ABSTRACT
BACKGROUND:
In more than 60 countries worldwide, laboratory testing plays a challenging and expensive role in trauma resuscitation. In 1995, the literature already suggested that routine laboratory testing may not be useful for most trauma patients. Our study hypothesized that still the need for some laboratory tests perhaps should be reconsidered. Therefore, the aim of this study was to create more insight in the distribution between normal and abnormal parameters for routine laboratory testing in trauma patient management.
METHODS:
This retrospective analysis was performed at Amsterdam UMC, location AMC, an academic level 1 trauma center. Data concerning age, gender, American Society of Anesthesiologists (ASA) physical state classification system (ASA), Injury Severity Scores, Glasgow Coma Scales, mechanism of injury, presence of high-energy trauma, and type of injury (blunt or penetrating) were obtained. Laboratory parameters included comprehensive hematology, coagulation, arterial blood gas, kidney, and liver blood panels. Analytical focus was paid to the patient’s vital status, the indication for an emergency intervention, and the risk of in-hospital mortality.
RESULTS:
A total of 1287 patients were included in the study. Patients with unstable vital signs or who required emergency intervention were most often dealing with abnormalities in pO2, glucose, D-dimer, creatinine, and alcohol values. Mean corpuscular volume (MCV), international normalized ratio (INR), fibrinogen, and amylase were obtained in more than 80% of the patients, but in specific patient groups only abnormal in less than 9%.
CONCLUSION:
Trauma patients suffer mainly from abnormal values of D-dimer, pO2, glucose, creatinine, and alcohol. By contrast, MCV, INR, amylase, fibrinogen, and thrombocytes are regularly obtained as well, but only abnormal in a small amount of trauma patients. These findings suggest reconsiderations and more accuracy in the performance of laboratory testing, especially for trauma patients with stable vital signs.
Keywords: Emergency care, routine laboratory testing, trauma patients, traumatic injury
INTRODUCTION
In more than 60 countries worldwide, laboratory testing plays a challenging and expensive role in trauma resuscitation.[1,2] Initial assessment protocols suggest routine laboratory testing to obtain enough information about the hemodynamic and respiratory condition of the patient, including the severity of occult blood loss and metabolic stability. These concern comprehensive blood sample analyses, including hematologic, arterial blood gas, coagulation, kidney, and liver panels.
The blood analyses are mainly performed based on the pre-existing evidence that traumatic injuries can generate biochemical responses that result in a modified metabolism of proteins, amino acids and other components of the human body.[3] These adverse pathophysiological changes can subsequently cause severe clinical deterioration, resulting in an emergency intervention or even mortality.[4,5] However, trauma patients are often treated with such urgency that the results of laboratory tests are commonly not available. Moreover, several results are in retrospect not even necessary and could, therefore, have been omitted in selected patients.[6–8]
In 1995, Tortella et al.[9] already suggested that routine laboratory testing may not be useful for most trauma patients. In the subsequent 20 years, additional studies investigated the (limited) utility of laboratory testing, but our study hypothesized that still the need for some laboratory tests perhaps should be reconsidered.[10–16] Therefore, the aim of this study was to create more insight in the distribution between normal and abnormal parameters for routine laboratory testing in trauma patient management.
MATERIALS AND METHODS
Study Design
This retrospective analysis was performed at (blinded manuscript), an academic level 1 trauma center. All patients presented at the trauma resuscitation room for 2 years were included in the study. The selected patients were identified from the regional trauma registry database. Patients under the age of 18 were excluded, as well as patients who were transferred to another hospital after trauma screening. Approval for this study was obtained from the Local Institutional Review Board (Medical Ethics Review Committee [blinded manuscript]).
Data Extraction
Data concerning age, gender, American Society of Anesthesiologists (ASA) physical state classification system (ASA), injury severity scores, Glasgow Coma Scores (GCSs), mechanism of injury, presence of high-energy trauma ,and type of injury (blunt or penetrating) were obtained.[17] Furthermore, differences between stable and unstable patients were analyzed. A stable patient (with normal hemodynamic- and respiratory status) was defined by the following criteria: Non-intubated, heart rate <100/min, systolic blood pressure >90 mmHg, respiratory rate >10 or <29/min, O2-saturation >94% (without supplemental oxygen), and GCS >13. In addition, patients that required an emergency intervention (within 6 hours after admission) were compared with patients that did not need any interventions or that could await an elective operation. Emergency interventions included, for example, thoracotomy, laparotomy, external fixation, open reduction internal fixation, coiling, spondylodesis, and neurosurgical interventions. Finally, the risk of in-hospital mortality was analyzed.
Laboratory Analysis
The primary blood tests obtained at the trauma room were used for this analysis. The obtained laboratory results included:
Hematology panel: Hemoglobin, hematocrit, mean corpuscular volume (MCV), leukocytes, and thrombocytes
Arterial blood gas panel: Potential of hydrogen (pH), partial pressure of carbon dioxide (pCO2), bicarbonate, base excess (BE), partial pressure of O2 (pO2), and blood oxygen saturation (O2-saturation)
Coagulation panel: International normalized ratio (INR), prothrombin time (PT), aPTT, fibrinogen, and D-dimer
Kidney panel: Sodium, potassium, urea, and creatinine
Liver panel: Aspartate transaminase, alanine transaminase, lactate, and amylase
Others: Creatine kinase-mb (CKMB), glucose, and alcohol.
Statistical Analysis
Demographic characteristics were determined, and cross tabs were used to identify, in which percentage the laboratory values deviate from the reference values in the different patient groups (stable/unstable, emergency intervention/no emergency intervention, and in-hospital mortality/survival). Since this was a retrospective study, no sample size analysis was performed. Statistical analyses were performed using IBM SPSS Statistics version 26.0.
RESULTS
A total of 1287 patients were included in the study. The study group consisted predominantly of men (66.0%), with a mean age of 46 years old. More than 80% of the patients were in good health condition or had a mild systemic disease before the injury occurred (ASA I-II). Less than a quarter of the patients (n=302) had to undergo an operation, with 140 patients (10.9%) operated in <6 hours after trauma (emergency intervention). Additional patient characteristics are shown in Table 1. Details about the laboratory parameters (including reference values) are shown in Appendix 1.
Table 1.
Patient characteristics
| Patient characteristics | Total population (n=1287) |
|---|---|
| Age, mean (SD; range) | 46 (18.9; 18–104) |
| Gender, male, n (%) | 850 (66.0) |
| ASA I-II, n (%)a | 1,038 (80.6) |
| ASA III-IV, n (%)a | 175 (13.6) |
| ISS, median (interquartile range) | 5 (1–16) |
| GCS in-hospital ≤13, n (%) | 218 (16.9) |
| Type of injury, blunt, n (%) | 1230 (95.6) |
| Mechanism of injury | |
| Falling accident, n (%) | 365 (28.4) |
| Motor vehicle collision, n (%) | 740 (57.5) |
| Stabbing or shooting accident, n (%) | 49 (3.8) |
| Other, n (%) | 133 (10.3) |
| High Energy Trauma (HET), n (%) | 917 (71.3) |
| Stable based on ATLS, n (%) | 829 (64.4) |
| Blood transfusion at the ED, n (%) | 10 (0.8) |
| Operating room, n (%) | 302 (23.5) |
| Operation within 6 hours after | 140 (10.9) |
| admission, n (%) | |
| Mortality at the ED, n (%) | 9 (0.7) |
| Overall mortality, n (%) | 66 (5.1) |
Sum is not equal to total population, due to missing ASA scores (n=74). SD: Standard deviation; ASA: ASA physical state classification system; ISS: Injury Severity Scores; GCS: Glasgow Coma Scales; ATLS: Advanced trauma life support; ED: Emergency department.
Patient’s Vital Status
As shown in Figure 1, patients who were stable at arrival had mainly abnormalities in respectively pO2, D-dimer, glucose, creatinine, and alcohol. Patients with unstable vital signs were also often dealing with abnormal pO2, D-dimer, glucose, and creatinine values. By contrast, especially in patients with stable vital signs, MCV, INR, fibrinogen, and amylase were obtained in more than 700 patients, but only abnormal in <7% of these patients (n≤52). The greatest variances in abnormal values between stable and unstable patients were seen in hemoglobin, base excess, potassium, and liver enzyme values.
Figure 1.
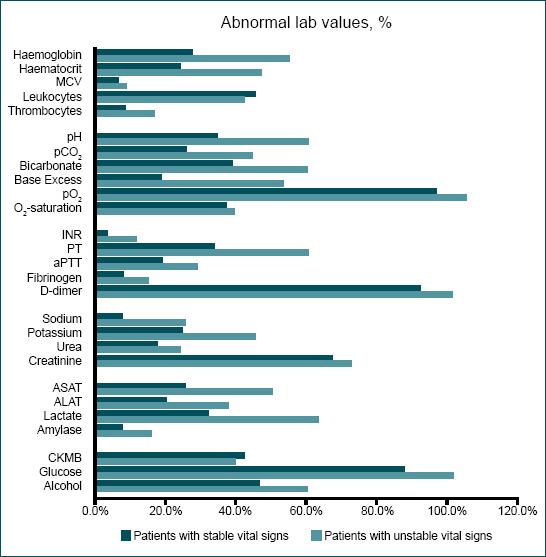
Patients with stable and unstable vital signs based on the ATLS.
Emergency Intervention
Figure 2 shows an overview of patients who underwent an intervention within 6 hours after admission. Here as well, major variation was seen in the amount of abnormal hemoglobin, potassium, and liver enzyme values between the two compared patient groups. For patients who required emergency interventions, pO2, D-dimer, glucose, creatinine, and alcohol values were most often abnormal. Patients that did not need any interventions or that could await an elective operation were undergoing extensive laboratory tests (≥75%) including INR, MCV, fibrinogen, amylase, and thrombocyte determination. However, these values were abnormal in <8% of the total population.
Figure 2.

Patients with an emergency intervention within 6 hours after admission to the hospital stable and patients that did not need any intervention or could await an elective operation.
Mortality
Of the deceased patients, all the obtained D-dimer samples were abnormal (n=41) (Fig. 3). Glucose, pO2, pH, and lactate were also deteriorated in the majority of these patients, but MCV, amylase, CKMB, O2-saturation, and fibrinogen values were scarcely changed. Furthermore, patients who survived their injury did likewise barely suffer from abnormal MCV, INR, fibrinogen, amylase, and thrombocyte values. Nevertheless, these laboratory parameters were sampled in more than 80% of these patients (n≥1052).
Figure 3.

Patients who died within the hospital and patients who survived the trauma.
DISCUSSION
This retrospective analysis, including 1287 patients who were admitted to a level 1 trauma center, shows that trauma patients suffered mainly from abnormalities in pO2, glucose, D-dimer, creatinine, and alcohol values. By contrast, values regarding MCV, INR, amylase, fibrinogen, and thrombocytes were extensively obtained, but remained often normal. These results are broadly in line with the previous literature, suggesting more accurate considerations regarding laboratory testing than currently is taken.[9–14] Thus, the outcomes might raise concerns, because the previous studies date from almost a decade ago, implying that only minimal improvement in diagnostic accuracy have occurred in the past years.
Furthermore, the major differences in laboratory values between deceased patients and survivors are prominent (Fig. 3). Liver enzymes are almost twice as often abnormal in patients who died, as well as hemoglobin, thrombocyte, and blood gas values, pointing out the necessity to stabilize these values in patients with traumatic injuries. Similar results can be seen between the other compared patient groups, namely, “stable versus unstable patients” and “the need for emergency interventions within 6 hours after admission versus no need for emergency interventions” (Figs. 1 and 2). However, several important points should be taken into account prior to any conclusions being reached.
First, it is remarkable that more than 57% of the patients suffered from abnormal glucose values, but this can be explained by the hyperglycemic-induced stress response after traumatic injuries.[18–20] Besides, it is important to notice that, despite the large quantities of abnormal laboratory values, its effect on treatment strategy or trauma patient management is not clear yet.
At the other end of the spectrum, laboratory values including INR, MCV, fibrinogen, amylase, and thrombocytes were very often determined, but almost never abnormal. Evidently, MCV values are often not affected since trauma patients suffer often from normocytic anemia due to acute blood loss. Apart from that, several previous studies found that abnormalities in amylase levels do not have substantial meaning in traumatic injuries, since it is not accurate enough to identify nor exclude injuries of the pancreas.[21,22] We are aware of the challenge that laboratory requests entail in urgent cases. Nonetheless, these findings encourage reconsideration for the implementation of laboratory testing, especially since most of the blood results are not directly available.
In addition, major hemodynamic instabilities can be detected faster and more accurately through other diagnostic modalities. For most of the patients with suspected intra-abdominal injuries, low-threshold use of Focused Assessment with Sonography in Trauma, computed tomography (CT), and CT angiography is implemented. These diagnostic imaging techniques can be used to visualize, for example, pancreatic injuries and splenic ruptures, together with other intra-abdominal hemorrhages.[23] The increased utilization of these innovative techniques might enable the omission of some laboratory values. Nonetheless, one should not misinterpret that laboratory evaluation should be completely avoided, since selected blood parameters are still crucial for certain patient groups. The blood analyses can also be used for the determination of clinical deterioration, for example, the level of shock and coagulopathy. However, patient characteristics for these specific patient groups are thus far not defined.
Another point of consideration includes the fact that INR is determined in most of the patients, but only abnormal in a small proportion. Nevertheless, it is important to notice that still more than 32% of the patients who died, had abnormal INR values. We were limited in analyzing anticoagulant use, like we are in the emergency setting, but we could hypothesize increasing importance for patients with these medicines. The differences in number between INR and PT values can be explained by the variety in coagulometers.[24,25]
Limitations
A limitation of this study includes the fact that the data are obtained within an academic medical center, where – next to medical professionals – residents play a big role in the care for the injured patients. With the variability in clinical experience, differences in the initial assessment might create inequality in laboratory approach. Furthermore, we were unable to determine the effect on clinical decision making and trauma patient management. In addition, we acknowledge that the retrospective aspect of this study, the relatively outdated data, and the lack of long-term follow-up restricts our level of evidence. As far as we know, this study includes the most recent available literature regarding this subject, but for more accurate results, multivariate analyses with additional factors and comparison groups are preferred.
Conclusion
We can state that laboratory values are often obtained for trauma patients, but its effect on treatment or trauma patient management is not clear yet. These patients suffer mainly from abnormal values of D-dimer, pO2, glucose, creatinine, and alcohol. By contrast, MCV, INR, amylase, fibrinogen, and thrombocytes are regularly obtained as well, but only abnormal in a small amount of trauma patients. These findings suggest reconsiderations and more accuracy in the performance of laboratory testing than currently is performed. Especially for trauma patients with stable vital signs, a more selective approach toward laboratory analyses should be considered. However, further research with more specific patient groups is recommended.
Appendix 1.
Laboratory reference values with the total number of obtained and abnormal values
| Reference value | Obtained, n (%) | Abnormal values, n (%) | |
|---|---|---|---|
| Haematology | |||
| Haemoglobin | 8.5–10.5 mmol/L* | 1.249 (97.0) | 389 (30.2) |
| Haematocrit | 0.40–0.50 L/L* | 1.184 (92.0) | 320 (24.9) |
| MCV | 80–100 fL | 1.185 (92.1) | 75 (5.8) |
| Leukocytes | 8.5–10.5 109/L* | 1.190 (92.5) | 440 (34.2) |
| Thrombocytes | 150–400 109/L | 1.198 (93.1) | 117 (9.1) |
| Arterial blood gas | |||
| pH | 7.35–7.45 | 972 (75.5) | 362 (28.1) |
| pCO2 | 4.4–6.3 kPa | 978 (76.0) | 272 (21.1) |
| Bicarbonate | 23–29 mmol/L | 978 (76.0) | 385 (29.9) |
| Base Excess | -3.0–+3.0 mmol/L | 985 (76.5) | 266 (20.7) |
| pO2 | 10.0–13.3 kPa | 976 (75.8) | 812 (63.1) |
| O2-saturation | >95% | 968 (75.2) | 308 (23.9) |
| Coagulation | |||
| INR | <1.3 | 1.132 (88.0) | 64 (5.0) |
| PT | 9.7–11.6 sec | 1.154 (89.7) | 420 (32.6) |
| aPTT | 22–30 sec | 1.148 (89.2) | 219 (17.0) |
| Fibrinogen | 1.5–4.0 g/L | 1.094 (85.0) | 98 (7.6) |
| D-dimeer | <0.5 mg/L | 1.084 (84.2) | 862 (67.0) |
| Kidney panel | |||
| Sodium | 135–145 mmol/L | 1.030 (80.0) | 129 (10.0) |
| Potassium | 3.5–4.5 mmol/L | 1.031 (80.1) | 283 (22.0) |
| Urea | 2.1–7.1 mmol/L | 1.199 (93.2) | 203 (15.8) |
| Creatinine | 75–110 µmol/L* | 1.238 (96.2) | 91 (7.1) |
| Liver panel | |||
| ASAT | <40 U/L | 1.031 (80.1) | 296 (23.0) |
| ALAT | <45 U/L* | 1.199 (93.2) | 267 (20.7) |
| Lactate | 0.4–2.0 mmol/L | 934 (72.6) | 342 (26.6) |
| Amylase | <220 U/L | 1.193 (92.7) | 108 (8.4) |
| Others | |||
| CKMB | <5.2 µg/L | 197 (15.3) | 130 (10.1) |
| Glucose | 4.1–5.6 mmol/L | 956 (74.3) | 740 (57.5) |
| Alcohol | >0.1 ‰ | 1.170 (90.9) | 501 (38.9) |
Female reference: Hemoglobin 7.5–10 mmol/L; Hematocrit 0.35–0.45 L/L; Leukocytes 7.5–10.0 mmol/L; Creatinine 65–95 µmol/L; ALAT <34 U/L. MCV: Mean corpuscular volume; pH: Potential of hydrogen; pO2: Partial pressure of O2; O2-saturation: Blood oxygen saturation; INR: International normalized ratio; PT: Prothrombin time; aPTT: Activated partial thromboplastin time; ASAT: Aspartate transaminase; ALAT: Alanine transaminase; CKMB: Creatine kinase-mb.
Footnotes
Ethics Committee Approval: The study design was not within the scope of the Act on Medical Research with People (WMO) and therefore, ethical approval was waived by the Local Institutional Review Board (Medical Ethics Reviews Committee (blinded manuscript), reference number W14_185 # 14.17.0230). This article does not contain any studies with animals performed by any of the authors.
Peer-review: Internally peer-reviewed.
Authorship Contributions: Concept: Z.P., P.V.S., J.H.; Design: Z.P., T.S., P.V.S., G.G., J.H.; Supervision: T.S., G.G., J.H.; Data: Z.P., P.V.S.; Analysis: Z.P., T.S., P.V.S., G.G., J.H.; Literature search: Z.P., T.S., P.V.S.; Writing: Z.P., T.S., P.V.S., G.G., J.H.; Critical revision: T.S., P.V.S., G.G, J.H.
Conflict of Interest: None declared.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Chandler WL, Ferrell C, Trimble S, Moody S. Development of a rapid emergency hemorrhage panel. Transfusion. 2010;50:2547–52. doi: 10.1111/j.1537-2995.2010.02753.x. [DOI] [PubMed] [Google Scholar]
- 2.Callum JL, Rizoli S, Pendergrast J. Rapid laboratory testing for trauma patients:Where a perfect result may not be in the best interests of the patient. Transfusion. 2010;50:2529–32. doi: 10.1111/j.1537-2995.2010.02934.x. [DOI] [PubMed] [Google Scholar]
- 3.Hill AG, Hill GL. Metabolic response to severe injury. Br J Surg. 1998;85:884–90. doi: 10.1046/j.1365-2168.1998.00779.x. [DOI] [PubMed] [Google Scholar]
- 4.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure:A bimodal phenomenon. J Trauma Inj Infect Crit Care. 1996;40:501–10. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Nast-Kolb D, Waydhas C, Gippner-Steppert C, Schneider I, Trupka A, Ruchholtz S, et al. Indicators of the posttraumatic inflammatory response correlate with organ failure in patients with multiple injuries. J Trauma Inj Infect Crit Care. 1997;42:446–54. doi: 10.1097/00005373-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Trisorio Liuzzi MP, Attolini E, Quaranta R, Paparella D, Cappabianca G, Di Serio F, et al. Laboratory request appropriateness in emergency:Impact on hospital organization. Clin Chem Lab Med. 2006;44:760–4. doi: 10.1515/CCLM.2006.131. [DOI] [PubMed] [Google Scholar]
- 7.Chu UB, Clevenger FW, Imami ER, Lampard SD, Frykberg ER, Tepas JJ. The impact of selective laboratory evaluation on utilization of laboratory resources and patient care in a level-I trauma center. Am J Surg. 1996;172:558–62. doi: 10.1016/S0002-9610(96)00234-6. [DOI] [PubMed] [Google Scholar]
- 8.Alonso-Cerezo MC, Martín JS, Montes MA, De La Iglesia VM. Appropriate utilization of clinical laboratory tests. Clin Chem Lab Med. 2009;47:1461–5. doi: 10.1515/CCLM.2009.335. [DOI] [PubMed] [Google Scholar]
- 9.Tortella BJ, Lavery RF, Rekant M. Utility of routine admission serum chemistry panels in adult trauma patients. Acad Emerg Med. 1995;2:190–4. doi: 10.1111/j.1553-2712.1995.tb03194.x. [DOI] [PubMed] [Google Scholar]
- 10.Namias N, McKenney MG, Martin LC. Utility of admission chemistry and coagulation profiles in trauma patients:A reappraisal of traditional practice. J Trauma Inj Infect Crit Care. 1996;41:21–5. doi: 10.1097/00005373-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Tasse JL, Janzen ML, Ahmed NA, Chung RS. Screening laboratory and radiology panels for trauma patients have low utility and are not cost effective. J Trauma Inj Infect Crit Care. 2008;65:1114–6. doi: 10.1097/TA.0b013e318184b4f2. [DOI] [PubMed] [Google Scholar]
- 12.Duggan S, Tillotson L, McCann P. Routine laboratory tests in adult trauma:Are they necessary? Bull R Coll Surg Engl. 2011;93:266–72. [Google Scholar]
- 13.Köksal Ö, Eren Çevik Ş, Akköse Aydin Ş, Özdemir F. Acil servise başvuran travma hastalarinda rutin testlerin gerekliliğinin analizi. Ulus Travma ve Acil Cerrahi Derg. 2012;18:23–30. doi: 10.5505/tjtes.2011.84748. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs IA, Kelly K, Valenziano C, Chevinsky AH, Pawar J, Jones C. Cost savings associated with changes in routine laboratory tests ordered for victims of trauma. Am Surg. 2000;66:579–84. [PubMed] [Google Scholar]
- 15.Munro J, Booth A, Nicholl J. Routine preoperative testing:A systematic review of the evidence. Health Technol Assess. 1997;1:1–62. [PubMed] [Google Scholar]
- 16.Velanovich V. The value of routine preoperative laboratory testing in predicting postoperative complications:A multivariate analysis. Surgery. 1991;109:236–43. [PubMed] [Google Scholar]
- 17.Ringdal KG, Skaga NO, Steen PA, Hestnes M, Laake P, Jones JM, et al. Classification of comorbidity in trauma:The reliability of pre-injury ASA physical status classification. Injury. 2013;44:29–35. doi: 10.1016/j.injury.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Kopelman TR, O'Neill PJ, Kanneganti SR, Davis KM, Drachman DA. The relationship of plasma glucose and glycosylated hemoglobin A1C levels among nondiabetic trauma patients. J Trauma Inj Infect Crit Care. 2008;64:30–3. doi: 10.1097/TA.0b013e318161b0ab. [DOI] [PubMed] [Google Scholar]
- 19.Kerby JD, Griffin RL, MacLennan P, Rue LW. Stress-induced hyperglycemia, not diabetic hyperglycemia, is associated with higher mortality in trauma. Ann Surg. 2012;14:1340. doi: 10.1097/SLA.0b013e3182654549. [DOI] [PubMed] [Google Scholar]
- 20.Sung J, Bochicchio GV, Joshi M, Bochicchio K, Tracy K, Scalea TM. Admission hyperglycemia is predictive if outcome in critically ill trauma patients. J Trauma. 2005;59:80–3. doi: 10.1097/01.ta.0000171452.96585.84. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M, Maemura K, Sawada Y, Yoshioka T, Sugimoto T. Hyperamylasemia in critically injured patients. J Trauma. 1980;20:951–5. doi: 10.1097/00005373-198011000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Olsen WR. The serum amylase in blunt abdominal trauma. J Trauma. 1973;13:200–4. doi: 10.1097/00005373-197303000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Vela JH, Wertz CI, Onstott KL, Wertz JR. Trauma imaging:A literature review. Radiol Technol. 2017;88:263–76. [PubMed] [Google Scholar]
- 24.Poller L, Ibrahim S, Jespersen J, Pattison A. Coagulometer international sensitivity index (ISI) derivation, a rapid method using the prothrombin time/international normalized ratio (PT/INR) Line:A multicenter study. J Thromb Haemost. 2012;10:1379–84. doi: 10.1111/j.1538-7836.2012.04751.x. [DOI] [PubMed] [Google Scholar]
- 25.D'Angelo A, Seveso MP, D'Angelo SV, Gilardoni F, Macagni A, Manotti C, et al. Comparison of two automated coagulometers and the manual tilt-tube method for the determination of prothrombin time. Am J Clin Pathol. 1989;92:321–8. doi: 10.1093/ajcp/92.3.321. [DOI] [PubMed] [Google Scholar]


