Query (Q) fever, due to Coxiella burnetii, is a ubiquitous zoonosis. It was first described by Derrick (29) in 1935 in Queensland, Australia, during an outbreak of a febrile illness among abattoir workers. Subsequently, Burnet and Freeman (19) isolated a fastidious intracellular bacterium from guinea pigs that had been injected with blood or urine from Derrick’s patients and named it Rickettsia burnetii. This bacterium was morphologically and biochemically similar to other gram-negative bacteria. On the basis of cultural and biochemical characteristics, Philip (137) classified R. burnetii in a new genus, Coxiella, named after Herald R. Cox, who first isolated this microorganism in the United States. This genus contained only one species, C. burnetii. Since then, it has been isolated from several mammals and from ticks, and it may persist in the environment. The disease may be acquired by the respiratory or digestive route. The incidence of Q fever is unknown and may be underestimated. Over the last few years, the apparent increase in the incidence of this disease may be due to increased reporting or diagnosis. The clinical presentation of Q fever is polymorphic and nonspecific and may be acute, most often pneumonia or hepatitis, or chronic, most often endocarditis. Inapparent and subclinical infections are common. The diagnosis of Q fever relies mainly upon serology, the most commonly used method being the immunofluorescence assay. Serological testing for Q fever should always be done for a patient with a febrile illness and negative blood cultures.
BACTERIOLOGY
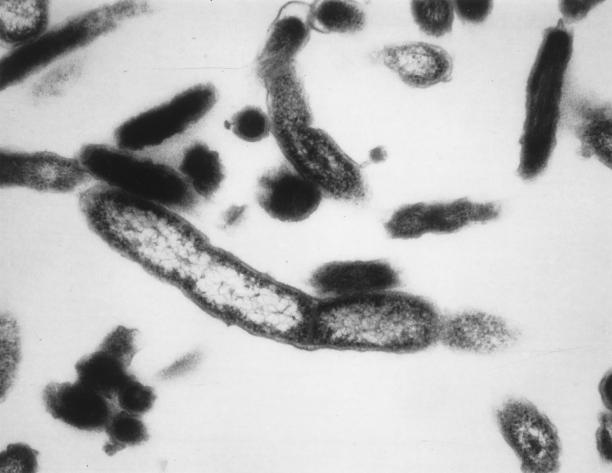
C. burnetii is a short (0.3 to 1.0 μm), pleomorphic rod that possesses a membrane similar to that of gram-negative bacteria (Fig. 1). It shares characteristics with bacteria included in the genus Rickettsia, including a small genome (65), staining by the Gimenez method (54), strict intracellular growth in eukaryotic cells, and association with arthropods (196). C. burnetii can be cultivated on cell layers from clinical or animal samples and can persist in daughter cells without affecting the viability of these persistently infected cells. On the other hand, C. burnetii demonstrates particular characteristics, such as a G+C content of 43% (196), a sporulation-like process conferring resistance to harsh environmental conditions (113), passive entry into host cells by phagocytosis, and a survival in phagolysosomes where a low pH (pH 4.5) is necessary for its metabolism (61, 62, 116). Moreover, contrary to the rickettsia, classified in the α1 subdivision of the family Proteobacteria, C. burnetii has recently been classified into the γ subdivision of this family, close to Rickettsiella grylli, Legionella spp., and Francisella spp., on the basis of a comparison of the sequences of the 16S rRNA-encoding gene (195). Another major characteristic of C. burnetii is its antigenic variation, called phase variation. This phenomenon, similar to the rough-smooth variation in enterobacteria, is due to partial loss of lipopolysaccharide (LPS) (60, 186). The LPS represents a major virulence determinant of C. burnetii (59). When isolated from animals or humans, C. burnetii expresses phase I antigens and is very infectious (a single bacterium may infect a human). Phase I LPS, with its extended carbohydrate structure, sterically blocks access of antibody to surface proteins (59). This may explain, at least in part, why the bacterium persists at unknown sites after recovery from acute cases of Q fever, accompanied by lifelong seropositivity. After subculture in cells or embryonated eggs, modification of the LPS results in an antigenic shift to the phase II form, which is less infectious. This LPS modification makes surface proteins accessible for antibodies (59, 186). LPS seems to be the only antigen and immunogen differing between phase I and II in C. burnetii (3). This antigenic peculiarity is extremely valuable for the serological differentiation between acute and chronic Q fever.
FIG. 1.
C. burnetii. Transmission electron micrograph showing a gram-negative-like wall cell. Magnification, ×75,000.
C. burnetii expresses a low degree of genetic heterogeneity among strains. However, variation in the composition of LPS has been demonstrated (60, 185). Moreover, 20 different genotypes have been delineated by pulsed-field gel electrophoresis (65) and/or restriction fragment length polymorphism analysis (178). The 1.6 × 106-bp genome comprises a chromosome and four copies of a plasmid. Four different plasmids have been described for the Nine Mile strain, and they vary in their size (117, 160, 162, 190). Besides host factors which probably play a major role in the clinical form of the disease (143), the roles of LPS type, plasmid type (161, 173, 177, 203), and the route of infection (111) are still controversial.
EPIDEMIOLOGY
Q fever is a worldwide zoonosis. The reservoirs are extensive but only partially known and include mammals, birds, and arthropods, mainly ticks. While an important reservoir seems to be small wild rodents, the most commonly identified sources of human infection are farm animals such as cattle, goats, and sheep. Pets, including cats (67), rabbits, and dogs, have also been demonstrated to be potential sources of urban outbreaks. Cats are suspected as an important reservoir of C. burnetii in urban areas and may be the source of urban outbreaks (90, 108, 118). In Canada, 6 to 20% of cats have anti-C. burnetii antibodies (67). Wild rats have been suspected as an important reservoir in Great Britain (193). All these mammals, when infected, shed the desiccation-resistant organisms in urine, feces, milk, and, especially, birth products (80, 106). Reactivation of infection occurs in female mammals during pregnancy. Q fever causes abortions in goats and, less frequently, sheep and causes reproductive problems in cattle (7, 192, 204). High concentrations of C. burnetii (up to 109 bacteria per g of tissue) are found in the placentas of infected animals (4). Due to its resistance to physical agents, probably related to its sporulation process (89, 113), C. burnetii survives for long periods in the environment.
In humans, infection results from inhalation of contaminated aerosols from amniotic fluid or placenta or contaminated wool. Therefore, Q fever is an occupational hazard. At greatest risk are persons in contact with farm animals, but also at risk are laboratory personnel who work with infected animals (75). When looking for the source of C. burnetii exposure, the investigator should search for contact with a parturient or newborn animal. Mammals also shed C. burnetii in milk, and thus, consumption of raw milk could be a source of infection (47, 118, 180). Sexual transmission of Q fever has been demonstrated in the mouse (86) and has been suspected in humans (100). Sporadic cases of human-to-human transmission following contact with an infected parturient woman have been reported and have been suspected to occur by direct aerosol transmission. It has also been proven to occur via transplacental transmission, resulting in congenital infections (98, 153), via intradermal inoculation (14, 39), and via blood transfusion (21, 151). Ticks transmit C. burnetii to domestic mammals but not to humans (80). C. burnetii may persist asymptomatically in humans throughout life. However, pregnancy, a cardiac valvular abnormality, a vascular aneurysm or prosthesis, hemodialysis (91), and immunodeficiency, including AIDS (64, 77, 97, 143, 147), may promote reactivation of dormant C. burnetii.
In Europe, acute Q fever cases are more frequently reported in spring and early summer. They may occur at all ages, but they are more frequent in men than in women. Q fever is usually benign, but mortality occurs in 1 to 11% of patients with chronic Q fever (143). C. burnetii is endemic in every part of the world except New Zealand (68, 78). Since the clinical presentation is very pleomorphic and nonspecific, the incidence of Q fever among humans is probably underestimated, and diagnosis particularly relies upon the physician’s awareness of the symptoms of Q fever and the presence of a reliable diagnostic laboratory. In southern France, 5 to 8% of cases of endocarditis are due to C. burnetii, and the prevalence of acute Q fever is 50 cases per 100,000 inhabitants (180). Seroepidemiological surveys have shown that 18.3% of blood donors in Morocco, 26% in Tunisia (125), 37% in Zimbabwe (82), 44% in Nigeria (11), 10 to 37% in northeast Africa, and 14.6 to 36.6% in different areas of Canada (109, 112) had anti-C. burnetii antibodies. Large outbreaks of Q fever have also been reported in the Basque country in Spain (1), in Switzerland (37), in Great Britain (57), in Berlin, Germany (164), and more recently, in southern France (unpublished data).
CLINICAL MANIFESTATIONS
Clinical signs of Q fever are often subclinical or extremely mild. For example, during a Q fever outbreak in Switzerland (37), of the 415 patients diagnosed with Q fever, 224 were seropositive but asymptomatic (54%) and only 2% of those affected were hospitalized. C. burnetii infections may be acute or chronic.
Acute infection.
The incubation period has been estimated to be approximately 20 days (range, 14 to 39 days) (37). There is no typical form of acute Q fever. The clinical signs vary greatly from patient to patient. The most important diagnostic clue is the epidemiological circumstance. Typically, three major presentations are described. These are as follows.
(i) Self-limited flu-like syndrome.
A self-limited flu-like syndrome is the most common manifestation of Q fever. In Spain, this form of Q fever has been demonstrated as the cause of 21% of episodes of fever lasting for more than 1 week and less than 3 weeks. The most frequent symptoms, usually following a sudden onset, are high-grade fever (104°F or 40°C), fatigue, headache, and myalgias. In 1973 Derrick (30) described the course of infection in 173 patients infected with C. burnetii. The duration of fever was 5 to 57 days, with a median of 10 days. He noted that the duration of fever increased with increasing age and in 28% of cases fever relapsed (30).
(ii) Pneumonia.
Atypical pneumonia is one of the most commonly recognized forms of acute Q fever. This is the major manifestation of acute Q fever in Nova Scotia, Canada (103), in the Basque country in Spain (1), and in Switzerland (37), while in France (180), Ontario, California (23), and Australia (168), hepatitis is the predominant form of acute Q fever. Marrie et al. (107) demonstrated that 3.7% of all patients with community-acquired pneumonia admitted to a tertiary-care teaching hospital in Nova Scotia over a 5-year period were due to C. burnetii, which is similar to the findings of Lieberman et al. (93) in Israel (5.8%). Most cases are clinically asymptomatic or mild, characterized by a nonproductive cough, fever, and minimal auscultatory abnormalities, but some patients present with acute respiratory distress (105). Pleural effusion can also be present. Findings on the chest radiograph are nonspecific.
The duration of symptoms varies from 10 to 90 days. The mortality rate ranges from 0.5 to 1.5%, depending upon the series (30, 180).
(iii) Hepatitis.
Three major forms of hepatitis may be encountered: an infectious hepatitis-like form of hepatitis with hepatomegaly but seldom with jaundice, clinically asymptomatic hepatitis, and prolonged fever of unknown origin with characteristic granulomas on liver biopsy (35, 131, 140, 157, 194).
(iv) Other manifestations.
Many other clinical manifestations of acute Q fever are possible: maculopapular or purpuric exanthema in 10% of patients (180), pericarditis and/or myocarditis (which is frequently fatal), and severe headache. Aseptic meningitis and/or encephalitis, which occur in 0.2 to 1.3% of patients with Q fever (15, 28, 34, 55, 63, 102, 110, 156), are rarely accompanied by seizures and coma (34, 156). Polyradiculoneuritis (12), optic neuritis (165), hemophagocytosis (43), hemolytic anemia (22), transient hypoplastic anemia (69), thyroiditis, gastroenteritis (95), pancreatitis, lymphadenopathy mimicking lymphoma (142), erythema nodosum (24), bone marrow necrosis (13, 58), inappropriate secretion of antidiuretic hormone (9), mesangioproliferative glomerulonephritis related to antiphospholipid antibodies (184), and splenic rupture (6) are uncommon manifestations of acute Q fever.
Chronic infection.
Chronic Q fever was initially described as lasting for more than 6 months after the onset (151). It occurs in approximately 5% of patients infected with C. burnetii and may develop insidiously months to years after the acute disease. In the chronic form of Q fever, C. burnetii multiplies in macrophages, and a permanent rickettsemia results in very high levels of persistent antibodies. Typically, the heart is the most commonly involved organ, followed by arteries, bones, and liver (18). Endocarditis usually occurs in patients with previous valvular damage or those who are immunocompromised (143, 147, 149, 182). Chronic Q fever represents 3% of all cases of endocarditis in England and Lyon, France (129), and 15% in Marseille, France (48), and its annual incidence is 0.75 cases per 1 million population in Israel (167). Clinically, the disease usually presents as a subacute or acute blood culture-negative endocarditis (18, 150, 152, 187). Symptoms are not specific. Arterial embolism occurs in about 20% of patients (174). Vegetations are only rarely seen by transthoracic cardiac ultrasonography. They are usually smooth and nodular (76). Because of the lack of specificity of symptoms, the diagnosis is often delayed 12 to 24 months, resulting in an increased mortality rate. Other manifestations of chronic Q fever include infections of aneurysms or vascular grafts (49), isolated hepatitis possibly complicated by hepatic fibrosis and cirrhosis (18, 187), and osteoarthritis and osteomyelitis (13, 40, 146). Rare cases of pericardial effusion (189), pulmonary interstitial fibrosis (2), pseudotumor of the lung (72, 96), lymphoma-like presentation (17), amyloidosis (79), and mixed cryoglobulinemia (42) have been reported in the literature.
Q fever during pregnancy.
Both acute and chronic Q fever have been described during pregnancy. In mammals, C. burnetii undergoes reactivation during pregnancy and thus is responsible for higher rates of abortion, prematurity, and low birth weight (27, 141, 192, 204). In humans, it has been isolated from the placenta of a woman who became pregnant 2 years after an episode of acute Q fever (139, 175), but few cases have been reported (8, 31, 98, 104, 153, 155, 175). Clinically, although most cases seem to be asymptomatic (98), complications may complicate the course of the disease, such as in utero fetal death (153), placentitis (8), or thrombocytopenia (155). Although intrauterine transmission of C. burnetii has been documented, the consequences of congenital Q fever remain to be determined.
NONSPECIFIC LABORATORY DIAGNOSIS
Acute Q fever.
The leukocyte count in patients with acute Q fever is usually normal (18). However, 25% of patients have an elevated leukocyte count, ranging from 14 × 109 to 21 × 109/liter. The erythrocyte sedimentation rate may be elevated. Thrombocytopenia is noted in 25% of patients. Liver enzyme levels are elevated in as many as 85% of patients. The increase in transaminase levels is usually moderate, ranging from 2 to 10 times normal values. During an episode of prolonged fever, the association of a normal leukocyte count, thrombocytopenia, and elevated hepatic enzyme levels are evocative of Q fever. However, thrombocytosis (>400 × 109 liter) may be encountered during convalescence. Twenty percent of patients have an elevated creatine phosphokinase level. In Q fever meningoencephalitis, a mild lymphocytic pleiocytosis is frequently noted in the spinal fluid.
Autoantibodies are commonly found during the course of Q fever, but their real significance is still unknown. They are probably an epiphenomenon of the disease but can be responsible for severe complications. A variety of these autoantibodies have been described in acute Q fever, including antimitochondrial antibodies (41, 92) and anti-smooth muscle antibodies (92). A moderate level of anti-smooth muscle antibodies is frequent at the time of seroconversion (151). Other antibodies, such as antibodies to phospholipids, especially anticardiolipin antibodies (74, 138, 159, 166, 184) or lupus anticoagulant (126), should be detected prior to an hepatic biopsy. Although they had been suspected to be identical, anti-phase II antibodies to C. burnetii and anticardiolipin antibodies have been demonstrated to be different (126). It is possible that the antibodies to phospholipids could be triggered by an increased level of exposure of anionic phospholipids from endothelial cells damaged by C. burnetii (126). These antibodies are usually transient and disappear during convalescence (184). Poux et al. (138) have detected both immunoglobulin G (IgG) and IgM anticardiolipin antibodies in a patient with acute Q fever and observed an early disappearance of IgM in the first 2 months of convalescence. An acquired inhibitor of blood coagulation factor IX (antihemophilia B) has also been reported (5).
Chronic Q fever.
In Q fever endocarditis, clinical and biological symptoms are related to the predominantly cell-mediated inflammatory response to the microorganism (18, 150, 182). Conventional blood cultures remain negative. The laboratory manifestations of an inflammatory syndrome are usual, including anemia, elevated erythrocyte sedimentation rate, and polyclonal hypergammaglobulinemia. The leukocyte count may be normal, increased, or decreased. Thrombocytopenia and elevated hepatic enzyme levels are commonly found. Renal involvement is common, characterized by an elevated creatinine level and microhematuria. Monoclonal immunoglobulins are rarely observed (42), whereas cryoglobulins are frequently found. Autoantibodies are also frequent in chronic Q fever, particularly rheumatoid factor, anti-smooth muscle (92), or antinuclear antibodies. Antimitochondrial antibodies (41, 92), circulating anticoagulant antibodies, and a positive Coombs’ test may also be observed (92).
SPECIFIC LABORATORY DIAGNOSIS
Collection and storage of specimens.
C. burnetii is a very infectious disease. Thus, only biosafety level 3 laboratories and experienced personnel should be allowed to manipulate contaminated specimens and cultivate this microorganism from clinical samples. Several human specimens are suitable for the detection of C. burnetii, but their availability depends on the clinical presentation. DNA amplification may be performed from blood, cerebrospinal fluid, bone marrow, cardiac valve biopsy, vascular aneurysm or graft, bone biopsy, or liver biopsy specimens; milk; placenta; fetal specimens in case of an abortion; and cell culture supernatants. Blood should be collected on EDTA or sodium citrate, and the leukocyte layer should be saved for the amplification. Solid specimens should be kept frozen at −80°C before testing. C. burnetii may be cultivated from the leukocyte layer of a heparinized blood, whole blood, plasma, bone marrow, cerebrospinal fluid, cardiac valve biopsy, vascular aneurysm or graft, bone biopsy, or liver biopsy specimen; milk; placenta; and fetal specimens in case of an abortion but not from blood collected on EDTA or sodium citrate. All specimens, excluding whole blood, should be stored at −80°C and should be forwarded on dry ice to the diagnostic laboratory. Whole blood should be kept at 4°C. Furthermore, erythrocytes should not be inoculated onto shell vials because they lead to a high background at the time of the examination with UV light.
Pathological examination.
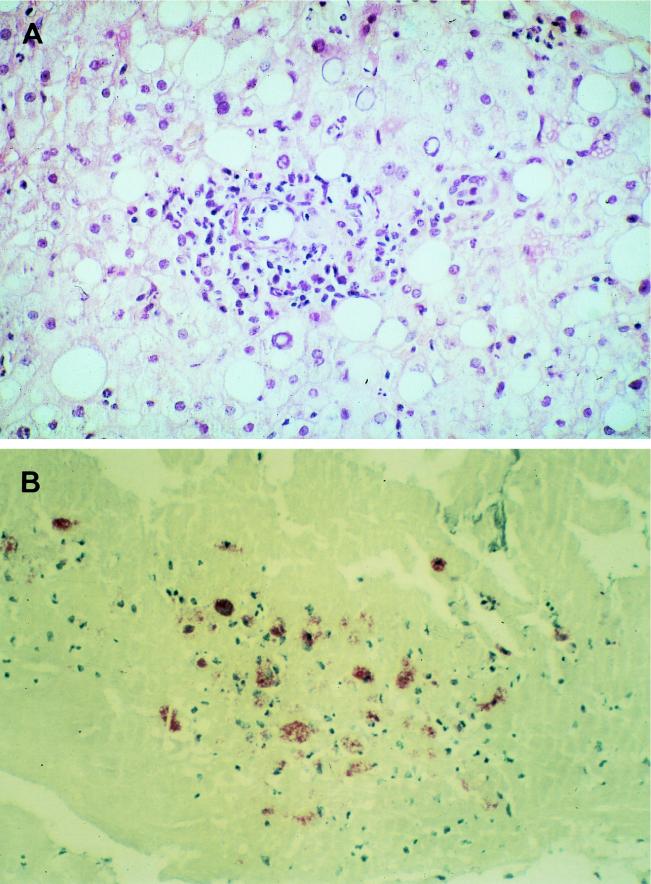
The immune response during Q fever is associated with an inflammatory reaction, which results in the formation of granulomatous lesions most commonly involving the lungs, liver, and bone marrow. La Scola et al. (87) have demonstrated that the route of infection may influence the pathological changes. The histology of Q fever pneumonia in humans has rarely been studied (72, 73, 94, 132). Macroscopically, red or gray hepatization may be present. Microscopically, interstitial edema and infiltration by lymphocytes and macrophages occur. Alveolar spaces are filled with histiocytes, and intra-alveolar focal necrosis and hemorrhage have been described. A necrotizing bronchitis and bronchiolitis may be encountered. Microorganisms are usually lacking in these lesions. Giant cells and plasma cells were seen in a pulmonary pseudotumor due to C. burnetii (72). The hepatic lesions are different in acute and chronic Q fever. In acute cases, the characteristic findings are granulomatous lesions containing the so-called doughnut granuloma, which consist of dense fibrin rings surrounding a central lipid vacuole (35, 56, 131, 140, 169) (Fig. 2A). Granulomatous changes and necrosis were noted in bone marrow (25, 124, 169). In chronic cases, pathological findings are nonspecific: lymphocytic infiltration and foci of spotty necrosis (72). The vegetations in Q fever endocarditis are often smooth and nodular. The valve is often infiltrated with foamy macrophages, which are filled with C. burnetii cells.
FIG. 2.
(A) Liver in acute Q fever. A liver biopsy specimen was stained with hematoxylin-phloxin-saffron. One granuloma is seen within the fatty liver parenchyma. The lesions consist of inflammatory infiltrates made of epithelioid cells, polymorphonuclear leukocytes, and histiocytes. Magnification, ×400. (B) Immunoperoxidase staining of a cardiac valve biopsy specimen showing fibrous valvular tissue comprising inflammatory infiltrates made of histiocytes. Magnification, ×40.
Immunodetection of C. burnetii in tissues.
The detection of C. burnetii in tissues is especially informative in patients who are undergoing treatment for chronic Q fever. Samples can be tested fresh or after formalin fixation and paraffin embedding. Valvular or vascular samples are most valuable. Several techniques are available, including immunoperoxidase staining (16) (Fig. 2B), a capture enzyme-linked immunosorbent assay system (ELISA) or enzyme-linked immunosorbent fluorescence assay system (176), or tests with monoclonal antibodies (114, 119, 176). The last technique can also be used for detection of antigen in paraffin-embedded tissues (148). Brouqui et al. (16) examined the valves of 17 patients with Q fever endocarditis using immunohistochemical methods. The organisms were clustered as a single intracytoplasmic mass within mononuclear cells and usually occupied the entire cytoplasm. Kocianova and Lukacova (83) could detect C. burnetii in the hemolymph of ticks using an immunodot blot test.
DNA amplification.
PCR has successfully been used to detect C. burnetii DNA in cell cultures and clinical samples (171) (Table 1). Initially, methods used specific hybridization of labelled DNA probes to nucleic acid amplified from clinical samples (50, 51, 99, 199). These methods were very sensitive and specific but were available only in specialized research laboratories. The availability of primers derived from genes specific to C. burnetii has allowed a simple and reliable method for the detection of this bacterium, even in paraffin-embedded tissues (171, 172). Moreover, PCR has proven to be more sensitive than standard culture techniques for retrospective diagnosis with frozen samples and for the follow-up of patients treated for chronic Q fever (171). In our experience, specimens kept frozen at −80°C are suitable for PCR for several years. Fritz et al. (52) have used PCR to quantify the amount of C. burnetii in tissue. In our laboratory, we routinely use primers derived from the htpAB-associated repetitive element (70, 197). This element exists in at least 19 copies in the C. burnetii Nine Mile I genome, and PCR based upon this gene is very sensitive (197).
TABLE 1.
Genes and derived primers available for PCR amplification of C. burnetii
| Gene | Reference(s) | Primers (sequences) |
|---|---|---|
| 16S rRNA | 199 | 16S1 (5′-CTC CTG GCG GCG AGA GTG GC-3′) |
| 16S2N (5′-GTT AGC TTC GCT ACT AAG AAG GGA ACT TCC C-3′) | ||
| 23S rRNA | 71 | 976F (5′-AGG TCC TGG TGG AAA GGA ACG-3′) |
| 1446R (5′-TCT CAT CTG CCG AAC CCA TTG C-3′) | ||
| 16S-23S rRNA internal transcribed spacer | 170, 179 | 16SF (5′-TTG TAC ACA CCG CCC GTC A-3′) |
| 23SR (5′-GGG TT (CGT) CCC CAT TCG G-3′) | ||
| 16SS (5′-GAA GTC GTA ACA AGG TA-3′) | ||
| 23SS (5′-TCT CGA TGC CAA GGC ATC CAC C-3′) | ||
| Superoxide dismutase | 171 | CB1 (5′-ACT CAA CGC ACT GGA ACG GC-3′) |
| CB2 (5′-TAG CTG AAG CCA ATT CGC C-3′) | ||
| Plasmid QpRS | 199 | QpRS01 (5′-CTC GTA CCC AAA GAC TAT GAA TAT ATC-3′) |
| QpRS02 (5′-CAC ATT GGG TAT CGT ACT GTC CCT-3′) | ||
| Plasmid QpH1 | 199 | QpH11 (5′-TGA CAA ATA GAA TTT CTT CAT TTT GAT-3′) |
| QpH12 (5′-GCT TAT TTT CTT CCT CGA ATC TAT GAA T-3′) | ||
| cbbE | 199 | G4131 (5′-CTG ATG TGT CAA GTA ATG TCG G-3′) |
| G4132 (5′-CTT CAT GGT TAT GAT TCT GCG-3′) | ||
| htpAB | 197 | Trans1 (5′-TAT GTA TCC ACC GTA GCC AGT C-3′) |
| Trans2 (5′-CCC AAC AAC ACC TCC TTA TTC-3′) |
Culture of C. burnetii.
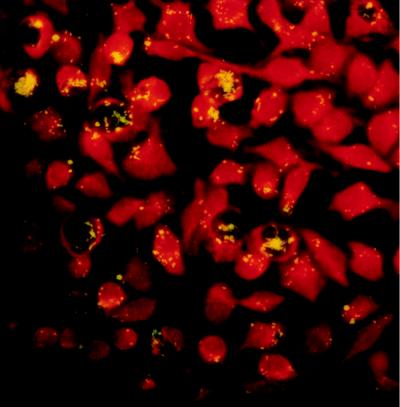
The isolation of C. burnetii must be done only in biosafety level 3 laboratories due to its extreme infectivity. This microorganism can be isolated by inoculation of specimens onto conventional cell cultures (monkey kidney cells, Vero cells) or into embryonated hen yolk sacs (127) or laboratory animals, such as mice or guinea pigs (200). Embryonated eggs die 7 to 9 days after inoculation. Guinea pigs develop fever 5 to 8 days after intraperitoneal inoculation. The spleen is the most valuable organ for the recovery of C. burnetii. Ground spleen extracts should subsequently be inoculated into embryonated eggs. Although they are now used less often, these methods remain helpful in cases requiring isolation from tissues contaminated with multiple bacteria or in order to obtain phase I Coxiella antigens from phase II cells. The recent development of a cell microculture system from a commercially available method for virus culture, the shell vial cell culture system, has allowed for improvement in the isolation of intracellular bacteria, especially C. burnetii (101, 154). Specimens are inoculated onto human embryonic lung fibroblasts grown on a 1-cm2 coverslip within a shell vial. A 1-h centrifugation step enhances the attachment and penetration of bacteria into the cells. After an incubation period of 6 days, detection of C. burnetii within the cells is achieved by microscopic examination after staining. The organism appears as a short rod which is not stained by Gram staining but which is visible after Giemsa or Gimenez staining (54). The identification of C. burnetii within the cells is performed by a direct immunofluorescence assay with polyclonal or monoclonal anti-C. burnetii antibodies conjugated to fluorescein isothiocyanate (154) (Fig. 3). In our laboratory, we systematically amplify shell vial supernatants by PCR. Although Mühlemann et al. (119) could isolate C. burnetii from the heart valves of patients treated for Q fever endocarditis, better results are obtained if clinical specimens are collected prior to the initiation of antibiotic therapy (121). Fifteen percent of untreated patients with Q fever pneumonia have positive blood cultures by this method, as do 53% of patients with endocarditis (53, 121).
FIG. 3.
Shell vial cell culture system: direct immunofluorescence assay incorporating monoclonal anti-C. burnetii antibodies conjugated to fluorescein isothiocyanate. The C. burnetii isolates appear as short rods. Magnification, ×870.
Serological diagnosis of Q fever.
Since the clinical diagnosis is difficult, in most instances, the diagnosis of Q fever relies upon serology. Several methods have been described: microagglutination (46, 81, 123), complement fixation (66, 120, 134), radioimmunoassay (33), indirect immunofluorescence antibody tests (immunofluorescence assay) (44, 134), indirect haemolysis test (183), ELISA (84, 133, 188, 191, 201), enzyme-linked immunosorbent fluorescence assay (163), dot immunoblotting, and Western immunoblotting (10, 198). Criteria to be taken into account in choosing a diagnostic test include its specificity, sensitivity, positive predictive value, cost, and the amount of antigen required. The most reliable and commonly used methods are indirect immunofluorescence, complement fixation, ELISA, and microagglutination. Only the first two are commercially available. The sensitivity, specificity, and positive predictive value of immunofluorescence assay, complement fixation, and ELISA are presented in Table 2.
TABLE 2.
Sensitivity, specificity, and positive and negative predictive values of different serological tests for diagnosis of Q fever
| Test | Form of disease | Titer | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Reference |
|---|---|---|---|---|---|---|---|
| Microimmunofluorescence | Acute | Anti-phase II IgG, ≥1:200; anti-phase II IgM, ≥1:50 | 58.4 | 92.2 | NAa | 88–96.6 | 181 |
| Chronic | Anti-phase I IgG, ≥1:800 | 100 | NA | 98.6 | NA | 181 | |
| Anti-phase I IgG, ≥1:1,600 | NA | NA | 100 | NA | 181 | ||
| ELISA | Acute | Anti-phase II IgG, ≥1:1,024 | 80 | >99 | NA | NA | 191 |
| Anti-phase II IgM, ≥1:512 | 84 | ||||||
| Acute (screening) | Anti-phase II IgG, ≥1:128; anti-phase II IgM, ≥1:128 | NA | 97.3–98.7 | NA | NA | 191 | |
| Microagglutination | Acute | 1:64 | 81.6 | 98.6 | NA | NA | 123 |
NA, not available.
(i) Indirect immunofluorescence.
Currently, the immunofluorescence assay is the reference method for the serodiagnosis of Q fever (130). It is the simplest and one of the most accurate serological techniques. In order to prepare antigens for this test, phase II C. burnetii Nine Mile reference strain is grown in confluent layers of L929 mouse fibroblasts, while phase I antigens are obtained from the spleens of mice inoculated with phase II organisms (181). This method of preparation has been demonstrated to yield antigens with the highest sensitivity for C. burnetii antibody detection (85). In our laboratory, we use a microimmunofluorescence technique, which requires very small amounts of antigens. Sera are diluted in phosphate-buffered saline with 3% nonfat powdered milk to avoid the nonspecific fixation of antibodies. This method can be used to determine antibodies to phases I and II in the IgG, IgM, and IgA fractions. However, test results can be confounded by the presence of a rheumatoid factor. Thus, a rheumatoid factor absorbant is used in order to remove IgG before the determination of IgM and IgA (181). The choice of a negative cutoff titer depends upon the source and purity of the antigen and the amount of background antigen stimulation in the population to be studied. We use a 1:50 dilution as our first positive dilution (181). Screening is performed with anti-phase II anti-immunoglobulins with a 1:50 dilution for the tested sera. Positive sera are then serially diluted and tested for the presence of anti-phase I and II IgG, IgM, and IgA. Seroconversion is usually detected 7 to 15 days after the onset of clinical symptoms. About 90% of patients have detectable antibodies by the third week.
(ii) Complement fixation.
Complement fixation is very specific, although it is less specific than the immunofluorescence assay, but it lacks sensitivity (134). Sera are heat inactivated before testing against phase II antigens (66). This method detects both anti-phase I and II antibodies. However, a prozone phenomenon may be present with serum specimens from patients with chronic Q fever, and this phenomenon could result in a false-negative test. It is also more time-consuming than the immunofluorescence assay (136). Moreover, cross-reaction with hen egg antigens may result in false-positive results. The interpretation of results requires acute- and convalescent-phase serum samples. Seroconversion is detected later by the complement fixation test than by the immunofluorescence assay or ELISA (between 10 and 20 days after the onset of symptoms) (57, 134).
(iii) ELISA.
First described by Field et al. (44), ELISA was described as more specific and sensitive than complement fixation for the diagnosis of Q fever. It was then proposed as a good method for seroepidemiological surveys (135). Peter et al. (133) and Cowley et al. (26) have demonstrated that this technique was even more sensitive than the immunofluorescence assay and could serve for the serodiagnosis of Q fever. However, it is a more laborious technique than the immunofluorescence assay and it requires a considerable experience in interpreting the results. Therefore, its application to Q fever diagnosis is still limited. Technically, microtiter plates are coated with purified C. burnetii antigens. Serially diluted sera in phosphate-buffered saline containing 0.1% Tween 20 are then incubated with the antigens, and antibodies are detected with alkaline phosphatase-conjugated rabbit anti-human IgG, IgM, and IgA. Both anti-phase I and II antibodies are detected.
(iv) Western blotting.
Western immunoblotting has been reported as both a specific and a sensitive method for the diagnosis of Q fever (10). However, in our experience (several thousands of tests), the results were not reproducible and the test lacked specificity (unpublished data). Furthermore, it was time-consuming and was not suitable for screening. It is based on the entire spectrum of antigens of C. burnetii and allows a differentiation of the humoral response to a number of different antigenic components of this microorganism. The molecular masses of the different antigens ranges from 10 to 100 kDa.
(v) Dot immunoblotting.
Cowley et al. (26) demonstrated that dot immunoblotting was as sensitive and as specific as ELISA and immunofluorescence assay but more sensitive than complement fixation. They also proposed this method as a screening test.
(vi) Microagglutination.
Microagglutination is simple and sensitive and can detect an early antibody response to C. burnetii (81). It has a major disadvantage, especially when compared to the immunofluorescence assay, in that it requires large amounts of antigens. More recently, high-density particle agglutination has been demonstrated to be highly specific and much more sensitive than microagglutination (123).
(vii) Indirect hemolysis test.
Tokarevich et al. (183) proposed an indirect hemolysis test as early as 1990. They suggested that this test could be highly sensitive and specific and could serve for the follow-up of chronic Q fever.
(viii) Radioimmunoassay.
Doller et al. (33) proposed a radioimmunoassay for the diagnosis of Q fever. This technique, based on the IgM antibody-capture principle, uses 125I and therefore must be used in radioactivity-equipped laboratories.
Cross-adsorption.
Cross-adsorption is used for the detection of antibodies cross-reacting with other bacteria. This cross-reactivity will vary depending on the serological technique used and the host animal from which the antiserum is obtained. Cross-reactions against Legionella pneumophila (38, 45), Legionella micdadei (32, 122), and Bartonella quintana and Bartonella henselae (88) have been reported. Confirmation of antigenic cross-reactivity is made by cross-adsorption and Western immunoblotting. A cross-adsorption study is carried out by mixing separately the serum to be tested with the bacteria involved in the cross-reaction. Cross-adsorption of the serum sample being studied results in the disappearance of homologous antibodies when adsorption is performed with the bacterium responsible for the disease, whereas disappearance of only homologous antibodies is observed when adsorption is performed with antigens of the bacteria responsible for the cross-reaction. One major limitation of this method is that it requires large amounts of antigens.
Interpretation of results of serological testing.
The predictive value of positive and negative results is affected by the prevalence of the disease, creating a certain degree of background antigen stimulation in the studied population (115). Therefore, the determination of cutoff values is very important for the interpretation of the serological results (Table 3). For example, in Marseille we use a 1:50 serum dilution for immunofluorescence assay screening, whereas in Halifax we use a 1:8 dilution (109). While a diagnostic test needs to be very sensitive, a seroepidemiology test needs to be very specific to prevent false-positive results due to cross-reacting antibodies. The antigenic variation of C. burnetii is extremely useful in differentiating acute and chronic illness. In acute Q fever, antibodies to phase II antigens predominate, and their titer is higher than the phase I antibody titer. As with many other infectious diseases, IgM antibodies are the first to appear. On the other hand, in chronic forms of the disease, such as endocarditis, elevated anti-phase I antibodies are uniformly detected. As cutoff values in the immunofluorescence assay, Tissot-Dupont et al. (181) recommend titers of anti-phase II IgG of ≥200 and titers of anti-phase II IgM of ≥50 for the diagnosis of acute Q fever and titers of anti-phase I IgG ≥800 for the diagnosis of chronic Q fever. They have also demonstrated that anti-phase I IgA titers did not contribute to the diagnosis of chronic Q fever. Recently, we showed that an anti-phase I IgG C. burnetii titer of ≥1:800 was diagnostic of Q fever in case of an endocarditis, and thus, we have modified the Duke criteria for the diagnosis of Q fever endocarditis to include one positive blood culture for C. burnetii and a phase I IgG titer of ≥1:800 as major criteria for the diagnosis of Q fever endocarditis (48). A complement fixation titer of 1:40 is diagnostic for acute Q fever (57), while a 1:200 titer of antibody to phase I is diagnostic for chronic Q fever (136). With the ELISA, a fourfold or greater increase in the humoral immune response to C. burnetii antigens is highly predictive of Q fever. Waag et al. (191) proposed cutoff values of ≥1,024 for anti-phase II IgG, ≥512 for anti-phase II IgM, and ≥128 for anti-phase I IgG and IgM antibodies. With Western immunoblotting, chronic Q fever results in the detection of a greater number of protein antigens. The presence of antibodies reacting with 50-, 80-, and 160-kDa antigens is indicative of a chronic form of the disease (10).
TABLE 3.
Interpretation of C. burnetii serology resultsa
| Form of disease | Microimmunofluorescence assay inverse titer
|
Complement fixation assay titer
|
||||||
|---|---|---|---|---|---|---|---|---|
| Anti-phase II
|
Anti-phase I
|
Anti-phase II | Anti-phase I | |||||
| IgG | IgM | IgA | IgG | IgM | IgA | |||
| Acute | 400 | 200 | 50 | 50 | 200 | 0 | ≥1:40 | 0 |
| Acute | 400 | 100 | 0 | 0 | 0 | 0 | ||
| Treated | 400 | 0 | 0 | 200 | 0 | 0 | 1:1 | 0 |
| Treated | 100 | 0 | 0 | 100 | 0 | 0 | ||
| Chronic | 3,200 | 0 | 800 | 3,200 | 0 | 800 | ≥1:200 | ≥1:200 |
| Chronic | 6,400 | 800 | 400 | 6,400 | 800 | 400 | ||
The titers reported here are characteristic examples of the antibody responses observed during Q fever.
Prognosis.
In acute Q fever, immunofluorescence assay titers reach their maximum levels 4 to 8 weeks after the onset of disease and then decrease gradually over the following 12 months (57). Dupuis et al. (36) have shown that IgM titers declined to undetectable levels after 10 to 12 weeks, while it was 17 weeks in the study by Field et al. (44). The ELISA technique could even detect specific antibodies for more than 5 years after an acute episode (191). Guigno et al. (57) have tried to correlate the evolution of acute Q fever and the IgG/IgM ratio based on a single serum, but these data were too imprecise to be useful. Outschoorn et al. (128) have developed an ELISA for the detection of IgG subclass antibodies and have suggested that IgG2 could play a protective role or limit chronic disease. Complement fixation and immunofluorescence assay can be combined for the follow-up. A drop in the complement fixation titer often implies resolution and will occur before an immunofluorescence assay titer drop. The persistence of high levels of anti-phase I antibodies, despite appropriate treatment, or the reappearance of such antibodies should raise suspicion of possible chronic Q fever. Patients with valvular or vascular abnormalities, those who are immunodeficient, and pregnant women should have repeated C. burnetii serology tests if they have a medical history of acute Q fever or a prolonged and unexplained febrile episode. In case of acute Q fever in such a patient, an immunofluorescence assay should be done monthly for at least 6 months. The follow-up of patients treated for chronic Q fever should also be done serologically. During therapy, serological testing should be carried out once monthly for 6 months and every 3 months thereafter. The levels of antibodies decrease very slowly. IgM antibodies, when present, disappear first, and then the IgA antibodies disappear, but the IgG titers remain positive for years. Antimicrobial treatment can be stopped after 18 months to 3 years if the anti-phase I IgG titer by immunofluorescence assay is below 1:400 and anti-phase I IgA is undetectable (144, 151). Other attempts to predict the course of chronic Q fever have been made. Camacho et al. (20) used radial diffusion to detect the different IgG subclasses. They demonstrated that high IgG1 and IgG3 levels were observed in patients with chronic Q fever, and they suggested that the determination of IgG subclasses could be used to differentiate acute from chronic infection and that during long-term follow-up such testing could be used to detect a relapse. The same investigators (20), using an ELISA, noted that IgA2 antibodies were mostly found in acute Q fever, while IgG1 seemed to be specific for chronic forms of the disease. Sabatier et al. (158) suggested that the determination of lymphocyte subclasses could be predictive of the course of Q fever, with a CD4/CD8 ratio <1 being predictive of a possible relapse.
CONCLUSION
Although described 60 years ago, Q fever is still a poorly understood disease (116, 145). Its reservoirs seem to be related to any mammal, but ticks may also be reservoirs. The clinical presentation is very pleomorphic and includes severe forms with a poor prognosis. Most often, acute cases present as asymptomatic infections, as a flu-like syndrome, as a pneumonia, or as hepatitis. Host factors probably play an important role in the development of chronic disease, which may present as a blood culture-negative endocarditis or as an infected aneurysm. Although its exact prevalence is unknown, it is likely that the number of cases of Q fever is underestimated. Therefore, the diagnosis must be considered in the case of an unexplained fever, especially if the fever recurred following contact with possibly contaminated mammals. The best tests for diagnosis are those which permit the direct detection of bacteria. They include shell vial cell culture, PCR amplification, and immunodetection with tissue biopsy specimens. All these techniques require a level 3 biosafety laboratory and trained personnel due to the extreme infectivity of C. burnetii. In chronic cases, the techniques that allow the direct detection of C. burnetii in blood or tissues should be used before the beginning of therapy. As for indirect specific diagnosis, the technique to be used should be very sensitive and should detect antibodies early in the course of the disease. Although many techniques have been described, immunofluorescence assay is the reference method. It is both very specific and sensitive. In case of acute Q fever, diagnosis would be confirmed by an immunofluorescence assay titer greater than or equal to the cutoff value (which must be determined for each geographical area) or by a fourfold increase in the antibody titer detected by immunofluorescence assay, complement fixation, ELISA, or microagglutination. The presence of cross-reacting antibodies should be investigated by cross-adsorption followed by Western immunoblotting.
We recommend that all patients with blood culture-negative endocarditis, febrile patients with aortic aneurysms, and those with prolonged fever, granulomatous hepatitis, or atypical pneumonia in areas where Q fever is endemic should at the very least undergo serological testing for Q fever.
REFERENCES
- 1.Aguirre Errasti C, Montejo Baranda M, Hernandez Almaraz J L, de la Hoz Torres C, Martinez Gutierrez E, Villate Navarro J L, Sobradillo Pena V. An outbreak of Q fever in the Basque country. Can Med Assoc J. 1984;131:48–49. [PMC free article] [PubMed] [Google Scholar]
- 2.Aitken I D, Bogel K, Cracea E, Edlinger E, Houwers D, Krauss H, Rady M, Rehacek J, Schiefer H G, Schmeer N, et al. Q fever in Europe: current aspects of aetiology, epidemiology, human infection, diagnosis and therapy. Infection. 1987;15:323–327. doi: 10.1007/BF01647731. [DOI] [PubMed] [Google Scholar]
- 3.Amano K I, Williams J C. Chemical and immunological characterization of lipopolysaccharides from phase I and phase II Coxiella burnetii. J Bacteriol. 1984;160:994–1002. doi: 10.1128/jb.160.3.994-1002.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babudieri B. Q fever: a zoonosis. Adv Vet Sci. 1959;5:81–182. [Google Scholar]
- 5.Bachaud M, Massip P, Boneu B, Armengaud M. Inhibiteur acquis du facteur anti-hémophilique B (facteur IX) au cours d’une fièvre Q. Rev Med Toulouse. 1983;1:229–231. [Google Scholar]
- 6.Baumbach A, Brehm B, Sauer W, Doller G, Hoffmeister H M. Spontaneous splenic rupture complicating acute Q fever. Am J Gastroenterol. 1992;87:1651–1653. [PubMed] [Google Scholar]
- 7.Behymer D, Riemann H P. Coxiella burnetii infection (Q fever) J Am Vet Med Assoc. 1989;194:764–767. [PubMed] [Google Scholar]
- 8.Bental T, Fejgin M, Keysary A, Rzotkiewicz S, Oron C, Nachum R, Beyth Y, Lang R. Chronic Q fever of pregnancy presenting as Coxiella burnetii placentitis: successful outcome following therapy with erythromycin and rifampin. Clin Infect Dis. 1995;21:1318–1321. doi: 10.1093/clinids/21.5.1318. [DOI] [PubMed] [Google Scholar]
- 9.Biggs B A, Douglas J G, Grant I W, Crompton G K. Prolonged Q fever associated with inappropriate secretion of anti-diuretic hormone. J Infect. 1984;8:61–63. doi: 10.1016/s0163-4453(84)93381-4. [DOI] [PubMed] [Google Scholar]
- 10.Blondeau J M, Williams J C, Marrie T J. The immune response to phase I and phase II Coxiella burnetii antigens as measured by Western immunoblotting. Ann N Y Acad Sci. 1990;590:187–202. doi: 10.1111/j.1749-6632.1990.tb42220.x. [DOI] [PubMed] [Google Scholar]
- 11.Blondeau J M, Yates L, Martin R, Marrie T, Ukoli P, Thomas A. Q fever in Sokoto, Nigeria. Ann N Y Acad Sci. 1990;590:281–282. doi: 10.1111/j.1749-6632.1990.tb42233.x. [DOI] [PubMed] [Google Scholar]
- 12.Bonetti B, Monaco S, Ferrari S, Tezzon F, Rizzuto N. Demyelinating polyradiculoneuritis following Coxiella burnetii infection (Q fever) Ital J Neurol Sci. 1991;12:415–417. doi: 10.1007/BF02335782. [DOI] [PubMed] [Google Scholar]
- 13.Brada M, Bellingham A J. Bone-marrow necrosis and Q fever. Br Med J. 1980;281:1108–1109. doi: 10.1136/bmj.281.6248.1108-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.British Medical Journal. Experimental Q fever in man. Br Med J. 1950;1:1000. . (Editorial.) [Google Scholar]
- 15.Brooks R G, Licitra C M, Peacock M G. Encephalitis caused by Coxiella burnetii. Ann Neurol. 1986;20:91–93. doi: 10.1002/ana.410200116. [DOI] [PubMed] [Google Scholar]
- 16.Brouqui P, Dumler J S, Raoult D. Immunohistologic demonstration of Coxiella burnetii in the valves of patients with Q fever endocarditis. Am J Med. 1994;97:451–458. doi: 10.1016/0002-9343(94)90325-5. [DOI] [PubMed] [Google Scholar]
- 17.Brouqui P, Raoult D, Gabriel B. Chronic Coxiella burnetii infection mimicking malignant hematologic disease. Am J Hematol. 1992;39:309. doi: 10.1002/ajh.2830390415. [DOI] [PubMed] [Google Scholar]
- 18.Brouqui P, Tissot-Dupont H, Drancourt M, Berland Y, Etienne J, Leport C, Goldstein F, Massip P, Micoud M, Bertrand A, Raoult D. Chronic Q fever. Ninety-two cases from France, including 27 cases without endocarditis. Arch Intern Med. 1993;153:642–648. doi: 10.1001/archinte.153.5.642. [DOI] [PubMed] [Google Scholar]
- 19.Burnet F M, Freeman M. Experimental studies on the virus of “Q” fever. Med J Aust. 1937;2:299–305. [Google Scholar]
- 20.Camacho M T, Outschoorn I, Kovacova E, Tellez A. IgA subclasses in Q fever. In: Kazar J, Toman R, editors. Rickettsiae and rickettsial diseases. Bratislava, Slovakia: Slovak Academy of Sciences; 1996. pp. 455–457. [Google Scholar]
- 21.Canadian Diseases Weekly Report. Comment on Q fever transmitted by blood transfusion-United States. Can Dis Wkly Rep. 1977;3:210. . (Editorial.) [Google Scholar]
- 22.Cardellach F, Font J, Agusti A G, Ingelmo M, Balcells A. Q fever and hemolytic anemia. J Infect Dis. 1983;148:769. doi: 10.1093/infdis/148.4.769. [DOI] [PubMed] [Google Scholar]
- 23.Clark W H, Lennette E H, Railback O C, Romer M S. Q fever in California—clinical features in one hundred eighty cases. Arch Intern Med. 1951;88:155–161. doi: 10.1001/archinte.1951.03810080023003. [DOI] [PubMed] [Google Scholar]
- 24.Conget I, Mallolas J, Mensa J, Rovira M. Erythema nodosum and Q fever. Arch Dermatol. 1987;123:867. . (Letter.) [PubMed] [Google Scholar]
- 25.Constans J, Mathieu F, Bergeaud S, Ducloux G, Fleury B, De Mascarel A, Conri C. Q fever: diagnostic contribution of bone marrow biopsy. Apropos of a case. Rev Med Intern. 1987;8:511–512. doi: 10.1016/s0248-8663(87)80202-3. [DOI] [PubMed] [Google Scholar]
- 26.Cowley R, Fernandez F, Freemantle W, Rutter D. Enzyme immunoassay for Q fever: comparison with complement fixation and immunofluorescence tests and dot immunoblotting. J Clin Microbiol. 1992;30:2451–2455. doi: 10.1128/jcm.30.9.2451-2455.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowther R W, Spicer A J. Abortion in sheep and goats in Cyprus caused by Coxiella burnetii. Vet Rec. 1976;99:29–30. doi: 10.1136/vr.99.2.29. [DOI] [PubMed] [Google Scholar]
- 28.Dano P, Gayraud D, Martet G, Drancourt M, Raoult D. Méningoencéphalite due àCoxiella burnetii. Rev Neurol (Paris) 1990;146:511–513. [PubMed] [Google Scholar]
- 29.Derrick E H. “Q” fever, a new fever entity: clinical features, diagnosis and laboratory investigation. Med J Aust. 1937;2:281–299. doi: 10.1093/clinids/5.4.790. [DOI] [PubMed] [Google Scholar]
- 30.Derrick E H. The course of infection with Coxiella burneti. Med J Aust. 1973;1:1051–1057. [PubMed] [Google Scholar]
- 31.Dindinaud G, Aigius G, Burucoa C, Senet J M, Deshayes M, Magnin G, Castets M. Fièvre Q et mort foetale in-utero. J Gynecol Obstet Biol Reprod. 1991;20:969–972. [PubMed] [Google Scholar]
- 32.Dobija-Domaradzki M, Hausser J L, Gosselin F. Coexistence of Legionnaires’ disease and Q fever in a single patient. Can Med Assoc J. 1984;130:1022–1023. [PMC free article] [PubMed] [Google Scholar]
- 33.Doller G, Doller P C, Gerth H J. Early diagnosis of Q fever: detection of immunoglobulin M by radioimmunoassay and enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 1984;3:550–553. doi: 10.1007/BF02013617. [DOI] [PubMed] [Google Scholar]
- 34.Drancourt M, Raoult D, Xeridat B, Milandre L, Nesri M, Dano P. Q fever meningoencephalitis in five patients. Eur J Epidemiol. 1991;7:134–138. doi: 10.1007/BF00237356. [DOI] [PubMed] [Google Scholar]
- 35.DuPont H L, Hornick R B, Levin H S, Rapoport M I, Woodward T E. Q fever hepatitis. Ann Med Intern. 1971;74:198–206. doi: 10.7326/0003-4819-74-2-198. [DOI] [PubMed] [Google Scholar]
- 36.Dupuis G, Peter O, Peacock M, Burgdorfer W, Haller E. Immunoglobulin responses in acute Q fever. J Clin Microbiol. 1985;22:484–487. doi: 10.1128/jcm.22.4.484-487.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dupuis G, Petite J, Peter O, Vouilloz M. An important outbreak of human Q fever in a Swiss Alpine valley. Int J Epidemiol. 1987;16:282–287. doi: 10.1093/ije/16.2.282. [DOI] [PubMed] [Google Scholar]
- 38.Dwyer D E, Gibbons V L, Brady L M, Cunningham A L. Serological reaction to Legionella pneumophila group 4 in a patient with Q fever. J Infect Dis. 1988;158:499–500. . (Letter.) [PubMed] [Google Scholar]
- 39.Eklund C M, Parker R R, Lackman D B. Case of Q fever probably contracted by exposure to ticks in nature. Public Health Rep. 1947;62:1413. [PubMed] [Google Scholar]
- 40.Ellis M E, Smith C C, Moffat M A. Chronic or fatal Q-fever infection: a review of 16 patients seen in North-East Scotland (1967–80) Q J Med. 1983;52:54–66. [PubMed] [Google Scholar]
- 41.Elouaer R, Blanc L, Andre C. Anticorps antimitochondries donnant un aspect d’anti M1 au cours de la fièvre Q. Gastroenterol Clin Biol. 1985;8:980. [PubMed] [Google Scholar]
- 42.Enzenauer R J, Arend W P, Emlen J W. Mixed cryoglobulinemia associated with chronic Q fever. J Rheumatol. 1991;18:76–78. [PubMed] [Google Scholar]
- 43.Estrov Z, Bruck R, Shtalrid M, Berrebi A, Resnitzky P. Histiocytic hemophagocytosis in Q fever. Arch Pathol Lab Med. 1984;108:7. . (Letter.) [PubMed] [Google Scholar]
- 44.Field P R, Hunt J G, Murphy A M. Detection and persistence of specific IgM antibody to Coxiella burnetii by enzyme-linked immunosorbent assay: a comparison with immunofluorescence and complement fixation tests. J Infect Dis. 1983;148:477–487. doi: 10.1093/infdis/148.3.477. [DOI] [PubMed] [Google Scholar]
- 45.Finidori J P, Raoult D, Bornstein N, Fleurette J. Study of cross reaction between Coxiella burnetii and Legionella pneumophila using indirect immunofluorescence assay and immunoblotting. Acta Virol. 1992;36:459–465. [PubMed] [Google Scholar]
- 46.Fiset P, Ormsbee R A, Silberman R, Peacock M, Spielman S H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969;13:60–66. [PubMed] [Google Scholar]
- 47.Fishbein D B, Raoult D. A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg. 1992;47:35–40. doi: 10.4269/ajtmh.1992.47.35. [DOI] [PubMed] [Google Scholar]
- 48.Fournier P E, Casalta J P, Habib G, Messana T, Raoult D. Modification of the diagnostic criteria proposed by the duke endocarditis service to permit improved diagnosis of Q fever endocarditis. Am J Med. 1996;100:629–633. doi: 10.1016/s0002-9343(96)00040-x. [DOI] [PubMed] [Google Scholar]
- 49.Fournier P E, Casalta J P, Piquet P, Tournigand P, Branchereau A, Raoult D. Coxiella burnetii infection of aneurysms or vascular grafts: report of seven cases and review. Clin Infect Dis. 1998;26:116–121. doi: 10.1086/516255. [DOI] [PubMed] [Google Scholar]
- 50.Frazier M E, Heinzen R A, Mallavia L P, Baca O G. DNA probes detecting Coxiella burnetii strains. Acta Virol. 1992;36:83–89. [PubMed] [Google Scholar]
- 51.Frazier M E, Mallavia L P, Samuel J E, Baca O G. DNA probes for the identification of Coxiella burnetti strains. Ann N Y Acad Sci. 1990;590:445–458. doi: 10.1111/j.1749-6632.1990.tb42253.x. [DOI] [PubMed] [Google Scholar]
- 52.Fritz E, Thiele D, Willems H, Wittenbrink M M. Quantification of Coxiella burnetii by polymerase chain reaction (PCR) and a colorimetric microtiter plate hybridization assay (CMHA) Eur J Epidemiol. 1995;11:549–557. doi: 10.1007/BF01719307. [DOI] [PubMed] [Google Scholar]
- 53.Gil-Grande R, Aguado J M, Pastor C, Garcia-Bravo M, Gomez-Pellico C, Soriano F, Noriega A. Conventional viral cultures and shell vial assay for diagnosis of apparently culture-negative Coxiella burnetii endocarditis. Eur J Clin Microbiol Infect Dis. 1995;14:64–67. doi: 10.1007/BF02112624. [DOI] [PubMed] [Google Scholar]
- 54.Gimenez D F. Staining rickettsiae in yolk-sac cultures. Stain Technol. 1964;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- 55.Gomez-Aranda F, Pachon Diaz J, Romero Acebal M, Lopez Cortes L, Navarro Rodriguez A, Maestre Moreno J. Computed tomographic brain scan findings in Q fever encephalitis. Neuroradiology. 1984;26:329–332. doi: 10.1007/BF00339780. [DOI] [PubMed] [Google Scholar]
- 56.Greiner T C, Mitros F A, Stapleton J, Van Rybroek J. Fine-needle aspiration findings of the liver in a case of Q fever. Diagn Cytopathol. 1992;8:181–184. doi: 10.1002/dc.2840080218. [DOI] [PubMed] [Google Scholar]
- 57.Guigno D, Coupland B, Smith E G, Farrell I D, Desselberger U, Caul E O. Primary humoral antibody response to Coxiella burnetii, the causative agent of Q fever. J Clin Microbiol. 1992;30:1958–1967. doi: 10.1128/jcm.30.8.1958-1967.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hackstadt T. Antigenic variation in the phase I lipopolysaccharide of Coxiella burnetii isolates. Infect Immun. 1986;52:337–340. doi: 10.1128/iai.52.1.337-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hackstadt T. The role of lipopolysaccharides in the virulence of Coxiella burnetii. Ann N Y Acad Sci. 1990;590:27–32. doi: 10.1111/j.1749-6632.1990.tb42203.x. [DOI] [PubMed] [Google Scholar]
- 60.Hackstadt T, Peacock M G, Hitchcock P J, Cole R L. Lipopolysaccharide variation in Coxiella burnetii: intrastrain heterogeneity in structure and antigenicity. Infect Immun. 1985;48:359–365. doi: 10.1128/iai.48.2.359-365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hackstadt T, Williams J C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci USA. 1981;78:3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hackstadt T, Williams J C. pH dependence of the Coxiella burnetii glutamate transport system. J Bacteriol. 1983;154:598–603. doi: 10.1128/jb.154.2.598-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrell G T. Rickettsial involvement of the central nervous system. Med Clin N Am. 1953;37:395–422. doi: 10.1016/s0025-7125(16)35021-0. [DOI] [PubMed] [Google Scholar]
- 64.Heard S R, Ronalds C J, Heath R B. Coxiella burnetii infection in immunocompromised patients. J Infect. 1985;11:15–18. doi: 10.1016/s0163-4453(85)90870-9. [DOI] [PubMed] [Google Scholar]
- 65.Heinzen R A, Stiegler G L, Whiting L L, Schmitt S A, Mallavia L P, Frazier M E. Use of pulsed field gel electrophoresis to differentiate Coxiella burnetii strains. Ann N Y Acad Sci. 1990;590:504–513. doi: 10.1111/j.1749-6632.1990.tb42260.x. [DOI] [PubMed] [Google Scholar]
- 66.Herr S, Huchzermeyer H F, Te Brugge L A, Williamson C C, Roos J A, Schiele G J. The use of a single complement fixation test technique in bovine brucellosis, Johne’s disease, dourine, equine piroplasmosis and Q fever serology. Onderstepoort J Vet Res. 1985;52:279–282. [PubMed] [Google Scholar]
- 67.Higgins D, Marrie T J. Seroepidemiology of Q fever among cats in New Brunswick and Prince Edward Island. Ann N Y Acad Sci. 1990;590:271–274. doi: 10.1111/j.1749-6632.1990.tb42231.x. [DOI] [PubMed] [Google Scholar]
- 68.Hilbink F, Penrose M, Kovacova E, Kazar J. Q fever is absent from New Zealand. Int J Epidemiol. 1993;22:945–949. doi: 10.1093/ije/22.5.945. [DOI] [PubMed] [Google Scholar]
- 69.Hitchins R, Cobcroft R G, Hocker G. Transient severe hypoplastic anemia in Q fever. Pathology. 1986;18:254–255. doi: 10.3109/00313028609059470. [DOI] [PubMed] [Google Scholar]
- 70.Hoover T A, Vodkin M H, Williams J. A Coxiella burnetii repeated DNA element resembling a bacterial insertion sequence. J Bacteriol. 1992;174:5540–5548. doi: 10.1128/jb.174.17.5540-5548.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ibrahim A, Norlander L, Macellaro A, Sjöstedt A. The potential of the 23S rRNA gene for rapid detection of Coxiella burnetii in clinical samples. In: Kazar J, Toman R, editors. Rickettsiae and rickettsial diseases. Bratislava, Slovakia: Slovak Academy of Sciences; 1996. pp. 441–445. [Google Scholar]
- 72.Janigan D T, Marrie T J. An inflammatory pseudotumor of the lung in Q fever pneumonia. N Engl J Med. 1983;308:86–87. doi: 10.1056/NEJM198301133080207. [DOI] [PubMed] [Google Scholar]
- 73.Janigan D T, Marrie T J. Pathology of Q fever pneumonia. In: Marrie T J, editor. Q fever, the disease. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 162–170. [Google Scholar]
- 74.Janowski M, Peltier J Y. Unexplained prolonged fever and antiphospholipid syndrome. Rev Med Intern. 1997;16:249–250. doi: 10.1016/0248-8663(96)80847-2. [DOI] [PubMed] [Google Scholar]
- 75.Johnson J E, III, Kadull P J. Laboratory acquired Q fever. A report of fifty cases. Am J Med. 1966;41:391–403. doi: 10.1016/0002-9343(66)90085-4. [DOI] [PubMed] [Google Scholar]
- 76.Jortner R, Demopoulos L A, Bernstein N E, Tunick P A, Shapira Y, Shaked Y, Kronzon I. Transesophageal echocardiography in the diagnosis of Q-fever endocarditis. Am Heart J. 1994;128:827–831. doi: 10.1016/0002-8703(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 77.Kanfer E, Farrag N, Price C, MacDonald D, Coleman J, Barrett A J. Q fever following bone marrow transplantation. Bone Marrow Transplant. 1988;3:165–166. [PubMed] [Google Scholar]
- 78.Kaplan M M, Bertagna P. The geographical distribution of Q fever. Bull W H O. 1955;13:829–860. [PMC free article] [PubMed] [Google Scholar]
- 79.Kayser K, Wiebel M, Schulz V, Gabius H J. Necrotizing bronchitis, angiitis, and amyloidosis associated with chronic Q fever. Respiration. 1995;62:114–116. doi: 10.1159/000196404. [DOI] [PubMed] [Google Scholar]
- 80.Kazar J. Q fever. In: Kazar J, Toman R, editors. Rickettsiae and rickettsial diseases. Bratislava, Slovakia: Slovak Academy of Sciences; 1996. pp. 353–362. [Google Scholar]
- 81.Kazar J, Brezina R, Schramek S, Palanova A, Tvrda B. Suitability of the microagglutination test for detection of post-infection and post-vaccination Q fever antibodies in human sera. Acta Virol. 1981;25:235–240. [PubMed] [Google Scholar]
- 82.Kelly P J, Matthewman L A, Mason P R, Raoult D. Q fever in Zimbabawe. S Afr Med J. 1993;83:21–25. [PubMed] [Google Scholar]
- 83.Kocianova E, Lukacova M. Detection of Coxiella burnetii antigen in the hemolymph of ixodid ticks by the immunodot blot test. In: Kazar J, Toman R, editors. Rickettsiae and rickettsial diseases. Bratislava, Slovakia: Slovak Academy of Sciences; 1996. pp. 469–471. [Google Scholar]
- 84.Kovacova E, Gallo J, Schramek S, Kazar J, Brezina R. Coxiella burnetii antigens for detection of Q fever antibodies by ELISA in human sera. Acta Virol. 1987;31:254–259. [PubMed] [Google Scholar]
- 85.Kovacova E, Kazar J, Spanelova D. Analysis of antibody response in humans and goats with the use of different Coxiella burnetii antigenic preparations. In: Kazar J, Toman R, editors. Rickettsiae and rickettsial diseases. Bratislava, Slovakia: Slovak Academy of Sciences; 1996. pp. 463–468. [Google Scholar]
- 86.Kruszweska D, Wirzbanowska-Tylewska S. Influence of Coxiella burnetii infection of male mice on their offspring. Acta Virol. 1991;35:79–82. [PubMed] [Google Scholar]
- 87.La Scola B, Lepidi H, Raoult D. Pathologic changes during acute Q fever: influence of the route of infection and inoculum size in infected guinea pigs. Infect Immun. 1997;65:2443–2447. doi: 10.1128/iai.65.6.2443-2447.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.La Scola B, Raoult D. Serological cross reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J Clin Microbiol. 1996;34:2270–2274. doi: 10.1128/jcm.34.9.2270-2274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lang G H. Coxiellosis (Q fever) in animals. In: Marrie T J, editor. Q fever, the disease. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 23–48. [Google Scholar]
- 90.Langley J M, Marrie T J, Covert A, Waag D M, Williams C. Poker players’ pneumonia. An urban outbreak of Q fever following exposure to a parturient cat. N Engl J Med. 1988;319:354–355. doi: 10.1056/NEJM198808113190607. [DOI] [PubMed] [Google Scholar]
- 91.Leonetti F, Raoult D, Dussol B, Brunet P, Berland Y. Chronic Q fever hemodialysis patients. Nephron. 1995;67:231–233. doi: 10.1159/000187934. [DOI] [PubMed] [Google Scholar]
- 92.Levy P Y, Raoult D, Razongles J J. Q-fever and autoimmunity. Eur J Epidemiol. 1989;5:447–453. doi: 10.1007/BF00140139. [DOI] [PubMed] [Google Scholar]
- 93.Lieberman D, Boldur I, Manor E, Hoffman S, Schlaeffer F, Porath A. Q-fever pneumonia in the negev region of Israel: a review of 20 patients hospitalised over a period of one year. J Infect. 1995;30:135–140. doi: 10.1016/s0163-4453(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 94.Lille R D, Perrin T L, Armstrong C. An institutional outbreak of pneumonitis. III. Histopathology in man and rhesus monkeys in the pneumonitis due to the virus of “Q fever.”. Public Health Rep. 1941;56:1419–1425. [Google Scholar]
- 95.Lim K C, Kang J Y. Q fever presenting with gastroenteritis. Med J Aust. 1980;1:327. doi: 10.5694/j.1326-5377.1980.tb134887.x. [DOI] [PubMed] [Google Scholar]
- 96.Lipton J H, Fong T C, Gill M J, Burgess K, Elliott P D. Q fever inflammatory pseudotumor of the lung. Chest. 1987;92:756–757. doi: 10.1378/chest.92.4.756. [DOI] [PubMed] [Google Scholar]
- 97.Loudon M M, Thompson E N. Severe combined immunodeficiency syndrome, tissue transplant, leukaemia, and Q fever. Arch Dis Child. 1988;63:207–209. doi: 10.1136/adc.63.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ludlam H, Wreghitt T G, Thornton S, Thomson B J, Bishop N J, Coomber S, Cunniffe J. Q fever in pregnancy. J Infect. 1997;34:75–78. doi: 10.1016/s0163-4453(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 99.Mallavia L P, Whiting L L, Minnick M F, Heinzen R A, Reschke D, Foreman M, Baca O G, Frazier M E. Strategy for detection and differentiation of Coxiella burnetii strains using the polymerase chain reaction. Ann N Y Acad Sci. 1990;590:572–581. doi: 10.1111/j.1749-6632.1990.tb42268.x. [DOI] [PubMed] [Google Scholar]
- 100.Mann J S, Douglas J G, Inglis J M, Leitch A G. Q fever: person to person transmission within a family. Thorax. 1986;41:974–975. doi: 10.1136/thx.41.12.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marrero M, Raoult D. Centrifugation-shell vial technique for rapid detection of Mediterranean spotted fever rickettsia in blood culture. Am J Trop Med Hyg. 1989;40:197–199. doi: 10.4269/ajtmh.1989.40.197. [DOI] [PubMed] [Google Scholar]
- 102.Marrie T J. Pneumonia and meningo-encephalitis due to Coxiella burnetii. J Infect. 1985;11:59–61. doi: 10.1016/s0163-4453(85)91066-7. [DOI] [PubMed] [Google Scholar]
- 103.Marrie T J. Q fever, 1979–1987—Nova Scotia. Can Dis Weekly Rep. 1988;14:69–70. [PubMed] [Google Scholar]
- 104.Marrie T J. Q fever in pregnancy: report of two cases. Infect Dis Clin Pract. 1993;2:207–209. [Google Scholar]
- 105.Marrie T J. Coxiella burnetii (Q fever) pneumonia. Clin Infect Dis. 1995;21:S253–S264. doi: 10.1093/clind/21.supplement_3.s253. [DOI] [PubMed] [Google Scholar]
- 106.Marrie T J, Durant H, Williams J C, Mintz E, Waag D M. Exposure to parturient cats: a risk factor for acquisition of Q fever in maritime Canada. J Infect Dis. 1988;158:101–108. doi: 10.1093/infdis/158.1.101. [DOI] [PubMed] [Google Scholar]
- 107.Marrie T J, Durant H, Yates L. Community-acquired pneumonia requiring hospitalization: 5-year prospective study. Rev Infect Dis. 1989;11:586–599. doi: 10.1093/clinids/11.4.586. [DOI] [PubMed] [Google Scholar]
- 108.Marrie T J, MacDonald A, Durant H, Yates L, McCormick L. An outbreak of Q fever probably due to contact with a parturient cat. Chest. 1988;93:98–103. doi: 10.1378/chest.93.1.98. [DOI] [PubMed] [Google Scholar]
- 109.Marrie T J, Pollak P T. Seroepidemiology of Q fever in Nova Scotia: evidence for age dependent cohorts and geographical distribution. Eur J Epidemiol. 1995;11:47–54. doi: 10.1007/BF01719945. [DOI] [PubMed] [Google Scholar]
- 110.Marrie T J, Raoult D. Rickettsial infections of the central nervous system. Semin Neurol. 1992;12:213–224. doi: 10.1055/s-2008-1041178. [DOI] [PubMed] [Google Scholar]
- 111.Marrie T J, Stein A, Janigan D, Raoult D. Route of infection determines the clinical manifestations of acute Q fever. J Infect Dis. 1996;173:484–487. doi: 10.1093/infdis/173.2.484. [DOI] [PubMed] [Google Scholar]
- 112.Marrie T J, Yates L. Incidence of Q fever: pilot studies in two areas in Nova Scotia. Ann N Y Acad Sci. 1990;590:275–280. doi: 10.1111/j.1749-6632.1990.tb42232.x. [DOI] [PubMed] [Google Scholar]
- 113.McCaul T F. The developmental cycle of Coxiella burnetii. In: Williams J C, Thompson H A, editors. Q fever: the biology of Coxiella burnetii. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 223–258. [Google Scholar]
- 114.McCaul T F, Williams J C. Localization of DNA in Coxiella burnetii by post-embedding immunoelectron microscopy. Ann N Y Acad Sci. 1990;590:136–147. doi: 10.1111/j.1749-6632.1990.tb42216.x. [DOI] [PubMed] [Google Scholar]
- 115.McDade J E. Diagnosis of rickettsial diseases. A perspective. Eur J Epidemiol. 1991;7:270–275. doi: 10.1007/BF00145676. [DOI] [PubMed] [Google Scholar]
- 116.Mege J L, Maurin M, Capo C, Raoult D. Coxiella burnetii: the “query” fever bacterium a model of immune subversion by a strictly intracellular microorganism. FEMS Microbiol Rev. 1997;19:209–217. doi: 10.1111/j.1574-6976.1997.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 117.Minnick M F, Heinzen R A, Douthart R, Mallavia L P, Frazier M E. Analysis of QpRS-specific sequences from Coxiella burnetii. Ann N Y Acad Sci. 1990;590:514–522. doi: 10.1111/j.1749-6632.1990.tb42261.x. [DOI] [PubMed] [Google Scholar]
- 118.Morita C, Katsuyama J, Yanase T, Ueno H, Muramatsu Y, Hohdatsu T, Koyama H. Seroepidemiological survey of Coxiella burnetii in domestic cats in Japan. Microbiol Immunol. 1994;38:1001–1003. doi: 10.1111/j.1348-0421.1994.tb02159.x. [DOI] [PubMed] [Google Scholar]
- 119.Mühlemann K, Matter L, Meyer B, Schopfer K. Isolation of Coxiella burnetii from heart valves of patients treated for Q fever endocarditis. J Clin Microbiol. 1995;33:428–431. doi: 10.1128/jcm.33.2.428-431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Murphy A M, Field P R. The persistence of complement-fixing antibodies to Q-fever (Coxiella burnetii) after infection. Med J Aust. 1970;1:1148–1150. doi: 10.5694/j.1326-5377.1970.tb84481.x. [DOI] [PubMed] [Google Scholar]
- 121.Musso D, Raoult D. Coxiella burnetii blood cultures from acute and chronic Q-fever patients. J Clin Microbiol. 1995;33:3129–3132. doi: 10.1128/jcm.33.12.3129-3132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Musso D, Raoult D. Serological cross-reactions between Coxiella burnetii and Legionella micdadei. Clin Diagn Lab Immunol. 1997;4:208–212. doi: 10.1128/cdli.4.2.208-212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nguyen S V, Otsuka H, Zhang G Q, To H, Yamaguchi T, Fukushi H, Noma A, Hirai K. Rapid method for detection of Coxiella burnetii antibodies using high-density particle agglutination. J Clin Microbiol. 1996;34:2947–2951. doi: 10.1128/jcm.34.12.2947-2951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Okun D B, Sun N C, Tanaka K R. Bone marrow granulomas in Q fever. Am J Clin Pathol. 1979;71:117–121. doi: 10.1093/ajcp/71.1.117. [DOI] [PubMed] [Google Scholar]
- 125.Omezzine-Letaief A, Yacoub S, Tissot-Dupont H, Le Cam C, Ghachem L, Letaief J, Raoult D. Seroepidemiological survey of rickettsial infections among blood donors in central Tunisia. Trans R Soc Trop Med Hyg. 1995;89:266–268. doi: 10.1016/0035-9203(95)90531-6. [DOI] [PubMed] [Google Scholar]
- 126.Ordi-Ros J, Selva-O’Callaghan A, Monegal-Ferran F, Monasterio-Aspiri Y, Juste-Sanchez C, Vilardell-Tarres M. Prevalence, significance, and specificity of antibodies to phospholipids in Q fever. Clin Infect Dis. 1994;18:213–218. doi: 10.1093/clinids/18.2.213. [DOI] [PubMed] [Google Scholar]
- 127.Ormsbee R A. The growth of Coxiella burnetii in embryonated eggs. J Bacteriol. 1952;63:73–86. doi: 10.1128/jb.63.1.73-86.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Outschoorn I, Camacho M T, Kovacova E, Tellez A. Role of some minor IgG subclasses (IgG2 and IgG3) in Q fever. In: Kazar J, Toman R, editors. Rickettsiae and rickettsial diseases. Bratislava, Slovakia: Slovak Academy of Sciences; 1996. pp. 459–462. [Google Scholar]
- 129.Palmer S R, Young S E. Q fever endocarditis in England and Wales, 1975–81. Lancet. 1982;ii:1448–1449. doi: 10.1016/s0140-6736(82)91341-1. [DOI] [PubMed] [Google Scholar]
- 130.Peacock M G, Philip R N, Williams J C, Faulkner R S. Serological evaluation of O fever in humans: enhanced phase I titers of immunoglobulins G and A are diagnostic for Q fever endocarditis. Infect Immun. 1983;41:1089–1098. doi: 10.1128/iai.41.3.1089-1098.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pellegrin M, Delsol G, Auvergnat J C, Familiades J, Faure H, Guiu M, Voigt J J. Granulomatous hepatitis in Q fever. Hum Pathol. 1980;11:51–57. doi: 10.1016/s0046-8177(80)80105-5. [DOI] [PubMed] [Google Scholar]
- 132.Perin T L. Histopathologic observations in a fatal case of Q fever. Arch Pathol. 1949;47:361–365. [PubMed] [Google Scholar]
- 133.Peter O, Dupuis G, Bee D, Luthy R, Nicolet J, Burgdorfer W. Enzyme-linked immunosorbent assay for diagnosis of chronic Q fever. J Clin Microbiol. 1988;26:1978–1982. doi: 10.1128/jcm.26.10.1978-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peter O, Dupuis G, Burgdorfer W, Peacock M. Evaluation of the complement fixation and indirect immunofluorescence tests in the early diagnosis of primary Q fever. Eur J Clin Microbiol Infect Dis. 1985;4:394–396. doi: 10.1007/BF02148690. [DOI] [PubMed] [Google Scholar]
- 135.Peter O, Dupuis G, Peacock M G, Burgdorfer W. Comparison of enzyme-linked immunosorbent assay and complement fixation and indirect fluorescent-antibody tests for detection of Coxiella burnetii antibody. J Clin Microbiol. 1987;25:1063–1067. doi: 10.1128/jcm.25.6.1063-1067.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peter O, Flepp M, Bestetti G, Nicolet J, Luthy R, Dupuis G. Q fever endocarditis: diagnostic approaches and monitoring of therapeutic effects. Clin Invest. 1992;70:932–937. doi: 10.1007/BF00180442. [DOI] [PubMed] [Google Scholar]
- 137.Philip C B. Comments on the name of the Q fever organism. Public Health Rep. 1948;63:58–59. [Google Scholar]
- 138.Poux J M, Jauberteau M O, Boudet R, Leroux-Robert C, Liozon F. Anti-cardiolipin antibodies in acute Coxiella burnetii infection. Value of the search and course of different isotypes. Presse Med. 1997;24:416. [PubMed] [Google Scholar]
- 139.Prasad B N, Chandiramani N K, Wagle A. Isolation of Coxiella burnetii from human sources. Int J Zoonoses. 1986;13:112–117. [PubMed] [Google Scholar]
- 140.Qizilbash A H. The pathology of Q fever as seen on liver biopsy. Arch Pathol Lab Med. 1983;107:364–367. [PubMed] [Google Scholar]
- 141.Raju N R, Collings D F, Saville P H. Abortion in black belly Barbodos sheep in Fiji caused by Coxiella burnetii. Aust Vet J. 1988;65:225–226. doi: 10.1111/j.1751-0813.1988.tb14465.x. [DOI] [PubMed] [Google Scholar]
- 142.Ramos H S, Hodges R E, Meroney W. Q fever: report of a case simulating lymphoma. Ann Intern Med. 1957;47:1030–1035. doi: 10.7326/0003-4819-47-5-1030. [DOI] [PubMed] [Google Scholar]
- 143.Raoult D. Host factors in the severity of Q fever. Ann N Y Acad Sci. 1990;590:33–38. doi: 10.1111/j.1749-6632.1990.tb42204.x. [DOI] [PubMed] [Google Scholar]
- 144.Raoult D. Treatment of Q fever. Antimicrob Agents Chemother. 1993;37:1733–1736. doi: 10.1128/aac.37.9.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Raoult D. Q fever: still a query after all these years. J Med Microbiol. 1996;44:77–78. doi: 10.1099/00222615-44-2-77. [DOI] [PubMed] [Google Scholar]
- 146.Raoult D, Bollini G, Gallais H. Osteoarticular infection due to Coxiella burnetii. J Infect Dis. 1989;159:1159–1160. doi: 10.1093/infdis/159.6.1159. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 147.Raoult D, Brouqui P, Marchou B, Gastaut J A. Acute and chronic Q fever in patients with cancer. Clin Infect Dis. 1992;14:127–130. doi: 10.1093/clinids/14.1.127. [DOI] [PubMed] [Google Scholar]
- 148.Raoult D, Laurent J C, Mutillod M. Monoclonal antibodies to Coxiella burnetii for antigenic detection in cell cultures and in paraffin embedded tissues. Am J Clin Pathol. 1994;101:318–320. doi: 10.1093/ajcp/101.3.318. [DOI] [PubMed] [Google Scholar]
- 149.Raoult D, Levy P, Tissot-Dupont H, Chicheportiche C, Tamalet C, Gastaut J A, Salducci J. Q fever and HIV infection. AIDS. 1993;7:81–86. doi: 10.1097/00002030-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 150.Raoult D, Levy P Y, Harle J R, Etienne J, Massip P, Goldstein F, Micoud M, Beytout J, Gallais H, Remy G, Capron J P. Chronic Q fever: diagnosis and follow up. Ann N Y Acad Sci. 1990;590:51–60. doi: 10.1111/j.1749-6632.1990.tb42206.x. [DOI] [PubMed] [Google Scholar]
- 151.Raoult D, Marrie T. Q fever. Clin Infect Dis. 1995;20:489–496. doi: 10.1093/clinids/20.3.489. [DOI] [PubMed] [Google Scholar]
- 152.Raoult D, Raza A, Marrie T J. Q fever endocarditis and other forms of chronic Q fever. In: Marrie T J, editor. Q fever, the disease. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 179–199. [Google Scholar]
- 153.Raoult D, Stein D. Q fever during pregnancy—a risk for women, fetuses, and obstetricians. N Engl J Med. 1994;330:371. doi: 10.1056/nejm199402033300518. [DOI] [PubMed] [Google Scholar]
- 154.Raoult D, Vestris G, Enea M. Isolation of 16 strains of Coxiella burnetii from patients by using a sensitive centrifugation cell culture system and establishment of strains in HEL cells. J Clin Microbiol. 1990;28:2482–2484. doi: 10.1128/jcm.28.11.2482-2484.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Reichman N, Raz R, Keysary A, Goldwasser R, Flatau E. Chronic Q fever and severe thrombocytopenia in a pregnant woman. Am J Med. 1988;85:253–254. doi: 10.1016/s0002-9343(88)80355-3. [DOI] [PubMed] [Google Scholar]
- 156.Reilly S, Northwood J L, Caul E O. Q fever in Plymouth, 1972–88. A review with particular reference to neurological manifestations. Epidemiol Infect. 1990;105:391–408. doi: 10.1017/s095026880004797x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rice P S, Kudesia G, McKendrick M W, Cullen D R. Coxiella burnetii serology in granulomatous hepatitis. J Infect. 1993;27:63–66. doi: 10.1016/0163-4453(93)93803-c. [DOI] [PubMed] [Google Scholar]
- 158.Sabatier F, Dignat-George F, Mege J L, Brunet C, Raoult D, Sampol J. CD4+ T-cell lymphopenia in Q fever endocarditis. Clin Diagn Lab Immunol. 1997;4:89–92. doi: 10.1128/cdli.4.1.89-92.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Salva A, Porcel J M, Ordi J. Anticardiolipin antibodies and rickettsial disease. Clin Exp Rheumatol. 1990;8:228. [Google Scholar]
- 160.Samuel J E, Frazier M E, Kahn M L, Thomashow L S, Mallavia L P. Isolation and characterization of a plasmid from phase I Coxiella burnetii. Infect Immun. 1983;41:488–493. doi: 10.1128/iai.41.2.488-493.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Samuel J E, Frazier M E, Mallavia L P. Correlation of plasmid type and disease caused by Coxiella burnetii. Infect Immun. 1985;49:775–779. doi: 10.1128/iai.49.3.775-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Savinelli E A, Mallavia L P. Comparison of Coxiella burnetii plasmids to homologous chromosomal sequences present in a plasmidless endocarditis-causing isolate. Ann N Y Acad Sci. 1990;590:523–533. doi: 10.1111/j.1749-6632.1990.tb42262.x. [DOI] [PubMed] [Google Scholar]
- 163.Schmeer N, Muller H P, Baumgartner W, Wieda J, Krauss H. Enzyme-linked immunosorbent fluorescence assay and high-pressure liquid chromatography for analysis of humoral immune responses to Coxiella burnetii proteins. J Clin Microbiol. 1988;26:2520–2525. doi: 10.1128/jcm.26.12.2520-2525.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schneider T, Jahn H U, Steinhoff D, Guschoreck H M, Liesenfeld O, Mater-Bohm H, Wesirow A L, Lode H, Ludwig W D, Dissmann T. A Q fever epidemic in Berlin. The epidemiological and clinical aspects. Dtsch Med Wochenschr. 1993;118:689–695. doi: 10.1055/s-2008-1059379. [DOI] [PubMed] [Google Scholar]
- 165.Schuil J, Richardus J H, Baarsma G S, Schaap G J. Q fever as a possible cause of bilateral optic neuritis. Br J Ophthalmol. 1985;69:580–583. doi: 10.1136/bjo.69.8.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Selva A, Ordi-Ros J, Cucurull E, Monegal F. Antiphospholipid antibodies and Q fever. Med Clin (Barcelona) 1997;105:359. [PubMed] [Google Scholar]
- 167.Siegman-Igra Y, Kaufman O, Keysary A, Rzotkiewicz S, Shalit I. Q fever endocarditis in Israel and a worldwide review. Scand J Infect Dis. 1997;29:41–49. doi: 10.3109/00365549709008663. [DOI] [PubMed] [Google Scholar]
- 168.Spelman D W. Q fever: a study of 111 consecutive cases. Med J Aust. 1982;1:547–553. doi: 10.5694/j.1326-5377.1982.tb124169.x. [DOI] [PubMed] [Google Scholar]
- 169.Srigley J R, Vellend H, Palmer N, Phillips M J, Geddie W R, Van Nostrand A W, Edwards V D. Q fever. The liver and bone marrow pathology. Am J Surg Pathol. 1985;9:752–758. [PubMed] [Google Scholar]
- 170.Stein A, Kruszewska D, Gouvernet J, Raoult D. Study of the 16S–23S ribosomal DNA internal spacer of Coxiella burnetii. Eur J Epidemiol. 1997;13:471–475. doi: 10.1023/a:1007389315808. [DOI] [PubMed] [Google Scholar]
- 171.Stein A, Raoult D. Detection of Coxiella burnetii by DNA amplification using polymerase chain reaction. J Clin Microbiol. 1992;30:2462–2466. doi: 10.1128/jcm.30.9.2462-2466.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stein A, Raoult D. A simple method for amplification of DNA from paraffin-embedded tissues. Nucleic Acids Res. 1992;20:5237–5238. doi: 10.1093/nar/20.19.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stein A, Raoult D. Lack of pathotype specific gene in human Coxiella burnetii isolates. Microb Pathog. 1993;15:177–185. doi: 10.1006/mpat.1993.1068. [DOI] [PubMed] [Google Scholar]
- 174.Stein A, Raoult D. Q fever endocarditis. Eur Heart J. 1995;16:19–23. doi: 10.1093/eurheartj/16.suppl_b.19. [DOI] [PubMed] [Google Scholar]
- 175.Syrucek L, Sobeslavsky O, Gutvirth I. Isolation of Coxiella burnetii from human placentas. J Hyg Epidemiol Microbiol Immunol. 1958;2:29–35. [PubMed] [Google Scholar]
- 176.Thiele D, Karo M, Krauss H. Monoclonal antibody based capture Elisa/Elifa for detection of Coxiella burnetii in clinical specimens. Eur J Epidemiol. 1992;8:568–574. doi: 10.1007/BF00146378. [DOI] [PubMed] [Google Scholar]
- 177.Thiele D, Willems H. Is plasmid based differentiation of Coxiella burnetii in “acute” and “chronic” isolates still valid? Eur J Epidemiol. 1994;10:427–434. doi: 10.1007/BF01719667. [DOI] [PubMed] [Google Scholar]
- 178.Thiele D, Willems H, Kopf G, Krauss H. Polymorphism in DNA restriction patterns of Coxiella burnetii isolates investigated by pulsed field gel electrophoresis and image analysis. Eur J Epidemiol. 1993;9:419–425. doi: 10.1007/BF00157400. [DOI] [PubMed] [Google Scholar]
- 179.Thiele D, Willems H, Krauss H. The 16S/23S ribosomal spacer region of Coxiella burnetii. Eur J Epidemiol. 1994;10:421–426. doi: 10.1007/BF01719666. [DOI] [PubMed] [Google Scholar]
- 180.Tissot-Dupont H, Raoult D, Brouqui P, Janbon F, Peyramond D, Weiller P J, Chicheportiche C, Nezri M, Poirier R. Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients—323 French cases. Am J Med. 1992;93:427–434. doi: 10.1016/0002-9343(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 181.Tissot-Dupont H, Thirion X, Raoult D. Q fever serology: cutoff determination for microimmunofluorescence. Clin Diagn Lab Immunol. 1994;1:189–196. doi: 10.1128/cdli.1.2.189-196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tobin M J, Cahill N, Gearty G, Maurer B, Blake S, Daly K, Hone R. Q fever endocarditis. Am J Med. 1982;72:396–400. doi: 10.1016/0002-9343(82)90495-8. [DOI] [PubMed] [Google Scholar]
- 183.Tokarevich N K, Schramek S, Daiter A B. Indirect haemolysis test in Q fever. Acta Virol. 1990;34:358–360. [PubMed] [Google Scholar]
- 184.Tolosa-Vilella C, Rodriguezjornet A, Fontrocabanyera J, Andreunavarro X. Mesangioproliferative glomerulonephritis and antibodies to phospholipids in a patient with acute Q fever: case report. Clin Infect Dis. 1995;21:196–198. doi: 10.1093/clinids/21.1.196. [DOI] [PubMed] [Google Scholar]
- 185.Toman R, Kazar J. Evidence for the structural heterogeneity of the polysaccharide component of Coxiella burnetii strain Nine Mile lipopolysaccharide. Acta Virol. 1991;35:531–537. [PubMed] [Google Scholar]
- 186.Toman R, Skultety L. Structural study on a lipopolysaccharide from Coxiella burnetii strain Nine Mile in avirulent phase II. Carbohydr Res. 1996;283:175–185. doi: 10.1016/0008-6215(96)87610-5. [DOI] [PubMed] [Google Scholar]
- 187.Turck W P, Howitt G, Turnberg L A, Fox H, Longson M, Matthews M B, Das Gupta R. Chronic Q fever. Q J Med. 1976;45:193–217. [PubMed] [Google Scholar]
- 188.Uhaa I J, Fishbein D B, Olson J G, Rives C C, Waag D M, Williams J C. Evaluation of specificity of indirect enzyme-linked immunosorbent assay for diagnosis of human Q fever. J Clin Microbiol. 1994;32:1560–1565. doi: 10.1128/jcm.32.6.1560-1565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Valero F, Degroote P, Millaire A, Raoult D, Wattre P, Ducloux G. Pericardial effusion as the initial feature of Q fever. Am Heart J. 1995;130:1308–1309. doi: 10.1016/0002-8703(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 190.Valkova D, Kazar J. A new plasmid (QpDV) common to Coxiella burnetii isolates associated with acute and chronic Q fever. FEMS Microbiol Lett. 1995;125:275–280. doi: 10.1111/j.1574-6968.1995.tb07368.x. [DOI] [PubMed] [Google Scholar]
- 191.Waag D, Chulay J, Marrie T, England M, Williams J. Validation of an enzyme immunoassay for serodiagnosis of acute Q fever. Eur J Clin Microbiol Infect Dis. 1995;14:421–427. doi: 10.1007/BF02114898. [DOI] [PubMed] [Google Scholar]
- 192.Waldhalm D G, Stoenner H G, Simmons R E, Thomas L A. Abortion associated with Coxiella burnetii infection in dairy goats. J Am Vet Med Assoc. 1978;173:1580–1581. [PubMed] [Google Scholar]
- 193.Webster J P, LLoyd G, Macdonald D W. Q fever (Coxiella burnetii) reservoir in wild brown rat (Rattus norvegicus) populations in the UK. Parasitology. 1995;110:31–35. doi: 10.1017/s0031182000081014. [DOI] [PubMed] [Google Scholar]
- 194.Weir W R, Bannister B, Chambers S, De Cock K, Mistry H. Chronic Q fever associated with granulomatous hepatitis. J Infect. 1984;8:56–60. doi: 10.1016/s0163-4453(84)93354-1. [DOI] [PubMed] [Google Scholar]
- 195.Weisburg W G, Dobson M E, Samuel J E, Dasch G A, Mallavia L P, Baca O, Mandelco L, Sechrest J E, Weiss E, Woese C R. Phylogenetic diversity of the rickettsiae. J Bacteriol. 1989;171:4202–4206. doi: 10.1128/jb.171.8.4202-4206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Weiss E, Moulder J W. Order I Rickettsiales, Gieszczkiewicz 1939. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 687–703. [Google Scholar]
- 197.Willems H, Thiele D, Frolich-Ritter R, Krauss H. Detection of Coxiella burnetii in cow’s milk using the polymerase chain reaction. J Vet Med B. 1994;41:580–587. doi: 10.1111/j.1439-0450.1994.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 198.Willems H, Thiele D, Glas-Adollah-Baik Kashi M, Krauss H. Immunoblot technique for Q fever. Eur J Epidemiol. 1992;8:103–107. doi: 10.1007/BF03334980. [DOI] [PubMed] [Google Scholar]
- 199.Willems H, Thiele D, Krauss H. Plasmid based differentiation and detection of Coxiella burnetii in clinical samples. Eur J Epidemiol. 1993;9:411–417. doi: 10.1007/BF00157399. [DOI] [PubMed] [Google Scholar]
- 200.Williams J C, Thomas L A, Peacock M G. Humoral immune response to Q fever: enzyme-linked immunosorbent assay antibody response to Coxiella burnetii in experimentally infected guinea pigs. J Clin Microbiol. 1986;24:935–939. doi: 10.1128/jcm.24.6.935-939.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Williams J C, Thomas L A, Peacock M G. Identification of phase-specific antigenic fractions of Coxiella burnetti by enzyme-linked immunosorbent assay. J Clin Microbiol. 1986;24:929–934. doi: 10.1128/jcm.24.6.929-934.1986. . (Erratum, 25:588, 1987.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Reference deleted.
- 203.Yeaman M R, Baca O G. Unexpected antibiotic susceptibility of a chronic isolate of Coxiella burnetii. Ann N Y Acad Sci. 1990;590:297–305. doi: 10.1111/j.1749-6632.1990.tb42236.x. [DOI] [PubMed] [Google Scholar]
- 204.Zeman D H, Kirkbride C A, Leslie-Steen P, Duimstra J R. Ovine abortion due to Coxiella burnetii infection. J Vet Diagn Invest. 1989;1:178–180. doi: 10.1177/104063878900100218. [DOI] [PubMed] [Google Scholar]