed increased blood glucose, body temperature, energy expenditure, and locomotion. These parameters peak in 20–30 min and remain elevated for up to 4 h. The mice also experience a faster decrease in RER as greater fat oxidation is required to fuel the increased energy expenditure in the fasting state. A positional heat map reveals greater physical activity during fasting, with typical occupancy occurring near the empty food hopper (Figure 3G). In the first hour after food withdrawal, we observed a 10.5 ± 13 mg/dl increase in glucose, a 1.5 ± 0.4 °C increase in body temperature, a 10-fold increase in physical activity, and a 34% increase in energy expenditure. We find that food removal, rather than reducing the glucose variability, has the opposite effect of increasing glucose variability (Figure 3A–F).
To better quantify the intra-mouse glycemic variability under each condition, CGM data from each group were analyzed using three frequently used statistical methods, continuous overlapping net glycemic action (CONGA), mean amplitude of glucose excursion (MAGE), and mean absolute difference (MAD) [[13], [14], [15]]. CONGA, a method used for the analysis of short-term glycemic variability in the order of minutes to hours, was increased during the 4-h fast and compared to mice with ad libitum access to food for the same duration. MAGE, another measure that assesses glycemic variability on shorter time scales, is inversely correlated with insulin resistance and was similarly elevated in the 4-h fasted mice compared to the ad libitum mice. Finally, we assessed the MAD, which was originally developed for the analysis of 24-h data and is essentially a variation on the standard deviation of intermittent glucose measurements, and found results concordant with CONGA and MAGE (Figure 3H–J). Overall, these results argue that the inter-mouse glycemic variability experienced during fasting correlates with intra-mouse glycemic variability and that this variability is not an artifact of the stress of intermittent blood sampling.
To test whether our findings of increased glycemic variability generalized beyond healthy, wild-type mice, mice fed a high fat diet were observed during ad libitum feeding or a 4 h fast. Similar overall trends were observed in male mice fed a high-fat (HFD) for 7 days (Supplemental Figure 1). During the first 6 h of the light photoperiod, ad libitum HFD mice had higher average glucose levels and more glycemic variability than chow-fed mice (156 ± 20 mg/dl vs 136 ± 15 mg/dl (mean ± SD)). Consistent with the greater energy density of the HFD, we observed a trend towards greater caloric intake in HFD compared to chow-fed conditions (1.47 ± 0.749 kcal vs 1.35 ± 0.868 kcal). As expected, the diet's high-fat content produced greater fat oxidation rates (lower RER) when compared to chow-fed mice. In addition, the effect of food withdrawal in HFD mice promoted an exaggerated response compared to chow-fed mice. As a result, we observe greater increases in HFD vs chow glucose (13.0 vs 10.5 mg/dl), body temperature (1.6 vs 1.4 °C), and locomotor activity (14-fold vs 10-fold) (Supplemental Figures 1 and 4). Increased glucose variability caused by fasting was seen consistently across both chow and HFD conditions.
2.4. Effect of protocol-associated environmental stress on glycemic variability
Postulating that these physiologic findings were due at least in part to the stress of food withdrawal, we assessed levels of corticosterone, a hormone of the pituitary adrenocortical axis elevated in response to environmental stress. We found that 4 h of fasting triggered a 3-fold rise of corticosterone (Figure 3K), supporting the hypothesis that stress was playing a significant role in the observed metabolic shifts and variability. Given that several protocol-related events other than food withdrawal itself could cause the mice stress, we set out to isolate the effect of each of these perturbations on our measured metabolic parameters.
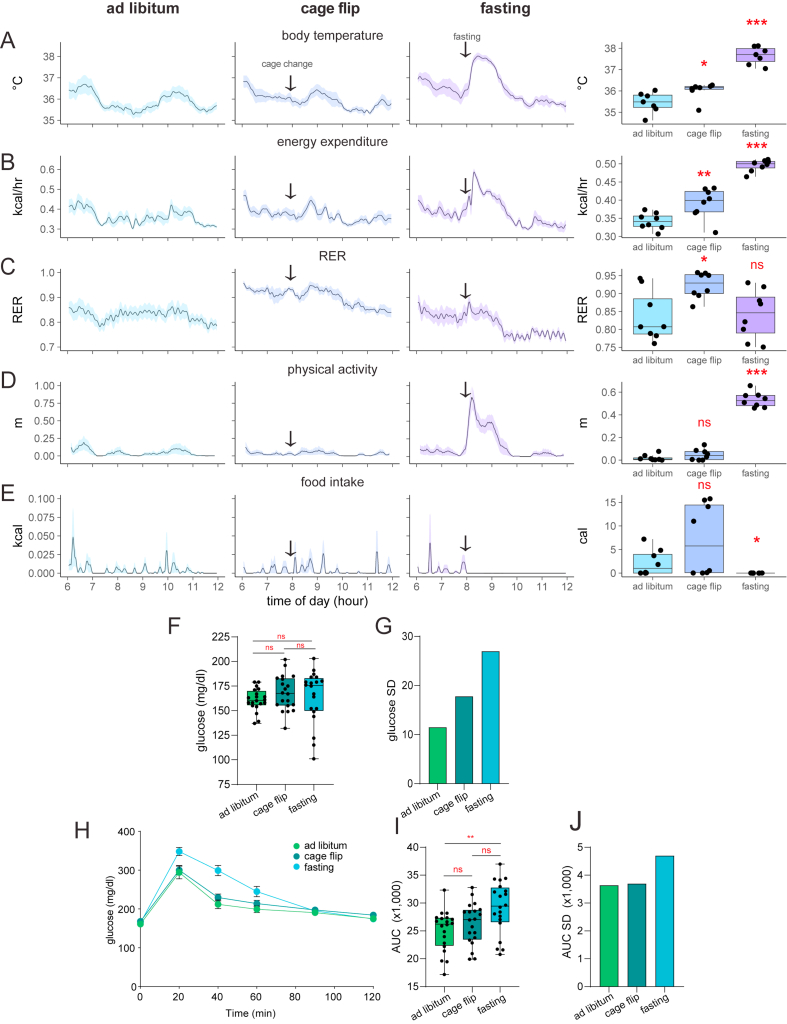
We first assessed the effects of the cage change itself in the absence of food withdrawal. We monitored 8 healthy female mice on a standard chow diet during the first 6 h of the light photoperiod under three separate conditions: ad libitum food access without cage change, ad libitum food access with a new cage with new woodchip bedding, and with a standard fasting protocol—food withdrawal accompanied by change to a new cage with woodchip bedding. We found that the cage change alone was not associated with an increase in physical activity as seen in the fasting group but was associated with small increases in body temperature and energy expenditure that were smaller in magnitude than the same increases seen in the fasting group (Supplemental Figure 3A–D). There was significant inter-mouse variability in food intake in the mice that underwent cage change alone (Supplemental Figure 3E), and there was a sustained increase in RER not seen in the fasting group (Supplemental Figure 3C) with no corresponding increase in food intake (Supplemental Figure 3E).
We next addressed whether the stress of the cage change could contribute to the variation in the glucose tolerance test. In an independent cohort of WT C57Bl/6J male mice that have not undergone surgeries, we examined the effects of the cage change compared to ad libitum conditions or fasting with a cage change. Baseline glucose levels prior to the GTT were similar in all three groups, although fasting mice have greater variation in glucose levels (Supplemental Figure 3F–G). As before, fasting mice exhibit a greater glucose excursion, greater area under the curve, and higher standard deviation of the AUC. The mice experiencing a cage change exhibit a similar phenotype to mice with ad libitum access to food (Supplemental Figure 3H–J). Overall, these results suggest that while the stress of the cage change contributes a minor role to the observed shift in metabolic state of mice undergoing fasting for a GTT, the physiologic and environmental stress of fasting itself are likely larger contributors to these changes.
Another concern we wished to address was whether the results obtained with the CGM GTT were consistent with the traditional, non-CGM GTT. In the latter, the repeated blood sampling involved may impose stress that could confound results or the results observed with CGM could be due to the invasive nature of the implanted device. Using a handheld glucometer, we confirmed the transient hyperglycemia following food restriction in an independent cohort of wild type C57Bl/6J male mice without any surgical intervention. In these naïve mice, we found an increase in blood glucose when sampled 20 min prior to and 20 min following food withdrawal (Figure 3L). However, this effect was not observed in a cohort of C57Bl/6 female mice (Supplemental Figure 2). The absence of an increase in serum glucose levels in the fasting female mice may reflect either the lack of a stress-related release of glycogen or more efficient glucose clearance in females compared to male mice.
These studies validate that non-invasive CGM technologies and well-established handheld glucometer technologies deliver similar results in measuring the effects of acute fasting on increased glucose levels in male mice. Furthermore, these findings suggest that the stress of blood sampling during the GTT does not render baseline glucose levels significantly different from telemetric monitoring.
2.5. Fasting during the dark photoperiod drives entry into torpor
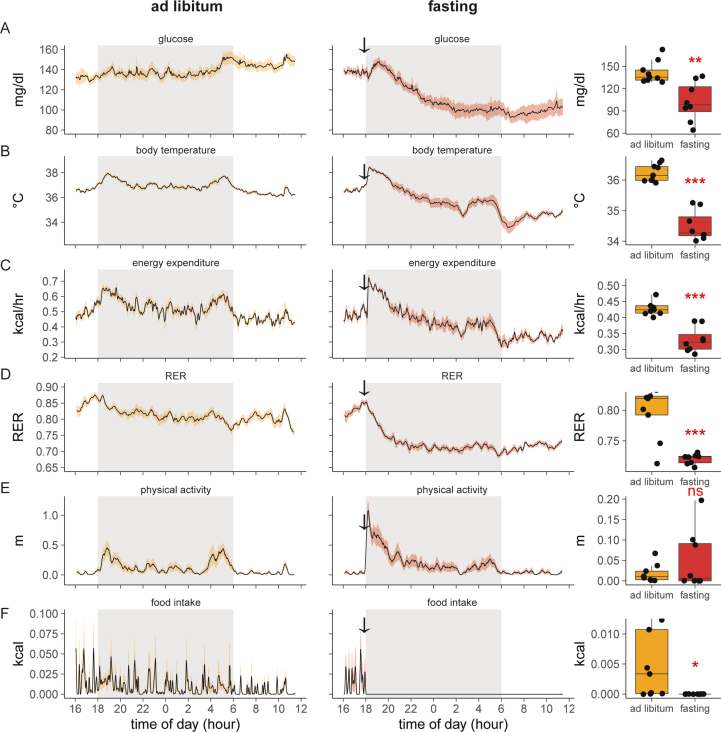
After observing an unexpectedly large effect of food removal during the light photoperiod, we next explored the effect of fasting in the dark photoperiod. Over 18 h, mice with ad libitum access to standard chow maintained tight control of glucose levels at an average of 136 ± 13 mg/dl. Other physiological parameters during the dark period are bimodal. Body temperature, physical activity, energy expenditure, and food intake reach peak levels shortly after onset and before the conclusion of the dark photoperiod. In addition, as quantified by RER, carbohydrate oxidation reaches a daily maximum concurrent with the first peak of food intake at the initiation of the dark photoperiod (Figure 4A–F).
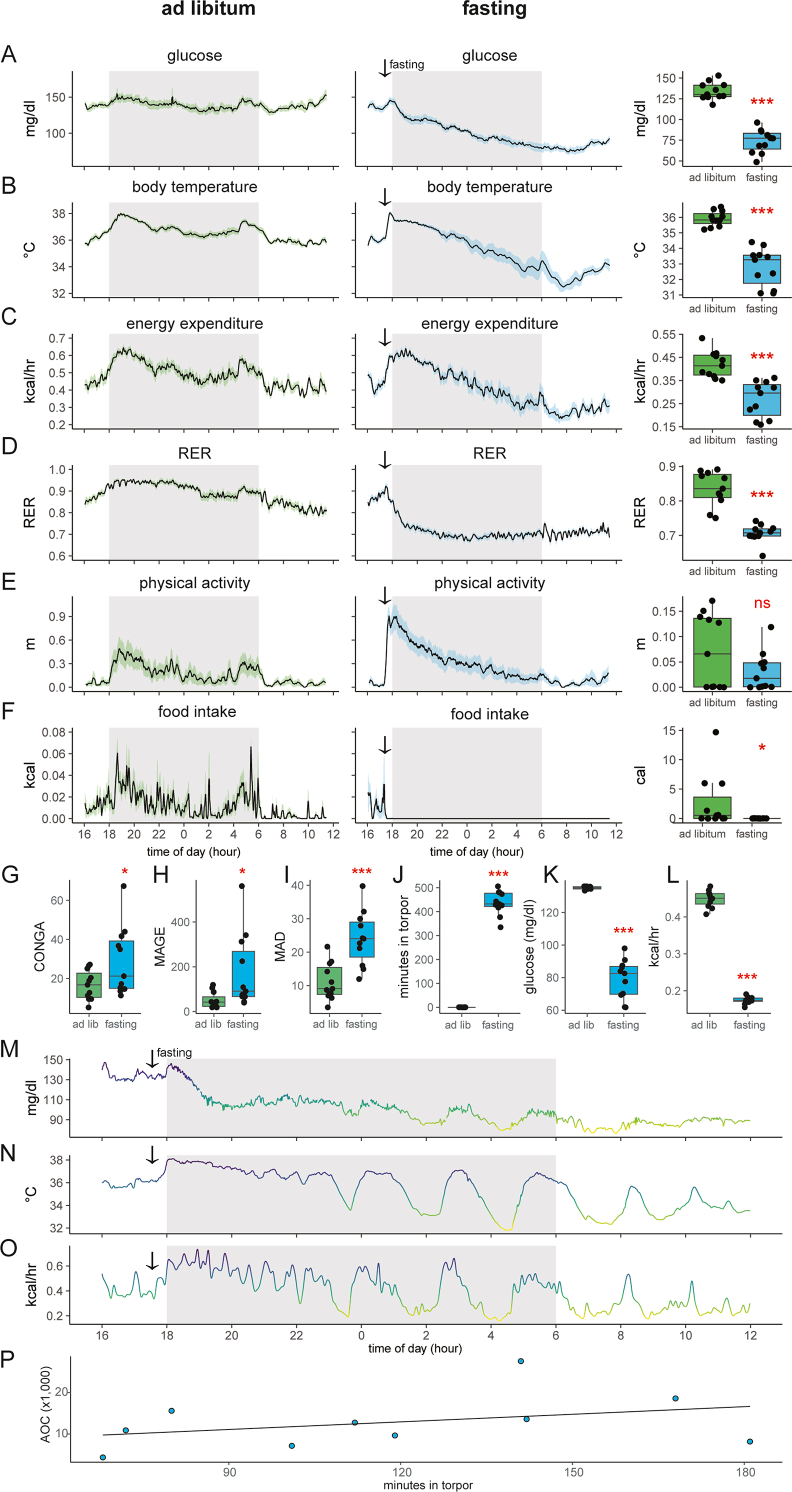
Figure 4.
Entry into torpor following food withdrawal during the dark photoperiod. A–F) recordings of chow fed and 18-h fasted mice. Left/green: ad libitum fed mice; center/blue: the same animals two days later fasted at 17:30 (arrow). Right: mean differences values for each animal between 0800 and 0900. A) Blood glucose, B) body temperature, C) metabolic rate, D) respiratory exchange ratio, E) in-cage physical activity, F) food intake. The solid black line represents the mean value at each minute, shaded area represents the SEM. Arrows denote the time of food removal. G–I) Estimates of glycemic variability including G) CONGA, H) MAGE, I) MAD. J–L) quantifying fasting-induced torpor. J) Time spent in torpor as defined by body temperature threshold (<34.5 °C). K) Glucose levels or L) energy expenditure in mice during bouts of fasting-induced torpor or during the corresponding intervals under ad libitum conditions. M–O) Recordings for one mouse during overnight fasting with plots of M) blood glucose, N) body temperature, and O) metabolic rate. P) Regression analysis of glucose AOC for 18-h fasted mice vs time spent in torpor during the 4-h prior to GTT. ns, not significant; ∗,p < 0.05; ∗∗∗p < 0.001.
As mice eat most (74%) of their food in the dark photoperiod (Figure 1C), we examined the effect of food withdrawal 30 min before the onset of darkness. The acute response observed to food removal is a spike in blood glucose and body temperature coinciding with increased physical activity levels. After the initial peak, average glucose levels drop 45% to 73.5 mg/dl at the end of the fasting period. In addition, core body temperature drops 3 °C, and energy expenditure decreased by approximately 30% during this period. Physical activity initially increases but ultimately drops to near 0. Low RER levels reflect a fasting state and oxidation of fat stores. Overall, this phenotypic pattern is consistent with torpor—a hibernation-like adaptive state used by rodents to conserve energy during conditions of food scarcity (Figure 4).
To address intra-mouse glycemic variability during the prolonged fast during the dark photoperiod, we computed the CONGA, MAGE, and MAD. Using the CGM data recorded from mice undergoing an 18 h overnight fast or during the corresponding times under ad libitum conditions. Similar to the findings for the mice undergoing a 4-h fast, we observed a significant increase in all three measures in the mice undergoing an 18-h fast compared to the ad libitum condition (Figure 4G–I). Notably, the MAGE was much higher in the 18-h overnight fasted mice compared to the 4-h fasted mice, likely driven by the large fluctuations in glucose levels corresponding to bouts of torpor.
To quantify the time spent in torpor we measured the time each animal held a body temperature below 34.5 °C [16]. With these criteria, mice spend more than 7 h in torpor during the 18-h fast (Figure 4J). During the corresponding period, the body temperature of ad libitum-fed mice was consistently higher than this threshold, i.e., 0 min in torpor. During these bouts of torpor, blood glucose levels were 56 mg/dl (42%) lower than during the corresponding times in ad libitum conditions (79 ± 12 vs 135 ± 1 mg/dl, Figure 4K). Energy expenditure levels were 61% lower during torpor (Figure 4L). We also plotted individual animals to examine the metabolic dynamics during torpor. We find a strong relationship between glucose, body temperature, and metabolic rates (Figure 4M−O). As bouts of torpor coincide with intervals of low blood glucose levels, we next examined whether the time spent in torpor might affect the response to a glucose bolus. The glucose excursion curves for these mice (Figure 2F) positively correlate to the time spent in torpor (Figure 4P). The correlation indicates that the more time mice spent in torpor before the GTT, the greater their glucose excursion.
As entry into torpor is a process integrating information on ambient temperature, food availability, photoperiod, and adipose tissue stores, we similarly examined whether mice would enter torpor on a high-fat diet. We observe strikingly similar effects for mice on HFD fasted overnight compared to ad libitum-fed mice. Under both dietary conditions, we see that while fasting reduced blood glucose levels, it introduced more physiological variability than in unfasted mice (Supplemental Figure 4).
3. Discussion
The availability of continuous glucose monitoring has paved the way for new insights into metabolic regulation and into better understanding glycemic variability in humans and now in mice. The first-line assay of glycemic control, the GTT, measures the ability of an organism to clear a bolus of glucose from the bloodstream and provides a method to detect disorders of glucose metabolism. Though simple to perform, the response to this perturbation integrates a number of complex physiological processes including the rates of endogenous glucose production, glucose-stimulated insulin secretion, insulin sensitivity, and other factors [4]. Therefore, meaningful interpretation of the results depends heavily on the baseline physiological state of the organism as well as environmental factors, and we here apply CGM to better understand and optimize the GTT.
Many consider fasting as an essential component of the GTT in preclinical studies to achieve stable baseline glucose measurements and monitor responses [6,[17], [18], [19], [20]] from the post-absorptive state. However, we show here that fasting paradoxically contributes to the variability of the GTT due to the contribution of endogenous glucose production. Previous work has suggested that environmental stressors associated with fasting include the entry of the investigator into the room, changing cage bedding, or the novel environment of placing the animals in a fresh cage [19,21]. We confirm and extend these findings in showing that fasting can contribute to GTT variability.
We find a surprisingly tight regulation of glucose levels in healthy mice between light and dark photoperiods despite the considerable circadian differences in food intake and other metabolic parameters. Using noninvasive monitoring of blood glucose, food intake, physical activity, and metabolic rate, we show two distinct fasting patterns depending on the photoperiod. In mice fasted during the light photoperiod, we observe a hyperglycemic and hyper-metabolic stress response as well as an increase in physical activity consistent with food-seeking behavior (Figure 3). These effects are more prominent at the beginning of the fast. In contrast, mice fasted for the duration of the dark photoperiod enter a shallow state of torpor (Figure 4). Prolonged fasting has been shown to reduce blood glucose and insulin levels, consistent with a state of insulin sensitivity [22]. It is therefore not surprising that torpor's effects on insulin sensitivity may be contributing to the variation in the GTT (Figure 3P).
We find consistent results from CGM and handheld glucometers, suggesting that the stress of repeated tail blood glucose measurement may not be a large source of variation in the GTT when performed by a skilled investigator. Of note, collecting larger blood volumes for the measurement of circulating hormones can introduce stress and variability [1,5]. To minimize stress on the animals, we did not collect blood for insulin or other hormone measurements during the procedures. However, prior studies show that 6-h fasting in chow fed mice is associated with higher glucose levels and lower insulin levels [2].
We observed a hyper-metabolic, hyperglycemic response of mice to food removal. These results are consistent with the phenomenon of stress-induced hyperthermia (SIH) [23]. In response to multiple different stressors, animals show autonomic reactions including elevated body temperature. In humans, this type of stress is also associated with increased temperature and blush responses. In mice, the cage switch is a paradigm of SIH whereby the animals transferred to a new cage will experience a transient elevation in temperature and physical activity consistent with our observations. The anxiolytic effects of benzodiazepines attenuate these responses [24,25]. Effects of cage switch SIH on metabolic rate and glycemic variability have not previously been reported. Our data would suggest that increased glucose levels, glycemic variability, and rates of energy expenditure are previously unappreciated aspects of SIH.
We have previously preferred overnight fasting prior to performing a GTT [[26], [27], [28]]. In our experience, mice fasted for 16–18 h during the dark photoperiod were calmer and less likely to bite. In the context of the current study, we re-evaluate these behaviors as consistent with mice in a state of torpor. Torpor allows animals to survive periods of low food availability by rapidly decreasing metabolic energy demands and body temperature [20]. Mice enter into progressively deeper periods of hypometabolic states with brief periods of arousal to search for food. Using CGM, we demonstrate that periods of torpor are associated with sharp decreases in blood glucose levels. Similar, although less pronounced effects on glucose are also observed while mice are housed at thermoneutral temperatures [29]. The extent of torpor, i.e., the degree of decrease in body temperatures and metabolic rates, introduces considerable variability in blood glucose compared to the tight regulation of glucose in ad libitum-fed mice. Although the goal of overnight fasting prior to a GTT is to reduce glucose variability, this paradigm also has the opposite effect of introducing unwanted glucose variation.
However, it is important to emphasize that intra-subject glycemic variability, while undesirable from the perspective of experimental reproducibility and minimization of the number of experimental animals needed to achieve the desired statistical power, may be an inherent feature of the post-absorptive state. Indeed, glycemic variability a measure that carries physiologic and clinical significance. For example, high glycemic variability predicts mortality in populations of critically ill patients and is thought to be the driver of vascular complications of diabetes [[30], [31], [32], [33]]. It has also been suggested that measures of glycemic variability may be more reliably diagnostic of impaired glucose tolerance than commonly used clinical measures including the hemoglobin A1c and the oral glucose tolerance test [31,34]. It should therefore not be concluded that eradication of glycemic variability in experiments will necessarily improve the insights derived from those experiments, and there are certainly situations in which variability should be embraced as meaningful rather than as a mere nuisance. CGM offers a unique opportunity to obtain and utilize the rich information of intra-subject glycemic variability.
These results are possible through the technical innovation allowed by combining simultaneous indirect calorimetry and continuous glucose monitoring with telemetry. While these studies were performed in wild-type mice, this demonstrated feasibility, and when paired together with the genetic tractability of the mouse as a model organism, there lies immense potential. It remains to be determined how best to analyze the rich CGM datasets from preclinical research.
In sum, using CGM we find that mice on a standard chow diet or short-term HFD exhibit exceptionally stable blood glucose control. While many physiological parameters exhibit substantial circadian variation, including food intake and physical activity, glycemia remains consistent through light and dark photoperiods. We find that the fasting methods tested do not reduce variability in blood glucose levels or decrease the variability of the GTT. Rather we would like to draw attention to the tradeoffs between fasting and non-fasting experimental designs.
3.1. Limitations
Our study does not exhaustively test whether a fasting paradigm might exist that could reduce the variability of the glucose tolerance test. We examined CGM in only a single strain of male mice on either a chow diet or short-term HFD. We would expect an increase in glycemic variability in diabetic mice. The use of continuous glucose monitoring is surgically challenging to achieve and is expensive, with each single-use probe costing approximately 1000 USD. These factors suggest that CGM in mice may have limited adoption in the research community. Indeed, we found quantitatively similar results of the GTT with a handheld glucometer. In addition, our study demonstrates that some experimental variation is derived just from the cage change prior to the glucose tolerance test. Regardless, introduction of these new protocols may complicate comparison with previous data. Specifically, this paradigm of ad libitum glucose tolerance tests may produce different results in mice under altered environmental, genetic, or behavioral conditions.
4. Methods
4.1. Animal husbandry
All animal experiments were performed with approval from the Institutional Animal Care and Use Committees (IACUC) of The Beth Israel Deaconess Medical Center. Male and female C57BL/6 wildtype mice at 18–20 weeks of age were ordered from the Jackson Laboratory. Mice were held at 12 h/12 h light/dark cycles 0600:1800, 22 ± 2 °C room temperature, and 30%–70% humidity with ad libitum access to food and water; special treatments (e.g. fasting, GTT) are individually specified. Cages and corn cob bedding were changed once a week, and mice were monitored regularly for their health status for the duration of this study. Additionally, mice were monitored on a standard chow diet for 23 days before switching to HFD.
4.2. Continuous glucose monitoring
The CGM system utilized here differs from those used in clinical practice as these mouse probes record glucose from circulating blood rather than subcutaneous interstitial fluid.
Mouse-sized HD-XG probes record glucose and body temperature (Data Sciences International). The glucose sensor is implanted in the aortic arch, while the probe's body is implanted in the peritoneal cavity to record core body temperature. W.B.R. attended the DSI surgical skills course (DSI, St Paul, MN) to ensure the surgery was performed according to the manufacturer's recommendations. Of the mice undergoing surgery, 11 of 16 mice survived the CGM implantation procedure. Following 12–16 days of surgical recovery, glucose probes were powered on by proximity to a magnetic field. After 4 h, probes were calibrated with a 2-point calibration at ad libitum baseline and 20 min following a 2 g glucose per kg body weight bolus. For calibrations, the tail tip was nicked to elicit a drop of blood, and the average of two measurements with hand-held glucometers was used. For the remainder of the experiment, single-point calibrations were taken 2× weekly ad libitum. The calibration measurements were repeated if the glucometers showed a difference of more than 10%.
4.3. Indirect calorimetry
Metabolic cage data was collected on individually housed mice placed in a Promethion indirect calorimeter (Sable Systems) with a temperature-controlled cabinet (Pol-Eco) and provided with ad libitum food (Labdiet 5008, metabolizable energy 3.23 kcal/g or Research Diets D12492i, 60% kcal from fat, 5.21 kcal/g) and water purified by reverse osmosis. DSI or Starr Scientific telemetry receiver bases were placed beneath the Promethion Cages to match the implanted probes. Mice were maintained under 12-h/12-h light/dark photoperiods (0600–1800) at an ambient temperature of 23 ± 0.2 °C. Position and physical activity were collected every second. Rates of oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured every 2 min. Data were imputed to a 1-min resolution to match the glucose and body temperature recordings. Male mice for chow and HFD experiments were run in two staggered cohorts of n = 6 and n = 5. Mice not receiving glucose probes were implanted with Starr scientific E-Mitter body temperature telemetry probes in the peritoneal cavity. All mice survived the body temperature surgeries, however one probe failed to transmit data. We observed poor agreement between locomotor activity as recorded by the implanted telemetry probe and the infrared (IR) beam break measurements. Many episodes of physical activity due to beam breaks were not detected by telemetry. We, therefore solely used the IR measurements for physical activity. Torpor was quantified at a body temperature below 34.5, as described [35].
4.4. Fasting, cage change/cage flip, or ad libitum monitoring
Due to the caloric content of residual corn kernels, corn cob bedding was replaced with wood chip-based bedding for the fast [36]. No interventions were performed on mice monitored in the ad libitum condition: neither the corn cob bedding nor the cage was changed. For the cage flip, the mice were placed in a clean cage with fresh wood chip bedding at 08:00. The mice had a momentary lack of access to food and water for under 2 min during the cage change. After the monitoring period (12:00) or following the glucose tolerance test (14:30) the cage was changed again to clean corn cob bedding. Fasting was performed in conjunction with a complete cage change. For the duration of the fast mice retained access to drinking water.
4.5. Data analysis
Data analysis and plots were generated in the R programming language version 4.3.0 [37] using the tidyverse package or GraphPad Prism software [38]. Indirect calorimetry data were exported with Macro Interpreter, macro 13 (Sable Systems) prior to analysis in CalR version 1.3 (19). Rates of energy expenditure were calculated with the Weir equation [39]. RER is calculated as VCO2/VO2. Telemetry recordings contain short periods of signal dropout. Missing values were imputed using the imputeTS R package [40]. As indirect calorimetry and CGM were recorded on the same PC, data files were aligned by the system time to the nearest minute. The two cohorts of male mice were aligned by experimental start time. Data visualization used a 5- to 15-min rolling mean function to reduce visual noise. All comparisons of fasting versus non-fasting were performed in the same animals with an interval of no more than 3 days. As a result, starting body weights did not significantly differ. Fasting vs ad libitum-fed comparisons were performed with ANOVA. Three-way comparisons were performed with one way ANOVA and Tukey's HSD post-hoc test.
4.6. Corticosterone assay
Serum or urine was collected from mice for the corticosterone assay. For serum, whole blood samples were collected by cardiac puncture after carbon dioxide euthanasia and placed on ice for 30 min. After centrifugation at 2000 × g 4 °C for 10 min, the supernatant was collected and frozen before the corticosterone assay. For urine, mice were transferred to single cages without bedding. After 1 h, urine on the cage bottom was collected. The test was performed using the Corticosterone Parameter Assay Kit (KGE009, R&D Systems) according to the manufacturer's protocol.
4.7. Glucose tolerance testing
The glucose tolerance tests were performed with a sterile-filtered 20% glucose solution at 2 g of glucose per one kilogram bodyweight. The animals were aged 21–23 weeks at the time of the tests for chow fed mice, and 25–27 weeks for mice on HFD. Blood was collected by nicking the tail vein for handheld glucometer readings. Glucose concentrations were measured by using the CONTOUR NEXT EZ meter and CONTOUR NEXT test strips from Bayer AG according to the manufacturer's protocol. For all experiments, the GTT was performed at 1200.
Acknowledgments
We would like to thank Amelia Douglass, Brad Lowell, and members of the Banks laboratory for stimulating discussions. Technical assistance was provided by Zongfang Yang. Financial support was provided through NIH grants R01DK133948, R01DK107717, S10OD028635, P30DK034854, and P30DK135043.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2023.101795.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
Supplemental Figure 1. Effect of fasting on HFD mice during the light photoperiod. Left/orange: ad libitum (ad libitum) fed mice, center/red: the same animals two days later fasted at 0800 (arrow). Right: mean differences for each animal between 0800 and 0900. A) blood glucose, B) body temperature, C) metabolic rate, D) respiratory exchange ratio, E) in-cage distance traveled F) food intake. The solid black line represents the mean value at each minute, orange or red shaded area represents the SEM. ns, not significant; ∗∗∗p<0.001
figs2.
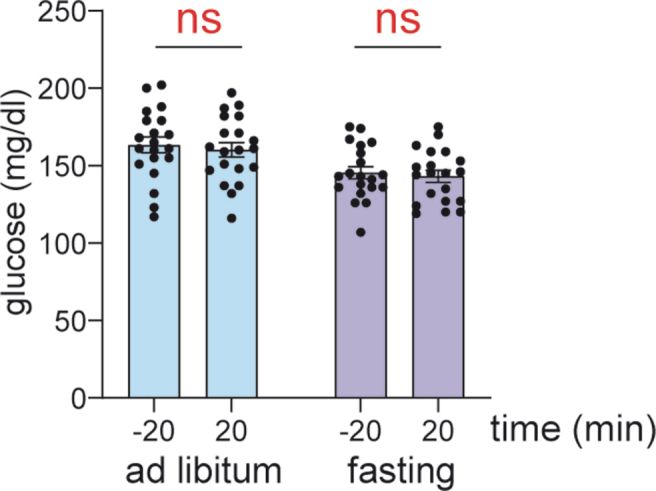
Supplemental Figure 2. Effect of fasting on blood glucose in female mice. Measurements of glucose 20 minutes before and 20 minutes after food removal with a hand-held glucometer. For mice with ad libitum access to food, measurements were taken at the same time of day without food removal (n=20 female mice).
figs3.
Supplemental Figure 3. Relative contribution of cage change or fasting to glycemic variability. A-F) Female mice were fasted, disturbed by a cage change (cage flip) with ad libitum access to food, or were unperturbed (ad libitum) during the light photoperiod on a standard chow diet. WT C57Bl/6 female mice on a chow diet (n=8) were implanted with body temperature probes while monitored with indirect calorimetry. Sky blue: ad libitum fed mice, periwinkle: cage flip, mice placed in a new cage with new bedding, lavender: fasted at 0800. Right: mean differences values for each animal between 0800 and 0900. A) Body temperature, B) metabolic rate, C) respiratory exchange ratio, D) in-cage physical activity E) food intake. The solid black line represents the mean value at each minute, shaded area represents the SEM. F-J.) Male mice were similarly fasted, or experienced a cage change, or were left undisturbed prior to a GTT with a handheld glucometer (n=20). F) baseline glucose and G) standard deviation of baseline glucose prior to the start of the GTT. H) Glucose excursion curves. I-J) AUC analysis and standard deviation of the GTT. ns, not significant; ∗,p<0.05; ∗∗,p<0.01; ∗∗∗p<0.001
figs4.
Supplemental Figure 4. Entry into torpor on HFD food withdrawal during the dark photoperiod. Orange: ad libitum fed mice, center/red: the same animals two days later fasted at 17:30. Right: mean differences values for each animal between 0800 and 0900. A) Blood glucose, B) body temperature, C) metabolic rate, D) respiratory exchange ratio, E) in-cage physical activity F) food intake. The solid black line represents the mean value at each minute, orange or red shaded area represents SEM. ns, not significant; ∗,p<0.05; ∗∗,p<0.01; ∗∗∗p<0.001
Data availability
Data will be made available upon reasonable request.
References
- 1.Ayala J.E., Samuel V.T., Morton G.J., Obici S., Croniger C.M., Shulman G.I., et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3(9–10):525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrikopoulos S., Blair A.R., Deluca N., Fam B.C., Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295(6):E1323–E1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 3.Bowe J.E., Franklin Z.J., Hauge-Evans A.C., King A.J., Persaud S.J., Jones P.M. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. J Endocrinol. 2014;222(3):G13–G25. doi: 10.1530/JOE-14-0182. [DOI] [PubMed] [Google Scholar]
- 4.Virtue S., Vidal-Puig A. GTTs and ITTs in mice: simple tests, complex answers. Nat Metab. 2021;3(7):883–886. doi: 10.1038/s42255-021-00414-7. [DOI] [PubMed] [Google Scholar]
- 5.Ayala J.E., Bracy D.P., McGuinness O.P., Wasserman D.H. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006;55(2):390–397. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- 6.Carper D., Coué M., Laurens C., Langin D., Moro C. Reappraisal of the optimal fasting time for insulin tolerance tests in mice. Mol Metab. 2020;42:101058. doi: 10.1016/j.molmet.2020.101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglass A.M., Resch J.M., Madara J.C., Kucukdereli H., Yizhar O., Grama A., et al. Neural basis for fasting activation of the hypothalamic-pituitary-adrenal axis. Nature. 2023;620(7972):154–162. doi: 10.1038/s41586-023-06358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evers S.S., Kim K.-S., Bozadjieva N., Lewis A.G., Farris D., Sorensen M.J., et al. Continuous glucose monitoring reveals glycemic variability and hypoglycemia after vertical sleeve gastrectomy in rats. Mol Metab. 2020;32:148–159. doi: 10.1016/j.molmet.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X.D., Pechter D., Yang L., Ping X., Yao Z., Zhang R., et al. Decreased complexity of glucose dynamics preceding the onset of diabetes in mice and rats. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0182810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wuyts C., Simoens C., Pinto S., Philippaert K., Vennekens R. Continuous glucose monitoring during pregnancy in healthy mice. Sci Rep. 2021;11(1):4450. doi: 10.1038/s41598-021-83901-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Group, J.D.R.F.C.G.M.S. Variation of interstitial glucose measurements assessed by continuous glucose monitors in healthy, nondiabetic individuals. Diabetes Care. 2010;33(6):1297–1299. doi: 10.2337/dc09-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korstanje R., Ryan J.L., Savage H.S., Lyons B.L., Kane K.G., Sukoff Rizzo S.J. Continuous glucose monitoring in female NOD mice reveals daily rhythms and a negative correlation with body temperature. Endocrinology. 2017;158(9):2707–2712. doi: 10.1210/en.2017-00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonnell C., Donath S., Vidmar S., Werther G., Cameron F. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther. 2005;7(2):253–263. doi: 10.1089/dia.2005.7.253. [DOI] [PubMed] [Google Scholar]
- 14.Service F.J., Nelson R.L. Characteristics of glycemic stability. Diabetes Care. 1980;3(1):58–62. doi: 10.2337/diacare.3.1.58. [DOI] [PubMed] [Google Scholar]
- 15.Rodbard D. The challenges of measuring glycemic variability. J Diabetes Sci Technol. 2012;6(3):712–715. doi: 10.1177/193229681200600328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fjelldal M.A., Stawski C., Sørås R., Wright J. Determining the different phases of torpor from skin-or body temperature data in heterotherms. J Therm Biol. 2023;111:103396. doi: 10.1016/j.jtherbio.2022.103396. [DOI] [PubMed] [Google Scholar]
- 17.Fontaine D. Common questions for diabetic models. Jackson Lab.
- 18.Benedé-Ubieto R., Estévez-Vázquez O., Ramadori P., Cubero F.J., Nevzorova Y.A. Guidelines and considerations for metabolic tolerance tests in mice. Diabetes Metab Syndr Obes. 2020;13:439–450. doi: 10.2147/DMSO.S234665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennard M.R., Nandi M., Chapple S., King A.J. The glucose tolerance test in mice: sex, drugs and protocol. Diabetes Obes Metab. 2022;24(11):2241–2252. doi: 10.1111/dom.14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen T.L., Kiersgaard M., Sørensen D.B., Mikkelsen L. Fasting of mice: a review. Lab Anim. 2013;47(4):225–240. doi: 10.1177/0023677213501659. [DOI] [PubMed] [Google Scholar]
- 21.Sukoff Rizzo S.J., Silverman J.L. Methodological considerations for optimizing and validating behavioral assays. Curr Protoc Mouse Biol. 2016;6(4):364–379. doi: 10.1002/cpmo.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavrilova O., Leon L.R., Marcus-Samuels B., Mason M.M., Castle A.L., Refetoff S., et al. Torpor in mice is induced by both leptin-dependent and-independent mechanisms. Proc Natl Acad Sci U S A. 1999;96(25):14623–14628. doi: 10.1073/pnas.96.25.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oka T., Oka K., Hori T. Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosom Med. 2001;63(3):476–486. doi: 10.1097/00006842-200105000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Vinkers C.H., Groenink L., van Bogaert M.J., Westphal K.G., Kalkman C.J., van Oorschot R., et al. Stress-induced hyperthermia and infection-induced fever: two of a kind? Physiol Behav. 2009;98(1–2):37–43. doi: 10.1016/j.physbeh.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Machado N.L.S., Abbott S.B.G., Resch J.M., Zhu L., Arrigoni E., Lowell B.B., et al. A Glutamatergic hypothalamomedullary circuit mediates thermogenesis, but not heat conservation, during stress-induced hyperthermia. Curr Biol. 2018;28(14):2291–2301 e2295. doi: 10.1016/j.cub.2018.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banks A.S., Li J., McKeag L., Hribal M.L., Kashiwada M., Accili D., et al. Deletion of SOCS7 leads to enhanced insulin action and enlarged islets of Langerhans. J Clin Investig. 2005;115(9):2462–2471. doi: 10.1172/JCI23853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banks A.S., Kim-Muller J.Y., Mastracci T.L., Kofler N.M., Qiang L., Haeusler R.A., et al. Dissociation of the glucose and lipid regulatory functions of FoxO1 by targeted knockin of acetylation-defective alleles in mice. Cell Metab. 2011;14(5):587–597. doi: 10.1016/j.cmet.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall J.A., Ramachandran D., Roh H.C., DiSpirito J.R., Belchior T., Zushin P.H., et al. Obesity-linked PPARgamma S273 phosphorylation promotes insulin resistance through Growth Differentiation Factor 3. Cell Metab. 2020;32(4):665–675 e666. doi: 10.1016/j.cmet.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo Martire V., Valli A., Bingaman M.J., Zoccoli G., Silvani A., Swoap S.J. Changes in blood glucose as a function of body temperature in laboratory mice: implications for daily torpor. Am J Physiol Endocrinol Metab. 2018;315(4):E662–E670. doi: 10.1152/ajpendo.00201.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S.H., Kim J.Y., Kim E.S., Park I.R., Ha E.Y., Chung S.M., et al. Early glycaemic variability increases 28-day mortality and prolongs intensive care unit stay in critically ill patients with pneumonia. Ann Med. 2022;54(1):2736–2743. doi: 10.1080/07853890.2022.2128399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch I.B. Glycemic variability and diabetes complications: does it matter? Of course it does. Diabetes Care. 2015;38(8):1610–1614. doi: 10.2337/dc14-2898. [DOI] [PubMed] [Google Scholar]
- 32.Esposito K., Giugliano D., Nappo F., Marfella R., Campanian Postprandial Hyperglycemia Study, G Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110(2):214–219. doi: 10.1161/01.CIR.0000134501.57864.66. [DOI] [PubMed] [Google Scholar]
- 33.Saisho Y. Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int J Mol Sci. 2014;15(10):18381–18406. doi: 10.3390/ijms151018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libman I.M., Barinas-Mitchell E., Bartucci A., Robertson R., Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab. 2008;93(11):4231–4237. doi: 10.1210/jc.2008-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hudson J.W., Scott I.M. Daily torpor in the laboratory mouse, Mus musculus var. Albino. Physiol Zool. 1979;52(2):205–218. [Google Scholar]
- 36.Sveeggen T.M., Isakson B.E., Straub A.C., Bagher P. Bedding as a variable affecting fasting blood glucose and vascular physiology in mice. Am J Physiol Heart Circ Physiol. 2023;325(2):H338–H345. doi: 10.1152/ajpheart.00168.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.R Core Team . 4.2.2 ed. R Foundation for Statistical Computing; Vienna, Austria: 2023. R: a language and environment for statistical computing. [Google Scholar]
- 38.Wickham H. Tidy data. J Stat Softw. 2014;59(10):1–23. [Google Scholar]
- 39.Weir J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109(1–2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moritz S., Bartz-Beielstein T. imputeTS: time series missing value imputation in R. R J. 2017;9(1):207. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.