Abstract
Background and Aims
The Diverticular Inflammation and Complication Assessment (DICA) classification and the Combined Overview on Diverticular Assessment (CODA) were found to be effective in predicting the outcomes of Diverticular Disease (DD). We ascertain whether fecal calprotectin (FC) can further aid in improving risk stratification.
Methods
A three‐year international, multicentre, prospective cohort study was conducted involving 43 Gastroenterology and Endoscopy centres. Survival methods for censored observations were used to estimate the risk of acute diverticulitis (AD) in newly diagnosed DD patients according to basal FC, DICA, and CODA. The net benefit of management strategies based on DICA, CODA and FC in addition to CODA was assessed with decision curve analysis, which incorporates the harms and benefits of using a prognostic model for clinical decisions.
Results
At the first diagnosis of diverticulosis/DD, 871 participants underwent FC measurement. FC was associated with the risk of AD at 3 years (HR per each base 10 logarithm increase: 3.29; 95% confidence interval, 2.13–5.10) and showed moderate discrimination (c‐statistic: 0.685; 0.614–0.756). DICA and CODA were more accurate predictors of AD than FC. However, FC showed high discrimination capacity to predict AD at 3 months, which was not maintained at longer follow‐up times. The decision curve analysis comparing the combination of FC and CODA with CODA alone did not clearly indicate a larger net benefit of one strategy over the other.
Conclusions
FC measurement could be used as a complementary tool to assess the immediate risk of AD. In all other cases, treatment strategies based on the CODA score alone should be recommended.
Keywords: acute diverticulitis, CODA score, DICA score, diverticular disease, diverticulosis, fecal calprotectin
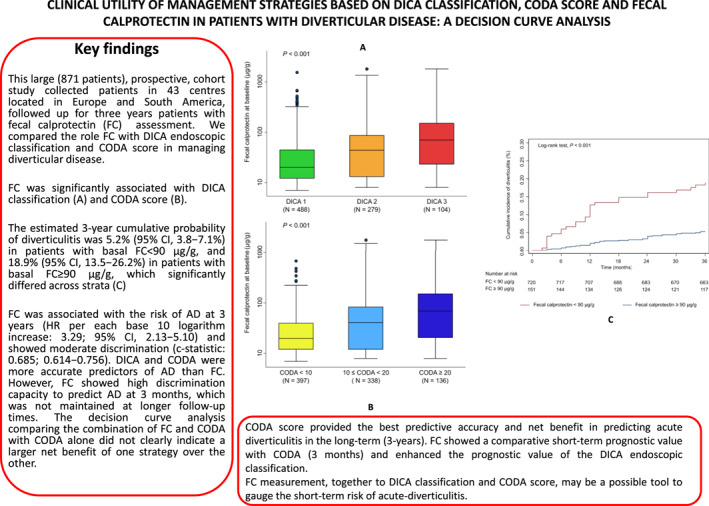
Key summary.
Summarize the established knowledge on this subject
The Diverticular Inflammation and Complication Assessment (DICA) classification and the Combined Overview on Diverticular Assessment (CODA) score are validated prognostic tools for diverticulitis.
However, the role of Fecal Calprotectin (FC) in the decision‐making process in Diverticular Disease (DD) in comparison with the available validated tools is unknown.
What are the significant and/or new findings of this study?
This large (871 patients) prospective cohort study collected FC from DD patients inEurope and South America, who were prospectively followed up for three years.
The CODA score provided the best predictive accuracy and net benefit in predicting acute diverticulitis (AD) in the long‐term (3‐year). FC showed a comparative short‐term prognostic value with CODA (3 months) and enhanced the prognostic value of the DICA endoscopic classification.
FC measurement, together with the DICA classification and CODA score, may be a possible tool to gauge the short‐term risk of acute‐diverticulitis.
INTRODUCTION
Although diverticulosis of the colon is the most frequently recognized anatomical alteration during colonoscopy, 1 for many years, an endoscopic classification has been absent, and only imaging‐based 2 , 3 , 4 or clinically based 5 , 6 , 7 classifications have been available. Nonetheless, endoscopic diverticular inflammation is detected in up to 2% of patients undergoing colonoscopy. 8 , 9 , 10
In 2015, the first endoscopic classification of diverticulosis/diverticular disease, called “Diverticular Inflammation and Complication Assessment” (DICA), was presented. 11 After its validation in two studies, 12 , 13 a recent large prospective study confirmed that this classification has a significant impact in predicting disease outcomes. 14 Its clinical evolution, a prognostic score named “Combined Overview on Diverticular Assessment” (CODA), enhanced the predicting value of this classification. 14
Fecal calprotectin (FC) is a cytoplasmic antimicrobial protein mainly detected in granulocytes, monocytes, and macrophages. This protein, which is released during cell activation or death, is stable in feces for several days after excretion. 15 FC has shown to be a sensitive marker of activity in inflammatory bowel diseases 16 and in predicting disease relapses. 16
Increased levels of FC may also be found in systemic diseases involving the gastrointestinal tract, 17 such as diverticular disease (DD). FC was found to increase in symptomatic uncomplicated diverticular diseases (SUDD). 18 It was able to discriminate SUDD from Irritable Bowel Syndrome, 18 and dropped significantly after treatment. 19 , 20 , 21 However, we do not know whether and when FC may be included in the decision‐making process for DD.
In this study, we performed a prospective investigation and applied decision curve analysis to assess the role of FC in patients with DD according to the DICA classification and CODA score. We compared the net benefit of management strategies based on the DICA classification and CODA score with a strategy based on baseline FC alone or in combination with the CODA score. The results of the current study may improve and guide decision making in patients with DD.
METHODS
Study design and study aims
This is a post‐hoc analysis of an international multicentre prospective cohort study that included 871 patients with DD in 43 centres located in Europe and South America. A centralized laboratory analysis of FC was not planned in our initial study protocol. To homogenize and increase the comparability across centres, we considered as valid FC measurements only those deriving from quantitative assays expressed in μg/g.
Our aims were (i) to evaluate the prognostic capacity of FC for predicting diverticulitis in patients with diverticulosis/DD, (ii) to compare the prognostic performance of FC with validated prognostic tools available (i.e., DICA classification and CODA score), 14 and (iii) to compare the clinical utility of management strategies based on the CODA classification, the DICA score, and the combination of CODA score with FC by comparing the net benefit of management strategies based on the three different prognostic approaches.
Only patients at the first diagnosis of diverticulosis/DD (i.e., newly diagnosed DD patients) were enrolled during a consecutive period of 6 months. A common database was built to collect demographic and clinical data. Symptoms were assessed using a 10‐point visual scale, from 0 (absence) to 10 (worst).
Patients were clinically assessed at entry, after 3, 6, 9 and then every 6 months for 3 years. The duration of follow‐up was estimated on the available data about AD occurrence/recurrence. AD occurrence is generally low in diverticulosis, 22 and generally occurs within 2 years in patients with SUDD. 23 Moreover, diverticulitis usually recurred within 18 months mainly. 10 As DICA 1 is a diverticulosis without a sign of inflammation but can be associated with symptoms, similar to the ones occurring in SUDD (1), and DICA 2 and 3 are diverticulosis with a sign of present (DICA 2) or present/past inflammation (DICA 3), we thought that a 3‐year follow‐up was the right follow‐up time to observe the outcome of the diverticulosis/DD according to the DICA classification. Detailed eligibility criteria are reported in the extended method section.
Statistical analysis
Descriptive statistics included medians and interquartile ranges (IQR) for continuous variables and frequency analyses for categorical variables. The two‐sample Wilcoxon rank‐sum test or the Kruskal–Wallis test were used to compare continuous variables across groups.
We assessed the association between FC measured at baseline and diverticulitis by means of time‐to‐event (survival) methods for censored observations. Time to event was defined as the time from the baseline visit until the date of event or censoring. Kaplan–Meier estimates were employed to plot cumulative incidence curves, compared by log‐rank tests and univariate Cox regression.
The value of any new candidate prognostic biomarker should be evaluated against the best available prognostic tools. We fit four Cox regression models considering as predictors: (i) the DICA classification; (ii) the CODA score (i.e., a multivariable model including component predictors DICA classification, age, and pain score, as appropriate) 24 ; (iii) DICA classification and FC; (iv) CODA score (i.e., component predictors) and FC. To assess whether the model fit was significantly improved by adding FC to the validated prognostic tools available (i.e., the DICA classification and CODA score), we applied a likelihood ratio (LR) test. For all models, a linear combination of predictors and individual predicted probability was computed.
The base 10 logarithm of baseline FC treated as a continuous variable was used. To display the predictive potential of FC, we plotted Kaplan–Meier curves stratified by FC dichotomized at a threshold of 90 μg/g. This threshold was selected by maximally selected rank statistics (R package: survminer).
We assessed model discrimination using the Harrell's c‐statistic. 24 We computed the Brier score for right censored data to gauge the predictive accuracy of these models, and calculated the Nagelkerke's R‐squared (as a measure of overall model performance). 24 Regarding the univariable model including FC as the only predictor, we assessed model calibration by plotting the observed proportion versus predicted risk of diverticulitis and reporting a smoothed calibration curve with 95% confidence intervals across the risk spectrum. 24 We also reported a calibration plot for the model consisting of a combination of CODA score and FC.
To assess whether the discrimination capacity of FC varies over time (i.e., prediction in the short‐term vs. long term), we used time‐dependent receiver operating characteristic (ROC) curve analysis by means of inverse probability of censoring weighting (R package: timeROC). 25
Decision curve analysis
When a new candidate biomarker is added to an existing validated score, changes in the discrimination capacity are usually minimal. For this reason, using discrimination measures to establish the usefulness of a new biomarker has been duly criticized. We compared the clinical utility of the existing prognostic tools (i.e., DICA classification and CODA score) with that of the addition of FC to the CODA score by quantifying the net benefit of these management strategies when different threshold probabilities for prognosis of diverticulitis were considered (i.e., decision‐curve analysis; see eAppendices 1 and 2). 26 , 27
Stata software (Stata Corp., College Station, TX, USA) and the R software were used for statistical analysis.
RESULTS
Table 1 reports the baseline demographic and clinical characteristics. Patients were predominantly men (50.4%) with a median age of 65 years (IQR, 56–72). They were slightly overweight (median BMI: 26; IQR 23.2–28.9), and 28.2% of them were smokers. Most patients (56.0%) had a DD corresponding to DICA 1 classification, 32.1% DICA 2, and 11.9% DICA 3, and a median CODA score of 10 (IQR, 7–16). Basal FC varied widely across patients and ranged from 8 to 1800 μg/g with a median of 25 μg/g (IQR, 12–70).
TABLE 1.
Baseline demographic and clinical characteristics of the study population (N = 871).
| Median (IQR) or N (%) | |
|---|---|
| Age, years | 65 (56–72) |
| ≥65 | 451 (51.8) |
| Gender, male | 439 (50.4) |
| BMI, kg/m2 | 26 (23.2–28.9) |
| ≥30 | 166 (19.1) |
| Smoking | |
| Smokers | 246 (28.2) |
| Non‐smokers | 513 (58.9) |
| Ex‐smokers | 112 (12.8) |
| Appendectomy | 224 (25.7) |
| Presence of co‐morbidities | |
| Charlson's score | 3 (2–4) |
| Charlson's score >3 | 220 (25.2) |
| Presence of any symptom | 682 (78.3) |
| Cumulative symptom score a | 8 (2–13) |
| >7 | 441 (50.6) |
| Abdominal pain | 2 (0–5) |
| >2 | 404 (46.4) |
| Meteorism | 2 (0–4) |
| >2 | 371 (42.6) |
| Constipation | 0 (0–2) |
| >2 | 208 (23.9) |
| Diarrhea | 0 (0–2) |
| >2 | 199 (22.8) |
| DICA classification | |
| 1 | 488 (56.0) |
| 2 | 279 (32.1) |
| 3 | 104 (11.9) |
| CODA score | 10 (7–16) |
| Fecal calprotectin, μg/g | 25 (12–70) |
| >90 b | 151 (17.3) |
Note: Values are expressed as number (percentage) for categorical variables and as median (interquartile range) for continuous variables.
Abbreviations: BMI, body mass index; IQR, interquartile range.
The cumulative symptom score ranges from 0 to 40 points. It is obtained by adding the points regarding abdominal pain, meteorism, constipation, and diarrhea as measured on a 10‐points visual analog scale.
This threshold was selected by the maximally selected rank statistic (see Methods section).
A total of 65 diverticulitis events and 19 surgeries due to complications occurred during an average follow‐up of 2.8 years. The cumulative incidence of diverticulitis was 26.5 per 1000 person‐years, corresponding to an estimated 3‐year risk of 7.6% (95% CI, 6.0–9.6%). The cumulative incidence of surgery due to complications was 7.7 per 1000 person‐years, corresponding to an estimated 3‐year risk of 2.3% (95% CI, 1.4–3.5%).
Prognostic significance of fecal calprotectin
Higher baseline FC levels, expressed as the base 10 logarithms, were significantly associated with increased hazard of developing AD over the 3‐year follow‐up (HR, per each log unit increase: 3.29; 95% CI, 2.13–5.10; p < 0.001). The estimated 3‐year cumulative probability of diverticulitis was 5.2% (95% CI, 3.8–7.1%) in patients with basal FC < 90 μg/g, and 18.9% (95% CI, 13.5–26.2%) in patients with basal FC ≥ 90 μg/g, which significantly differed across strata (log‐rank test, p < 0.001; Figure 1). Baseline FC levels were significantly (p < 0.001) higher in patients who later developed AD (median: 77 μg/g; IQR, 30–178 μg/g) as compared to those who did not (median: 24 μg/g; IQR, 12–66 μg/g; Figure 2a). FC increased with increasing DICA classification (Kwallis test, p < 0.001; Figure 2b) and increasing CODA scores (Kwallis test, p < 0.001; Figure 2c). FC showed good apparent calibration (Appendix Figure 1), and moderate discrimination (c‐statistic: 0.685; 95% CI, 0.614–0.756). Table 2 summarizes other measures of performance.
FIGURE 1.

Kaplan–Meier curves of cumulative incidence of diverticulitis from patients categorized into high and low fecal calprotectin (FC) levels at baseline. The threshold (90 μg/g) was selected by maximally selected rank statistics. This threshold is used purely for illustrative reasons and should not be considered as the best threshold to adopt for long‐term decision making.
FIGURE 2.

Box plots displaying (a) baseline fecal calprotectin (FC) in patients who developed diverticulitis versus those who did not; (b) by Diverticular Inflammation and Complication Assessment (DICA) endoscopic classification levels; (c) and by Combined Overview on Diverticular Assessment (CODA) score. The p‐values reported correspond to a two‐sample Wilcoxon rank‐sum test (a) and the Kruskal–Wallis test (b and c).
TABLE 2.
Performance measures of fecal calprotectin, DICA classification, CODA score, and the combination of fecal calprotectin with these two prognostic tools in predicting subsequent acute diverticulitis.
| Harrel's c‐statistic (95% CI) | Brier score (95% CI) | Nagerlkerke's R 2 (95% CI) | |
|---|---|---|---|
| Fecal calprotectin a | 0.685 (0.614–0.756) | 0.0610 (0.0466–0.0754) | 0.226 (0.091–0.409) |
| DICA classification | 0.779 (0.728–0.830) | 0.0525 (0.0396–0.0653) | 0.571 (0.399–0.734) |
| DICA classification + fecal calprotectin a | 0.806 (0.755–0.857) | 0.0507 (0.0381–0.0633) | 0.619 (0.448–0.778) |
| CODA score | 0.827 (0.786–0.868) | 0.0498 (0.0373–0.0623) | 0.659 (0.525–0.798) |
| CODA score + fecal calprotectin a | 0.832 (0.793–0.871) | 0.0486 (0.0362–0.0609) | 0.689 (0.568–0.834) |
Abbreviations: CI, confidence interval; CODA, Combined Overview on Diverticular Assessment; DICA, Diverticular Inflammation and Complication Assessment; FC, fecal calprotectin.
The base 10 logarithm of baseline FC as a continuous variable.
Comparison of fecal calprotectin with the DICA classification and CODA score
The DICA classification and the CODA score are validated prognostic tools for predicting the clinical outcomes of DD. 1 We confirmed that DICA classification and CODA score were significant predictors of the risk of diverticulitis in the study population as shown in Table 2, and Appendix Tables 1 and 2. The c‐statistics of the DICA classification (0.779; 95% CI, 0.728–0.830) and CODA score (0.827; 95% CI, 0.786–0.868) were significantly higher compared with FC (p‐values for the difference: 0.020 and 0.001, respectively). As apparent from the other measures of performance, both DICA classification and CODA score were better prognostic tools than FC alone.
Combination of fecal calprotectin with DICA classification and CODA score
FC and DICA classifications were both fitted in a multivariable Cox model (Appendix Table 3). An LR test indicated an improved model fit over a Cox model using DICA classification as the only predictor (LR test, p = 0.003). The combination of FC and DICA classification showed a slight but significant increase in discrimination capacity over DICA classification alone (c‐statistic: 0.806 vs. 0.779, p‐value for the difference = 0.022; Table 2).
The FC and CODA score (i.e., its predicting components) 24 were fitted in a multivariable Cox model (Appendix Table 4). The LR test indicated an improved model fit over a Cox model using the CODA score alone (LR test, p = 0.010). The discrimination capacity of the combination of FC and CODA score did not differ in comparison to that of CODA score alone (c‐statistic: 0.832 vs. 0.827, p‐value for the difference = 0.41; Table 2). The individual probability of developing diverticulitis for each of the 871 patients according to the linear combination of CODA score and FC is displayed in Appendix Figure 2, and its calibration is reported in Appendix Figure 3.
Clinical utility of management strategies based on DICA classification, CODA score, and the combination of CODA score and fecal calprotectin
To evaluate and compare the clinical utility of management strategies based on the CODA classification, the DICA score and the combination of the CODA score and FC, we used the decision curve analysis. Decision curve analysis inherently incorporates the consequences of clinical action that is the result of risk stratification after applying a prognostic score or rule. It is important to show that taking clinical actions on the basis of a certain prognostic score offers a larger net benefit over other existing strategies in a range of plausible thresholds. For extended guidance on how to interpret the decision curve analysis, please refer to eAppendix 2.
We examined the relationship between a range of threshold probabilities for predicting diverticulitis in patients with DD and the relative value of false‐positive and false‐negative results (i.e., the net benefit). The net benefit of the three management strategies was plotted and compared (Figure 3). Here, we compared strategies based on DICA classification (strategy 1), CODA score (strategy 2), and the linear combination of CODA score and FC (strategy 3). All three scores offered a better net benefit than a strategy of “treating all” and “treating none” over a large range of plausible threshold probabilities. Among the three management strategies, strategy 1 based on the DICA classification offered the lowest net benefit at most threshold probabilities. Between strategies 2 and 3, no score offered a clearly larger net benefit over the other in the entire spectrum of plausible threshold probabilities. More in detail, strategy 2 based on the CODA score offered the largest net benefit at decision thresholds from 16% to 22% and from 29% to 37%, whereas strategy 3 based on the CODA score and FC offered a larger benefit at other threshold probabilities except for those below 8%, where strategies 2 and 3 offered a similar net benefit. Under these circumstances, a simpler and less expensive strategy should be recommended (i.e., a treatment strategy based on the CODA score alone).
FIGURE 3.

Decision curve analysis plotting the net benefit of management strategies adopted on the basis of three prognostic tools predicting the 3‐year risk of diverticulitis in patients with Diverticular Disease (DD). The net benefit corresponding to using the Diverticular Inflammation and Complication Assessment (DICA) classification (in orange), the Combined Overview on Diverticular Assessment (CODA) score (in green) and the CODA score plus fecal calprotectin (FC) are compared to strategies to “treat all” (diagonal dashed line) and “treat none” (horizontal dashed line). The net benefit, plotted on the y axis, is a metric representing the benefit of a certain intervention minus its harms multiplied by an exchange rate (x axis). The unit of net benefit is true positive. A net benefit of 0.05, for instance, means to find “5 true positives for every 100 patients in the target population” with no harms (i.e., benefit is “net”). The net benefit is plotted over a range of possible decision thresholds/exchange rates (i.e., individual predicted probabilities derived by applying the prognostic tool). The net benefit also incorporates any consequence (i.e., clinical actions taken) of knowing the individual risk of a subsequent diverticulitis. The net benefit of five different strategies is compared. The two extreme—default—strategies are “treat all” (diagonal dashed line) and “treat none” (horizontal dashed line), meaning enacting clinical actions as if all patients with DD will develop diverticulitis (i.e., “treat all”), or as if nobody of them will develop diverticulitis (i.e., “treat none”). The x axis can also be renamed preference: clinicians more worried about the harms of a missed diverticulitis (i.e., true positive) will adopt thresholds closer to a predicted probability of zero (i.e., left side of the graph), while clinicians more worried about the harms/costs of unnecessary interventions/visits (i.e., on false positives) will adopt higher thresholds (i.e., right side of the graph). The x axis is also called “exchange rate”, which is an odds ratio and represents how many false positives are worth one true positive (i.e., adopting a threshold probability of 20% means that a patient with a predicted probability over 20% will be considered likely to develop diverticulitis—and treated accordingly—and by adopting this classification rule/threshold, one accepts that one true positive is worth four false positives). Interventions associated with different harms/costs may need the adoption of different thresholds/exchange rates. The prognostic tool corresponding to the highest net benefit over the largest range of threshold probabilities should be adopted. When this is unclear, the simpler prognostic tool or the one corresponding to lower costs/harms/inconvenience should be used. For a comprehensive guidance regarding interpreting the decision curve analysis, please see eAppendix 2.
Short‐term prediction of clinical outcomes
FC is a non‐invasive intervention that may be used for the short‐term management of patients with DD. We investigated its prognostic value in a timeframe of 3 months instead of the previous analyses conducted at 3 years of follow‐up.
The time‐dependent area under receiving operator curves at 3 months showed very high discrimination capacity for FC (time‐dependent AUC3months = 0.976, 95% CI: 0.966–0.986), CODA score (AUC3months = 0.962, 95% CI: 0.950–0.974), and DICA classification (AUC3months = 0.943, 95% CI: 0.933–0.954). The discrimination capacity of FC at 3 months was comparable to that of the CODA score (p for the difference = 0.10) and significantly higher than that of the DICA score (p < 0.001). The Brier score at this time‐frame was virtually the same for the two scores and FC (0.009; 95% CI: 0.003–0.015).
The discrimination capacity of FC decreased quickly with longer follow‐up times, while the discrimination by DICA and CODA was more stable and higher at longer follow‐up times (Appendix Figure 4). FC demonstrated a significant short‐term prognostic value, which, however, deteriorated quickly after 3 months. Accordingly, Figure 4 describes the possible short‐term (3‐month) risk stratification of patients with newly diagnosed colonic diverticulosis detected on endoscopy.
FIGURE 4.

Flow‐chart suggesting the possible short‐term (3‐month) risk stratification of patients with newly diagnosed colonic diverticulosis detected on endoscopy. CODA, Combined Overview on Diverticular Assessment; DICA, Inflammation and Complication Assessment; FC, fecal calprotectin.
DISCUSSION
The field of gastroenterology moves forward in the evolving landscape of personalized medicine. Collecting biomarkers through non‐invasive methods to predict the evolution of a disease has become increasingly common. FC is an easily collected biomarker that correlates with the severity of inflammatory bowel diseases (IBD) (mainly ulcerative colitis), may predict the course of these diseases, and harbours immune‐regulatory functions. 28 These properties render it an interesting marker of inflammation in DD.
In this context, FC has been used mainly to differentiate DD from irritable bowel syndrome as well as non‐invasive marker of inflammation in response to treatment or following an episode of AD. 1 , 29 However, the extent to which FC could predict the course of DD was rather uncertain before the current study. We recently found that the DICA endoscopic score, its clinical evolution, and the CODA score accurately predict the evolution of DD. 14 Both these prognostic tools have been developed and validated in a wide international prospective study, but the role of FC in comparison with DICA and CODA is unknown.
We found FC to be a moderately accurate predictor of the 3‐year risk of diverticulitis. FC levels were strongly associated with the DICA classification and classes of CODA score. Despite this, DICA classification and CODA score were both more accurate predictors of AD in the long‐term. This finding is not surprising. Although calprotectin is a useful marker of inflammation, both DICA and CODA rely on a structured endoscopic evaluation; thus, they can provide a more exhaustive picture of the risk of complications in patients with DD, which is characterized by extensive structural and functional changes of the bowel wall. 1
In personalized medicine, it is important to use up‐to‐date methodology to guide decisions in clinical practice. As recently reported, 30 , 31 decision analytic methods are useful tools to determine under which circumstances prognostic scores and biomarkers provide the net benefit for a population of patients. In this study, we used the decision curve analysis to compare the net benefit of management strategies based on the DICA classification and CODA score (i.e., the two existing validated prognostic scores for predicting clinical outcomes of DD) with a strategy based on baseline FC alone or in combination with the CODA score.
FC enhanced the predictive value of DICA classification over the course of DD; however, this was not the same for the CODA score. When used in combination with CODA, there was no clear net benefit in comparison to CODA alone. In other words, adding two clinical parameters or adding FC to the DICA classification provided similar net benefits for patients with DD over the spectrum of plausible thresholds used for decision making. FC seems to be an important but not a mandatory tool in the decision making in patients suffering from DD, limiting its use in predicting the long‐term evolution of the disease. Moreover, FC has some limitations regarding its use in real life: lack of standardized assessment, 32 no easy availability even in developed countries, 33 and high variability across well‐known diseases. 34
Irrespectively of the role of FC, the DICA classification has been confirmed as the keystone to build up a reliable predicting score for the outcome of DD. Adding clinical (CODA score) or laboratory (FC) findings enhances the prognostic value of DICA, but assessing the colon harboring diverticula with this classification is a necessary step to set up reliable decision‐making in these patients. Of course, the DICA classification has some limitations. It is useful when the patient is diagnosed first with diverticulosis/DD, but we do not know whether it may be useful in the same way in patients with well‐known diverticulosis/DD and, probably, already treated. Moreover, we do not know yet whether DICA‐based risk stratification can improve treatment choices. Considering this, new therapeutic trials should be designed according to these classifications in order to identify patients with DD who need prophylactic treatment.
However, updating the DICA classification or the CODA score of a patient over time would require new colonoscopy; thus, it cannot be performed often, especially in fragile patients. FC may be a non‐invasive tool providing useful short‐term information when the DICA classification of a patient may be outdated (i.e., >3 years). The discrimination capacity of FC in predicting AD in the short term (i.e., 3 months) was very high with no significant differences as compared to CODA. This finding may support the complementary use of FC to gauge the short‐term risk of acute‐diverticulitis and perhaps as a surrogate marker to assess treatment effectiveness. This novel finding, however, should be considered in light of the study limitations. This study is a post‐hoc analysis of an international multicentre prospective cohort study. A centralized laboratory analysis of FC was not planned in our initial study protocol, which is a weakness. Although we took steps to increase the comparability across centres, further studies using a standardized and centralized assessment of FC are warranted to confirm our findings on the use of FC to assess the short‐term risk of AD.
In conclusion, we believe that the results of the current study can contribute to improving decision making in patients with DD. Our data suggest that FC may predict clinical outcomes in the short‐term. If adequately confirmed in further ad hoc studies, this biomarker could be used as a complementary tool to assess the immediate risk of diverticulitis in patients with longstanding DD in whom performing further colonoscopy is deemed inconvenient or unfeasible.
DICA INTERNATIONAL GROUP
Marco Astegiano, Division of Gastroenterology, “Molinette” University Hospital, Torino, Italy; Francesco Bachetti, Department of Medicine and Surgery, Gastroenterology and Hepatology Unit, “Santa Maria della Misericordia” University Hospital, University of Perugia, Perugia, Italy; Gianluca Baldassarre, Digestive Endoscopy Unit, ULSS7 Alto Vicentino, Santorso (VI), Italy; Fabio Baldi †, Digestive Endoscopy Unit, Civil Hospital, Tarquinia (VT), Italy; Edoardo Borsotti, Division of Gastroenterology, “San Donato” Hospital, San Donato Milanese (MI), Italy; Claudio Cassieri, Division of Internal Medicine and Gastroenterology, “Cristo Re” Hospital, Rome, Italy; Alessia Cazzato, Division of Gastroenterology, “Santa Caterina Novella” Hospital, Galatina (LE), Italy; Stefania Chiri, Division of Gastroenterology, “Santa Caterina Novella” Hospital, Galatina (LE), Italy; Antonio Ciccone, Division of Gastroenterology, Hepatology and Nutrition, San Salvatore Hospital, Department of Life, Health & Environmental Sciences, University of L'Aquila, L'Aquila, Italy; Alberto Damiani, Service of Digestive Endoscopy, “Villa dei Pini” Home Care, Civitanova Marche (MC), Italy; Patrizia De Colibus, Division of Gastroenterology, “T. Maresca” Hospital, Torre del Greco (NA), Italy; Roberto Faggiani, Division of Gastroenterology, “S. Camillo” Hospital, Rome, Italy; Fabio Finocchiaro, Division of Gastroenterology, “Molinette” University Hospital, Torino, Italy; Serafina Fiorella, Division of Gastroenterology, “San Pio da Pietrelcina” Hospital, Vasto (CH), Italy; Francesca Foschia, Territorial Gastroenterology Service, ASL Roma 2, Rome, Italy; Federica Furfaro, IBD Unit, “Humanitas” University Hospital, Rozzano (MI), Italy; Sara Gallina, Division of Gastroenterology, “Belcolle” Hospital, Viterbo, Italy; Gian Marco Giorgetti, Digestive Endoscopy and Nutritional Unit, “S. Eugenio” Hospital, Rome, Italy; Simona Grad, 2nd Medical Department, “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj‐Napoca, Romania; Giuseppe Grande, Digestive Endoscopy Unit, “Sant'Agostino Estense” Hospital, Baggiovara (MO), Italy; Antonio Grandolfo, Division of Gastroenterology, “S. Paolo” Hospital, Bari, Italy; Maria Antonia Lai, Division of Gastroenterology, “Monserrato” University Hospital, University of Cagliari, Cagliari, Italy; Piera Giuseppina Lecca, Division of Internal Medicine and Gastroenterology, “Cristo Re” Hospital, Rome, Italy; Daniele Lisi, Territorial Gastroenterology Service, ASL Roma 2, Rome, Italy; Loris Riccardo Lopetuso, Division of Internal Medicine and Gastroenterology, IRCCS “A. Gemelli” Hospital, Fondazione Policlinico Gemelli, Catholic University, Rome, Italy; Department of Medicine and Ageing Sciences, “G. d'Annunzio” University of Chieti‐Pescara, Chieti, Italy; Center for Advanced Studies and Technology (CAST), “G. d'Annunzio” University of Chieti‐Pescara, Chieti, Italy; Antonio Penna, Teerritorial Gastroenterology Service, ASL Bari, Bari, Italy; Flavia Pigò, Digestive Endoscopy Unit, “Sant'Agostino Estense” Hospital, Baggiovara (MO), Italy; Piero Portincasa, Internal Medicine Unit, “A. Murri” Medical Clinic, Policlinico University Hospital, Bari, Italy; Giannenrico Rizzatti, Division of Internal Medicine and Gastroenterology, IRCCS “A. Gemelli” Hospital, Fondazione Policlinico Gemelli, Catholic University, Rome, Italy; Giovanni Luca Rizzo, Division of Gastroenterology, “Perrino” Hospital, Brindisi, Italy; Stefania Scanni, Territorial Gastroenterology Service, ASL Roma 2, Rome, Italy; Luigi Schiffino, Division of Gastroenterology and Surgical Endoscopy, “Grassi” Hospital, Ostia, Rome, Italy; Ieva Stundiene, Institute of Clinical Medicine, Vilnius University Hospital, Vilnius, Lithuania; Antonino Tesoriere, Digestive Endoscopy Unit, Civil Hospital, Tarquinia (VT), Italy; Riccardo Urgesi, Division of Gastroenterology and Digestive Endoscopy “S. Giovanni‐Addolorata” Hospital, Rome, Italy; Paolo Usai, Division of Gastroenterology, “Monserrato” University Hospital, University of Cagliari, Cagliari, Italy.
AUTHOR CONTRIBUTIONS
Guarantor of the Article: Antonio Tursi. Conception and design: Antonio Tursi; Francesco Di Mario, Giovanni Brandimarte, and Erasmo Spaziani. Acquisition and collection of data: Antonio Tursi, Walter Elisei, Marcello Picchio, Giovanni Brandimarte, Leonardo Allegretta, Maria Laura Annunziata, Marco Astegiano, Francesco Bachetti, Mauro Bafutto, Gabrio Bassotti, Maria Antonia Bianco, Raffaele Colucci, Rita Conigliaro, Dan L. Dumitrascu, Ricardo Escalante, Luciano Ferrini, Serafina Fiorella, Giacomo Forti, Marilisa Franceschi, Maria Giovanna Graziani, Frank Lammert, Giovanni Latella, Giovanni Maconi, Gerardo Nardone, Lucia Camara De Castro Oliveira, Enio Chaves Oliveira, Alfredo Papa, Savvas Papagrigoriadis, Anna Pietrzak, Stefano Pontone, Piero Portincasa, Tomas Poskus, Giuseppe Pranzo, Matthias Christian Reichert, Giovanni Luca Rizzo, Stefano Rodinò, Jaroslaw Regula, Giuseppe Scaccianoce, Franco Scaldaferri, Luigi Schiffino, Roberto Vassallo, Costantino Zampaletta, Angelo Zullo, Silvio Danese, Gianluca Baldassarre, Fabio Baldi†, Edoardo Borsotti, Claudio Cassieri, Alessia Cazzato, Stefania Chiri, Antonio Ciccone, Debora Compare, Alberto Damiani, Patrizia De Colibus, Roberto Faggiani, Fabio Finocchiaro, Francesca Foschia, Federica Furfaro, Sara Gallina, Gianmarco Giorgetti, Simona Grad, Giuseppe Grande, Antonio Grandolfo, Maria Antonia Lai, Piera Giuseppina Lecca, Daniele Lisi, Loris Riccardo Lopetuso, Antonio Penna, Flavia Pigò, Giannenrico Rizzatti, Stefania Scanni, Ieva Stundiene, Antonino Tesoriere, Riccardo Urgesi, and Paolo Usai. Analysis and interpretation of data: Antonio Tursi, Daniele Piovani, Walter Elisei, Marcello Picchio, Stefanos Bonovas, and Silvio Danese. Final approval of the version to be published: Antonio Tursi, Daniele Piovani, Walter Elisei, Marcello Picchio, Giovanni Brandimarte, Francesco Di Mario, Leonardo Allegretta, Maria Laura Annunziata, Marco Astegiano, Mauro Bafutto, Gabrio Bassotti, Maria Antonia Bianco, Raffaele Colucci, Rita Conigliaro, Dan L. Dumitrascu, Ricardo Escalante, Luciano Ferrini, Serafina Fiorella, Giacomo Forti, Marilisa Franceschi, Maria Giovanna Graziani, Frank Lammert, Giovanni Latella, Giovanni Maconi, Gerardo Nardone, Lucia Camara De Castro Oliveira, Enio Chaves Oliveira, Alfredo Papa, Savvas Papagrigoriadis, Anna Pietrzak, Stefano Pontone, Piero Portincasa, Tomas Poskus, Giuseppe Pranzo, Matthias Christian Reichert, Giovanni Luca Rizzo, Stefano Rodinò, Jaroslaw Regula, Giuseppe Scaccianoce, Franco Scaldaferri, Luigi Schiffino, Roberto Vassallo, Costantino Zampaletta, Angelo Zullo, Stefanos Bonovas, Silvio Danese, Marco Astegiano, Francesco Bachetti, Gianluca Baldassarre, Fabio Baldi†, Edoardo Borsotti, Claudio Cassieri, Alessia Cazzato, Stefania Chiri, Antonio Ciccone, Debora Compare, Alberto Damiani, Patrizia De Colibus, Roberto Faggiani, Fabio Finocchiaro, Francesca Foschia, Federica Furfaro, Sara Gallina, Gianmarco Giorgetti, Simona Grad, Giuseppe Grande, Antonio Grandolfo, Maria Antonia Lai, Piera Giuseppina Lecca, Daniele Lisi, Loris Riccardo Lopetuso, Antonio Penna, Flavia Pigò, Giannenrico Rizzatti, Stefania Scanni, Erasmo Spaziani, Ieva Stundiene, Antonino Tesoriere, Riccardo Urgesi, Paolo Usai.
CONFLICT OF INTEREST STATEMENT
Silvio Danese, MD, PhD, served as speaker, consultant, and/or advisory board member for Abbvie, Allergan, Alfa Wassermann, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Gilead, Hospira, Johnson and Johnson, Merck, MSD, Mundipharma, Pfizer Inc., Sandoz, Takeda, Tigenix, UCB Pharma, Vifor: Giovanni Maconi, MD, served as speaker and/or advisory board fees for AlfaSigma, Arena, Janssen, Gilead, Roche; Gerardo Nardone, MD, PhD, received funding for target projects from Apharm and Sofar; Anna Pietrzak, MD, served as lecturer for AlfaSigma and Polpharma; Jaroslaw Regula, MD, PhD, served as lecturer for AlfaSigma, Takeda, Ipsen and Servier; Franco Scaldaferri, MD, PhD, served as lecturer for Sanofi; The remaining authors declare no competing interests.
ETHICS APPROVAL
The study was conducted according to the World Medical Association Declaration of Helsinki of 1975. It was approved by the Ethic Committee of the coordinator centre and all participating centres. The study was recorded at www.ClinicalTrials.gov (NCT02758860).
PATIENTS CONSENT STATEMENT
All study participants provided informed written consent prior to endoscopic investigation and to take part in this study.
Supporting information
Supporting Information S1
Supporting Information S2
ACKNOWLEDGMENTS
No funding was obtained for this study.
Tursi A, Piovani D, Brandimarte G, Di Mario F, Elisei W, Picchio M, et al. Diverticular Inflammation and Complication Assessment classification, CODA score and fecal calprotectin in clinical assessment of patients with diverticular disease: a decision curve analysis. United European Gastroenterol J. 2023;11(7):642–653. 10.1002/ueg2.12369
†The co‐author Fabio Baldi is deceased.
Contributor Information
Antonio Tursi, Email: antotursi@tiscali.it.
DICA International Group:
Marco Astegiano, Francesco Bachetti, Gianluca Baldassarre, Fabio Baldi, Edoardo Borsotti, Claudio Cassieri, Alessia Cazzato, Stefania Chiri, Antonio Ciccone, Alberto Damiani, Patrizia De Colibus, Roberto Faggiani, Fabio Finocchiaro, Serafina Fiorella, Francesca Foschia, Federica Furfaro, Sara Gallina, Gian Marco Giorgetti, Simona Grad, Giuseppe Grande, Antonio Grandolfo, Maria Antonia Lai, Piera Giuseppina Lecca, Daniele Lisi, Loris Riccardo Lopetuso, Antonio Penna, Flavia Pigò, Piero Portincasa, Giannenrico Rizzatti, Giovanni Luca Rizzo, Stefania Scanni, Luigi Schiffino, Ieva Stundiene, Antonino Tesoriere, Riccardo Urgesi, and Paolo Usai
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Tursi A, Scarpignato C, Strate LL, Lanas A, Kruis W, Lahat A, et al. Colonic diverticular disease. Nat Rev Dis Primers. 2020;6(1):20. 10.1038/s41572-020-0153-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lohrmann C, Ghanem N, Pache G, Makowiec F, Kotter E, Langer M. CT in acute perforated sigmoid diverticulitis. Eur J Radiol. 2005;56(1):78–83. 10.1016/j.ejrad.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 3. Wasvary H, Turfah F, Kadro O, Beauregard W. Same hospitalization resection for acute diverticulitis. Am Surg. 1999;65(7):632–635. 10.1177/000313489906500706 [DOI] [PubMed] [Google Scholar]
- 4. Ambrosetti P, Becker C, Terrier F. Colonic diverticulitis: impact of imaging on surgical management ‐ a prospective study of 542 patients. Eur Radiol. 2002;12(5):1145–1149. 10.1007/s00330-001-1143-y [DOI] [PubMed] [Google Scholar]
- 5. Köhler L, Sauerland S, Neugebauer E, Caprilli R, Fingerhut A, Haboubi NY, et al. Diagnosis and treatment of diverticular disease: results of a consensus development conference. The Scientific Committee of the European Association for Endoscopic Surgery. Surg Endosc. 1999;13(4):430–436. 10.1007/s004649901007 [DOI] [PubMed] [Google Scholar]
- 6. Sheth AA, Longo W, Floch MH. Diverticular disease and diverticulitis. Am J Gastroenterol. 2008;103(6):1550–1556. 10.1111/j.1572-0241.2008.01879.x [DOI] [PubMed] [Google Scholar]
- 7. Hansen O, Graupe F, Stock W. Prognostic factors in perforating diverticulitis of the large intestine. Chirurg. 1998;69(4):443–449. 10.1007/s001040050436 [DOI] [PubMed] [Google Scholar]
- 8. Ghorai S, Ulbright TM, Rex DK. Endoscopic findings of diverticular inflammation in colonoscopy patients without clinical acute diverticulitis: prevalence and endoscopic spectrum. Am J Gastroenterol. 2003;98(4):802–806. 10.1111/j.1572-0241.2003.07383.x [DOI] [PubMed] [Google Scholar]
- 9. Tursi A, Elisei W, Giorgetti GM, Aiello F, Brandimarte G. Inflammatory manifestations at colonoscopy in patients with colonic diverticular disease. Aliment Pharmacol Ther. 2011;33(3):358–365. 10.1111/j.1365-2036.2010.04530.x [DOI] [PubMed] [Google Scholar]
- 10. Hall JF, Roberts PL, Ricciardi R, Read T, Scheirey C, Wald C, et al. Long‐term follow‐up after an initial episode of diverticulitis: what are the predictors of recurrence? Dis Colon Rectum. 2011;54(3):283–288. 10.1007/dcr.0b013e3182028576 [DOI] [PubMed] [Google Scholar]
- 11. Tursi A, Brandimarte G, Di Mario F, Andreoli A, Annunziata ML, Astegiano M, et al. Development and validation of an endoscopic classification of diverticular disease of the colon: the DICA classification. Dig Dis. 2015;33:68–76. [DOI] [PubMed] [Google Scholar]
- 12. Tursi A, Brandimarte G, di Mario F, Nardone G, Scarpignato C, Picchio M, et al. The “DICA” endoscopic classification for diverticular disease of the colon shows a significant interobserver agreement among community endoscopists. J Gastrointestin Liver Dis. 2019;28(1):23–27. 10.15403/jgld.2014.1121.281.dic [DOI] [PubMed] [Google Scholar]
- 13. Tursi A, Brandimarte G, Di Mario F, Lanas A, Scarpignato C, Bafutto M, et al. The DICA endoscopic classification for diverticular disease of the colon shows a significant interobserver agreement among community endoscopists: an international study. J Gastrointestin Liver Dis. 2019;28(Suppl 4):39–44. 10.15403/jgld-558 [DOI] [PubMed] [Google Scholar]
- 14. Tursi A, Brandimarte G, Di Mario F, Elisei W, Picchio M, Allegretta L, et al. Prognostic performance of the ‘DICA’ endoscopic classification and the ‘CODA’ score in predicting clinical outcomes of diverticular disease: an international, multicentre, prospective cohort study. Gut. 2022;71(7):1350–1358. 10.1136/gutjnl-2021-325574 [DOI] [PubMed] [Google Scholar]
- 15. Tibble JA, Sigthorsson G, Foster R, Forgacs I, Bjarnason I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123(2):450–460. 10.1053/gast.2002.34755 [DOI] [PubMed] [Google Scholar]
- 16. Khaki‐Khatibi F, Qujeq D, Kashifard M, Moein S, Maniati M, Vaghari‐Tabari M. Calprotectin in inflammatory bowel disease. Clin Chim Acta. 2020;510:556–565. 10.1016/j.cca.2020.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alibrahim B, Aljasser MI, Salh B. Fecal calprotectin use in inflammatory bowel disease and beyond: a mini‐review. Can J Gastroenterol Hepatol. 2015;29(3):157–163. 10.1155/2015/950286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Štimac D, Nardone G, Mazzari A, Crucitti A, Maconi G, Elisei W, et al. What's new in diagnosing diverticular disease. J Gastrointestin Liver Dis. 2019;28(Suppl 4):17–22. 10.15403/jgld-553 [DOI] [PubMed] [Google Scholar]
- 19. Tursi A, Brandimarte G, Elisei W, Giorgetti GM, Inchingolo CD, Aiello F. Faecal calprotectin in colonic diverticular disease: a case‐control study. Int J Colorectal Dis. 2009;24(1):49–55. 10.1007/s00384-008-0595-9 [DOI] [PubMed] [Google Scholar]
- 20. Kvasnovsky CL, Leong LEX, Choo JM, Abell GCJ, Papagrigoriadis S, Bruce KD, et al. Clinical and symptom scores are significantly correlated with fecal microbiota features in patients with symptomatic uncomplicated diverticular disease: a pilot study. Eur J Gastroenterol Hepatol. 2018;30(1):107–112. 10.1097/meg.0000000000000995 [DOI] [PubMed] [Google Scholar]
- 21. Brandimarte G, Frajese GV, Bargiggia S, Castellani D, Cocco A, Colucci R, et al. Performance of a multi‐compounds nutraceutical formulation in patients with symptomatic uncomplicated diverticular disease. Minerva Gastroenterol (Torino). 2022;68(2):216–222. 10.23736/S2724-5985.22.03132-1 [DOI] [PubMed] [Google Scholar]
- 22. Shahedi K, Fuller G, Bolus R, Cohen E, Vu M, Shah R, et al. Long‐term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol. 2013;11(12):1609–1613. 10.1016/j.cgh.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tursi A, Franceschi M, Elisei W, Picchio M, Di Mario F, Brandimarte G. The natural history of symptomatic uncomplicated diverticular disease: a long‐term follow‐up study. Ann Gastroenterol. 2021;34:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–W73. 10.7326/m14-0698 [DOI] [PubMed] [Google Scholar]
- 25. Kamarudin AN, Cox T, Kolamunnage‐Dona R. Time‐dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol. 2017;17(1):53. 10.1186/s12874-017-0332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565–574. 10.1177/0272989x06295361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ. 2016;352:i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jukic A, Bakiri L, Wagner EF, Tilg H, Adolph TE. Calprotectin: from biomarker to biological function. Gut. 2021;70(10):1978–1988. 10.1136/gutjnl-2021-324855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tursi A, Elisei W, Picchio M, Brandimarte G. Increased faecal calprotectin predicts recurrence of colonic diverticulitis. Int J Colorectal Dis. 2014;29(8):931–935. 10.1007/s00384-014-1884-0 [DOI] [PubMed] [Google Scholar]
- 30. Stidham RW, Vickers A, Singh K, Waljee AK. From clinical trials to clinical practice: how should we design and evaluate prediction models in the care of IBD? Gut. 2022;71(6):1046–1047. 10.1136/gutjnl-2021-324712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ. 2016;352:i6. 10.1136/bmj.i6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. D'Amico F, Rubin DT, Kotze PG, Magro F, Siegmund B, Kobayashi T, et al. International consensus on methodological issues in standardization of fecal calprotectin measurement in inflammatory bowel diseases. United Eur Gastroenterol J. 2021;9(4):451–460. 10.1002/ueg2.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kennedy NA, Hansen R, Younge L, Mawdsley J, Beattie RM, Din S, et al. Organisational changes and challenges for inflammatory bowel disease services in the UK during the COVID‐19 pandemic. Frontline Gastroenterol. 2020;11(5):343–350. 10.1136/flgastro-2020-101520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Calafat M, Cabré E, Mañosa M, Lobatón T, Marín L, Domènech E. High within‐day variability of fecal calprotectin levels in patients with active ulcerative colitis: what is the best timing for stool sampling? Inflamm Bowel Dis. 2015;21(5):1072–1076. 10.1097/mib.0000000000000349 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Supporting Information S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


