Summary
We present a protocol for measuring the pH of cell-free bacterial-conditioned media based on changes in the ultraviolet-visible (UV-Vis) absorbance spectrum using the pH indicator dye litmus. This protocol includes detailed procedures for performing bacterial culturing, examining bacterial growth, collecting cell-free supernatant, litmus dye addition, and pH-based calibration curve preparations. This assay has been designed for flexible formatting that can accommodate both high-volume and low-volume sample sets.
Subject areas: High Throughput Screening, Microbiology
Graphical abstract
Highlights
-
•
Existing UV-vis spectrophotometers can determine solution pH of cell-free cultures
-
•
Optical cuvette-based format can integrate into existing microbiology workflows
-
•
96-well plate sample formatting allows for high-throughput sample analysis
-
•
Litmus dye pH dynamic range (4.4–8.8) is suitable for microbiological applications
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
We present a protocol for measuring the pH of cell-free bacterial-conditioned media based on changes in the ultraviolet-visible (UV-vis) absorbance spectrum using the pH indicator dye litmus. This protocol includes detailed procedures for performing bacterial culturing, examining bacterial growth, collecting cell-free supernatant, litmus dye addition, and pH-based calibration curve preparations. This assay has been designed for flexible formatting that can accommodate both high-volume and low-volume sample sets.
Before you begin
High-throughput screening (HTS) has emerged as a valued technology for many scientific applications. Recently, precise HTS systems have been implemented for the examination of bacterial growth,1 but at present, the measurement of solution pH for the high volumes of cell-free conditioned media samples generated by these HTS systems remains a challenge. Conditioned media pH determinations are highly relevant to downstream applications, such as cell treatment or co-culture, organoid microinjection, or metabolite analysis.2,3,4,5,6 The traditional method for measuring the solution pH of conditioned media samples is to use a calibrated pH sensitive electrode. However, this approach is not compatible with HTS-based microbiology applications based on the use of microplates. First, microplate well volumes (∼300–400 μL) are typically too small to accommodate the displacement volume for most pH electrodes. Second, it is not possible to perform pH measurements in all wells simultaneously using a single electrode, and pH electrode arrays designed for 96-well plate formats do not yet exist. Third, the use of electrodes could introduce well-to-well contamination and influence downstream analysis. Finally, electrode-based pH measurement systems are also prone to protein layer build-up on the surface of the pH sensitive glass membrane on the electrode, which can result in measurement errors.7 Commercial kits have been designed to measure the pH of samples contained in a 96-well plate format using fluorescent dyes.8 Fluorescent indicators are a valuable tool for measuring the pH of transparent media solutions. Unfortunately, some components present in a bacterial growth medium are highly fluorescent, making fluorescence-based pH measurements challenging. Moreover, these kits and fluorescent dyes are expensive and often not available to laboratories with limited access to a highly developed supply chain. These types of assays also require fluorescent plate readers, which are not available at all institutions, and tend to be expensive. To overcome these limitations, we have developed a simple, high-throughput assay to measure the solution pH of conditioned bacterial growth media using the pH-sensitive indicator litmus dye. Litmus dye is commonly available and relatively cheap. Litmus dye solutions exhibit a gradual color transition from orange (in acidic conditions) to purple (in basic conditions) over a pH range of approximately 4.4 to 8.8 (see Figure 1A). In this study, we describe a high-throughput and accessible method to monitor pH changes in conditioned bacterial media samples using either a high volume (∼1 mL optical cuvettes) format using a spectrophotometer, or a high-throughput 96-well plate-based low volume (∼ 0.100 mL) format using a standard microplate reader. We employed a 10 mg/mL stock solution of litmus dye to perform these studies. This method allows for rapid and accurate pH measurement which can easily be applied to multiple organisms. The absorption spectroscopy measurements described in this protocol can be performed using UV-Vis spectrophotometers typically found in microbiology labs.
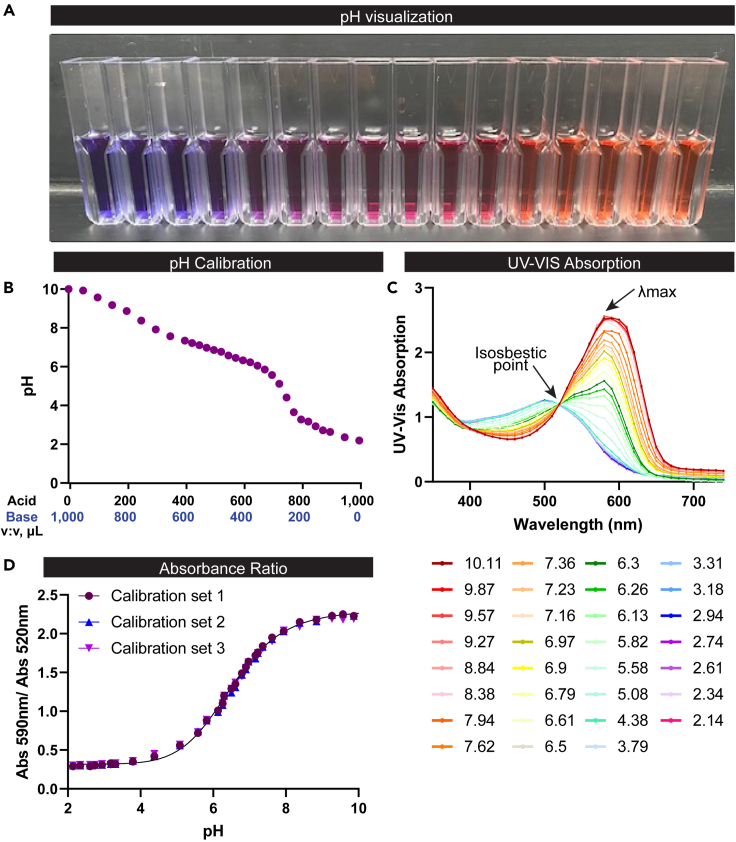
Figure 1.
The pH of ZMB1 containing litmus dye can be measured by UV-Vis absorption spectrophotometry by cuvette
(A) Representative image of the color of ZMB1 solutions over a pH range that spans from blue at pH 10.11 (left-side) to red at pH 2.14 (right-side).
(B) Plot of the measured pH for a series of ZMB1 calibrator solutions that were prepared at different ratios of ZMB1 acidic (pH 2) and basic (pH 10) stock solutions.
(C) UV-Vis absorption spectrum for the pH-sensitive litmus dye produced over a range of pHs. The isosbestic point – a wavelength where the light absorption of an indicator dye is independent of solution pH – for litmus (520 nm) is indicated by an arrow in the plot. The wavelength of maximum light absorption, or the lambda max (λmax), is indicated with a separate arrow at 590 nm.
(D) The Calibration Curve was fit using the referenced Python code for sigmoid-based calibration curve fitting with the absorption ratio (Abs590 nm / Abs520 nm) plotted as a function of solution pH.
Preparation of the litmus dye pH indicator solution
Timing: 1 day
The litmus dye stock solution preparation described here should be prepared immediately prior to use, and will serve as the UV-Vis absorption-based pH indicator in all cell-free bacterial-conditioned culture samples. It is critical that the litmus stock solution is filtered to remove undissolved dye prior to use.
Litmus stock solution
-
1.
Weigh out 1 g of litmus, and transfer the dye into a 250 mL glass bottle.
-
2.
Add a 50 mL volume of water to the litmus dye.
-
3.
Allow the dye to incubate at room temperature overnight.
-
4.
The following day, filter the solution.
-
5.
Add a 30 mL volume of 95% ethanol to the filtrate.
-
6.
Fill to 100 mL total volume with water.
-
7.
The concentration of litmus dye in this stock solution is 10 mg/mL.
Preparation of the chemically defined bacterial medium ZMB1
Timing: 3 h
ZMB1 is chemically defined bacterial growth medium that is comprised of nine different solution preparations – each of which has been described in detail directly below. Prior to use, the ZMB1 growth medium needs to be sterile-filtered using a 0.2 μm filter apparatus in a biosafety cabinet, and the bottle needs to be wrapped in aluminum foil to protect the growth medium from light. Sterile ZMB1 growth medium can be used for up to 4 months when protected from light and stored at +4°C.
ZMB1 preparation
-
8.
Add a 900 mL volume of deionized water into a clean glass 1 L bottle.
-
9.
Add a magnetic stir bar into the bottle and place the bottle on a magnetic plate. Set the plate to medium speed (500–1,000 rpm) to mix the solution.
-
10.
Weigh out the following amino acids, buffers and salts and add them to the water to create the Base Medium.
-
11.
For a typical experiment, weigh out an appropriate mass of glucose and add it to the Base Medium. If the goal of the experiment is to analyze bacterial growth in response to different carbohydrates, then do not include the glucose to the base medium.
Base Medium
| Reagent | Final concentration | Amount |
|---|---|---|
| L- Alanine | 5.0 mM | 0.40 g |
| L-Arginine | 4.6 mM | 0.72 g |
| L-asparagine | 4.2 mM | 0.50 g |
| Aspartic Acid | 0.42 mM | 0.05 g |
| L-Cysteine hydrochloride monohydrate | 1.2 mM (L-Cys) | 0.20 g |
| L-Glutamine | 4.6 mM | 0.60 g |
| L-Glutamic Acid | 4.5 mM | 0.60 g |
| Glycine | 4.4 mM | 0.30 g |
| L-Histidine | 1.2 mM | 0.17 g |
| L-Isoleucine | 2.0 mM | 0.24 g |
| L-Leucine | 8.4 mM | 1.00 g |
| L-Lysine mono hydrochloride | 3.0 mM (L-Lys) | 0.50 g |
| L-Methionine | 0.44 mM | 0.06 g |
| L-Phenylalanine | 2.6 mM | 0.40 g |
| L-Proline | 6.8 mM | 0.70 g |
| L-Serine | 5.2 mM | 0.50 g |
| L-Threonine | 4.6 mM | 0.50 g |
| L-Tryptophan | 1.1 mM | 0.20 g |
| L-Tyrosine | 1.8 mM | 0.30 g |
| L-Valine | 6.6 mM | 0.70 g |
| Potassium phosphate monobasic | 25.3 | 3.10 g |
| Sodium phosphate dibasic | 50.1 | 6.40 g |
| Sodium chloride | 57.0 | 3.00 g |
| ∗Glucose | 92.5 | 15.0 g |
| Cobalt(II) chloride hexahydrate | 0.88 μM (Co2+ ) | 0.00019 g |
| Calcium chloride dihydrate | 0.30 mM (Ca2+) | 0.04 g |
| Ferrous sulfate heptahydrate | 0.16 mM (Fe2+) | 0.04 g |
| Copper(II) sulfate pentahydrate | 0.84 μM (Cu2+) | 0.00019 g |
| Ammonium molybdate tetrahydrate | 0.34 μM | 0.00038 g |
| Magnesium sulfate heptahydrate | 4.5 mM (Mg2+) | 1.00 g |
| Zinc Sulfate heptahydrate | 0.019 mM (Zn2+) | 0.005 g |
| Ammonium Sulfate | 8.4 mM | 1.00 g |
| Boric Acid | 0.013 mM | 0.00075 g |
| Potassium Sulfate | 0.14 mM | 0.023 g |
| Potassium Iodide | 0.00074 mM | 0.00011 g |
| Nitrilotriacetic acid | 0.044 mM | 0.0075 g |
| MOPS | 79.6 mM | 15.0 g |
| Tricine | 9.30 mM | 1.5 g |
| Glutathione | 0.054 mM | 0.015 |
| Water | N/A | 900 mL |
| Total Volume | N/A | 900 mL |
Note: The Base Medium can be stored at 4°C for 4 months protected from light.
Note: We recommend making the ZMB1 without glucose and then adding the desired carbohydrate source. However, if no other nutrient sources will be tested, glucose can be added at this step. Most bacteria will not grow well in ZMB1 without glucose.
Water-Soluble Vitamin Solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Thiamine hydrochloride | 0.17 mM | 5.60 mg |
| D-Pantothenic acid hemicalcium salt | 0.22 mM | 12.0 mg |
| Nicotinamide | 0.74 mM | 9.00 mg |
| Pyridoxine hydrochloride | 2.4 mM | 48.0 mg |
| p-Aminobenzoic acid | 0.041 mM | 0.56 mg |
| Vitamin B12 (cobalamin) | 0.074 mM | 10.0 mg |
| Inositol | 1.11 mM | 20.0 mg |
| Water | N/A | 100 mL |
| Total Volume | N/A | 100 mL |
Note: The Water-Soluble Vitamin Solution can be stored at 4°C for 4 months protected from light.
-
12.
Prepare a 1 M NaOH solution by adding 3.97 g of anhydrous NaOH pellets to 100 mL of water.
Note: If NaOH solutions are to be stored in glass bottles, then a plastic cap or plastic stopper must be used to cap the solution - a glass stopper and a glass bottle will fuse in the presence of NaOH, and the bottle cannot be opened.
CRITICAL: Concentrated NaOH solutions are caustic; see the reagent safety statements Section for instructions on handling this solution.
Folic Acid Solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Folic acid | 126 mM | 0.56 g |
| 1 M NaOH solution | N/A | 10 mL |
| Total Volume | N/A | 10 mL |
Note: The Folic Acid Solution can be stored at 4°C for 4 months protected from light.
Biotin Solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Biotin | 2.4 mM | 0.06 g |
| Ethanol | 0.150–0.250 mL | 3–5 drops |
| Water | N/A | 100 mL |
| Total Volume | N/A | ∼100 mL |
Note: The Biotin Solution can be stored at 4°C for 4 months protected from light.
Note: Add 3–5 drops of 100% (200 proof ethanol) to the solution and swirl the contents to dissolve the biotin. If needed, the solution can be warmed to 40°C to aid in the dissolution of biotin.
-
13.
Prepare a 0.02 M acetic acid solution by adding a 57-μL volume of glacial acetic acid into 12.5 mL deionized water.
Note: Acetic acid is volatile, and these solutions can be stored for a period of several months when the solution is stored in a container with a well-secured cap.
CRITICAL: Glacial acetic acid is volatile and corrosive; see the reagent safety statements Section for instructions on handling this solution.
Riboflavin Solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Riboflavin | 0.531 mM | 0.005 g |
| 0.02 M Acetic Acid Solution | N/A | 25 mL |
| Total Volume | N/A | 25 mL |
Note: The Riboflavin Solution can be stored at 4°C for 4 months protected from light.
Note: If needed, the solution can be warmed to 40°C to aid in the dissolution of riboflavin.
Lipoic Acid Solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Lipoic acid | 0.48 mM | 0.001 g |
| Potassium acetate | 916 mM | 0.900 g |
| Water | N/A | 10 mL |
| Total Volume | N/A | 10 mL |
Note: Prepare the Lipoic Acid Solution fresh for each use.
Note: Heat this solution to 60°C to aid in the dissolve of the materials.
-
14.
Prepare a 10 M NaOH solution by dissolving 40 g of NaOH pellets in 80 mL water, stirring continuously. Fill to 100 mL with water once in solution.
CRITICAL: Concentrated NaOH solutions are caustic; see the reagent safety statements Section for instructions on handling this solution.
10× Nucleic Acid Base Group Solution
| Ingredient | Final concentration | Amount |
|---|---|---|
| Uracil | 205 mM | 0.23 g |
| Xanthine | 25.0 mM | 0.038 g |
| 10 M NaOH | N/A | 10 mL |
| Total Volume | N/A | 10 mL |
Note: The 10× Nucleic Acid Base Group Solution can be stored at 4°C for 4 months.
Note: Concentrated NaOH solutions are caustic, see the reagent safety statements Section for instructions on handling this solution.
100× Nucleic Acid Base Group Solution
| Ingredient | Final concentration | Amount |
|---|---|---|
| Guanine | 370 mM | 0.56 g |
| 10 M Sulfuric Acid (H2SO4) | N/A | 10 mL |
| Total Volume | N/A | 10 mL |
Note: The 100× Nucleic Acid Base Group Solution can be stored at 4°C for 4 months.
Note: Sulfuric acid is volatile, and these solutions can be stored for a period of several months when the solution is stored in a container with a well-secured cap.
Note: Concentrated sulfuric acid solutions are volatile and corrosive; see the reagent safety statements Section for instructions on handling this solution.
1× Nucleic Acid Base Group Solution
| Ingredient | Final concentration | Amount |
|---|---|---|
| Adenine | 8.14 mM | 0.011 g |
| Water | N/A | 10 mL |
| Total Volume | N/A | 10 mL |
Note: Prepare the 1× Nucleic Acid Base Group Solution fresh for each use.
Note: Heat this solution to 60°C to aid in the dissolve the adenine component.
ZMB1 Growth Medium
| Solution | Amount |
|---|---|
| Base Medium | 900 mL |
| Water-Soluble Vitamin Solution | 10 mL |
| Folic Acid Solution | 0.1 mL |
| Biotin Solution | 1 mL |
| Riboflavin Solution | 5 mL |
| Lipoic Acid Solution | 10 mL |
| 10× Nucleic Acid Base Group Solution | 1 mL |
| 100× Nucleic Acid Base Group Solution | 1 mL |
| 1× Nucleic Acid Base Group Solution | 10 mL |
| Adjust to pH 7 with 10 M NaOH | A few drops |
| Fill to volume with deionized water | 1,000 mL |
Note: The ZMBI Growth Medium can be stored at 4°C for 4 months.
-
15.
In a biosafety cabinet, sterile filter the solution through a 0.2 μm filter unit.
-
16.
Wrap the bottle containing the solution in foil to protect it from light and store the medium at 4°C for 4 months.
Reagent safety statements
CRITICAL: Metal salts can be toxic by all routes of entry (skin absorption, ingestion, or inhalation), and can be corrosive or caustic depending on the anion present in the salt, and can cause severe burns to the skin, eyes, mouth, or respiratory and GI tract. Proper gloves, goggles, and a chemical-resistant smock should be worn when handling these materials.
CRITICAL: Anhydrous NaOH pellets are a caustic material, and can cause severe burns to the skin and eyes. Proper gloves, goggles, and a chemical-resistant smock should be worn when working with this material. Dissolving anhydrous NaOH pellets in water is exothermic, and a tremendous amount of heat can be generated – only use containers that are thermally stable.
CRITICAL: Concentrated sulfuric acid solutions are volatile and corrosive, and can cause severe burns to the skin, eyes, mouth, respiratory and GI tract during even short exposures. Proper gloves, goggles, and a chemical-resistant smock should be worn, and a chemical fume hood should be used when working with these solutions.
CRITICAL: Concentrated glacial acetic acid solutions are volatile and corrosive, and can cause severe burns to the skin, eyes, mouth, respiratory and GI tract during even short exposures. Proper gloves, goggles, and a chemical-resistant smock should be worn, and a chemical fume hood should be used when working with these solutions.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| Lactobacillus acidophilus | ATCC | ATCC 4796 |
| Lactococcus lactis | Carolina Biologicals | CB1 |
| Enterococcus faecalis | ATCC | 29212 |
| Chemicals, peptides, and recombinant proteins | ||
| 3-morpholinopropane-1-sulfonic acid (MOPS) | Millipore Sigma | M1254 |
| Glacial acetic acid | Fisher Scientific | A491-212 |
| Adenine | Fisher Scientific | A0149 |
| Ammonium molybdate tetrahydrate | Millipore Sigma | M1019 |
| Ammonium sulfate | Millipore Sigma | A4418 |
| Arabinose | Millipore Sigma | A3256 |
| Biotin | Fisher Scientific | B0463 |
| Boric acid | Fisher Scientific | AA3677136 |
| Calcium chloride dehydrate | Fisher Scientific | BP510 |
| Cobalt (II) chloride hexahydrate | Millipore Sigma | 255599 |
| Copper (II) sulfate pentahydrate | Millipore Sigma | 209198 |
| D-pantothenic acid hemicalcium salt | Fisher Scientific | 18-602-346 |
| de Man, Rogosa and Sharpe (MRS) growth media | Difco, VWR | 90004–082 |
| Ethanol | Fisher Scientific | BP2818100 |
| Ferrous sulfate heptahydrate | Millipore Sigma | F-8633 |
| Folic acid | Millipore Sigma | F87758 |
| Fructose | Fisher Scientific | A17718 |
| Galactose | Fisher Scientific | DF0163-17-4 |
| Glucose | Fisher Scientific | AC170080010 |
| Glutathione | Millipore Sigma | G-4251 |
| Glycine | Millipore Sigma | G8790 |
| Guanine | Fisher Scientific | G46-500 |
| Hydrochloric acid (ACS reagent; 37%) | Millipore Sigma | 320331 |
| Inositol | Fisher Scientific | A13586 |
| L-alanine | Fisher Scientific | A0179 |
| L-arginine | Fisher Scientific | A14730 |
| L-asparagine | Fisher Scientific | A0542 |
| L-aspartic acid | Fisher Scientific | A0546 |
| L-cysteine hydrochloride monohydrate | Fisher Scientific | BP376-100 |
| L-glutamic acid | Fisher Scientific | AC156212500 |
| L-glutamine | Fisher Scientific | G0063 |
| L-histidine | Millipore Sigma | H8000 |
| L-isoleucine | Fisher Scientific | BP384-100 |
| L-leucine | Fisher Scientific | A12311 |
| L-lysine hydrochloride | Millipore Sigma | L8662 |
| L-methionine | Fisher Scientific | AAA1031836 |
| L-phenylalanine | Fisher Scientific | A13238 |
| L-proline | Fisher Scientific | P0481 |
| L-serine | Fisher Scientific | AAA1117936 |
| L-threonine | Fisher Scientific | J63709 |
| L-tryptophan | Fisher Scientific | T0541 |
| L-tyrosine | Fisher Scientific | AAA1114130 |
| L-valine | Millipore Sigma | V0500 |
| Lipoic acid | Millipore Sigma | T5625 |
| Magnesium sulfate heptahydrate | Fisher Scientific | M63-500 |
| Mannose | Fisher Scientific | AAA1084214 |
| Nicotinamide | Millipore Sigma | N0636 |
| Nitrilotriacetic acid | Fisher Scientific | 415742500 |
| P-aminobenzoic acid | Fisher Scientific | ICN10256990 |
| Litmus dye | Fisher Scientific | S25747A |
| Phosphate-buffered saline (PBS) 1× | VWR | 76371 |
| Potassium acetate | Millipore Sigma | 236497 |
| Potassium iodide | Fisher Scientific | A12704 |
| Potassium phosphate monobasic | Millipore Sigma | P5655 |
| Potassium sulfate | Fisher Scientific | P305-500 |
| Pyridoxine hydrochloride | Fisher Scientific | P0561 |
| Raffinose | Millipore Sigma | R-0250 |
| Riboflavin | Millipore Sigma | 555682 |
| Sodium chloride | VWR | BDH9286 |
| Sodium hydroxide (NaOH; anhydrous, 98% pure) | Millipore Sigma | S5881 |
| Sodium phosphate dibasic | Millipore Sigma | 94046 |
| Sucrose | Fisher Scientific | BP220-1 |
| Sulfuric acid | Millipore Sigma | 258105 |
| Thiamine hydrochloride | Fisher Scientific | A19560 |
| Tricine | Millipore Sigma | T0682250G |
| Uracil | Millipore Sigma | U-0750 |
| Vitamin B12 (cobalamin) | Fisher Scientific | C0449 |
| Xanthine | Fisher Scientific | X0004 |
| Zinc sulfate heptahydrate | Fisher Scientific | A12915 |
| de Man, Rogosa and Sharpe (MRS) medium | Fisher Scientific | BD 288110 |
| de Man, Rogosa and Sharpe (MRS) agar | Fisher Scientific | BD 288210 |
| Brain Heart Infusion (BHI) medium | Fisher Scientific | R452472 |
| Brain Heart Infusion (BHI) medium agar | Fisher Scientific | R452452 |
| Software and algorithms | ||
| Spyder-IDE (version# 4.2.5) | ||
| Python (version# 3.7.4) | ||
| Python code for sigmoid-based calibration curve fitting Lukmen-lab/pHSigma: Fitting UV-VIS pH indicator litmus absorbance spectrum ratio calibration | Zenodo https://doi.org/10.5281/zenodo.8206410 |
||
| Other | ||
| Anaerobic growth chamber | Anaerobe Systems | AS-150 |
| Hand-held pH meter | Oakton | 35634–30 |
| Spectrophotometer (for OD600nm for microbial growth measurements) | Bio-Rad | SmartSpec 3000 |
| NanoDrop spectrophotometer | Thermo Fisher | One/OneC |
| 96-well DeepWell polypropylene (PP) microplates (2 mL) | Fisher Scientific | 12-566-120 |
| 96-well conical bottom polystyrene (PS) microplates (300 μL) | Thermo Fisher | 277143 |
| 96-well flat bottom polystyrene (PS) microplates (300 μL) | Fisher Scientific | 12-565-501 |
| 96-well filter plate containing a 0.2 μm PVDF filter | Fisher Scientific | MSIPS4510 |
| 1.5 mL optical polymethyl methacrylate (PMMA) cuvettes (1.5–3.0 mL) | VWR | 97000-586 |
| Sterile loop | Millipore Sigma | I8388 |
| Petri dishes | Fisher Scientific | FB0875712 |
| 96-well plate silicon mat | Fisher Scientific | 03-396-49 |
Step-by-step method details
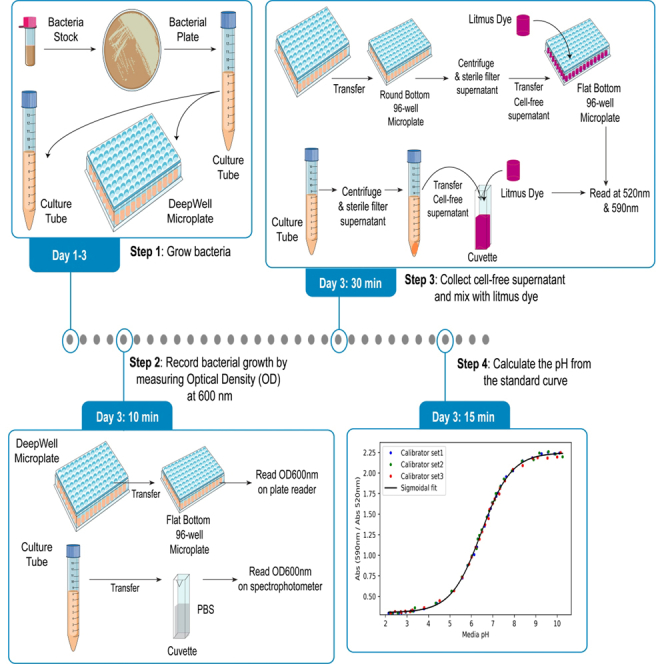
Grow bacteria in a rich medium
Timing: 2 days
A small amount of bacteria cells are pulled from frozen stock and are cultured overnight on an appropriate agar plate that contains a rich growth medium such as De man, Rogosa, and Sharpe (MRS) or brain heart infusion (BHI). After confirming contaminant-free growth of the desired microbe, a second overnight culture is performed in liquid rich medium culture by removing a single colony and dispersing it into a 5 mL volume of the rich growth medium.
-
1.Create an agar plate with single isolates of Lactobacillus acidophilus ATCC 4796 on a sterile MRS agar plate.
-
a.Remove a frozen L. acidophilus glycerol stock from the −80°C freezer and transfer the vial on dry ice to a sterile biosafety cabinet or lab bench with a Bunsen burner.
-
b.Keep the stock on dry ice throughout the inoculation. Do not allow the stock to thaw.
-
c.Using the pointed end of a sterile loop, collect a small amount of bacteria (around the size of a sunflower seed) from the frozen stock and streak bacteria on the MRS agar plate using a four-quadrant method.
-
d.Incubate the plate at 37°C aerobically.
-
a.
-
2.Create an agar plate with single isolates of Lactococcus lactis CB and Enterococcus faecalis ATCC 29212 on a sterile BHI agar plate.
-
a.Remove frozen L. lactis and E. faecalis glycerol stock from the −80°C freezer and transfer the vial on ice to a sterile biosafety cabinet or lab bench with a Bunsen burner.
-
b.Keep the stock on ice throughout the inoculation.
-
c.Using the pointed end of a sterile loop, collect a small amount of bacteria (around the size of a sunflower seed) from the frozen stock and streak bacteria on the BHI agar plate using a four-quadrant method.
-
d.Incubate the plate at 37°C aerobically.
-
a.
-
3.
The next morning, inspect the MRS and BHI agar plates to confirm growth, and assess the culture for the existence of microbial contaminants.
-
4.Grow an overnight culture of bacteria in rich medium.
-
a.Using a sterile loop, select a single L. acidophilus colony from the MRS agar plate and add the loop to 5 mL volume of MRS growth medium in a 14 mL culture tube.
-
b.Using a sterile loop, select a single L. lactis or E. faecalis colony from the BHI agar plates and add the loop to 5 mL volume of BHI growth medium in individual 14 mL culture tubes.
-
c.Loosely cap the inoculated medium culture and incubate aerobically overnight at 37°C.
-
d.The next morning, examine the culture under a light microscope at 40× to ensure there are no contaminants.
-
e.Measure the growth of the bacteria by examining the optical density as follows:
-
i.Vortex-mix the culture vigorously to ensure a homogenous microbial suspension.
-
ii.Transfer a 100 μL volume of the bacterial culture into an optical cuvette that contains a 900 μL volume of phosphate-buffered saline (PBS) to create a 1:10 dilution.
-
iii.Add 100 μL volume of uninoculated MRS medium or BHI medium into an optical cuvette that contains a 900 μL volume of phosphate-buffered saline (PBS) to serve as a blank for the spectrophotometer.
-
iv.The microbial density is determined by measuring the optical density at 600 nm (OD600nm) against a blank consisting of a 100 μL volume of MRS with a 900 μL volume of PBS using a SmartSpec 3000 spectrophotometer, or equivalent instrument.
-
i.
-
a.
CRITICAL: Bacterial medium selection depends on the microbe used in the analysis. As an example, we have selected L. acidophilus, L. lactis, and E. faecalis because these are all lactic acid bacteria that can significantly lower the pH of a solution. However, other bacteria and their agar and preferred rich medium can be used. If strict anaerobic organisms are selected, an anaerobic workstation is required for cultivating those microbes. Facultative anaerobes and strict aerobes can be grown in traditional incubators. The examples used in this protocol are facultative anaerobes and can be grown aerobically.
Grow bacteria in a chemically defined bacterial medium ZMB1
Timing: 1 day
The chemically defined bacterial growth medium ZMB1 is used for this work because this growth medium can be used to grow multiple organisms (i.e., Bacteroides, Clostridium, Lactobacillus, Streptococcus, Bifidobacteria, Escherichia, Klebsiella, Blautia, etc) and is amiable to downstream analysis such as liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based bioanalysis and pH assessment.2,9
-
5.Sub-culture bacteria from the rich culture into the chemically defined growth medium ZMB1 and aliquot the solution into a 96-well DeepWell polypropylene microplate.
-
a.Using the OD600nm measurement as a reference, inoculate an appropriate volume the cultures into the appropriate volume of ZMB1 to final create a solution with an OD600nm = 0.1.
-
b.Vortex mix vigorously the inoculated ZMB1 medium to ensure a homogenous microbial suspension.
-
c.Aliquot 2 mL volume of the inoculated ZMB1 medium into individual wells in a 96-well DeepWell polypropylene microplate. Chemicals or nutrient sources can be added to this solution for testing.
-
d.Cover the 96-well DeepWell polypropylene microplate with a silicon mat, and culture the bacteria aerobically with rotational mixing for 20 h at 37°C.
-
a.
CRITICAL: The ZMB1 medium can be made with or without glucose. If you want to compare bacterial growth on different substrates (like galactose, mannose, arabinose, fructose, etc), do not add glucose to the ZMB1 stock solution. We recommend adding 100 mM of carbohydrate to test different nutrient sources. If growing anaerobic bacteria, DeepWell microplate plate can be grown anaerobically for 20 h at 37°C.
Assess bacterial growth by measuring optical density on a plate reader
Timing: 10 min
The Optical Density at 600 nm (OD600nm) is obtained to determine the degree of bacterial growth during the overnight culture.
-
6.After the incubation is complete, examine the bacterial growth by measuring the optical density:
-
a.Using a multi-channel pipette, mix the wells of the DeepWell polypropylene microplate containing L. acidophilus, L. lactis and E. faecalis cultures and transfer a 100 μL volume from each into a 96-well flat bottom polystyrene (PS) microplate
-
b.Measure the OD600nm using a Synergy H1 plate reader employing path correction, or an equivalent instrument
-
a.
CRITICAL: If no bacterial growth is observed, there may be components missing from the ZMB1 that are required for the growth of the selected organism. These components can be added to the ZMB1 for optimal growth.
Generate a calibration curve
Timing: 10 min
A 12-point calibration curve can be prepared for day-to-day UV-Vis spectrophotometer calibrations for routine microbiology work. The 31-point calibration curve described below was prepared specifically to determine the quality of the sigmoidal fitting algorithm, and to assess the accuracy of pH measurements across the entire dynamic range of litmus dye. Further, we used this experimental accuracy data to define a dynamic pH range limit (pH range of 4.4–8.8) for Litmus dye that is accurate and useful for microbiological applications.
-
7.
To create a ZMB1 solution at a basic pH, add 10 M NaOH stepwise to a 40 mL volume of uninoculated ZMB1 medium until the pH is ∼10. This solution is the basic ZMB1 media stock solution for bulk Calibration Standard preparations.
-
8.
To create a ZMB1 solution at an acidic pH, add 10 M HCl stepwise to a 40 mL volume of uninoculated ZMB1 medium until the pH is ∼2. This solution is the acidic ZMB1 media stock solution for bulk calibration standard preparations.
-
9.Mix the acidic and basic ZMB1 media stocks at set volume proportions in order to prepare the calibration standards for a 12-point calibration curve at 1 mL solution volumes.
-
a.Calibration #1 1: 0 μL acidic ZMB1 (pH 2) + 1000 μL basic ZMB1 (pH 10).
-
b.Calibration #2: 150 μL acidic ZMB1 (pH 2) + 850 μL basic ZMB1 (pH 10).
-
c.Calibration #3: 300 μL acidic ZMB1 (pH 2) + 700 μL basic ZMB1 (pH 10).
-
d.Calibration #4: 450 μL acidic ZMB1 (pH 2) + 550 μL basic ZMB1 (pH 10).
-
e.Calibration #5: 600 μL acidic ZMB1 (pH 2) + 400 μL basic ZMB1 (pH 10).
-
f.Calibration #6: 625 μL acidic ZMB1 (pH 2) + 375 μL basic ZMB1 (pH 10).
-
g.Calibration #7: 675 μL acidic ZMB1 (pH 2) + 325 μL basic ZMB1 (pH 10).
-
h.Calibration #8: 700 μL acidic ZMB1 (pH 2) + 300 μL basic ZMB1 (pH 10).
-
i.Calibration #9: 750 μL acidic ZMB1 (pH 2) + 250 μL basic ZMB1 (pH 10).
-
j.Calibration #10: 800 μL acidic ZMB1 (pH 2) + 200 μL basic ZMB1 (pH 10).
-
k.Calibration #11: 900 μL acidic ZMB1 (pH 2) + 100 μL basic ZMB1 (pH 10).
-
l.Calibration #12: 1000 μL acidic ZMB1 (pH 2) + 0 μL basic ZMB1 (pH 10).
-
a.
-
10.
Measure the solution pH for each calibration standard using a pH electrode.
-
11.
Transfer a 900 μL volume for each calibration standard into 1.5 mL polymethyl methacrylate (PMMA) optical cuvette.
-
12.
Add a 100 μL volume of a litmus dye stock (stock concentration 10 mg/mL) to each cuvette containing a 900 μL volume of calibration standard.
-
13.
Record the absorption values for 520 nm (isosbestic point of litmus) and 590 nm (λmax of litmus) on a standard spectrophotometer.
-
14.
Calculate the UV-Vis absorption ratio (Abs590nm/Abs520nm) for each of the calibration standards.
-
15.
Import the values of pH (recorded with the hand-held pH meter) and the UV-Vis absorption ratio (Abs590nm/Abs520nm) into the custom Python script (Lukmen-lab/pHSigma: Fitting UV-VIS pH indicator litmus absorbance spectrum ratio calibration | Zenodo) to construct a sigmoidal curve. The sigmoidal calibration curve fit is performed using the following equation:
| (Equation 1) |
In this equation, the variables x and y are the experimental solution pH and the UV-Vis absorption ratio (Abs590nm/Abs520nm) for each calibration standard, respectively. The coefficients from the sigmoidal curve fit using the Python script include A is the amplitude, b is the offset, xo is the inflection point, and k is the slope of the inflection point – see Table 1 for experimental values for these coefficients determined.
-
16.
Calculate the solution pH values for each calibration standard and conditioned bacterial media specimen using the following equation.
| (Equation 2) |
-
17.
The accuracy of the method can be back calculated using the percent bias (%Bias) equation below, and the accuracy results for each of the calibration standards contained in the three calibration curves are tabulated in Table 2.
| (Equation 3) |
Note: We generated a series of 31 dilutions in triplicate to confirm the accuracy of the method and ensure the fit of the curve (See Figure 1B). For daily experiments, we recommend a 12-point curve in duplicate to ensure an optimal calibration curve. Alternatively, individuals can simply use the 31-point calibration curve available online (Lukmen-lab/pHSigma: Fitting UV-VIS pH indicator litmus absorbance spectrum ratio calibration | Zenodo) to back-calculate the pH of their bacterial cultures (See Figure 1D). To identify the isosbestic point and λmax for litmus, we examined the UV-Vis absorption spectra over an optical wavelength range of 350–750 nm (See Figure 1C). This method identified that the isosbestic point occurred at 520 nm and λmax occurred at 590 nm. For individuals using this protocol, it is not necessary to examine a range of spectra; measuring 520 nm and 590 nm is sufficient to calculate pH.
Table 1.
Sigmoidal curve fit parameters obtained for fitting Equation 1 to experimental data
| Coefficient | Value |
|---|---|
| A | 1.9430 |
| xo | 6.4614 |
| k | 1.4235 |
| b | 0.3039 |
Table 2.
Accuracy assessment for calibration curves prepared in triplicate and back calculating the pH based on the sigmoidal curve fit parameters (Table 1) and Equation 2
| Curve #1 |
Curve #2 |
Curve #3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. pH | Abs. Ratio (590 nm/520 nm) | Calc. pH | Accuracy (% bias) | Exp. pH | Abs. Ratio (590 nm/520 nm) | Calc. pH | Accuracy (% bias) | Exp. pH | Abs. Ratio (590 nm/520 nm) | Calc. pH | Accuracy (% bias) |
| 8.84 | 2.183 | 8.84 | 100.0% | 8.90 | 2.162 | 8.63 | 97.0% | 8.86 | 2.179 | 8.79 | 99.2% |
| 8.38 | 2.148 | 8.52 | 101.6% | 8.36 | 2.144 | 8.49 | 101.5% | 8.39 | 2.090 | 8.17 | 97.4% |
| 7.94 | 2.028 | 7.91 | 99.6% | 7.89 | 2.027 | 7.91 | 100.2% | 7.94 | 2.044 | 7.97 | 100.4% |
| 7.62 | 1.955 | 7.68 | 100.8% | 7.52 | 1.927 | 7.60 | 101.1% | 7.56 | 1.904 | 7.54 | 99.8% |
| 7.36 | 1.841 | 7.40 | 100.5% | 7.35 | 1.833 | 7.38 | 100.4% | 7.33 | 1.812 | 7.33 | 100.1% |
| 7.23 | 1.757 | 7.22 | 99.9% | 7.14 | 1.750 | 7.21 | 101.0% | 7.30 | 1.746 | 7.20 | 98.7% |
| 7.16 | 1.717 | 7.15 | 99.9% | 7.09 | 1.675 | 7.08 | 99.8% | 7.09 | 1.686 | 7.10 | 100.1% |
| 6.97 | 1.640 | 7.02 | 100.7% | 6.98 | 1.641 | 7.02 | 100.5% | 7.00 | 1.629 | 7.00 | 100.0% |
| 6.90 | 1.569 | 6.90 | 100.0% | 6.83 | 1.542 | 6.86 | 100.4% | 6.85 | 1.558 | 6.88 | 100.5% |
| 6.79 | 1.487 | 6.77 | 99.7% | 6.72 | 1.466 | 6.74 | 100.3% | 6.80 | 1.446 | 6.71 | 98.7% |
| 6.61 | 1.354 | 6.58 | 99.5% | 6.48 | 1.308 | 6.51 | 100.4% | 6.64 | 1.372 | 6.60 | 99.4% |
| 6.50 | 1.287 | 6.48 | 99.7% | 6.37 | 1.242 | 6.41 | 100.7% | 6.50 | 1.286 | 6.48 | 99.6% |
| 6.30 | 1.198 | 6.35 | 100.8% | 6.30 | 1.197 | 6.35 | 100.8% | 6.38 | 1.195 | 6.35 | 99.5% |
| 6.26 | 1.099 | 6.20 | 99.1% | 6.24 | 1.081 | 6.18 | 99.0% | 6.20 | 1.097 | 6.20 | 100.0% |
| 6.13 | 1.008 | 6.06 | 98.9% | 6.02 | 0.992 | 6.04 | 100.3% | 6.01 | 0.976 | 6.01 | 100.1% |
| 5.82 | 0.876 | 5.85 | 100.5% | 5.84 | 0.879 | 5.85 | 100.2% | 5.89 | 0.893 | 5.88 | 99.8% |
| 5.58 | 0.724 | 5.56 | 99.6% | 5.56 | 0.736 | 5.58 | 100.4% | 5.58 | 0.732 | 5.57 | 99.9% |
| 5.08 | 0.559 | 5.13 | 101.1% | 5.08 | 0.563 | 5.15 | 101.3% | 5.19 | 0.568 | 5.16 | 99.5% |
| 4.38 | 0.416 | 4.50 | 102.7% | 4.34 | 0.419 | 4.52 | 104.1% | 4.52 | 0.448 | 4.69 | 103.7% |
Tabulated pH data spans a range of 4.38–8.90 because the precision (%Bias) over this pH range was between 97.0%–104.1%.
Assess pH from cell-free supernatant using the low-volume format using 96-well plates
Timing: 30 min
The low-volume format is amiable for use in 96-well plate formats that can be used in high-throughput microbiology applications that use micro-scale organism cultures.
-
18.
To calculate the pH, transfer 200 μL volume from each DeepWell polypropylene microplate well into a 96-well round bottom polystyrene (PS) microplate.
-
19.
Centrifuge the plate at 2,000 × g for 10 min to pellet the bacteria.
-
20.
Without disrupting the pellet, transfer a 120 μL volume to a Corning 96 well filter plate containing a 0.2 μm polyvinylidene difluoride (PVDF) filter.
-
21.
Place a new 96-well flat bottom PS microplate to the bottom of a vacuum apparatus and place the 96-well filter plate containing the cell-free supernatant on the top.
-
22.
Use the vacuum to sterile filter the media into the new 96-well plate.
-
23.
Transfer a 90 μL volume of the sterile cell-free supernatant to another 96-well plate using a multiple-channel pipette.
-
24.
Add a 100 μL volume of each calibrator (with Litmus dye) prepared in the 12-point standard curve in duplicate to the plate (see generate a calibration curve Step 9).
-
25.
Add a 10 μL volume of litmus dye stock (stock concentration 10 mg/mL) into each well of the 96-well plate containing the 90 μL volume of sterile cell-free supernatant using a multi-channel pipette
-
26.
Measure the wavelengths 520 nm (isosbestic point) and 590 nm (λmax) on a plate reader, such as a Biotek Synergy H1.
-
27.
Use the procedures described in steps 12–15 (generate a calibration curve) to calculate the solution pH values for the calibration standards and conditioned bacterial media samples.
CRITICAL: It is critical that no bacteria be transferred in the cell-free supernatant. Bacteria in the solution can shift the 590 nm wavelength and skew the pH calculations with the litmus dye. If a vacuum apparatus is not available, wells can be transferred to Eppendorf tubes, centrifuged at 2,000 × g for 10 min in a microfuge and sterile filtered by hand using syringes.
Assess pH from cell-free supernatant using the high-volume format using optical cuvettes
Timing: 30 min
The high-volume format is used to perform solution pH determinations of cell-free bacterial cultures with traditional microbiology workflows that produce large volume cultures.
-
28.
To calculate the pH of a standard 5 mL bacterial culture in 14 mL conical tube, centrifuge the tube at 6,000 × g for 10 min to pellet the bacteria.
-
29.
Transfer 3 mL of the cell-free supernatant into a syringe with a 0.2 μm PVDF filter and sterile filter the solution.
-
30.
Transfer a 900 μL volume of the sterile bacterial supernatant into 1.5 mL PMMA optical cuvettes.
-
31.
Add a 100 μL volume of litmus dye stock (stock concentration 10 mg/mL) to each cuvette containing the 900 μL volume of sample.
-
32.
Create a 12-point standard curve in duplicate (see generate a calibration curve Step 9) and transfer a 900 μL volume for each calibration standard into 1.5 mL PMMA optical cuvettes containing a 100 μL volume of litmus dye stock (stock concentration 10 mg/mL).
-
33.
Record the absorption values for 520nm (isosbestic point of litmus) and 590nm (λmax of litmus) on a standard spectrophotometer.
-
34.
Calculate the UV-Vis absorption ratio (Abs590nm/Abs520nm) for each of the calibration standards.
-
35.
Use the procedures described in steps 12–15 (generate a calibration curve) to calculate the solution pH values for the calibration standards and conditioned bacterial media samples.
CRITICAL: In the traditional cuvette format, it is also critical that no bacteria be transferred in the cell-free supernatant as bacteria can shift the 590 nm wavelength.
Expected outcomes
As a proof of concept of the technique, we first created a set of standards by adjusting 40 mL of ZMB1 to pH 10 using 5 M NaOH and adjusting another 40 mL of ZMB1 to pH 2 using 5 M HCl. We blended these two ZMB1 together to generate a standard pH curve that ranged from pH ∼2 to 10 (see Figure 1; Table 1). We measured the pH of each of these standards using a hand-held pH meter and transferred 1 mL of the standard to cuvettes. We measured the full-spectrum UV-Vis absorption spectrum (350–750 nm) of each standard in the cuvette using a NanoDrop spectrophotometer. A 1 mL volume of PBS was used to blank the run. The color of the standards ranged from blue at pHs > 8, purple at neutral pH, and red at acidic pHs (Figure 1A). The pH changes can also be visualized as a reflection of blending in the calibration curve (Figure 1B). The UV-Vis absorption spectra for each Calibrator was normalized to a consistent absorption value at 520 nm, which is in close proximity to the isosbestic point for litmus: a wavelength where the light absorption of an indicator dye is independent of solution pH (Figure 1C). The λmax was identified at 590 nm. Calibration curves generated by the ratio of these two wavelengths (590 nm/520 nm) revealed a sigmoidal curve, which could be fit using the referenced Python code for sigmoid-based calibration curve fitting (Figure 1D).
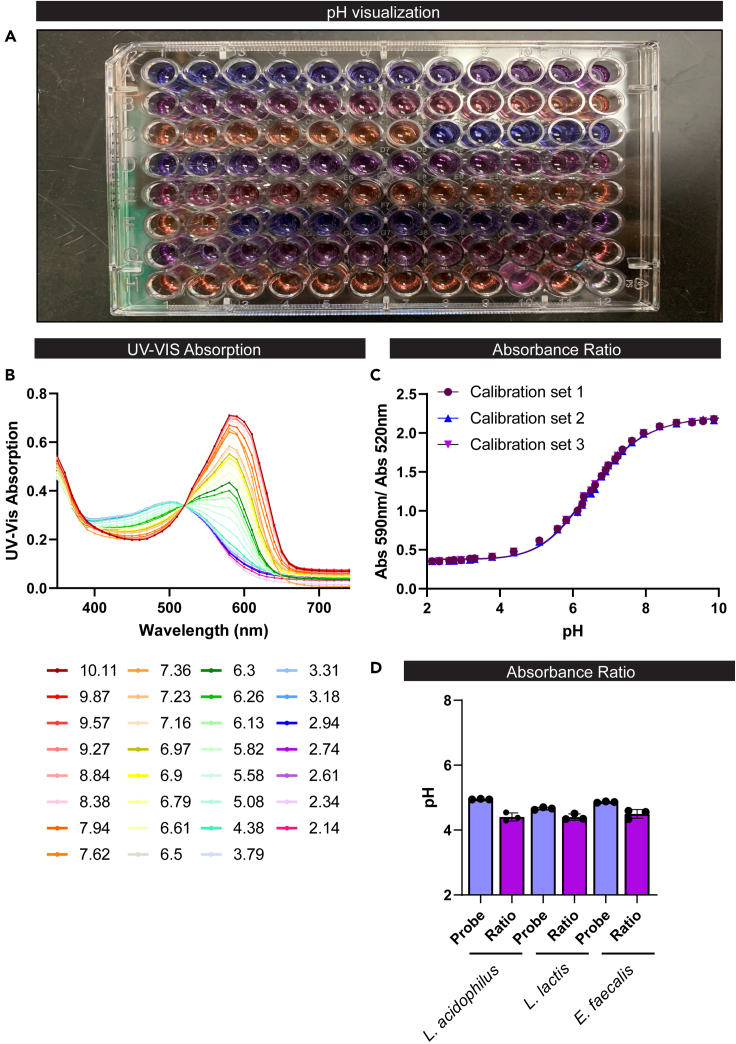
To confirm the ability of this method to work in a high throughput manner, we transferred these standards to a 96-well plate and examined the UV-Vis absorption spectrum on a Biotek Synergy H1 Plate Reader (Figure 2A). Like the cuvettes, we observed the isosbestic point of litmus was 520 nm and the λmax was 590 nm (Figure 2B). The standards on the 96-well plate could also be fit using the referenced Python code for sigmoid-based calibration curve fitting (Figure 2C). To validate the use of this assay for bacterial cultures, we grew 5 mL cultures of L. acidophilus, L. lactis and E. faecalis in ZMB1 overnight at 37°C. The bacteria all grew in the ZMB1 medium (L. acidophilus OD600nm = 4.6 ± 0.8, L. lactis OD600nm = 3.5 ± 0.6, E. faecalis OD600nm = 6.1 ± 0.5). After centrifuging the bacteria and sterile filtering the supernatant, we measured the pH using a hand-held pH. We also mixed the supernatant with litmus dye (1:10 dilution), measured the absorption at 520 nm and 590 nm on the plate reader and calculated the pH using the ratio of 590 nm/520 nm back calculated to the standard curve. The calculated pH values closely mirrored those of the hand-held pH measurement (Figure 2D) and had an accuracy of 92%–95%.
Figure 2.
The pH of ZMB1 containing litmus dye can be measured by UV-Vis absorption spectrophotometry by plate reader and bacterial pH can be calculated using these curves
(A) a photograph that shows the differences of the observable color of litmus dye when present in calibration standards or cell-free bacterial cultures of varying solution pH.(B) UV-Vis absorption spectrum for the pH-sensitive litmus dye produced over a range of pHs. The isosbestic point was at 520 nm and the lambda max (λmax) was at 590 nm.
(C) The Calibration Curve was fit using the referenced Python code for sigmoid-based calibration curve fitting with the absorption ratio (Abs590 nm / Abs520 nm) plotted as a function of solution pH.
(D) pH analysis of lactic acid bacteria in ZMB1 by hand-held pH (probe) and plate reader method (ratio).
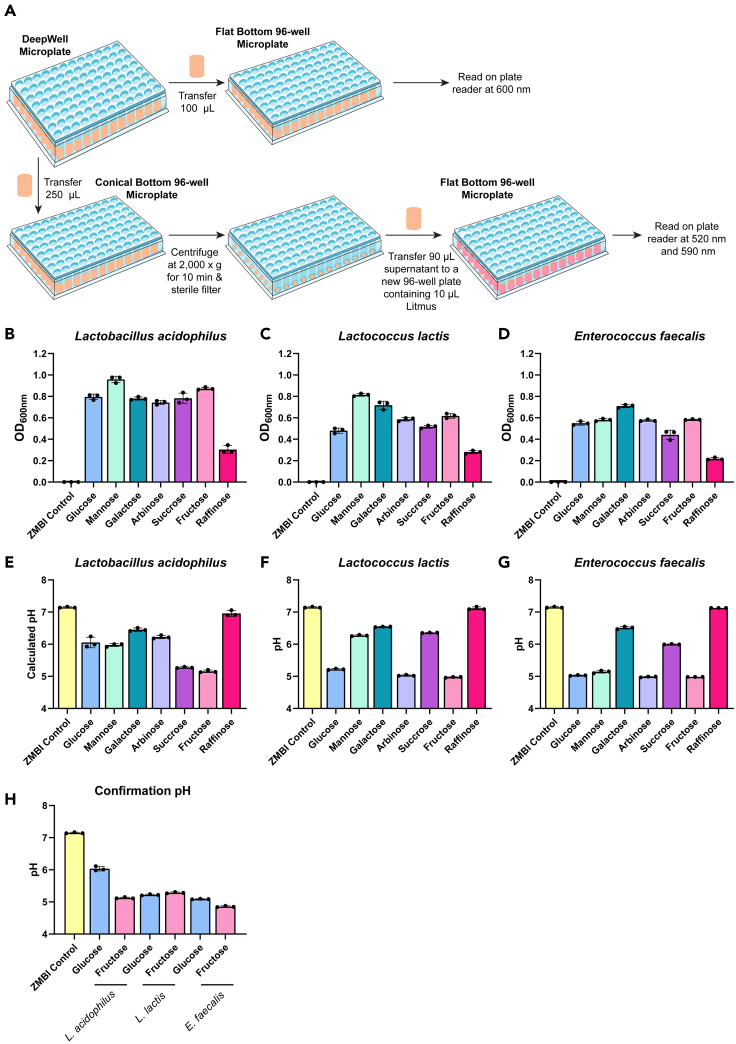
Next, we used this assay to generate a more high-throughput method for examining bacterial pH (Figure 3A). We selected lactic acid bacteria L. acidophilus, L. lactis and E. faecalis because these microbes are all known to generate lactic acid and lower the pH. We first grew these microbes in their respective rich media (L. acidophilus in MRS and L. lactis and E. faecalis in BHI). We made our ZMB1 medium without glucose so we could test the growth of our lactic acid bacteria in response to different nutrient sources. We added 100 mM glucose, mannose, galactose, arabinose, sucrose, fructose or raffinose to the ZMB1 to create ZMB1 with different nutrients. We then subcultured the bacteria into ZMB1 containing these different nutrients. These cultures were grown in 2 mL volumes in triplicate in a Deep 96-well plate overnight at 37°C. After the incubation, the wells were mixed with a multi-channel pipette and 100 μL of the solution was transferred to a flat bottomed 96-well plate. The OD600nm was measured on a Biotek plate reader. We observed varying levels of growth of L. acidophilus, L. lactis and E. faecalis in response to different carbohydrate sources (Figures 3B–3D). In general, all the microbes grew well with glucose, mannose, galactose, arabinose, sucrose, and fructose. Next, we transferred 250 μL of the wells to a conical bottom 96-well plate and centrifuged the plate at 2,000 × g for 10 min to pellet the bacteria. We added 150 μL of supernatant (being careful not to disturb the pellet) to a 0.2 μm 96-well filter plate and sterile filtered the supernatant using a vacuum. We transferred 90 μL of the sterile bacterial supernatant to a new 96-well plate. To create a standard curve, we adjusted ZMB1 to pH 8, 7, 6, 5, 4, 3, and 2 and added 90 μL of these standards to the plate. 10 μL of the litmus stock was added to all the wells and the absorption at 520 and 590 nm was examined on a Synergy H1 plate reader. The standards were compared to the larger set of standards and the supernatant pH was calculated from the using the referenced Python code for sigmoid-based calibration curve fitting. We found that L. acidophilus, L. lactis and E. faecalis significantly lowered the pH of the ZMB1 in the presence of multiple carbohydrates, especially fructose (Figures 3E–3G). Using a hand-held pH, we confirmed that L. acidophilus, L. lactis and E. faecalis lowered the pH of ZMB1 in response to fructose (L. acidophilus pH = 5.1 ± 0.1, L. lactis pH = 5.3 ± 0.1, E. faecalis pH = 4.9 ± 0.1) (Figure 3H). Collectively these data indicate that we can use litmus dye to successfully measure bacterial pH changes in a high-throughput manner. This protocol can be applied to a wide range of microbes and provides an inexpensive method of examining bacterial pH.
Figure 3.
UV-Vis absorption spectrophotometry can be applied to high throughput DeepWell plates
(A–G) (A) Schematic of the procedure. OD600nm values, reflecting the growth of individual microbes, from the DeepWell plates growth with (B) Lactobacillus acidophilus ATCC 4796, (C) Lactococcus lactis CB, and (D) Enterococcus faecalis ATCC 29212 in response to 100 mM of different carbohydrate sources. Calculated pH based on the ratio of 590 nm/520 nm wavelengths of cultures with (E) Lactobacillus acidophilus ATCC 4796, (F) Lactococcus lactis CB, and (G) Enterococcus faecalis ATCC 29212.
(H) pH analysis of lactic acid bacteria by hand-held pH (probe).
Quantification and statistical analysis
The standard curve was calculated using the referenced Python code used to perform sigmoid-based calibration curve fitting - see the software and algorithms section of the key resources table for the web address to download the code.
Limitations
We selected litmus as the pH indicator dye because it is inexpensive, the dye possesses an isosbestic point that is useful for auto-normalizing UV-Vis-based absorption measurements, and the optical properties are well known. However, other pH sensitive dyes or bacterial growth media may be selected as long as the medium selected do not interfere with the optical absorption of the pH sensing dye.
This protocol amenable to adaption. We have selected DeepWell microplates because these plates allow for a larger volume than traditional 96-well flat bottom microplates (2 mL vs. 300 μL). Some versions of these DeepWell microplates are able to be centrifuged as well; although they require centrifuge adaptors. Using multi-channel pipettes, we describe the easy transfer bacterial cultures to flat bottom 96-well plates for optical density analysis and round bottom 96-well plates for removal of bacteria. This method is optimal since it only requires an absorbance plate reader. The method described uses 96 well plates, but other plates (i.e., 384 well, 24 well plates, etc.) could be used if the plate reader allows for multiple formats. The simplicity and rapidity in setting up this method provides a realistic and cost-effective alternative to measure bacterial pH from 96-well plates. Another benefit of this method is that multiple time points could be collected. If the culture volume in the DeepWell plates were amplified to 2 mL, 6 time points could be easily collected. These potential applications make this protocol highly valuable for researchers.
Lactic acid bacteria are well documented for their ability to lower the pH of solution through the production of lactate. These microbes can dramatically alter the pH, creating environments as low as pH 3–4. As a result, these microbes are ideal candidates for this type of high-throughput analysis. However, other microbes, such as Escherichia, Klebsiella, Clostridium, Proteus, and Bacteroides may not lower the pH of the medium or the changes may be very slight. For these types of microbes, we recommend pilot studies using a hand-held pH meter.
Troubleshooting
Problem 1: Bacteria does not grow well in ZMB1
Corresponding Step: Grow bacteria in a chemically defined bacterial medium ZMB1.
Although we have found that multiple types of bacteria grow in ZMB1, there are a few bacteria that require additional medium components. For example, Veillonella parvula requires hemin to be added to ZMB1 for robust growth.
Potential solution
If the desired bacteria do not grow, we suggest performing a pilot study and adding supplements stepwise to achieve a solution with maximal growth. Additionally, whether the bacteria are grown with rotational mixing or not is based upon the type of bacteria. Many bacteria have improved growth with rotational mixing (shaking at 100–250 rpm) due to the increased exposure to nutrients. A pilot study can be performed to determine if shaking is necessary for the optimal growth of the desired bacteria.
Problem 2: Questionable pH values
Corresponding Step: Assess pH from cell-free supernatant using the low-volume format using 96-well plates.
If the calculated pH values appear to deviate from the expected values, there may be bacteria in the solution that have gotten through the filtration that might be skewing the pH values.
Potential solution
Since bacteria can be detected at the ODs used to calculate the pH, it is very important to ensure that no bacteria are present in the solution use for pH measurement. To determine if there are any bacteria in the solution, place the 96-well on an inverted microscope and examine at 40× or 60×. Alternatively, remove 10 μL of the solution and transfer to a clean microscope slide and examine under a microscope. If bacteria are observed, refilter the solution and read on the plate reader again.
Alternative growth conditions
Corresponding Step: Grow bacteria in a rich medium.
The type of bacteria selected for analysis will dictate which type of rich medium is used. Common rich media include MRS, BHI, nutrient broth, Luria-Bertani, Columbia, Tryptic Soy Broth. The type of rich medium used does not influence the procedure and can be selected based on whatever medium will yield the highest density of cells.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Thomas Horvath, thomas.horvath2@bcm.edu.
Materials availability
No newly generated materials are associated with this protocol.
Data and code availability
The referenced Python code used to perform sigmoid-based calibration curve fitting and experimental dataset supporting this current protocol have been uploaded to the following website: https://github.com/Lukmen-lab/pHSigma and Zenodo: https://doi.org/10.5281/zenodo.8206410.
Acknowledgments
This work was supported by NIH F32 DK130288 (K.A.E.), K01DK123195 (M.A.E.), and P20 GM120457 (M.A.E.). The Texas Children’s Hospital Department of Pathology provides salary support to Texas Children’s Microbiome Center-Metabolomics and Proteomics and Mass Spectrometry Laboratory staff.
Author contributions
Conceptualization, methodology, writing – review and editing, and funding acquisition, K.A.E., M.A.E., and T.D.H.; investigation, K.A.E., H.G., C.D., and R.K.S.; software, N.O.; formal analysis and validation, K.A.E., N.O., M.A.E., and T.D.H.; visualization, K.A.E., N.O., and M.A.E.; writing – original draft, K.A.E.; supervision, M.A.E. and T.D.H.
Declaration of interests
T.D.H. is an Editorial Advisory Board member and is contracted as an Associate Academic Editor for a Cell Press journal called STAR Protocols.
References
- 1.Kurokawa M., Ying B.W. Precise, High-throughput Analysis of Bacterial Growth. J. Vis. Exp. 2017;10 doi: 10.3791/56197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horvath T.D., Haidacher S.J., Engevik M.A., Luck B., Ruan W., Ihekweazu F., Bajaj M., Hoch K.M., Oezguen N., Spinler J.K., et al. Interrogation of the mammalian gut–brain axis using LC–MS/MS-based targeted metabolomics with in vitro bacterial and organoid cultures and in vivo gnotobiotic mouse models. Nat. Protoc. 2023;18:490–529. doi: 10.1038/s41596-022-00767-7. [DOI] [PubMed] [Google Scholar]
- 3.Engevik M., Ruan W., Visuthranukul C., Shi Z., Engevik K.A., Engevik A.C., Fultz R., Schady D.A., Spinler J.K., Versalovic J. Limosilactobacillus reuteri ATCC 6475 metabolites upregulate the serotonin transporter in the intestinal epithelium. Benef. Microbes. 2021;12:583–599. doi: 10.3920/bm2020.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engevik M.A., Herrmann B., Ruan W., Engevik A.C., Engevik K.A., Ihekweazu F., Shi Z., Luck B., Chang-Graham A.L., Esparza M., et al. Bifidobacterium dentium-derived y-glutamylcysteine suppresses ER-mediated goblet cell stress and reduces TNBS-driven colonic inflammation. Gut Microb. 2021;13:1–21. doi: 10.1080/19490976.2021.1902717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engevik M.A., Luck B., Visuthranukul C., Ihekweazu F.D., Engevik A.C., Shi Z., Danhof H.A., Chang-Graham A.L., Hall A., Endres B.T., et al. Human-Derived Bifidobacterium dentium Modulates the Mammalian Serotonergic System and Gut-Brain Axis. Cell. Mol. Gastroenterol. Hepatol. 2021;11:221–248. doi: 10.1016/j.jcmgh.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engevik M.A., Ruan W., Esparza M., Fultz R., Shi Z., Engevik K.A., Engevik A.C., Ihekweazu F.D., Visuthranukul C., Venable S., et al. Immunomodulation of dendritic cells by Lactobacillus reuteri surface components and metabolites. Physiol. Rep. 2021;9 doi: 10.14814/phy2.14719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMillan G. Understand some basic truths of pH measurement. Chem. Eng. Prog. 1991;87:30–37. [Google Scholar]
- 8.John G.T., Goelling D., Klimant I., Schneider H., Heinzle E. PH-sensing 96-well microtitre plates for the characterization of acid production by dairy starter cultures. J. Dairy Res. 2003;70:327–333. doi: 10.1017/s0022029903006344. [DOI] [PubMed] [Google Scholar]
- 9.Zhang G., Mills D.A., Block D.E. Development of chemically defined media supporting high-cell-density growth of lactococci, enterococci, and streptococci. Appl. Environ. Microbiol. 2009;75:1080–1087. doi: 10.1128/AEM.01416-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The referenced Python code used to perform sigmoid-based calibration curve fitting and experimental dataset supporting this current protocol have been uploaded to the following website: https://github.com/Lukmen-lab/pHSigma and Zenodo: https://doi.org/10.5281/zenodo.8206410.