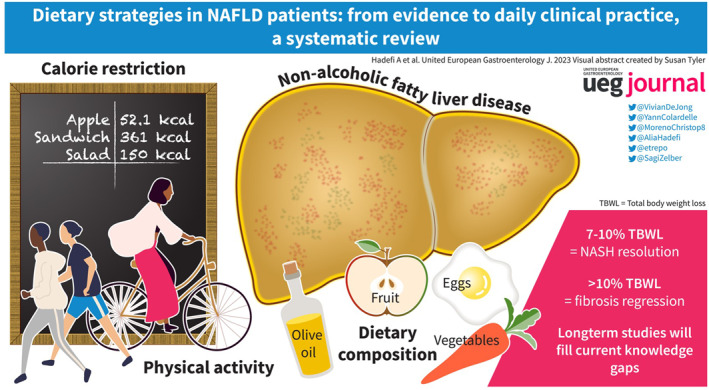
Abstract
Lifestyle modification comprising calorie restriction (CR) and increased physical activity enabling weight loss is the first‐line of treatment for non‐alcoholic fatty liver disease (NAFLD). However, CR alone is not optimal and evidence suggests that dietary pattern and composition are also critical in NAFLD management. Accordingly, high consumption of red and processed meat, saturated fat, added sugar, and sweetened beverages are associated with an increased risk of developing NAFLD and hepatocellular carcinoma, while other foods and compounds such as fish, olive oil, and polyphenols are, in contrast, beneficial for metabolic disorders. Therefore, several dietary interventions have been studied in order to determine which strategy would be the most beneficial for NAFLD. The evidence regarding the effectiveness of different dietary interventions such as low carbohydrate/low‐fat diet, time‐restricted eating diet, CR, and the well‐studied Mediterranean diet is summarized.
Keywords: dietary pattern, Mediterranean diet, NAFLD, physical activity, weight loss
INTRODUCTION
Non‐alcoholic fatty liver disease (NAFLD) affects 30% of the global population. 1 , 2 , 3 , 4 The rising global burden of NAFLD parallels the increasing prevalence of type 2 diabetes mellitus (T2DM) and obesity, resulting in high healthcare resource utilization and costs. 5 , 6 NAFLD is a spectrum of liver diseases ranging from liver steatosis to non‐alcoholic steatohepatitis (NASH), and fibrosis, which may lead to cirrhosis and hepatocellular carcinoma. 7 Furthermore, NAFLD is associated with an increased risk of incident T2DM, chronic kidney disease, extrahepatic cancers, and cardiovascular morbidity and mortality, 8 , 9 , 10 , 11 underscoring the multi‐systemic pattern of this disease. Similarly, evidence suggests that the visceral adipose tissue (VAT), one of the hallmarks of NAFLD, is associated with increased atherosclerosis and cardiometabolic risk, 12 , 13 , 14 , while its reduction is correlated with hepatic histologic improvements, independently of liver fat reduction. 15 Therefore, considering the aforementioned findings, NAFLD management requires a multidisciplinary care team to mitigate negative liver‐related and extra‐hepatic‐related outcomes.
Currently, no pharmaceutical treatments are approved for NAFLD therapy. Therefore, comprehensive lifestyle modification interventions, including calorie restriction (CR) and increased energy expenditure, remain the cornerstones of NAFLD treatment. 16 , 17 Although the amount of weight loss is the most important determinant of liver histological feature outcomes 18 , 19 and the most well‐validated treatment, healthy eating patterns and dietary composition can also have a beneficial impact on the risk of new‐onset NAFLD, 20 and can provide additional benefits such as the reduction of cardiovascular disease risk, 21 , 22 improvements in metabolic outcomes, 23 , 24 and reductions in mortality. 25 However, only a few patients reach the significant and sustained weight loss needed for a positive effect on liver damage, and maintaining long‐term adherence to lifestyle modification remains a challenge.
The evidence in the literature is growing with regard to the specific dietary patterns associated with greater cardiovascular and metabolic benefits, but is scarce regarding strategies to adjust the pattern to individual patients based on socio‐economic, cultural background, and personal preferences for promoting long‐term adherence. Recent research has demonstrated that the Mediterranean diet (MD) is beneficial for the prevention of cardiovascular disease, 26 and the management of NAFLD. 27 , 28 However, various other dietary strategies exist that are less well studied in the area of NAFLD. This systematic review aims to summarize the effects of different dietary strategies and exercise interventions on liver function in patients with NAFLD.
METHODS
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 29
Data sources and search strategy
We searched four databases, MEDLINE, EMBASE, Web of SCIENCE, and Cochrane Central Register of Controlled Trials (CENTRAL), from 2010 to September 2022. The formulation of search terms was designed and conducted jointly by a medical librarian with study investigators. The list of search terms is provided in Supporting Information S1. Reference lists of previously published systematic reviews and meta‐analyses were examined to find additional relevant studies. Only articles published in English were considered.
Eligibility criteria
We included only randomized controlled trials (RCTs) and clinical controlled trials in this systematic review. The inclusion criteria were as follows: (a) adult patients (>18 years old); (b) dietary and exercise interventions on surrogate markers of NAFLD; (c) interventions: MD intervention and/or CR intervention and/or time‐restricted eating and/or low‐fat diet (LFD) and/or low‐carbohydrate diet (LCD) and/or physical activity (PA); (e) surrogate markers of NAFLD: histology (NAFLD activity score [NAS], individual scoring of ballooning, lobular inflammation, and steatosis) and/or liver function tests (LFTs) (including alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), and/or non‐invasive markers of liver fibrosis (NAFLD fibrosis score, fibrosis 4 index [FIB‐4], elastography, FibroScan‐AST [FAST] score), and/or non‐invasive markers of liver steatosis evaluated either by imaging: controlled attenuation parameter (CAP), magnetic resonance imaging or spectroscopy (MRI/MRS) proton density fat fraction (PDFF), ultrasonography, or serologically: fatty liver index (FLI), hepatic steatosis index (HIS); (f) minimal sample size of 30 patients (total); (g) human studies.
The following were exclusion criteria: (a) lab‐based feeding trials; (b) animal studies; (c) in vitro studies; and (d) other study designs.
Primary outcomes included surrogate markers of NAFLD (histological features and/or LFTs, and/or non‐invasive assessment of liver fibrosis and steatosis) and secondary outcomes included total body weight loss (TBWL), waist circumference, quality of life, cardiometabolic parameters (blood pressure, lipid profile, cardiovascular risk) and glycated hemoglobin (HbA1C).
Two reviewers (AH, MA) independently assessed relevant studies for eligibility. The final study selection was reached by a mutual agreement between the two reviewers.
RESULTS
A total of 4374 studies were identified, 20 of which were duplicates. After title and abstract review, 241 studies were scanned for full‐text review. The full‐text review resulted in 61 studies, of which 10 were systematic reviews and meta‐analyses used for checking reference lists. Finally, 51 studies were included in this narrative systematic review (Figure 1).
FIGURE 1.
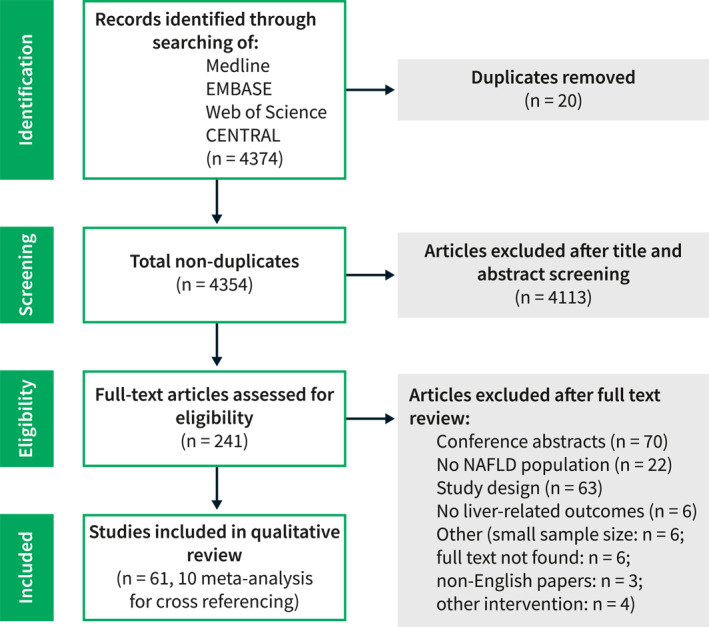
Preferred Reporting Items for Systematic Reviews (PRISMA) flow diagram.
Table 1 outlines the characteristics and efficacy outcomes of different lifestyle interventions in NAFLD.
TABLE 1.
Characteristics and main results of clinical trials testing the effects of different lifestyle interventions on liver outcomes.
| Author | Design | Sample size | Group | Mean age (years) | Mean BMI (kg/m2) | Intervention and comparator/control groups | Intervention characteristics | Intervention duration | Primary outcome |
|---|---|---|---|---|---|---|---|---|---|
| Male n (%) | |||||||||
| Mediterranean diet (MD) | |||||||||
| Properzi 2018, Australia 30 | RCT |
|
NAFLD | 53 | 30.9 |
|
|
12 weeks |
|
| Katsagoni 2018, Greece 31 | RCT |
|
NAFLD | 44 (median) | 31.7 (median) |
|
|
6 months |
|
| Misciagna 2017, Italy 32 | RCT |
|
NAFLD | ‐ | ‐ |
|
6 months |
|
|
| Marin‐Alejandre 2019, Spain 33 | RCT |
|
NAFLD | 49.2 | 33.3 |
|
|
6 months |
|
| Nourian 2020, Iran 34 | RCT |
|
NAFLD | 49.4 | 32.3 |
|
|
2 months |
|
| Abbate, 2021, Spain 35 | RCT |
|
NAFLD | 52.3 | 34.3 |
|
|
6 months |
|
| Yaskolka Meir 2021, Israel 36 | RCT |
|
Central obesity/dyslipidaemia NAFLD (62%) | 50.5 | 31.3 |
|
|
18 months |
|
| Gepner 2018, Israel 37 | RCT |
|
|
47.4 | 30.9 |
|
|
18 months | ↓ HS (intervention vs. comparator) |
| Mazzotti 2018, Italy 38 | CCT |
|
NAFLD | 46 | 33.7 |
|
|
24 months |
|
| Dorosti 2020, Iran 39 | RCT |
|
NAFLD | 43.1 | 32.5 |
|
No advice on calorie allowances, physical activity or behavior changes (both). | 12 weeks | ↓ HS, ALT, and AST (intervention vs. control) |
| Shidfar 2018, Iran 40 | RCT |
|
NAFLD | 46.1 | 29.6 |
|
Calorie restriction: Personalized calorie deficit (both). Olive oil dosage supplied (MD component). | 12 weeks |
|
| Rezaei 2019, Iran 41 | RCT |
|
NAFLD | 46.3 | 30.6 |
|
|
12 weeks |
|
| Naimimohasses 2022, Iran 42 | CCT |
|
NAFLD | 58 | 33.9 |
|
|
12 weeks |
|
| George 2022, Australia 43 | RCT |
|
NAFLD | 52.6 | 31.6 |
|
|
12 weeks | ↓ IHL and LSM (ns between intervention and comparator) |
| Ghetti 2019, Brazil 44 | RCT |
|
NASH | 48.3 | 30.1 |
|
Calorie restriction: 500–750 kcal/d deficit | 3 months |
|
| Marin‐Alejandre 2021, Spain 45 | RCT |
|
NAFLD | 49.2 | 33.3 |
|
6 months |
|
|
| Calorie restriction diet (CR) | |||||||||
| Wong, 2013, Hong Kong 46 | RCT |
|
NAFLD | 51 | 25.5 |
|
|
12 months |
|
| Dong 2016, China 47 | RCT |
|
NAFLD | 56.7 | 26 |
|
|
2 years |
|
| Promrat 2010, USA 48 | RCT |
|
NASH | 48.9 | 33.9 |
|
|
48 weeks |
|
| Cheng 2017, China 49 | RCT |
|
NAFLD | 60 | 26.4 |
|
|
8.6 months |
|
| Johari 2019, Malaysia 50 | RCT |
|
NAFLD | 45.3 | 31.6 |
|
Calorie restriction: Restrict 70% of their calorie requirements between 2 and 8 p.m. (on fast days) and ad libitum (on non‐fast days). | 8 weeks |
|
| Shojasaadat 2019, Iran 51 | RCT |
|
NAFLD | 41 | 31.7 |
|
Calorie restriction: 350–700 kcal/d deficit. | 12 weeks | ↓ AST (intervention) |
| Atefi 2022, Iran 52 | RCT |
|
NAFLD | 38.9 | 30.9 |
|
Dietary pattern: 30 g of oil/day. | 12 weeks | ↓ Fatty liver grade (both) (intervention vs. control) |
| Asghari 2022, Iran 53 | RCT |
|
NAFLD | 40.1 | 31.3 |
|
12 weeks | ↓ ALT and AST only in intervention | |
| Arefhosseini 2011, Iran 54 | RCT |
|
NAFLD | 38 | 28.9 |
|
↓ Of grade of hepatic steatosis (both) | ||
| Garousi 2021, Iran 55 | RCT |
|
NAFLD | 43.5 | 32 |
|
|
3 months | ↓ ALT, AST (both) (intervention vs. control) |
| Wong 2018, Hong Kong 56 | RCT |
|
NAFLD |
|
‐ |
|
12 months |
|
|
| Time‐restricted eating (TRE) | |||||||||
| Cai 2019, China 57 | RCT |
|
NAFLD | 33.6 | 26.8 |
|
No physical activity or behavioral advice provided. | 12 weeks | No change in LSM (both) |
| Varkaneh HK 2022, Iran 58 | RCT |
|
NAFLD | 46.4 | 30.4 |
|
12 weeks |
|
|
| Mari 2021, Israel 59 | CCT |
|
NAFLD | 51.8 | 36.7 |
|
4 weeks | ↓ NFS | |
| Low carbohydrate (LCH)—Low fat diet (LFD) | |||||||||
| Jang 2018, South Korea 60 | RCT |
|
NAFLD |
|
|
|
Booklet with informations regarding calorie intake, macronutrient composition and specific food consumption patterns was proposed. | 8 weeks |
|
| Kani AH 2014, Iran 61 | RCT |
|
NAFLD | 48.5 | 31.3 |
|
Moderate daily physical activity of 30 min | 8 weeks | ↓ ALT (all) |
| Eckard 2013, USA 62 | RCT |
|
NAFLD (biopsy‐proven) | 44 | 32.7 |
|
|
6 months | ↓ NAS score (ns for control) |
| Razavi 2016, Iran 63 | RCT |
|
NAFLD | 39.7 | 28.5 |
|
|
8 weeks |
|
| Sun 2012, China 64 | RCT |
|
NAFLD | 39.9 | 37.7 |
|
Physical activity: Walking, jogging, stair climbing. | 12 months | ↓ ALT |
| Sun 2022, China 65 | RCT |
|
NAFLD | 39.8 | 28.6 |
|
12 weeks |
|
|
| Rodriguez‐Hernandez 2011, Spain 66 | RCT (not controlled) |
|
NAFLD | 46.3 | 38.7 |
|
6 months | ↓ ALT (both) | |
| Internet‐based approaches | |||||||||
| Vilar‐Gomez 2019, USA 67 | CCT |
|
NAFLD | 53.8 | 40.4 |
|
CCI: Remote personal health coach and medical providers, either on site or via web‐based educational content. | 12 months |
|
| Physical activity approaches | |||||||||
| Zelber‐Sagi 2014, Israel 68 | RCT |
|
NAFLD | 46.3 | 30.8 |
|
|
3 months | ↓ Liver steatosis (intervention vs. control) |
| Abdelbasset 2019, Saudi Arabia 69 | RCT |
|
NAFLD | 54.4 | 36.3 |
|
HII: Performed on a cycle Ergometer. Five minutes warm‐up followed by three sets of 4‐min cycling sessions at 80%–85% of the VO2 max with 2‐min interval at 50% of the VO2 max between sets. | 8 weeks | ↓ IHTG (MRI) |
| Bacchi 2013, Italy 70 | RCT |
|
NAFLD | 56 | 28.8 |
|
|
4 months | ↓ hepatic fat (both) |
| Rezende 2016, Brazil 71 | RCT |
|
NAFLD | 56.2 | 31.1 |
|
Exercise training: Treadmill aerobic exercise. | 24 weeks | No significant decrease of liver fat |
| Zhang 2016, China 72 | RCT |
|
NAFLD | 53.2 | 27.9 |
|
|
12 months |
|
| Abd El‐Kader 2016, Saudi Arabia 73 | RCT |
|
NAFLD | 50.8 | 32.4 |
|
AE: Treadmill‐based training program at 65%–75% of the maximum heart rate. | 3 months | ↓ ALT, AST (only intervention) |
| Cuthbertson 2016, UK 74 | RCT |
|
NAFLD | 50 | 30.6 |
|
AE: Treadmill, cross‐trainer, bike ergometer, rower. | 16 weeks | ↓ IHTG (intervention vs. control) |
| Shamsoddini 2015, Iran 75 | RCT |
|
NAFLD | 45.9 | 30.6 |
|
|
8 weeks | ↓ hepatic fat in intervention and comparator |
| Takahashi 2015, Japan 76 | CCT |
|
NAFLD | 55.5 | 28.5 |
|
RT: Push‐ups (3 sets of 10) + squats (3 sets of 10). | 12 weeks | ↓ ALT, hepatic steatosis (only intervention) |
| Oh 2021, Japan 77 | CCT |
|
NAFLD | 49.7 | 28.1 |
|
AE: Incremental increase over the time. Fast walking and/or light jogging. | 3 months | ↓ Liver steatosis, liver stiffness, FAST score |
| Nath 2020, India 78 | CCT |
|
NAFLD | 37.3 | 26.9 |
|
AE: Walking, jogging, marching drill, “lathi drill,” and yoga. | 6 months | ↓ ALT, AST (only significant in the intervention group) |
| OH 2017, Japan 79 | RCT |
|
NAFLD | 51.2 | 27.2 |
|
|
12 weeks |
|
| Babu 2022, Finland 80 | RCT |
|
NAFLD | 59.9 | 29.7 |
|
HIIT: Five bouts of 2–4 min work intervals (at 85% of max W4) interspersed by 3 min of active recovery. | 12 weeks | No change regarding liver outcomes. |
Note: Number of males, mean age and BMI are data related to the intervention group.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUD, australian dollars; BW, body weight; CAP, controlled attenuated parameter; CCT, clinical controlled trial; CHO, carbohydrate; FAST score, Fibroscan‐AST score; FIB‐4, fibrosis‐4 index; FLI, fatty liver index; HS, hepatic steatosis; IHL, intrahepatic lipid content; IHTG, intrahepatic triglyceride; LS, liver stiffness; LSM, liver stiffness measurement; MLG, Mediterranean lifestyle group; MRS‐PDFF, magnetic resonance‐spectroscopy‐measured proton density fat fraction; MUFA, monounsaturated fat; NAFLD, non‐alcoholic fatty liver disease; NAS, non‐alcoholic fatty liver disease activity score; NFS, NAFLD fibrosis score; N‐LFS, NAFLD liver fat score; ns, non‐significant; PRO, protein; PUFA, polyunsaturated fat; RCT, randomized controlled trial; RR, relative reduction; SFA, saturated fat.
Table 2 depicts the characteristics and efficacy outcomes of different lifestyle interventions on liver histology in NAFLD patients.
TABLE 2.
Characteristics of the studies and effects of different lifestyle interventions on liver histology in NAFLD patients.
| Author | Design | Sample size | Group | Mean age (years) | Mean BMI (kg/m2) | Intervention and comparator/control groups | Intervention characteristics | Intervention duration | Primary outcome |
|---|---|---|---|---|---|---|---|---|---|
| Male n (%) | |||||||||
| Calorie restriction diet (CR) | |||||||||
| Promrat 2010, USA 48 | RCT |
|
NASH | 48.9 | 33.9 |
|
|
48 weeks |
|
| Low carbohydrate (LCH)—Low fat diet (LFD) | |||||||||
| Eckard 2013, USA 62 | RCT |
|
NAFLD (biopsy‐proven) | 44 | 32.7 |
|
|
6 months | ↓ NAS score (ns for control) |
Note: Number of males, mean age and BMI are data related to the intervention group.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BW, body weight; CCT, clinical controlled trial; CHO, carbohydrate; HS, hepatic steatosis; NAFLD, non‐alcoholic fatty liver disease; NAS, non‐alcoholic fatty liver disease activity score; PRO, protein; RCT, randomized controlled trial.
Mediterranean diet (with or without calorie‐restriction)
MD is characterized by a high intake of olive oil, vegetables, fruits, nuts, legumes, whole grains, fish, and seafood, while reducing the intake of red meat (mainly processed meat), added sugars, and refined carbohydrates characterized by a high glycemic index, all of which lower nutrients and fiber content resulting in low nutritional values. 81
MD is designed to have a low intake of saturated fat versus a high intake of mono‐unsaturated fat and omega‐3 poly‐unsaturated fat 28 (Figure 2a).
FIGURE 2.
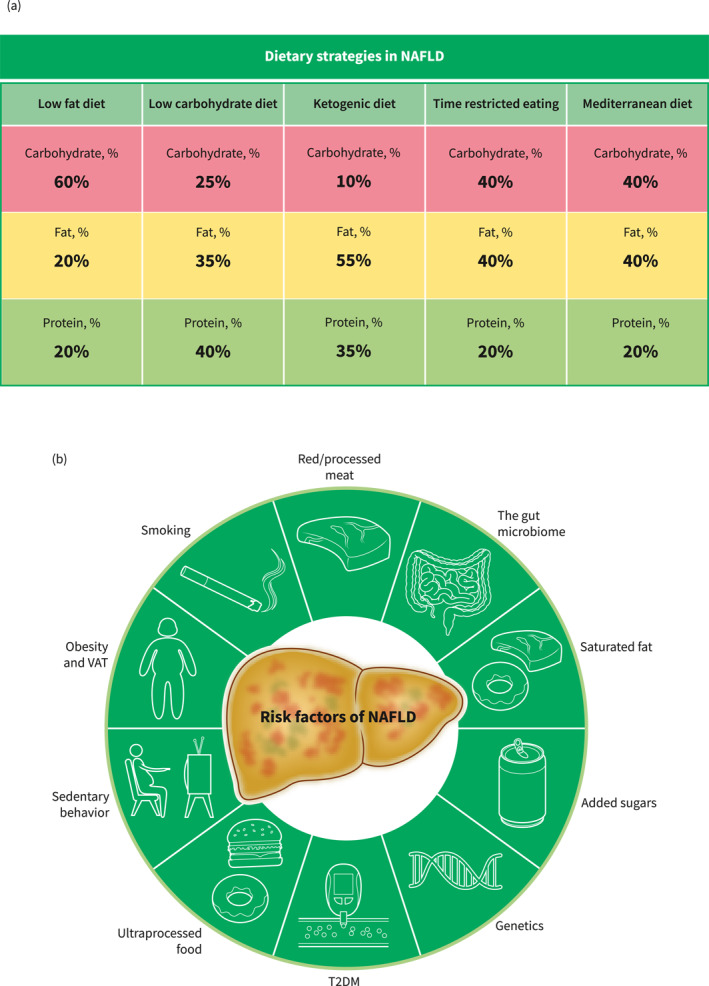
Macronutrient distribution of different dietary interventions and modifiable and non‐modifiable risk factors of NAFLD onset and progression. Macronutrients are presented as percentages of total energy intake. We recommend that the macronutrient distribution of the time‐restricted eating be in accordance with the Mediterranean diet, but this can vary. The modifiable and non‐modifiable risk factors of NAFLD onset and progression should be taken into consideration in any dietary strategy chosen. (A) Dietary strategies in NAFLD. (B) Risk factors of NAFLD. NAFLD, non‐alcoholic fatty liver disease; T2DM, type 2 diabetes mellitus; VAT, visceral adipose tissue. Source: Figures created with BioRender.com.
Due to the fact that weight loss achieved by a CR LFD (Figure 2A) along with exercise has been shown to provide resolution of NASH and even regression of fibrosis when TBWL is >10%, 18 several RCTs 30 , 43 have been conducted to compare the effects of the two aforementioned diets in NAFLD patients. In two short‐term RCTs 30 , 43 of 12‐week dietary interventions (MD vs. LFD), no significant differences in terms of liver steatosis and metabolic outcomes between the two strategies were demonstrated. Hepatic steatosis (evaluated by MRS), was significantly reduced only in the LFD group in the MEDINA trial. 43 However, the Framingham risk score (a validated tool for evaluating 10‐year cardiovascular risk 82 ) was only significantly improved in the MD intervention, and there was a greater adherence to the MD compared with the LFD. 30
In order to determine the long‐term effect of these two interventions, an 18‐month RCT 37 was performed among patients with central obesity (N = 278, 53% had NAFLD) assessing the effects on liver fat content and visceral adiposity of four different lifestyle modification strategies: (i) isocaloric LFD with/without moderate PA, and (ii) isocaloric MD‐low carbohydrate (LC) diet with/without moderate PA. This study showed that, independent of weight loss, PA (with either diet) had a significantly greater effect on VAT, whereas the MD‐LC diet was superior to LFD in terms of liver fat improvement. Similarly, studies 34 , 39 , 42 comparing the isocaloric MD diet to standard of care (healthy lifestyle advice) have also observed significant decreases in liver fat content, non‐invasive markers of liver fibrosis, and LFTs.
Since CR plays a role in weight loss, personalizing the dietary pattern of an MD diet to ensure greater adherence and weight loss could be advantageous. In this regard, two RCTs 33 , 35 have recently assessed the influence of increasing meal frequency (7 meals/day) of a MD‐LC calorie‐restricted dietary intervention along with PA on liver surrogate outcomes in NAFLD patients. Although this intervention induced significant improvements in liver steatosis and LFTs, no significant difference was found between the higher meal frequency MD diet, the classic MD intervention (5 meals/day), and the control group who was advised to follow a healthy lifestyle diet. Several other studies 31 , 32 , 40 , 41 assessed CR MD diet strategies in the setting of NAFLD, and showed that this strategy was associated with improvements in non‐invasive markers of liver fibrosis, steatosis, and LFTs.
On the other hand, it has been recently shown that polyphenol intake (abundant in food such as berries, nuts, coffee, tea, and whole grains) improves not only glucose and lipid metabolism 83 , 84 but also may have a protective effect on NAFLD. 85 The DIRECT plus RCT trial 36 assessed the effect of a green MD diet enriched with polyphenols (28 g/day of walnuts, 3–4 cups per day of green tea, 100 g per day of Mankai strain and a green shake) combined with PA on liver steatosis and liver function tests as compared to either a classic MD diet (with PA) or a control group following the standard of care. Both MD groups were restricted in processed and red meat. Two hundred and ninety‐four patients (of which 62% had NAFLD) were included and followed for 18 months. The modified green MD diet led to greater hepatic fat loss (−38.9%) as compared to MD (−19.6%, p = 0.035) and the control group (−12.2%, p < 0.001), adjusted for weight loss. Interestingly, the following factors were independently associated with greater hepatic fat loss: high intake of Mankai and walnuts, reduction of red and processed meat consumption, improved serum folate and adipokine/lipid biomarkers, changes in the microbiome composition (beta‐diversity), and specific bacteria (p < 0.05 for all).
Lastly, a comprehensive CR MD web‐based structured motivational program 38 implemented for 24 months did not seem to be inferior to a group‐based MD intervention (5 weekly meetings) in terms of reduction of liver fibrosis (FIB‐4) and steatosis (FLI). This strategy is likely more suited and tailor‐made for younger patients.
Calorie restriction
Numerous studies 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 have evaluated the effect of a CR diet with or without PA on NAFLD. In most of these studies, the CR consisted of a 500–1000 kcal deficit of total energy requirements and was usually adjusted to body weight. Although the studies were heterogeneous regarding intervention and outcome assessment modalities, there was compelling evidence supporting a dose‐response relationship between the degree of CR and improvements in liver histological features and weight loss.
Time‐restricted eating
Intermittent fasting (IF) is deemed to be associated with several positive metabolic benefits by depleting the body's glycogen stores and activating lipolysis within adipocytes. Consequently, several signaling pathways are activated (such as peroxisome proliferator activated receptor alpha [PPAR‐α] and activating transcription factor 4 [ATF4]), resulting in improvement in insulin resistance and inhibition of hepatic lipogenesis. 86 , 87 , 88 , 89 Different modalities of IF exist daily time‐restricted feeding regimen (TRF) (18‐h fasting period and 6‐h eating period), alternate‐day fasting (ADF) (24‐h of fasting at 25% of baseline energy), and the 5:2 intermittent regimen, which consists of fasting 2 days a week (intake of 500 calories). The effect of an ADF regimen in NAFLD patients has been compared to TRF (16 h of fasting) and a control group where patients consumed 80% of their daily energy requirement. 57 After 12 weeks, both ADF and TRF were associated with a reduction in weight, fat mass, and serum triglycerides. However, there was no change in terms of liver stiffness, albeit, regression of liver fibrosis usually occurs at later stages. Conversely, the 5:2 intermittent regimen also administered for a short‐term (12 weeks) 58 induced reductions in liver stiffness and steatosis as compared to a control group (standard of care), but similar to a LCD following an equal weight reduction of about 7 kg. Similar findings were found in a retrospective comparative study comparing NAFLD patients who had fasted during Ramadan to a control group. 59 These discrepancies regarding liver fibrosis could be explained by the fact that these studies used surrogate endpoints of liver fibrosis (liver stiffness or NAFLD fibrosis score) and, therefore, a reduction does not particularly correlate with histological fibrosis stage reduction if the value is still within the same cut‐off range.
Low carbohydrate diet‐Low fat diet
Several studies 60 , 61 , 62 , 63 , 64 , 65 , 66 sought to compare the effectiveness of LCD and LFD (Figure 2A) on surrogate liver outcomes for NAFLD. Similar to time‐restricted eating studies, most of these studies were of short‐term course and LCD seemed to be superior to LFD in terms of liver fat reduction and LFTs, adjusted for equal weight loss. Nevertheless, these studies presented several drawbacks such as small sample size, various LCD dietary type compositions, and different modalities for assessing liver fat content, making it difficult to draw any convincing conclusions. Results from a long‐term study 66 comparing LCD to LFD diets showed a decrease in ALT in both interventions. This was also confirmed by a recent meta‐analysis showing that there was no significant difference between the LCD and LFD diets on liver fat reduction and LFTs in NAFLD patients. 90
Very low carbohydrate ketogenic diet
Very low carbohydrate ketogenic diet (Figure 2A) (VLCKD) is characterized by a low intake of carbohydrates (<10% of total daily energy, <20–50 g/day), 1.2–1.5 g of protein/kg of ideal body weight (hence preserving lean body mass), and a high fat macronutrient composition (70%–80% of total daily energy). 91 A few short and small studies 92 , 93 have evaluated the effect of VLCKD on NAFLD compared with a standard CR diet and found significant reductions in liver fat content. Despite the substantial weight loss induced in a short‐term course by this type of dietary approach, its long‐term maintenance is not sustainable or recommended due to the lack of long‐term data on efficacy and safety.
DISCUSSION
NAFLD is likely a result of the interplay between genetic predisposition, and environmental, behavioral, and health factors including diet, T2DM, and obesity (Figure 2B). 94 , 95 Compelling evidence suggests that overconsumption of added sugars (especially fructose containing sugars), 96 , 97 and saturated fat, 98 , 99 or specific foods such as processed/red meat, 100 ultra‐processed food, 101 and sugar sweetened beverages is associated with an increased risk of developing NAFLD. In addition, sugar‐sweetened beverage consumption is strongly linked to the risk of hepatocellular carcinoma. 102 , 103
Since dietary pattern and composition drive NAFLD development, different dietary strategies, highlighted in this review, have been studied in order to determine which approach could be more beneficial in NAFLD patients. To date, the most studied dietary intervention is the MD diet, which combines moderately reduced intake of carbohydrates and minimal consumption of added sugars, and has been found to be achievable and acceptable by patients. 104 However, despite the convincing results of MD diet effectiveness on surrogate markers of NAFLD, the impact of this dietary strategy on liver histological features as well as clinical outcomes of NAFLD still needs to be addressed. Furthermore, economical, geographical, and cultural barriers 105 could jeopardize adherence to this dietary intervention, which emphasizes the importance of personalizing our approach to not only the patient's needs but also their socio‐economic status. Ultimately, as previously mentioned, CR and increased energy expenditure remain the cornerstones of NAFLD treatment. Therefore, the approach of lifestyle modification should be holistic (Figure 3A), encompassing recommendations regarding dietary pattern and composition but also promoting PA 106 and behavioral strategies to ensure greater adherence and benefits in terms of mortality. In this regard, comprehensive structured web‐based programs maintaining NAFLD awareness 38 , 67 (Figure 3B) could not only be an asset for managing attrition, and hence, adherence, but also a way to reduce cost and health care resource utilization. Some patients will find web‐based interventions convenient and some patients will need the in‐person interventions or a combination of both. Other actions for increasing compliance to lifestyle modification are summarized in Figure 3B.
FIGURE 3.
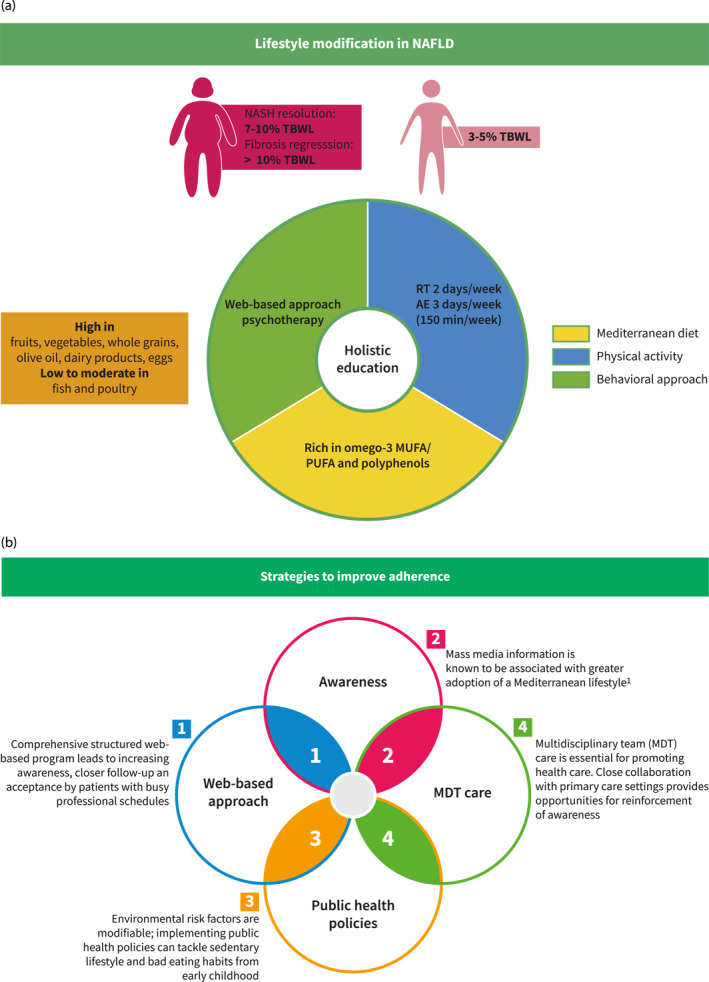
Holistic education in NAFLD and strategies to improve adherence. (A) Holistic education in NAFLD and strategies to improve adherence. (B) Strategies to improve adherence. AE, aerobic exercise; MUFA, monounsaturated fatty acid; NAFLD, non‐alcoholic fatty liver disease; NASH, non‐alcoholic steatohepatitis; PUFA, polyunsaturated fatty acid; RT, resistance training; TBWL, total body weight loss.
PERSPECTIVES AND CONCLUSION
Since NAFLD is a chronic disease requiring life‐long therapy, there are several unmet needs to address in order to better characterize an effective diet and lifestyle intervention in NAFLD patients. Considering that NAFLD is a heterogeneous disease, patient stratification is crucial for establishing a personalized and tailored dietary approach. Nutrigenomics and nutrigenetics could be a promising tool to decipher the effect of different dietary strategies on the modulation of different NAFLD variants. 107 Additionally, more robust data are needed in the assessment of dietary intake and in the characterization of the interventions associated with greater adherence. Long‐term longitudinal studies will likely help to fill these knowledge gaps.
In conclusion, lifestyle modification comprising CR, increased PA, and changes in dietary composition remain the cornerstones of NAFLD management. Since most dietary and sedentary lifestyle environmental risk factors are modifiable, health policies are essential to tackle obesity, unhealthy eating, and sedentary lifestyle. Finally, multidisciplinary care teams led by primary care healthcare providers should be implemented in order to provide to best structured care to NAFLD patients.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Supporting information
Supporting Information S1
Hadefi A., Arvanitakis M., Trépo E., Zelber‐Sagi S.. Dietary strategies in non‐alcoholic fatty liver disease patients: from evidence to daily clinical practice, a systematic review. United European Gastroenterol J. 2023;11(7):663–689. 10.1002/ueg2.12443
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology. 2020;72(5):1605–1616. 10.1002/hep.31173 [DOI] [PubMed] [Google Scholar]
- 2. Estes C, Anstee QM, Arias‐Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904. 10.1016/j.jhep.2018.05.036 [DOI] [PubMed] [Google Scholar]
- 3. Golabi P, Paik JM, Harring M, Younossi E, Kabbara K, Younossi ZM. Prevalence of high and moderate risk nonalcoholic fatty liver disease among adults in the United States, 1999–2016. Clin Gastroenterol Hepatol. 2022;20(12):2838–2847.e7. 10.1016/j.cgh.2021.12.015 [DOI] [PubMed] [Google Scholar]
- 4. Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77(4):1335–1347. 10.1097/hep.0000000000000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Hara J, Finnegan A, Dhillon H, Ruiz‐Casas L, Pedra G, Franks B, et al. Cost of non‐alcoholic steatohepatitis in Europe and the USA: the GAIN study. JHEP Rep. 2020;2(5):100142. 10.1016/j.jhepr.2020.100142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol. 2022;19(1):60–78. 10.1038/s41575-021-00523-4 [DOI] [PubMed] [Google Scholar]
- 7. Sanyal AJ, Van Natta ML, Clark J, Neuschwander‐Tetri BA, Diehl A, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385(17):1559–1569. 10.1056/nejmoa2029349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non‐alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta‐analysis of 501 022 adult individuals. Gut. 2021;70(5):962–969. 10.1136/gutjnl-2020-322572 [DOI] [PubMed] [Google Scholar]
- 9. Mantovani A, Csermely A, Tilg H, Byrne CD, Targher G. Comparative effects of non‐alcoholic fatty liver disease and metabolic dysfunction‐associated fatty liver disease on risk of incident cardiovascular events: a meta‐analysis of about 13 million individuals. Gut. 2022;72(7):1433–1436. 10.1136/gutjnl-2022-328224 [DOI] [PubMed] [Google Scholar]
- 10. Mantovani A, Petracca G, Beatrice G, Csermely A, Tilg H, Byrne CD, et al. Non‐alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta‐analysis of observational cohort studies. Gut. 2022;71(4):778–788. 10.1136/gutjnl-2021-324191 [DOI] [PubMed] [Google Scholar]
- 11. Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Schattenberg JM, et al. Non‐alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta‐analysis. Gut. 2022;71(1):156–162. 10.1136/gutjnl-2020-323082 [DOI] [PubMed] [Google Scholar]
- 12. Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8(7):616–627. 10.1016/s2213-8587(20)30110-8 [DOI] [PubMed] [Google Scholar]
- 13. Neeland IJ, Turer AT, Ayers CR, Powell‐Wiley TM, Vega GL, Farzaneh‐Far R, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308(11):1150–1159. 10.1001/2012.jama.11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–725. 10.1016/s2213-8587(19)30084-1 [DOI] [PubMed] [Google Scholar]
- 15. Shen W, Middleton MS, Cunha GM, Delgado TI, Wolfson T, Gamst A, et al. Changes in abdominal adipose tissue depots assessed by MRI correlate with hepatic histologic improvement in non‐alcoholic steatohepatitis. J Hepatol. 2023;78(2):238–246. 10.1016/j.jhep.2022.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. 10.1016/j.jhep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 17. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 18. Vilar‐Gomez E, Martinez‐Perez Y, Calzadilla‐Bertot L, Torres‐Gonzalez A, Gra‐Oramas B, Gonzalez‐Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378.e5. 10.1053/j.gastro.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 19. Lassailly G, Caiazzo R, Ntandja‐Wandji LC, Gnemmi V, Baud G, Verkindt H, et al. Bariatric surgery provides long‐term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159(4):1290–1301.e5. 10.1053/j.gastro.2020.06.006 [DOI] [PubMed] [Google Scholar]
- 20. Ma J, Hennein R, Liu C, Long MT, Hoffmann U, Jacques PF, et al. Improved diet quality associates with reduction in liver fat, particularly in individuals with high genetic risk scores for nonalcoholic fatty liver disease. Gastroenterology. 2018;155(1):107–117. 10.1053/j.gastro.2018.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. Circulation. 2014;129(25 Suppl 2):S76–S99. 10.1161/01.cir.0000437740.48606.d1 [DOI] [PubMed] [Google Scholar]
- 22. Martínez‐González MA, García‐Arellano A, Toledo E, Salas‐Salvadó J, Buil‐Cosiales P, Corella D, et al. A 14‐item Mediterranean diet assessment tool and obesity indexes among high‐risk subjects: the PREDIMED trial. PLoS One. 2012;7(8):e43134. 10.1371/journal.pone.0043134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non‐alcoholic fatty liver disease. J Hepatol. 2013;59(1):138–143. 10.1016/j.jhep.2013.02.012 [DOI] [PubMed] [Google Scholar]
- 24. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low‐carbohydrate, Mediterranean, or low‐fat diet. N Engl J Med. 2008;359(3):229–241. 10.1097/01.ogx.0000334740.32446.f3 [DOI] [PubMed] [Google Scholar]
- 25. Sotos‐Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, et al. Association of changes in diet quality with total and cause‐specific mortality. N Engl J Med. 2017;377(2):143–153. 10.1056/nejmoa1613502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Estruch R, Ros E, Salas‐Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra‐virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. 10.1056/nejmoa1800389 [DOI] [PubMed] [Google Scholar]
- 27. Suárez M, Boqué N, Del Bas JM, Mayneris‐Perxachs J, Arola L, Caimari A. Mediterranean diet and multi‐ingredient‐based interventions for the management of non‐alcoholic fatty liver disease. Nutrients. 2017;9(10):1052. 10.3390/nu9101052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zelber‐Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non‐alcoholic fatty liver disease: evidence and plausible mechanisms. Liver Int. 2017;37(7):936–949. 10.1111/liv.13435 [DOI] [PubMed] [Google Scholar]
- 29. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1016/j.ijsu.2021.105906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Properzi C, O'Sullivan TA, Sherriff JL, Ching HL, Jeffrey GP, Buckley RF, et al. Ad libitum Mediterranean and low‐fat diets both significantly reduce hepatic steatosis: a randomized controlled trial. Hepatology. 2018;68(5):1741–1754. 10.1002/hep.30076 [DOI] [PubMed] [Google Scholar]
- 31. Katsagoni CN, Papatheodoridis GV, Ioannidou P, Deutsch M, Alexopoulou A, Papadopoulos N, et al. Improvements in clinical characteristics of patients with non‐alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: a randomised controlled clinical trial. Br J Nutr. 2018;120(2):164–175. 10.1017/s000711451800137x [DOI] [PubMed] [Google Scholar]
- 32. Misciagna G, Diaz MD, Caramia DV, Bonfiglio C, Franco I, Noviello MR, et al. Effect of a low glycemic index Mediterranean diet on non‐alcoholic fatty liver disease. A randomized controlled clinici trial. J Nutr Health Aging. 2017;21(4):404–412. 10.1007/s12603-016-0809-8 [DOI] [PubMed] [Google Scholar]
- 33. Marin‐Alejandre BA, Abete I, Cantero I, Monreal JI, Elorz M, Herrero JI, et al. The metabolic and hepatic impact of two personalized dietary strategies in subjects with obesity and nonalcoholic fatty liver disease: the fatty liver in obesity (FLiO) randomized controlled trial. Nutrients. 2019;11(10):2543. 10.3390/nu11102543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nourian M, Askari G, Golshiri P, Miraghajani M, Shokri S, Arab A. Effect of lifestyle modification education based on health belief model in overweight/obese patients with non‐alcoholic fatty liver disease: a parallel randomized controlled clinical trial. Clin Nutr ESPEN. 2020;38:236–241. 10.1016/j.clnesp.2020.04.004 [DOI] [PubMed] [Google Scholar]
- 35. Abbate M, Mascaró CM, Montemayor S, Barbería‐Latasa M, Casares M, Gómez C, et al. Energy expenditure improved risk factors associated with renal function loss in NAFLD and MetS patients. Nutrients. 2021;13(2):629. 10.3390/nu13020629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meir AY, Rinott E, Tsaban G, Zelicha H, Kaplan A, Rosen P, et al. Effect of green‐Mediterranean diet on intrahepatic fat: the DIRECT plus randomised controlled trial. Gut. 2021;70(11):2085–2095. 10.1136/gutjnl-2020-323106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gepner Y, Shelef I, Schwarzfuchs D, Zelicha H, Tene L, Yaskolka Meir A, et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: CENTRAL magnetic resonance imaging randomized controlled trial. Circulation. 2018;137(11):1143–1157. 10.1161/circulationaha.117.030501 [DOI] [PubMed] [Google Scholar]
- 38. Mazzotti A, Caletti MT, Brodosi L, Di Domizio S, Forchielli ML, Petta S, et al. An internet‐based approach for lifestyle changes in patients with NAFLD: two‐year effects on weight loss and surrogate markers. J Hepatol. 2018;69(5):1155–1163. 10.1016/j.jhep.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 39. Dorosti M, Jafary Heidarloo A, Bakhshimoghaddam F, Alizadeh M. Whole‐grain consumption and its effects on hepatic steatosis and liver enzymes in patients with non‐alcoholic fatty liver disease: a randomised controlled clinical trial. Br J Nutr. 2020;123(3):328–336. 10.1017/s0007114519002769 [DOI] [PubMed] [Google Scholar]
- 40. Shidfar F, Bahrololumi SS, Doaei S, Mohammadzadeh A, Gholamalizadeh M, Mohammadimanesh A. The effects of extra virgin olive oil on alanine aminotransferase, aspartate aminotransferase, and ultrasonographic indices of hepatic steatosis in nonalcoholic fatty liver disease patients undergoing low calorie diet. Can J Gastroenterol Hepatol. 2018;2018:1–7. 10.1155/2018/1053710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rezaei S, Akhlaghi M, Sasani MR, Boldaji RB. Olive oil lessened fatty liver severity independent of cardiometabolic correction in patients with non‐alcoholic fatty liver disease: a randomized clinical trial. Nutrition. 2019;57:154–161. 10.1016/j.nut.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 42. Naimimohasses S, O’gorman P, Wright C, Fhloinn DN, Holden D, Conlon N, et al. Differential effects of dietary versus exercise intervention on intrahepatic MAIT cells and histological features of NAFLD. Nutrients. 2022;14(11):2198. 10.3390/nu14112198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. George ES, Reddy A, Nicoll AJ, Ryan MC, Itsiopoulos C, Abbott G, et al. Impact of a Mediterranean diet on hepatic and metabolic outcomes in non‐alcoholic fatty liver disease: the MEDINA randomised controlled trial. Liver Int. 2022;42(6):1308–1322. 10.1111/liv.15264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghetti FF, Oliveira DG, de Oliveira JM, Ferreira LEVVC, Cesar DE, Moreira APB. Effects of dietary intervention on gut microbiota and metabolic‐nutritional profile of outpatients with non‐alcoholic steatohepatitis: a randomized clinical trial. J Gastrointest Liver Dis. 2019;28(3):279–287. 10.15403/jgld-197 [DOI] [PubMed] [Google Scholar]
- 45. Marin‐Alejandre BA, Cantero I, Perez‐Diaz‐del‐Campo N, Monreal JI, Elorz M, Herrero JI, et al. Effects of two personalized dietary strategies during a 2‐year intervention in subjects with nonalcoholic fatty liver disease: a randomized trial. Liver Int. 2021;41(7):1532–1544. 10.1111/liv.14818 [DOI] [PubMed] [Google Scholar]
- 46. Wong VWS, Chan RSM, Wong GLH, Cheung BHK, Chu WCW, Yeung DKW, et al. Community‐based lifestyle modification programme for non‐alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59(3):536–542. 10.1016/j.jhep.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 47. Dong FY, Zhang Y, Huang YQ, Wang YQ, Zhang GS, Hu XN, et al. Long‐term lifestyle interventions in middle‐aged and elderly men with nonalcoholic fatty liver disease: a randomized controlled trial. Sci Rep. 2016;6(1):8. 10.1038/srep36783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–129. 10.1002/hep.23276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cheng SL, Ge J, Zhao C, Le SL, Yang YF, Ke DD, et al. Effect of aerobic exercise and diet on liver fat in pre‐diabetic patients with non‐alcoholic‐fatty‐liver‐disease: a randomized controlled trial. Sci Rep. 2017;7(1):11. 10.1038/s41598-017-16159-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Johari MI, Yusoff K, Haron J, Nadarajan C, Ibrahim KN, Wong MS, et al. A randomised controlled trial on the effectiveness and adherence of modified alternate‐day calorie restriction in improving activity of non‐alcoholic fatty liver disease. Sci Rep. 2019;9(1):11232. 10.1038/s41598-019-47763-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shojasaadat F, Ayremlou P, Hashemi A, Mehdizadeh A, Zarrin R. A randomized controlled trial comparing effects of a low‐energy diet with n‐3 polyunsaturated fatty acid supplementation in patients with non‐alcoholic fatty liver disease. J Res Med Sci. 2019;24:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Atefi M, Entezari MH, Vahedi H, Hassanzadeh A. Sesame oil ameliorates alanine aminotransferase, aspartate aminotransferase, and fatty liver grade in women with nonalcoholic fatty liver disease undergoing low‐calorie diet: a randomized double‐blind controlled trial. Int J Clin Pract. 2022;2022:4982080. 10.1155/2022/4982080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Asghari S, Rezaei M, Rafraf M, Taghizadeh M, Asghari‐Jafarabadi M, Ebadi M. Effects of calorie restricted diet on oxidative/antioxidative status biomarkers and serum fibroblast growth factor 21 levels in nonalcoholic fatty liver disease patients: a randomized, controlled clinical trial. Nutrients. 2022;14(12):2509. 10.3390/nu14122509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arefhosseini SR, Ebrahimi‐Mameghani M, Farsad Naeimi A, Khoshbaten M, Rashid J. Lifestyle modification through dietary intervention: health promotion of patients with non‐alcoholic fatty liver disease. Health Promot Perspect. 2011;1(2):147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Garousi N, Tamizifar B, Pourmasoumi M, Feizi A, Askari G, Clark CCT, et al. Effects of lacto‐ovo‐vegetarian diet vs. standard‐weight‐loss diet on obese and overweight adults with non‐alcoholic fatty liver disease: a randomised clinical trial. Arch Physiol Biochem. 2021;129(4):1–9. 10.1080/13813455.2021.1890128 [DOI] [PubMed] [Google Scholar]
- 56. Wong VWS, Wong GLH, Chan RSM, Shu SST, Cheung BHK, Li LS, et al. Beneficial effects of lifestyle intervention in non‐obese patients with non‐alcoholic fatty liver disease. J Hepatol. 2018;69(6):1349–1356. 10.1016/j.jhep.2018.08.011 [DOI] [PubMed] [Google Scholar]
- 57. Cai H, Qin YL, Shi ZY, Chen JH, Zeng MJ, Zhou W, et al. Effects of alternate‐day fasting on body weight and dyslipidaemia in patients with non‐alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol. 2019;19(1):219. 10.1186/s12876-019-1132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kord Varkaneh H, Salehi Sahlabadi A, Gaman MA, Rajabnia M, Sedanur Macit‐Celebi M, Santos HO, et al. Effects of the 5:2 intermittent fasting diet on non‐alcoholic fatty liver disease: a randomized controlled trial. Front Nutr. 2022;9:948655. 10.3389/fnut.2022.948655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mari A, Khoury T, Baker M, Said Ahmad H, Abu Baker F, Mahamid M. The impact of Ramadan fasting on fatty liver disease severity: a retrospective case control study from Israel. Isr Med Assoc J. 2021;23(2):94–98. [PubMed] [Google Scholar]
- 60. Jang EC, Jun DW, Lee SM, Cho YK, Ahn SB. Comparison of efficacy of low‐carbohydrate and low‐fat diet education programs in non‐alcoholic fatty liver disease: a randomized controlled study. Hepatol Res. 2018;48(3):E22–E29. 10.1111/hepr.12918 [DOI] [PubMed] [Google Scholar]
- 61. Kani AH, Alavian SM, Esmaillzadeh A, Adibi P, Azadbakht L. Effects of a novel therapeutic diet on liver enzymes and coagulating factors in patients with non‐alcoholic fatty liver disease: a parallel randomized trial. Nutrition. 2014;30(7‐8):814–821. 10.1016/j.nut.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 62. Eckard C, Cole R, Lockwood J, Torres DM, Williams CD, Shaw JC, et al. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Ther Adv Gastroenterol. 2013;6(4):249–259. 10.1177/1756283x13484078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Razavi Zade M, Telkabadi MH, Bahmani F, Salehi B, Farshbaf S, Asemi Z. The effects of DASH diet on weight loss and metabolic status in adults with non‐alcoholic fatty liver disease: a randomized clinical trial. Liver Int. 2016;36(4):563–571. 10.1111/liv.12990 [DOI] [PubMed] [Google Scholar]
- 64. Sun WH, Song MQ, Jiang CQ, Xin YN, Ma JL, Liu YX, et al. Lifestyle intervention in non‐alcoholic fatty liver disease in Chengyang District, Qingdao, China. World J Hepatol. 2012;4(7):224–230. 10.4254/wjh.v4.i7.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sun P, Huang L, Shuai P, Wan Z, Liu Y, Xue J, et al. Effect of a high protein, low glycemic index dietary intervention on metabolic dysfunction‐associated fatty liver disease: a randomized controlled trial. Front Nutr. 2022;9:863834. 10.3389/fnut.2022.863834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rodríguez‐Hernández H, Cervantes‐Huerta M, Rodríguez‐Moran M, Guerrero‐Romero F. Decrease of aminotransferase levels in obese women is related to body weight reduction, irrespective of type of diet. Ann Hepatol. 2011;10(4):486–492. 10.1016/s1665-2681(19)31517-0 [DOI] [PubMed] [Google Scholar]
- 67. Vilar‐Gomez E, Athinarayanan SJ, Adams RN, Hallberg SJ, Bhanpuri NH, McKenzie AL, et al. Post hoc analyses of surrogate markers of non‐alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open‐label, non‐randomised controlled study. BMJ Open. 2019;9(2):e023597. 10.1136/bmjopen-2018-023597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zelber‐Sagi S, Buch A, Yeshua H, Vaisman N, Webb M, Harari G, et al. Effect of resistance training on non‐alcoholic fatty‐liver disease a randomized‐clinical trial. World J Gastroenterol. 2014;20(15):4382–4392. 10.3748/wjg.v20.i15.4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Soliman GS. A randomized controlled trial on the effectiveness of 8‐week high‐intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health‐related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Medicine. 2019;98(12):8. 10.1097/md.0000000000014918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bacchi E, Negri C, Targher G, Faccioli N, Lanza M, Zoppini G, et al. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 randomized trial). Hepatology. 2013;58(4):1287–1295. 10.1002/hep.26393 [DOI] [PubMed] [Google Scholar]
- 71. Rezende REF, Duarte SMB, Stefano JT, Roschel H, Gualano B, De Sá Pinto AL, et al. Randomized clinical trial: benefits of aerobic physical activity for 24 weeks in postmenopausal women with nonalcoholic fatty liver disease. Menopause. 2016;23(8):876–883. 10.1097/gme.0000000000000647 [DOI] [PubMed] [Google Scholar]
- 72. Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: a randomized clinical trial. JAMA Intern Med. 2016;176(8):1074–1082. 10.1001/jamainternmed.2016.3202 [DOI] [PubMed] [Google Scholar]
- 73. Abd El‐Kader SM, Al‐Shreef FM, Al‐Jiffri OH. Biochemical parameters response to weight loss in patients with non‐alcoholic steatohepatitis. Afr Health Sci. 2016;16(1):242–249. 10.4314/ahs.v16i1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cuthbertson DJ, Shojaee‐Moradie F, Sprung VS, Jones H, Pugh CJ, Richardson P, et al. Dissociation between exercise‐induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non‐alcoholic fatty liver disease. Clin Sci. 2016;130(2):93–104. 10.1042/cs20150447 [DOI] [PubMed] [Google Scholar]
- 75. Shamsoddini A, Sobhani V, Ghamar Chehreh ME, Alavian SM, Zaree A. Effect of aerobic and resistance exercise training on liver enzymes and hepatic fat in Iranian men with Nonalcoholic fatty liver disease. Hepat Mon. 2015;15(10):e31434. 10.5812/hepatmon.31434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Takahashi A, Abe K, Usami K, Imaizumi H, Hayashi M, Okai K, et al. Simple resistance exercise helps patients with non‐alcoholic fatty liver disease. Int J Sports Med. 2015;36(10):848–852. 10.1055/s-0035-1549853 [DOI] [PubMed] [Google Scholar]
- 77. Oh S, Tsujimoto T, Kim B, Uchida F, Suzuki H, Iizumi S, et al. Weight‐loss‐independent benefits of exercise on liver steatosis and stiffness in Japanese men with NAFLD. JHEP Rep. 2021;3(3):100253. 10.1016/j.jhepr.2021.100253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nath P, Panigrahi MK, Sahu MK, Narayan J, Sahoo RK, Patra AA, et al. Effect of exercise on NAFLD and its risk factors: comparison of moderate versus low intensity exercise. J Clin Translational Hepatol. 2020;8(2):120–126. 10.14218/jcth.2019.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Oh S, So R, Shida T, Matsuo T, Kim B, Akiyama K, et al. High‐intensity aerobic exercise improves both hepatic fat content and stiffness in sedentary obese men with nonalcoholic fatty liver disease. Sci Rep. 2017;7:1–12. 10.1038/srep43029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Babu AF, Csader S, Männistö V, Tauriainen MM, Pentikäinen H, Savonen K, et al. Effects of exercise on NAFLD using non‐targeted metabolomics in adipose tissue, plasma, urine, and stool. Sci Rep. 2022;12(1):6485. 10.1038/s41598-022-10481-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yki‐Järvinen H, Luukkonen PK, Hodson L, Moore JB. Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2021;18(11):770–786. 10.1038/s41575-021-00472-y [DOI] [PubMed] [Google Scholar]
- 82. Jahangiry L, Farhangi MA, Rezaei F. Framingham risk score for estimation of 10‐years of cardiovascular diseases risk in patients with metabolic syndrome. J Health Popul Nutr. 2017;36(1):36. 10.1186/s41043-017-0114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Annuzzi G, Bozzetto L, Costabile G, Giacco R, Mangione A, Anniballi G, et al. Diets naturally rich in polyphenols improve fasting and postprandial dyslipidemia and reduce oxidative stress: a randomized controlled trial. Am J Clin Nutr. 2014;99(3):463–471. 10.3945/ajcn.113.073445 [DOI] [PubMed] [Google Scholar]
- 84. Bozzetto L, Prinster A, Annuzzi G, Costagliola L, Mangione A, Vitelli A, et al. Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care. 2012;35(7):1429–1435. 10.2337/dc12-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Salomone F, Ivancovsky‐Wajcman D, Fliss‐Isakov N, Webb M, Grosso G, Godos J, et al. Higher phenolic acid intake independently associates with lower prevalence of insulin resistance and non‐alcoholic fatty liver disease. JHEP Rep. 2020;2(2):100069. 10.1016/j.jhepr.2020.100069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19(2):181–192. 10.1016/j.cmet.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mattson MP, Moehl K, Ghena N, Schmaedick M, Cheng A. Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci. 2018;19(2):63–80. 10.1038/nrn.2017.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Memel ZN, Wang J, Corey KE. Intermittent fasting as a treatment for nonalcoholic fatty liver disease: what is the evidence? Clin Liver Dis. 2022;19(3):101–105. 10.1002/cld.1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381(26):2541–2551. 10.1056/nejmra1905136 [DOI] [PubMed] [Google Scholar]
- 90. Ahn J, Jun DW, Lee HY, Moon JH. Critical appraisal for low‐carbohydrate diet in nonalcoholic fatty liver disease: review and meta‐analyses. Clin Nutr. 2019;38(5):2023–2030. 10.1016/j.clnu.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 91. Kirkpatrick CF, Bolick JP, Kris‐Etherton PM, Sikand G, Aspry KE, Soffer DE, et al. Review of current evidence and clinical recommendations on the effects of low‐carbohydrate and very‐low‐carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. 2019;13(5):689–711.e1. 10.1016/j.jacl.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 92. Haghighatdoost F, Salehi‐Abargouei A, Surkan PJ, Azadbakht L. The effects of low carbohydrate diets on liver function tests in nonalcoholic fatty liver disease: a systematic review and meta‐analysis of clinical trials. J Res Med Sci. 2016;21(1):53. 10.4103/1735-1995.187269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Luukkonen PK, Dufour S, Lyu K, Zhang XM, Hakkarainen A, Lehtimäki TE, et al. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2020;117(13):7347–7354. 10.1073/pnas.1922344117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Anstee QM, Seth D, Day CP. Genetic factors that affect risk of alcoholic and nonalcoholic fatty liver disease. Gastroenterology. 2016;150(8):1728–1744.e7. 10.1053/j.gastro.2016.01.037 [DOI] [PubMed] [Google Scholar]
- 95. Update on NAFLD genetics: from new variants to the clinic; 2020. [DOI] [PubMed]
- 96. Simons N, Veeraiah P, Simons P, Schaper NC, Kooi ME, Schrauwen‐Hinderling VB, et al. Effects of fructose restriction on liver steatosis (FRUITLESS); a double‐blind randomized controlled trial. Am J Clin Nutr. 2021;113(2):391–400. 10.1093/ajcn/nqaa332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Geidl‐Flueck B, Hochuli M, Németh Á, Eberl A, Derron N, Köfeler HC, et al. Fructose‐ and sucrose‐ but not glucose‐sweetened beverages promote hepatic de novo lipogenesis: a randomized controlled trial. J Hepatol. 2021;75(1):46–54. 10.1016/j.jhep.2021.02.027 [DOI] [PubMed] [Google Scholar]
- 98. Zelber‐Sagi S, Ivancovsky‐Wajcman D, Fliss Isakov N, Webb M, Orenstein D, Shibolet O, et al. High red and processed meat consumption is associated with non‐alcoholic fatty liver disease and insulin resistance. J Hepatol. 2018;68(6):1239–1246. 10.1016/j.jhep.2018.01.015 [DOI] [PubMed] [Google Scholar]
- 99. He K, Li Y, Guo X, Zhong L, Tang S. Food groups and the likelihood of non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. Br J Nutr. 2020;124(1):1–13. 10.1017/s0007114520000914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ivancovsky‐Wajcman D, Fliss‐Isakov N, Grinshpan LS, Salomone F, Lazarus JV, Webb M, et al. High meat consumption is prospectively associated with the risk of non‐alcoholic fatty liver disease and presumed significant fibrosis. Nutrients. 2022;14(17):3533. 10.3390/nu14173533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ivancovsky‐Wajcman D, Fliss‐Isakov N, Webb M, Bentov I, Shibolet O, Kariv R, et al. Ultra‐processed food is associated with features of metabolic syndrome and non‐alcoholic fatty liver disease. Liver Int. 2021;41(11):2635–2645. 10.1111/liv.14996 [DOI] [PubMed] [Google Scholar]
- 102. Ivancovsky‐Wajcman D, Fliss‐Isakov N, Salomone F, Webb M, Shibolet O, Kariv R, et al. Dietary vitamin E and C intake is inversely associated with the severity of nonalcoholic fatty liver disease. Dig Liver Dis. 2019;51(12):1698–1705. 10.1016/j.dld.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 103. Li Y, Guo L, He K, Huang C, Tang S. Consumption of sugar‐sweetened beverages and fruit juice and human cancer: a systematic review and dose‐response meta‐analysis of observational studies. J Cancer. 2021;12(10):3077–3088. 10.7150/jca.51322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Haigh L, Kirk C, El Gendy K, Gallacher J, Errington L, Mathers JC, et al. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non‐alcoholic fatty liver disease (NAFLD): a systematic review and meta‐analysis. Clin Nutr. 2022;41(9):1913–1931. 10.1016/j.clnu.2022.06.037 [DOI] [PubMed] [Google Scholar]
- 105. Haigh L, Bremner S, Houghton D, Henderson E, Avery L, Hardy T, et al. Barriers and facilitators to Mediterranean diet adoption by patients with nonalcoholic fatty liver disease in Northern Europe. Clin Gastroenterol Hepatol. 2019;17(7):1364–1371.e3. 10.1016/j.cgh.2018.10.044 [DOI] [PubMed] [Google Scholar]
- 106. Henry A, Paik JM, Austin P, Eberly KE, Golabi P, Younossi I, et al. Vigorous physical activity provides protection against all‐cause deaths among adults patients with nonalcoholic fatty liver disease (NAFLD). Aliment Pharmacol Ther. 2022;57(6):709–722. 10.1111/apt.17308 [DOI] [PubMed] [Google Scholar]
- 107. Ramos‐Lopez O. Multi‐omics nutritional approaches targeting metabolic‐associated fatty liver disease. Genes. 2022;13(11):2142. 10.3390/genes13112142 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


