Abstract
Background
General interest and incidence are increasing in wild-type transthyretin amyloidosis (ATTRwt) in recent time. As patient population increases, further knowledge of the management of the frequently encountered interacting cardiac comorbidities is requested to improve treatment of ATTRwt patients.
Case summary
A 73-year-old male ATTRwt patient presented to the outpatient clinic (Day 0) with dyspnoea, leg swelling, and palpitations. At diagnosis, 3 years prior to presentation, he exhibited only minor signs of ATTRwt. At Day 0, clinical examination revealed atrial fibrillation and mild peripheral oedema. Anticoagulant and symptomatic treatment with beta-blocker and diuretics was initiated, and the patient was planned for sub-acute direct cardioversion, and the patient was discharged with a Holter monitor to outpatient care. At Day 7, analysis of the monitoring demonstrated spontaneous conversion to sinus rhythm and, unexpectedly, episodes of high-rate self-remittent sustained monomorphic ventricular tachycardia (VT) and frequent ventricular ectopic beats. At Day 8, a sub-acute coronary angiography was performed which revealed a significant proximal left anterior descending artery stenosis which was treated with percutaneous coronary intervention (PCI) and subsequently an internal defibrillator was implanted. Following visits at 1- and 3-month post-PCI at the outpatient clinic revealed no VT and suppression of ventricular ectopic beats.
Discussion
The case illustrates some of the frequently encountered cardiac comorbidities (e.g. atrial fibrillation, ventricular arrhythmia, and ischaemic heart disease) associated with ATTRwt. A high level of suspicion is warranted to identify treatable cardiac conditions [atrial fibrillation, atrioventricular (AV) block, and ischaemic heart disease] and to uncover potentially fatal cardiac conditions in patients with ATTRwt.
Keywords: Case report, ATTRwt, Ventricular tachycardia, Ischaemic heart disease, Atrial fibrillation, ICD
Learning points.
Co-existing cardiac comorbidities (e.g. atrial fibrillation, ventricular arrhythmia, and ischaemic heart disease) are frequent in wild-type transthyretin amyloidosis (ATTRwt) disease.
Ischaemic heart disease might be exacerbated by ATTRwt and can result in ventricular tachyarrhythmia.
A high level of clinical suspicion is recommended to identify important cardiac comorbidity and manifestations of ATTRwt (conduction disease, ischaemic heart disease, and atrial fibrillation).
Introduction
Wild-type transthyretin amyloidosis (ATTRwt) is characterized by progressive amyloid myocardial infiltration leading to the development of restrictive cardiomyopathy.1 The prevalence of ATTRwt increases with age, affects predominantly males, and presents most often with heart failure symptoms, and the disease is associated with increased morbidity and mortality.2 Non-invasive diagnostics (bone scintigraphy) and greater awareness of ATTRwt have led to an increase in incidence in recent years,3 in which new pharmacological disease-modifying treatments, i.e. tafamidis, also have become available.4 Consequently, attention has increased on the clinical management of co-existing cardiac comorbidities among ATTRwt patients whose life expectancy is expected to improve.5
In this case report, we present an early disease stage ATTRwt patient and illustrate the emerging issue of managing comorbidities in ATTRwt.
Summary figure
| Year | Event |
|---|---|
| Mar 2016 | Surgical intervention: unilateral carpal tunnel syndrome. |
| Nov 2019 | Diagnosis of wild-type transthyretin amyloidosis (ATTRwt) (screening study) (scintigraphy and endomyocardial biopsy positive for ATTRwt). |
| Day 0 Aug 2022 | Current presentation: shortness of breath, palpitation (atrial fibrillation), and mild swelling of lower legs. Treatment with diuretics, beta-blocker, anti-coagulation, and planned sub-acute direct cardioversion. Discharged for outpatient care. |
| Day 7 | Result of 48-h Holter monitoring: paroxysmal atrial fibrillation (2 h). Three episodes of ventricular tachycardia (VT), ∼20% ventricular ectopic beats. |
| Day 8 | Coronary angiography: proximal left anterior descending artery stenosis—percutaneous coronary intervention performed simultaneously. Anti-thrombotic and anti-cholesterol treatment added. |
| Day 13 | Implantation of a dual chamber implantable cardioverter defibrillator (DDD-ICD). |
| Day 42 Sep 2022 |
At 1 month follow-up: New York Heart Association (NYHA) 1, no VT, and reduction of ventricular ectopy. |
| Day 104 Nov 2022 |
Three-month DDD-ICD monitoring: no evidence of VT. |
Case presentation
At Day 0 (2022), a 73-year-old male with known ATTRwt presented to the outpatient clinic with shortness of breath, mild swelling of the lower legs, and palpitations with onset within the recent 2 weeks.
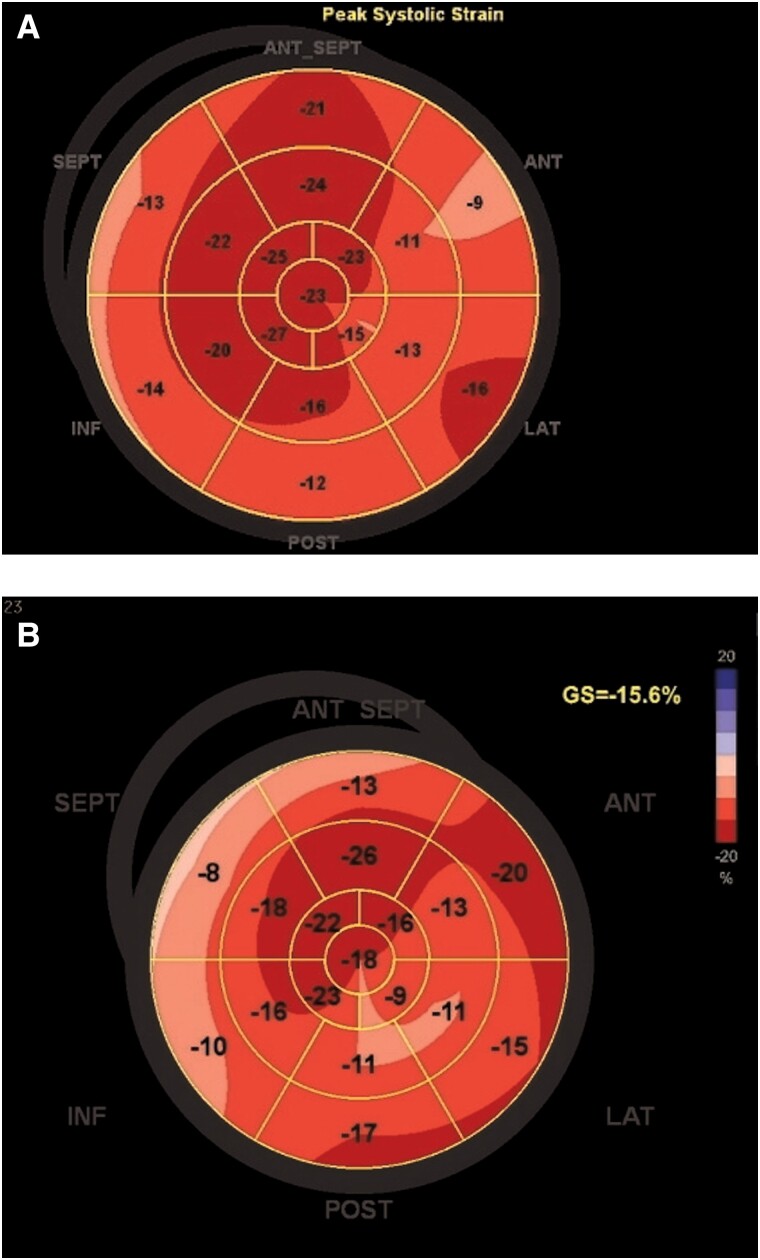
Three years prior, he was diagnosed with ATTRwt following participation in a screening trial for transthyretin amyloidosis (ATTR) in patients with previous carpal tunnel surgery.6 At diagnosis, he was asymptomatic without any history of cardiovascular disease. His electrocardiogram (ECG) showed sinus rhythm with borderline low voltage in the limb leads, and N-terminal prohormone of brain natriuretic peptide (NT-proBNP) was slightly elevated at 390 ng/L (normal < 300 ng/L). Echocardiography demonstrated mild increased left ventricular (LV) wall thickness of 12 mm, LV ejection fraction (LVEF) 70%, and normal LV global longitudinal strain (LVGLS) −18.8% with borderline apical sparring pattern (Figure 1A and Supplementary Material S1A). The diagnosis of ATTRwt was established by a positive bone scintigraphy—Perugini grade 2 (see Supplementary Material S2)—and by endomyocardial biopsy. Genetic testing was negative for variant ATTR. He was subsequently followed untreated for 3 years with stable parameters.
Figure 1.
Global longitudinel strain plot at diagnosis (A) and at 3-month follow-up (B) after contact to outpatient clinic.
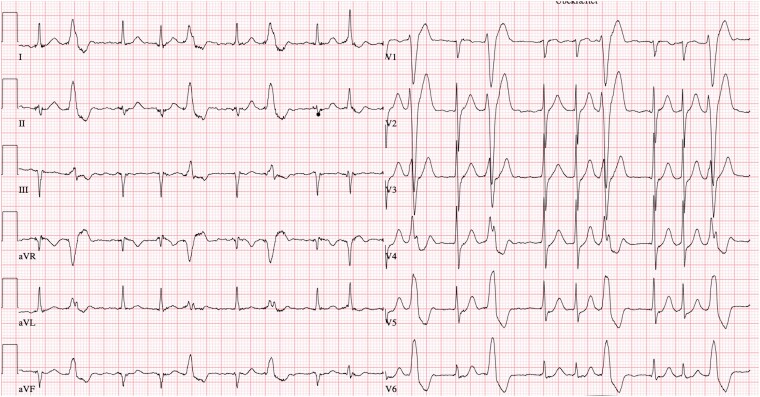
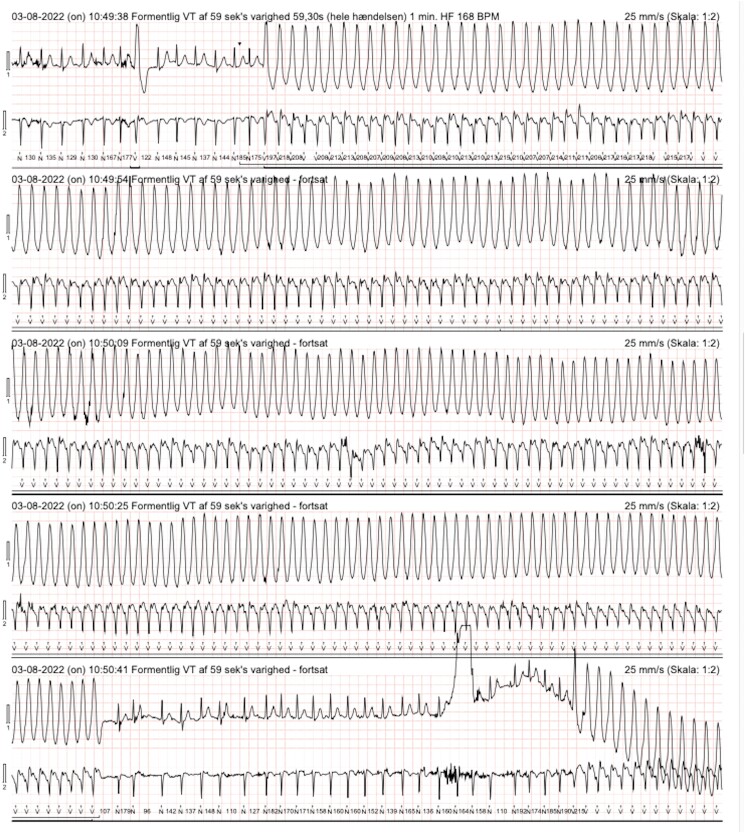
At Day 0, cardiac examination of the patient showed mild leg oedema, no heart murmurs, irregular heart rate of 110 b.p.m., elevated blood pressure (150/80 mmHg), ECG with atrial fibrillation, and frequent ventricular ectopy (Figure 2). Echocardiography revealed LVEF 57%, increased wall thickness of 14 mm, and tricuspid regurgitant gradient of 18 mmHg. Serum NT-proBNP was 436 ng/L. No further imaging was ordered. Furosemide (40 mg daily), low dosage of metoprolol (50 mg daily), and dabigatran (150 mg × 2 daily) were initiated immediately. Following discharge, a 48-h Holter monitoring was performed and a direct current cardioversion (DCC) preceded by transoesophageal echocardiography was planned within 3–4 weeks. However, at Day 7, the Holter monitoring analysis revealed that the atrial fibrillation ceased after 2 h and sinus rhythm remained subsequently. Unexpectedly, three episodes of symptomatic (palpitations) monomorphic ventricular tachycardia (VT) was observed with a frequency of 255 b.p.m. and a duration of 40–60 s (Figure 3). In addition, ∼20% of total beats were ectopic unifocal ventricular beats. At Day 8, the coronary angiography demonstrated a highly significant stenosis of the proximal left anterior descending artery (LAD) which was treated by direct percutaneous coronary intervention (PCI) (see Supplementary Material S3A and B). Clopidogrel 75 mg × 1 was added with a treatment duration of 12 months. The unifocal ventricular ectopy vanished immediately after the PCI as observed by in-hospital cardiac telemetry, and the patient was asymptomatic at same-day discharge. At Days 12–14, as a consequence of the characteristics of the monomorphic VT, the ATTRwt diagnosis, and the presence of paroxysmal atrial fibrillation, it was decided by consensus to implant a dual chamber implantable cardioverter defibrillator (DDD-ICD). Tafamidis was not considered as it is not currently approved by Danish Health Authorities.
Figure 2.
ECG showing atrial fibrillation and frequent monomorphic ventricular ectopy.
Figure 3.
Holter monitoring showing atrial fibrillation interrupted by episodes of ventricular tachycardia.
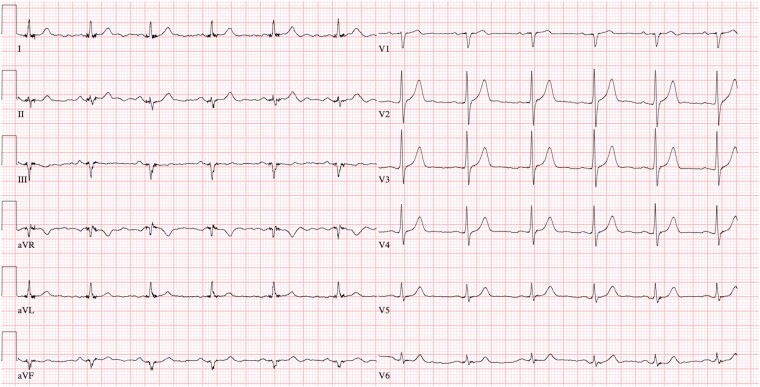
At 1- and 3-month follow-up (Day 42/110), the patient was well-being and asymptomatic. The ECG (Figure 4), a 48-h Holter monitor examination and ICD interrogation revealed persistence of sinus rhythm, no recurrent VT, and a marked reduction of ventricular ectopic beats to <1% of total beats. Echocardiography demonstrated minor subclinical changes with a reduction of LVEF to 60% and LVGLS to −15.6% with local mildly reduced LV strain values in the anterolateral segments (Figure 1B) (see Supplementary Material S1B). N-terminal prohormone of brain natriuretic peptide was 537 ng/L.
Figure 4.
ECG showing sinus rhythm without ventricular ectopy.
Discussion
The present case demonstrates the plethora of clinical considerations encountered when managing patients with ATTRwt. Firstly, atrial fibrillation is common in ATTRwt with a prevalence of 40–70%.7 Wild-type transthyretin amyloidosis patients with symptomatic atrial fibrillation are likely to benefit clinically, haemodynamically, and prognostically from maintenance of sinus rhythm.8 However, high rates of recurrence,8 safety concerns with DCC (atrial thrombi),9 and occasional intolerance to amiodarone complicate treatment. In this patient, an initial rhythm control strategy was planned due to symptoms, mild disease stage (National Amyloidosis Centre stage 1), and first-time occurrence of atrial fibrillation. Until DCC, a low dosage of metoprolol was initiated to regulate the atrial fibrillation and to reduce the ventricular ectopy. Nonetheless, beta-blockade should, if possible, be avoided in ATTRwt as the ATTRwt haemodynamics is characterized by a low stroke volume and chronotropic dependency to maintain normal cardiac output.10
Disease-modifying treatment, i.e. tafamidis, was not initiated as Danish Health Authorities have not financially approved tafamidis for the treatment of ATTRwt. Irrespectively, this patient would have been an ideal candidate for tafamidis treatment with initiation at a very early disease stage.4
Monomorphic VT, which was present in this patient, can be observed in most cardiomyopathies, in patients with scar tissue after myocardial infarction,11 and can occasionally be observed during acute myocardial ischaemia.12 The monomorphic VT in this case was likely worsening in chronic myocardial ischaemia caused by the LAD stenosis and a component of intramyocardial ischaemia often seen in patients with ATTRwt cardiomyopathy.13,14 The interstitial amyloid myocardial infiltration is also believed to have potential toxic effects on the myocytes and could theoretically facilitate re-entrant circuits and thus predisposition of ventricular arrhythmias.1,11 In addition to VT suppression after PCI, the frequent unifocal ventricular ectopy stopped and was not present at follow-up. This indicates that myocardial ischaemia was likely the triggering factor for the unifocal ventricular ectopic activity and sustained VT. In general, ventricular arrhythmias are frequent in ATTRwt, but sustained VT is assumed to be rare.15 However, the evidence is scarce and inadequate for any definite conclusion.
The effect of ICD therapy as secondary prevention in patients with ATTRwt cardiomyopathy is uncertain as no randomized controlled trials have been performed and are unlikely to be carried out due to the relative rarity of the disease, age characteristics of the patients, and the general prognosis. The indication for ICD therapy in rare diseases is most often evaluated on an individual basis as exemplified with the present case. Even though the LAD lesion was treated by PCI and the VT was subsequently suppressed, it was considered that the cause of the monomorphic VT could be multi-factorial involving the amyloid cardiomyopathy itself. As the present patients was relatively young, in an early disease stage, and without major comorbidities, it was by consensus decided to implant an ICD.
Conclusion
In this case report, we present a patient with ATTRwt with several clinical issues including significant arrhythmias, ischaemic heart disease, and progressive infiltrative cardiomyopathy. The present case illustrates that clinicians need to be aware of the frequently occurring cardiovascular comorbidities and the potential interaction with the ATTRwt cardiomyopathy to ensure appropriate treatment.
Further insights are warranted to address the effect and necessity of various medical treatments and interventions in ATTRwt patients.
Supplementary Material
Contributor Information
Jens Kæstel Skov, Department of Clinical Epidemiological Department, Aarhus University Hospital and Aarhus University, Aarhus N, Denmark; Department of Clinical Medicine, Aarhus University Hospital and Aarhus University, Aarhus N, Denmark.
Bertil Ladefoged, Department of Clinical Medicine, Aarhus University Hospital and Aarhus University, Aarhus N, Denmark.
Tor Skibsted Clemmensen, Department of Clinical Medicine, Aarhus University Hospital and Aarhus University, Aarhus N, Denmark.
Steen Hvitfeldt Poulsen, Department of Clinical Medicine, Aarhus University Hospital and Aarhus University, Aarhus N, Denmark.
Lead author biography
 Dr Jens Kæstel Skov has achieved his medical studies at Aarhus University, Denmark, in 2018. He has a particular interest in cardiology and has a 1-year residency at the Cardiology Department of Herning, Denmark, and Cardiology Department at Aarhus University Hospital, Denmark. He is currently working in a PhD programme with a focus on a trial of screening of ATTRwt amyloidosis among patients with atrioventricular (AV) block.
Dr Jens Kæstel Skov has achieved his medical studies at Aarhus University, Denmark, in 2018. He has a particular interest in cardiology and has a 1-year residency at the Cardiology Department of Herning, Denmark, and Cardiology Department at Aarhus University Hospital, Denmark. He is currently working in a PhD programme with a focus on a trial of screening of ATTRwt amyloidosis among patients with atrioventricular (AV) block.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Consent: The patient has consented to the publishing of the case report in line with the COPE best practice guidelines.
Funding: None declared.
Data availability
The data underlying this article are available in the article and in its online Supplementary material.
References
- 1. Griffin JM, Rosenblum H, Maurer MS. Pathophysiology and therapeutic approaches to cardiac amyloidosis. Circ Res 2021;128:1554–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pinney JH, Whelan CJ, Petrie A, Dungu J, Banypersad SM, Sattianayagam P, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc 2023;2:e000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Westin O, Butt JH, Gustafsson F, Schou M, Salomo M, Køber L, et al. Two decades of cardiac amyloidosis. JACC CardioOncol 2021;3:522–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 5. Giancaterino S, Urey MA, Darden D, Hsu JC. Management of arrhythmias in cardiac amyloidosis. JACC Clin Electrophysiol 2020;6:351–361. [DOI] [PubMed] [Google Scholar]
- 6. Ladefoged B, Clemmensen T, Dybro A, Hartig-Andreasen C, Kirkeby L, Gormsen LC, et al. Identification of wild-type transthyretin cardiac amyloidosis in patients with carpal tunnel syndrome surgery (CACTuS). ESC Heart Fail 2022;10:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donnellan E, Wazni OM, Hanna M, Elshazly MB, Puri R, Saliba W, et al. Atrial fibrillation in transthyretin cardiac amyloidosis. JACC Clin Electrophysiol 2020;6:1118–1127. [DOI] [PubMed] [Google Scholar]
- 8. Donnellan E, Wazni OM, Hanna M, Elshazly MB, Puri R, Saliba W, et al. Atrial fibrillation in transthyretin cardiac amyloidosis: predictors, prevalence, and efficacy of rhythm control strategies. JACC Clin Electrophysiol 2020;6:1118–1127. [DOI] [PubMed] [Google Scholar]
- 9. El-Am EA, Dispenzieri A, Melduni RM, Ammash NM, White RD, Hodge DO, et al. Direct current cardioversion of atrial arrhythmias in adults with cardiac amyloidosis. J Am Coll Cardiol 2019;73:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clemmensen TS, Mølgaard H, Sörensen J, Eiskjaer H, Andersen NF, Mellemkjaer S, et al. Inotropic myocardial reserve deficiency is the predominant feature of exercise haemodynamics in cardiac amyloidosis. Eur J Heart Fail 2017;19:1457–1465. [DOI] [PubMed] [Google Scholar]
- 11. Meja Lopez E, Malhotra R. Ventricular tachycardia in structural heart disease. J Innov Card Rhythm Manag 2019;10:3762–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demidova MM, Úlfarsson ÆÖ, Carlson J, Erlinge D, Platonov PG. Relation of early monomorphic ventricular tachycardia to long-term mortality in ST-elevation myocardial infarction. Am J Cardiol 2022;163:13–19. [DOI] [PubMed] [Google Scholar]
- 13. Clemmensen TS, Eiskjær H, Mølgaard H, Larsen AH, Soerensen J, Andersen NF, et al. Abnormal coronary flow velocity reserve and decreased myocardial contractile reserve are main factors in relation to physical exercise capacity in cardiac amyloidosis. J Am Soc Echocardiogr 2018;31:71–78. [DOI] [PubMed] [Google Scholar]
- 14. Clemmensen TS, Soerensen J, Hansson NH, Tolbod LP, Harms HJ, Eiskjær H, et al. Myocardial oxygen consumption and efficiency in patients with cardiac amyloidosis. J Am Heart Assoc 2018;7:e009974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knoll K, Fuchs P, Weidmann I, Altunkas F, Voss S, Lennerz C, et al. Incidence and predictors of ventricular arrhythmias in transthyretin amyloid cardiomyopathy. J Clin Med 2023;12:4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary material.