Summary
High-grade serous ovarian cancer (HGSOC) is a hormone-related cancer with high mortality and poor prognosis. Based on the transcriptome of 57,444 cells in ascites from 10 patients with HGSOC (including 5 pre-menopausal and 5 post-menopausal patients), we identified 14 cell clusters which were further classified into 6 cell types, including T cells, B cells, NK cells, myeloid cells, epithelial cells, and stromal cells. We discovered an increased proportion of epithelial cells and a decreased proportion of T cells in pre-menopausal ascites compared with post-menopausal ascites. GO analysis revealed the pre-menopausal tumor microenvironments (TME) are closely associated with viral infection, while the post-menopausal TME are mostly related to the IL-17 immune pathway. SPP1/CD44-mediated crosstalk between myeloid cells and B cells, NK cells, and stromal cells mainly present in the pre-menopausal group, while SPP1/PTGER4 -mediated crosstalk between myeloid cells and epithelial cells mostly present in the post-menopausal group.
Subject areas: Microenvironment, Cancer
Graphical abstract

Highlights
-
•
Single-cell atlas of pre- and post-menopausal HGSOC ascites
-
•
SPP1+ macrophages are related to the prognosis of HGSOC
-
•
SPP1/CD44 receptor ligand pairs exist in the pre-menopausal HGSOC ascites cells
-
•
SPP1/PTGER4 receptor ligand pairs exist in the post-menopausal HGSOC ascites cells
Microenvironment; Cancer
Introduction
High-grade serous ovarian cancer (HGSOC) is the most common histological subtype of Epithelial ovarian cancer (EOC), which is the most lethal gynecologic malignancy worldwide.1,2,3 More than 75% of patients with HGSOC are initially diagnosed at advanced stage, and have a relatively low 5-year survival rate (30%) compared with 93% in patients with localized disease.1,4 The occurrence of HGSOC is affected by many factors including aging, family history, chromosomal instability, and genetic risk factors, and so forth.5,6,7 Menopause is the typical sign of women’s senescence,8 and most patients with HGSOC are diagnosed after menopause with a median age of 63∼65 years.7,9,10
As hormone-related diseases, both ovarian cancer and breast cancer are influenced by various hormonal signaling pathways.11,12,13 The burden of breast cancer, both pre-menopausal and post-menopausal, has been significantly rising globally with distinct morbidity, suggesting that it is important to distinguish the breast cancer burden by menopausal status.14 However, it remains unclear how the menopause status is associated with HGSOC. Hormone-related ovarian cancer has different etiologies in the pre-menopausal and post-menopausal age groups.9,11,15 A connection between incessant ovulation, pelvic inflammatory disease related to sex activity and epithelial ovarian carcinogenesis is plausible during premenopausal status,16,17,18 while the accumulation of senescent cells, decrease in sex hormone levels, and age-related physiology variations such as poor immunologic surveillance, excess body weight and obesity contribute to post-menopausal carcinogenesis of ovary.11,19,20,21,22,23,24 Thus, the investigation of ovarian cancer in terms of menopausal status is important to inform prevention and detection initiatives and health care planning.
Ascites is the hallmark symptom of ovarian cancer, which contains complex tumor microenvironments (TME) including cellular and acellular components originating from blood, lymph, or tissue space. The cellular components of ascites include ovarian cancer cells (which may be present as individual cells or spheroids), immune cells (including myeloid cells, lymphocytes, and others), and stromal cells (such as fibroblasts, cancer-associated fibroblasts, endothelial cells, and pericyte), while the non-cellular components include various cytokines and proteins. Interactions between these TME components may contribute to the development and progression of ovarian cancer and lead to pathogenic phenotypes such as metastasis, recurrence, and chemo-resistance.25,26
With the recent advances in next-generation sequencing technology at single-cell resolution, subsequent focus studies of ascites can provide a deeper understanding of intra-tumoral heterogeneity and molecular mechanisms related to ovarian cancer progression, metastasis, and drug-resistance.26,27,28,29,30,31 Here, we explored the tumor microenvironment by detecting 57,444 single-cell transcriptomes from 5 pre-menopausal and 5 patients with post-menopausal HGSOC. We observed that pre-menopausal HGSOC ascites had reduced the proportion of T cells in the immune component and an increased proportion of malignant epithelial cells, compared to post-menopausal tumors. We also showed extensive intercellular communication between epithelial cells and stromal cells in HGSOC, especially in post-menopausal patients. Interestingly, we have found aggregated different immune signaling pathways between the pre- and post-menopausal HGSOC, which may enlighten us in understanding the origination and development of ovarian cancer, and ultimately guide future immunotherapy.
Results
A cell atlas of ascites in patients with pre-menopausal and post-menopausal high-grade serous ovarian cancer
The reliable epidemiological estimates for the menopause give a median age at natural menopause of 48–52 years among women in developed nations.8 Menopausal status was defined using age as an index, whereby female cancer cases or deaths at age 50 years or older were regarded as postmenopausal according to previous study.14 We then analyzed the prognostic data of pre- and post-menopausal women in the cBioPortal Ovarian Serous Cystadenocarcinoma (TCGA, Firehose Legacy) database (http://www.cbioportal.org/) divided by 50 years old and excluded data from patients over 70 years old to eliminate prognostic errors caused by multiple comorbidities in elderly patients. We found that the probability of overall survival of post-menopausal women was significantly lower than that of pre-menopausal women (Figure 1A). As the main symptom of patients with HGSOC, ascites represents the microenvironment within the abdominal cavity, and are associated with recurrent drug resistance. We performed scRNA-seq on individual ascites from 10 treatment-naive patients, including 5 pre-menopausal patients and 5 post-menopausal patients (Figure 1A; Table S1), and the ages of the patients were ranged from 41 to 68 years.
Figure 1.
ScRNA-seq profiling of the pre- and post-menopausal HGSOC ascites environments
(A) Overall survival (OS) of ovarian serous adenocarcinoma between pre- and post-menopausal (50-year-old cutoff value) in the cBioPortal database, and Schematic representation of the experimental strategy.
(B) Uniform Manifold Approximation and Projection (UMAP) plot, showing the annotation and color codes for cell types in the HGSOC ecosystem. and 6 cell types and 14 clusters are shown in (B).
(C) UMAP plot showing patient origins by color.
(D) UMAP plot showing pre- and post-menopause origins by color.
(E) Violin plot showing specific gene expression in various cell types.
(F) Heatmap showing the expression of marker genes in the indicated cell types.
(G) Histogram indicating the proportion of cells in ascites of each analyzed patient.
(H) Histogram showing the fraction of cells in pre-menopausal (green) and post-menopausal (blue) ascites. Data are represented as mean ± SEM.
A total of 57,444 cells were acquired from these samples following standard procedures (see STAR Methods). We identified 14 cell clusters (0–13) which were further classified into 6 cell types (Figure 1B) in ten patients (Figure 1C). All these cell subtypes were shared among the patients of both pre-menopausal and post-menopausal samples (Figures 1D, S1A, and S1B). Based on the expression of known marker genes, we identified cells including myeloid cells (LYZ and C1QB), T cells (CD2 and CD3D/G), B cells (CD79A/B), natural killer (NK) cells (GZMB), epithelial cells (MUC16 and EPCAM) and stromal cells (PDGFRA and COL1A2) (Figures 1E and S1C–S1H). All cell types (both immune and non-immune fractions) expressed the corresponding specific high level of genes (Figure 1F).
We analyzed the proportion of various cells, the proportion of myeloid cells was relatively high in all the patients. The differences in the corresponding cell types and proportions of each cluster of different patients revealed substantial inter-patient heterogeneity of immune cell compositions among HGSOC ascites (Figure 1G). We found that pre-menopausal patients have more epithelial cells and fewer T cells in their ascites compared to post-menopausal patients (Figure 1H). In summary, we have identified the major cell types in pre-menopausal and post-menopausal HGSOC samples, and revealed the differences in the proportions of immune and non-immune cells, which clearly reflects the heterogeneity of HGSOC before and after menopause status.
SPP1+ macrophage and G5 neutrophils play major roles in high-grade serous ovarian cancer ascites
The interaction between myeloid cells and adaptive immunity is also emerging as an important regulator of cancer progression, and tumor-associated myeloid cells (TAMs) probably have an important role in cancer immune evasion.32 But the similarity and difference of their essential properties across different tumors remain elusive. We then performed unsupervised graph-based clustering on myeloid cells and then identified five major cell types (macrophages, neutrophils, DCs, monocytes, and myeloid-cycling) based on canonical cell markers, DCs, and macrophages were further divided into multiple sub-populations (Figure 2A). All cell sub-populations are evenly distributed between different patients and between pre- and post-menopausal groups (Figures 2B and 2C). Macrophages, neutrophils, DCs, monocytes, and cell cycle regulation were characterized by specific high expression of MARCO/CD163, APOBEC3A, HLA/FCER1A, and IDO1, respectively (Figure 2D). Two classical DC subsets were identified, including CD1C+ FCER1A+ DC1 and HLA+ CLEC9A+ DC2. Further clustering of the macrophages gave rise to 3 sub-populations with specific gene signatures, including SPP1+ macrophage, CCL5+ macrophage, and KRT+ macrophage. SPP1+ macrophage and neutrophils account for the largest proportion of myeloid cells in ascites from pre- and post-menopausal patients, accounting for more than 50% of all patients, but the overall difference between groups is not significant, except for the Myeloid-cycling sub-population (Figure 2E). we used slingshot to perform a pseudotime analysis of the Myeloid subgroup without a specified starting point and there are three different trajectories, with the starting subgroup Monocytes, one of them passes through SPP1+macrohage, and end at the subgroup DC2 (Figure S2A). We also depicted the pseudotime distribution of this developmental trajectory, with colors ranging from red to blue indicating the developmental time from early to late stage. It can be seen that SPP1+macrophage is in a later stage of development (Figure S2B). Previous studies have shown that the high expression of SPP1 in ovarian cancer ascites and plasma have been shown to be correlated to poor prognosis and shortened survival.33,34,35 As macrophages can be classically divided into M1 (CD68+) and M2 (CD163+), there are no cell clusters with expression profile similar to M1 cells in our existing data. However, we identified the sub-population of SPP1+ macrophages as M2 by some specifically expressed genes (STAB1, CD180, TREM2), a group of cells that may play a role in promoting tumor progression and may be a subpopulation associated with poor prognosis in ovarian cancer.36,37 SPP1+ macrophage was expressed in both pre- and post-menopausal patients, and accounted for the largest proportion of macrophages, suggesting that M2 and SPP1+ macrophage (the main cause of HGSOC ascitic dissemination and metastasis) might play a major role in HGSOC ascites. The macrophage subpopulation, especially SPP1+ macrophage, showed a more important role compared with other myeloid cells. The presence of M1 (CD68+), M2 (CD163+), and SPP1+ macrophages were confirmed by IF staining (Figures 2F and 2G). Through Gene Ontology and KEGG analysis, we found that myeloid cells in ascites from pre-menopausal patients tend to have functional enrichment related to viral infection, while post-menopausal cells mostly converge in the NF-κB signaling pathway, Toll-such as receptor signaling pathway and IL-17 signaling pathway (Figures 2H and S2C). In the comparison of different genes between groups, we found that the SPP1 was enriched in macrophages from ascites of pre-menopausal patients (Figure 2I),
Figure 2.
Myeloid-derived cell components in pre- and post-menopausal HGSOC ascites
(A) UMAP plot showing the subtypes of myeloid-derived cells derived from HGSOC ascites. Each cluster is color-coded according to cell type. Cluster annotations are indicated in the figure.
(B) UMAP plot showing HGSOC ascites myeloid cells, clustered and color-coded, according to patient. Data are represented as mean ± SEM.
(C) UMAP plot, showing pre- and post-menopausal myeloid cells by color.
(D) Heatmap showing the expression of marker genes in each subtype of myeloid-derived cells.
(E) Histogram indicating the proportion of myeloid subgroups in ascites of pre- and post-menopausal patients.
(F and G) Representative images of IF staining in Formalin-fixed paraffin-embedded (FFPE) ascites cells, indicating CD68+ and CD163+ cells (F), and SPP1+ CD163+ cells (G) in paired pre- and post-menopausal ascites. Scale bar, 10 μm. Bar chart based on IF staining results shows the relative fluorescence intensity of CD163, CD68, and SPP1 in cells. Data are represented as mean ± SEM. All statistical analyses were conducted using paired t-test. n represents the number of cells.
(H) Gene ontology (GO) analysis of pre- (green) and post-menopausal (blue) myeloid cells.
(I) The heatmap indicating the gene expression in the myeloid cell subtypes of pre- and post-menopausal HGSOC ascites.
Meanwhile, we found that the content of neutrophils also accounted for a large part of the whole myeloid cells, next abundant to SPP1+ macrophage. Neutrophils are the first cells to be recruited to the injured or infected sites, and are the main component of the innate immune system. According to the study of Xie et al.,38 neutrophils have been classified into eight subsets (G0-G5c) in bone marrow, tissue, and peripheral blood. Using the genetic markers present in the ref.38,39, we annotated the function of neutrophils in HGSOC ascites, the original neutrophils subgroup was reclassified into G5b and G5c (Figure S2D). G5b subgroup highly expressed interferon-related genes, including (IFI6, IFIT2, IFIT3, IFIT1, and ISG15, which may play important roles in resisting virus infection and inducing cell apoptosis. During the differentiation and maturation process of neutrophils, the expression level of the aging marker CXCR4 was increased in the more mature subset G5c neutrophils. Besides, CCL4, TNFAIP3, CXCL8, and CCL4C2, which code chemokines and inflammatory factors, were expressed at a high level in G5c cells as well, and there are differentially expressed genes between G5b and G5c neutrophils in premenopausal and postmenopausal ascites (Figure S2E).
Lymphocytes cellular profile of the ascites of patients with pre- and post-menopausal high-grade serous ovarian cancer
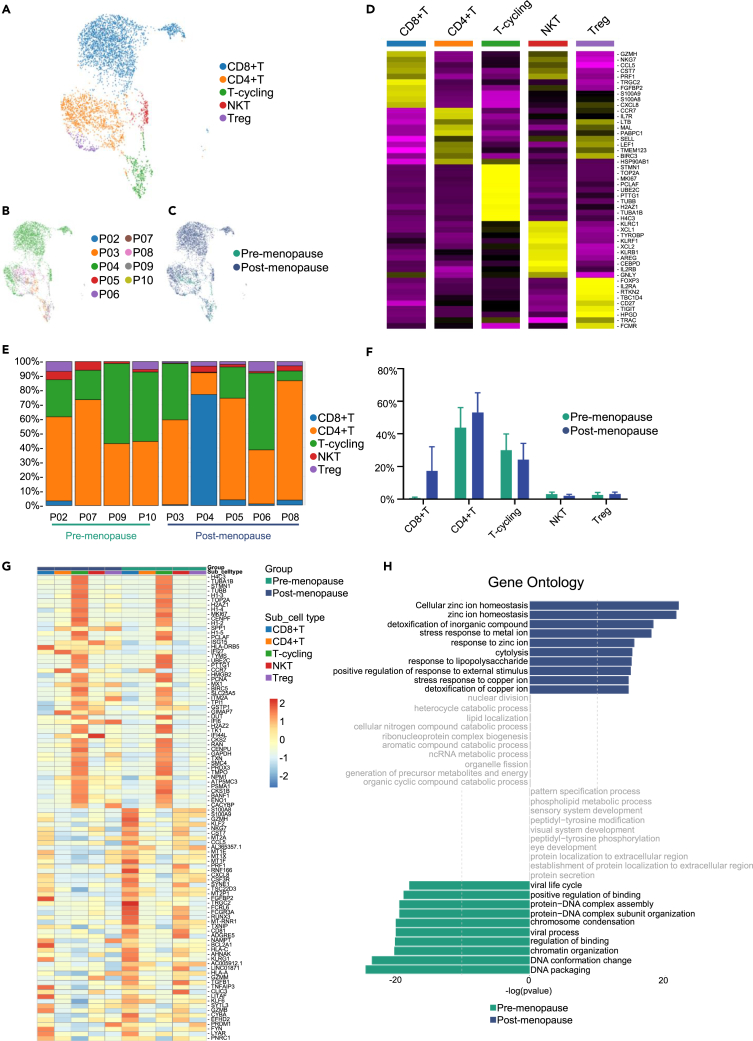
Given the key roles of lymphocytes in tumor immunity, we focused on the characterization of lymphocytes. We subclustered the transcriptomic profiles of 4,556 T cells, 740 B cells and 1,117 NK cells. T cells were most abundant and were divided into five subgroups (CD8+ T, CD4+ T, Treg, NKT, and T-cycling) (Figure 3A). T cells exhibit different proportions between patients and between pre- and post-menopausal groups (Figures 3B and 3C). High expression of CD8A and CD8B was defined as CD8+T, and high expression level of CD4, CCR7, IL7R were defined as CD4+T cells, while Treg cells exhibited high expression level of FOXP3 and IL2RA, another cluster exhibited high expression of NKG7, and GZMH, which was defined as NKT cells, T-cycling cells were defined with high expression of cell-cycling genes such as MKI67, TOP2A, and STMN1, the heatmap of highly expressed genes in each T cells subgroups is shown in Figure 3D. Then, we compared the numbers of T cells between different groups, we demonstrated that much more T cells, especially CD8+T cells were shown in the ascites of post-menopausal patients (Figures 3E and 3F). There are also differences in the transcriptome of T cells in pre- and post-menopausal ascites, as shown in Figure 3G. GO analysis showed stress response to metal ion and KEGG analysis showed natural killer cell-mediated cytotoxicity and antigen processing and presentation of T cell receptor signaling pathway in post-menopausal patients. These results suggested that ascites of post-menopausal HGSOC may activate more immune response (Figures 3H and S3). These results suggest that T cells, especially CD8+T cells, may function in the ascitic immune microenvironment of patients with postmenopausal HGSOC.
Figure 3.
T cells in pre- and post-menopausal HGSOC ascites
(A) UMAP plot showing the subtypes of T cells derived from HGSOC ascites. Each cluster is color-coded according to cell type. Cluster annotations are indicated in the figure.
(B) UMAP plot showing HGSOC ascites T cells, clustered and color-coded, according to patient.
(C) UMAP plot, showing pre- and post-menopausal T cells by color.
(D) Heatmap showing the expression of marker genes in each subtype of T cells.
(E) Histogram indicating the proportion of T cell subgroups in ascites of each analyzed patient.
(F) Histogram of the proportion of T cell subgroups in pre- and post-menopausal HGSOC ascites. Data are represented as mean ± SEM.
(G) The heatmap indicating the gene expression in the T cell subtypes of pre- and post-menopausal HGSOC ascites.
(H) Gene ontology (GO) analysis of pre- (green) and post-menopausal (blue) T cells.
To investigate the B cell subtypes in our samples, B cells were clustered yielding five clusters (Plasma cell, B-cycling, B cell1, B cell 2and B cell3) (Figure 4A) and existed heterogeneity in various subgroups of B cells between patients and between pre- and post-menopausal ascites (Figures 4B and S4A). There is no significant difference in the content and distribution of B cells in pre- and post-menopausal patients (Figure 4C). In the post-menopausal group, gene ontology analysis is mainly reflected in cytoplasmic translation and KEGG results showed that related to ribosomes and COVID-19 immune response. On the contrary, in the pre-menopausal group, we found that gene ontology was clustered in ion-related reactions, and KEGG confirmed that it was associated with mineral absorption (Figures 4D and S4B). The plasma cell cluster highly expressed antibody-related genes (IGHG1, IGHG4, IGKC, and IGHG2), the B-cycling cluster highly expressed STMN1 and HMGB, B cell1 cluster highly expressed FCER1G, TYROBP and S100A family genes, B cell2 cluster highly expressed IL7R and B cell3 cluster expressed KRT family genes (Figure 4E). These results suggest that B cells may contribute to the immune response within the tumor microenvironment. There are significant transcriptome differences between pre- and post-menopausal B cells, and the B cells of post-menopausal group highly express RPS family genes, related to protein translation activity (Figure S4C).
Figure 4.
B cells and NK cells in pre- and post-menopausal HGSOC ascites
(A) UMAP plot showing the subtypes of B cells derived from HGSOC ascites. Each cluster is color-coded according to cell type. Cluster annotations are indicated in the figure.
(B) UMAP plot, showing pre- and post-menopausal B cells by color.
(C) Histogram indicating the proportion of B cell subgroups in ascites of each analyzed patient.
(D) Gene ontology (GO) analysis of pre- (green) and post-menopausal (blue) B cells.
(E) Heatmap showing the expression of marker genes in each subtype of B cells.
(F) UMAP plot showing the subtypes of NK cells derived from HGSOC ascites. Each cluster is color-coded according to cell type. Cluster annotations are indicated in the figure.
(G) UMAP plot, showing pre- and post-menopausal NK cells by color.
(H) Histogram indicating the proportion of NK cell subgroups in ascites of each analyzed patient.
(I) Heatmap showing the expression of marker genes in each subtype of NK cells.
(J) Gene ontology (GO) analysis of pre- (green) and post-menopausal (blue) NK cells.
In HGSOC ascites, four NK subtypes (NK cell1, NK-cycling, NKT, and NK cell2) were defined (Figure 4F) and each subgroup was distributed across different patients and groups (Figures 4G and S4D). Of the four subgroups, NK cell 1 has the largest number, and all subgroups have extensive interpatient and intra-patient heterogeneity (Figure 4H). one cluster showed high expression of MT2A, MT1G, and MT1X, defined as NK cell 1, NK-cycling cells were defined with high expression level of ILI44L and SOX4. NKT cluster exhibited high expression of CD3D and GZMA. NK cell 2 was defined with high expression of LYZ, CD14, and CCL2 (Figure 4I). The gene ontology of NK cells in the post-menopausal group mainly includes cellular response to cadmium ion and KEGG results showed that Th17 cell differentiation and IL-17 signaling pathway are enriched, while both GO and KEGG analysis demonstrated the association of viral infection with the premenopausal status of the patients (Figures 4J and S4E). The differences in the transcriptome of NK cells in pre- and post-menopausal ascites are shown in Figure S4F.
Ecosystematic epithelial and stromal cell features in pre- and post-menopausal patients
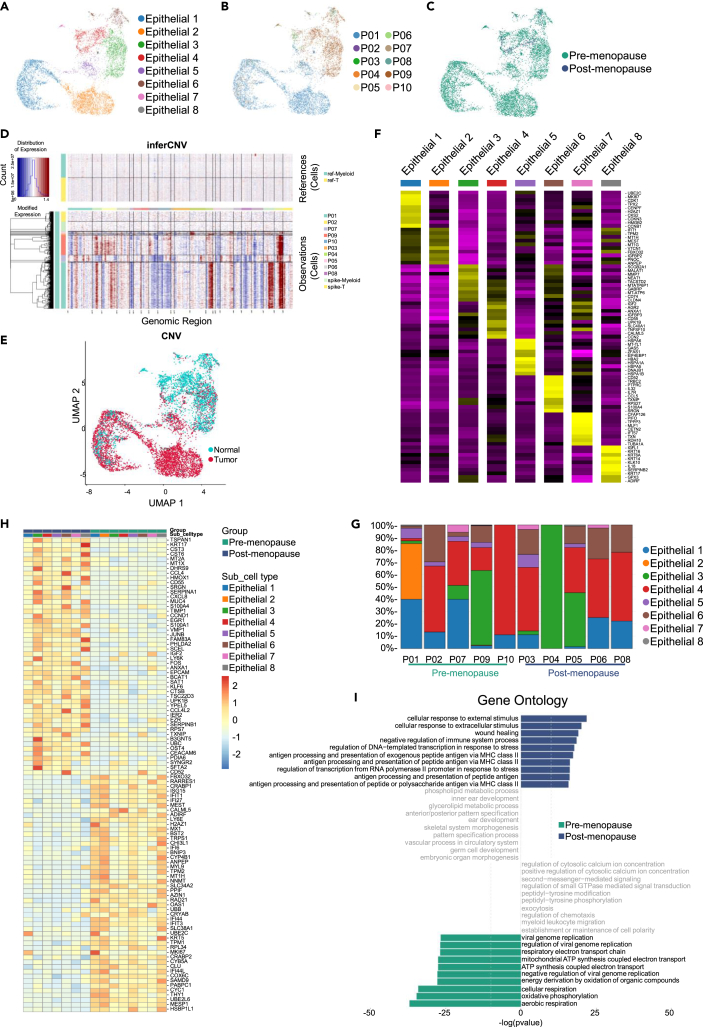
Next, we extracted cells from the epithelial cells and performed hierarchical clustering. A total of 7,870 epithelial cells were collected across all ascites and divided into a set of 8 clusters (Figure 5A), and most epithelial cells are present in the ascites of premenopausal patients (Figures 5B and 5C). Somatic copy-number variations (CNV) for each cell type were analyzed using the R package inferCNV (v1.10.1), and malignant epithelial cells were determined with CNV correlations above 0.5 (Figures 5D and 5E). Our data indicated that most epithelial cells in ascites were malignant cells, and chromosomal region amplification was present in all subgroups. Based on the inferCNVscore results, we refer to Epithelial 1, 2, and 5 clusters as ‘‘cancer epithelial cells’’. MUC16, which is an indicator of advanced disease, was expressed by cluster epithelial 2.40 Epithelial 1 expressed MKI67 and epithelial 6 expressed VIM, indicative of a proliferating population. Epithelial 3 expressed FN1, markers of EMT. Ovarian cancer stem cells, marked by the co-expression of CD33, CD44, CD117, and CD24, were not observed.41 We did not observe clusters of stem cells, likely due to the fact that the cells shed from the primary tumor into the ascites are more likely to be well-differentiated tumor cells rather than stem cells. Ovarian cancer is known to metastasize through dissemination, and ascites plays a role as a flow medium in the process of ovarian cancer metastasis. Therefore, more tumor cells in the ascites of pre-menopausal patients may be inclined to a higher risk of disseminated metastasis. There are significant differentially expressed genes among various epithelial subpopulations (Figure 5F) and all subgroups had significant heterogeneity among patients, which was consistent with the biological characteristics of HGSOC (Figure 5G). The differentially expressed gene profiles between pre- and post-menopausal patients seem to indicate the diverse pathogenicity of HGSOC in different menopausal states (Figure 5H). GO and KEGG analysis revealed that the gene functions of pre-menopausal patients were viral genome repair and energy metabolism, and the functions in post-menopausal patients were mainly antigen presentation, immune and stress responses (Figures 5I and S5A). These results reveal a snapshot of the heterogeneous transcriptional state of pre- and post-menopausal HGSOC tumor cells during the later course of disease (International Federation of Gynecology and Obstetrics [FIGO] stages Ⅲc-Ⅳ).
Figure 5.
Epithelial cells in pre- and post-menopausal HGSOC ascites
(A) UMAP plot showing the subtypes of epithelial cells derived from HGSOC ascites. Each cluster is color-coded according to cell type. Cluster annotations are indicated in the figure.
(B) UMAP plot showing HGSOC ascites epithelial cells, clustered and color-coded, according to patient.
(C) UMAP plot, showing pre- and post-menopausal epithelial cells by color.
(D) The expression value of normal cells is drawn in the top heatmap, and that of ascites cells is drawn in the bottom heatmap. Genes are arranged from left to right on the whole chromosome. The expansion of the chromosome region is shown as red block, while the deletion of the chromosome region is shown as blue block.
(E) InferCNV score shows in UMAP plot and define 0.5 as cutoff judgment, define Normal (blue) and Tumor (red) groups in UMAP plot.
(F) Heatmap showing the expression of marker genes in each subtype of epithelial cells.
(G) Histogram indicating the proportion of epithelial cell subgroups in ascites of each analyzed patient.
(H) The heatmap indicating the gene expression in the epithelial cell subtypes of pre- and post-menopausal HGSOC ascites.
(I) Gene ontology (GO) analysis of pre- (green) and post-menopausal (blue) epithelial cells.
We further investigated the features of stromal cells. Of the 925 cells, 5 cellular clusters emerged (Figure 6A). In ten patients with HGSOC, no stromal cells were detected in the ascites of P09 patient and five subgroups were distributed between pre- and post-menopausal patients (Figures 6B and 6C). Mesenchymal stem cell (MSC) characteristics (CD73, CD90, CD105 e.g.,) were not observed in ascitic stromal cells, as the cells shed were all end-stage differentiated cells. Five subclusters of tumor associated stromal cells were grouped on the basis of each gene expression features. Stromal subclusters 1, 2, 3, 4, and 5 were referred to as FOSB/FOS/JUN+ stromal cells, KRT7/18/19+ stromal cells, TOP2A/CENPF+ stromal cells, GZMA/NKG7+ stromal cells and FCER1G/S100A+ stromal cells, respectively (Figure 6D). In terms of quantity, there was no significant statistical difference in the number of stromal cell subgroups in the ascites of pre- and post-menopausal patients, but only stromal 4 was detected in the ascites of P07 patient (Figures 6E and S5B). GO analysis showed that the gene function of pre-menopausal ascites stromal cells is mainly related to defense response to virus, while after menopause, it is related to the response to mechanical stimulus (Figure 6F). There were significant differences in the transcriptome of stromal cells in pre- and post-menopausal ascites (Figure 6G). To explore the relationship between different cells in HGSOC, we used CellPhoneDB to calculate potential ligand-receptor pairs of each cluster (Figure 7A). Notably, epithelial clusters possessed the most interaction pairs with stromal clusters, revealing the dominant role of stromal cells in tumor microenvironment.
Figure 6.
Stromal cells in pre- and post-menopausal HGSOC ascites
(A) UMAP plot showing the subtypes of stromal cells derived from HGSOC ascites. Each cluster is color-coded according to cell type. Cluster annotations are indicated in the figure.
(B) UMAP plot showing HGSOC ascites stromal cells, clustered and color-coded, according to patient.
(C) UMAP plot, showing pre- and post-menopausal stromal cells by color.
(D) Heatmap showing the expression of marker genes in each subtype of stromal cells.
(E) Histogram indicating the proportion of stromal cell subgroups in pre- and post-menopausal HGSOC ascites. Data are represented as mean ± SEM.
(F) Gene ontology (GO) analysis of pre- (green) and post-menopausal (blue) stromal cells.
(G) The heatmap indicating the gene expression in the stromal cell subtypes of pre- and post-menopausal HGSOC ascites.
Figure 7.
Intercellular communication in pre- and post-menopausal HGSOC ascites
(A–C) The number of acceptor ligand pairs for intercellular communication in pre- (A) and post-menopausal (B) HGSOC ascites cells, and shows the difference between the two groups (post-menopausal minus pre-menopausal) (C).
(D) SPP1-related ligand pairs and their expression differences in pre- and post-menopausal HGSOC ascites.
(E) Violin plot showing the average expression level of differentially expressed ligand genes in pre- and post-menopausal ascites cells.
(F) TNM-plot analysis showing the expression levels of SPP1, PTGER4, ITGA4, ITGB1, and CD44 in ovarian cancer and normal patients.
(G) KM-plot analysis showing the overall survival rate of patients in TCGA RNA-seq (left) and TCGA gene chip of stage Ⅲc-Ⅳ(right) database, characterized by either low (black) or high (red) expression of SPP1. Significance was calculated using the log rank test.
The antigen presentation pathway and IL-17 signaling pathway were activated in the post-menopausal high-grade serous ovarian cancer
In ascites microenvironment, upregulated genes in the pre-menopause and post-menopause patients were enriched related to the antigen presentation pathway and viral infection. However, the interleukin (IL)-17 signaling pathway was most enriched in the upregulated genes of myeloid cell, T cell, NK cell, and stromal cell in the comparison of post-menopause vs. pre-menopause, suggesting that the ascites microenvironment was involved in IL-17 cytokine responses in post-menopausal patients, and IL-17 signaling pathway has been reported prone to be associated with prognosis of ovarian cancer.42,43,44,45,46,47 In generally, the presented antigen during immune-mediated inflammation leads to the differentiation of CD4 helper T cells and stimulates Th17 cells to produce proinflammatory cytokines, such as TNF and IL-17. Many reports have described various cancers with elevated IL-17A-producing cell counts and IL-17A expression, and proved occupancy ratios of Th17 and γδT17 were increased in peripheral blood mononuclear cells (PBMCs) of patients with ovarian cancer. Moreover, neutrophil-to-lymphocyte ratio (NLR) correlated positively with the Th17 occupancy ratio and with both shorter overall survival (OS) and worse progression-free survival (PFS).46
Extensive intercellular communication exists in pre- and post-menopausal high-grade serous ovarian cancer ascites
To uncover the cellular crosstalk in pre- and post-menopausal ascites, we analyzed the receptor–ligand interactions using CellPhoneDB (Figures 7A and 7B). Myeloid cells, epithelial cells, and stromal cells are widely communicated with other types of cells (Figure 7C). And the communication was remarkably strengthened in pre-menopausal HGSOC, especially with epithelial cells (Figure 7C). This finding suggested the communication between macrophages and epithelial cells via receptor–ligand interactions. Results showed that macrophages communicated with all types of cells using SPP1, especially SPP1-a4b1/PTGER4/CD44 interaction, which was identified in pre- and post-menopausal ascites, suggesting a role of macrophage-derived SPP1 in the progression of HGSOC ascites (Figure 7D). We have identified the expression of corresponding receptor ligand genes in cells, where the expression of SPP1 in myeloid cells and CD44 in stromal cells was increased in pre-menopausal ascites. SPP1 is mainly expressed in the macrophage subgroup, indicating that macrophages may be the dominant cell group that plays the role of ligand acceptors (Figures 7E, S6A, and S7A). Although the overall results of B cells showed that CD44 expression was higher in post-menopausal groups, subgroup analysis revealed that CD44 expression was still higher in B cell 1–3 subgroups in pre-menopausal group (Figures 7E, S6B, and S7C). Analysis of RNA-seq data in normal and tumor tissues using the TNM-plot database (https://tnmplot.com/analysis/) revealed a significant increase in the expression of SPP1 in ovarian cancer tissues, suggesting that SPP1 may serve as a potential tumor marker for ovarian cancer (Figure 7F). We verified clinical data from two public databases, we found that overexpression of SPP1 gene is associated with low overall survival in ovarian cancer (Figure 7G), and further demonstrated that SPP1 plays an important role in the progression of ovarian cancer.
Discussion
Recently, steady progress has been made in the surveillance and treatment strategies of HGSOC, while tumor heterogeneity and complex TME resulting in recurrent relapse (including refractory ascites and ileus) still pose great challenge for HGSOC treatment. We still need more data to understand the ascites TME of HGSOC for precise and individualized treatment. A recent study has shown that patients with HGSOC at different ages have different genomic changes such as HRD status.48 Female aging occurs after menopause with significant hormonal alterations and immune system senescence, therefore the pelvic ascites microenvironment and cellular ecosystem variances between pre- and post-menopausal HGSOC are still an enigma to be deciphered.
Here, we provided a comprehensive single-cell transcriptome landscape to characterize the tumor ecosystem of pre- and post-menopausal HGSOC ascites. Our analysis revealed a unique tumor ecosystem of HGSOC ascites in pre-menopausal ascites, characterized by an increase in tumor cells and a decrease in T cells compared to post-menopausal ascites. Suggesting widespread dissemination and metastasis were more frequent in pre-menopausal HGSOC owing to much more exfoliated tumor cells in the ascites. Based on that, we have proposed a hypothesis that the patients with premenopausal HGSOC are likely to present with more extensive pelvic and peritoneal metastasis.
In immune cells of HGSOC ascites, we observed myeloid cell populations at the transcriptome level, in which macrophages predominated. Previous studies have reported the different roles of M1 and M2 macrophage subtypes in the progression of ovarian cancer, and M2 subtype macrophage cells play a role in chemotherapy resistance and metastasis.49 Our study found that M2-like macrophages (SPP1+ macrophages) account for 40% of myeloid cells, and there was no difference in the pre- and post-menopausal groups of HGSOC ascites. SPP1 (secreted phosphoprotein 1), also called OPN (osteopontin), expressed in various types of cells including macrophages, other immune cells, and epithelial cells. SPP1 has alternative splicing isoforms (OPN-a, OPN-b, OPN-c, OPN-d, OPN-e, and OPN-f), and the functions of its isoform are heterogeneous in a tumor-specific manner. OPN-c modulates cell cycle, apoptosis, adhesion, angiogenesis, invasion, and metastasis in breast, prostate, and ovarian cancer.50,51 As a related indicator of poor prognosis and shortened survival in ovarian cancer,33,34,35 SPP1 is also related with inflammation, tumor progression, bone metastasis and drug resistance as reported via cross talk with its receptors such as CD44 and αvβ3 mediated downstream signals.52,53,54 The interaction of SPP1 and CD44 can induce cell adhesion, movement and promote cancer stemness–like phenotypes to enhance tumor progression and metastases.55,56 Notably, we found that SPP1-CD44 is widely expressed in pre- and post-menopausal HGSOC ascites, especially higher in the pre-menopausal group; while SPP1-PTGER4 is higher in the post-menopausal group. Besides, SPP1 is mostly distributed in macrophages of the ascites, and SPP1-CD44 is highly expressed between myeloid cells and B cells, NK cells, and stromal cells in the pre-menopausal group, while SPP1-PTGER4 was highly expressed between myeloid cells and epithelial cells in the post-menopausal group. The role of neutrophils in tumors has also been extensively explored in recent studies. We found that neutrophils in HGSOC ascites are all differentiated and mature neutrophils, indicating that the inflammatory environment of ascites has a catalytic effect on the development of tumors and may be a target for the treatment of advanced HGSOC in the future.
According to GO and KEGG analysis, we found that cells of pre-menopausal HGSOC ascites are most related to the viral infection pathway, while cells in post-menopausal HGSOC ascites are related to the IL-17 immune pathway, suggesting that chronic infection and IL-17 pathway may be involved in the occurrence and development of ovarian cancer. Because pre-menopausal women have normal menstrual cycles and more active sexual activity, high occurrence of pelvic infection and fluctuating hormone levels might lead to malignant transformation of the pelvic microenvironment and carcinogenesis. While IL-17 produced by tumor microenvironment can promote the self-renewal of CD133+cancer stem cells in ovarian cancer.42 Therefore, IL-17 may be a potential therapeutic target for post-menopausal ovarian cancer. Although our study has shown significant differences in the proportion of ascitic tumor cells in patients with pre- and post-menopausal HGSOC, it may also be related to our small sample sizes. ScRNA-seq has revolutionized the way we understand cancer progression, so we used this powerful technology to explore the pathogenesis of HGSOC before and after menopause. Nevertheless, our results still face many challenges and limitations, and the conclusions still need to be verified by a large number of clinical samples owing to the heterogeneity of tumor cells.
In summary, these scRNA-Seq data of ascites collected from pre-menopausal and post-menopausal HGSOC cases represent the first attempt to characterize the impact of menopause status on the cell diversity of ovarian cancer. Clearly, more detailed research is required in a larger dataset, especially on the diversity of lymphocytes and epithelial cells. We propose that the comprehensive assessment of HGSOCs, especially those with different menopausal statuses, will open a new window for understanding the diagnosis, prevention, and treatment of HGSOCs.
Limitations of study
Our results still face some challenges and limitations. Firstly, our conclusions are based on a small sample, and need to be verified by a large clinical sample to eliminate the heterogeneity of tumor microenvironments from different patients. Secondly, a single-cell landscape of pre- and post-menopausal high-grade serous ovarian cancer tissues might also be performed to strengthen the comparison for our better understanding of HGSOC. Finally, the relationship of ascites tumor microenvironments of HGSOC with gene mutations such as BRCA1/2 should also be considered for the pre- and post-menopausal patients, as for the germline BRCA1 mutation were more likely found in the pre-menopausal patients. However, we can well understand the multiplexed tumor microenvironments of ovarian cancer from the technologies newly springing up in the future.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Monoclonal mouse anti-human CD68 | Invitrogen | Cat# 14-0688-82; RRID:AB_11151139 |
| Polyclonal rabbit anti-human CD163 | Invitrogen | Cat# PA5-109327; RRID:AB_2854738 |
| Monoclonal mouse anti-human Osteopontin | Invitrogen | Cat# MA5-17180; RRID:AB_2538651 |
| Biological samples | ||
| Single cell suspension of ascites | the First Affiliated Hospital of University of Science and Technology of China | N/A |
| Paraffin cell section of patients’ ascites | the First Affiliated Hospital of University of Science and Technology of China | N/A |
| Critical commercial assays | ||
| 4 0,6-diamidino-2-phenylindole (DAPI) | Sigma-Aldrich | Cat# D9542 |
| VECTASHIELD mounting medium | Vector lab | Cat# H-1000 |
| Single Cell RNA-seq Kit | Singleron iotechnologies | N/A |
| Deposited data | ||
| Processed expression data files for scRNA-seq | This paper | GSA: HRA004993 |
| Software and algorithms | ||
| Seurat (v4.1.1) | Stuart et al.57 | https://github.com/satijalab/seurat/releases/tag/v3.0.0 |
| inferCNV (v1.10.1) | Patel et al.58 | https://github.com/broadinstitute/inferCNV |
| CellphoneDB (v2.0.0) | Vento-Tormo et al.59 | https://github.com/Teichlab/cellphonedb |
| Monocle2 (v2.22.0) | Qiu et al.60 | https://github.com/cole-trapnell-lab/monocle-release |
| slingshot 2.2.1 | Street et al.61 | https://github.com/kstreet13/slingshot |
| R4.1.2 | R Core | https://www.r-roject.org |
| GraphPad Prism 8.0.2 software | GraphPad Prism Software Inc. | https://www.graphpad.com |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Ying Zhou (caddiezy@ustc.edu.cn).
Materials availability
This study did not generate new unique reagents and all materials in this study are commercially available.
Experimental model and study participant details
Ethics approval and consent to participate
This study has got ethics approval and consent from the First Affiliated Hospital of University of Science & Technology of China ethics committee (2021-KY108).
Patients and collection of samples
A total of 10 patients were pathologically diagnosed with primary HGSOC at stage Ⅲc-Ⅳ at the First Affiliated Hospital of University of Science and Technology of China and were enrolled in this study, and the demographics of the patients are shown in Table S1 (the definition of post-menopause is more than one year of natural menopause). Before surgery, informed consent for the collection of clinical information and tissue collection was obtained from each patient. De-identified patient information, including ovarian cancer histology, stage, and treatment history was collected. Specimens were collected from patients, and ascites were drained by gynecological surgeons and transferred for further processing in closed-vacuum bottles. These samples were paraffin-embedded for immunofluorescence. Ethical approvals were obtained from the ethics committee of the First Affiliated Hospital of the University of Science and Technology of China(2021-KY108).
Method details
Sample handling
The 10 cases of malignant ascites were centrifuged to collect the pelleted cells, which were then suspended in erythrocyte lysis buffer (Solarbio R1010) for 15 minutes to lyse the erythrocytes. Then the cell suspensions were pooled and pipetted into a new 50-ml conical tube through a 100 μm mesh, spun for 5 min at 500 g at 4°C and resuspended in 1×PBS to prepare for droplet-based scRNA-seq.
Immunofluorescence
Fixation of ascites cell precipitation with 4% Paraformaldehyde for paraffin embedding, FFPE cell blocks from 6 patients (Validation cohort, Table S2) who had HGSOC ascites were used for IF staining, all patients signed an informed consent form before the surgery to allow us to use their intraoperative specimens for subsequent experiments. FFPE blocks were cut into 3μm thick sections and dewaxed in xylene, and rehydrated in alcohol, followed by heat-induced antigen retrieval in high pressure and 95-99°C trisodium citrate dihydrate (3g/L) and citric acid monohydrate (0.4g/L) (pH 6.0) for 2 min. After washing, sections were incubated with 5% BSA in PBS for 30 min at room temperature to block nonspecific binding. Subsequently, slides were incubated overnight at 4 °C with the primary antibody, and then incubated at room temperature for 2 hours in a dark chamber with the fluorophore-conjugated secondary antibody diluted in PBS with 5% BSA. The following antibodies were used: Monoclonal mouse anti-human CD68 (1:1000, 14-0688-82, Invitrogen), Polyclonal rabbit anti-human CD163 (1:100, PA5-109327, Invitrogen), Monoclonal mouse anti-human Osteopontin (SPP1) (1:500, MA5-17180, Invitrogen), Alexa Fluor 546 cross-adsorbed goat anti-mouse (1:100, A-11003, Invitrogen), Alexa Fluor 488-conjugated goat anti-rabbit (1:100, ab150077, Abcam). Finally, slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (F6057, Sigma Aldrich). Drop mounting medium for fluorescence (VECTASHIELD H-1000, Vector lab) on the slide and mounted coverslips. We used ZEISS 980 for image acquisition, ZEN 2.3 (blue edition) image analysis software was used to separate positive from negative cells.
Single cell RNA library preparation and sequencing
Cell suspensions were barcoded through the Singleron Single Cell platform following the Singleron GEXSCOPEˆTM protocol32 using a microfluidic chip (GEXSCOPEˆTM Single Cell RNA-seq Kit, Singleron Biotechnologies). Single cell RNA libraries were constructed according to the manufacturer’s instructions (Singleron Biotechnologies). The resulting sequencing was performed on Illumina HiSeq X10 with 150 bp paired-end reads.
Single cell RNA-seq data processing
Sequencing data were aligned using Selescope (Singleron Biotechnologies) version 2.2.1, developed from Singleron, with default parameters against the GRCh38 human reference genome downloaded from the 10x Genomics official website.
We applied the Seurat package version 3 to import 10x data with default parameters and filtered genes expressed in no more than 3 cells. We also performed strict quality control to filter out cells that showed (1) a number of unique genes less than 200 or more than 7000, or (2) a percentage of reads mapping to the mitochondrial genome more than 20 percent. Additionally, we applied DoubletFinder33 to identify doublets with 15 principal components and default threshold. In order to recover sharper biological distribution, we normalize the count data using the function NormalizeData built in the R package Seruat.34
Data integration and batch effect correction
To remove sample-specific subgroups caused by batch effect, we used the Harmony Integration algorithm to integrate our data. We performed SCTransform normalization and selected 2000 most variable genes separately for each sample in the Seurat Integration process.
Data clustering, differential expression analysis and annotation
The procedures for dimensionality reduction, clustering and identifying differentially expressed genes were performed using the Seurat package. We first calculated the PCA matrix based on the integrated data using the RunPCA function in the Seurat standard workflow. Next, we computed the k-nearest neighbors using the FindNeighbors function with 20 components to construct a shared nearest neighbor graph between every cell and its 20 nearest neighbors. The resulting nearest neighbor graph was then used to find clusters by the Louvain algorithm35 using the FindClusters function with 0.1 resolution. Uniform Manifold Approximation and Projection (UMAP) was analyzed using the RunUMAP function with 20 components for the visualization of the clustering result.
To identify the differentially expressed genes (DEGs) in different clusters or metastasis groups, we performed differential expression analysis. These DEGs or cluster-specific biomarkers were obtained using the FindAllMarkers function with default parameters and the following thresholds: at least 0.5-fold difference (log-scale) between the two groups of cells, minimum fraction of genes expressing in both clusters > 0.25, and only return positive markers.
We performed machine learning algorithm to predict the cell type of each cell. We annotated the cell type by analyzing the expression of the recognized markers, and obtained 6 major cell types including Myeloid cells (C1QB, LYZ), T cells (CD2, CD3G, CD3D), B cells (CD79B, CD79A), NK cells (GZMB), Epithelial cells (MUC16, EPCAM), and Stromal cells (PDGFRA, COL1A2). These major cell types were further classified into 35 sub-clusters using higher resolution in the Find Clusters function with relative cell lineages.
Gene ontology enrichment analysis
We defined specific differentially expressed genes (DEGs) in pre-menopause group and post-menopause group, respectively, of Myeloid /Epithelial /T/NK /Stromal and B sub-cell types. Based on these DEGs, enriched gene ontology (GO) terms or KEGG (Kyoto Encyclopedia of Genes and Genomes) were then acquired for each sub-cluster or group using the cluster Profiler package36 with default parameters. Annotation Database org. Hs. eg. db was performed to map these DEGs, and the visualization was shown by bar plot.
InferCNV and copyKAT
InferCNV inferred chromosomal variation by exploring the intensity of gene expression at different locations in the tumor genome compared to a reference set of normal cells. Specify healthy cells as a reference to calculate the amplification or deletion of chromosome regions in each sample. For the copy number change matrix generated by the inferCNV analysis, we normalized it and selected genes with copy numbers in the top95%, and then calculated the Pearson correlation coefficient between the copy numbers of the top95% genes in the cell and the mean values of all genes in the row. We calculated an additional inferCNVscore and artificially defined a correlation of 0.5 as the cutoff to determine whether the cell is a cancer cell. Visualize the above results by representing the correlation between a cell pair and cells with highly active chromosomal changes.
CopyKAT: Identifying genome-wide aneuploidy in single cells and identifying aneuploidy as cancer cells.37
Cytokine/receptor interaction analysis
To test whether the cell-cell interaction between pre-menopause group and post-menopause group was different, we applied the CellphoneDB38 algorithm to the single-cell RNA sequencing profiles between groups to evaluate the effects of ligand/receptor interactions based on the expression of ligands in one cell type and the corresponding receptor expression in another cell type. We focused on the significant interactions in pre-menopause group compared with post-menopause group and picked the relative cytokine/receptor pairs.
Quantification and statistical analysis
To identify differentially expressed genes between two groups of cells, we used a Wilcoxon Rank Sum test. To determine whether the fraction of cell types was significantly different between two conditions, we applied a one-way ANOVA test. We also used the Benjamini Hochberg algorithm to correct the p-values to identify the GO terms and KEGG. Survival curves were plotted using the Kaplan-Meier analysis, and the log rank test was utilized to examine significant differences. Statistical analyses were undertaken using GraphPad Prism 8.0.2 software (GraphPad Prism Software Inc., San Diego, CA, USA). p < 0.05 was considered statistically significant.
Acknowledgments
We would like to thank HanGene Biotech for technical assistance and grateful to all the patients who participated in this study.
Funding: This study was supported by the National Key R&D Program of China (2022YFC2403400 and 2019YFA0802600), the National Natural Science Foundation of China (82172773 ,82303588 and 32270590); Anhui Provincial Key Research and Development Projects (2022e07020013); and Natural Science Foundation of Anhui Province (2208085QH252), the 2020 USTC Affiliated Hospital Introduction Project to Medical Leading Technology (2020LXJS-05), Beijing Science & Technology Innovation Fund (KC2021-JX-0186-143), Natural Science Research Project of Colleges and Universities in Anhui Province (2022AH051255).
Author contributions
YZ, JWF, XYH, LC, YGZ, YJL, YYW, TJZ, and FN: study concept and design and critical revision of the article. WYC and HYL: drafting of the article. YYZ, JXY, and JWF: acquisition of data, analysis, and interpretation of data. WYC, LLQ, and YL: collection of clinical specimens. CG and YZ: study supervision.
All authors read and approved the final article.
Declaration of interests
The authors declare that they have no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: August 25, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107712.
Contributor Information
Chuang Guo, Email: gchuang@ustc.edu.cn.
Ying Zhou, Email: caddiezy@ustc.edu.cn.
Supplemental information
Data and code availability
-
•
Data: Our raw data has been uploaded to the National Genomics Data Center with the number HRA004993 (https://ngdc.cncb.ac.cn/), any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
-
•
Code: This study does not report any original code.
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Miller K.D., Nogueira L., Devasia T., Mariotto A.B., Yabroff K.R., Jemal A., Kramer J., Siegel R.L. Cancer treatment and survivorship statistics, 2022. CA A Cancer J. Clin. 2022;72:409–436. doi: 10.3322/caac.21731. [DOI] [PubMed] [Google Scholar]
- 3.Xia C., Dong X., Li H., Cao M., Sun D., He S., Yang F., Yan X., Zhang S., Li N., Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin. Med. J. 2022;135:584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lheureux S., Braunstein M., Oza A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA A Cancer J. Clin. 2019;69:280–304. doi: 10.3322/caac.21559. [DOI] [PubMed] [Google Scholar]
- 5.Sisodia R.C., Del Carmen M.G. Lesions of the Ovary and Fallopian Tube. N. Engl. J. Med. 2022;387:727–736. doi: 10.1056/NEJMra2108956. [DOI] [PubMed] [Google Scholar]
- 6.Natanzon Y., Goode E.L., Cunningham J.M. Epigenetics in ovarian cancer. Semin. Cancer Biol. 2018;51:160–169. doi: 10.1016/j.semcancer.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torre L.A., Trabert B., DeSantis C.E., Miller K.D., Samimi G., Runowicz C.D., Gaudet M.M., Jemal A., Siegel R.L. Ovarian cancer statistics, 2018. CA A Cancer J. Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis S.R., Lambrinoudaki I., Lumsden M., Mishra G.D., Pal L., Rees M., Santoro N., Simoncini T. Nat. Rev. Dis. Prim. 2015;1 doi: 10.1038/nrdp.2015.4. [DOI] [PubMed] [Google Scholar]
- 9.Matulonis U.A., Sood A.K., Fallowfield L., Howitt B.E., Sehouli J., Karlan B.Y. Ovarian cancer. Nat. Rev. Dis. Prim. 2016;2 doi: 10.1038/nrdp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuroki L., Guntupalli S.R. Treatment of epithelial ovarian cancer. BMJ. 2020;371:m3773. doi: 10.1136/bmj.m3773. [DOI] [PubMed] [Google Scholar]
- 11.Moorman P.G., Calingaert B., Palmieri R.T., Iversen E.S., Bentley R.C., Halabi S., Berchuck A., Schildkraut J.M. Hormonal risk factors for ovarian cancer in premenopausal and postmenopausal women. Am. J. Epidemiol. 2008;167:1059–1069. doi: 10.1093/aje/kwn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulkoyluoglu-Cotul E., Arca A., Madak-Erdogan Z. Crosstalk between Estrogen Signaling and Breast Cancer Metabolism. Trends Endocrinol. Metabol. 2019;30:25–38. doi: 10.1016/j.tem.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Henderson B.E., Feigelson H.S. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 14.Heer E., Harper A., Escandor N., Sung H., McCormack V., Fidler-Benaoudia M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Global Health. 2020;8:e1027–e1037. doi: 10.1016/S2214-109X(20)30215-1. [DOI] [PubMed] [Google Scholar]
- 15.Wentzensen N., Poole E.M., Trabert B., White E., Arslan A.A., Patel A.V., Setiawan V.W., Visvanathan K., Weiderpass E., Adami H.O., et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J. Clin. Oncol. 2016;34:2888–2898. doi: 10.1200/JCO.2016.66.8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming J.S., Beaugié C.R., Haviv I., Chenevix-Trench G., Tan O.L. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: revisiting old hypotheses. Mol. Cell. Endocrinol. 2006;247:4–21. doi: 10.1016/j.mce.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Idahl A., Le Cornet C., González Maldonado S., Waterboer T., Bender N., Tjønneland A., Hansen L., Boutron-Ruault M.C., Fournier A., Kvaskoff M., et al. Serologic markers of Chlamydia trachomatis and other sexually transmitted infections and subsequent ovarian cancer risk: Results from the EPIC cohort. Int. J. Cancer. 2020;147:2042–2052. doi: 10.1002/ijc.32999. [DOI] [PubMed] [Google Scholar]
- 18.Paavonen J., Turzanski Fortner R., Lehtinen M., Idahl A. Chlamydia trachomatis, Pelvic Inflammatory Disease, and Epithelial Ovarian Cancer. J. Infect. Dis. 2021;224:S121–S127. doi: 10.1093/infdis/jiab017. [DOI] [PubMed] [Google Scholar]
- 19.Jeon S.Y., Hwang K.A., Choi K.C. Effect of steroid hormones, estrogen and progesterone, on epithelial mesenchymal transition in ovarian cancer development. J. Steroid Biochem. Mol. Biol. 2016;158:1–8. doi: 10.1016/j.jsbmb.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Tung K.H., Wilkens L.R., Wu A.H., McDuffie K., Nomura A.M.Y., Kolonel L.N., Terada K.Y., Goodman M.T. Effect of anovulation factors on pre- and postmenopausal ovarian cancer risk: revisiting the incessant ovulation hypothesis. Am. J. Epidemiol. 2005;161:321–329. doi: 10.1093/aje/kwi046. [DOI] [PubMed] [Google Scholar]
- 21.Shafrir A.L., Babic A., Tamimi R.M., Rosner B.A., Tworoger S.S., Terry K.L. Reproductive and hormonal factors in relation to survival and platinum resistance among ovarian cancer cases. Br. J. Cancer. 2016;115:1391–1399. doi: 10.1038/bjc.2016.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maomao C., He L., Dianqin S., Siyi H., Xinxin Y., Fan Y., Shaoli Z., Changfa X., Lin L., Ji P., Wanqing C. Current cancer burden in China: epidemiology, etiology, and prevention. Cancer Biol. Med. 2022;19:1121–1138. doi: 10.20892/j.issn.2095-3941.2022.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davenport C., Rai N., Sharma P., Deeks J.J., Berhane S., Mallett S., Saha P., Champaneria R., Bayliss S.E., Snell K.I., Sundar S. Menopausal status, ultrasound and biomarker tests in combination for the diagnosis of ovarian cancer in symptomatic women. Cochrane Database Syst. Rev. 2022;7:CD011964. doi: 10.1002/14651858.CD011964.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Prieto M., Sánchez-Borrego R., Lubián-López D.M., Pérez-López F.R. Etiopathogenesis of ovarian cancer. An inflamm-aging entity? Gynecol. Oncol. Rep. 2022;42 doi: 10.1016/j.gore.2022.101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ford C.E., Werner B., Hacker N.F., Warton K. The untapped potential of ascites in ovarian cancer research and treatment. Br. J. Cancer. 2020;123:9–16. doi: 10.1038/s41416-020-0875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izar B., Tirosh I., Stover E.H., Wakiro I., Cuoco M.S., Alter I., Rodman C., Leeson R., Su M.J., Shah P., et al. A single-cell landscape of high-grade serous ovarian cancer. Nat. Med. 2020;26:1271–1279. doi: 10.1038/s41591-020-0926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornburg M., Desbois M., Lu S., Guan Y., Lo A.A., Kaufman S., Elrod A., Lotstein A., DesRochers T.M., Munoz-Rodriguez J.L., et al. Single-cell dissection of cellular components and interactions shaping the tumor immune phenotypes in ovarian cancer. Cancer Cell. 2021;39:928–944.e6. doi: 10.1016/j.ccell.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Olalekan S., Xie B., Back R., Eckart H., Basu A. Characterizing the tumor microenvironment of metastatic ovarian cancer by single-cell transcriptomics. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109165. [DOI] [PubMed] [Google Scholar]
- 29.Regner M.J., Wisniewska K., Garcia-Recio S., Thennavan A., Mendez-Giraldez R., Malladi V.S., Hawkins G., Parker J.S., Perou C.M., Bae-Jump V.L., Franco H.L. A multi-omic single-cell landscape of human gynecologic malignancies. Mol. Cell. 2021;81:4924–4941.e10. doi: 10.1016/j.molcel.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Z., Artibani M., Alsaadi A., Wietek N., Morotti M., Shi T., Zhong Z., Santana Gonzalez L., El-Sahhar S., Carrami E.M., et al. The Repertoire of Serous Ovarian Cancer Non-genetic Heterogeneity Revealed by Single-Cell Sequencing of Normal Fallopian Tube Epithelial Cells. Cancer Cell. 2020;37:226–242.e7. doi: 10.1016/j.ccell.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Nath A., Cosgrove P.A., Mirsafian H., Christie E.L., Pflieger L., Copeland B., Majumdar S., Cristea M.C., Han E.S., Lee S.J., et al. Evolution of core archetypal phenotypes in progressive high grade serous ovarian cancer. Nat. Commun. 2021;12:3039. doi: 10.1038/s41467-021-23171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engblom C., Pfirschke C., Pittet M.J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer. 2016;16:447–462. doi: 10.1038/nrc.2016.54. [DOI] [PubMed] [Google Scholar]
- 33.Bao L.H., Sakaguchi H., Fujimoto J., Tamaya T. Osteopontin in metastatic lesions as a prognostic marker in ovarian cancers. J. Biomed. Sci. 2007;14:373–381. doi: 10.1007/s11373-006-9143-1. [DOI] [PubMed] [Google Scholar]
- 34.Cerne K., Hadzialjevic B., Skof E., Verdenik I., Kobal B. Potential of osteopontin in the management of epithelial ovarian cancer. Radiol. Oncol. 2019;53:105–115. doi: 10.2478/raon-2019-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schorge J.O., Drake R.D., Lee H., Skates S.J., Rajanbabu R., Miller D.S., Kim J.H., Cramer D.W., Berkowitz R.S., Mok S.C. Osteopontin as an adjunct to CA125 in detecting recurrent ovarian cancer. Clin. Cancer Res. 2004;10:3474–3478. doi: 10.1158/1078-0432.CCR-03-0365. [DOI] [PubMed] [Google Scholar]
- 36.Cheng S., Li Z., Gao R., Xing B., Gao Y., Yang Y., Qin S., Zhang L., Ouyang H., Du P., et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell. 2021;184:792–809.e23. doi: 10.1016/j.cell.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Pittet M.J., Michielin O., Migliorini D. Clinical relevance of tumour-associated macrophages. Nat. Rev. Clin. Oncol. 2022;19:402–421. doi: 10.1038/s41571-022-00620-6. [DOI] [PubMed] [Google Scholar]
- 38.Xie X., Shi Q., Wu P., Zhang X., Kambara H., Su J., Yu H., Park S.Y., Guo R., Ren Q., et al. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat. Immunol. 2020;21:1119–1133. doi: 10.1038/s41590-020-0736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y., Huang J., Guo Z., Zhu Z., Shao Y., Li L., Yang Y., Yu Y., Liu L., Sun B. Primitive genotypic characteristics in umbilical cord neutrophils identified by single-cell transcriptome profiling and functional prediction. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.970909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thériault C., Pinard M., Comamala M., Migneault M., Beaudin J., Matte I., Boivin M., Piché A., Rancourt C. MUC16 (CA125) regulates epithelial ovarian cancer cell growth, tumorigenesis and metastasis. Gynecol. Oncol. 2011;121:434–443. doi: 10.1016/j.ygyno.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 41.Klemba A., Purzycka-Olewiecka J.K., Wcisło G., Czarnecka A.M., Lewicki S., Lesyng B., Szczylik C., Kieda C. Surface markers of cancer stem-like cells of ovarian cancer and their clinical relevance. Contemp. Oncol. 2018;22:48–55. doi: 10.5114/wo.2018.73885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiang T., Long H., He L., Han X., Lin K., Liang Z., Zhuo W., Xie R., Zhu B. Interleukin-17 produced by tumor microenvironment promotes self-renewal of CD133+ cancer stem-like cells in ovarian cancer. Oncogene. 2015;34:165–176. doi: 10.1038/onc.2013.537. [DOI] [PubMed] [Google Scholar]
- 43.Bellone M., Brevi A., Huber S. Microbiota-Propelled T Helper 17 Cells in Inflammatory Diseases and Cancer. Microbiol. Mol. Biol. Rev. 2020;84 doi: 10.1128/MMBR.00064-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bilska M., Pawłowska A., Zakrzewska E., Chudzik A., Suszczyk D., Gogacz M., Wertel I. Th17 Cells and IL-17 As Novel Immune Targets in Ovarian Cancer Therapy. JAMA Oncol. 2020;2020 doi: 10.1155/2020/8797683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Block M.S., Dietz A.B., Gustafson M.P., Kalli K.R., Erskine C.L., Youssef B., Vijay G.V., Allred J.B., Pavelko K.D., Strausbauch M.A., et al. Th17-inducing autologous dendritic cell vaccination promotes antigen-specific cellular and humoral immunity in ovarian cancer patients. Nat. Commun. 2020;11:5173. doi: 10.1038/s41467-020-18962-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aotsuka A., Matsumoto Y., Arimoto T., Kawata A., Ogishima J., Taguchi A., Tanikawa M., Sone K., Mori-Uchino M., Tsuruga T., et al. Interleukin-17 is associated with expression of programmed cell death 1 ligand 1 in ovarian carcinoma. Cancer Sci. 2019;110:3068–3078. doi: 10.1111/cas.14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Droeser R.A., Mechera R., Däster S., Weixler B., Kraljević M., Delko T., Güth U., Stadlmann S., Terracciano L., Singer G. MPO density in primary cancer biopsies of ovarian carcinoma enhances the indicative value of IL-17 for chemosensitivity. BMC Cancer. 2016;16:639. doi: 10.1186/s12885-016-2673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Filippova O.T., Selenica P., Pareja F., Vahdatinia M., Zhu Y., Pei X., Riaz N., Long Roche K., Chi D.S., Abu-Rustum N.R., et al. Molecular characterization of high-grade serous ovarian cancers occurring in younger and older women. Gynecol. Oncol. 2021;161:545–552. doi: 10.1016/j.ygyno.2021.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raghavan S., Mehta P., Xie Y., Lei Y.L., Mehta G. Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments. J. Immunother. Cancer. 2019;7:190. doi: 10.1186/s40425-019-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walaszek K., Lower E.E., Ziolkowski P., Weber G.F. Breast cancer risk in premalignant lesions: osteopontin splice variants indicate prognosis. Br. J. Cancer. 2018;119:1259–1266. doi: 10.1038/s41416-018-0228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chou C.F., Huang C.C., Bin Dabil N., Chang P.L. Assessing SPP1/Osteopontin (OPN) Splice Variants and Their Association to Nonmelanoma Skin Cancer by Absolute Quantification: Identification of OPN-5 Subvariants and Their Protein Coding Potential. Cancer Invest. 2021;39:559–570. doi: 10.1080/07357907.2021.1933015. [DOI] [PubMed] [Google Scholar]
- 52.Pang X., Gong K., Zhang X., Wu S., Cui Y., Qian B.Z. Osteopontin as a multifaceted driver of bone metastasis and drug resistance. Pharmacol. Res. 2019;144:235–244. doi: 10.1016/j.phrs.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 53.Carlinfante G., Vassiliou D., Svensson O., Wendel M., Heinegård D., Andersson G. Differential expression of osteopontin and bone sialoprotein in bone metastasis of breast and prostate carcinoma. Clin. Exp. Metastasis. 2003;20:437–444. doi: 10.1023/a:1025419708343. [DOI] [PubMed] [Google Scholar]
- 54.Chakraborty G., Jain S., Behera R., Ahmed M., Sharma P., Kumar V., Kundu G.C. The multifaceted roles of osteopontin in cell signaling, tumor progression and angiogenesis. Curr. Mol. Med. 2006;6:819–830. doi: 10.2174/156652406779010803. [DOI] [PubMed] [Google Scholar]
- 55.Moorman H.R., Poschel D., Klement J.D., Lu C., Redd P.S., Liu K. Osteopontin: A Key Regulator of Tumor Progression and Immunomodulation. Cancers. 2020;12 doi: 10.3390/cancers12113379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao H., Chen Q., Alam A., Cui J., Suen K.C., Soo A.P., Eguchi S., Gu J., Ma D. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018;9:356. doi: 10.1038/s41419-018-0391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M., III, Hao Y., Stoeckius M., Smibert P., Satija R. Comprehensive integration of single-cell data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel A.P., Tirosh I., Trombetta J.J., Shalek A.K., Gillespie S.M., Wakimoto H., Cahill D.P., Nahed B.V., Curry W.T., Martuza R.L., et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vento-Tormo R., Efremova M., Botting R.A., Turco M.Y., Vento-Tormo M., Meyer K.B., Park J.E., Stephenson E., Polański K., Goncalves A., et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature. 2018;563:347–353. doi: 10.1038/s41586-018-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu X., Mao Q., Tang Y., Wang L., Chawla R., Pliner H.A., Trapnell C. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods. 2017;14:979–982. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Street K., Risso D., Fletcher R.B., Das D., Ngai J., Yosef N., Purdom E., Dudoit S. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics. 2018;19:477. doi: 10.1186/s12864-018-4772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data: Our raw data has been uploaded to the National Genomics Data Center with the number HRA004993 (https://ngdc.cncb.ac.cn/), any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
-
•
Code: This study does not report any original code.