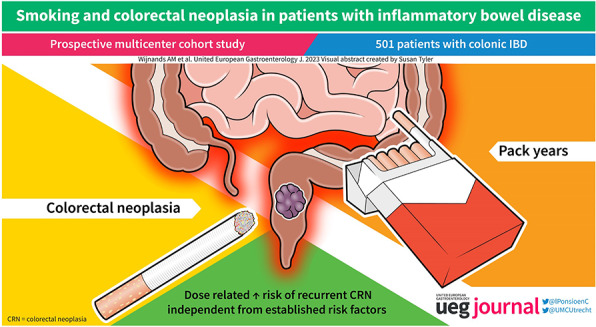
Abstract
Background and Aims
Prior studies on the effect of smoking on the risk of colitis‐associated colorectal neoplasia (CRN) have reported conflicting results. We aimed to further elucidate the association between smoking, including possible dose‐effects, and the development of colorectal neoplasia in patients with inflammatory bowel disease (IBD).
Methods
We performed a prospective multicenter cohort study including patients with colonic IBD enrolled in a surveillance program in four academic hospitals between 2011 and 2021. The effects of smoking status and pack‐years at study entry on subsequent recurrent events of CRN (including indefinite, low‐ and high‐grade dysplasia, and colorectal cancer [CRC]) were evaluated using uni‐ and multivariable Prentice, Williams, and Peterson total‐time Cox proportional hazard models. Adjustment was performed for extensive disease, prior/index dysplasia, sex, age, first‐degree relative with CRC, primary sclerosing cholangitis, and endoscopic inflammation.
Results
In 501 of the enrolled 576 patients, at least one follow‐up surveillance was performed after the study index (median follow‐up 5 years). CRN occurred at least once in 105 patients. Ever smoking was not associated with recurrent CRN risk (adjusted hazard ratio [aHR] 1.04, 95% confidence interval [CI] 0.75–1.44), but an increasing number of pack‐years was associated with an increased risk of recurrent CRN (aHR per 10 pack‐years 1.17, 95% CI 1.03–1.32; p < 0.05). Separate analyses per IBD type did not reveal differences.
Conclusions
This study found that an increase in pack‐years is associated with a higher risk of recurrent CRN in patients with IBD, independent of established CRN risk factors (NCT01464151).
Keywords: colorectal cancer, CRC, dysplasia, IBD, inflammation, pack‐years, PSC, smoking habit, surveillance, ulcerative colitis
Key summary.
Summarise the established knowledge on this subject
Previous studies report conflicting results on the impact of smoking on colorectal neoplasia (CRN) risk in patients with inflammatory bowel disease (IBD). None investigated a dose‐effect relationship nor adjusted for most known CRN risk factors.
What are the significant and/or new findings of this study?
We found a dose‐effect between smoking and recurrent risk of CRN in patients with IBD, independent of CRN risk factors (including endoscopic inflammation). No association was found for smoking status.
INTRODUCTION
Patients with colonic inflammatory bowel disease (IBD) have an increased risk of developing colorectal cancer (CRC). 1 , 2 , 3 , 4 The main driver for carcinogenesis in IBD is thought to be inflammation, that induces dysplastic changes leading to CRC. 5 , 6 Established risk factors for (advanced) colorectal neoplasia (CRN) in IBD patients include primary sclerosing cholangitis (PSC), 7 prior low‐grade dysplasia (LGD), 8 and cumulative inflammatory burden. 9 While smoking is an accepted risk factor for sporadic adenomas and CRC, 10 , 11 , 12 , 13 the effects of smoking on the risk of CRN in IBD patients are unclear.
Hypothetically, smoking could reduce the inflammatory burden in patients with ulcerative colitis (UC) and thereby lead to a decreased CRN risk. 14 , 15 Opposite effects might be expected in patients with Crohn's disease (CD) in whom smoking is associated with adverse outcomes. 15 Prior studies, examining the effect of smoking on (advanced) CRN risk in patients with IBD, reported conflicting results. 8 , 16 These studies were mostly based on retrospective observational study designs prone to biases caused by, amongst others, lack of predefined outcomes or variables. 17 Also, in few studies, the effect of smoking was investigated in a multivariable model and none reported on a dose‐effect relationship.
Here, we report the results of a multicenter prospective study, on the association of smoking—including possible dose‐effects—and the development of CRN in patients with longstanding IBD.
MATERIALS AND METHODS
We conducted a prospective, multicenter cohort study in four academic hospitals in the Netherlands. The study was registered in the Clinical Trials register (NCT01464151) and reported following the Strengthening The Reporting of Observational Studies in Epidemiology (STROBE) statement for cohort studies (checklist provided in Table S1). 18
Study population
Patients with longstanding IBD enrolled in a colonoscopic surveillance program between 2011 and 2017 at one of the participating centers were recruited. Surveillance procedures were scheduled in accordance with the Dutch surveillance guidelines, with annual, 3‐yearly, or 5‐yearly intervals. 19 The duration of prospective follow‐up was 5 years. Data collection was retrospective if prospective data was missing and to extent the follow‐up period up to July 2021 for all included patients.
Inclusion criteria were a diagnosis of colonic IBD (CD, UC, or IBD‐unclassified [IBD‐U] with ≥30% colonic involvement), and a disease duration ≥8 years or any duration in case of concomitant PSC in patients aged 18–70 years. Exclusion criteria included prior advanced CRN (defined as high‐grade dysplasia [HGD] or CRC), a history of (sub)total colectomy, contra‐indications for withholding anticoagulants prior to colonoscopy, coagulation disorders, short life expectancy, severe comorbidities, clinical or endoscopic disease activity (at discretion of treating physician as this leads to inadequate surveillance; once disease remission was achieved patients could be included), or referral for an endoscopic polypectomy.
Sample size
The original sample size of 700 patients was aimed at detecting a total of 100 cases of dysplasia during the prospective study period to enable the identification of tumor markers and risk factors for CRN. Based on an interim analysis, the sample size was adjusted to 600 patients because CRN rates were higher than anticipated. The present study describes results of the primary analysis (i.e. risk factors for CRN).
Data collection
Patients completed a questionnaire at the study index (i.e. first surveillance procedure after study enrollment) including questions on smoking status and pack‐years (number of cigarettes smoked per day divided by 20, multiplied by years of smoking), 20 family history of CRC, and IBD‐related medication (current and previous use, latter defined as use of ≥3 months). Patient demographics and disease characteristics were collected from electronic health records in the study index. Maximal colonic disease extent (endoscopic and histologic) was classified as limited (E2 for UC or IBD‐U and <50% for CD) or extensive (E3 for UC or IBD‐U and >50% for CD). For CD, colonic disease extent was estimated at <50% and >50% if, respectively, ≤2 or ≥3 segments were involved; if the number of inflamed segments was not reported, we estimated the maximal disease extent based on the endoscopy report. Data on colonic extent were considered missing if the maximal disease extent was not seen at diagnosis (i.e. incomplete colonoscopy) or if estimation of maximal disease extent was not possible. Histologic disease extent was only scored if biopsies were sampled from all colonic segments prior to or at the study index.
Endoscopists assessed the degree of endoscopic inflammation (no, mild, moderate, or severe), and recorded the presence of strictures and post‐inflammatory polyps at each procedure. Colonic segments were scored separately (ascending, transverse, descending/sigmoid, and rectum). Bowel preparation quality was scored from 1 (inadequate)–5 (excellent), scores ≥3 indicated adequate visualization of the colonic mucosa despite residual stool/staining. If the data collection was performed retrospectively, the Boston Bowel Preparation Scale (BBPS) score 21 or The Leiden Quality Score 22 were used in which, respectively, scores ≥6 and ≥3 indicated adequate bowel preparation. In most centers, chromoendoscopy was applied and two random biopsies were sampled per colonic segment. Macroscopic lesions were sampled separately. The presence of dysplasia (indefinite for dysplasia [IND], LGD, HGD, or CRC) was determined by an expert gastro‐intestinal pathologist and, if present, confirmed by a second pathologist as part of standard care.
If a (sub)total colectomy was performed during follow‐up, details on the indication and results from histopathological examination were documented.
Study endpoint
The study outcome was the occurrence of recurrent CRN events (IND, LGD, HGD, or CRC). We assumed a lesion to be the same if a local recurrence after endoscopic resection occurred or if the lesion was found at the exact same location in a consecutive procedure. These were not categorized as recurrent events. The end of study was defined as the third event of CRN during follow‐up (see next paragraph), the last available surveillance colonoscopy, or colectomy within the study period at the participating center.
Statistical analysis
All statistical analyses were performed using R statistical software, version 4.0.3 for Windows, particularly using functions from the packages survival (version 3.2–13), rms (version 6.2–0), and mice (version 3.14.0).
The relationship between smoking status or pack‐years and recurrent events of CRN was evaluated using Prentice, Williams, and Peterson (PWP)‐total time Cox proportional hazards models. A PWP‐total time Cox proportional hazards model is an extension of the classic Cox proportional hazards model, but in contrast to the latter, survival analysis can be performed on recurrent events and thereby provides estimates based on more information. A PWP‐total time Cox proportional hazards model particularly allows the baseline hazard to change for sequential events and thereby can accommodate that the occurrence of an event impacts the likelihood of a subsequent event. 23 , 24 If ≥ 1 CRN lesion was identified during a procedure, these were considered as one event. The maximum number of evaluated recurrent events was set at three because the subgroup of patients with more events was too small for meaningful analysis. 25 A stratified analysis per event was performed to investigate the impact of smoking status and pack‐years on the first and consecutive events. To account for left truncation, time since IBD diagnosis rather than time since the study index was used as the timescale in our model. Model assumptions were checked and multiple imputation was performed to replace missing values (details are provided in Supporting Information S1).
The effects of smoking status and pack‐years (continuous, per 10 pack‐year increase) at the study index on recurrent CRN risk were evaluated in a univariable and an adjusted multivariable model. In the multivariable model, adjustment for the most important risk factors for CRN was performed, 8 including extensive disease, dysplasia prior to or at the study index (including IND or LGD), PSC, sex, first‐degree relative with CRC, age (continuously), and mean endoscopic inflammation score (scale 0–3 based on visualized colonic segments, included as a time‐varying covariate). All analyses were performed for the total cohort and separately for UC/IBD‐U and CD.
A two‐sided p‐value of <0.05 was considered statistically significant.
RESULTS
Study population
Patients were recruited at one of the four participating centers between 2011 and 2017; follow‐up was completed by July 2021 for all patients. In total, 613 patients were included of whom 37 were excluded for various reasons (Figure 1). Of the remaining 576 patients, 316 (55%) were diagnosed with UC/IBD‐U and 260 (45%) with CD. Fifty percent were male and the median age was 50 years (interquartile interval [IQI] 39–58). At the study index, 275 patients reported smoking or had formerly smoked (48%) with a median number of 9 pack‐years (IQI 4–20, range 78). For UC/IBD‐U patients, the percentages of patients with extensive disease were 55% and 61% in patients ever and never smoking, respectively. In CD disease, extent was classified as extensively in 62% of patients ever smoking and 65% for never smokers. The number of patients diagnosed with CRN prior to or at the study index was 134 (23%) (all IND or LGD except for one patient with HGD). The study index was equal to the first real‐life surveillance in 58 patients (10%). Table 1 provides additional data on patient and disease characteristics.
FIGURE 1.
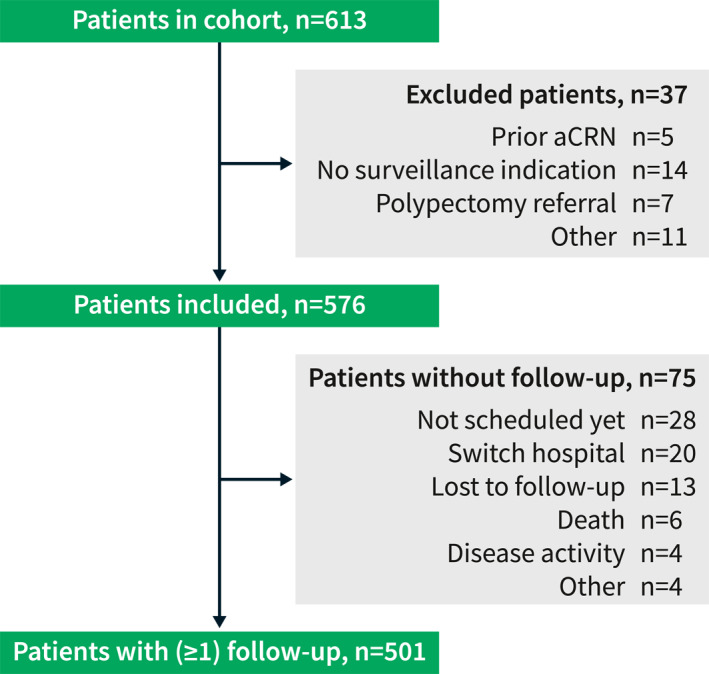
Flowchart of patients included in the prospective multicenter surveillance cohort. For 75 patients, no follow‐up information was available. In four patients, this was due to the presence of continuous (severe) disease activity. In another four patients, no follow‐up was available because of co‐morbidities or unwillingness to undergo surveillance. aCRN, advanced colorectal neoplasia; n, number.
TABLE 1.
Characteristics of 576 Dutch IBD patients included in a prospective multicenter surveillance cohort study.
| Characteristic | Total cohort n = 576 | Ever smokers n = 275 | Never smokers n = 273 | Missing data (n) |
|---|---|---|---|---|
| Male sex, n (%) | 289 (50) | 129 (47) | 147 (54) | |
| Age (y), median (IQI) | 50 (39–58) | 54 (44–61) | 45 (34–53) | |
| IBD disease duration (y), median (IQI) | 18 (11–26) | 18 (13–27) | 17 (11–25) | 2 |
| IBD type, n (%) | ||||
| UC/IBD‐U | 316 | 145 (53) | 155 (57) | |
| CD | 260 | 130 (47) | 118 (43) | |
| Endoscopic disease extent, n (%) | 29 | |||
| Limited (E2 a /<50%) | 159 (28) | 84 (31) | 72 (26) | |
| Extensive (E3 a />50%) | 388 (67) | 177 (64) | 189 (69) | |
| Histologic disease extent, n (%) | 89 | |||
| E2 a /<50% | 81 (14) | 41 (15) | 40 (15) | |
| E3 a />50% | 406 (70) | 185 (67) | 198 (73) | |
| PSC, n (%) | 49 (9) | 11 (4) | 35 (13) | |
| Prior or index CRN (IND or LGD), n (%) | 134 (23) | 83 (30) | 46 (17) | |
| Surveillance history, n (%) | 410 (71) | 195 (71) | 197 (72) | |
| Positive family history of CRC, n (%) | 169 (29) | 95 (35) | 73 (27) | 33 |
| First degree relative | 78 (14) | 45 (16) | 33 (12) | |
| Smoking status, n (%) | 28 | |||
| Current | 72 (13) | 72 (26) | ‐ | |
| Quit | 203 (35) | 203 (74) | ‐ | |
| Never | 273 (47) | ‐ | 273 (100) | |
| Pack‐years, median (IQI) | 0 (0–8) | 9 (4–20) | ‐ | 50 |
| Current medication, n (%) | ||||
| 5‐ASA | 322 (56) | 149 (54) | 157 (58) | 1 |
| Thiopurine | 205 (36) | 84 (31) | 109 (40) | 1 |
| MTX | 27 (5) | 10 (4) | 15 (5) | 1 |
| Biological | 128 (22) | 64 (23) | 57 (21) | 1 |
| Previous medication use, n (%) | ||||
| 5‐ASA | 486 (84) | 231 (84) | 238 (87) | 9 |
| MTX or thiopurines | 354 (61) | 160 (58) | 175 (64) | 9 |
| Biological | 159 (28) | 73 (27) | 75 (27) | 11 |
| Risk category b , n (%) | 14 | |||
| High | 143 (25) | 72 (26) | 68 (25) | |
| Intermediate | 283 (49) | 130 (47) | 136 (50) | |
| Low | 136 (24) | 65 (24) | 64 (23) | |
Abbreviations: 5‐ASA, 5‐aminosalicylic acid; CD, Crohn’s disease; CRC, colorectal carcinoma; IBD‐U, inflammatory bowel disease unclassified; IND, indefinite for dysplasia; IQI, interquartile interval; LGD, low‐grade dysplasia; MTX, methotrexate; PSC, primary sclerosing cholangitis; UC, ulcerative colitis; y, years.
According to the Montreal classification. E2, left‐sided disease; E3, extensive disease.
According to the Dutch IBD surveillance guideline, high‐risk category: PSC, stricture (UC), prior dysplasia in <5 years, first‐degree relative with CRC (age <50 years); intermediate risk category: post‐inflammatory polyps, first‐degree relative with CRC (age >50 years), chronic disease activity, extensive disease; or low‐risk category in case of left‐sided UC or CD with <50% of colonic involvement.
Surveillance colonoscopies and colectomy procedures
In total, 1672 surveillance procedures were performed in 576 patients (798 procedures in patients ever smoking, 811 in patients never smoking, and 63 in patients with unknown smoking status). Data collection was carried out prospectively in 63% of procedures and was completed by chart review for the remainder. Chromoendoscopy was applied in 66% of the surveillance procedures; in the remaining procedures, the surveillance was performed using high‐definition white‐light examination only. In 64% of all colonoscopy, conditions for surveillance were considered adequate (no or only mild endoscopic inflammation in one colonic segment and sufficient bowel preparation quality). Supporting Information S1 provides additional information on procedure characteristics.
Follow‐up details and events of colorectal neoplasia
In 501 patients (87%), at least one follow‐up procedure was performed (median follow‐up duration 5.0 years [IQI 3.1–6.3]). Thirty‐three patients were lost to follow‐up (of whom 20 switched hospital); these patients were younger and were more often assigned to a lower risk group based on the Dutch surveillance guideline (data not shown). 19
In 125 patients (25%), CRN occurred prior to or at the study index, of whom 61 patients also developed CRN during follow‐up. CRN occurred at least once during the follow‐up in 105 patients (21%), of whom 62 experienced a single event, 29 patients had two consecutive events, and in 14 patients ≥3 events were observed during follow‐up.
In three patients, surgical resection was performed for LGD lesions that were considered endoscopically non‐resectable. Seven patients were diagnosed with advanced CRN (1 HGD and 6 CRC), of whom six were treated with subtotal colectomy. One patient, diagnosed with CRC, had a limited life expectancy due to cholangiocarcinoma and did not undergo surgery. Supporting Information S1 provides detailed information on CRC tumor stages and treatment.
During follow‐up, a colectomy was performed for therapy‐refractory disease (n = 10) and after the diagnosis of a neuroendocrine tumor (n = 1).
Recurrent event analysis
Univariable analysis showed that smoking status (ever vs. never) was not associated with an increased risk of recurrent CRN in our total cohort (hazard ratio [HR] 1.19, 95% confidence interval [CI] 0.88–1.61; p = 0.27). After adjustment for the most important risk factors for CRN, ever smoking was not associated with recurrent CRN risk (adjusted HR [aHR] 1.04, 95% CI 0.75–1.44; p = 0.82). An increase in pack‐years was associated with an increased risk of recurrent CRN in univariable analysis (HR per 10 pack‐year increase 1.24, 95% CI 1.10–1.40; p < 0.05). This association remained present after adjustment for the most important risk factors for CRN (aHR per 10 pack‐year increase 1.17, 95% CI 1.03–1.32; p < 0.05). In Figure 2a,b, per event and total‐time univariable HRs are depicted. Heterogeneity in the direction of the estimate of the effect was found to be present for smoking status (Figure 2a).
FIGURE 2.
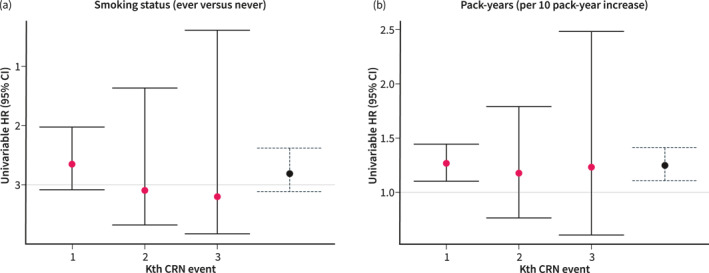
Univariable HRs per event and total‐time (blue/dotted) (a) smoking status and (b) pack‐years. Interpretation: Heterogeneity is present for smoking status (Figure 2a) in the direction of the estimate of the effect for the first and consecutive events. Therefore, summary results should be interpreted with caution. Homogeneity in the direction of the estimate of the effect for consecutive events is present for pack‐years (Figure 2b). The broad confidence intervals for the second and third events represent uncertainty caused by smaller risk sets. CI, confidence interval; CRN, colorectal neoplasia; HR, hazard ratio.
Separate analysis per IBD type did not show an association between ever smoking and the risk of recurrent CRN. An increase in pack‐years was associated with recurrent CRN risk of UC/IBD‐U (HR per 10 pack‐year increase 1.22, 95% CI 1.04–1.42; p < 0.05) but not for CD (HR per 10 pack‐year increase 1.18, 95% CI 0.95–1.48; p = 0.14). The adjusted analyses per IBD type for smoking status and pack‐years did not identify an association with the risk of recurrent CRN (Table 2). Likewise, the inclusion of an interaction term between IBD type (UC/IBD‐U vs. CD) and smoking status or pack‐years did not add more value in the total cohort adjusted analysis (p‐value interaction terms 0.89 and 0.86).
TABLE 2.
Smoking and pack‐years and the risk of recurrent CRN based on univariable and adjusted PWP‐total time Cox proportional hazards models.
| Total cohort (n = 501 of whom 105 ≥1 CRN event) | UC or IBD‐U (n = 277 of whom 66 ≥1 CRN event) | CD (n = 224 of whom 39 ≥1 CRN event) | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Univariable analyses | ||||||
| Ever smoking (reference = never) | 1.19 (0.88–1.61) | 0.27 | 1.27 (0.84–1.93) | 0.26 | 1.17 (0.69–1.98) | 0.57 |
| Pack‐years (per 10 pack‐year increase) | 1.24 (1.10–1.40) | <0.05 | 1.22 (1.04–1.42) | <0.05 | 1.18 (0.95–1.48) | 0.14 |
| Adjusted analyses a | ||||||
| Ever smoking (reference = never) | 1.04 (0.75–1.44) | 0.82 | 1.03 (0.64–1.64) | 0.91 | 0.91 (0.49–1.66) | 0.74 |
| Pack‐years (per 10 pack‐year increase) | 1.17 (1.03–1.32) | <0.05 | 1.08 (0.91–1.28) | 0.36 | 1.08 (0.86–1.37) | 0.49 |
Note: p‐values of interaction terms between IBD type and smoking status or pack‐years are, respectively, 0.89 and 0.86 (Wald test).
Abbreviations: CD, Crohn's disease; CI, confidence interval; CRN, colorectal neoplasia; HR, hazard ratio; IBD‐U, inflammatory bowel disease‐unclassified; n, number; PWP, Prentice, Williams, and Peterson; UC, ulcerative colitis.
HR adjusted for extensive disease, first‐degree relative with colorectal cancer, primary sclerosing cholangitis, male sex, age (per year increase, restricted cubic spline with four knots), prior or index colorectal neoplasia, and mean endoscopic inflammation (scale 0–3, included as time‐varying covariate).
Results from the adjusted analyses did not change if mean endoscopic inflammation was excluded from the model and the interaction terms between smoking status or pack‐years and mean endoscopic inflammation were non‐significant (data not shown).
DISCUSSION
In this multicenter prospective cohort study of 576 patients with IBD undergoing colonoscopic CRN surveillance, a dose‐effect relationship of smoking on recurrent CRN risk in patients was found. An increase in pack‐years was associated with an increased risk of recurrent CRN, which remained significant after adjustment for established CRN risk factors. Smoking status alone (ever vs. never) was not associated with recurrent CRN risk and heterogeneity was present in the direction of the estimate of the effect for the first and consecutive events (Figure 2a). Separate analyses did not reveal differences per IBD type.
Prior studies on the effect of smoking status on (advanced) CRN risk in patients with IBD reported conflicting results. A recent retrospective cohort study found an increased CRN risk in ever smoking (CD), active smoking (CD), and former smoking (UC) patients, compared with patients who never smoked (aHRs ranging from 1.73 to 2.20). 16 In the remaining categories no statistically significant associations were found, although CD patients with a former smoking status showed a trend towards an increased risk (aHR 2.16, 95% CI 1.00–4.70; p = 0.051). Of note, adjustment was performed only for age and sex and smoking status was determined at the time of last follow‐up. In a meta‐analysis, ever smoking was associated with a decreased risk of advanced CRN in univariable pooled analysis (odds ratio 0.66, 95% CI 0.49–0.88; p < 0.05) and no effect was found in the pooled analysis of multivariable results (odds ratio 1.27, 95% CI 0.75–2.13; p = 0.37). 8 In our study, we did not find an association between smoking status (ever vs. never) at the study index and recurrent CRN risk. Thus, given all evidence, we conclude that smoking status as a categorical parameter leads to a loss of information and is therefore a suboptimal way to describe the relationship.
This is the first study examining smoking and CRN risk in patients with IBD in a dose‐effect relationship. In our study, an increase in pack‐years was associated with a higher risk of recurrent CRN in both univariable and multivariable analysis (aHR per 10 pack‐year increase 1.17, 95% CI 1.03–1.32; p < 0.05). This dose‐effect relationship underscores that smoking affects the risk of CRN in patients with IBD. These findings are in line with studies from the general population that report an association of pack‐years with sporadic adenoma (pooled relative risk per 10 pack‐year 1.13, 95% CI 1.09–1.18; p < 0.001) 12 and CRC (20 pack‐years vs. zero, pooled relative risk 1.15). 13
Currently, inflammation is thought to be the main driver of the development of colitis‐associated CRC. 5 , 6 There is a large body of evidence showing that smoking has anti‐inflammatory properties in the setting of UC 14 and it can therefore be postulated that smoking reduces the risk of CRN development in those patients. In our total cohort analysis, we found that an increase in pack‐years was associated with an increased risk of recurrent CRN, even after adjustment for mean endoscopic inflammation scores measured during surveillance procedures. This finding did not differ between UC/IBD‐U and CD (non‐significant interaction term). For sporadic CRC, previous studies on tumor characteristics suggest that the effect of smoking on carcinogenesis is probably caused by microsatellite instability rather than through the chromosomal instability pathway. 13 Since colitis‐associated CRC seems to be characterized by chromosomal instability, 5 , 26 , 27 it can be questioned how these pathways are balanced in smoking IBD patients. Our database did not enable a distinction between colitis‐associated and sporadic CRN lesions, although we can assume most lesions in patients with extensive disease or pancolitis (67% of total cohort) to originate from (previously) inflamed mucosa.
This study has several strengths. First, the prospective design of this cohort enabled the use of predefined definitions and accurate data collection and thereby the analysis of the effect of smoking on CRN in a dose‐effect relationship. In addition, this allowed for adjustment for the most important risk factors of IBD‐related CRN, including mean endoscopic inflammation scores. Second, only few patients were lost to follow‐up. The impact of potential bias caused by patients lost to follow‐up (including switch of hospital) can be expected to be small as this involved only 6% of our total cohort. 28 Third, recurrent event analysis was used to estimate the effect of smoking status and pack‐years on CRN risk. In doing so, the estimates of the effect derived from this study are more comprehensive as they also incorporate information after the first event of CRN.
The study has some limitations that should be acknowledged. The relatively short follow‐up period and small cohort size lead to some uncertainty in our results that is reflected in the broad confidence intervals. Nevertheless, the results of this study are based on one of the largest, carefully conducted prospective surveillance cohort studies published. Also, while interpreting our results, it should be noted that the first event of CRN evaluated in this study was not equal to the first event in real life, as 23% of the included patients had developed CRN prior to or at the study index. We corrected for prior CRN or CRN at the study index in the adjusted models. Furthermore, the information for mean endoscopic inflammation scores was solely based on surveillance procedures generally performed during remission, potentially leading to lower scores, and information on the degree of histologic inflammation was not included. In addition, we did not adjust for lifestyle factors such as heavy alcohol consumption and obesity, although these factors impact CRC risk in the general population, 29 , 30 nor adjusted for potential chemo preventive effects of medication. Finally, the generalisability of our study results might be hampered due to the academic setting.
CONCLUSION
In conclusion, a higher number of pack‐years is associated with an increased risk of recurrent CRN in patients with IBD independent of established CRN risk factors. Smoking status was not associated with recurrent CRN risk. The suggestion of a dose‐effect relationship provides additional impetus, next to other health outcomes, 31 for patients to quit smoking.
AUTHOR CONTRIBUTIONS
Anouk M. Wijnands: design of the study, acquisition of data, analysis and interpretation of data, and drafting the article. Sjoerd G. Elias and Bas B. L. Penning de Vries: analysis and interpretation of data. Evelien Dekker, Herma H. Fidder, Frank Hoentjen, Jeroen Maljaars, Andrea E. van der Meulen‐de Jong, Renske J. Ouwehand, Cyriel Y. Ponsioen, Fiona D. M. van Schaik: acquisition of data. Joren R. ten Hove and Erik Mooiweer: conception and design of the study and acquisition of data. Bas Oldenburg: conception and design of the study, acquisition of data, and analysis and interpretation of data. All authors critically revised the article for important intellectual content and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
CP: has received research grants from Gilead and Perspectum and consultancy fees from Shire and Pliant. FvS has served as consultant for Takeda, Galapagos and Dr Falk. ED: has endoscopic equipment on loan from FujiFilm and Olympus, and receives a research grant from FujiFilm. ED has also received honorarium for consultancy from FujiFilm, Olympus, GI Supply, CPP‐FAP, PAION and Ambu, and speakers' fees from Olympus, GI Supply, Norgine, IPSEN, PAION and FujiFilm. FH has served on advisory boards or as a speaker for Abbvie, Janssen‐Cilag, MSD, Takeda, Celltrion, Teva, Sandoz and Dr Falk. Funding (Grants/Honoraria): Takeda, Janssen‐Cilag, and Abbvie; Consulting Fees: Celgene. All other authors declare no conflicts of interest.
ETHICS APPROVAL
This study was approved by the medical ethics committee at UMC Utrecht (protocol number 11‐050). Informed consent was obtained from all participants. The study was conducted in accordance with the Dutch Medical Research Involving Human Subjects Act (WMO) and the Declaration of Helsinki.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
We thank Johannes P.D. Schultheiss and Mirjam Severs for their help in data collection for this study. This work was supported by unrestricted funding from Merck Sharp & Dohme Corp and Ferring Pharmaceuticals.
Wijnands AM, Elias SG, Dekker E, Fidder HH, Hoentjen F, ten Hove JR, et al. Smoking and colorectal neoplasia in patients with inflammatory bowel disease: dose‐effect relationship. United European Gastroenterol J. 2023;11(7):612–620. 10.1002/ueg2.12426
The abstract of this manuscript was presented at the UEG week 2022 and Digestive Disease Days 2022 (Dutch Gastroenterology congress).
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly due to the privacy of individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta‐analysis of population‐based cohort studies. Inflamm Bowel Dis. 2013;19(4):789–799. 10.1097/mib.0b013e31828029c0 [DOI] [PubMed] [Google Scholar]
- 2. Jess T, Simonsen J, Jorgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143(2):375–381.e371; quiz e313‐374. 10.1053/j.gastro.2012.04.016 [DOI] [PubMed] [Google Scholar]
- 3. Olen O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population‐based cohort study. Lancet. 2020;395(10218):123–131. 10.1016/s0140-6736(19)32545-0 [DOI] [PubMed] [Google Scholar]
- 4. Olen O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Colorectal cancer in Crohn's disease: a Scandinavian population‐based cohort study. Lancet Gastroenterol Hepatol. 2020;5(5):475–484. 10.1016/s2468-1253(20)30005-4 [DOI] [PubMed] [Google Scholar]
- 5. Itzkowitz SH. Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35(3):553–571. 10.1016/j.gtc.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 6. Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14(11):931–968. 10.1016/s0046-8177(83)80175-0 [DOI] [PubMed] [Google Scholar]
- 7. Shah SC, Ten Hove JR, Castaneda D, Palmela C, Mooiweer E, Colombel JF, et al. High risk of advanced colorectal neoplasia in patients with primary sclerosing cholangitis associated with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2018;16(7):1106–1113.e1103. 10.1016/j.cgh.2018.01.023 [DOI] [PubMed] [Google Scholar]
- 8. Wijnands AM, de Jong ME, Lutgens M, Hoentjen F, Elias SG, Oldenburg B. Prognostic factors for advanced colorectal neoplasia in inflammatory bowel disease: systematic review and meta‐analysis. Gastroenterology. 2021;160(5):1584–1598. 10.1053/j.gastro.2020.12.036 [DOI] [PubMed] [Google Scholar]
- 9. Choi CR, Al Bakir I, Ding NJ, Lee GH, Askari A, Warusavitarne J, et al. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single‐centre study. Gut. 2019;68(3):414–422. 10.1136/gutjnl-2017-314190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta‐analysis. Int J Cancer. 2009;124(10):2406–2415. 10.1002/ijc.24191 [DOI] [PubMed] [Google Scholar]
- 11. Cheng J, Chen Y, Wang X, Wang J, Yan Z, Gong G, et al. Meta‐analysis of prospective cohort studies of cigarette smoking and the incidence of colon and rectal cancers. Eur J Cancer Prev. 2015;24(1):6–15. 10.1097/cej.0000000000000011 [DOI] [PubMed] [Google Scholar]
- 12. Botteri E, Iodice S, Raimondi S, Maisonneuve P, Lowenfels AB. Cigarette smoking and adenomatous polyps: a meta‐analysis. Gastroenterology. 2008;134(2):388–395. 10.1053/j.gastro.2007.11.007 [DOI] [PubMed] [Google Scholar]
- 13. Botteri E, Borroni E, Sloan EK, Bagnardi V, Bosetti C, Peveri G, et al. Smoking and colorectal cancer risk, overall and by molecular subtypes: a meta‐analysis. Am J Gastroenterol. 2020;115(12):1940–1949. 10.14309/ajg.0000000000000803 [DOI] [PubMed] [Google Scholar]
- 14. van der Heide F, Dijkstra A, Weersma RK, Albersnagel FA, van der Logt EM, Faber KN, et al. Effects of active and passive smoking on disease course of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2009;15(8):1199–1207. 10.1002/ibd.20884 [DOI] [PubMed] [Google Scholar]
- 15. Cosnes J. Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice. Best Pract Res Clin Gastroenterol. 2004;18(3):481–496. 10.1016/j.bpg.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 16. van der Sloot KWJ, Tiems JL, Visschedijk MC, Festen EAM, van Dullemen HM, Weersma RK, et al. Cigarette smoke increases risk for colorectal neoplasia in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2021. [DOI] [PubMed] [Google Scholar]
- 17. Sedgwick P. Retrospective cohort studies: advantages and disadvantages. BMJ. 2014;348(jan24 1):g1072. 10.1136/bmj.g1072 [DOI] [PubMed] [Google Scholar]
- 18. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. 10.1016/s0140-6736(07)61602-x [DOI] [PubMed] [Google Scholar]
- 19. Nederlandse Vereniging van Maag‐, Darm‐ en Leverartsen, Handleiding behandeling IBD ‐ 2014‐2015. https://www.mdl.nl/sites/www.mdl.nl/files/richlijnen/Document_volledig_Handleiding_met_literatuur_def.pdf. Accessed 8 Apr 2021.
- 20. National Cancer Institute . Pack year. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/pack-year?redirect=true. Accessed 18 July 2022.
- 21. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy‐oriented research. Gastrointest Endosc. 2009;69(3 Pt 2):620–625. 10.1016/j.gie.2008.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boonstra JJ, de Vos Tot Nederveen Cappel WH, Langers AM, van der Sluis H, Hardwick JH, Vasen HF. Colonoscopy in Lynch syndrome: the need for a new quality score. Fam Cancer. 2017;16(2):239–241. 10.1007/s10689-016-9950-0 [DOI] [PubMed] [Google Scholar]
- 23. Amorim LD, Cai J. Modelling recurrent events: a tutorial for analysis in epidemiology. Int J Epidemiol. 2015;44(1):324–333. 10.1093/ije/dyu222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yadav CP, Sreenivas V, Khan MA, Pandey RM. An overview of statistical models for recurrent events analysis: a review. Epidemiology. 2018;8:4. [Google Scholar]
- 25. Kelly PJ, Lim LL. Survival analysis for recurrent event data: an application to childhood infectious diseases. Stat Med. 2000;19(1):13–33. [DOI] [PubMed] [Google Scholar]
- 26. Wanders LK, Cordes M, Voorham Q, Sie D, de Vries SD, d’Haens GRAM, et al. IBD‐associated dysplastic lesions show more chromosomal instability than sporadic adenomas. Inflamm Bowel Dis. 2020;26(2):167–180. 10.1093/ibd/izz171 [DOI] [PubMed] [Google Scholar]
- 27. Hirsch D, Hardt J, Sauer C, Heselmeyer–Hadded K, Witt SH, Kienle P, et al. Molecular characterization of ulcerative colitis‐associated colorectal carcinomas. Mod Pathol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sacket DL, Richardson WS, Rosenberg W, Haynes RB. Evidence‐based medicine: how to practice and teach EBM. New York: Churchill Livingstone; 1997. [Google Scholar]
- 29. Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. 10.1136/bmj.j477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cai S, Li Y, Ding Y, Chen K, Jin M. Alcohol drinking and the risk of colorectal cancer death: a meta‐analysis. Eur J Cancer Prev. 2014;23(6):532–539. 10.1097/cej.0000000000000076 [DOI] [PubMed] [Google Scholar]
- 31. Collaborators GBDT, Kendrick PJ, Ababneh E, Abbafati C, Abbasi‐Kangevari M, Abdoli A, et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990‐2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021;397(10292):2337–2360. 10.1016/s0140-6736(21)01169-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.


