Abstract
The levels of non-coding RNAs (ncRNAs) are regulated by transcription, RNA processing, and RNA degradation pathways. One mechanism for the degradation of ncRNAs involves the addition of oligo(A) tails by non-canonical poly(A) polymerases, which then recruit processive sequence-independent 3′ to 5′ exonucleases for RNA degradation. This pathway of decay is also regulated by three 3′ to 5′ exoribonucleases, USB1, PARN, and TOE1, which remove oligo(A) tails and thereby can protect ncRNAs from decay in a manner analogous to the deubiquitination of proteins. Loss-of-function mutations in these genes lead to premature degradation of some ncRNAs and lead to specific human diseases such as Poikiloderma with Neutropenia (PN) for USB1, Dyskeratosis Congenita (DC) for PARN and Pontocerebellar Hypoplasia type 7 (PCH7) for TOE1. Herein, we review the biochemical properties of USB1, PARN, and TOE1, how they modulate ncRNA levels, and their roles in human diseases.
Keywords: PARN, USB1, TOE1, non-coding RNA regulation, deadenylases
Gene expression in eukaryotes is regulated at multiple levels. While transcription regulation plays an important role, the regulation of RNA processing and degradation are also important in modulating the levels of both mRNAs and non-coding RNAs (ncRNAs) (1).
After transcription, 3′-end modifications of mRNA molecules can modulate the fate of newly synthesized RNAs. The 3′ ends of mRNAs are dynamically changed by the opposing effects of poly(A) polymerases and exonucleases, which can affect all aspects of mRNA metabolism (2). For example, the addition of poly(A) tails to mRNAs promotes their processing, export, and translation (3, 4, 5, 6, 7). Moreover, 3′ poly(A) tails on mRNAs can increase their stability by reducing the rate of decapping and/or inhibiting access to 3′ to 5′ exonucleases (8).
Similarly, 3′-end modifications of ncRNAs can regulate RNA processing and/or degradation. For instance, the addition of CCA to tRNA and uridylation of U6 small nuclear RNA (snRNA) are involved in RNA maturation (9, 10, 11), and the monouridylation of let-7 pre-miRNA by TUT4/TUT7 enhances its processing into mature let-7 (12). In contrast, other polymerases can promote degradation. For example, non-canonical poly(A) polymerases of the TRAMP complex interact with the nuclear exosome complex and are involved in the 3′-end processing and degradation of rRNAs and snoRNAs (13, 14, 15). Likewise, the uridylation of target RNAs, such as uridylation of let-7 pre-miRNA by TUT4/TUT7, or histone mRNAs (16), can recruit exonucleases for RNA degradation (13, 14, 15, 17, 18, 19, 20, 21).
Oligo(A) tail addition can also promote the degradation of some ncRNAs. For example, oligoadenylation by PAPD5/PAPD7 of miRNAs, human telomerase RNA (hTR), Y RNAs, ribosomal RNAs (rRNAs), small nucleolar RNAs (snoRNAs), small Cajal body-specific RNAs (scaRNAs), and small nuclear RNAs (snRNAs) can recruit exonucleases to degrade the RNAs (22, 23, 24, 25, 26, 27, 28, 29, 30). Similarly, PAPD5-mediated adenylation has been proposed to destabilize miR-21 and hTR RNAs in human cancer cell lines (22, 25, 26, 27, 28).
As oligo(A) tails can promote RNA degradation, it is not surprising that a set of deadenylases can remove oligo(A) tails and thereby stabilize some ncRNAs. To date, there are three such ncRNA deadenylases that can regulate ncRNAs in this manner including the poly(A) specific ribonuclease (PARN), USB1 (also called Mpn1), and Target of Erg1 (TOE1, also called Caf1z). Recent studies have shown that these enzymes regulate the stability of several ncRNAs in mammalian cells, such as hTR, Y RNAs, piwi-interacting RNAs (piRNAs), and miRNAs by removing poly(A) tails added by PAPD5/7. The poly(A) tail removal limits the recruitment of exonucleases DIS3L, DIS3L2, and/or the nuclease exosome (22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37).
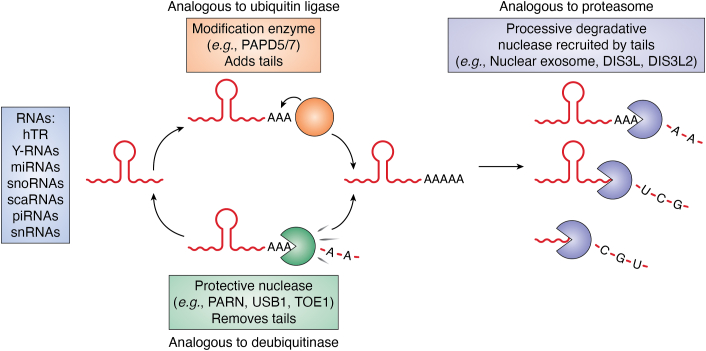
The process of oligo(A) tail addition and removal regulating the degradation of ncRNAs can be considered analogous to the control of protein degradation by ubiquination and deubiquination (Fig. 1). In this analogy, the oligo(A) polymerases such as PAPD5/7 are analogous to E3 ubiquitin ligases. Moreover, the recent results showing that PARN, TOE1, and USB1 stabilize different ncRNAs by removing these oligo(A) tails suggest this group of enzymes functions analogously to deubiquitinases. This suggests an emerging and unappreciated commonality of function for the USB1, TOE1, and PARN enzymes.
Figure 1.
Analogy of adenylation and deadenylation of ncRNAs to ubiquination and deubiquination. The process of regulating ncRNA degradation by addition and removal of the poly(A) tail can be considered analogous to the control of protein degradation by ubiquination and deubiquination.
Consistent with a commonality of function, USB1, PARN, and TOE1 are each connected to a specific human disease (32, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48). For example, loss-of-function mutations in USB1 (USB1 mutants) lead to the genetic disorder Poikiloderma with Neutropenia (PN), an autosomal-recessive bone marrow failure (BMF) syndrome with marked clinical overlap with Dyskeratosis Congenita (DC) (49). In contrast, loss-of-function mutations in PARN (PARN mutants) cause a severe form of DC called Hoyeraal-Hreidarsson syndrome, which causes abnormally short telomeres and congenital defects (38, 39, 40, 41) and idiopathic pulmonary fibrosis (IPF) (40). Finally, biallelic loss-of-function mutations in TOE1 (TOE1 mutants) cause Pontocerebellar Hypoplasia type 7 (PCH7), a unique recessive syndrome characterized by neurodegeneration with ambiguous genitalia (32, 45).
Herein, we review the properties of USB1, PARN, and TOE1, and their roles in RNA regulation. As mutations in these enzymes lead to human diseases, understanding their functions and targets may provide insights into treatments for these diseases.
The USB1, PARN, and TOE1 enzymes
USB1 is a member of the 2H phosphodiesterase superfamily, which can be subdivided into H x T and H x S enzymes, the latter of which contains USB1 (50). The 2H phosphodiesterase superfamily is an enzyme family with 2′,3′-cyclic or 1′,2′-cyclic phosphodiesterase (CPDase) activity, 3′-5′ or 2′-5′ phosphodiesterase activity, or 2′,5′-RNA ligase activity. The active sites of these enzymes all utilize two catalytic histidines within the central H x S/T tetrapeptide motifs that act as a general acid and base, while the serine or threonine residues help coordinate substrates and assist in transition state stabilization (51, 52, 53, 54, 55, 56, 57). In the USB1 family members, these key histidines are His120 and His208 in human USB1 (PDB ID 6D30 and 6D2Z) (Fig. 2), His109 and His199 in fission yeast (S. pombe) USB1, and His133 and His231 in budding yeast (Saccharomyces cerevisiae) (PDB ID 5UQJ). Although the overall sequence conservation can be rather low between family members, all known crystal structures of 2H family members, including USB1, display a characteristic fold with conserved terminal and transit lobes and the H x S/T motifs centrally positioned in a substrate binding cleft (53, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66).
Figure 2.
Cartoons illustrating the domains of USB1, PARN, and TOE1.
In contrast, PARN and TOE1 belong to the DEDD protein superfamily, one of the six exoribonuclease superfamilies (67, 68). Exoribonucleases in the DEDD superfamily are part of the much larger exonuclease superfamily, containing the proofreading domains of many DNA polymerases as well as other DNA exonucleases (69, 70). DEDD-type nucleases are named after their conserved catalytic Asp and Glu residues and include several other conserved residues distributed in three separate sequence motifs (70). The nucleases of this superfamily coordinate two metal ions essential to their catalytic mechanism (71). The DEDD superfamily can be divided into two subgroups, DEDDy and DEDDh, which are distinguished by whether the fifth conserved residue is a tyrosine or a histidine (67). Based on its crystal structure, PARN was determined to belong to the DEDDh subfamily (67).
PARN consists of three domains: an RNA recognition motif domain (RRM), a nuclease domain, and an R3H domain (Fig. 2). Mutational analyses showed that four conserved DEDD residues in PARN (Asp28, Glu30, Asp292, and Asp382) are essential for the catalytic activity of PARN and are required for the binding of divalent metal ions to PARN (72). Moreover, when substituting His377 by Ala in C-terminal truncated PARN, PARN’s activity is inhibited, suggesting that His377, as the fifth conserved residue, is also essential for the catalytic activity of PARN (73).
There are modest studies on TOE1 biochemical properties compared to PARN. Initial inspection of the human genome revealed TOE1 as a distant homolog of yeast Caf1p, which encodes an mRNA deadenylase subunit (74, 75). However, through evolutionary distance analysis, TOE1 shows greater similarity to PARN than to Caf1 (76), which led to TOE1 being classified as a member of the DEDDh family. The TOE1 protein consists of a unique C3H-type zinc finger domain, a DEDD nuclease domain, and an arginine-rich predicted basic nuclear localization signal (NLS) (Fig. 2) (77). Point mutations Asp64Ala and Glu66Ala in the nuclease domain of TOE1 showed little deadenylase activity in vitro, demonstrating these residues are important for TOE1’s enzymatic activity (77).
Biochemical roles of USB1, PARN, and TOE1 in RNA regulation
Biochemical roles of USB1
USB1 was first shown to affect U6 snRNA maturation (57). U6 snRNA is a part of the spliceosome, a large and highly dynamic complex that acts to remove introns from precursor mRNAs (43, 51, 78, 79). Nascent human U6 and U6atac snRNA transcripts are transcribed with a heterogeneous polyuridine 3′ end, owing to the stochastic nature of RNA polymerase III terminations (80, 81). While TUT1 catalyzes 3′ polyuridylation of U6 and U6atac snRNAs, USB1 removes 3′ uridines from U6 (57). More specifically, in vitro experiments showed that USB1 can remove uridine nucleotides from the 3′ end of U6 snRNA and catalyzes a formation of terminal 2′, 3′ cyclic phosphate, which stimulates the binding of Lsm2-8 and leads to the formation of U6 snRNPs (58, 64, 82, 83, 84).
Human and budding yeast USB1 appear to process U6 snRNAs differently, despite sharing highly similar structures (57, 58, 64, 85). In vitro analyses showed that the human USB1 post-transcriptionally removes uridine and adenosine nucleosides from the 3′ ends of spliceosomal U6 snRNAs, and can catalyze terminal 2′, 3′-cyclic phosphate formation (57, 58, 64, 85). USB1 measures the appropriate length of the U6 oligo(U) tail by reading the position of a key adenine nucleotide (A102) and pausing five uridine residues downstream (51, 57, 58, 64, 86). In S. cerevisiae, an unbiased genetic screen revealed that the yeast ortholog of USB1 is essential for U6 snRNA biogenesis and cell viability (78). In S. cerevisiae, USB1 mainly removes a single nucleotide from U6, leaving a 3′ monophosphate which strongly inhibits further processing (64). This argues that S. cerevisiae USB1 has 2′-CPDase activity that hydrolyzes the 2′,3′-cyclic phosphate product into a 3′ monophosphate, which is not exhibited in human USB1.
Crystallography studies showed that even with low similarity in sequence identity (<20%), humans and S. cerevisiae USB1 share highly similar structures (PDB ID 6D31, 4H7W, and 5UQJ) (57, 64). Moreover, these structures of human USB1 bound to nucleotides and intact RNA, along with kinetic analyses, and QM/MM simulations, which is a type of molecular stimulation method (87), have provided insights into its catalytic mechanism (57, 64). However, despite their similar structure, the mechanism of S. cerevisiae USB1’s 2′-CPDase activity remained poorly understood. By comparing S. cerevisiae USB1 (PDB ID 5UQJ) and K. marxianus USB1 structures (PDB ID 6PFQ), it has been suggested that the CPDase activity comes from a loop structure that is conserved in yeast and forms a distinct penultimate (n-1) nucleotide binding site (85). This suggested that the CPDase activity in yeast USB1 is related to the loop architecture that many yeast species possess but is absent from the human USB1.
Despite the related role for USB1 in U6 and U6atac snRNA processing, USB1 defects show distinct phenotypes in different species (Fig. 3). In budding yeast, USB1 deletion leads to cell death (58, 78), while in the fission yeast strand, the ΔUSB1 cells only showed reduced proliferation (51). In both yeast species, cells with USB1 depletions (where USB1 is expressed from a glucose-repressible galactose promoter) show global defects in pre-mRNA splicing, and this can be reversed by over-expressing U6 (51, 58, 78). This suggests that these yeast phenotypes are probably linked to U6 processing by USB1, leading to a subsequent defect in proper pre-mRNA splicing.
Figure 3.
Substrates of USB1 and USB1-related phenotypes. USB1 removes poly(U) tails from U6 snRNAs and poly(A) tails from miRNAs (31, 57, 58, 64, 78, 85). USB1-deficiency leads to pre-mRNA splicing defects and miRNA destabilization, resulting in developmental defects of various species and hematopoiesis (31, 42, 43, 51, 58, 78, 88).
In more complex organisms, USB1 can affect other processes including hematopoiesis (Fig. 3) (31, 87, 88). This is relevant for USB1 since people with loss-of-function alleles of USB1 have PN syndrome, which includes defects in the production of neutrophils (88). In PN-modeled zebrafish, morpholino USB1 suppression causes pigmentation and osteochondral defects, severe morphological defects, including a bent tail, thin yolk extension, reduced body length, and a significantly decreased number of neutrophils (87, 88). Thus, USB1-deficient embryos display developmental abnormalities recapitulating some of the properties of the human syndrome (88). In this zebrafish model, USB1 KD also causes splicing defects and a decrease in the formation of aberrant transcripts (87, 88). Specifically, the splicing of genes involved in neutrophil differentiation and development was aberrant in the morphant. Real-time PCR analyses of stage-specific markers showed defects of primitive hematopoiesis (87, 88). Together, these studies demonstrate the intrinsic requirement of USB1 for hematopoiesis and raise the possibility this could be affected by changes in pre-mRNA splicing.
In humans, USB1 also regulates hematopoietic development, but this appears to be primarily through deadenylating miRNAs instead of directly affecting splicing (Figs. 3 and 6A) (31, 58). The possibility that USB1 affects miRNAs comes from several observations in experiments done with a pathogenic USB1 mutation introduced into hESCs (31). First, while most ncRNAs, mRNAs, and U6 snRNA levels were not affected by the USB1 mutation, some key miRNAs affecting hematopoietic development were decreased. Second, when these miRNAs were over-expressed, the USB1-dependent defect in hematopoiesis was rescued. Third, there was an increase in short A tails on miRNAs in the USB1 mutant, and those tails were reduced by inhibition of the PAPD5/7 adenylase. Finally, inhibition of PAPD5/7 adenylase rescued miRNA levels and the defect in development seen in the USB1 mutant cells. While USB1 generally acts on U-tails, it can remove poly(A) tails in vitro (31, 57). These results suggest that one function of USB1 is to remove oligo(A) tails of miRNAs and thereby regulate human hematopoiesis.
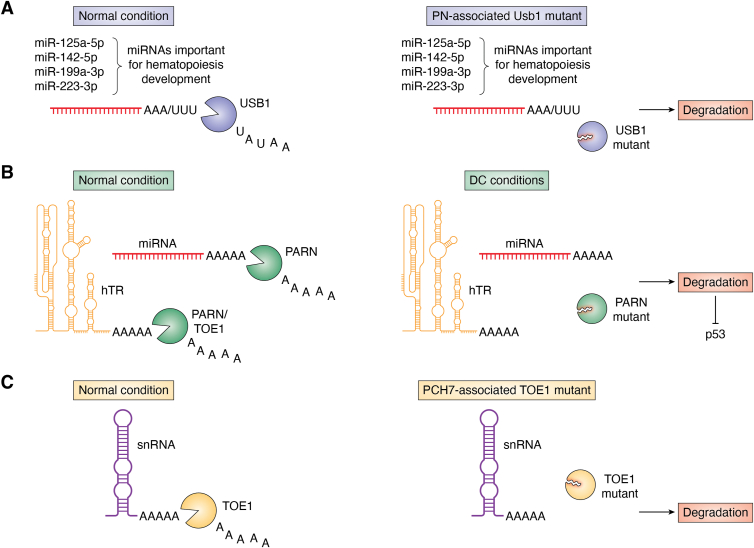
Figure 6.
Model depicting the regulation of non-coding RNA stability through ribonucleases in different diseases.A, miRNAs that are important for hematopoiesis development are stabilized by USB1 in normal conditions while are targeted for degradation in Poikiloderma with Neutropenia disease where USB1 is mutated (31, 42, 78). B, hTR and miRNAs are stabilized by PARN and/or TOE1 in normal conditions while are targeted for degradation in Dyskeratosis Congenita disease where PARN is mutated (22, 24, 34, 38, 41, 46). C, snRNAs are stabilized by TOE1 in normal condition while are targeted for degradation in Pontocerebellar Hypoplasia type 7 where TOE1 is mutated (32, 45).
USB1 may regulate hematopoiesis in a tissue-specific manner. USB1 is required for U6 and U6atac snRNA processing and there are observed defects in pre-mRNA splicing in PN-modeled zebrafish (51, 78, 87, 88). However, in PN-modeled zebrafish, there was no difference in U6 snRNA levels between wild-type and USB1-deficient embryos and only the genes involved in neutrophil differentiation and development showed splicing defects, not the hematopoietic precursors and erythroid-specific genes (87). These observations are consistent with the data from human PN that lymphoblastoid cells do not exhibit reduced U6 snRNA levels and have normal pre-mRNA splicing (58) and USB1 acts as a miRNA deadenylase to regulate hematopoiesis (31). These data suggested that USB1 might affect both splicing by U6 processing and miRNA stability by removing 3′-end adenylated tails added by PAPD5 to regulate hematopoiesis in a tissue-specific manner, which is supported by high levels of USB1 expression in hemopoietic tissue (31).
Biochemical roles of PARN
PARN is a poly(A) specific nuclease, first identified in extracts of Xenopus oocytes (89). PARN removes poly(A) tails from RNAs and releases 5′ AMP as a reaction product. It is suggested that the homodimer of PARN functions as a structural unit for its enzymatic activity since the substitution of Ile113, Phe123, or Phe127, important residues for homodimerization, inactivated or reduced PARN’s activity significantly (73). PARN has been demonstrated to preferentially cleave a poly(A) substrate with a free 3′-OH group (89, 90). This conclusion was supported by crystal structure showing that Glu30 specifically interacts with the 3′ hydroxyl group of the ribose (PDB ID 2A1R) (73). This observation suggested that Glu30 may act both as the catalytic residue and also confer specificity for the recognition of the 3′ poly(A) tail.
Among all deadenylases, PARN is unique as it can bind both the cap structure and the poly(A) tail during deadenylation (91, 92, 93, 94, 95). PARN recognizes and binds m7GpppG through Trp residue in the RRM domain (Trp475 in human and Trp468 in mouse) while the R3H domain helps to stabilize PARN (95, 96, 97). PARN’s activity was higher when processing RNA with 5′-cap structure compared to noncapped RNA substrates and the addition of free m7GpppG cap analog inhibited poly(A) degradation in vitro, suggesting that 3′-end poly(A) removal is linked to 5′ end cap structure of the RNA substrates (90, 91, 93). One unclear functional property of PARN is its specificity for poly(A). Biochemical studies have shown that PARN degrades poly(A) most efficiently and poly(U) under certain conditions but with essentially no activity on poly(C) or poly(G) (89, 90). How PARN achieves specificity for poly(A) is unclear as there are no H-bonding interactions between adenine bases and the protein (PDB ID 2A1R) (73). Interestingly, the DEDD deadenylase enzyme Pan2 in complex with RNA also does not exhibit any canonical base-specific contacts (PDB ID 6R9O), and instead achieves poly(A) specificity through intrinsic stacked, helical confirmation of poly(A) RNA (98). This suggests that instead of the canonical mechanisms of sequence-specific RNA recognition, PARN may utilize a structure-based mechanism similar to Pan2 to achieve specificity for poly(A) tails.
Recent studies have identified several targets of PARN. Although PARN was suspected as a key regulator for mRNAs due to its preference for m7G-cap and its role in global poly(A) shortening during Xenopus oocyte (89, 99, 100), PARN was shown to predominantly localize to the nucleolus and cytoplasmic foci and process ncRNAs in HeLa cells, such as 18S rRNAs, snoRNAs, hTR, scaRNAs, piRNAs, Y RNAs, and miRNAs (Fig. 4) (22, 23, 24, 25, 26, 27, 29, 30, 98, 101, 102, 103, 104, 105, 106).
Figure 4.
Substrates of PARN and PARN-related phenotypes. PARN removes poly(A) tails from mRNAs, miRNAs, hTR, piRNAs, 18S rRNAs, snoRNAs, scaRNAs, and Y RNAs (22, 23, 24, 25, 26, 27, 29, 30, 37, 106, 107, 114). PARN mutants show dysregulation of these RNAs and lead to developmental defects in higher plants, zebrafish, C.elegans, and DC and pulmonary fibrosis in humans (37, 38, 39, 40, 41, 46, 47, 89).
PARN also plays important role in development (Fig. 4). PARN is essential in plant development (107, 108, 109). Specifically, PARN mutant embryos showed developmental defects in higher plants, which has been proposed to be due to a failure to remove the 3′ poly(A) tails on specific subsets of mRNAs (110). In C. elegans, PARN mutants show reduced brood size and fertility compared to wild-type animals and accumulated untrimmed piRNAs with 3′ extensions (37, 76). Moreover, during Xenopus oocyte maturation, PARN was shown to cause global poly(A) shortening (89, 99, 100). These studies indicated that PARN deadenylase activity is crucial for the development of various species.
PARN deadenylase activity is involved in cancers and human diseases. A recent study found that in acute lymphocytic leukemia and acute myeloid leukemia, PARN’s expression levels were increased compared to non-malignant clinical samples (111, 112). In lung cancer, expression of PARN is associated with increased overall survival (110). Moreover, mutations in PARN are associated with DC and the familial form of idiopathic pulmonary fibrosis (IPF), an age-related disease featuring processive lung scarring (38, 39, 40, 41, 47). Both of these diseases are characterized by defects in telomerase activity and shorter telomeres (38, 39, 40, 41). Since PARN acts as a deadenylase to remove 3′ oligoadenylated ends of hTR, this suggests a link between PARN, hTR deadenylation, and the causes of DC and IPF (Figs. 4 and 6B) (22, 29, 111).
PARN requires binding partners for proper regulation in cells. The binding partners can either repress or activate the enzyme’s activities. For example, the interaction of the poly(A) tail with poly(A) binding proteins (PABPs) and/or the interaction of cap-binding proteins such as eIF4E and CBP80 with the 5′ cap structure has been shown to repress PARN and protect RNA substrates (113, 114). On the other hand, several RNA-binding proteins help to recruit PARN to the target RNAs for poly(A) removal. For instance, the CUG-binding protein (CUG-BP) can bind to cFos and TNFα mRNA and stimulate the poly(A) tail shortening of these RNAs by recruiting PARN (115). Similarly, miR-125b-loaded miRISC has been suggested to contribute to the specific recruitment of PARN to TP53 mRNA to regulate p53 levels (116). Finally, human tristetraproline (TTP) activates PARN activity when transfected in HEK293 extracts and this activation requires the binding of TTP to RNAs (117).
Biochemical roles of TOE1
TOE1 is a deadenylase that was first identified as a target of Erg1, an immediate early transcription factor. One function of Erg1 is to decrease the growth and tumorigenic potential of several tumor cell types (118, 119) and TOE1 was shown to be accountable for the growth inhibitory effect of Erg1 (74). Since TOE1 shows high similarity to PARN through evolutionary distance analysis (76), one could hypothesize that TOE1 may act redundantly with PARN in removing 3′ end tails of RNAs. However, in HeLa cells, TOE1 was shown to localize to Cajal bodies, while PARN is primarily in the nucleoli, indicating that TOE1 and PARN have distinct subcellular locations (29, 30, 98).
TOE1 exhibits deadenylation on RNA substrates and shows a preference for poly(A) (77). However, TOE1 catalyzes rapid deadenylation followed by a slower 3′-to-5′ exonucleolytic decay of non-poly(A) sequences, which is distinct from PARN (77).
TOE1 has been shown to deadenylate several ncRNAs, including snRNAs (32). More specifically, TOE1 promotes the maturation of all regular RNA polymerase II transcribed snRNAs of the major and minor spliceosomes by removing 3′ oligo(A) tails and preventing nuclear exosome targeting (33). However, TOE1 removal of oligo(A) tails on snRNAs and snoRNAs can provide a mechanism for RNA quality control (33). TOE1 does show some overlap of substrates with PARN, and together both these enzymes can act on snoRNAs, scaRNAs, and hTR (Fig. 5) (30, 34)).
Figure 5.
Substrates of TOE1 and TOE1-related phenotypes. TOE1 removes poly(A) tails from snRNAs, hTR, snoRNAs, and scaRNAs (30, 32, 34). TOE1 mutants in PCH7 patients and PCH7-modeled mice and zebrafish lead to extended adenylated 3′ ends of hTR and snRNAs, resulting in developmental defects and defects in brain structures (32, 45).
TOE1 expression also shows effects on development. Over-expression of TOE1 negatively affects the growth of HEK293 and H4 cells by altering the cell cycle through the induction of p21 (34, 74). On the contrary, TOE1 KD causes developmental arrest during the morula-to-blastocyst transition in mice by upregulating p21 (120). These studies suggest that TOE1 regulating p21 may be tissue-specific and further investigation of the molecular mechanism of TOE1 is needed for a better understanding of TOE1’s role in development.
TOE1 is involved in PCH7 (Figs. 5 and 6C). This was first suggested by the identification of various TOE1 mutations in PCH7 patients (32, 45). Moreover, mouse embryos with homozygous TOE1 frameshift mutations showed uniform lethality demonstrating TOE1 is required for mouse development. Furthermore, in a PCH7-disease model using zebrafish, knockdown of the single TOE1 orthologue led to reproducible microcephaly, small eye, and curly tail phenotype in 90% of embryos with defects in the brain structure of the midbrain, cerebellum, and hindbrain by 48 h post-fertilization. This phenotype was rescued by co-injection of human TOE1 mRNA but not the catalytically inactive DE mutant- or patient mutation-encoding mRNAs, suggesting that reduced expression of TOE1 leads to neurodegeneration and PCH-like brain defects in vivo (32). Moreover, TOE1 DE-associated snRNAs are predominantly enriched for untemplated 3′ adenosine (32), suggesting a link between PCH7 and TOE1 deadenylase activity on the processing of snRNA 3′ ends.
TOE1 is also suggested to modulate HIV-1 infection (Fig. 5). TOE1 was shown to directly interact with the HIV viral transactivation response element as part of an inhibitory mechanism (121). Moreover, TOE1 can be secreted following immune response activation and exhibits the ability to spontaneously cross the plasma membrane and penetrate cells in culture (121). Interestingly, exogenously added TOE1 can also inhibit HIV-1 LTR (long terminal repeat) transactivation, retaining HIV-1 inhibitory activity (121). Both TOE1’s antiviral potency and its cell-penetrating capability have been identified to lie within the 35-amino-acid region containing the nuclear localization signal (NLS) (121). This suggests that TOE1 may have other functions besides ncRNA deadenylation.
TOE1 was also shown to be involved in tauopathy. In C. elegans, loss of parn-2, an ortholog of TOE1, partially suppressed tauopathy (122). Moreover, this might be relevant to human disease since Alzheimer’s disease patients with low TOE1 levels exhibit significantly increased pathological tau deposits (122). Interestingly, blocking 3′ end poly(A) extensions with cordycepin exacerbated tauopathy in human cultured cells (122). Together, these data suggest that there is a link between tauopathy, TOE1, and poly(A) RNA metabolism.
Similar to PARN, TOE1 has been shown to associate with several proteins, including hCcr4d, DKC1 (TERC subunit), and spliceosomal proteins (32, 34, 77); however, whether these interactions modulate TOE1 function has not been determined.
Human diseases are caused by failure to deadenylate ncRNAs
A striking hallmark is that loss-of-function mutations in USB1, PARN, and TOE1 are all involved in human diseases featuring abnormal 3′ end extensions of ncRNAs (32, 38, 39, 40, 41, 42, 44, 45, 46, 47, 48). Thus, an understanding of these disease mechanisms may suggest possible manipulations to restore ncRNAs for therapeutic improvements.
Loss-of-functions mutations in C16orf57, which encodes USB1, in humans leads to PN, which is a rare, autosomal recessive skin condition (OMIM 604173). To date, there are 38 PN patients reported with 19 different mutations in C16orf57 (43, 49, 123). Based on the bioinformatic prediction, all C16orf57 mutations impair the protein structure by either removing one or both tetrapeptide motifs, or destroying the symmetry of the native folding. Mutations in C16orf57 gene also produce phenotypes with marked clinical overlap with DC and Rothmund-Thomson syndrome (RTS), a poikiloderma that is sometimes confused with PN (49). However, unlike patients with DC, which is often caused by mutations in factors involving telomere maintenance, telomeres in patients with PN are not significantly shortened, and therefore telomere length represents a clear distinguishable feature for the correct diagnosis of these different BMF syndromes (48). RTS can also be distinguished from PN since RTS patients harbor mutations in the RECQL4 DNA helicase, which is involved in DNA repair and replication (124, 146). Thus, mutations in C16orf57 can be used as a molecular marker for the precise diagnosis of PN.
In humans, it appears USB1 acts as a miRNA deadenylase to regulate hematopoietic development (Fig. 6A). A previous study showed that lymphoblastoid cells from PN patients do not exhibit reduced U6 snRNA levels and have normal pre-mRNA splicing (58). Recently, the analysis of human embryonic stem cells harboring PN-associated mutation c.531_delA in C16orf57 showed that this mutation severely impairs human hematopoietic development (31). In this model system, it was demonstrated that hematopoietic failure in USB1 mutants is caused by dysregulated miRNA levels in hematopoiesis, due to a failure to remove destabilizing 3′ end oligo(A) tails added by PAPD5/7. This phenotype can be reversed by inhibition of PAPD5/7 with a PAPD5/7 inhibitor, RG7834 (31). Despite the widely appreciated role of USB1 in U6 maturation, this study identified a new role for USB1 in miRNA regulation and in PN disease.
Loss-of-function mutations in PARN also lead to a type of BMF. Specifically, mutations in PARN were found in a severe form of DC known as Hoyeraal-Hreidarsson syndrome and IPF (38, 39, 40, 41, 46, 47). DC is an inherited, life-threatening bone marrow failure disorder, caused by genetic defects in components of the telomerase holoenzyme in human cells and leads to age-related bone marrow failure and cancer. Most mutations associated with DC are found in genes encoding components of the telomerase enzyme complex including hTR, the telomerase RNP component dyskerin (DCK1), and the catalytic subunit TERC (124, 125). Moreover, it was shown that loss of PARN leads to defective 3′ end maturation of hTR, suggesting the link between PARN, hTR maintenance, and the causes of DC and IPF (22, 25, 26, 27, 30, 41, 111).
PARN deficiency negatively affects the stability of hTR and miRNAs, which is likely to cause severe phenotypes in DC and IPF (Fig. 6B). Several studies have shown that PARN inhibition leads to a failure to remove the poly(A) tail of hTR, leading to the recruitment of nuclear 3′ to 5′ exonuclease EXOSC10, which leads to hTR degradation (22, 25, 26, 27, 29, 30, 41, 111). Moreover, it was shown that PARN deficiency also leads to the accumulation of longer oligo(A) tails and affects the stability of several miRNAs, which upregulate p53 expression (24). Since chronic upregulation of p53 signaling would negatively affect cell growth and development, this could explain the severe phenotype of PARN deficiency in DC patients (24).
Mutations in TOE1 were found in PCH7 (32, 45). Pontocerebellar hypoplasias (PCH) are a group of autosomal recessive neurodegenerative disorders with prenatal onset, mainly affecting the growth and survival of neurons in the cerebellar cortex, the dentate, inferior olivary, and ventral pontine nuclei (126). To date, 10 subtypes of PCH have been identified (32, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141). PCH7 is characterized by neurological deterioration, astrophy/hypoplasia of the pons and cerebellum, muscular hypotonia, and breathing abnormalities, combined with hypogonadism (142).
Patients with PCH7 harbor biallelic, loss-of-function mutations in TOE1, resulting in the accumulation of incompletely processed snRNAs (Fig. 6C). TOE1 is associated with pre-snRNAs and snRNAs associated with TOE1 catalytically inactive mutant, U1, U2, and U5 snRNAs contained longer tails than those associated with WT TOE1, suggesting that TOE1 plays a role as a 3′-5′ exonuclease for specific snRNA processing (32). There was an increase in the fraction of U1 and U2 snRNAs, and to a less extent, U5 snRNAs containing tails in patient-derived TOE1 defective fibroblast and NPC lines compared to unaffected relatives, suggesting that there might be a link between the cause of PCH7 and a key factor involved in incompletely processed snRNAs which are likely caused by loss-of-function TOE1 mutations (32).
TOE1 and PARN may act non-redundantly on regulating and maintaining hTR biogenesis in diseases (Fig. 6B). It is interesting to note that cerebellum hypoplasia also manifests in patients with telomere-related diseases, such as Hoyeraal-Hreidarsson and Revesz syndromes (142, 143). It has been shown that TOE1 deficiency leads to the accumulation of hTR precursors, including oligoadenylated and 3′ end extended forms. However, TOE1 deficiency only affects telomerase activity and shortening, but not hTR levels (34). The current model speculated that most of the hTR poly(A) tails are removed by PARN in the nucleoli, then hTR transits to Cajal bodies for further processing by TOE1 and/or PARN, cooperative or sequentially (34). This explains why when TOE1 is deficient, there are more immature hTR precursors but no decrease in total hTR levels. Thus, understanding the precise mechanisms of how these deadenylases function may give insights into the development and pathogenesis of diseases such as DC and PCH7.
Inhibition of PAPD5/7 may be a potential therapy for PN and DC. Dysregulated miRNA and hTR levels in USB1 and PARN mutants contribute to hematopoietic failure, due to a failure to remove 3′ end adenylated tails added by PAPD5/7. Previous studies showed that modulation of miRNA 3′ end adenylation through genetic or chemical inhibition of PAPD5/7 rescues hematopoietic in USB1 mutants (31). Likewise, PAPD5 KD can rescue telomerase activity in PARN-deficient cells and restore defects in hematopoiesis (22, 144). Though there is no data of how PAPD5/7 affects snRNAs and PCH7 phenotypes, these studies suggest that inhibitors of PAPD5/7 might be a potential treatment for PN and DC, and possibly PCH7.
Future outlooks
It is now appreciated that the balance of the addition and removal of oligo(A) tails can serve as a common pathway for regulating the stability of some ncRNAs. In this context, the poly(A) specific exonucleases USB1, PARN, and TOE1 have emerged as playing important roles in controlling the stability of ncRNAs. Looking forward there are outstanding issues to address. First, it will be important to understand why these enzymes are specifically required for some developmental transitions in both plants and mammals. Understanding these areas should shed new light on the roles of their biological roles and may identify new RNA substrates for these enzymes. Second, it will be important to determine whether therapeutic intervention in the ncRNA adenylation/deadenylation dynamic can be useful in therapeutic contexts. Currently, inhibition of adenylation by PAPD5/7 inhibitor has been suggested as possible therapy for DC and PN (31, 145). Alternatively, there may be other contexts where inhibition of ncRNA deadenylation might have therapeutic benefits (24), although testing these ideas will require the development of robust chemical inhibitors of PARN, USB1, and TOE1.
Conflict of interest
The authors declared no conflict of interest for this review.
Acknowledgments
This work was supported by funds from the Howard Hughes Medical Institute and funds from the Department of Defense (W81XWH2110447). All the figures were created with BioRender.com.
Author contributions
T. N. H. and R. P. writing - original draft.
Reviewed by members of the JBC Editorial Board. Edited by Karin Musier-Forsyth
References
- 1.Lu P., Vogel C., Wang R., Yao X., Marcotte E.M. Absolute protein expression profiling estimates the relative contributions of transcriptional and translational regulation. Nat. Biotechnol. 2007;25:117–124. doi: 10.1038/nbt1270. [DOI] [PubMed] [Google Scholar]
- 2.Yan Y.B. Deadenylation: enzymes, regulation, and functional implications. Wiley Interdiscip. Rev. RNA. 2014;5:421–443. doi: 10.1002/wrna.1221. [DOI] [PubMed] [Google Scholar]
- 3.Darnell J.E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971;174:507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- 4.Edmonds M., Vaughan M.H., Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc. Natl. Acad. Sci. U. S. A. 1971;68:1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niwa M., Rose S.D., Berget S.M. In vitro polyadenylation is stimulated by the presence of an upstream intron. Genes Dev. 1990;4:1552–1559. doi: 10.1101/gad.4.9.1552. [DOI] [PubMed] [Google Scholar]
- 6.Wassarman K.M., Steitz J.A. Association with terminal exons in pre-mRNAs: a new role for the U1 snRNP? Genes Dev. 1993;7:647–659. doi: 10.1101/gad.7.4.647. [DOI] [PubMed] [Google Scholar]
- 7.Dreyfus M., Régnier P. The poly(A) tail of mRNAs: bodyguard in eukaryotes, scavenger in Bacteria. Cell. 2002;111:611–613. doi: 10.1016/s0092-8674(02)01137-6. [DOI] [PubMed] [Google Scholar]
- 8.Łabno A., Tomecki R., Dziembowski A. Cytoplasmic RNA decay pathways - enzymes and mechanisms. Biochim. Biophys. Acta. 2016;1863:3125–3147. doi: 10.1016/j.bbamcr.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Sprinzl M., Cramer F. The -C-C-A end of tRNA and its role in protein biosynthesis. Prog. Nucleic Acid Res. Mol. Biol. 1979;22:1–69. doi: 10.1016/s0079-6603(08)60798-9. [DOI] [PubMed] [Google Scholar]
- 10.Deutscher M.P., Ni R.C. Purification of a low molecular weight form of rat liver arginyl-tRNA synthetase. J. Biol. Chem. 1982;257:6003–6006. [PubMed] [Google Scholar]
- 11.Reddy R., Henning D., Das G., Harless M., Wright D. The capped U6 small nuclear RNA is transcribed by RNA polymerase III. J. Biol. Chem. 1987;262:75–81. [PubMed] [Google Scholar]
- 12.Heo I., Joo C., Cho J., Ha M., Han J., Kim V.N. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol. Cell. 2008;32:276–284. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 13.LaCava J., Houseley J., Saveanu C., Petfalski E., Thompson E., Jacquier A., et al. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell. 2005;121:713–724. doi: 10.1016/j.cell.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Vanácová S., Wolf J., Martin G., Blank D., Dettwiler S., Friedlein A., et al. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol. 2005;3:e189. doi: 10.1371/journal.pbio.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyers F., Rougemaille M., Badis G., Rousselle J.C., Dufour M.E., Boulay J., et al. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell. 2005;121:725–737. doi: 10.1016/j.cell.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 16.Marzluff W.F., Koreski K.P. Birth and death of histone mRNAs. Trends Genet. 2017;33:745–759. doi: 10.1016/j.tig.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia H., Wang X., Liu F., Guenther U.P., Srinivasan S., Anderson J.T., et al. The RNA helicase Mtr4p modulates polyadenylation in the TRAMP complex. Cell. 2011;145:890–901. doi: 10.1016/j.cell.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagan J.P., Piskounova E., Gregory R.I. Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat. Struct. Mol. Biol. 2009;16:1021–1025. doi: 10.1038/nsmb.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang H.M., Triboulet R., Thornton J.E., Gregory R.I. A role for the Perlman syndrome exonuclease Dis3l2 in the Lin28-let-7 pathway. Nature. 2013;497:244–248. doi: 10.1038/nature12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ustianenko D., Hrossova D., Potesil D., Chalupnikova K., Hrazdilova K., Pachernik J., et al. Mammalian DIS3L2 exoribonuclease targets the uridylated precursors of let-7 miRNAs. RNA. 2013;19:1632–1638. doi: 10.1261/rna.040055.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rissland O.S., Norbury C.J. Decapping is preceded by 3' uridylation in a novel pathway of bulk mRNA turnover. Nat. Struct. Mol. Biol. 2009;16:616–623. doi: 10.1038/nsmb.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukla S., Schmidt J.C., Goldfarb K.C., Cech T.R., Parker R. Inhibition of telomerase RNA decay rescues telomerase deficiency caused by dyskerin or PARN defects. Nat. Struct. Mol. Biol. 2016;23:286–292. doi: 10.1038/nsmb.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukla S., Parker R. PARN modulates Y RNA stability and its 3'-end formation. Mol. Cell. Biol. 2017;37:e00264–e00317. doi: 10.1128/MCB.00264-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shukla S., Bjerke G.A., Muhlrad D., Yi R., Parker R. The RNase PARN controls the levels of specific miRNAs that contribute to p53 regulation. Mol. Cell. 2019;73:1204–1216.e1204. doi: 10.1016/j.molcel.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen D., Grenier St-Sauveur V., Bergeron D., Dupuis-Sandoval F., Scott M.S., Bachand F. A polyadenylation-dependent 3' end maturation pathway is required for the Synthesis of the human telomerase RNA. Cell Rep. 2015;13:2244–2257. doi: 10.1016/j.celrep.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Tseng C.K., Wang H.F., Burns A.M., Schroeder M.R., Gaspari M., Baumann P. Human telomerase RNA processing and quality control. Cell Rep. 2015;13:2232–2243. doi: 10.1016/j.celrep.2015.10.075. [DOI] [PubMed] [Google Scholar]
- 27.Moon D.H., Segal M., Boyraz B., Guinan E., Hofmann I., Cahan P., et al. Poly(A)-specific ribonuclease (PARN) mediates 3'-end maturation of the telomerase RNA component. Nat. Genet. 2015;47:1482–1488. doi: 10.1038/ng.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boele J., Persson H., Shin J.W., Ishizu Y., Newie I.S., Søkilde R., et al. PAPD5-mediated 3' adenylation and subsequent degradation of miR-21 is disrupted in proliferative disease. Proc. Natl. Acad. Sci. U. S. A. 2014;111:11467–11472. doi: 10.1073/pnas.1317751111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berndt H., Harnisch C., Rammelt C., Stöhr N., Zirkel A., Dohm J.C., et al. Maturation of mammalian H/ACA box snoRNAs: PAPD5-dependent adenylation and PARN-dependent trimming. RNA. 2012;18:958–972. doi: 10.1261/rna.032292.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Son A., Park J.E., Kim V.N. PARN and TOE1 Constitute a 3' end maturation Module for nuclear non-coding RNAs. Cell Rep. 2018;23:888–898. doi: 10.1016/j.celrep.2018.03.089. [DOI] [PubMed] [Google Scholar]
- 31.Jeong H.C., Shukla S., Fok W.C., Huynh T.N., Batista L.F.Z., Parker R. USB1 is a miRNA deadenylase that regulates hematopoietic development. Science. 2023;379:901–907. doi: 10.1126/science.abj8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lardelli R.M., Schaffer A.E., Eggens V.R., Zaki M.S., Grainger S., Sathe S., et al. Biallelic mutations in the 3' exonuclease TOE1 cause pontocerebellar hypoplasia and uncover a role in snRNA processing. Nat. Genet. 2017;49:457–464. doi: 10.1038/ng.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lardelli R.M., Lykke-Andersen J. Competition between maturation and degradation drives human snRNA 3' end quality control. Genes Dev. 2020;34:989–1001. doi: 10.1101/gad.336891.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng T., Huang Y., Weng K., Lin S., Li Y., Shi G., et al. TOE1 acts as a 3' exonuclease for telomerase RNA and regulates telomere maintenance. Nucleic Acids Res. 2019;47:391–405. doi: 10.1093/nar/gky1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ustianenko D., Pasulka J., Feketova Z., Bednarik L., Zigackova D., Fortova A., et al. TUT-DIS3L2 is a mammalian surveillance pathway for aberrant structured non-coding RNAs. EMBO J. 2016;35:2179–2191. doi: 10.15252/embj.201694857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izumi N., Shoji K., Sakaguchi Y., Honda S., Kirino Y., Suzuki T., et al. Identification and functional analysis of the pre-piRNA 3' Trimmer in Silkworms. Cell. 2016;164:962–973. doi: 10.1016/j.cell.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang W., Tu S., Lee H.C., Weng Z., Mello C.C. The RNase PARN-1 Trims piRNA 3' ends to promote transcriptome surveillance in C. elegans. Cell. 2016;164:974–984. doi: 10.1016/j.cell.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burris A.M., Ballew B.J., Kentosh J.B., Turner C.E., Norton S.A., Giri N., et al. Hoyeraal-hreidarsson syndrome due to PARN mutations: fourteen years follow-up. Pediatr. Neurol. 2016;56:62–68.e61. doi: 10.1016/j.pediatrneurol.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhanraj S., Gunja S.M., Deveau A.P., Nissbeck M., Boonyawat B., Coombs A.J., et al. Bone marrow failure and developmental delay caused by mutations in poly(A)-specific ribonuclease (PARN) J. Med. Genet. 2015;52:738–748. doi: 10.1136/jmedgenet-2015-103292. [DOI] [PubMed] [Google Scholar]
- 40.Stuart B.D., Choi J., Zaidi S., Xing C., Holohan B., Chen R., et al. Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat. Genet. 2015;47:512–517. doi: 10.1038/ng.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tummala H., Walne A., Collopy L., Cardoso S., de la Fuente J., Lawson S., et al. Poly(A)-specific ribonuclease deficiency impacts telomere biology and causes dyskeratosis congenita. J. Clin. Invest. 2015;125:2151–2160. doi: 10.1172/JCI78963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Concolino D., Roversi G., Muzzi G.L., Sestito S., Colombo E.A., Volpi L., et al. Clericuzio-type poikiloderma with neutropenia syndrome in three sibs with mutations in the C16orf57 gene: delineation of the phenotype. Am. J. Med. Genet. A. 2010;152A:2588–2594. doi: 10.1002/ajmg.a.33600. [DOI] [PubMed] [Google Scholar]
- 43.Volpi L., Roversi G., Colombo E.A., Leijsten N., Concolino D., Calabria A., et al. Targeted next-generation sequencing appoints c16orf57 as clericuzio-type poikiloderma with neutropenia gene. Am. J. Hum. Genet. 2010;86:72–76. doi: 10.1016/j.ajhg.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka A., Morice-Picard F., Lacombe D., Nagy N., Hide M., Taïeb A., et al. Identification of a homozygous deletion mutation in C16orf57 in a family with Clericuzio-type poikiloderma with neutropenia. Am. J. Med. Genet. A. 2010;152A:1347–1348. doi: 10.1002/ajmg.a.33455. [DOI] [PubMed] [Google Scholar]
- 45.Wang C., Ge Y., Li R., He G., Lin Y. Novel compound heterozygous missense variants in TOE1 gene associated with pontocerebellar hypoplasia type 7. Gene. 2023;862 doi: 10.1016/j.gene.2023.147250. [DOI] [PubMed] [Google Scholar]
- 46.Zhang D., Zhou Z., Abu-Hijleh M., Batra K., Xing C., Garcia C.K. Homozygous rare PARN missense mutation in familial pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019;199:797–799. doi: 10.1164/rccm.201809-1632LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kropski J.A., Reiss S., Markin C., Brown K.K., Schwartz D.A., Schwarz M.I., et al. Rare genetic variants in PARN are associated with pulmonary fibrosis in families. Am. J. Respir. Crit. Care Med. 2017;196:1481–1484. doi: 10.1164/rccm.201703-0635LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walne A.J., Vulliamy T., Beswick R., Kirwan M., Dokal I. Mutations in C16orf57 and normal-length telomeres unify a subset of patients with dyskeratosis congenita, poikiloderma with neutropenia and Rothmund-Thomson syndrome. Hum. Mol. Genet. 2010;19:4453–4461. doi: 10.1093/hmg/ddq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walne A.J., Collopy L., Cardoso S., Ellison A., Plagnol V., Albayrak C., et al. Marked overlap of four genetic syndromes with dyskeratosis congenita confounds clinical diagnosis. Haematologica. 2016;101:1180–1189. doi: 10.3324/haematol.2016.147769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazumder R., Iyer L.M., Vasudevan S., Aravind L. Detection of novel members, structure-function analysis and evolutionary classification of the 2H phosphoesterase superfamily. Nucleic Acids Res. 2002;30:5229–5243. doi: 10.1093/nar/gkf645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shchepachev V., Wischnewski H., Missiaglia E., Soneson C., Azzalin C.M. Mpn1, mutated in poikiloderma with neutropenia protein 1, is a conserved 3'-to-5' RNA exonuclease processing U6 small nuclear RNA. Cell Rep. 2012;2:855–865. doi: 10.1016/j.celrep.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 52.Arn E.A., Abelson J.N. The 2'-5' RNA ligase of Escherichia coli. Purification, cloning, and genomic disruption. J. Biol. Chem. 1996;271:31145–31153. doi: 10.1074/jbc.271.49.31145. [DOI] [PubMed] [Google Scholar]
- 53.Hofmann A., Zdanov A., Genschik P., Ruvinov S., Filipowicz W., Wlodawer A. Structure and mechanism of activity of the cyclic phosphodiesterase of Appr>p, a product of the tRNA splicing reaction. EMBO J. 2000;19:6207–6217. doi: 10.1093/emboj/19.22.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kato M., Shirouzu M., Terada T., Yamaguchi H., Murayama K., Sakai H., et al. Crystal structure of the 2'-5' RNA ligase from Thermus thermophilus HB8. J. Mol. Biol. 2003;329:903–911. doi: 10.1016/s0022-2836(03)00448-0. [DOI] [PubMed] [Google Scholar]
- 55.Sawaya R., Schwer B., Shuman S. Genetic and biochemical analysis of the functional domains of yeast tRNA ligase. J. Biol. Chem. 2003;278:43928–43938. doi: 10.1074/jbc.M307839200. [DOI] [PubMed] [Google Scholar]
- 56.Remus B.S., Jacewicz A., Shuman S. Structure and mechanism of E. coli RNA 2',3'-cyclic phosphodiesterase. RNA. 2014;20:1697–1705. doi: 10.1261/rna.046797.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nomura Y., Roston D., Montemayor E.J., Cui Q., Butcher S.E. Structural and mechanistic basis for preferential deadenylation of U6 snRNA by Usb1. Nucleic Acids Res. 2018;46:11488–11501. doi: 10.1093/nar/gky812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hilcenko C., Simpson P.J., Finch A.J., Bowler F.R., Churcher M.J., Jin L., et al. Aberrant 3' oligoadenylation of spliceosomal U6 small nuclear RNA in poikiloderma with neutropenia. Blood. 2013;121:1028–1038. doi: 10.1182/blood-2012-10-461491. [DOI] [PubMed] [Google Scholar]
- 59.Gao Y.G., Yao M., Okada A., Tanaka I. The structure of Pyrococcus horikoshii 2'-5' RNA ligase at 1.94 A resolution reveals a possible open form with a wider active-site cleft. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006;62:1196–1200. doi: 10.1107/S1744309106046616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hofmann A., Grella M., Botos I., Filipowicz W., Wlodawer A. Crystal structures of the semireduced and inhibitor-bound forms of cyclic nucleotide phosphodiesterase from Arabidopsis thaliana. J. Biol. Chem. 2002;277:1419–1425. doi: 10.1074/jbc.M107889200. [DOI] [PubMed] [Google Scholar]
- 61.Gold M.G., Smith F.D., Scott J.D., Barford D. AKAP18 contains a phosphoesterase domain that binds AMP. J. Mol. Biol. 2008;375:1329–1343. doi: 10.1016/j.jmb.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Myllykoski M., Raasakka A., Han H., Kursula P. Myelin 2',3'-cyclic nucleotide 3'-phosphodiesterase: active-site ligand binding and molecular conformation. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Myllykoski M., Raasakka A., Lehtimäki M., Han H., Kursula I., Kursula P. Crystallographic analysis of the reaction cycle of 2',3'-cyclic nucleotide 3'-phosphodiesterase, a unique member of the 2H phosphoesterase family. J. Mol. Biol. 2013;425:4307–4322. doi: 10.1016/j.jmb.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Didychuk A.L., Montemayor E.J., Carrocci T.J., DeLaitsch A.T., Lucarelli S.E., Westler W.M., et al. Usb1 controls U6 snRNP assembly through evolutionarily divergent cyclic phosphodiesterase activities. Nat. Commun. 2017;8:497. doi: 10.1038/s41467-017-00484-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raasakka A., Myllykoski M., Laulumaa S., Lehtimäki M., Härtlein M., Moulin M., et al. Determinants of ligand binding and catalytic activity in the myelin enzyme 2',3'-cyclic nucleotide 3'-phosphodiesterase. Sci. Rep. 2015;5 doi: 10.1038/srep16520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Myllykoski M., Kursula P. Structural aspects of nucleotide ligand binding by a bacterial 2H phosphoesterase. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuo Y., Deutscher M.P. Exoribonuclease superfamilies: structural analysis and phylogenetic distribution. Nucleic Acids Res. 2001;29:1017–1026. doi: 10.1093/nar/29.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldstrohm A.C., Wickens M. Multifunctional deadenylase complexes diversify mRNA control. Nat. Rev. Mol. Cell Biol. 2008;9:337–344. doi: 10.1038/nrm2370. [DOI] [PubMed] [Google Scholar]
- 69.Moser M.J., Holley W.R., Chatterjee A., Mian I.S. The proofreading domain of Escherichia coli DNA polymerase I and other DNA and/or RNA exonuclease domains. Nucleic Acids Res. 1997;25:5110–5118. doi: 10.1093/nar/25.24.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bernad A., Blanco L., Lázaro J.M., Martín G., Salas M. A conserved 3'----5' exonuclease active site in prokaryotic and eukaryotic DNA polymerases. Cell. 1989;59:219–228. doi: 10.1016/0092-8674(89)90883-0. [DOI] [PubMed] [Google Scholar]
- 71.Steitz T.A., Steitz J.A. A general two-metal-ion mechanism for catalytic RNA. Proc. Natl. Acad. Sci. U. S. A. 1993;90:6498–6502. doi: 10.1073/pnas.90.14.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ren Y.G., Martínez J., Virtanen A. Identification of the active site of poly(A)-specific ribonuclease by site-directed mutagenesis and Fe(2+)-mediated cleavage. J. Biol. Chem. 2002;277:5982–5987. doi: 10.1074/jbc.M111515200. [DOI] [PubMed] [Google Scholar]
- 73.Wu M., Reuter M., Lilie H., Liu Y., Wahle E., Song H. Structural insight into poly(A) binding and catalytic mechanism of human PARN. EMBO J. 2005;24:4082–4093. doi: 10.1038/sj.emboj.7600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Belle I., Wu J.X., Sperandio S., Mercola D., Adamson E.D. In vivo cloning and characterization of a new growth suppressor protein TOE1 as a direct target gene of Egr1. J. Biol. Chem. 2003;278:14306–14312. doi: 10.1074/jbc.M210502200. [DOI] [PubMed] [Google Scholar]
- 75.Tucker M., Valencia-Sanchez M.A., Staples R.R., Chen J., Denis C.L., Parker R. The transcription factor associated Ccr4 and Caf1 proteins are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell. 2001;104:377–386. doi: 10.1016/s0092-8674(01)00225-2. [DOI] [PubMed] [Google Scholar]
- 76.Nousch M., Techritz N., Hampel D., Millonigg S., Eckmann C.R. The Ccr4-Not deadenylase complex constitutes the main poly(A) removal activity in C. elegans. J. Cell Sci. 2013;126:4274–4285. doi: 10.1242/jcs.132936. [DOI] [PubMed] [Google Scholar]
- 77.Wagner E., Clement S.L., Lykke-Andersen J. An unconventional human Ccr4-Caf1 deadenylase complex in nuclear cajal bodies. Mol. Cell. Biol. 2007;27:1686–1695. doi: 10.1128/MCB.01483-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mroczek S., Krwawicz J., Kutner J., Lazniewski M., Kuciński I., Ginalski K., et al. C16orf57, a gene mutated in poikiloderma with neutropenia, encodes a putative phosphodiesterase responsible for the U6 snRNA 3' end modification. Genes Dev. 2012;26:1911–1925. doi: 10.1101/gad.193169.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fica S.M., Nagai K. Cryo-electron microscopy snapshots of the spliceosome: structural insights into a dynamic ribonucleoprotein machine. Nat. Struct. Mol. Biol. 2017;24:791–799. doi: 10.1038/nsmb.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kunkel G.R., Maser R.L., Calvet J.P., Pederson T. U6 small nuclear RNA is transcribed by RNA polymerase III. Proc. Natl. Acad. Sci. U. S. A. 1986;83:8575–8579. doi: 10.1073/pnas.83.22.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arimbasseri A.G., Rijal K., Maraia R.J. Transcription termination by the eukaryotic RNA polymerase III. Biochim. Biophys. Acta. 2013;1829:318–330. doi: 10.1016/j.bbagrm.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Didychuk A.L., Montemayor E.J., Brow D.A., Butcher S.E. Structural requirements for protein-catalyzed annealing of U4 and U6 RNAs during di-snRNP assembly. Nucleic Acids Res. 2016;44:1398–1410. doi: 10.1093/nar/gkv1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Achsel T., Brahms H., Kastner B., Bachi A., Wilm M., Lührmann R. A doughnut-shaped heteromer of human Sm-like proteins binds to the 3'-end of U6 snRNA, thereby facilitating U4/U6 duplex formation in vitro. EMBO J. 1999;18:5789–5802. doi: 10.1093/emboj/18.20.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rader S.D., Guthrie C. A conserved Lsm-interaction motif in Prp24 required for efficient U4/U6 di-snRNP formation. RNA. 2002;8:1378–1392. doi: 10.1017/s1355838202020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nomura Y., Montemayor E.J., Virta J.M., Hayes S.M., Butcher S.E. Structural basis for the evolution of cyclic phosphodiesterase activity in the U6 snRNA exoribonuclease Usb1. Nucleic Acids Res. 2020;48:1423–1434. doi: 10.1093/nar/gkz1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shchepachev V., Wischnewski H., Soneson C., Arnold A.W., Azzalin C.M. Human Mpn1 promotes post-transcriptional processing and stability of U6atac. FEBS Lett. 2015;589:2417–2423. doi: 10.1016/j.febslet.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 87.Patil P., Uechi T., Kenmochi N. Incomplete splicing of neutrophil-specific genes affects neutrophil development in a zebrafish model of poikiloderma with neutropenia. RNA Biol. 2015;12:426–434. doi: 10.1080/15476286.2015.1017240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Colombo E.A., Carra S., Fontana L., Bresciani E., Cotelli F., Larizza L. A zebrafish model of poikiloderma with neutropenia recapitulates the human syndrome hallmarks and traces back neutropenia to the myeloid progenitor. Sci. Rep. 2015;5 doi: 10.1038/srep15814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Körner C.G., Wahle E. Poly(A) tail shortening by a mammalian poly(A)-specific 3'-exoribonuclease. J. Biol. Chem. 1997;272:10448–10456. doi: 10.1074/jbc.272.16.10448. [DOI] [PubMed] [Google Scholar]
- 90.Martinez J., Ren Y.G., Thuresson A.C., Hellman U., Astrom J., Virtanen A. A 54-kDa fragment of the Poly(A)-specific ribonuclease is an oligomeric, processive, and cap-interacting Poly(A)-specific 3' exonuclease. J. Biol. Chem. 2000;275:24222–24230. doi: 10.1074/jbc.M001705200. [DOI] [PubMed] [Google Scholar]
- 91.Dehlin E., Wormington M., Körner C.G., Wahle E. Cap-dependent deadenylation of mRNA. EMBO J. 2000;19:1079–1086. doi: 10.1093/emboj/19.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martînez J., Ren Y.G., Nilsson P., Ehrenberg M., Virtanen A. The mRNA cap structure stimulates rate of poly(A) removal and amplifies processivity of degradation. J. Biol. Chem. 2001;276:27923–27929. doi: 10.1074/jbc.M102270200. [DOI] [PubMed] [Google Scholar]
- 93.Gao M., Fritz D.T., Ford L.P., Wilusz J. Interaction between a poly(A)-specific ribonuclease and the 5' cap influences mRNA deadenylation rates in vitro. Mol. Cell. 2000;5:479–488. doi: 10.1016/s1097-2765(00)80442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Opyrchal M., Anderson J.R., Sokoloski K.J., Wilusz C.J., Wilusz J. A cell-free mRNA stability assay reveals conservation of the enzymes and mechanisms of mRNA decay between mosquito and mammalian cell lines. Insect Biochem. Mol. Biol. 2005;35:1321–1334. doi: 10.1016/j.ibmb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 95.Monecke T., Schell S., Dickmanns A., Ficner R. Crystal structure of the RRM domain of poly(A)-specific ribonuclease reveals a novel m(7)G-cap-binding mode. J. Mol. Biol. 2008;382:827–834. doi: 10.1016/j.jmb.2008.07.073. [DOI] [PubMed] [Google Scholar]
- 96.Liu W.F., Zhang A., He G.J., Yan Y.B. The R3H domain stabilizes poly(A)-specific ribonuclease by stabilizing the RRM domain. Biochem. Biophys. Res. Commun. 2007;360:846–851. doi: 10.1016/j.bbrc.2007.06.139. [DOI] [PubMed] [Google Scholar]
- 97.Nagata T., Suzuki S., Endo R., Shirouzu M., Terada T., Inoue M., et al. The RRM domain of poly(A)-specific ribonuclease has a noncanonical binding site for mRNA cap analog recognition. Nucleic Acids Res. 2008;36:4754–4767. doi: 10.1093/nar/gkn458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fong K.W., Li Y., Wang W., Ma W., Li K., Qi R.Z., et al. Whole-genome screening identifies proteins localized to distinct nuclear bodies. J. Cell Biol. 2013;203:149–164. doi: 10.1083/jcb.201303145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Copeland P.R., Wormington M. The mechanism and regulation of deadenylation: identification and characterization of Xenopus PARN. RNA. 2001;7:875–886. doi: 10.1017/s1355838201010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim J.H., andRichter J.D. Opposing polymerase-deadenylase activities regulate cytoplasmic polyadenylation. Mol. Cell. 2006;24:173–183. doi: 10.1016/j.molcel.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 101.Virtanen A., Henriksson N., Nilsson P., Nissbeck M. Poly(A)-specific ribonuclease (PARN): an allosterically regulated, processive and mRNA cap-interacting deadenylase. Crit. Rev. Biochem. Mol. Biol. 2013;48:192–209. doi: 10.3109/10409238.2013.771132. [DOI] [PubMed] [Google Scholar]
- 102.Yoda M., Cifuentes D., Izumi N., Sakaguchi Y., Suzuki T., Giraldez A.J., et al. Poly(A)-specific ribonuclease mediates 3'-end trimming of Argonaute2-cleaved precursor microRNAs. Cell Rep. 2013;5:715–726. doi: 10.1016/j.celrep.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ding D., Liu J., Dong K., Midic U., Hess R.A., Xie H., et al. PNLDC1 is essential for piRNA 3' end trimming and transposon silencing during spermatogenesis in mice. Nat. Commun. 2017;8:819. doi: 10.1038/s41467-017-00854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ishikawa H., Yoshikawa H., Izumikawa K., Miura Y., Taoka M., Nobe Y., et al. Poly(A)-specific ribonuclease regulates the processing of small-subunit rRNAs in human cells. Nucleic Acids Res. 2017;45:3437–3447. doi: 10.1093/nar/gkw1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Montellese C., Montel-Lehry N., Henras A.K., Kutay U., Gleizes P.E., O'Donohue M.F. Poly(A)-specific ribonuclease is a nuclear ribosome biogenesis factor involved in human 18S rRNA maturation. Nucleic Acids Res. 2017;45:6822–6836. doi: 10.1093/nar/gkx253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nishimura T., Nagamori I., Nakatani T., Izumi N., Tomari Y., Kuramochi-Miyagawa S., et al. PNLDC1, mouse pre-piRNA Trimmer, is required for meiotic and post-meiotic male germ cell development. EMBO Rep. 2018;19 doi: 10.15252/embr.201744957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nishimura N., Yoshida T., Murayama M., Asami T., Shinozaki K., Hirayama T. Isolation and characterization of novel mutants affecting the abscisic acid sensitivity of Arabidopsis germination and seedling growth. Plant Cell Physiol. 2004;45:1485–1499. doi: 10.1093/pcp/pch171. [DOI] [PubMed] [Google Scholar]
- 108.Nishimura N., Kitahata N., Seki M., Narusaka Y., Narusaka M., Kuromori T., et al. Analysis of ABA hypersensitive germination2 revealed the pivotal functions of PARN in stress response in Arabidopsis. Plant J. 2005;44:972–984. doi: 10.1111/j.1365-313X.2005.02589.x. [DOI] [PubMed] [Google Scholar]
- 109.Reverdatto S.V., Dutko J.A., Chekanova J.A., Hamilton D.A., Belostotsky D.A. mRNA deadenylation by PARN is essential for embryogenesis in higher plants. RNA. 2004;10:1200–1214. doi: 10.1261/rna.7540204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maragozidis P., Papanastasi E., Scutelnic D., Totomi A., Kokkori I., Zarogiannis S.G., et al. Poly(A)-specific ribonuclease and Nocturnin in squamous cell lung cancer: prognostic value and impact on gene expression. Mol. Cancer. 2015;14:187. doi: 10.1186/s12943-015-0457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roake C.M., Chen L., Chakravarthy A.L., Ferrell J.E., Raffa G.D., Artandi S.E. Disruption of telomerase RNA maturation kinetics precipitates disease. Mol. Cell. 2019;74:688–700.e683. doi: 10.1016/j.molcel.2019.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maragozidis P., Karangeli M., Labrou M., Dimoulou G., Papaspyrou K., Salataj E., et al. Alterations of deadenylase expression in acute leukemias: evidence for poly(a)-specific ribonuclease as a potential biomarker. Acta Haematol. 2012;128:39–46. doi: 10.1159/000337418. [DOI] [PubMed] [Google Scholar]
- 113.Gao M., Wilusz C.J., Peltz S.W., Wilusz J. A novel mRNA-decapping activity in HeLa cytoplasmic extracts is regulated by AU-rich elements. EMBO J. 2001;20:1134–1143. doi: 10.1093/emboj/20.5.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Balatsos N.A., Nilsson P., Mazza C., Cusack S., Virtanen A. Inhibition of mRNA deadenylation by the nuclear cap binding complex (CBC) J. Biol. Chem. 2006;281:4517–4522. doi: 10.1074/jbc.M508590200. [DOI] [PubMed] [Google Scholar]
- 115.Moraes K.C., Wilusz C.J., Wilusz J. CUG-BP binds to RNA substrates and recruits PARN deadenylase. RNA. 2006;12:1084–1091. doi: 10.1261/rna.59606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang X., Devany E., Murphy M.R., Glazman G., Persaud M., Kleiman F.E. PARN deadenylase is involved in miRNA-dependent degradation of TP53 mRNA in mammalian cells. Nucleic Acids Res. 2015;43:10925–10938. doi: 10.1093/nar/gkv959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lai W.S., Kennington E.A., Blackshear P.J. Tristetraprolin and its family members can promote the cell-free deadenylation of AU-rich element-containing mRNAs by poly(A) ribonuclease. Mol. Cell. Biol. 2003;23:3798–3812. doi: 10.1128/MCB.23.11.3798-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang R.P., Fan Y., de Belle I., Niemeyer C., Gottardis M.M., Mercola D., et al. Decreased Egr-1 expression in human, mouse and rat mammary cells and tissues correlates with tumor formation. Int. J. Cancer. 1997;72:102–109. doi: 10.1002/(sici)1097-0215(19970703)72:1<102::aid-ijc15>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 119.de Belle I., Huang R.P., Fan Y., Liu C., Mercola D., Adamson E.D. p53 and Egr-1 additively suppress transformed growth in HT1080 cells but Egr-1 counteracts p53-dependent apoptosis. Oncogene. 1999;18:3633–3642. doi: 10.1038/sj.onc.1202696. [DOI] [PubMed] [Google Scholar]
- 120.Wang H., Ming X., Zhang S., Chen J., Liu X., Wu X., et al. Knockdown of Toe1 causes developmental arrest during the morula-to-blastocyst transition in mice. Theriogenology. 2022;194:154–161. doi: 10.1016/j.theriogenology.2022.10.011. [DOI] [PubMed] [Google Scholar]
- 121.Sperandio S., Barat C., Cabrita M.A., Gargaun A., Berezovski M.V., Tremblay M.J., et al. TOE1 is an inhibitor of HIV-1 replication with cell-penetrating capability. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E3392–E3401. doi: 10.1073/pnas.1500857112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kow R.L., Strovas T.J., McMillan P.J., Jacobi A.M., Behlke M.A., Saxton A.D., et al. Distinct Poly(A) nucleases have differential impact on sut-2 dependent tauopathy phenotypes. Neurobiol. Dis. 2021;147 doi: 10.1016/j.nbd.2020.105148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Colombo E.A., Bazan J.F., Negri G., Gervasini C., Elcioglu N.H., Yucelten D., et al. Novel C16orf57 mutations in patients with Poikiloderma with Neutropenia: bioinformatic analysis of the protein and predicted effects of all reported mutations. Orphanet J. Rare Dis. 2012;7:7. doi: 10.1186/1750-1172-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mason P.J., Bessler M. The genetics of dyskeratosis congenita. Cancer Genet. 2011;204:635–645. doi: 10.1016/j.cancergen.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kirwan M., Dokal I. Dyskeratosis congenita, stem cells and telomeres. Biochim. Biophys. Acta. 2009;1792:371–379. doi: 10.1016/j.bbadis.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Namavar Y., Barth P.G., Kasher P.R., van Ruissen F., Brockmann K., Bernert G., et al. Clinical, neuroradiological and genetic findings in pontocerebellar hypoplasia. Brain. 2011;134:143–156. doi: 10.1093/brain/awq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.NORMAN R.M. Cerebellar hypoplasia in Werdnig-Hoffmann disease. Arch. Dis. Child. 1961;36:96–101. doi: 10.1136/adc.36.185.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goutières F., Aicardi J., Farkas E. Anterior horn cell disease associated with pontocerebellar hypoplasia in infants. J. Neurol. Neurosurg. Psychiatry. 1977;40:370–378. doi: 10.1136/jnnp.40.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barth P.G. Pontocerebellar hypoplasias. An overview of a group of inherited neurodegenerative disorders with fetal onset. Brain Dev. 1993;15:411–422. doi: 10.1016/0387-7604(93)90080-r. [DOI] [PubMed] [Google Scholar]
- 130.Barth P.G., Blennow G., Lenard H.G., Begeer J.H., van der Kley J.M., Hanefeld F., et al. The syndrome of autosomal recessive pontocerebellar hypoplasia, microcephaly, and extrapyramidal dyskinesia (pontocerebellar hypoplasia type 2): compiled data from 10 pedigrees. Neurology. 1995;45:311–317. doi: 10.1212/wnl.45.2.311. [DOI] [PubMed] [Google Scholar]
- 131.Barth P.G. Pontocerebellar hypoplasia--how many types? Eur. J. Paediatr. Neurol. 2000;4:161–162. doi: 10.1053/ejpn.2000.0294. [DOI] [PubMed] [Google Scholar]
- 132.Barth P.G., Aronica E., de Vries L., Nikkels P.G., Scheper W., Hoozemans J.J., et al. Pontocerebellar hypoplasia type 2: a neuropathological update. Acta Neuropathol. 2007;114:373–386. doi: 10.1007/s00401-007-0263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Renbaum P., Kellerman E., Jaron R., Geiger D., Segel R., Lee M., et al. Spinal muscular atrophy with pontocerebellar hypoplasia is caused by a mutation in the VRK1 gene. Am. J. Hum. Genet. 2009;85:281–289. doi: 10.1016/j.ajhg.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Albrecht S., Schneider M.C., Belmont J., Armstrong D.L. Fatal infantile encephalopathy with olivopontocerebellar hypoplasia and micrencephaly. Report three siblings. Acta Neuropathol. 1993;85:394–399. doi: 10.1007/BF00334450. [DOI] [PubMed] [Google Scholar]
- 135.Budde B.S., Namavar Y., Barth P.G., Poll-The B.T., Nürnberg G., Becker C., et al. tRNA splicing endonuclease mutations cause pontocerebellar hypoplasia. Nat. Genet. 2008;40:1113–1118. doi: 10.1038/ng.204. [DOI] [PubMed] [Google Scholar]
- 136.Rajab A., Mochida G.H., Hill A., Ganesh V., Bodell A., Riaz A., et al. A novel form of pontocerebellar hypoplasia maps to chromosome 7q11-21. Neurology. 2003;60:1664–1667. doi: 10.1212/01.wnl.0000068548.58498.41. [DOI] [PubMed] [Google Scholar]
- 137.Durmaz B., Wollnik B., Cogulu O., Li Y., Tekgul H., Hazan F., et al. Pontocerebellar hypoplasia type III (CLAM): extended phenotype and novel molecular findings. J. Neurol. 2009;256:416–419. doi: 10.1007/s00415-009-0094-0. [DOI] [PubMed] [Google Scholar]
- 138.Patel M.S., Becker L.E., Toi A., Armstrong D.L., Chitayat D. Severe, fetal-onset form of olivopontocerebellar hypoplasia in three sibs: PCH type 5? Am. J. Med. Genet. A. 2006;140:594–603. doi: 10.1002/ajmg.a.31095. [DOI] [PubMed] [Google Scholar]
- 139.Edvardson S., Shaag A., Kolesnikova O., Gomori J.M., Tarassov I., Einbinder T., et al. Deleterious mutation in the mitochondrial arginyl-transfer RNA synthetase gene is associated with pontocerebellar hypoplasia. Am. J. Hum. Genet. 2007;81:857–862. doi: 10.1086/521227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rankin J., Brown R., Dobyns W.B., Harington J., Patel J., Quinn M., et al. Pontocerebellar hypoplasia type 6: a british case with PEHO-like features. Am. J. Med. Genet. A. 2010;152A:2079–2084. doi: 10.1002/ajmg.a.33531. [DOI] [PubMed] [Google Scholar]
- 141.Anderson C., Davies J.H., Lamont L., Foulds N. Early pontocerebellar hypoplasia with vanishing testes: a new syndrome? Am. J. Med. Genet. A. 2011;155A:667–672. doi: 10.1002/ajmg.a.33897. [DOI] [PubMed] [Google Scholar]
- 142.Bakar Ö., Işik U., Canpolat C., Alanay Y. Hoyeraal-Hreidarsson Syndrome: an extremely rare Dyskeratosis Congenita Phenotype. Pediatr. Dermatol. 2015;32:e263–266. doi: 10.1111/pde.12693. [DOI] [PubMed] [Google Scholar]
- 143.Vogiatzi P., Perdigones N., Mason P.J., Wilson D.B., Bessler M. A family with Hoyeraal-Hreidarsson syndrome and four variants in two genes of the telomerase core complex. Pediatr. Blood Cancer. 2013;60:E4–E6. doi: 10.1002/pbc.24389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nagpal N., Agarwal S. Telomerase RNA processing: implications for human health and disease. Stem Cells. 2020 doi: 10.1002/stem.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Batista L.F.Z., Dokal I., Parker R. Telomere biology disorders: time for moving towards the clinic? Trends Mol. Med. 2022;28:882–891. doi: 10.1016/j.molmed.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Larizza L., Roversi G., Volpi L. Rothmund-thomson syndrome. Orphanet J. Rare Dis. 2010;5:2. doi: 10.1186/1750-1172-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]