Summary
Background
The strategy of dual blockade of TGF-β and PD-L1 pathways has not been previously tested in platinum-refractory recurrent or metastatic nasopharyngeal cancer (R/M NPC) patients. This study aimed to evaluate the safety and efficacy of bintrafusp alfa in refractory R/M NPC patients.
Methods
In this single-arm, single-centre phase II clinical trial, 38 histologically confirmed R/M NPC patients were enrolled and administered with bintrafusp alfa every 2 weeks. Primary endpoint was objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1). Secondary endpoints included progression-free survival (PFS), overall survival (OS), duration of response (DOR), and safety.
Findings
Thirty-eight patients were accrued (33 men; median age, 54 years). ORR was 23.7% (complete response, n = 2; partial response, n = 7). The median DOR was 19.2 months, median PFS was 2.3 months, median OS was 17.0 months, and 1-year OS rate was 63.2%. Unfortunately, 25 patients (65.7%) progressed within 8 weeks of treatment, 15 patients (39.5%) and 8 patients (21.1%) developed hyper-progressive disease (HPD) per RECIST v1.1 and tumor growth rate (TGR) ratio respectively. Sixteen patients (42.4%) experienced ≥ grade 3 treatment-related adverse events (TRAEs), most commonly anemia (n = 9, 23.7%) and secondary malignancies (n = 4, 10.5%). TRAEs led to permanent treatment discontinuation in 7 patients. Patients with strong suppression of plasma TGFβ1 level at week 8 were unexpectedly associated with worse ORR (9.1% vs 44.4%, P = 0.046) and development of HPD. There was no correlation between PD-L1 expression and ORR.
Interpretation
Bintrafusp alfa demonstrated modest activity in R/M NPC but high rates of HPD and treatment discontinuation secondary to TRAEs are concerning.
Funding
The project was supported by Alice Ho Miu Ling Nethersole Charity Foundation Professorship Endowed Fund and Merck KGaA.
Keywords: Efficacy, Safety, Correlative biomarkers, Bintrafusp alfa, Recurrent or metastatic nasopharyngeal cancer
Research in context.
Evidence before this study
While anti-PD-1/PD-L1 inhibitor is one of the treatments in patients with recurrent or metastatic (R/M) nasopharyngeal cancer (NPC), there has been growing interest in using combination approach to enhance its therapeutic effect. Preclinical data suggested the promising potential of the strategy of dual blockade of TGF-β and PD-L1 pathways. We searched PubMed from Jan 1, 2010, to Dec 31, 2021, with the following terms: “NPC”, “immunotherapy”, “TGFβ” and “bintrafusp alfa”. A phase I study showed that bintrafusp alfa is efficacious and safe in heavily pre-treated squamous cell carcinoma of head and neck cancer. However, we did not identify any study that addressed the TGF-β and PD-L1 dual blockage strategy in patients with NPC.
Added value of this study
In this phase II trial, bintrafusp alfa demonstrated modest activity in R/M NPC but high rates of hyper-progression (HPD) and treatment discontinuation secondary to TRAEs. Unexpectedly, our biomarker analyses showed that patients with stronger suppression of plasma TGFβ1 during treatment were associated with worse response, a higher incidence of HPD, and inferior survival outcomes.
Implications of all the available evidence
Our report raised safety concerns on the strategy of TGF-β and PD-L1 dual blockage in patients with NPC. Biomarker correlative studies are urgently needed to understand the biology and guide future drug development.
Introduction
Nasopharyngeal cancer (NPC) is one of the most common head and neck cancers (HNC) in Western Pacific.1 In this region, the predominant subtypes are non-keratinizing differentiated (WHO class II) and undifferentiated carcinoma (WHO class III).2 The management of NPC remains challenging, with 15%–30% of patients experiencing failure after radical treatment and 5% presenting with distant metastasis.3 Cisplatin-based chemotherapy is the standard of care for patients with recurrent or metastatic (R/M) NPC and is associated with promising survival outcomes.4 However, treatment outcome is dismal for those who are refractory to platinum-based chemotherapy.5 Therefore, there is an unmet need to establish better therapy especially in platinum resistant cases.
NPC is closely associated with Epstein–Barr virus (EBV) infection.2 The tumor microenvironment is characterised by a high programmed cell death-ligand 1 (PD-L1) expression and dense lymphocytic infiltration – a classic example of an immune-hot environment.6,7 These features render immune checkpoint inhibitors (ICIs) as potentially promising therapies. There is growing evidence regarding the clinical efficacy of anti-PD-1/PD-L1 inhibitors in patients with chemo-refractory NPC. Still, the number of patients who could benefit from PD-1 checkpoint inhibition remains low (17–34%).8, 9, 10, 11, 12, 13, 14 Emphasis is now focused on using a combination approach to enhance the therapeutic effect observed in the ICI trials.
The transforming-growth factor beta (TGF-β) pathway is important for tumorigenesis in many cancers. TGF-β promotes tumor progression via stromal modification, angiogenesis, and induction of epithelial-mesenchymal transition.15 TGF-β can inhibit the T-cell invasion and modulate anti-PD-1/PD-L1 antibody resistance.16 Further, there is a link between dysregulation of TGF-β pathway and tumorigenesis in EBV-positive cancers17; TGF-β1 was up-regulated in EBV-positive NPC tissues,18 TGF-β levels are increased in patients with NPC compared to healthy individuals with levels positively correlating with disease staging and tumor aggressiveness.19, 20, 21, 22 Thus, sequestering TGF-β from the tumor micro-environment while concurrently blocking the PD-1/PD-L1 pathway may provide a novel therapeutic approach.
Bintrafusp alfa is a first-in-kind bifunctional fusion protein composed of an extracellular domain of the human TGF-β receptor II (TGF-βRII or TGF-β' trap') fused via a flexible linker to the C-terminus of each heavy chain of an IgG1 antibody blocking PD-L1 (anti-PD-L1).23 Preclinical studies demonstrated that bintrafusp alfa effectively suppressed tumor growth and metastasis more than a TGF-β trap or an anti-PD-L1 antibody alone.23,24 Bintrafusp alfa has been tested to be efficacious and safe in several phase I studies24, 25, 26, 27, 28, 29, 30; however, to date, there is scanty published data from phase II/III studies.31
Here, we report the result of phase II clinical trial examining the efficacy and safety of Bintrafusp alfa in previously untreated recurrent and metastatic NPC. The primary objective of the study was tumor objective response rate while secondary objectives included survival outcomes, disease control and treatment response, toxicities and patient-reported quality of life during treatment. Biomarkers were also evaluated to identify the potential treatment responders.
Methods
Patients and study design
This is a single-arm, single-centre phase II investigator-initiated trial conducted at Queen Mary Hospital (NCT04396886). Eligible patients had histologically or cytologically confirmed metastatic or distant recurrent NPC who were not amenable to curative treatment. Patients with isolated locally recurrent or progressive disease were excluded. The target lesions had to be measurable by the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. All patients had to receive at least one prior line of platinum-based chemotherapy for metastatic disease. A full list of eligibility criteria can be found in the appendix.
Treatment and assessments
Patients were treated with bintrafusp alfa at a dosage of 1200 mg intravenously every 2 weeks until disease progression, unacceptable toxicities, or upon withdrawal of consent.
Eligible patients underwent a baseline contrast-enhanced computed tomography (CT) of the head, neck, chest, abdomen, and pelvis. Radiological assessment was performed every 8 weeks for the first 12 months and then every 12 weeks thereafter by independent radiologist (YL) and investigators using RECIST version 1.1 criteria. Adverse events were graded according to National Cancer Institute Common Terminology Criteria version 4.0. The quality-of-life (QOL) of patients were measured using the EORTC QLQ-C30 and H&N-35 every 12 weeks in the first year.
Biomarker study
Plasma EBV DNA level was determined using a real-time quantitative polymerase chain reaction with probes against EBV DNA genes before treatment and every 2 weeks until disease progression.32 PD-L1 expression was determined by the immunohistochemistry (IHC) staining with 22C3 antibody and evaluated by certified pathologists. PD-L1 expression levels on tumor cells (cut-off ≥1%) and immune cells (cut-off ≥25%) were evaluated.
Plasma TGFβ1 level was measured using a TGFβ specific Enzyme-Linked Immunosorbent Assay every 8 weeks until disease progression. Extracellular vesicles were purified from the supernatants using the exosome isolation kit. Flow cytometer Cytoflex S was then used to examine the exosome size and purity. The concentration of PD-L1 in the exosome was measured by an ELISA kit every 2 weeks until disease progression.
Study end points
The primary endpoint of this study was the objective response rate (ORR) per independent review of radiologist according to RECIST version 1.1 criteria. The secondary endpoints included progression-free survival (PFS), time-to-progression (TTP), overall survival (OS), duration of response (DOR), disease control rate (DCR), time to response (TTR), safety, responses per immune-RECIST (iRECIST), correlation of plasma EBV DNA and clinical outcomes, and QOL measurement. Exploratory end points included PD-L1 expression, exosome PD-L1 (exo PD-L1), and TGFβ1 level as potential efficacy biomarkers. Post-hoc analyses on the incidence of hyper-progression (HPD) was assessed by (a) the RECIST 1.1 criteria as progressive disease in the first 8 weeks after treatment initiation and a minimum increase in the measurable lesions by 10 mm plus: (1) increase of ≥40% in the sum of target lesions compared with baseline or (2) increase of ≥20% in the sum of target lesions compared with baseline plus the appearance of new lesions in at least two different organs,33 and (b) the two-fold increase in the tumor growth rate (TGR) after initiation of the study treatment, which was assessed according to the definition by Ferte etg al; tumor growth (TG) is equal to TG = 3 Log (Dt/D0)/t, where D is tumor size defined as the sum of the longest diameters of the target lesions per RECIST 1.1. TGR were expressed as a percentage increase in tumor volume during 1 month using the following transformation formula: TGR = 100 (exp (TG) −1), where exp (TG) represents the exponential of TG.34 Further analysis was done to identify the predictors of HPD by evaluating the clinical factors and previously reported predictors of HPD.35
Statistics and sample size calculation
The sample size assumed a 40% ORR to bintrafusp alfa compared with 20% for checkpoint inhibitors. Modified Simon two-stage optimal design was used (power, 80%; a = 0.05; P0 = 0.20; P1 = 0.40; n1 = 18; n = 33 with an additional five patients to allow for ineligibility or other reasons). Because four responses were observed during the first stage, enrolment was continued until a total of 38 patients was reached.
Time-to-event variables were estimated using the Kaplan–Meier method, and survival rates were compared using a log-rank test. Two-sided P values < 0.05 were considered statistically significant. The Fisher exact test was used to correlate the binary clinical and biomarker data. In general, all available data will be included in the data listings and tabulations. Where appropriate, imputations of values for missing data for primary and secondary efficacy analyses will be performed. The cut-off values of baseline and changes in exo-PD-L1 and TGFβ1 level were calculated by the Receiver Operating Characteristic (ROC) curve analyses. All QOL scores were calculated using the EORTC QLQ-C30 and QLQ-H&N 35 methods, and the mean QOL with 95% CI at each time point was tabulated and compared by ANOVA. Data were analyzed using R version 3.25 (Vienna, Austria). R packages of “stats”, “survival”, “survminer” were adopted for our analyses.
Role of the funding source
The financial supporters of this study had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report. All the authors had full access to all the data and accepted responsibility to submit for publication.
All relevant data are presented in the paper or included as Supplementary Figs. and Tables. Raw data generated in this study are available upon reasonable request from the corresponding authors.
Results
Patient population
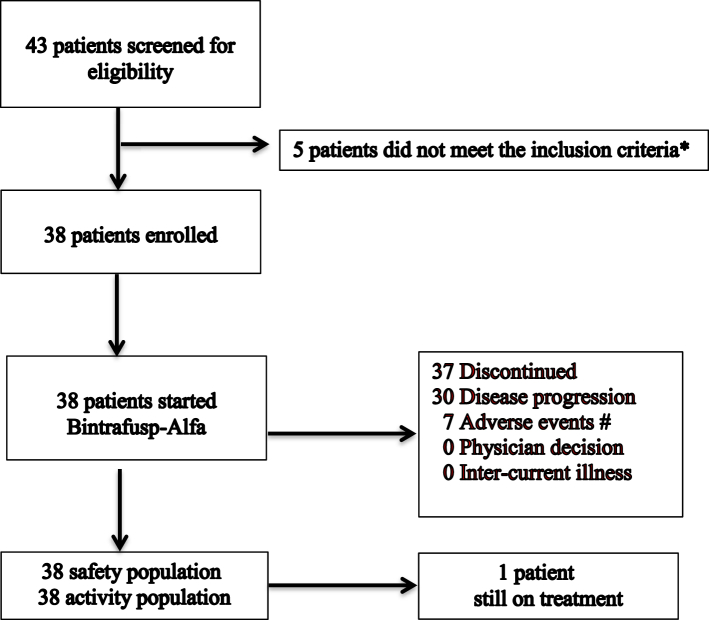
A total of 43 patients were screened between March 5, 2020 and December 8, 2020, of whom 38 patients were recruited (Fig. 1). Thirty-three patients (86.8%) are male, and 34 patients (89.5%) had Eastern Cooperative Oncology Group (ECOG) performance status 1. Seventeen patients (44.7%) presented with primary metastases; 36 patients (94.7%) had undifferentiated carcinoma. Twenty-nine patients (76.3%) had received at least two prior lines of therapy. Thirty-three patients (86.8%) had a history of radical or high-dose palliative radiotherapy to the head and neck region (2 Gy equivalent dose ≥60 Gy) (Table 1).
Fig. 1.
Trial Profile. ∗Among 5 patients did not meet eligibility criteria: Active brain metastasis (n = 1), no target lesion (n = 3), and isolated local relapse (n = 1). # Among 7 patients withdrawn due to adverse events: Secondary malignancy (n = 4), skin reaction (n = 2), epistaxis (n = 1).
Table 1.
Baseline demographics and clinical characteristics.
| Characteristics | N = 38 (%) |
|---|---|
| Age, median (range), years | 54 (18–72) |
| Sex | |
| Male | 33 (86.8) |
| Female | 5 (13.2) |
| Race | |
| Asian | 38 (100) |
| Others | 0 (0) |
| ECOG performance status | |
| 0 | 4 (10.5) |
| 1 | 34 (89.5) |
| 2 | 0 (0) |
| Histology (WHO) | |
| WHO I, squamous cell carcinoma | 0 (0) |
| WHO II, non-keratinizing carcinoma | 1 (2.6) |
| WHO III, undifferentiated carcinoma | 36 (94.7) |
| Unknown | 1 (2.6) |
| Stage | |
| Primary metastases | 17 (44.7) |
| Recurrence with nodal/distant metastases | 18 (47.4) |
| Recurrence with local and nodal/distant metastases | 3 (7.9) |
| Location of recurrent/metastatic diseases | |
| Bone | 23 (60.5) |
| Liver | 27 (71.1) |
| Lung | 15 (39.5) |
| Neck lymph nodes | 13 (34.2) |
| Distant lymph nodes | 23 (60.5) |
| Nasopharynx | 9 (23.7) |
| Others | 6 (15.8) |
| Previous radiotherapy to head and neck | |
| Radical treatment (66 Gy or above)a | 20 (52.6) |
| High dose palliation (60 Gy or above)a | 13 (34.2) |
| No | 5 (13.2) |
| Previous lines of therapy for recurrent/metastatic disease | |
| 1 | 9 (23.7) |
| 2 | 14 (36.8) |
| 3 | 5 (13.2) |
| 4 or more | 10 (26.3) |
| Previous chemotherapy for advanced disease | |
| Cisplatin | 31 (81.6) |
| Paclitaxel | 12 (31.6) |
| Gemcitabine | 38 (100) |
| 5-fluorouracil | 7 (18.4) |
| Carboplatin | 31 (81.6) |
| Docetaxel | 3 (7.9) |
| Cyclophosphamide | 2 (5.3) |
| Cetuximab | 1 (2.6) |
| Others (nadeplatin, tegafur, capecitabine) | 27 (71.1) |
ECOG, Eastern Cooperative Oncology Group; WHO, World Health Organization.
Radiation dose in 2 Gy equivalent.
Efficacy
As of December 31, 2021, the median follow-up was 15.6 months (range, 1.6–21.9 months), 19 patients (50%) died, and 1 patient (2.6%) remained on treatment. Median treatment duration was 1.8 months (range, 0.5–14.3 months) (Supplementary Fig. S1). The reasons for treatment discontinuation were progressive disease (PD) (n = 30, 78.9%) and adverse events (n = 7, 18.4%) (Fig. 1).
The confirmed ORR by independent review was 23.7% (95% CI, 12.4%–38.8%), with two patients (5.3%) having complete response (CR) and seven patients (18.4%) having a partial response (PR). The DCR was 28.9% (95% CI, 15.4%–45.2%). The median DOR was 19.2 months (range, 4.3–21.9 months) and the median TTR was 3.0 months (95% CI, 2.1–5.7 months). The median PFS was 2.3 months (95% CI, 1.9–2.4 months), TTP was 2.1 months (95% CI, 1.9–2.4 months), and the 12-month PFS rate was 30.1% (95% CI, 18.4%–45.3%). The median OS was 17.0 months (95% CI, 13.4–20.6 months) and the 12-month OS rate was 63.2% (95% CI, 47.3%–77.1%) (Table 2; Supplementary Fig. S1). The responses and survivals per immune-RECIST (iRECIST) were shown in Supplementary Table S1. As per investigator review, the ORR and DCR were 23.7% (95% CI, 12.4%–38.8%) and 31.6% (95% CI, 18.6%–47.3%) respectively. The median PFS was 2.1 months (95% CI, 1.8–2.4 months) and the median OS was 17.0 months (95% CI, 13.4–20.6 months). The median DOR and TTP were 18.5 months (95% CI, 1.7–21.9 months) and 2.1 months (95% CI, 2.0–2.5 months), respectively (Table 2).
Table 2.
Clinical activity of bintrafusp Alfa by RECIST criteria (N = 38).
| Clinical activity endpoint | Per investigator review (n = 38) | Per independent review (n = 38) |
|---|---|---|
| Confirmed BOR (CR + PR), No; % (95% CI) | 9; 23.7 (12.4–38.8) | 9; 23.7 (12.4–38.8) |
| CR, No; % (95% CI) | 2; 5.3 (1.1–15.8) | 2; 5.3 (1.1–15.8) |
| PR, No; % (95% CI) | 7; 18.4 (8.6–32.8) | 7; 18.4 (8.6–32.8) |
| SD, No; % (95% CI) | 3; 7.9 (8.6–32.8) | 4; 10.5 (3.7–23.1) |
| PD, No; % (95% CI) | 26; 68.4 (2.3–19.6) | 25; 65.8 (50–79.3) |
| DCR (CR + PR + SD ≥ 6 months), No; % (95% CI) | 12; 31.6 (18.6–47.3) | 11; 28.9 (15.4–45.9) |
| Median DOR, No; months (range) | 18.5 (1.7–21.9) | 19.2 (4.3–21.9) |
| Median TTP, No; months (range) | 2.1 (2.0–2.5) | 2.1 (1.9–2.4) |
| Median PFS (95% CI), months | 2.1 (1.8–2.4) | 2.3 (1.9–2.4) |
| 6-month PFS rate (95% CI), % | 31.6 (18.6–47.3) | 33.2 (19.8–46.5) |
| 12-month PFS rate (95% CI), % | 28.9 (16.5–44.5) | 30.1 (18.4–45.3) |
| Median OS (95% CI), months | 17.0 (13.4–20.6) | 17.0 (13.4–20.6) |
| 6-month OS rate (95% CI), % | 76.3 (61.2–87.6) | 76.3 (61.2–87.6) |
| 12-month OS rate (95% CI), % | 63.2 (47.3–77.1) | 63.2 (47.3–77.1) |
BOR, best of response; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate; DOR, duration of response; TTP, time to progression; PFS, progression-free survival; OS, overall survival.
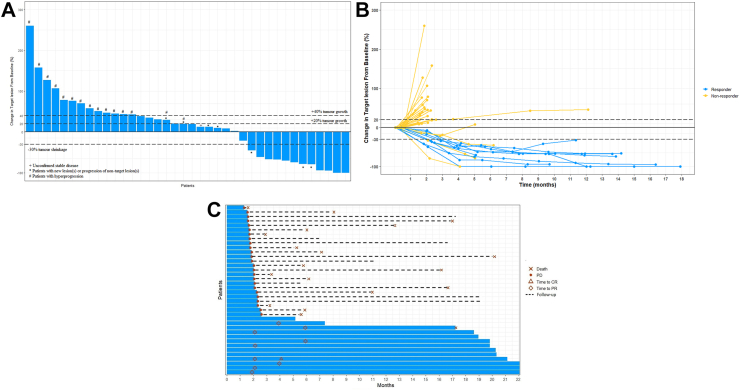
Notably, a total of 15 patients (39.5%) and 8 patients (21.1%) fulfilled the criteria of HPD per RECIST and TGR ratio respectively (Fig. 2).33 Among 26 patients with disease progression, 96.2% (25/26) of patients developed PD within the first 2 months of treatment (Fig. 2). Patients who had HPD per RECIST (n = 15, 39.5%) were associated with significantly worse survival compared with non-HPD progressors (n = 10, 26.3%) and non-progressors (n = 13, 34.2%) (12-month OS rate: 26.7% versus 80% versus 100%, P < 0.001) (Supplementary Fig. S1C). Similarly, patients who had HPD per TGR ratio (n = 8, 21.1%) were associated with significantly worse survival compared with non-HPD progressors (n = 17, 44.7%) and non-progressors (n = 13, 34.2%) (12-month OS rate: 25% versus 47.1% versus 100%, P < 0.001) (Supplementary Fig. S1D). The details of individual patients developed HPD and representative images of HPD were presented in Supplementary Fig. S2 and Table S2.
Fig. 2.
Tumor response assessment based on RECIST v1.1 per independent review. (A) Waterfall plot of change from baseline in tumor size (n = 38). Baseline was defined as the last measurement taken before the randomisation date. For each patient, the best (minimum) percentage change from baseline in the sum of diameters for all target lesions was represented by a vertical line, plotted in order of greatest percentage increase to greatest percentage decrease. Only patients with measurable disease at baseline and at least one post-baseline assessment were included in the waterfall plots. (B) Spider plot of the longitudinal change from baseline in tumor size (n = 38). The blue line represents responder; yellow line represents non-responder. (C) Swimmer plot on treatment exposure and response duration (n = 38).
The presence of liver metastases is the only significant factor in predicting HPD, while it was not correlated with age, sex, previous radiation, number of metastatic sites, and neutrophil-lymphocyte ratio (Supplementary Tables S3 and S4).
Safety and tolerability
All enrolled patients received a median of 4 cycles of bintrafusp alfa (range, 1 to 31 cycles). Thirty-four patients (89.4%) experienced treatment-related AEs (TRAEs); of these, 16 patients (42.4%) experienced grade 3 TRAE, which included anemia (n = 9, 23.7%), secondary malignancies (n = 4, 10.5%), and epistaxis (n = 3, 7.9%) (Table 3). Supplementary Table S5 illustrated the grade 3 or above TRAEs in different time periods. TRAEs led to the permanent treatment discontinuation in 7 patients (n = 3, Squamous cell carcinoma [SCC] skin; n = 2, skin reactions; n = 1, SCC base of tongue; n = 1, epistaxis) and dose interruption in 9 patients (23.7%). Treatment duration of >6 months was more commonly associated with permanent treatment discontinuation due to TRAE (Supplementary Table S6). No treatment related death was reported. Immune-related AEs (irAEs) were observed in 11 patients (28.9%), and 2 (5.3%) experienced grade 3 irAEs. Adverse events of special interest, including potential TGFβ-related skin lesions or reactions occurred in 7 patients (n = 3, SCC skin; n = 2, keratoacanthoma; n = 2, pruritus). Fifteen patients (39.5%) experienced treatment-emergent bleeding, total 6 ≥ grade 3 bleeding events were developed in 4 patients, and 12 out of them also had concomitant anemia. There was no significant deterioration in the quality-of-life scores throughout the treatment (Supplementary Fig. S3 and S4).
Table 3.
Common Treatment-related adverse events (TRAEs).
| All grade | Grade 1–2 n, % | Grade 3–4 n, % | Grade 5 n, % | |
|---|---|---|---|---|
| Any | 34 (89.4%) | 18 (47.4%) | 16 (42.1%) | 0 (0%) |
| AEs led to permanent discontinuation | 7 (18.4%) | 0 (0%) | 7 (18.4%) | 0 (0%) |
| AEs led to temporary discontinuation | 9 (23.7%) | 3 (7.9%) | 6 (15.8%) | 0 (0%) |
| Adverse events | ||||
| Constitutional | ||||
| Asthenia | 6 (15.8%) | 6 (15.8%) | 0 (0%) | 0 (0%) |
| Fatigue | 5 (13.2%) | 5 (13.2%) | 0 (0%) | 0 (0%) |
| Fever | 4 (10.5%) | 4 (10.5%) | 0 (0%) | 0 (0%) |
| Infusion-related reaction | 1 (2.6%) | 1 (2.6%) | 0 (0%) | 0 (0%) |
| Musculoskeletal pain | 4 (10.5%) | 4 (10.5%) | 0 (0%) | 0 (0%) |
| Edema | 3 (7.9%) | 3 (7.9%) | 0 (0%) | 0 (0%) |
| Skin-related | ||||
| Pruritus | 14 (36.8%) | 12 (31.6%) | 2 (5.2%) | 0 (0%) |
| Rash, maculopapular | 12 (31.6%) | 12 (31.6%) | 0 (0%) | 0 (0%) |
| Rash, acneiform | 6 (15.8%) | 5 (13.2%) | 1 (2.6%) | 0 (0%) |
| Herpes zoster | 2 (5.3%) | 2 (5.3%) | 0 (0%) | 0 (0%) |
| Keratoacanthoma | 2 (5.3%) | 2 (5.3%) | 0 (0%) | 0 (0%) |
| Secondary malignancy | ||||
| SCC of skin | 3 (7.9%) | 0 (0%) | 3 (7.9%) | 0 (0%) |
| SCC of base of tongue | 1 (2.6%) | 0 (0%) | 1 (2.6%) | 0 (0%) |
| Gastro-intestinal | ||||
| Decreased appetite | 6 (15.8%) | 6 (15.8%) | 0 (0%) | 0 (0%) |
| Nausea | 1 (2.6%) | 1 (2.6%) | 0 (0%) | 0 (0%) |
| Vomiting | 1 (2.6%) | 1 (2.6%) | 0 (0%) | 0 (0%) |
| Diarrhea | 3 (7.9%) | 3 (7.9%) | 0 (0%) | 0 (0%) |
| Stomatitis | 2 (5.3%) | 2 (5.3%) | 0 (0%) | 0 (0%) |
| Dry mouth | 5 (13.2%) | 5 (13.2%) | 0 (0%) | 0 (0%) |
| Laboratory | ||||
| ALT increased | 5 (13.2%) | 5 (13.2%) | 0 (0%) | 0 (0%) |
| AST increased | 11 (28.9%) | 9 (23.7%) | 2 (5.3%) | 0 (0%) |
| Anemia | 19 (50%) | 10 (26.3%) | 9 (23.7%) | 0 (0%) |
| Leukopenia | 1 (2.6%) | 1 (2.6%) | 0 (0%) | 0 (0%) |
| Neutropenia | 1 (2.6%) | 1 (2.6%) | 0 (0%) | 0 (0%) |
| Thrombocytopenia | 4 (10.5%) | 3 (7.9%) | 1 (2.6%) | 0 (0%) |
| Hypothyroidism | 6 (15.8%) | 6 (15.8%) | 0 (0%) | 0 (0%) |
| Amylase increased | 1 (2.6%) | 1 (2.6%) | 0 (0%) | 0 (0%) |
| Lipase increased | 1 (2.6%) | 1 (2.6%) | 0 (0%) | 0 (0%) |
| Immune-related | ||||
| Hepatitis | 2 (5.3%) | 0 (0%) | 2 (5.3%) | 0 (0%) |
| Pneumonitis | 1 (2.6%) | 1 (2.6%) | 0 (0%) | 0 (0%) |
| Colitis | 1 (2.6%) | 1 (2.6%) | 0 (0%) | 0 (0%) |
| Dermatitis | 4 (10.5%) | 4 (10.5%) | 0 (0%) | 0 (0%) |
| Adrenal insufficiency | 1 (2.6%) | 1 (2.6%) | 0 (0%) | 0 (0%) |
| Bleeding | ||||
| Epistaxis | 12 (31.6%) | 9 (23.7%) | 3 (7.9%) | 0 (0%) |
| Gingival bleeding | 8 (21.1%) | 8 (21.1%) | 0 (0%) | 0 (0%) |
| Hemoptysis | 2 (5.3%) | 2 (5.3%) | 0 (0%) | 0 (0%) |
| Gastric bleeding | 1 (2.6%) | 0 (0%) | 1 (2.6%) | 0 (0%) |
| Fresh per rectal bleeding | 1 (2.6%) | 0 (0%) | 1 (2.6%) | 0 (0%) |
Note: Definitely related, probably related, and possibly related were classified as treatment-related adverse events. Possibly unrelated and definitely unrelated were classified as treatment unrelated.
TRAE, treatment-related adverse events; SCC, Squamous cell carcinoma; ALT, Alanine transaminase; AST, Aspartate transaminase.
Plasma TGFβ1
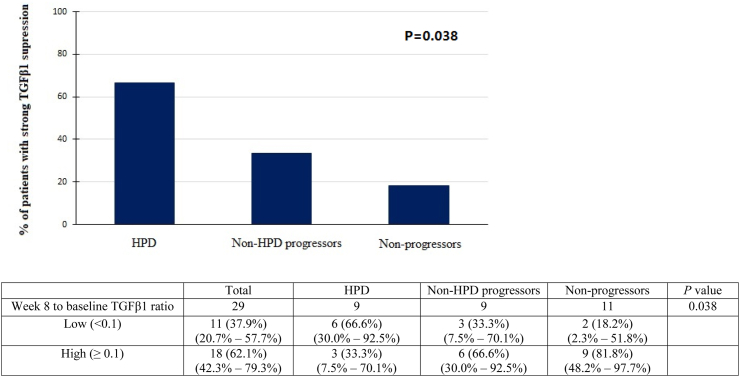
Plasma TGFβ1 levels were measured in 36 patients (Supplementary Table S7). Plasma TGFβ1 levels decreased significantly following 8 weeks of treatment (P < 0.001) (Supplementary Fig. S5). Patients who had a strong suppression of TGFβ1 level at week 8 (low week 8/baseline TGFβ1 ratio <0.1) were associated with worse ORR (9.1% versus 44.4%, P = 0.046) and PFS (2.3 months versus 5.6 months, P = 0.04) (Supplementary Fig. S5 and Table S8). Also, strong suppression of TGFβ1 was significantly more common among HPD per RECIST patients than PD and non-PD patients (HPD: 66.6% versus PD: 33.3% versus non-PD: 18.2%, P = 0.038) (Fig. 3). Similar trend was noted in patients with HPD per TGR ratio (Supplementary Fig. S1E). However, with a cut-off value of 1500 ng/ml at baseline, there was no significant difference between the groups in terms of ORR and survivals (Supplementary Fig. S5 and Table S8).
Fig. 3.
Bar chart showing the percentage of patients with strong TGFβ1 suppression (low week 8/baseline level) among patients with HPD vs non-HPD progressors vs non-progressors (per RECIST v1.1). Abbreviations: HPD: Hyper-progression; Non-HPD progressors, progressive disease not fulfilling the definition of HPD; Non-progressors = complete response (CR) + partial response (PR) + stable disease (SD).
PD-L1 expression
PD-L1 expression status in TC and IC samples were available in 31 patients (Supplementary Table S7). Two out of eight (25%) patients with PD-L1 TC negative tumors responded to the bintrafusp alfa. Six out of 23 (26.1%) patients with PD-L1 TC positive tumors responded, which was not statistically significant (P = 0.95). PD-L1 IC positive (n = 11, 35.5%) patients had an ORR of 27.3% whereas PD-L1 IC negative (n = 20, 64.5%) patients had an ORR of 25% (P = 0.89) (Supplementary Table S8).
Exosome PD-L1 (exo-PD-L1)
Plasma exo-PD-L1 was evaluated in 31 patients (Supplementary Table S7). At baseline, patients with an exo-PD-L1 expression ≥3.5 pg/ml and those with exo-PD-L1 expression <3.5 pg/ml had no significant differences in ORR, PFS, and OS. However, patients with the changes of exo-PD-L1 level ≥60 pg/ml at week 4 were associated with significantly worse ORR (5.3% versus 44.4%), median PFS (2.0 months versus not reached [NR]), and median OS (6.2 months versus NR) (Supplementary Fig. S5 and Table S8).
Plasma EBV DNA
Plasma EBV DNA was detected at baseline in all 38 patients (Supplementary Table S7). Patients with EBV DNA ≥10,000 copies/ml (n = 31) had significantly worse ORR [16.1% versus 57.1% (P = 0.02)] and survivals than those with EBV DNA <10,000 IU/ml (Supplementary Fig. S5 and Table S8). The dynamic monitoring of the EBV DNA titers were performed in 36 patients. Patients who demonstrated a decreasing trend in EBV DNA during the first 4 weeks of treatment were associated with significantly better ORR (40% versus 6.3%, P = 0.02) and PFS (Supplementary Fig. S5 and Table S8).
Discussion
Our results indicated that bintrafusp alfa had modest anti-tumour activity with an ORR of 24% in the unselected refractory NPC patients. The treatment responses appeared to be durable, with median DOR of 19.2 months (compared to chemotherapy 5–6 months) and a median OS of 17.0 months.5,10 Unfortunately, around 40% and 20% of patients developed HPD per RECIST and per TGR ratio criteria, respectively. Although little is known on rate of HPD in patients with R/M NPC treated with ICI,8, 9, 10, 11, 12, 13, 14 the incidences of HPD in current study were almost doubled to that reported in a recent meta-analysis that 9.4% and 20.6% developed HPD when treated with ICI based on TGR ratio and early tumor burden increase criteria, respectively.36 In addition, there is a high rate of treatment discontinuation secondary to the TRAEs and secondary malignancies.
Our report did not support the benefit of bintrafusp alfa concurrent blockade of the TGF-β and PD-L1 pathways in advanced NPC patients. This ORR of 23.7%, along with 1-year PFS rate of 30.1%, and 1-year OS rate of 63.2% among 38 patients treated with bintrafusp alfa were similar to that observed in a meta-analysis on anti-PD-1/PD-L1 inhibitors (ORR: 27%, DCR: 63%, 1-year PFS rate of 25%, and 1-year OS rate of 61%).37 Although cross-trial comparison should be interpreted with caution, numerically, DCR associated with bintrafusp alfa (28.9%) was worse than that reported in the anti-PD-1/PD-L1 clinical trials (63%).37 Two possible reasons for this phenomenon were the high rate of early disease progression and treatment discontinuation due to severe TRAEs.
In consistent with the pre-clinical data, our study showed that bintrafusp alfa was highly effective in reducing the plasma TGF-β1 level.24 However, in contrary to our hypothesis, patients with stronger suppression of TGF-β signalling was associated with worse ORR, survival, and the development of HPD. There are several postulations for this unexpected finding: the dual roles of TGF-β pathway in mediating the metastatic process; the inhibition of TGF-β pathway in late-stage cancer may paradoxically promote mesenchymal-epithelial transition and metastases,38, 39, 40, 41 the paracrine secretion of TGF-β in tumor microenvironment to activate downstream signalling pathway,42,43 and paradoxical effect of EBV-infected cells towards TGF-β inhibition.17 In-depth biomarker analyses are now underway to explore the mechanistic explanations behind and gain insights on the cancer biology of TGF-β pathway. Meantime, many prospective studies are now ongoing to evaluate the approach of dual TGF-β and PD-L1 axis inhibition in unselected population,44 the detrimental effects of TGF-β inhibition described in our study pose an important safety warning.
The presence of liver metastases was the only predictor of HPD in current study. Similar finding has been previously reported.45 Liver is characterized by distinctive immune-suppressive and immune-tolerance environment. Such a skewed immune micro-environment potentially fuels the growth of cancer cells and forms a safe niche for them to escape from the checking of host immune system. Further, the resident hepatic macrophages have tumor-promoting roles including immune suppression, metastasis, angiogenesis, and drug resistance. These features may attribute our findings of preponderance of HPD in patients with liver metastases.46
The high treatment discontinuation rate secondary to the TRAEs was another significant concern. In our study, seven patients had permanently discontinued bintrafusp alfa due to the development of TGF-β related toxicities (secondary malignancies, n = 4, skin reaction, n = 2, epistaxis, n = 1). Notably, all 7 patients demonstrated clinical benefit from bintrafusp alfa treatment, six patients had PR, and one had SD for more than 6 months. The tolerability of prolonged treatment of bintrafusp alfa was concerning; in our study, half of the patients received the treatment over six months turned out permanently discontinued the drug due to TRAEs. Dose interruption was also common (n = 9, 23.7%), which might have compromised the overall dose intensity and impacted the ORR. While the incidence and type of irAEs related to bintrafusp alfa were comparable to those observed with ICIs,47 the high toxicity rate was mainly driven by the inhibition of the TGF-β pathway which increased the risks of anemia, bleeding, secondary SCC, and skin lesions.48, 49, 50 The toxicities reported in this study were also observed in other bintrafusp alfa clinical trials.24, 25, 26, 27, 28, 29, 30 Noteworthy, in our study, 40% of the patients developed treatment-emergent bleeding. Most of the bleeding events originated from the head and neck mucosal area (epistaxis, 31.6%; gingival bleeding, 21.1%) previously received high dose radiation, while hemoptysis was uncommon (∼5%).
It was alarming that four patients had developed secondary SCC (10.5%) and all the secondary cancers were located in the head and neck region where previously had received high-dose radiation. Although these patients are inherently at risk in developing secondary malignancy,51 the blockage of tumor suppressive action of TGF-β in early-stage tumor may exacerbate the risk. As TGF-β is a pleiotropic cytokine with complex and often contradictory roles in carcinogenesis; in early carcinogenesis, TGF-β might act as a tumor suppressor with its cytostatic effect in the epithelial cells, while the loss of TGF-β was a late-stage event.52 Besides, TGF-β signalling played an important role to fine tone the balance between latent and lytic infection in EBV oncogenesis.17 EBV latent proteins, including LMP1 and LMP2A, were expressed during tumour progression.53 These proteins suppress TGF-β induced apoptosis which contributed to the maintenance of cancer stem-cell like population in NPC.54 Suppressing TGF-β level might disrupt the stem cells niche resulting in releasing cancer stem cells from dormancy.55 The therapeutic role and safety profile of bifunctional anti-PD-L1/TGFβ Trap fusion protein should be re-examined, in particular those who had previously received high dose irradiation.56,57
Our results supported the emerging evidence that EBV-DNA, but not PD-L1 expression status, is a predictive marker of patients with NPC receiving immunotherapy.9,11,58 Recent studies have revealed that exo-PD-L1 plays a key role in immunosuppression by targeting T lymphocytes in the tumor microenvironment.59,60 In our cohort, a sharp increase in the level of exo-PD-L1 was significantly more commonly observed in non-responders. Measuring exo-PD-L1 could provide real-time tracking of the systemic immune status and act as an early biomarker of immunotherapy response.
Several limitations of our study merit discussion. The major limitation is the lack of validation with randomization and the small sample size. Secondly, there is still no consensus on the best evaluation criteria of HPD. TGR ratio is the most used definition; however, it was being criticized for its clinical applicability as at least three radiological assessments (pre-baseline, baseline, post-treatment) are needed to calculate the ratio, also TGR ratio criteria does not consider the development of new lesions. On the other hand, the RECIST definition may risk overestimate the incidence of HPD.36 Therefore, we adopted the approach of using both criteria.61 Our study showed that the incidence of HPD in patients receiving dual blockage of TGF-β and PD-L1 was significantly higher than the historical data of ICI, irrespective of the criterion used.33 However, the high incidence of HPD was an unexpected finding, therefore our post-hoc analyses might subject to bias. Nevertheless, our findings on high rates of HPD associated with dual blockage was an important safety warning and warranted further validation in large-scale prospective cohort. Thirdly, around 95% of patients came from an endemic area where WHO III NPC are the predominant subtypes. The generalizability of our results into the global application remains to be determined. Finally, several statistical issues may potentially bias the results. For example, the impact of the treatment duration and follow-up time on the safety and toxicity data could not be fully addressed even we have reported the rates of severe TRAEs in different time periods. Also, statistical type I error may be introduced which is unavoidable for a small sample size with multiple comparisons like our study. Besides, in our primary and secondary analyses especially in biomarker analyses, a significant proportion of patients recurred or died before the extraction of biomarkers at pre-specified follow-up time-point, leading to missingness of data. In such cases, data could not be predicted based on the available data and thus imputation which was not considered necessary may introduce bias. A study with a larger sample size may further enhance the robustness.
Conclusions
Bintrafusp alfa has demonstrated modest clinical activity in refractory NPC patients but was associated with unusual high rate of HPD and TGF-β related complications. The results raised safety concerns on the strategy of TGF-β and PD-L1 dual blockage. Biomarker correlative studies are urgently needed to understand the biology and guide future drug development.
Contributors
Conception and Design: CL Chiang, TC Lam.
Provision of study materials or patients: CL Chiang, TC Lam, JCN Li, KSK Chan.
Data collection and assembly: CL Chiang, TC Lam, YYP Lee, LHT Law, D Zheng, AWI Lo.
Data analysis: KSK Chan.
Data interpretation: CL Chiang, TC Lam, JCB Li, YYP Lee.
Manuscript writing: All authors.
Manuscript approval: All authors.
Data sharing statement
Individual patient data will not be available for sharing. The study protocol can be available on request to the corresponding authors.
Ethics approval
This study was approved by The University of Hong Kong/Hospital Authority Hong Kong West Cluster Institutional Review Board (IRB number: UW 19–675). The patients/participants provided their written informed consent to participate in this study.
Declaration of interests
All the authors declare no potential conflicts of interest.
Acknowledgements
The project was supported by the Alice Ho Miu Ling Nethersole Charity Foundation Professorship Endowed Fund. Merck KGaA provided the study drug and financing of the study. We thank the patients who participated in the trial. We appreciate the efforts of John KS Fong, JP Hingley, and Dickson Poon for data collection and assembly. We acknowledge the Hong Kong NPC Study Group for their support in patient recruitment.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100898.
Contributor Information
Chi Leung Chiang, Email: chiangcl@hku.hk.
Tai Chung Lam, Email: taichung.lam@lthtr.nhs.uk.
Appendix A. Supplementary data
References
- 1.Carioli G., Negri E., Kawakita D., Garavello W., La Vecchia C., Malvezzi M. Global trends in nasopharyngeal cancer mortality since 1970 and predictions for 2020: focus on low-risk areas. Int J Cancer. 2017;140:2256–2264. doi: 10.1002/ijc.30660. [DOI] [PubMed] [Google Scholar]
- 2.Chua M.L.K., Wee J.T.S., Hui E.P., Chan A.T.C. Nasopharyngeal carcinoma. Lancet. 2016;387:1012–1024. doi: 10.1016/S0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 3.Lee A.W., Ma B.B., Ng W.T., Chan A.T. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33:3356–3364. doi: 10.1200/JCO.2015.60.9347. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L., Huang Y., Hong S., et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388:1883–1892. doi: 10.1016/S0140-6736(16)31388-5. [DOI] [PubMed] [Google Scholar]
- 5.Ma B.B., Chan A.T. Recent perspectives in the role of chemotherapy in the management of advanced nasopharyngeal carcinoma. Cancer. 2005;103:22–31. doi: 10.1002/cncr.20768. [DOI] [PubMed] [Google Scholar]
- 6.Bruce J.P., Yip K., Bratman S.V., Ito E., Liu F.F. Nasopharyngeal cancer: molecular landscape. J Clin Oncol. 2015;33:3346–3355. doi: 10.1200/JCO.2015.60.7846. [DOI] [PubMed] [Google Scholar]
- 7.Chen B.J., Chapuy B., Ouyang J., et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu C., Lee S.H., Ejadi S., et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol. 2017;35:4050–4056. doi: 10.1200/JCO.2017.73.3675. [DOI] [PubMed] [Google Scholar]
- 9.Ma B.B.Y., Lim W.T., Goh B.C., et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the mayo clinic phase 2 consortium (NCI-9742) J Clin Oncol. 2018;36:1412–1418. doi: 10.1200/JCO.2017.77.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang W., Yang Y., Ma Y., et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19:1338–1350. doi: 10.1016/S1470-2045(18)30495-9. [DOI] [PubMed] [Google Scholar]
- 11.Wang F.H., Wei X.L., Feng J., et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II clinical trial (POLARIS-02) J Clin Oncol. 2021;39:704–712. doi: 10.1200/JCO.20.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Even C., Wang H.M., Li S.H., et al. Phase II, randomized study of spartalizumab (PDR001), an anti-PD-1 antibody, versus chemotherapy in patients with recurrent/metastatic nasopharyngeal cancer. Clin Cancer Res. 2021;27:6413–6423. doi: 10.1158/1078-0432.CCR-21-0822. [DOI] [PubMed] [Google Scholar]
- 13.Shen L., Guo J., Zhang Q., et al. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colevas A.D., Bahleda R., Braiteh F., et al. Safety and clinical activity of atezolizumab in head and neck cancer: results from a phase I trial. Ann Oncol. 2018;29:2247–2253. doi: 10.1093/annonc/mdy411. [DOI] [PubMed] [Google Scholar]
- 15.Wrzesinski S.H., Wan Y.Y., Flavell R.A. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13:5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 16.Mariathasan S., Turley S.J., Nickles D., et al. TGFβ attenuates tumor response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velapasamy S., Dawson C.W., Young L.S., et al. The dynamic roles of TGF-β signalling in EBV-associated cancers. Cancers (Basel) 2018;10:247. doi: 10.3390/cancers10080247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu C., Wei W., Chen X., et al. A global view of the oncogenic landscape in nasopharyngeal carcinoma: an integrated analysis at the genetic and expression levels. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J., Menezes J., Prasad U., Ahmad A. Elevated serum levels of transforming growth factor beta1 in Epstein-Barr virus-associated nasopharyngeal carcinoma patients. Int J Cancer. 1999;84:396–399. doi: 10.1002/(sici)1097-0215(19990820)84:4<396::aid-ijc11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 20.Xia Y.Y., Yin L., Jiang N., et al. Downregulating HMGA2 attenuates epithelial-mesenchymal transition-induced invasion and migration in nasopharyngeal cancer cells. Biochem Biophys Res Commun. 2015;463:357–363. doi: 10.1016/j.bbrc.2015.05.068. [DOI] [PubMed] [Google Scholar]
- 21.Wang L., Tian W.D., Xu X., et al. Epstein-Barr virus nuclear antigen (EBNA1) protein induction of epithelial-mesenchymal transition in nasopharyngeal carcinoma cells. Cancer. 2014;120:363–372. doi: 10.1002/cncr.28418. [DOI] [PubMed] [Google Scholar]
- 22.Cao S., Cui Y., Xiao H., et al. Up-regulation of flotillin-1 promotes invasion and metastasis by activating TGF-beta signaling in nasopharyngeal carcinoma. Oncotarget. 2016;7:4252–4264. doi: 10.18632/oncotarget.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strauss J., Heery C.R., Schlom J., et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clin Cancer Res. 2018;24:1287–1295. doi: 10.1158/1078-0432.CCR-17-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lan Y., Zhang D., Xu C., et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan5488. [DOI] [PubMed] [Google Scholar]
- 25.Paz-Ares L., Kim T.M., Vicente D., et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line treatment of patients with NSCLC: results from an expansion cohort of a phase 1 trial. J Thorac Oncol. 2020;15:1210–1222. doi: 10.1016/j.jtho.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauss J., Gatti-Mays M.E., Cho B.C., et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus-associated malignancies. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho B.C., Daste A., Ravaud A., et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in advanced squamous cell carcinoma of the head and neck: results from a phase I cohort. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang Y.K., Bang Y.J., Kondo S., et al. Safety and tolerability of bintrafusp alfa, a bifunctional fusion protein targeting TGFβ and PD-L1, in asian patients with pre-treated recurrent or refractory gastric cancer. Clin Cancer Res. 2020;26:3202–3210. doi: 10.1158/1078-0432.CCR-19-3806. [DOI] [PubMed] [Google Scholar]
- 29.Yoo C., Oh D.Y., Choi H.J., et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strauss J., Gatti-Mays M.E., Redman J., et al. Safety and activity of M7824, a bifunctional fusion protein targeting PD-L1 and TGF-β, in patients with HPV associated cancers. J Clin Orthod. 2018;36:3007. [Google Scholar]
- 31.Yoo C., Javle M.M., Verdaguer Mata H., et al. Phase 2 trial of bintrafusp alfa as second-line therapy for patients with locally advanced/metastatic biliary tract cancers. Hepatology. 2023;78(3):758–770. doi: 10.1097/HEP.0000000000000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo Y.M., Leung S.F., Chan L.Y., et al. Kinetics of plasma Epstein-Barr virus DNA during radiation therapy for nasopharyngeal carcinoma. Cancer Res. 2000;60:2351–2355. [PubMed] [Google Scholar]
- 33.Matos I., Martin-Liberal J., García-Ruiz A., et al. Capturing hyper progressive disease with immune-checkpoint inhibitors using RECIST 1.1 criteria. Clin Cancer Res. 2020;26:1846–1855. doi: 10.1158/1078-0432.CCR-19-2226. [DOI] [PubMed] [Google Scholar]
- 34.Ferte C., Fernandez M., Hollebecque A., et al. Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin Cancer Res. 2014;20:246–252. doi: 10.1158/1078-0432.CCR-13-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan S.L. Hyperprogression in hepatocellular carcinoma: illusion or reality? J Hepatol. 2021;74:269–271. doi: 10.1016/j.jhep.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 36.Park H.J., Kim K.W., Won S.E., et al. Definition, incidence, and challenges for assessment of hyperprogressive disease during cancer treatment with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B.C., Cao R.B., Fu C., et al. The efficacy and safety of PD-1/PD-L1 inhibitors in patients with recurrent or metastatic nasopharyngeal carcinoma: a systematic review and meta-analysis. Oral Oncol. 2020;104 doi: 10.1016/j.oraloncology.2020.104640. [DOI] [PubMed] [Google Scholar]
- 38.Hao Y., Baker D., Ten Dijke P. TGF-β-Mediated epithelial-mesenchymal transition and cancer metastasis. Int J Mol Sci. 2019;20:2767. doi: 10.3390/ijms20112767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong Z., Carroll K.D., Policarpio D., et al. Anti-transforming growth factor beta receptor II antibody has therapeutic efficacy against primary tumor growth and metastasis through multieffects on cancer, stroma, and immune cells. Clin Cancer Res. 2010;16:1191–1205. doi: 10.1158/1078-0432.CCR-09-1634. [DOI] [PubMed] [Google Scholar]
- 40.Chaffer C.L., Brennan J.P., Slavin J.L., Blick T., Thompson E.W., Williams E.D. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 41.Biswas T., Gu X., Yang J., Ellies L.G., Sun L.Z. Attenuation of TGF-β signaling supports tumor progression of a mesenchymal-like mammary tumor cell line in a syngeneic murine model. Cancer Lett. 2014;346:129–138. doi: 10.1016/j.canlet.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Y., Xiao C.H., Tan L.D., et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br J Cancer. 2014;110:724–732. doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L., Pang Y., Moses H.L. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teixeira A.F., Ten Dijke P., Zhu H.J. On-target anti-TGF-β therapies are not succeeding in clinical cancer treatments: what are remaining challenges? Front Cell Dev Biol. 2020;8:605. doi: 10.3389/fcell.2020.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasaki A., Nakamura Y., Mishima S., et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. 2019;22:793–802. doi: 10.1007/s10120-018-00922-8. [DOI] [PubMed] [Google Scholar]
- 46.Donne R., Lujambio A. The liver cancer immune microenvironment: therapeutic implications for hepatocellular carcinoma. Hepatology. 2023;77:1773–1796. doi: 10.1002/hep.32740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khoja L., Day D., Chen T.W.W., Siu L.L., Hansen A.R. Tumor- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377–2385. doi: 10.1093/annonc/mdx286. [DOI] [PubMed] [Google Scholar]
- 48.Wang C.J., Lamping E., Strauss J., Gulley J.L. 14937 Keratoacanthomas associated with anti–TGF-β immunotherapy. JAAD. 2020;83 [Google Scholar]
- 49.Cammareri P., Rose A.M., Vincent D.F., et al. Inactivation of TGFβ receptors in stem cells drives cutaneous squamous cell carcinoma. Nat Commun. 2016;7 doi: 10.1038/ncomms12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goumans M.J., Liu Z., ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 51.Wang B., Wei J., Meng L., et al. Advances in pathogenic mechanisms and management of radiation-induced fibrosis. Biomed Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109560. [DOI] [PubMed] [Google Scholar]
- 52.Lo A.K., Dawson C.W., Lo K.W., Yu Y., Young L.S. Upregulation of Id1 by Epstein-Barr Virus-encoded LMP1 confers resistance to TGFβ-mediated growth inhibition. Mol Cancer. 2010;9:155. doi: 10.1186/1476-4598-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hau P.M., Lung H.L., Wu M., et al. Targeting epstein-barr virus in nasopharyngeal carcinoma. Front Oncol. 2020;10:600. doi: 10.3389/fonc.2020.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kondo S., Wakisaka N., Muramatsu M., et al. Epstein-Barr virus latent membrane protein 1 induces cancer stem/progenitor-like cells in nasopharyngeal epithelial cell lines. J Virol. 2011;85:11255–11264. doi: 10.1128/JVI.00188-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akhurst R.J., Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meng W., Xia Q., Wu L., et al. Downregulation of TGF-beta receptor types II and III in oral squamous cell carcinoma and oral carcinoma-associated fibroblasts. BMC Cancer. 2011;11:88. doi: 10.1186/1471-2407-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu S.L., Herrington H., Reh D., et al. Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 2006;20:1331–1342. doi: 10.1101/gad.1413306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J.Y., Wei X.L., Ren C., et al. Association of plasma epstein-barr virus DNA with outcomes for patients with recurrent or metastatic nasopharyngeal carcinoma receiving anti-programmed cell death 1 immunotherapy. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y., Li C.W., Chan L.C., et al. Exosomal PD-L1 harbors an active defence function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28:862–864. doi: 10.1038/s41422-018-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cordonnier M., Nardin C., Chanteloup G., et al. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracell Vesicles. 2020;9 doi: 10.1080/20013078.2019.1710899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim Y., Kim C.H., Lee H.Y., et al. Comprehensive clinical and genetic characterization of hyperprogression based on volumetry in advanced non-small cell lung cancer treated with immune checkpoint inhibitor. J Thorac Oncol. 2019;14:1608–1618. doi: 10.1016/j.jtho.2019.05.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.