Abstract
Background
Primary ciliary dyskinesia (PCD) is an inherited disorder in which dyskinetic cilia cause impaired mucociliary clearance of upper and lower airways. Airway ciliary movement can be indirectly tested in vivo after administration of a radiolabelled tracer to the lower airways for assessment of pulmonary mucociliary clearance or to the nose for assessing nasal mucociliary clearance (NMC). With this study, we investigated NMC as a quantifiable study outcome parameter in patients with PCD.
Material and methods
This single centre proof-of-concept study on NMC velocity investigated patients with PCD across different genotypes and nasal nitric oxide (nasal NO) levels. Healthy controls were used for comparison. NMC was determined as velocity in mm·min−1 of a nasally applied 99mTc-albumin colloid tracer. Using a gamma camera, repeated dynamic series of images each lasting 30 s were acquired during a 10-minute period and digitally stored.
Results
NMC velocity was investigated in seven patients with PCD (aged 9–31 years) and five adult healthy controls. Mean NMC velocity in healthy controls (8.5 mm·min−1) was significantly higher compared with people with PCD (0.00 mm·min−1, p<0.0001). NMC was completely absent in all included patients with PCD across different PCD genotypes and regardless of nasal NO values. The success rate of the test was 100% in both groups.
Conclusion
NMC velocity discriminated highly significantly between patients with PCD and healthy controls. We suggest here a fast and feasible set up for NMC measurements that is easily applicable for any clinical trial involving PCD medication aimed for the nasal compartment, a step before or parallel to conducting clinical trials investigating whole-lung ciliary function in PCD.
Tweetable abstract
99mTc-albumin colloid NMC velocity measurement is a quantitative test that is highly feasible, and highly discriminative between PCD and health, with very low radiation exposure, and is a potential outcome parameter for future clinical trials in PCD https://bit.ly/3Xbyn9C
Introduction
Primary ciliary dyskinesia (PCD) (MIM244400) is a multi-system disorder predominantly considered as a respiratory motile ciliopathy in which the dyskinetic cilia cause impairment of mucociliary clearance resulting in chronic destructive infectious and inflammatory disease of the upper and lower airways.
Currently, approximately 50 genes have been identified that harbour variants causing PCD [1, 2]. However, the number of characterised disease-causing PCD mutations is steadily increasing. Most mutations associated with PCD are autosomal recessive. Less frequently, X-chromosomal recessive or de novo autosomal dominant inheritance has been described [3].
Completed randomised clinical trials directed towards patients with PCD are sparse [4–6]. Pharmaceutical companies have become increasingly interested in developing precision medicine as a potential future treatment for PCD. This includes the development of mRNA transcript therapy, and designing correctors for ciliary proteins in patients with PCD related to their specific genotypes. Consequently, an even higher need for relevant outcomes for future clinical trials in patients with PCD has emerged. So far, there are no published studies on precision medicine in PCD.
A 99mTc tracer mucociliary clearance test can be used as an indirect in vivo functional test of ciliary movement in upper and lower airways and can be measured specifically in the nasal compartment as nasal mucociliary clearance (NMC) and separately in the pulmonary compartment as total pulmonary radioaerosol mucociliary clearance (PRMC). In previous studies on isolated NMC, only semi-quantitative measures have been applied [7, 8], whereas whole-lung PRMC has been investigated for quantitative diagnostic measures [9–11] and for whole-lung mucociliary clearance studies in healthy controls [12], and for outcome parameters in randomised clinical trials in patients with cystic fibrosis [13, 14].
NMC holds potential as an in vivo functional test that can deliver outcomes in future clinical trials for nasal PCD drugs, but so far quantitative references have been lacking.
Aim
In this proof-of-concept study, we show quantitative measurements of NMC velocity and the discrimination between NMC velocity in patients with PCD and healthy controls. The scope of the study was to investigate NMC as a potential outcome parameter, and not as a supplemental diagnostic test in PCD work-up.
Material and methods
Study design
Our study is a small prospective, single centre proof-of-concept pilot study investigating NMC velocity in a subset of patients with PCD from the cohort of the Danish PCD Centre and with healthy controls for comparison.
Patients
Child and adult patients from the cohort of the Danish PCD Centre and healthy adult controls were included for assessment of NMC velocity according to the following inclusion criteria: patients with PCD who were diagnosed according to the European Respiratory Society and American Thoracic Society (ATS) guidelines criteria [15], with a known biallelic pathogenic PCD gene defect and additional abnormal transmission electron microscopy (TEM), an abnormal ciliary function test and a nasal nitric oxide (nasal NO) test. Informed consent was collected from all the patients prior to inclusion. Healthy nonsmoking, nonpregnant and nonbreastfeeding adults who gave their informed consent to the NMC velocity study were included. All fertile female volunteers, healthy controls and patients with PCD, were obliged to provide a negative pregnancy test before inclusion in the study.
Subjects with acute upper respiratory infection were excluded from the study to avoid false low NMC velocity values as a consequence of secondary impaired nasal ciliary transport. Patients with PCD who reported stable chronic rhinitis symptoms were not excluded.
Determination of NMC velocity
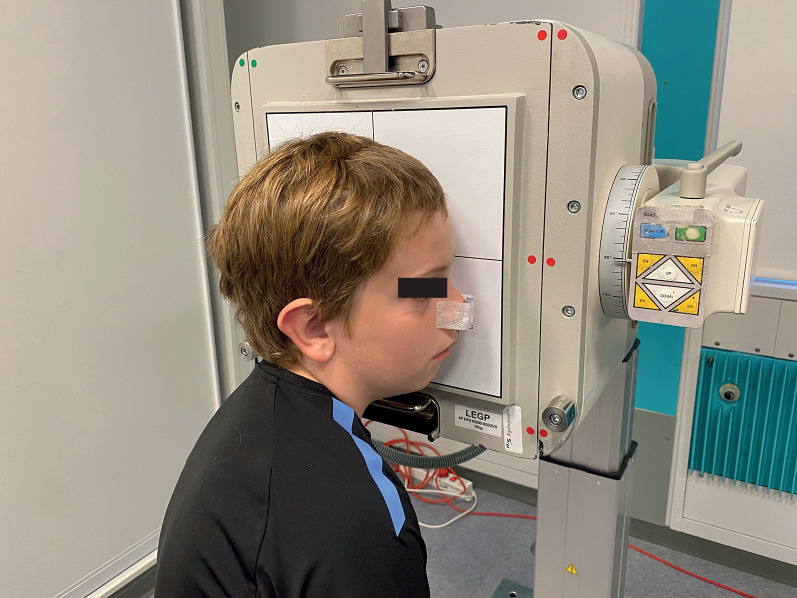
A 99mTc-albumin colloid tracer was used for the NMC velocity test, in which one small droplet of 1.85 MBq 99mTc-albumin colloid dissolved in isotonic saline was administered to the concha media by a Hamilton syringe. The small droplet size (2.5 μL) allowed the tracer to be administered precisely to the nasal concha media. The radiation dose for a full NMC measurement was 25 μSv. The subject had radioactive reference sources (57Co) attached to the nasal tip and anterior to the tragus, respectively, while the gamma camera faced the profile of the subject (figure 1). The reference sources served as points of direction and as references for movement of the head, which was corrected for to enable the velocity to be precisely calculated. The tracer movement was defined as the most forward front of the tracer.
FIGURE 1.
Photograph of a 10-year-old subject showing the gamma camera facing the profile of the subject during the 20 min dynamic acquisitions after administration of the radiolabelled tracer to the nasal concha media. A 57Co marker is attached to the tip of the nose and to the anterior tragus of the (left) ear leaning against the camera. The tragus marker is not visible on the photo.The photo is shown with consent from both parents and the child.
A dynamic series of 20 images each lasting 30 s was acquired during a 10-min period and digitally stored. The nasal mucociliary transport could be read directly from the screen, image by image, and the NMC velocity was calculated according to the following equation, since one image=0.5 min:
The resolution of the gamma camera was 4.66 mm/pixel (matrix: 128 × 128 pixels).
Nasal NO measurement
Nasal NO was measured using the stationary CLD88sp FeNO chemiluminescence analyser (ECO MEDICS AG, Duernten, Switzerland) and measurements were performed according to the recommended technical standards [16].
Nasal NO gas was aspirated via a nasal olive probe inserted into one nostril. Mean nasal NO concentration in parts per billion (ppb) was calculated from triple measurements in each subject. All subjects had nasal NO sampled during velum closure, preferentially by exhalation against resistance; if this was not possible for a subject, sampling was performed during breath-hold. The sampling flow rate was 0.33 L·min−1 and conversion from nasal NO concentration in ppb to nasal NO production rate (nL·min−1) was calculated as follows:
Ambient NO was recorded before each measurement. Nasal NO was measured on the same day as the NMC assessment.
PCD genetics
A next generation sequencing (NGS) panel including the following 34 PCD genes was used:
C21orf59, CCDC103, CCDC114, CCDC151, CCDC65, CCDC39, CCDC40, CCNO, CENPF, DNAH11, DNAH5, DNAH8, DNAH9, DNAI1, DNAI2, DNAL1, DNAAF1, DNAAF2, DNAAF3, DNAAF4, DRC1, GAS8, HYDIN, INVS, LRRC6, MCIDAS, OFD1, RSPH1, RSPH2, RSPH3, RSPH4A, RSPH9, SPAG1, ZMYND10.
High-speed video microscopy and transmission electron microscopy
Nasal ciliary beat pattern and beat frequency were determined from high-speed video microscopy (HSVM) ciliary function analysis of nasal brush biopsy material. Nasal ciliary ultrastructure was determined by TEM from a nasal curette scraping biopsy. HSVM and TEM were performed as standard diagnostic testing in all patients with PCD included in the study and according to guidelines [17–19].
Statistics
The Wilcoxon rank sum test was used to compare means of NMC velocity values between patients with PCD and healthy controls; a p-value <0.05 was considered statistically significant.
Ethics
The study was approved by the local ethics committee of Copenhagen, DK (journal number H-C-2007-0061). Informed consent was obtained from all participating subjects.
Results
We included seven patients with PCD (median age 14 years, range 9–31 years) and five healthy subjects (median age 39 years, range 20–50 years). In patients with PCD, NMC was shown to be completely absent, as NMC velocity was 0.0 mm·min−1 in all seven patients (100%), regardless of PCD genotype and nasal NO value. In healthy controls, the mean NMC velocity was 8.5 mm·min−1 and was significantly higher compared to the PCD group (p<0.0001) (table 1, video S1 and video S2). Patients with PCD genotypes associated with hallmark TEM outer dynein arm defects (N=3, 43%) had biallelic defects in DNAH5, DNAI1 and CCDC114, respectively. Four patients (57%) had PCD genotypes associated with no detectable TEM defects (DNAH11 and HYDIN) or minor, and sometimes not visible, TEM defects (RSPH9) (table 1). The success rate for a first conclusive test was 100% for both the PCD and healthy control groups.
TABLE 1.
Characteristics of patient genetics and diagnostics and the results of NMC velocity and nasal NO measurements for the included healthy controls and patients with PCD
| Subject number | Group | NMC velocity (mm min−1) | Age (years) | Gender | Nasal NO (ppb) | Nasal NO (nL·min−1) | Ciliary beat frequency by HSVM | Ciliary beat pattern by HSVM | Ciliary ultrastructure by TEM | PCD gene | Conclusive first test |
| 1 | HC | 6.8 | 38 | F | 660 | 217.8 | N/A | N/A | N/A | N/A | Yes |
| 2 | HC | 8.0 | 39 | M | 803 | 265.0 | N/A | N/A | N/A | N/A | Yes |
| 3 | HC | 8.4 | 50 | M | 1103 | 364.0 | N/A | N/A | N/A | N/A | Yes |
| 4 | HC | 8.7 | 20 | F | 1520 | 501.6 | N/A | N/A | N/A | N/A | Yes |
| 5 | HC | 10.4 | 47 | M | 667 | 220.1 | N/A | N/A | N/A | N/A | Yes |
| 6 | PCD | 0.0 | 14 | F | 208 | 68.4 | Slow (3–4 Hz) | Rotational | CP defect (9+0 CP loss/8+1 doublet transposition) | RSPH9 | Yes |
| 7 | PCD | 0.0 | 12 | M | 51 | 16.8 | Slow (3–4 Hz) | Rotational | CP defect (9+0 CP loss/8+1 doublet transposition) | RSPH9 | Yes |
| 8 | PCD | 0.0 | 9 | F | 31 | 10.2 | Slow (3–4 Hz) | Stiff and flickering | No abnormality | DNAH11 | Yes |
| 9 | PCD | 0.0 | 15 | M | 48 | 15.8 | Almost immotile (1–2 Hz) | Residual flickering | ODA defect | DNAI1 | Yes |
| 10 | PCD | 0.0 | 20 | F | 48 | 15.8 | Slow (3–4 Hz) | Asynchronous | No abnormality | HYDIN | Yes |
| 11 | PCD | 0.0 | 14 | F | 53 | 17.5 | Almost immotile (1–2 Hz) | Residual flickering | ODA defect | DNAH5 | Yes |
| 12 | PCD | 0.0# | 31 | F | 24 | 7.9 | Almost immotile (1–2 Hz) | Residual flickering | ODA defect | CCDC114 | Yes |
Nasal material for HSVM was obtained from nasal brush biopsy. Nasal material for TEM analysis was obtained from nasal curette scraping biopsy. NMC: nasal mucociliary clearance; HSVM: high-speed video microscopy; TEM: transmission electron microscopy; PCD: primary ciliary dyskinesia; HC: healthy control; N/A: not available; CP: central pair; ODA: outer dynein arm; #: post-measurement digital correction of head movement needed. From calculation of tragus reference movement, a false detectable clearance of 3.6 mm·min−1 was corrected to 0.0 mm·min−1.
The NMC film read outs showed the droplet area in the nose to be small and well defined, without spreading over a significant area, which made the tracer movement easy to read.
Post-measurement digital correction of the NMC velocity was necessary in one case only, a 31-year-old patient with PCD, where movement of the tragus reference indicated head movement during the 10 min of acquisitions. From determining the distance (mm) of the tragus reference movement, the nasal tracer movement could be re-calculated by subtracting the distance of the tragus reference movement. The correction resulted in a change from a false detectable NMC velocity of 3.6 mm·min−1 to a corrected NMC velocity of 0.0 mm·min−1.
Discussion
To our knowledge, this is the first study to show quantitative data on NMC velocity (mm·min−1) by use of a 99mTc-albumin colloid tracer as an in vivo nasal test of a radiolabelled tracer travelling from a fixed point in the nose towards a reference source.
Up to now, studies on NMC have been sparsely reported and with different outcome measures. In a previous study from De Boeck and colleagues [7], an abnormal NMC was defined semi-quantitatively as either no motion of a radioaerosol droplet towards a reference source or as the droplet travelling less than half of the expected distance, when applying a nasal 99mTc-albumin colloid tracer to patients with PCD and healthy individuals [7]. In a study by Naclerio and colleagues [8], NMC using a 99mTc labelled tracer was defined as the time for the amount of the tracer sprayed into the nose to be reduced by half.
Discrimination between healthy subjects and patients with PCD was highly significant for the applied NMC velocity test in this study. By use of a precise quantitative measure of NMC velocity, given in mm·min−1, we expect the method to be able to detect even smaller intermediate changes in NMC velocity in the spectrum from 0.0 mm·min−1 to normal (healthy) NMC values, when going from absent clearance in PCD to hopefully some measurable nasal clearance, if ciliary function in people with PCD could be medically partially restored.
In the lungs of patients with PCD, long-term lung function evolution has been shown to vary considerably between groups of patients with PCD [20] and growing evidence indicates that patients with specific genetic defects may have more severe lung disease [21–23]. Whether the geno-phenotypically more severe patients with PCD are also more likely to have severely reduced pulmonary mucociliary clearance has yet to be investigated.
Geno-phenotypical variation in the nasal compartments of patients with PCD is well established, as nasal ciliary beat pattern and nasal ciliary frequency obtained by HSVM varies considerably across different PCD genotypes [3]. Nasal NO values, which are usually very low in patients with PCD, may correlate with ciliary function [24–26] which also varies in patients with PCD. Normal nasal NO levels have been described in some but not all patients with PCD who harbour gene defects related to normal TEM, and higher nasal NO has been described in some but not all patients with PCD where ciliary function is less compromised [25, 27]. Correlating to this, NMC velocity could also vary across different PCD genotypes and possibly also increase if ciliary motility improved due to therapeutic PCD protein correction.
However, in this pilot study we found NMC to be completely absent in all tested patients with PCD regardless of nasal NO values and PCD genotype. Genotypes associated with classic TEM outer dynein arm defects (DNAH5, DNAI1 and CCDC114) and genotypes associated with no detectable TEM defects (DNAH11 and HYDIN) or minor, and sometimes not visible, TEM defects (RSPH9) [28] were all represented in this study (table 1).
All patients with PCD in our study had abnormal ciliary beat pattern and sub-normal range nasal NO production rates below the recommended cut-off value of 77 nl·min−1 to discriminate patients with PCD from healthy subjects [19, 29].
Limitations to the study
The study was a pilot study and, as such, had a limited number of patients. Even though not all PCD genotypes were represented in the patient group, the distribution between PCD genotypes related to classic TEM defects and nonhallmark TEM defects [18] was approximately 50% to 50%, and NMC velocity was absent in all patients regardless of genotype. Despite the small numbers, we found a highly significant discrimination between people with PCD and healthy controls. Future larger NMC studies to further investigate age effect and genotype effect are warranted.
Subjects were exposed to a radioactive tracer (99mTc-albumin colloid) but the diminutive tracer droplet size of 2.5 μL resulted in a very low radiation dose of 25 μSv for a full NMC measurement, only a quarter of the radiation exposure from a chest radiograph (0.1 mSv) and equivalent to 3 days of background radiation in Denmark.
Head movements during the 10 min of acquisitions causing movement of the nasal tracer may lead to false negative readings of NMC velocity. Assessment of tragus reference movement as an indicator of head movement is therefore essential at each post-measurement reading. In this study, head movement requiring post-measurement correction only occurred in one subject. Post-measurement digital correction was easily performed by subtracting the distance of the tragus reference movement from the total distance of the nasal tracer movement.
Nasal infections can cause secondary ciliary dysmotility and result in abnormal nasal mucociliary transport as previously shown by De Boeck and colleagues [7]. This should be taken into consideration when timing the NMC velocity measurement, and NMC measurement should be postponed if the patient suffers from an acute upper respiratory infection.
In summary, we found NMC velocity to be a feasible, painless and rapid in vivo measurement of the mucociliary clearance in the nasal compartment of the upper airways in both children and adults. NMC velocity measurement only required 10 min testing, and the amount of radiation exposure was minimal which favours the use of NMC velocity for repeated measurements; for example, at fixed time points before and during a clinical trial.
This quantitative test for NMC velocity could be an important tool in future clinical trials for nasal medication specifically directed towards patients with PCD, either by using NMC as a forerunner for further studies on whole-lung mucociliary clearance by PRMC, or for combined studies testing both nasal and lung medication in patients with PCD by NMC and PRMC, respectively.
Conclusion
With this proof-of-concept study we found 99mTc-albumin colloid NMC velocity measurement to be a quantitative test that is highly feasible and highly discriminative between PCD and health, and with a very low radiation exposure. We had an excellent success rate of performing the NMC test in 100% of both the group of patients with PCD and the group of healthy controls. NMC velocity measurement could be a potential important outcome parameter to test nasally administered drugs in future clinical trials designed specifically for patients with PCD.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: The authors J.K. Marthin, K.G. Nielsen and J. Mortensen have no conflicts of interests to disclose.
References
- 1.Horani A, Ferkol TW. Understanding primary ciliary dyskinesia and other ciliopathies. J Pediatr 2021; 230: 15–22. doi: 10.1016/j.jpeds.2020.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucas JS, Davis SD, Omran H, et al. Primary ciliary dyskinesia in the genomics age. Lancet Respir Med 2020; 8: 202–216. doi: 10.1016/S2213-2600(19)30374-1 [DOI] [PubMed] [Google Scholar]
- 3.Wallmeier J, Nielsen KG, Kuehni CE, et al. Motile ciliopathies. Nat Rev Dis Primers 2020; 6: 77. doi: 10.1038/s41572-020-0209-6 [DOI] [PubMed] [Google Scholar]
- 4.Kobbernagel HE, Buchvald FF, Haarman EG, et al. Efficacy and safety of azithromycin maintenance therapy in primary ciliary dyskinesia (bestcilia): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir Med 2020; 8: 493–505. doi: 10.1016/S2213-2600(20)30058-8 [DOI] [PubMed] [Google Scholar]
- 5.Paff T, Daniels JM, Weersink EJ, et al. A randomised controlled trial on the effect of inhaled hypertonic saline on quality of life in primary ciliary dyskinesia. Eur Respir J 2017; 49: 1601770. doi: 10.1183/13993003.01770-2016 [DOI] [PubMed] [Google Scholar]
- 6.Paff T, Omran H, Nielsen KG, et al. Current and future treatments in primary ciliary dyskinesia. Int J Mol Sci 2021; 22: 9834. doi: 10.3390/ijms22189834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Boeck K, Proesmans M, Mortelmans L, et al. Mucociliary transport using 99mTc-albumin colloid: a reliable screening test for primary ciliary dyskinesia. Thorax 2005; 60: 414–417. doi: 10.1136/thx.2004.027680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naclerio RM, Baroody FM, Bidani N, et al. A comparison of nasal clearance after treatment of perennial allergic rhinitis with budesonide and mometasone. Otolaryngol Head Neck Surg 2003; 128: 220–227. doi: 10.1067/mhn.2003.70 [DOI] [PubMed] [Google Scholar]
- 9.Marthin JK, Mortensen J, Pressler T, et al. Pulmonary radioaerosol mucociliary clearance in diagnosis of primary ciliary dyskinesia. Chest 2007; 132: 966–976. doi: 10.1378/chest.06-2951 [DOI] [PubMed] [Google Scholar]
- 10.Munkholm M, Nielsen KG, Mortensen J. Clinical value of measurement of pulmonary radioaerosol mucociliary clearance in the work up of primary ciliary dyskinesia. EJNMMI Res 2015; 5: 118. doi: 10.1186/s13550-015-0118-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker WT, Young A, Bennett M, et al. Pulmonary radioaerosol mucociliary clearance in primary ciliary dyskinesia. Eur Respir J 2014; 44: 533–535. doi: 10.1183/09031936.00011814 [DOI] [PubMed] [Google Scholar]
- 12.Bennett WD, Wu J, Fuller F, et al. Duration of action of hypertonic saline on mucociliary clearance in the normal lung. J Appl Physiol (1985) 2015; 118: 1483–1490. doi: 10.1152/japplphysiol.00404.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donaldson SH, Laube BL, Corcoran TE, et al. Effect of ivacaftor on mucociliary clearance and clinical outcomes in cystic fibrosis patients with G551D-CFTR. JCI Insight 2018; 3: e122695. doi: 10.1172/jci.insight.122695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donaldson SH, Laube BL, Mogayzel P, et al. Effect of lumacaftor-ivacaftor on mucociliary clearance and clinical outcomes in cystic fibrosis: results from the prospect MCC sub-study. J Cyst Fibros 2022; 21: 143–145. doi: 10.1016/j.jcf.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoemark A, Dell S, Shapiro A, et al. ERS and ATS diagnostic guidelines for primary ciliary dyskinesia: similarities and differences in approach to diagnosis. Eur Respir J 2019; 54: 1901066. doi: 10.1183/13993003.01066-2019 [DOI] [PubMed] [Google Scholar]
- 16.Beydon N, Kouis P, Marthin JK, et al. Nasal nitric oxide measurement in children for the diagnosis of primary ciliary dyskinesia: european respiratory society technical standard. Eur Respir J 2023; 61: 2202031. doi: 10.1183/13993003.02031-2022 [DOI] [PubMed] [Google Scholar]
- 17.Kempeneers C, Seaton C, Garcia Espinosa B, et al. Ciliary functional analysis: beating a path towards standardization. Pediatr Pulmonol 2019; 54: 1627–1638. doi: 10.1002/ppul.24439 [DOI] [PubMed] [Google Scholar]
- 18.Shoemark A, Boon M, Brochhausen C, et al. International Consensus Guideline for reporting transmission electron microscopy results in the diagnosis of primary ciliary dyskinesia (Beat PCD TEM Criteria). Eur Respir J 2020; 55: 1900725. doi: 10.1183/13993003.00725-2019 [DOI] [PubMed] [Google Scholar]
- 19.Lucas JS, Barbato A, Collins SA, et al. European respiratory society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J 2017; 49: 1601090. doi: 10.1183/13993003.01090-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marthin JK, Petersen N, Skovgaard LT, et al. Lung function in patients with primary ciliary dyskinesia: a cross-sectional and 3-decade longitudinal study. Am J Respir Crit Care Med 2010; 181: 1262–1268. doi: 10.1164/rccm.200811-1731OC [DOI] [PubMed] [Google Scholar]
- 21.Davis SD, Ferkol TW, Rosenfeld M, et al. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am J Respir Crit Care Med 2015; 191: 316–324. doi: 10.1164/rccm.201409-1672OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis SD, Rosenfeld M, Lee HS, et al. Primary ciliary dyskinesia: longitudinal study of lung disease by ultrastructure defect and genotype. Am J Respir Crit Care Med 2019; 199: 190–198. doi: 10.1164/rccm.201803-0548OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pifferi M, Bush A, Mulé G, et al. Longitudinal lung volume changes by ultrastructure and genotype in primary ciliary dyskinesia. Ann Am Thorac Soc 2021; 18: 963–970. doi: 10.1513/AnnalsATS.202007-816OC [DOI] [PubMed] [Google Scholar]
- 24.Walker WT, Jackson CL, Lackie PM, et al. Nitric oxide in primary ciliary dyskinesia. Eur Respir J 2012; 40: 1024–1032. doi: 10.1183/09031936.00176111 [DOI] [PubMed] [Google Scholar]
- 25.Raidt J, Krenz H, Tebbe J, et al. Limitations of nasal nitric oxide measurement for diagnosis of primary ciliary dyskinesia with normal ultrastructure. Ann Am Thorac Soc 2022; 19: 1275–1284. doi: 10.1513/AnnalsATS.202106-728OC [DOI] [PubMed] [Google Scholar]
- 26.Lindberg S, Cervin A, Runer T. Low levels of nasal nitric oxide (NO) correlate to impaired mucociliary function in the upper airways. Acta Otolaryngol 1997; 117: 728–734. doi: 10.3109/00016489709113468 [DOI] [PubMed] [Google Scholar]
- 27.Legendre M, Thouvenin G, Taytard J, et al. High nasal nitric oxide, cilia analyses, and genotypes in a retrospective cohort of children with primary ciliary dyskinesia. Ann Am Thorac Soc 2022; 19: 1704–1712. doi: 10.1513/AnnalsATS.202110-1175OC [DOI] [PubMed] [Google Scholar]
- 28.Castleman VH, Romio L, Chodhari R, et al. Mutations in radial spoke head protein genes Rsph9 and Rsph4a cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am J Hum Genet 2009; 84: 197–209. doi: 10.1016/j.ajhg.2009.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro AJ, Dell SD, Gaston B, et al. Nasal nitric oxide measurement in primary ciliary dyskinesia. a technical paper on standardized testing protocols. Ann Am Thorac Soc 2020; 17: e1–e12. doi: 10.1513/AnnalsATS.201904-347OT [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.