Abstract
Introduction
The relationship between cardiovascular disorders and nonalcoholic fatty liver disease (NAFLD) has been extensively studied. To better pool this data and make a more definite conclusion, we performed a meta‐analysis to evaluate the association between NAFLD and the thickness of media and intima of carotid artery (CIMT) and cardiovascular disorders.
Methods
We searched PubMed, Ovid, Scopus, ProQuest, Web of Science, and the Cochrane Library, and analyzed the pooled data using R studio and the “metafor” package.
Results
The final analysis included a total of 59 studies with 16,179 cases and 26,120 control individuals. NAFLD was shown to be associated with an increase of 0.1231 mm (20.6%) in carotid artery intima‐media thickness (CIMT) (p = 0.002, 95% confidence interval [CI]: 0.0462–0.2000) in individuals with NAFLD. The prevalence of atherosclerotic plaques in the carotid arteries and the occurrence of NAFLD are significantly correlated, according to a meta‐analysis based on 17 distinct studies (p = 0.001, 1.28–1.43, 95% CI, odds ratio = 1.356).
Conclusion
Patients with increased CIMT are considerably more likely to have NAFLD. Large prospective investigations are required to corroborate these findings and their prognostic significance, along with the effectiveness of the available interventions.
Keywords: atherosclerosis, cardiovascular diseases, carotid arteries, meta‐analysis, NAFLD
Key Points
Nonalcoholic fatty liver disease (NAFLD) is associated with increased carotid intima media thickness.
NAFLD is associated with increased prevalence of atherosclerotic plaques in the carotid arteries.
1. INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a liver condition marked by an excessive accumulation of fat and is brought on by causes other than alcohol and other particular liver‐damaging variables. 1 Its prevalence is rising in parallel with obesity and metabolic illness rates. 2 The prevalence of NAFLD varies by country and ranges from 10% to 24%, while obese patients show a frequency of 57% to 74%. 2.6% of children are also impacted, ranging from 22.5% to 52.8% among obese children. 3 In Asia, its prevalence has been increased; a 3–20‐fold increase in nations such as Japan in last two decades. 4 Obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome are among the main risk factors for NAFLD. 5 It is believed that NAFLD is likely a liver manifestation of metabolic syndrome, and clinical, epidemiological, and biochemical studies strongly support this hypothesis. 5 Moreover, recent studies have shown that NAFLD is associated with a variety of classic and non‐classic risk factors for cardiovascular disease disorders. In this case, different epidemiological studies have shown that cardiovascular disease is the primary cause of mortality in NAFLD patients. 6
One of the subclinical indicators of atherosclerosis is carotid intima‐media thickness (CIMT), which is strongly associated with coronary heart disease. 7 Numerous studies have been conducted to demonstrate the connection between NAFLD and atherosclerosis, and these studies show that there is a connection between NAFLD and an increase in the intima and media of carotid arteries. Aside from being strongly correlated with obesity and other elements of the metabolic syndrome, increased CIMT, plaque, and impaired endothelial flow‐mediated vasodilatation are additional indicators of subclinical atherosclerosis in nonalcoholic fatty liver patients. 8 , 9 A variety of chemicals are also released from fatty and inflamed liver in nonalcoholic fatty liver patients, particularly if they also have nonalcoholic steatohepatitis (NASH), which may have a pathogenic role in accelerating atherosclerosis. 10 Validation of these findings can turn NAFLD into a prognostic and therapeutical target for cardiovascular disorders. Despite several studies, this association has not yet been investigated in a systematic review approach. As a result, we aimed to conduct a systematic and comprehensive review on the association between NAFLD and cardiovascular disorders.
2. METHODS
2.1. Search strategy and eligibility criteria
This systematic review was conducted upon studies indexed in the PubMed, Ovid, Scopus, ProQuest, Web of science, and the Cochrane Library until July 2, 2023. Searching strategy to find the most relevant studies was (“hepatic steatosis” or “non‐alcoholic fatty liver disease” or “fatty liver”) and (“atherosclerosis” or “intima and media thickness” or “carotid artery” or “carotid plaque” or “cardiovascular disease”). In addition, our detailed search strategy is summarized in Table 1. The inclusion criteria were English‐language descriptive and cohort studies examining the association between adult patients with NAFLD and carotid artery atherosclerosis (over 18 years old). Moreover, studies that have not yet been published, or did not report the variables considered in this study as well as inclusion of patients with hepatic steatosis due to secondary causes (including alcoholic patients, intravenous nutrition, hepatitis B or C, or drugs) were excluded from our repertoire. Our Patient Intervention Comparator Outcome (PICO) was as follows: (1) P: patients with NAFLD, (2) I: ultrasound examination of the carotid artery, (3) C: normal control individuals, and (4) carotid‐intima thickness and its plaque amount. Two separate reviewers screened all the studies, and another investigator entered all the endpoints and measures obtained in the chosen studies in Excel. The data was then prepared and analyzed as explained in the statistical analysis section. No automation tool was used in this process.
Table 1.
Search strategy in PubMed database and keywords used for search.
| Search | Query | Items found |
|---|---|---|
| #1 | Search: “Atherosclerosis”[Mesh] Sort by: Most Recent | 56,032 |
| #2 | Search: ((Atherosclerosis[Title/Abstract]) OR (Atheroscleroses[Title/Abstract])) OR (Atherogenesis[Title/Abstract]) | 142,274 |
| #3 | Search: (“Atherosclerosis”[Mesh]) OR (((Atherosclerosis[Title/Abstract]) OR (Atheroscleroses[Title/Abstract])) OR (Atherogenesis[Title/Abstract])) | 163,244 |
| #4 | Search: “Carotid Arteries”[Mesh] Sort by: Most Recent | 62,693 |
| #5 | Search: (“Carotid Artery”[Title/Abstract]) OR (“Carotid Arteries”[Title/Abstract]) | 79,303 |
| #6 | Search: (“Carotid Arteries”[Mesh]) OR ((“Carotid Artery”[Title/Abstract]) OR (“Carotid Arteries”[Title/Abstract])) | 108,219 |
| #7 | Search: ((“Atherosclerosis”[Mesh]) OR (((Atherosclerosis[Title/Abstract]) OR (Atheroscleroses[Title/Abstract])) OR (Atherogenesis[Title/Abstract]))) AND ((“Carotid Arteries”[Mesh]) OR ((“Carotid Artery”[Title/Abstract]) OR (“Carotid Arteries”[Title/Abstract]))) | 13,290 |
| #8 | Search: “Carotid Artery Diseases”[Mesh] | 52,225 |
| #9 | Search: ((((((“Carotid Atherosclerosis”[Title/Abstract]) OR (“Carotid Atheroscleroses”[Title/Abstract])) OR (“Carotid Artery Disease*“[Title/Abstract])) OR (“Carotid Artery Disorder*“[Title/Abstract])) OR (“Carotid Arterial Disease*“[Title/Abstract])) OR (“Carotid artery atherosclerosis”[Title/Abstract])) OR (“Carotid Atherosclerotic Disease*“[Title/Abstract]) | 8489 |
| #10 | Search: (“Carotid Artery Diseases”[Mesh]) OR (((((((“Carotid Atherosclerosis”[Title/Abstract]) OR (“Carotid Atheroscleroses”[Title/Abstract])) OR (“Carotid Artery Disease*“[Title/Abstract])) OR (“Carotid Artery Disorder*“[Title/Abstract])) OR (“Carotid Arterial Disease*“[Title/Abstract])) OR (“Carotid artery atherosclerosis”[Title/Abstract])) OR (“Carotid Atherosclerotic Disease*“[Title/Abstract])) | 54,602 |
| #11 | Search: ((“Carotid Artery Diseases”[Mesh]) OR (((((((“Carotid Atherosclerosis”[Title/Abstract]) OR (“Carotid Atheroscleroses”[Title/Abstract])) OR (“Carotid Artery Disease*“[Title/Abstract])) OR (“Carotid Artery Disorder*“[Title/Abstract])) OR (“Carotid Arterial Disease*“[Title/Abstract])) OR (“Carotid artery atherosclerosis”[Title/Abstract])) OR (“Carotid Atherosclerotic Disease*“[Title/Abstract]))) OR (((“Atherosclerosis”[Mesh]) OR (((Atherosclerosis[Title/Abstract]) OR (Atheroscleroses[Title/Abstract])) OR (Atherogenesis[Title/Abstract]))) AND ((“Carotid Arteries”[Mesh]) OR ((“Carotid Artery”[Title/Abstract]) OR (“Carotid Arteries”[Title/Abstract])))) | 61,111 |
| #12 | Search: “Non‐alcoholic Fatty Liver Disease”[Mesh] | 23,321 |
| #13 | Search: ((((((NAFLD[Title/Abstract]) OR (“Non‐alcoholic Fatty Liver*“[Title/Abstract])) OR (“Nonalcoholic Steatohepatitis”[Title/Abstract])) OR (“Nonalcoholic Steatohepatitides”[Title/Abstract])) OR (“Nonalcoholic Fatty Liver Disease*“[Title/Abstract])) OR (“Non‐alcoholic Fatty Liver Disease*“[Title/Abstract])) OR (“Non alcoholic Fatty Liver Disease*“[Title/Abstract]) | 36,568 |
| #14 | Search: (“Nonalcoholic Fatty Liver Disease”[Mesh]) OR (((((((NAFLD[Title/Abstract]) OR (“Nonalcoholic Fatty Liver*“[Title/Abstract])) OR (“Nonalcoholic Steatohepatitis”[Title/Abstract])) OR (“Nonalcoholic Steatohepatitides”[Title/Abstract])) OR (“Non‐alcoholic Fatty Liver Disease*“[Title/Abstract])) OR (“Non‐alcoholic Fatty Liver Disease*“[Title/Abstract])) OR (“Non alcoholic Fatty Liver Disease*“[Title/Abstract])) | 39,599 |
| #15 | Search: ((“Non‐alcoholic Fatty Liver Disease”[Mesh]) OR (((((((NAFLD[Title/Abstract]) OR (“Nonalcoholic Fatty Liver*“[Title/Abstract])) OR (“Nonalcoholic Steatohepatitis”[Title/Abstract])) OR (“Nonalcoholic Steatohepatitides”[Title/Abstract])) OR (“Nonalcoholic Fatty Liver Disease*“[Title/Abstract])) OR (“Non‐alcoholic Fatty Liver Disease*“[Title/Abstract])) OR (“Non‐alcoholic Fatty Liver Disease*“[Title/Abstract]))) AND (((“Carotid Artery Diseases”[Mesh]) OR (((((((“Carotid Atherosclerosis”[Title/Abstract]) OR (“Carotid Atheroscleroses”[Title/Abstract])) OR (“Carotid Artery Disease*“[Title/Abstract])) OR (“Carotid Artery Disorder*“[Title/Abstract])) OR (“Carotid Arterial Disease*“[Title/Abstract])) OR (“Carotid artery atherosclerosis”[Title/Abstract])) OR (“Carotid Atherosclerotic Disease*“[Title/Abstract]))) OR (((“Atherosclerosis”[Mesh]) OR (((Atherosclerosis[Title/Abstract]) OR (Atheroscleroses[Title/Abstract])) OR (Atherogenesis[Title/Abstract]))) AND ((“Carotid Arteries”[Mesh]) OR ((“Carotid Artery”[Title/Abstract]) OR (“Carotid Arteries”[Title/Abstract]))))) | 184 |
| #16 | Search: “Carotid Stenosis”[Mesh] | 18,014 |
| #17 | Search: (((((“Carotid Stenosis”[Title/Abstract]) OR (“Carotid plaques”[Title/Abstract])) OR (“Carotid Stenoses”[Title/Abstract])) OR (“Carotid Ulcer*“[Title/Abstract])) OR (“Intima media thickness”[Title/Abstract])) OR (“Carotid intimal thickness”[Title/Abstract]) | 22,959 |
| #18 | Search: (“Carotid Stenosis”[Mesh]) OR ((((((“Carotid Stenosis”[Title/Abstract]) OR (“Carotid plaques”[Title/Abstract])) OR (“Carotid Stenoses”[Title/Abstract])) OR (“Carotid Ulcer*“[Title/Abstract])) OR (“Intima media thickness”[Title/Abstract])) OR (“Carotid intimal thickness”[Title/Abstract])) | 35,063 |
| #19 | Search: ((“Non‐alcoholic Fatty Liver Disease”[Mesh]) OR (((((((NAFLD[Title/Abstract]) OR (“Nonalcoholic Fatty Liver*“[Title/Abstract])) OR (“Nonalcoholic Steatohepatitis”[Title/Abstract])) OR (“Nonalcoholic Steatohepatitides”[Title/Abstract])) OR (“Nonalcoholic Fatty Liver Disease*“[Title/Abstract])) OR (“Non‐alcoholic Fatty Liver Disease*“[Title/Abstract])) OR (“Non alcoholic Fatty Liver Disease*“[Title/Abstract]))) AND ((“Carotid Stenosis”[Mesh]) OR ((((((“Carotid Stenosis”[Title/Abstract]) OR (“Carotid plaques”[Title/Abstract])) OR (“Carotid Stenoses”[Title/Abstract])) OR (“Carotid Ulcer*“[Title/Abstract])) OR (“Intima media thickness”[Title/Abstract])) OR (“Carotid intimal thickness”[Title/Abstract]))) | 268 |
| #20 | Search: “Cerebrovascular Disorders”[Mesh] | 427,170 |
| #21 | Search: ((((((((“Cardiovascular risk marker*“[Title/Abstract]) OR (“Surrogate markers of cardiovascular disease*“[Title/Abstract])) OR (“Cerebrovascular Disorder*“[Title/Abstract])) OR (“Intracranial Vascular Disease*“[Title/Abstract])) OR (“Intracranial Vascular Disorder*“[Title/Abstract])) OR (“Cerebrovascular Disease*“[Title/Abstract])) OR (“Brain Vascular Disorder*“[Title/Abstract])) OR (“Cerebrovascular Occlusion*“[Title/Abstract])) OR (“Cerebrovascular Insufficienc*“[Title/Abstract]) | 32,706 |
| #22 | Search: (“Cerebrovascular Disorders”[Mesh]) OR (((((((((“Cardiovascular risk marker*“[Title/Abstract]) OR (“Surrogate markers of cardiovascular disease*“[Title/Abstract])) OR (“Cerebrovascular Disorder*“[Title/Abstract])) OR (“Intracranial Vascular Disease*“[Title/Abstract])) OR (“Intracranial Vascular Disorder*“[Title/Abstract])) OR (“Cerebrovascular Disease*“[Title/Abstract])) OR (“Brain Vascular Disorder*“[Title/Abstract])) OR (“Cerebrovascular Occlusion*“[Title/Abstract])) OR (“Cerebrovascular Insufficienc*“[Title/Abstract])) | 441,976 |
| #23 | Search: ((“Non‐alcoholic Fatty Liver Disease”[Mesh]) OR (((((((NAFLD[Title/Abstract]) OR (“Nonalcoholic Fatty Liver*“[Title/Abstract])) OR (“Nonalcoholic Steatohepatitis”[Title/Abstract])) OR (“Nonalcoholic Steatohepatitides”[Title/Abstract])) OR (“Nonalcoholic Fatty Liver Disease*“[Title/Abstract])) OR (“Non‐alcoholic Fatty Liver Disease*“[Title/Abstract])) OR (“Non alcoholic Fatty Liver Disease*“[Title/Abstract]))) AND ((“Cerebrovascular Disorders”[Mesh]) OR (((((((((“Cardiovascular risk marker*“[Title/Abstract]) OR (“Surrogate markers of cardiovascular disease*“[Title/Abstract])) OR (“Cerebrovascular Disorder*“[Title/Abstract])) OR (“Intracranial Vascular Disease*“[Title/Abstract])) OR (“Intracranial Vascular Disorder*“[Title/Abstract])) OR (“Cerebrovascular Disease*“[Title/Abstract])) OR (“Brain Vascular Disorder*“[Title/Abstract])) OR (“Cerebrovascular Occlusion*“[Title/Abstract])) OR (“Cerebrovascular Insufficienc*“[Title/Abstract]))) | 220 |
2.2. Ethical considerations
The information about the patients was kept confidential since this research was done as a review. Only studies that addressed ethical concerns and were published in credible journals were included in this analysis. The findings of this research were disclosed without hindrance, in accordance with the confidentially principle, and by citing the relevant sources. The investigation was carried out with the greatest care to ensure that the conclusions are supported by the available scientific data. To prevent plagiarism, the scientific writing rules were also followed.
2.3. Statistical analysis
We have listed the studies that we utilized in along with their details in Table 2. After importing the data, and defining the effects as “the difference between carotid intima‐media thickness of subjects with NAFLD versus those without NAFLD; we performed the analysis using R. 79 The preparation was done using the “tidyverse” 80 package, and the meta‐analysis and visualizations were done using the “metafor” 81 package. For the meta‐analysis, we used the random‐effects model, and we checked for heterogeneity and ran sensitivity analysis. We performed the meta‐analysis both with the overall effect and by using sample number of the studies as weights. We then generated the forest plots and funnel plot to visualize the effects.
Table 2.
Summary of data obtained from articles.
| Carotid atherosclerosis plaque | CIMT(mm) | Number of NAFLD | Number of control patients | Total sample | NAFLD diagnosis | Published year | First author | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAFLD | CONTROL | NAFLD | CONTROL | ||||||||||
| Count | % | Count | % | Mean | SD | Mean | SD | ||||||
| 0.46 | 0.04 | 0.41 | 0.03 | 320 | 687 | 1007 | Sonography | 2017 | Xia Li 11 | ||||
| 35 | 31 | 0.78 | 0.15 | 0.75 | 0.15 | 636 | 484 | 898 | Sonography | 2020 | Hyeok‐Hee Lee 12 | ||
| 7 | 14.2 | 0 | 0 | 0.9 (no SD reported) | 0.6 (no SD reported) | 50 | 30 | 80 | Biopsy | 2014 | Nicoleta V. Leach 13 | ||
| 32.0% (high) | 22.10% (high) | 290 | 290 | 580 | Sonography | 2013 | Kamran B. Lankarani 14 | ||||||
| 0.4 | 0.19 | 0.27 | 0.18 | 117 | 44 | 161 | Sonography | 2013 | Metin Kucukazman 15 | ||||
| 0.818 | 0.006 | 0.818 | 0.008 | 747 | 464 | 1211 | Sonography | 2014 | Soo‐Kyung Kim 16 | ||||
| 0.75 | 0.06 | 0.74 | 0.08 | 180 | 1105 | 1285 | CT | 2014 | Nan Hee Kim 17 | ||||
| 0.72 | 0.06 | 0.619 | 0.04 | 103 | 50 | 153 | Sonography | 2019 | Cemal Kemaloglu 18 | ||||
| 0.68 | 0.1 | 0.65 | 0.1 | 40 | 26 | 66 | Sonography | 2009 | F. Karakurt 19 | ||||
| 109 | 34.1 | 59 | 18.8 | 0.79 | 0.18 | 0.73 | 0.13 | 320 | 313 | 633 | Sonography | 2012 | Ji Hoon Kang 20 |
| 0.09 | 0.19 | 0.8 | 0.1 | 29 | 22 | 51 | CT | 2013 | Pikkel Josef 21 | ||||
| 357 | 13.8 | 865 | 14.3 | 0.59 | 0.1 | 0.57 | 0.1 | 2590 | 6042 | 8632 | Sonography | 2012 | Yun Huang 22 |
| 0.66 | 0.04 | 0.64 | 0.04 | 342 | 613 | 955 | Sonography | 2016 | Ho Cheol Hong 23 | ||||
| 1.09 | 0.15 | 0.88 | 0.05 | 196 | 100 | 296 | Sonography | 2018 | Amr Shaaban Hanafy 24 | ||||
| 2452 | 56.5 | 1883 | 44.5 | 0.82 | 0.3 | 0.85 | 0.39 | 4349 | 4231 | 8571 | Sonography | 2017 | Kaifeng Guo 25 |
| 0.83 | 0.21 | 0.76 | 0.14 | 106 | 909 | 1015 | CT | 2018 | Anders Gummesson 26 | ||||
| 0.6 | 0.11 | 0.54 | 0.08 | 113 | 57 | 170 | Biopsy | 2013 | Halil Genc 27 | ||||
| 46 | 50 | 95 | 52 | 0.96 | 0.22 | 0.79 | 0.18 | 91 | 182 | 273 | Sonography/biopsy | 2016 | Anna Ludovica Fracanzani 28 |
| 0.89 | 0.26 | 0.64 | 0.14 | 125 | 350 | 375 | sonography/biopsy | 2008 | Anna Ludovica Fracanzani 29 | ||||
| 0.83 | 0.16 | 0.77 | 0.016 | 49 | 33 | 82 | Sonography | 2018 | Reza Fadaei 30 | ||||
| 0.6 | 0.13 | 0.5 | 0.08 | 67 | 35 | 102 | Biopsy | 2012 | Teoman Dogru 31 | ||||
| 0·60 | 0·50 | 115 | 74 | 189 | Sonography/biopsy | 2013 | Teoman Dogru 32 | ||||||
| 0.67 | 0.09 | 0.52 | 0.11 | 51 | 21 | 72 | biopsy | 2012 | Yasar Colaka 33 | ||||
| 0.68 | 0.15 | 0.68 | 0.14 | 93 | 37 | 130 | Sonography/biopsy | 2017 | Ibrahim Cetindaglı 34 | ||||
| 20 | 50 | 10 | 25 | 0.70 | 0.2 | 0.54 | 0.13 | 40 | 40 | 80 | Sonography | 2005 | Angel Brea 35 |
| 0.64 | 0.17 | 0.43 | 0.14 | 34 | 35 | 69 | Sonography | 2013 | Ö. Başar 36 | ||||
| 0.1 | 0.02 | 0.08 | 0.02 | 50 | 50 | 100 | Sonography | 2021 | Maha Assem 37 | ||||
| 0.64 | 0.1 | 0.52 | 0.10 | 57 | 30 | 87 | Sonography/biopsy | 2012 | Yasar Colak 38 | ||||
| 9 | 92.52 | 58 | 52.73 | 110 | 107 | 217 | Sonography | 2015 | Florin Casoinic 39 | ||||
| 0.646 | 0.091 | 0.544 | 0.067 | 40 | 40 | 80 | Biopsy | 2007 | Cem Aygun 40 | ||||
| 60 | 30.4 | 70 | 35.2 | 0.6 | 0 | 0.6 | 0 | 148 | 129 | 277 | Sonography | 2017 | Clarence Gill 41 |
| 31 | 24 | 0.79 | 0.22 | 0.73 | 0.15 | 452 | 453 | 905 | Sonography | 2012 | Xiaoming Li 42 | ||
| 0.81 | 0.14 | 0.58 | 0.15 | 250 | 85 | 335 | Sonography | 2011 | Afshin Mohammadi 43 | ||||
| 0.65 | 0.09 | 0.55 | 0.07 | 84 | 65 | 149 | Sonography | 2011 | Afshin Mohammadi 44 | ||||
| 38 | 25.3 | 8 | 5.3 | 0.62 | 0.19 | 0.5 | 0.13 | 150 | 150 | 300 | Sonography | 2019 | Ali Mohammadzadeh 45 |
| 0.56 | 0.11 | 0.45 | 0.13 | 99 | 52 | 151 | Sonography | 2014 | Maryam Zaare Nahandi 46 | ||||
| 0.44 | 0.07 | 0.4 | 0.05 | 61 | 41 | 102 | Biopsy | 2015 | Kadir Ozturk 47 | ||||
| 0.63 | 0.17 | 0.54 | 0.1 | 45 | 45 | 90 | Sonography | 2020 | Vijay Rampally 48 | ||||
| 0.79 | 0.18 | 0.67 | 0.13 | 23 | 28 | 51 | Biopsy | 2010 | Charalambos Vlachopoulos 49 | ||||
| 1.14 | 0.20 | 0.82 | 0.12 | 85 | 160 | 245 | Biopsy | 2006 | Giovanni Targher 50 | ||||
| 0.8 | 0.1 | 0.6 | 0.03 | 109 | 109 | 218 | Sonography | 2016 | Hafsa Riaz 51 | ||||
| 0.82 | 0.15 | 0.58 | 0.1 | 200 | 100 | 300 | Sonography | 2017 | Abid Rasool 52 | ||||
| 1.10 | 0.20 | 0.84 | 0.13 | 50 | 40 | 90 | Biopsy | 2005 | Giovanni Targher 53 | ||||
| 0.6 | 0.11 | 0.6 | 0.23 | 654 | 2770 | 3433 | Sonography | 2019 | Zhuojun Xin 54 | ||||
| 0.6 | 0.12 | 0.49 | 0.1 | 40 | 40 | 80 | Sonography | 2012 | Manik Lal Thakur 55 | ||||
| 1.2 | 0.14 | 0.9 | 0.12 | 37 | 35 | 72 | Sonography | 2016 | Radojica V. Stolic 56 | ||||
| 29.3 | 33.6 | 0.82 | 0.16 | 0.76 | 0.15 | 92 | 128 | 220 | Sonography | 2010 | Paolo Salvi 57 | ||
| 0.88 | 0.18 | 0.87 | 0.2 | 729 | 3394 | 4123 | Sonography | 2019 | Ebenezer Oni 58 | ||||
| 0.71 | 0.17 | 0.69 | 0.31 | 107 | 43 | 150 | Sonography | 2020 | Nurazam omar 59 | ||||
| 19.2 | 2.2 | 0.592 | 0.1 | 0.489 | 0.13 | 101 | 544 | 645 | Sonography | 2013 | Sandhya Mishra 60 | ||
| 57.8 | 37.5 | 0.84 | 0.1 | 0.72 | 0.1 | 90 | 64 | 154 | Sonography | 2009 | Stefano Ramilli 61 | ||
| 34.50% (high) | 19.10% (high) | 144 | 107 | 251 | Sonography | 2018 | Eugene Choon‐Li Tan 62 | ||||||
| 1.18 | 0.14 | 0.94 | 0.12 | 45 | 40 | 85 | Sonography | 2004 | Giovanni Targher 63 | ||||
| 1.09 | 0.77 | 0.98 | 0.68 | 38 | 18 | 56 | Sonography | 2011 | Laura I. Poanta 64 | ||||
| 31.6 | 40.1 | 0.82 | 0.2 | 0.64 | 0.14 | 73 | 51 | 124 | Sonography | 2018 | Sivabal Vanjiappan 65 | ||
| 27 | 71.1 | 4 | 8 | 1.1 | 0.1 | 0.8 | 0.1 | 38 | 50 | 88 | CT | 2014 | Ivana Mikolasevic 66 |
| 0.75 | 0.23 | 0.66 | 0.15 | 24 | 28 | 52 | Biopsy | 2012 | Binnur Pinarbasi 67 | ||||
| 0.67 | 0.15 | 0.63 | 0.13 | 394 | 421 | 815 | Sonography | 2015 | Seung Hwan Moon 68 | ||||
| 0.75 | 0.15 | 0.74 | 0.13 | 61 | 40 | 101 | CT | 2009 | Jean Michel Petit 69 | ||||
| 1 | 0.6 | 0.98 | 0.11 | 289 | 47 | 336 | Sonography | 2015 | Cristina Alina Silaghi 70 | ||||
| 20.4 | 0 | 0.75 | 0.15 | 0.58 | 0.09 | 77 | 15 | 92 | Biopsy | 2015 | Josep Puig 71 | ||
| 21.5 | 6 | 0.72 | 0.15 | 0.46 | 0.13 | 117 | 32 | 149 | Biopsy | 2015 | Josep Puig 71 | ||
| 645 | 40 | 967 | 59.9 | 43.84% (high) | 56.15% (high) | 1571 | 2541 | 4112 | Sonography | 2018 | Jilin Zheng 72 | ||
| 28.9 | 16.9 | 0.81 | 0.25 | 0.69 | 0.18 | 123 | 599 | 722 | Sonography | 2016 | Lie Zhang 73 | ||
| 21.9 | 15 | 30% (high) | 21.1% (high) | 1375 | 1237 | 2612 | Sonography | 2018 | Yu Chen Guo 74 | ||||
| 0.712 | 0.150 | 0.5875 | 0.088 | 76 | 24 | 100 | Sonography | 2022 | Shabbirhussain 75 | ||||
| 0.78 | 0.145 | 0.75 | 0.15 | 456 | 396 | 852 | Sonography | 2023 | Cho 76 | ||||
| 0.64 | 0.1 | 0.8 | 0.2 | 63 | 35 | 98 | Sonography | 2023 | Zhang 77 | ||||
| 31.8% high | 19.7% high | 384 | 506 | 890 | Sonography | 2022 | Bessho 78 | ||||||
Abbreviations: CT, computed tomography; NAFLD, nonalcoholic fatty liver disease; SD, standard deviation.
3. RESULTS
3.1. Data collection
PubMed, Ovid, Scopus, ProQuest, Web of Science, and the Cochrane Library databases were used in our study in which 495, 546, 566, 80, 683, and 4 articles were screened out, respectively. Strategy search for PubMed database is provided in Table 1. Consequently, a total of 2865 articles were screened out in which 1024 articles remained after eliminating duplicates from the total articles, while 159 items remained after initial screening using titles and abstracts. The whole texts of 159 articles were meticulously examined in the next stage. Then, 90 items were eliminated as being irrelevant and the data from 69 remained articles was collected, which is summarized in Table 2. There was a total of 56,582 patients in these studies, comprising 26,689 NAFLD cases and 38,584 control subjects. Finally, 10 articles were excluded due to reporting mean and percentage values rather than mean and standard deviation, and 59 articles remained for meta‐analysis. The details of included and excluded data can be shown in illustrated PRISMA flowchart (Figure 1).
Figure 1.
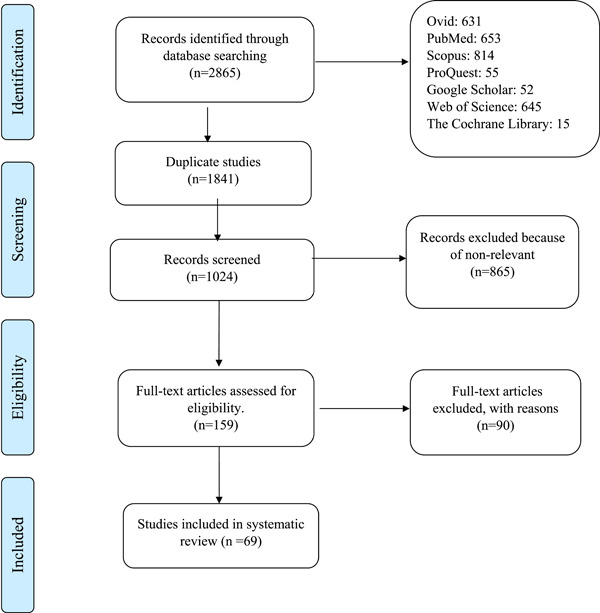
PRISMA flow diagram showing the selection process of studies in the systematic review and meta‐analysis.
3.2. Relationship between NAFLD and CIMT
The findings of the meta‐analysis based on 59 studies (after weighing based on their sample size) revealed that NAFLD was linked to an increase in CIMT associated with an increase of 0.1231 mm (20.6%) in CIMT (p = 0.002, 95% CI: 0.0462, 0.2000) in individuals with NAFLD. The forest plot in Figure 2 shows our pooled estimate, and the funnel plot in Figure 3 shows that the studies were symmetrically distributed.
Figure 2.
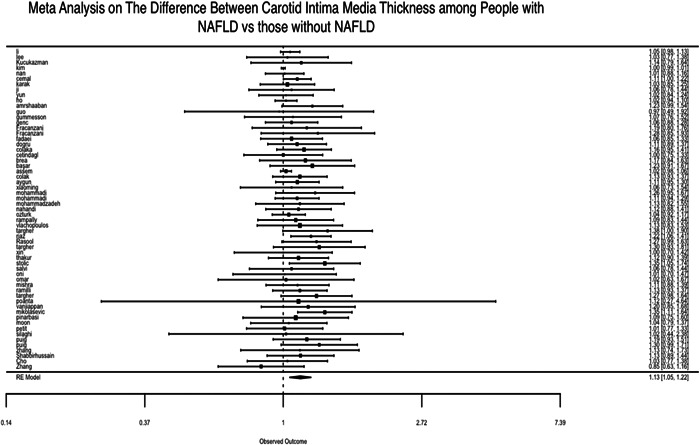
Results of a meta‐analysis of studies on the association of carotid artery intima‐media diameter (CIMT) with nonalcoholic fatty liver disease (NAFLD).
Figure 3.
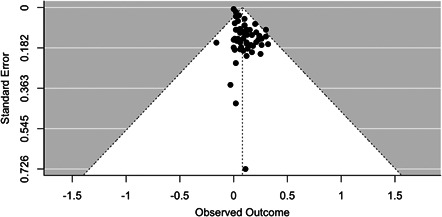
Funnel plot publication bias in the studies conducted on the relationship between carotid artery intima‐media diameter (CIMT) and nonalcoholic fatty liver disease (NAFLD).
At first we noted a significant heterogeneity among the studies, but after further exploration, we noted that one of the studies was causing a significant heterogeneity, and because of the lower sample size and the wide confidence interval in the study, 21 we excluded the study from the meta‐analysis. In the final analysis, using the sample size of the studies as weights, we noted no significant heterogeneity among the studies (Q = 67.49, and p = 0.18).
Also, we looked in several studies on patients suffering from diabetes, which had contributed to their NAFLD, and the outcome of a subgroup meta‐analysis of 7 studies that only involved diabetic patients—both the NAFLD group and the control group—showed us that the presence of NAFLD, while slightly increasing the odds, was not significantly correlated with increase in CIMT (p value = 0.557; 1.089–0.955 confidence interval [CI] 95%; odds ratio [OR] = 1.020). This analysis can be observed in Figure 4.
Figure 4.
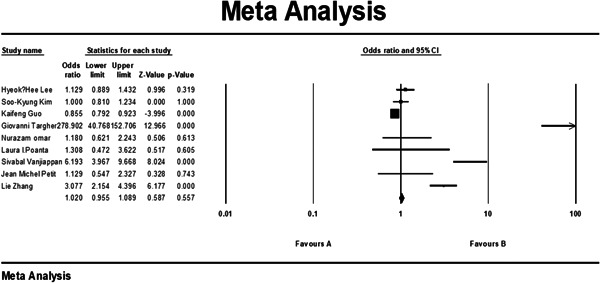
Subgroup results of meta‐analysis of studies conducted on diabetic patients regarding the relationship between carotid artery intima‐media diameter (CIMT) and nonalcoholic fatty liver disease (NAFLD). CI, confidence interval.
We also assessed for the effect of the method of diagnosis (ultrasound vs. CT scan vs. biopsy) and noticed that there was no significant effect of method of diagnosis on the association between NAFLD and increase in CIMT (CT vs. biopsy: p = 0.72; ultrasound vs. biopsy: p = 0.22).
3.3. Association between NAFLD and atherosclerotic plaque in carotid arteries
A meta‐analysis based on 17 separate studies revealed a significant association between the incidence of atherosclerotic plaques in the carotid arteries and the presence of NAFLD. They noted that in the patients with plaques, there was 1.35 times odds of having NAFLD versus those without plaques (p < 0.001, 1.28–1.43, 95% CI, OR = 1.356). The summary of this analysis can be viewed in Figure 5.
Figure 5.
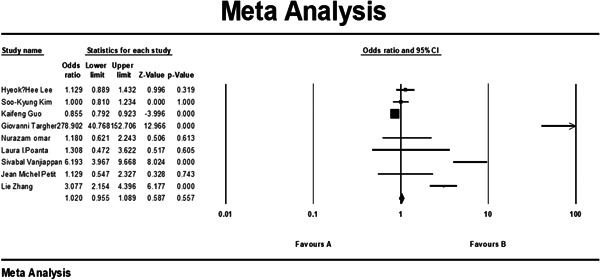
Results of a meta‐analysis of studies on the association of atherosclerotic plaque with NAFLD. CI, confidence interval; NAFLD, nonalcoholic fatty liver disease.
4. DISCUSSION
NAFLD has become a global public health issue since it is linked to metabolic risk factors such as obesity, diabetes mellitus, dyslipidemia, and metabolic syndrome, along with genetic, socioeconomic, and lifestyle components. 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 Increased endothelial dysfunction, 91 , 92 ischemic heart disease, 93 cardiovascular events, 93 , 94 , 95 peripheral vascular disease, cardiovascular morbidity, and cardiovascular mortality are all medical issues, which may be caused by NAFLD. 87 , 96 Other research has shown an independent link between NAFLD and cardiovascular disease, regardless of other metabolic risk factors. 97 This finding raises the possibility that NAFLD is a direct contributor to the pathogenesis of atherosclerosis rather than just a sign of cardiovascular disorder. 98 Numerous pathogenic mechanisms, including elevated levels of oxidative stress brought on by steatosis‐stimulated fatty acid oxidation, 87 systemic release of pro‐atherogenic molecules like tumor necrosis factor‐alpha, interleukin‐6, and oxidized low‐density lipoprotein cholesterol, 99 elevated insulin resistance, 100 and macrophage activation, 101 have been proposed as potential causes for the acceleration of atherosclerosis and the rise in the prevalence of cardiovascular diseases in NAFLD patients. The atherogenic impact of liver inflammation is further confirmed by the fact that NASH (nonalcoholic steatohepatitis) patients had more atherosclerosis than steatosis patients. 102 , 103
In the present study, 59 observational studies were reviewed and the association between NAFLD and increased CIMT as well as prevalence of atherosclerotic plaque in the carotid artery (both subclinical indicators of atherosclerosis) was evaluated. In a pooled analysis of 59 studies, NAFLD was shown to be linked with a higher CIMT rate. In a pooled analysis of 59 studies, NAFLD was shown to be linked with higher CIMT. Of note, CIMT was 0.12 mm more (20.6%) in those with NAFLD than in controls (without NAFLD). Meanwhile, this value was about 18.7% in a meta‐analysis conducted by Madan et al. on 20 observational studies examining the influence of NAFLD on CIMT in adults, 104 and 13% in a meta‐analysis performed by Sookoian et al. on 7 studies. 105 In addition, a meta‐analysis of 17 studies revealed that NAFLD was related with a higher incidence of carotid plaque found by ultrasonography. 104 In concordance to our findings, NAFLD was related with an elevated risk of carotid plaque (detected by ultrasonography) in the meta‐analysis of Madan et al. 104 (of 13 studies). However, in a subgroup meta‐analysis of seven studies comparing diabetic individuals with NAFLD to diabetic patients without NAFLD, the presence of NAFLD was not significantly correlated with elevated CIMT rate in these patients. NAFLD seemed to be connected with an elevated risk of cardiovascular disease in both diabetic and nondiabetic individuals (T2DM). 106 , 107 Despite the fact that multiple research projects have shown that NAFLD is substantially correlated with higher CIMT in nondiabetic individuals, the association between fatty liver and atherosclerosis in patients with T2DM is less obvious, and there are contradictory findings across investigations. 16 , 50 , 69 , 108 , 109 Targher et al. found that NAFLD measured by ultrasonography in T2DM patients on a restricted diet was related with an increased incidence of cardiovascular disease and CIMT. 109 However, in agreement with our findings, Petit et al. did not demonstrate a link between NAFLD and elevated CIMT in T2DM patients. 69 Similar to our investigation, Guo et al. also did not find correlation between NAFLD and elevated CIMT in T2DM patients in a Chinese hospitalized population controlling for multiple confounding factors. 25 In contrast to our findings, Kim et al. observed that NAFLD is related to higher CIMT in individuals with T2DM but is impacted by insulin resistance. 16 Guo et al., revealed that after controlling cardiovascular risk factors, there was an independent correlation between NAFLD and carotid and lower limb atherosclerotic plaques, which is indicative of the independent association between NAFLD and advanced atherosclerotic lesions in T2DM patients. 25 The association between NAFLD and carotid atherosclerosis may be obscured by diabetes, which is regarded as one of the most significant risk factors for cardiovascular disease and the progression of atherosclerosis in the body. Other possible explanations for this contradiction include the techniques for detecting fatty liver (using ultrasound, CT, or magnetic resonance spectroscopy), ethnic disparities, and sample size.
Although increased CIMT has been shown to be associated with an increased risk of stroke, 93 myocardial infarction, 93 , 94 and peripheral vascular disease, 93 a recent meta‐analysis study suggested that carotid plaques may be a better predictor of cardiovascular risk than CIMT. 110 In addition, comprehensive research revealed that carotid plaque area is a more accurate predictor of ischemic stroke in the first year than CIMT. Consequently, instead of CIMT, it is necessary to study the influence of NAFLD on the carotid plaque area in the next investigations. 111
A recent meta‐analysis by Madan et al. 104 of 28 studies revealed an increased risk of carotid atherosclerosis in adult and pediatric populations with NAFLD compared to groups without NAFLD. However, a number of other research have evaluated the connection between NAFLD and carotid disease. 11 , 23 , 24 , 25 , 26 , 28 , 30 , 34 , 41 , 45 , 48 , 51 , 52 , 56 , 58 , 65 , 73 As a result, we did an updated meta‐analysis to incorporate new research done in Asia and Europe over the last years on the connection between NAFLD and carotid atherosclerosis through measurements of the CIMT in millimeters.
The constraints of any meta‐analysis research, by its nature, include its effect on the reviewed papers' texts, the risk of publication bias (publication bias), and the comprehensive search approach. To prevent this, we conducted the meta‐analysis using a comprehensive search approach and unambiguous inclusion and exclusion criteria. In addition, owing to the inclusion of observational studies in the analysis, unmeasured and underreported confounding factors and errors are possible. However, one of the most important strengths of our study was the focus on the amount of difference, along with percentage of difference, in the thickness of carotid intima‐media, instead of just describing the association or the odds of difference.
5. CONCLUSION
Through this systematic review and meta‐analysis, we concluded that NAFLD is correlated with an increase of 20.6% (0.12 mm) in CIMT. We also observed that NAFLD is correlated with an increase in atherosclerotic plaques.
AUTHOR CONTRIBUTIONS
Manouchehr Khoshbaten: Conceptualization; data curation; formal analysis; writing—original draft. Sepideh Hadi Maleki: Data curation; formal analysis; writing—original draft. Sara Hadad: Data curation; formal analysis; validation; writing—original draft. Amrit Baral: Data curation; writing—original draft; writing—review & editing. Ana Vitoria Rocha: Data curation; investigation; writing—review & editing. Laxmi Poudel: Data curation; writing—original draft; writing—review & editing. Alireza Abdshah: Visualization; writing—original draft; writing—review & editing.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflicts of interest.
TRANSPARENCY STATEMENT
The lead author Alireza Abdshah affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
This study was funded by the authors.
Khoshbaten M, Maleki SH, Hadad S, et al. Association of non‐alcoholic fatty liver disease and carotid media‐intima thickness: a systematic review and a meta‐analysis. Health Sci Rep. 2023;6:e1554. 10.1002/hsr2.1554
DATA AVAILABILITY STATEMENT
The data set of the extracted measurements, along with R codes for analysis and plotting, can be made available upon request.
REFERENCES
- 1. Tilg H, Adolph TE, Dudek M, Knolle P. Non‐alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat Metab. 2021;3(12):1596‐1607. [DOI] [PubMed] [Google Scholar]
- 2. Barr J, Caballería J, Martínez‐Arranz I, et al. Obesity‐dependent metabolic signatures associated with nonalcoholic fatty liver disease progression. J Proteome Res. 2012;11(4):2521‐2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221‐1231. 10.1056/NEJMra011775 [DOI] [PubMed] [Google Scholar]
- 4. Das SK, Mukherjee S, Vasudevan D. Non‐alcoholic fatty liver disease: an under‐recognized cause with emerging importance. Curr Sci. 2006;90:659‐665. [Google Scholar]
- 5. Novakovic T, Inic‐Kostic B, Milinic S, Jovicevic L, Dzeletovic G. Cardiovascular disease risk factors in patients with non‐alcoholic fatty liver disease. Med Pregl. 2013;66(1‐2):24‐31. [DOI] [PubMed] [Google Scholar]
- 6. Pourshams A, Malekzadeh R, Monavvari A, et al. Prevalence and etiology of persistently elevated alanine aminotransferase levels in healthy Iranian blood donors. J Gastroenterol Hepatol. 2005;20(2):229‐233. [DOI] [PubMed] [Google Scholar]
- 7. Buckley AJ, Thomas EL, Lessan N, Trovato FM, Trovato GM, Taylor‐Robinson SD. Non‐alcoholic fatty liver disease: relationship with cardiovascular risk markers and clinical endpoints. Diabetes Res Clin Pract. 2018;144:144‐152. [DOI] [PubMed] [Google Scholar]
- 8. Grobbee DE, Bots ML. Carotid artery intima‐media thickness as an indicator of generalized atherosclerosis. J Intern Med. 1994;236(5):567‐573. [DOI] [PubMed] [Google Scholar]
- 9. Targher G. Non‐alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabetic Med. 2007;24(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 10. Bonora E, Targher G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat Rev Gastroenterol Hepatol. 2012;9(7):372‐381. [DOI] [PubMed] [Google Scholar]
- 11. Li X, Shi H, Wang Z, Chang L, Zhang M, Dong X. Arterial stiffness is increased in nondiabetic, nonhypertensive postmenopausal women with nonalcoholic fatty liver disease. J Hypertens. 2017;35(6):1226‐1234. [DOI] [PubMed] [Google Scholar]
- 12. Lee HH, Cho Y, Choi YJ, et al. Non‐alcoholic steatohepatitis and progression of carotid atherosclerosis in patients with type 2 diabetes: a Korean cohort study. Cardiovasc Diabetol. 2020;19(1):81. 10.1186/s12933-020-01064-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leach NV, Dronca E, Vesa SC, et al. Serum homocysteine levels, oxidative stress and cardiovascular risk in non‐alcoholic steatohepatitis. Eur J Intern Med. 2014;25(8):762‐767. [DOI] [PubMed] [Google Scholar]
- 14. Lankarani KB, Mahmoodi M, Lotfi M, et al. Common carotid intima‐media thickness in patients with non‐alcoholic fatty liver disease: a population‐based case‐control study. Korean J Gastroenterol. 2013;62(6):344‐351. [DOI] [PubMed] [Google Scholar]
- 15. Kucukazman M, Ata N, Yavuz B, et al. Evaluation of early atherosclerosis markers in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2013;25(2):147‐151. [DOI] [PubMed] [Google Scholar]
- 16. Kim S‐K, Choi YJ, Huh BW, et al. Nonalcoholic fatty liver disease is associated with increased carotid intima‐media thickness only in type 2 diabetic subjects with insulin resistance. J Clin Endocrinol Metab. 2014;99(5):1879‐1884. [DOI] [PubMed] [Google Scholar]
- 17. Kim NH, Park J, Kim SH, et al. Non‐alcoholic fatty liver disease, metabolic syndrome and subclinical cardiovascular changes in the general population. Heart. 2014;100(12):938‐943. [DOI] [PubMed] [Google Scholar]
- 18. Kemaloglu C, Kemaloglu MD. Non alcoholic fatty liver disease is a predictor of subclinical carotid atherosclerosis in the presence of metabolic syndrome. Nepalese Heart J. 2019;16(1):39‐45. [Google Scholar]
- 19. Karakurt F, Carlioglu A, Koktener A, et al. Relationship between cerebral arterial pulsatility and carotid intima media thickness in diabetic and non‐diabetic patients with non‐alcoholic fatty liver disease. J Endocrinol Invest. 2009;32:63‐68. [DOI] [PubMed] [Google Scholar]
- 20. Kang JH, Cho KI, Kim SM, et al. Relationship between nonalcoholic fatty liver disease and carotid artery atherosclerosis beyond metabolic disorders in non‐diabetic patients. J Cardiovasc Ultrasound. 2012;20(3):126‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Josef P, Ali I, Ariel P, Alon M, Nimer A. Relationship between retinal vascular caliber and coronary artery disease in patients with non‐alcoholic fatty liver disease (NAFLD). Int J Environ Res Public Health. 2013;10(8):3409‐3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang Y, Bi Y, Xu M, et al. Nonalcoholic fatty liver disease is associated with atherosclerosis in middle‐aged and elderly Chinese. Arterioscler Thromb Vasc Biol. 2012;32(9):2321‐2326. [DOI] [PubMed] [Google Scholar]
- 23. Hong HC, Hwang SY, Ryu JY, et al. The synergistic impact of nonalcoholic fatty liver disease and metabolic syndrome on subclinical atherosclerosis. Clin Endocrinol. 2016;84(2):203‐209. [DOI] [PubMed] [Google Scholar]
- 24. Shaaban Hanafy A, Abd‐Elsalam S, Ahmed AF, Dawoud MM. Multifocal fatty liver disease, insulin resistance and carotid atherosclerosis: exploring the interrelated relationship. J Ultrason. 2018;18(75):302‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo K, Zhang L, Lu J, et al. Non‐alcoholic fatty liver disease is associated with late but not early atherosclerotic lesions in Chinese inpatients with type 2 diabetes. J Diabetes Complications. 2017;31(1):80‐85. [DOI] [PubMed] [Google Scholar]
- 26. Gummesson A, Strömberg U, Schmidt C, et al. Non‐alcoholic fatty liver disease is a strong predictor of coronary artery calcification in metabolically healthy subjects: A cross‐sectional, population‐based study in middle‐aged subjects. PLoS One. 2018;13(8):e0202666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Genc H, Dogru T, Celebi G, et al. Non‐alcoholic fatty liver disease per se is not associated with carotid atherosclerosis. Gulhane Med J. 2013;55(2):84. [Google Scholar]
- 28. Fracanzani AL, Tiraboschi S, Pisano G, et al. Progression of carotid vascular damage and cardiovascular events in non‐alcoholic fatty liver disease patients compared to the general population during 10 years of follow‐up. Atherosclerosis. 2016;246:208‐213. [DOI] [PubMed] [Google Scholar]
- 29. Fracanzani AL, Burdick L, Raselli S, et al. Carotid artery intima‐media thickness in nonalcoholic fatty liver disease. Am J Med. 2008;121(1):72‐78. [DOI] [PubMed] [Google Scholar]
- 30. Fadaei R, Meshkani R, Poustchi H, et al. Association of carotid intima media thickness with atherogenic index of plasma, apo b/apo AI ratio and paraoxonase activity in patients with non‐alcoholic fatty liver disease. Arch Physiol Biochem. 2019;125(1):19‐24. [DOI] [PubMed] [Google Scholar]
- 31. Dogru T, Genc H, Tapan S, et al. Elevated asymmetric dimethylarginine in plasma: an early marker for endothelial dysfunction in non‐alcoholic fatty liver disease? Diabetes Res Clin Pract. 2012;96(1):47‐52. [DOI] [PubMed] [Google Scholar]
- 32. Dogru T, Genc H, Tapan S, et al. Plasma fetuin‐A is associated with endothelial dysfunction and subclinical atherosclerosis in subjects with nonalcoholic fatty liver disease. Clin Endocrinol. 2013;78(5):712‐717. [DOI] [PubMed] [Google Scholar]
- 33. Colak Y, Senates E, Yesil A, et al. Assessment of endothelial function in patients with nonalcoholic fatty liver disease. Endocrine. 2013;43:100‐107. [DOI] [PubMed] [Google Scholar]
- 34. Cetindağlı I, Kara M, Tanoglu A, et al. Evaluation of endothelial dysfunction in patients with nonalcoholic fatty liver disease: association of selenoprotein P with carotid intima‐media thickness and endothelium‐dependent vasodilation. Clin Res Hepatol Gastroenterol. 2017;41(5):516‐524. 10.1016/j.clinre.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 35. Brea A, Mosquera D, Martín E, Arizti A, Cordero L, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case–control study. Arterioscler Thromb Vasc Biol. 2005;25(5):1045‐1050. [DOI] [PubMed] [Google Scholar]
- 36. Başar Ö, Akbal E, Köklü S, et al. Increased H‐FABP concentrations in nonalcoholic fatty liver disease. Herz. 2013;38(4):417‐422. [DOI] [PubMed] [Google Scholar]
- 37. Assem M, Amin M, Khalafallah O, Hussien A, Saif A, Mousa S. Hypoadiponectinemia as a marker of increased cardiovascular risk in patients with non‐alcoholic fatty liver disease: correlation with albumin/creatinine ratio. Arch Endocrinol Metab. 2020;65:93‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Colak Y, Karabay CY, Tuncer I, et al. Relation of epicardial adipose tissue and carotid intima‐media thickness in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2012;24(6):613‐618. [DOI] [PubMed] [Google Scholar]
- 39. Casoinic F, Baston D, Sampelean D, Badau C, Buzoianu AD, Hancu N. Symptomatic and asymptomatic significant carotid artery disease in type 2 diabetes patients with nonalcoholic steatohepatitis. Human Veterinary Med. 2015;7(4):341‐345. [Google Scholar]
- 40. Aygun C, Kocaman O, Sahin T, et al. Evaluation of metabolic syndrome frequency and carotid artery intima‐media thickness as risk factors for atherosclerosis in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2008;53:1352‐1357. [DOI] [PubMed] [Google Scholar]
- 41. Gill C, Vatcheva KP, Pan J‐J, et al. Frequency of nonalcoholic fatty liver disease and subclinical atherosclerosis among young Mexican Americans. Am J Cardiol. 2017;119(11):1717‐1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li X, Xia M, Ma H, et al. Liver fat content is associated with increased carotid atherosclerosis in a Chinese middle‐aged and elderly population: the Shanghai Changfeng study. Atherosclerosis. 2012;224(2):480‐485. [DOI] [PubMed] [Google Scholar]
- 43. Mohammadi A, Bazazi A, Ghasemi‐Rad M. Evaluation of atherosclerotic findings in patients with nonalcoholic fatty liver disease. Int J Gen Med. 2011;717‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghasemi‐Rad M, Mohammadi HH, Habibpour M. Evaluation of carotid intima‐media thickness and flow‐mediated dilatation in middle‐aged patients with nonalcoholic fatty liver disease. Vasc Health Risk Manag. 2011;661‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mohammadzadeh A, Shahkarami V, Shakiba M, Sabetrasekh P, Mohammadzadeh M. Association of non‐alcoholic fatty liver disease with increased carotid intima‐media thickness considering other cardiovascular risk factors. Iran J Radiol. 2019;16(3):1182‐1186. [Google Scholar]
- 46. Nahandi MZ, Khoshbaten M, Ramazanzadeh E, et al. Effect of non‐alcoholic fatty liver disease on carotid artery intima‐media thickness as a risk factor for atherosclerosis. Gastroenterol Hepatol Bed Bench. 2014;7(1):55‐62. [PMC free article] [PubMed] [Google Scholar]
- 47. Ozturk K, Uygun A, Guler AK, et al. Nonalcoholic fatty liver disease is an independent risk factor for atherosclerosis in young adult men. Atherosclerosis. 2015;240(2):380‐386. [DOI] [PubMed] [Google Scholar]
- 48. Rampally V, Biri S, Nair I, Vadlakonda A. Determination of association between nonalcoholic fatty liver disease and carotid artery atherosclerosis among nondiabetic individuals. J Family Med Prim Care. 2020;9(2):1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vlachopoulos C, Manesis E, Baou K, et al. Increased arterial stiffness and impaired endothelial function in nonalcoholic fatty liver disease: a pilot study. Am J Hypertens. 2010;23(11):1183‐1189. [DOI] [PubMed] [Google Scholar]
- 50. Targher G, Bertolini L, Padovani R, et al. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006;29(6):1325‐1330. [DOI] [PubMed] [Google Scholar]
- 51. Riaz H, Iqbal J, Arif U. Association between non‐alcoholic fatty liver disease (NAFLD) and raised carotid intima‐media thickness (CIMT). Pak J Med Health Sci. 2016;10:1393‐1396. [Google Scholar]
- 52. Rasool A, Dar W, Latief M, Dar I, Sofi N, Khan M. Nonalcoholic fatty liver disease as an independent risk factor for carotid atherosclerosis. Brain Circ. 2017;3(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Targher G, Bertolini L, Padovani R, Zoppini G, Zenari L, Falezza G. Letter to the editor: associations between liver histology and carotid intima‐media thickness in patients with nonalcoholic fatty liver disease. Arterioscler Thromb Vasc Biol. 2005;25(12):2687‐2688. 10.1161/01.Atv.0000189299.61568.79 [DOI] [PubMed] [Google Scholar]
- 54. Xin Z, Zhu Y, Wang S, et al. Associations of subclinical atherosclerosis with nonalcoholic fatty liver disease and fibrosis assessed by non‐invasive score. Liver Int. 2020;40(4):806‐814. [DOI] [PubMed] [Google Scholar]
- 55. Thakur ML, Sharma S, Kumar A, et al. Nonalcoholic fatty liver disease is associated with subclinical atherosclerosis independent of obesity and metabolic syndrome in Asian Indians. Atherosclerosis. 2012;223(2):507‐511. [DOI] [PubMed] [Google Scholar]
- 56. Stolic RV, Trajkovic GZ, Kostic MM, et al. Correlation between nonalcoholic fatty liver and cardiovascular disease in elderly hemodialysis patients. Int Urol Nephrol. 2016;48:883‐889. [DOI] [PubMed] [Google Scholar]
- 57. Salvi P, Ruffini R, Agnoletti D, et al. Increased arterial stiffness in nonalcoholic fatty liver disease: the Cardio‐GOOSE study. J Hypertens. 2010;28(8):1699‐1707. [DOI] [PubMed] [Google Scholar]
- 58. Oni E, Budoff MJ, Zeb I, et al. Nonalcoholic fatty liver disease is associated with arterial distensibility and carotid intima‐media thickness:(from the multi‐ethnic study of atherosclerosis). Am J Cardiol. 2019;124(4):534‐538. [DOI] [PubMed] [Google Scholar]
- 59. Omar N, Koshy M, Hanafiah M, et al. Relationships between severity of steatosis with glycemic control and carotid intima‐media thickness among diabetic patients with ischemic heart disease. J Res Med Sci. 2020;25:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mishra S, Yadav D, Gupta M, Mishra H, Sharma P. A study of carotid atherosclerosis in patients with non‐alcoholic fatty liver disease. Indian J Clin Biochem. 2013;28:79‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ramilli S, Pretolani S, Muscari A, Pacelli B, Arienti V. Carotid lesions in outpatients with nonalcoholic fatty liver disease. World J Gastroenterol. 2009;15(38):4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tan ECL, Tai MLS, Chan WK, Mahadeva S. Association between non‐alcoholic fatty liver disease evaluated by transient elastography with extracranial carotid atherosclerosis in a multiethnic Asian community. JGH Open. 2019;3(2):117‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Targher G, Bertolini L, Padovani R, Zenari L, Zoppini G, Falezza G. Relation of nonalcoholic hepatic steatosis to early carotid atherosclerosis in healthy men. Diabetes Care. 2004;27(10):2498‐2500. 10.2337/diacare.27.10.2498 [DOI] [PubMed] [Google Scholar]
- 64. Poanta LI, Albu A, Fodor D. Association between fatty liver disease and carotid atherosclerosis in patients with uncomplicated type 2 diabetes mellitus. Med Ultrasonogr. 2011;13(3):215‐219. [PubMed] [Google Scholar]
- 65. Vanjiappan S, Hamide A, Ananthakrishnan R, Periyasamy SG, Mehalingam V. Nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and its association with cardiovascular disease. Diab Metab Syndr. 2018;12(4):479‐482. [DOI] [PubMed] [Google Scholar]
- 66. Mikolasevic I, Orlic L, Milic S, Zaputovic L, Lukenda V, Racki S. Non‐alcoholic fatty liver disease proven by transient elastography in hemodialysis patients: is it a new risk factor for adverse cardiovascular events? Blood Purif. 2014;37(4):259‐265. [DOI] [PubMed] [Google Scholar]
- 67. Pinarbasi B, Demir K, Oflaz H, et al. Measurement of the coronary flow velocity reserve in patients with non‐alcoholic fatty liver disease. Turk J Gastroenterol. 2012;23(6):720‐726. 10.4318/tjg.2012.0489 [DOI] [PubMed] [Google Scholar]
- 68. Moon SH, Hong S, Cho YS, et al. Hepatic FDG uptake is associated with future cardiovascular events in asymptomatic individuals with non‐alcoholic fatty liver disease. J Nucl Cardiol. 2017;24(3):892‐899. 10.1007/s12350-015-0297-y [DOI] [PubMed] [Google Scholar]
- 69. Petit JM, Guiu B, Terriat B, et al. Nonalcoholic fatty liver is not associated with carotid intima‐media thickness in type 2 diabetic patients. J Clin Endocrinol Metab. 2009;94(10):4103‐4106. [DOI] [PubMed] [Google Scholar]
- 70. Silaghi CA, Craciun AE, Cosma DT, et al. The impact of non‐alcoholic fatty liver disease on carotid artery intima‐media thickness as a risk factor for atherosclerosis. Bioscientifica. 2015;7(1):56‐62. [PMC free article] [PubMed] [Google Scholar]
- 71. Puig J, Blasco G, Daunis‐I‐Estadella J, et al. Nonalcoholic fatty liver disease and age are strong indicators for atherosclerosis in morbid obesity. Clin Endocrinol. 2015;83(2):180‐186. [DOI] [PubMed] [Google Scholar]
- 72. Zheng J, Zhou Y, Zhang K, et al. Association between nonalcoholic fatty liver disease and subclinical atherosclerosis: a cross‐sectional study on population over 40 years old. BMC Cardiovasc Disord. 2018;18(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang L, Guo K, Lu J, et al. Nonalcoholic fatty liver disease is associated with increased carotid intima‐media thickness in type 1 diabetic patients. Sci Rep. 2016;6(1):26805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guo Y‐C, Zhou Y, Gao X, et al. Association between nonalcoholic fatty liver disease and carotid artery disease in a community‐based Chinese population: a cross‐sectional study. Chin Med J. 2018;131(19):2269‐2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shabbirhussain BV, Singh S, Dixit VK, Verma A, Singh SK. Carotid intima media as predictor of liver fibrosis in type 2 diabetes mellitus with NAFLD. Diab Metab Syndr. 2022;16(7):102560. [DOI] [PubMed] [Google Scholar]
- 76. Cho Y, Park H‐S, Huh BW, et al. Non‐Alcoholic fatty liver disease with sarcopenia and carotid plaque progression risk in patients with type 2 diabetes mellitus. Diabetes Metab J. 2023;47(2):232‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang M, Tang L, Cui X, et al. Shear wave elastography in evaluation of carotid elasticity in the type 2 diabetes mellitus patients with nonalcoholic fatty liver disease. Int J Diabetes Dev Ctries. 2023;43(2):191‐198. [Google Scholar]
- 78. Bessho R, Kashiwagi K, Ikura A, et al. A significant risk of metabolic dysfunction‐associated fatty liver disease plus diabetes on subclinical atherosclerosis. PLoS One. 2022;17(5):e0269265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. R Core Team . R: A language and environment for statistical computing [Internet]. R Foundation for Statistical Computing; 2023. [Google Scholar]
- 80. Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. 10.21105/joss.01686 [DOI] [Google Scholar]
- 81. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw. 2010;36(3):1‐48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 82. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67(1):328‐357. [DOI] [PubMed] [Google Scholar]
- 83. Marchesini G, Moscatiello S, Di Domizio S, Forlani G. Obesity‐associated liver disease. J Clin Endocrinol Metab. 2008;93(11_suppl ment_1):74‐80. [DOI] [PubMed] [Google Scholar]
- 84. Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143(10):722‐728. [DOI] [PubMed] [Google Scholar]
- 85. Marchesini G. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917‐923. [DOI] [PubMed] [Google Scholar]
- 86. Adiels M, Taskinen M‐R, Borén J. Fatty liver, insulin resistance, and dyslipidemia. Curr Diab Rep. 2008;8(1):60‐64. [DOI] [PubMed] [Google Scholar]
- 87. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341‐1350. [DOI] [PubMed] [Google Scholar]
- 88. Asghari A, Jafari F, Jameshorani M, et al. Vitamin D role in hepatitis B: focus on immune system and genetics mechanism. Heliyon. 2022;8:e11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Talens M, Tumas N, Lazarus JV, Benach J, Pericàs JM. What do we know about inequalities in NAFLD distribution and outcomes? A scoping review. J Clin Med. 2021;10(21):5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cho J, Lee I, Park D‐H, Kwak H‐B, Min K. Relationships between socioeconomic status, handgrip strength, and non‐alcoholic fatty liver disease in middle‐aged adults. Int J Environ Res Public Health. 2021;18(4):1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Villanova N, Moscatiello S, Ramilli S, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42(2):473‐480. [DOI] [PubMed] [Google Scholar]
- 92. Wong MYZ, Yap JJL, Sultana R, Cheah M, Goh GBB, Yeo KK. Association between non‐alcoholic fatty liver disease and subclinical atherosclerosis in Western and Asian cohorts: an updated meta‐analysis. Open Heart. 2021;8(2):e001850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Targher G, Bertolini L, Poli F, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54(12):3541‐3546. [DOI] [PubMed] [Google Scholar]
- 94. Hamaguchi M. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13(10):1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Duell PB, Welty FK, Miller M, et al. Nonalcoholic fatty liver disease and cardiovascular risk: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42(6):e168‐e185. [DOI] [PubMed] [Google Scholar]
- 96. Cai J, Zhang S, Huang W. Association between nonalcoholic fatty liver disease and carotid atherosclerosis: a meta‐analysis. Int J Clin Exp Med. 2015;8(5):7673‐7678. [PMC free article] [PubMed] [Google Scholar]
- 97. Targher G, Arcaro G. Non‐alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191(2):235‐240. [DOI] [PubMed] [Google Scholar]
- 98. Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212‐1218. [DOI] [PubMed] [Google Scholar]
- 99. Targher G, Bertolini L, Padovani R, et al. Prevalence of non‐alcoholic fatty liver disease and its association with cardiovascular disease in patients with type 1 diabetes. J Hepatol. 2010;53(4):713‐718. [DOI] [PubMed] [Google Scholar]
- 100. Gaggini M, Morelli M, Buzzigoli E, DeFronzo R, Bugianesi E, Gastaldelli A. Non‐alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5(5):1544‐1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bieghs V, Rensen PCN, Hofker MH, Shiri‐Sverdlov R. NASH and atherosclerosis are two aspects of a shared disease: central role for macrophages. Atherosclerosis. 2012;220(2):287‐293. [DOI] [PubMed] [Google Scholar]
- 102. Ekstedt M, Franzén LE, Mathiesen UL, et al. Long‐term follow‐up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865‐873. [DOI] [PubMed] [Google Scholar]
- 103. Adams LA, Lymp JF, St. Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology. 2005;129(1):113‐121. [DOI] [PubMed] [Google Scholar]
- 104. Madan SA, John F, Pyrsopoulos N, Pitchumoni CS. Nonalcoholic fatty liver disease and carotid artery atherosclerosis in children and adults: a meta‐analysis. Eur J Gastroenterol Hepatol. 2015;27(11):1237‐1248. [DOI] [PubMed] [Google Scholar]
- 105. Sookoian S, Pirola CJ. Non‐alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49(4):600‐607. [DOI] [PubMed] [Google Scholar]
- 106. Targher G, Byrne CD. Clinical review: nonalcoholic fatty liver disease: a novel cardiometabolic risk factor for type 2 diabetes and its complications. J Clin Endocrinol Metab. 2013;98(2):483‐495. [DOI] [PubMed] [Google Scholar]
- 107. Luo J, Xu L, Li J, Zhao S. Nonalcoholic fatty liver disease as a potential risk factor of cardiovascular disease. Eur J Gastroenterol Hepatol. 2015;27(3):193‐199. [DOI] [PubMed] [Google Scholar]
- 108. Kim HC, Kim DJ, Huh KB. Association between nonalcoholic fatty liver disease and carotid intima‐media thickness according to the presence of metabolic syndrome. Atherosclerosis. 2009;204(2):521‐525. [DOI] [PubMed] [Google Scholar]
- 109. Targher G, Bertolini L, Padovani R, et al. Non‐alcoholic fatty liver disease is associated with carotid artery wall thickness in diet‐controlled type 2 diabetic patients. J Endocrinol Invest. 2006;29(1):55‐60. [DOI] [PubMed] [Google Scholar]
- 110. Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima‐media thickness, more accurately predicts coronary artery disease events: a meta‐analysis. Atherosclerosis. 2012;220(1):128‐133. [DOI] [PubMed] [Google Scholar]
- 111. Mathiesen EB, Johnsen SH, Wilsgaard T, Bønaa KH, Løchen M‐L, Njølstad I. Carotid plaque area and intima‐media thickness in prediction of first‐ever ischemic stroke: a 10‐year follow‐up of 6584 men and women: the Tromsø study. Stroke. 2011;42(4):972‐978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set of the extracted measurements, along with R codes for analysis and plotting, can be made available upon request.


