Abstract
Pseudomonas luteola, formerly known as Chryseomonas luteola, is an infrequently encountered aerobic gram-negative bacterium. While it has been identified as a potential human bacterial pathogen, its connection to specific clinical conditions remains limited. Here, we present an exceptional case of a 27-year-old immunocompetent man with acute tonsillitis, who developed P. luteola bacteremia. This unique correlation, not extensively documented in previous studies, sheds light on the potential pathogenicity of P. luteola in patients with acute tonsillitis.
Keywords: Pseudomonas luteola, Bacteremia, Acute tonsillitis
Introduction
Pseudomonas luteola, characterized by its distinctive yellow pigment, is a gram-negative, motile, and strictly aerobic rod. Differentiating from other yellow-pigmented non-fermenters due to its negative oxidase reaction, P. luteola is primarily found in environments with high moisture content [1], [2]. Although infrequently reported in the literature, P. luteola bacteremia has mostly been associated with immunocompromised individuals and those with indwelling catheters. We present a case of P. luteola bacteremia in an immunocompetent patient with acute tonsillitis, a unique occurrence not previously extensively studied [3].
Case report
A 27-year-old man with no known comorbidities; sexually active with single female partner and no history of illicit drug use or animal contact. Arrives to the emergency department (ED) complaining of fever and severe sore throat for three days. Notably, three years prior, he experienced frequent episodes of tonsillitis. The patient was hemodynamically stable at presentation and was discharged while on oral amoxicillin.
However, two days later, the patient experienced worsening symptoms. He returned to the ED and presented with the following vital signs: body temperature, 39 °C; blood pressure, 110/90 mmHg; and pulse rate, 125 beats per minute. Physical assessment revealed the presence of bilateral enlargement of the lymph nodes with erythematous and enlarged tonsils. Furthermore, he had a white blood cell count of 17,000/microliter, neutrophil count of 88%, C-reactive protein (CRP) level of 192 mg/L, erythrocyte sedimentation rate of 61 mm/h and HIV screening was negative. The chest radiograph showed normal findings. Additionally, one set of blood culture was collected on admission.
The patient was admitted to the hospital with severe tonsillitis and treated with 600 mg of clindamycin orally every 8 h. After 24 h, the patient remained febrile 38.4 oC and complained of severe neck pain. Computed tomography of the neck revealed nasopharyngeal adenoid and tonsillar hypertrophy, as well as multiple bilateral enlarged cervical lymph nodes. The patient’s fever persisted until the third day of hospitalization. The CRP levels increased to 256 mg/L.
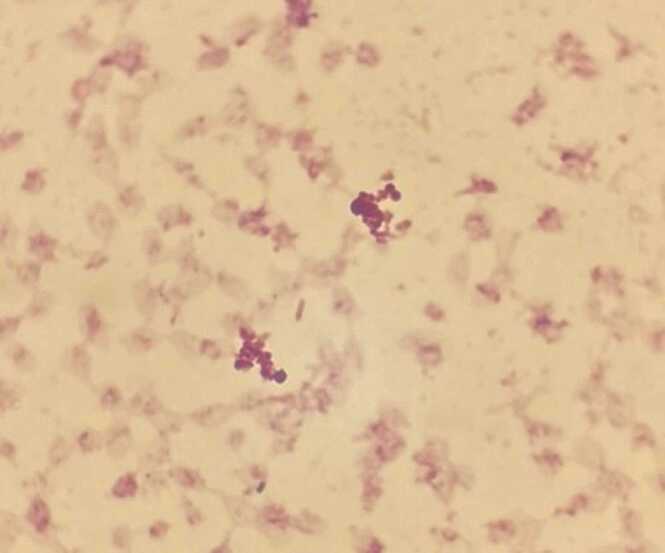
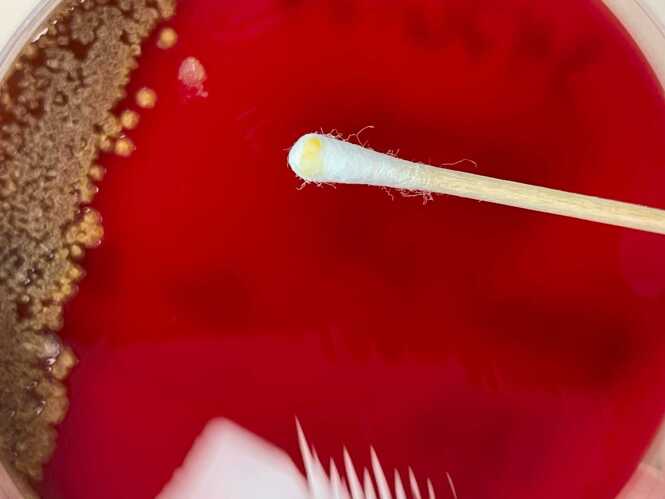
The aerobic and anaerobic bottles were fitted in the BACT/ALERT 3D blood culture system (Biomérieux, Durham, NC, USA). After 29 h, the aerobic bottle turned positive, and the gram stain revealed gram-negative bacilli (Fig. 1). Clindamycin was discontinued, and meropenem was initiated. 24 h later, yellow-colored colonies appeared on blood agar after 24 h (Fig. 2), non-lactose fermenter on Macconkey agar plate. The oxidase test was negative. Using the GN card Vitek 2 (Biomérieux, Inc., Hazelwood, MO), the strain was identified as P. luteola. The antibiotic susceptibility of this organism was examined using the E-test gradient MIC (Biomérieux, France). It demonstrated susceptibility to amikacin, ceftazidime, ciprofloxacin, piperacillin/tazobactam, meropenem and trimethoprim/sulfamethoxazole.
Fig. 1.
Gram stain revealed gram-negative bacilli.
Fig. 2.
Yellow-colored colonies appeared on Blood agar after 24 h.
The patient’s fever subsided 24 h after stating meropenem, and inflammatory biomarker levels began to decline. His condition stabilized, and he was eventually discharged with a 10-day course of ciprofloxacin. Two weeks after discharge, outpatient follow-up showed complete recovery and a plan for elective tonsillectomy with head and neck surgery.
Discussion
P. luteola, previously classified as a CDC group Ve-1, is a gram-negative, aerobic, non-spore-forming, motile, non-lactose fermenting, and oxidase-negative bacillus. This organism grows on MacConkey agar and produces yellow-pigmented colonies [1], [2].
P. luteola is a relatively rare opportunistic pathogen, commonly observed in their saprophytic form in various environments such as soil, water, and other areas with high moisture content. Moreover, they exhibit opportunistic pathogenicity in individuals who have preexisting medical conditions or have indwelling catheters [3].
The use of steroids and other immunosuppressive medications, the presence of a foreign body, and surgical instability predispose patients to develop P. luteola infection. Therefore, nosocomial infections are more common than community-acquired infections [3].
The documented cases encompass a range of conditions such as bacteremia [4], [5], empyema [6], endocarditis [7], peritonitis [8], and meningitis [9]. The number of healthy patients who develop these conditions is relatively few [10], [11]. To the best of our knowledge, this case report is the first to document the occurrence of P. Luteola bacteremia associated with acute tonsillitis in an immunocompetent patient.
A previous retrospective study by Bayhan et al., conducted in a Turkish tertiary care facility, reported an incidence of P. luteola bacteremia in seven pediatric patients over the course of a nine-year period of study. All have congenital diseases or primary immunodeficiencies. Among these patients, the infection was hospital acquired in six and community acquired in one [3].
According to some previous studies, P. luteola exhibits high resistance to trimethoprim-sulfamethoxazole, ceftriaxone, tetracycline, and ampicillin [11], and demonstrates susceptibility to other antibiotics, including ceftazidime, meropenem, aminoglycosides, and ciprofloxacin.
A study was previously conducted on febrile patients with cancer admitted to a referral hospital in Ethiopia to identify unusual bacterial pathogens that cause bloodstream infections, and their medication resistance profiles [12]. Of the 107 adult patients with cancer having fever, 13 (12.2%) had uncommon human infections. P. luteola was isolated from two neutropenic individuals with non-Hodgkin lymphoma. Both isolates were resistant to amoxicillin-clavulanic acid, ampicillin, chloramphenicol, ceftriaxone, and trimethoprim-sulfamethoxazole.
However, the study by Bayhan showed that all P. luteola strains were susceptible to amikacin, gentamicin, trimethoprim-sulfamethoxazole, and meropenem, but resistant to piperacillin-tazobactam, aztreonam, and colistin [3]. Therefore, the P. luteola isolates display different levels of susceptibility to antibiotics.
Conclusion
This unprecedented case underscores the potential for P. luteola to cause bacteremia in patients with acute tonsillitis, even in immunocompetent individuals. The rarity of such occurrences necessitates further research into the underlying mechanisms, treatment strategies, and potential impacts on healthcare systems and patients. This case report serves as a reminder that unusual pathogens can surface in unexpected clinical contexts, prompting a need for ongoing vigilance and research.
Funding
The authors received no specific grant from any funding agency.
Ethical approval
All authors have agreed to authorship, read and approved the manuscript, and given consent for publication of the manuscript.
Consent
Consent to publish was not obtained since the case report does not contain any personal identifiers.
CRediT authorship contribution statement
Sirine Ahmad: Drafted first version of the manuscript and medical care of the patient. Ahmad J. Alzahrani: Laboratory diagnosis and writing the microbiology section. Mohammed Alsaeed: Provided feedback on manuscript and medical care of patient.
Declaration of Competing Interest
All authors report no potential conflicts of interest.
References
- 1.Study.com; 2023 [cited 2023 Jul 25]. Available from: 〈https://study.com/academy/lesson/pseudomonas-luteola-habitat-identification.html〉.
- 2.Çiçek M., Hasçelik G., Müştak H.K., Diker K.S., Şener B. Rutin mikrobiyoloji laboratuvarında Pseudomonas luteola’nın doğru tanısı: İki suş nedeniyle [Accurate diagnosis of Pseudomonas luteola in routine microbiology laboratory: on the occasion of two isolates] Mikrobiyol Bul. 2016;50(4):621–624. doi: 10.5578/mb.27618. [DOI] [PubMed] [Google Scholar]
- 3.Bayhan G.I., Senel S., Tanir G., Ozkan S. Bacteremia caused by Pseudomonas luteola in pediatric patients. Jpn J Infect Dis. 2015;68(1):50–54. doi: 10.7883/yoken.JJID.2014.051. Epub 2014 Nov 25. [DOI] [PubMed] [Google Scholar]
- 4.Sabir M., Jamil B., Ahmed S. Bacteremia associated with central line infection by chryseomonas luteola in a case of recurrent meningiomas. Infect Dis J Pak. 2004;13(4):110–111. [Google Scholar]
- 5.Rastogi S., Sperber S.J. Facial cellulitis and Pseudomonas luteola bacteremia in an otherwise healthy patient. Diagn Microbiol Infect Dis. 1998;32(4):303–305. doi: 10.1016/s0732-8893(98)00082-0. [DOI] [PubMed] [Google Scholar]
- 6.Yousefi F., Shoja S., Honarvar N. Empyema caused by Pseudomonas luteola: a case report. Jundishapur J Microbiol. 2014;7(7) doi: 10.5812/jjm.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Leary T., Fong I.W. Prosthetic valve endocarditis caused by group Ve-1 bacteria (Chromobacterium typhiflavum) J Clin Microbiol. 1984;20(5):995. doi: 10.1128/jcm.20.5.995-.1984. PMID: 6511882; PMCID: PMC271493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor B.J., Kopecky R.T., Frymoyer P.A., Forbes B.A. Recurrent Pseudomonas luteola (CDC group Ve-1) peritonitis in a patient undergoing continuous ambulatory peritoneal dialysis. J Clin Microbiol. 1987;25(6):1113–1114. doi: 10.1128/jcm.25.6.1113-1114.1987. PMID: 3597754; PMCID: PMC269148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kostman J.R., Solomon F., Fekete T. Infections with Chryseomonas luteola (CDC group Ve-1) and Flavimonas oryzihabitans (CDC group Ve-2) in neurosurgical patients. Rev Infect Dis. 1991;13(2):233–236. doi: 10.1093/clinids/13.2.233. [DOI] [PubMed] [Google Scholar]
- 10.Dalamaga M., Karmaniolas K., Chavelas C., Liatis S., Matekovits H., Migdalis I. Pseudomonas luteola cutaneous abscess and bacteraemia in a previously healthy man. Scand J Infect Dis. 2004;36(6–7):495–497. doi: 10.1080/00365540310016196. [DOI] [PubMed] [Google Scholar]
- 11.Barry M. Pseudomonas luteola bacteremia in newly diagnosed systemic lupus erythematosus. Case Rep Infect Dis. 2021:4051378. doi: 10.1155/2021/4051378. PMID: 34434585; PMCID: PMC8382557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arega B., Wolde-Amanuel Y., Adane K., Belay E., Abubeker A., Asrat D. Rare bacterial isolates causing bloodstream infections in Ethiopian patients with cancer. Infect Agent Cancer. 2017 doi: 10.1186/s13027-017-0150-9. PMID: 28702079; PMCID: PMC5504797. [DOI] [PMC free article] [PubMed] [Google Scholar]