Key Points
Question
Among patients with uncontrolled hypertension, does the aldosterone synthase inhibitor lorundrostat safely lower blood pressure?
Findings
In this randomized clinical trial that included 200 participants, lorundrostat decreased blood pressure significantly more than placebo with 50-mg and 100-mg once-daily doses, and adverse events, including hyperkalemia, were uncommon.
Meaning
Aldosterone synthase inhibition with lorundrostat showed potential for blood pressure lowering in patients with hypertension that was inadequately controlled despite background antihypertensive treatment.
Abstract
Importance
Excess aldosterone production contributes to hypertension in both classical hyperaldosteronism and obesity-associated hypertension. Therapies that reduce aldosterone synthesis may lower blood pressure.
Objective
To compare the safety and efficacy of lorundrostat, an aldosterone synthase inhibitor, with placebo, and characterize dose-dependent safety and efficacy to inform dose selection in future trials.
Design, Setting, and Participants
Randomized, placebo-controlled, dose-ranging trial among adults with uncontrolled hypertension taking 2 or more antihypertensive medications. An initial cohort of 163 participants with suppressed plasma renin (plasma renin activity [PRA] ≤1.0 ng/mL/h) and elevated plasma aldosterone (≥1.0 ng/dL) were enrolled, with subsequent enrollment of 37 participants with PRA greater than 1.0 ng/mL/h.
Interventions
Participants were randomized to placebo or 1 of 5 dosages of lorundrostat in the initial cohort (12.5 mg, 50 mg, or 100 mg once daily or 12.5 mg or 25 mg twice daily). In the second cohort, participants were randomized in a 1:6 ratio to placebo or lorundrostat, 100 mg once daily.
Main Outcomes and Measures
The primary end point was change in automated office systolic blood pressure from baseline to study week 8.
Results
Between July 2021 and June 2022, 200 participants were randomized, with final follow-up in September 2022. Following 8 weeks of treatment in participants with suppressed PRA, changes in office systolic blood pressure of −14.1, −13.2, −6.9, and −4.1 mm Hg were observed with 100 mg, 50 mg, and 12.5 mg once daily of lorundrostat and placebo, respectively. Observed reductions in systolic blood pressure in individuals receiving twice-daily doses of 25 mg and 12.5 mg of lorundrostat were −10.1 and −13.8 mm Hg, respectively. The least-squares mean difference between placebo and treatment in systolic blood pressure was −9.6 mm Hg (90% CI, −15.8 to −3.4 mm Hg; P = .01) for the 50-mg once-daily dose and −7.8 mm Hg (90% CI, −14.1 to −1.5 mm Hg; P = .04) for 100 mg daily. Among participants without suppressed PRA, 100 mg once daily of lorundrostat decreased systolic blood pressure by 11.4 mm Hg (SD, 2.5 mm Hg), which was similar to blood pressure reduction among participants with suppressed PRA receiving the same dose. Six participants had increases in serum potassium above 6.0 mmol/L that corrected with dose reduction or drug discontinuation. No instances of cortisol insufficiency occurred.
Conclusions and Relevance
Among individuals with uncontrolled hypertension, use of lorundrostat was effective at lowering blood pressure compared with placebo, which will require further confirmatory studies.
Trial Registration
ClinicalTrials.gov Identifier: NCT05001945
This randomized clinical trial assesses the effect of 5 dosages of lorundrostat treatment vs placebo on 8-week systolic blood pressure change among adults with uncontrolled hypertension.
Introduction
Hypertension is the leading contributor to cardiovascular morbidity and mortality worldwide, and the majority of individuals in the United States with hypertension have inadequately controlled blood pressure (BP).1,2,3 An increased global prevalence of hypertension has paralleled increasing rates of obesity.4 Excess aldosterone production contributes to uncontrolled BP in patients with obesity and other associated diseases, such as obstructive sleep apnea and metabolic syndrome.5,6,7,8,9,10,11
Contemporary guidance for treatment of resistant hypertension advocates for use of mineralocorticoid receptor antagonists (MRAs) following treatment with a 3-drug regimen of a thiazide-type diuretic, a dihydropyridine calcium channel blocker, and an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker.12 However, antiandrogenic- and progestogenic-related adverse effects are common barriers to wider MRA use, particularly use of spironolactone. Additionally, MRAs do not block nongenomic effects of aldosterone, which may result in increased sympathetic activation, negatively affect glucose homeostasis, and stimulate vascular contractility and stiffness.13,14
Decreasing aldosterone production via inhibition of aldosterone synthase, rather than blocking the mineralocorticoid receptor, may avoid these adverse effects. Lorundrostat is a selective inhibitor of aldosterone synthase currently under investigation. The Trial on the Safety and Efficacy of MLS-101 (Lorundrostat) in Patients With Uncontrolled Hypertension (Target-HTN) was designed to examine the safety and efficacy of lorundrostat at 5 different doses in participants with uncontrolled hypertension, with a specific aim of BP reduction among participants with obesity or suppressed renin.
Methods
Study Design, Organization, and Oversight
Target-HTN was a multicenter, prospective, randomized, placebo-controlled, dose-ranging clinical trial performed at 43 sites within the United States. The trial protocol was designed by Mineralys Therapeutics. The institutional review boards at the clinical trial sites approved the study, and all participants provided written informed consent. An independent data monitoring committee oversaw patient safety. Cytel performed the statistical analysis, and the Cleveland Clinic Coordinating Center for Clinical Research (C5Research) independently confirmed the primary end-point analysis. The first author (L.J.L.) wrote the first version of the manuscript and made revisions following input from coauthors and the sponsor.
Study Population
Patients were eligible for trial enrollment if they were older than 18 years with a systolic automated office BP (AOBP) of 130 mm Hg or greater while taking 2 or more antihypertensive medications for at least 4 weeks at maximally tolerated doses. Cohort 1 of the study enrolled participants with suppressed plasma renin (plasma renin activity [PRA] ≤1.0 ng/mL/h) and serum aldosterone level of 1.0 ng/dL or greater based on morning measurements, while cohort 2 enrolled participants with PRA of greater than 1.0 ng/mL/h. Key exclusion criteria included orthostatic hypotension, type 1 diabetes, concomitant use of an epithelial sodium channel inhibitor or MRA, estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2, morning serum cortisol level of less than 3 µg/dL, serum sodium level of less than 135 mmol/L, or hemoglobin A1c of 9% or greater. A systolic AOBP of 175 mm Hg or greater, diastolic BP of 100 mm Hg or greater, or serum potassium level greater than 5.2 mmol/L was exclusionary in cohort 1, whereas exclusion criteria in cohort 2 were a systolic AOBP greater than 160 mm Hg, diastolic BP greater than 100 mm Hg, or serum potassium level greater than 4.8 mmol/L. Full inclusion and exclusion criteria are provided in the trial protocol (Supplement 1).
Study Procedures
Participants underwent 2 to 4 weeks of prescreening, a 2-week placebo run-in period to ensure eligibility, and then randomization and treatment for 8 weeks. A final visit was conducted 2 to 4 weeks following completion of the double-blind treatment (eFigure 1 in Supplement 2). Cohort 1 participants were randomly assigned in a 1:1:1:1:1:1 ratio to placebo or 1 of 5 lorundrostat doses (12.5 mg, 50 mg, or 100 mg once daily or 12.5 mg or 25 mg twice daily). An independent data and safety monitoring board performed an interim analysis in January 2022, and randomization to the 2 lowest doses of lorundrostat (12.5 mg once daily and 12.5 mg twice daily) was discontinued due to lack of consistent meaningful reduction of BP. A second cohort randomized participants to placebo or 100 mg once daily of lorundrostat in a 1:6 ratio. An additional interim analysis was performed following the last participant visit from cohort 1 and full enrollment of cohort 2 to guide dose selection in future studies. Following randomization, all participants’ study visits were conducted at weeks 1 through 8. Efficacy assessment with AOBP (average of the last 2 of 5 unattended measurements using an automated oscillometric sphygmomanometer device after approximately 5 minutes of rest in a seated position) was measured weekly throughout the study. Twenty-four-hour ambulatory BP monitoring was measured at baseline and once again prior to the 8-week visit.
Study End Points
The primary efficacy end point was change in systolic AOBP from baseline to the end of study week 8. Secondary efficacy end points included changes in diastolic AOBP, 24-hour ambulatory BP monitoring changes, and the proportion of participants achieving an AOBP less than 130/80 mm Hg at week 7 or 8. Additional safety and pharmacodynamic biomarker end points included changes in PRA, serum aldosterone, cortisol, potassium, and eGFR during the study. Prespecified adverse events of special interest were incidence of hyperkalemia with dose modification, hypotension with symptoms, and adrenal insufficiency. Adverse events were deemed treatment related if, in the opinion of the site investigator, there was a reasonable possibility that the event may have been caused by the study drug.
Sample Size Calculation and Power
The study was designed to provide information regarding a safe and effective dose of lorundrostat for subsequent efficacy trials. The sample size was based on the point and interval estimation of the difference in means between each dose group and placebo in change from baseline at week 8 in systolic AOBP. A sample size of 30 participants per group was estimated to provide the half-width of the 2-sided 90% CI of 3.8 mm Hg with a common standard deviation assumed to be 9 mm Hg. This was considered to have adequate precision to guide the evaluation of systolic BP changes across different dose levels for selection of doses and regimens to be investigated in future studies. All analyses of cohort 2 of the study were exploratory in nature, and no formal sample size considerations were undertaken for this cohort.
Statistical Analysis
Analyses were based on the full analysis set, defined as all randomized participants who received at least 1 dose of study medication. A safety analysis population was defined as all participants who took at least 1 dose of study drug. Continuous variables were summarized by means and standard deviations for normally distributed data. Baseline characteristics, medical history, medications, and laboratory measurements were summarized by study groups.
The primary efficacy analysis was performed using a mixed-model repeated-measures approach with fixed effects of categorical terms for treatment, week, and treatment by week interaction, and baseline systolic AOBP as a fixed continuous covariate. In this model, the participant is considered as a random effect, where an unstructured variance/covariance matrix is assumed for describing the correlation of repeated measurements over time. Analysis of the primary end point was repeated in each prespecified subgroup, and these analyses were considered exploratory. The statistical analysis plan outlined intercurrent events (ICE) that would result in imputation of week 8 BP. Specifically, if rescue BP medications were initiated, the observed systolic BP at time of rescue medication initiation was imputed. Additionally, if participant withdrawal occurred before completion of the 8-week period for a treatment-related reason or worsening, the baseline systolic BP value was imputed as the week 8 measurement. All secondary efficacy end-point analyses are considered supportive, and any inferential statistics are considered descriptive in nature. No adjustment for multiplicity was performed due to the phase 2 exploratory intent of the trial. All analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc). Additional analytic methods are described in the statistical analysis plan (Supplement 3).
Results
Study Participants
From July 2021 through June 2022, a total of 380 patients were screened (n = 307 in cohort 1; n = 73 in cohort 2) and 200 participants underwent randomization (n = 163 in cohort 1; n = 37 in cohort 2). The flow of trial participants is shown in Figure 1. Trial demographic and clinical characteristics are summarized in Table 1. Among randomized participants, the mean age was 65.7 (SD, 10.2) years, 60% were women, 36% were Black or African American, and 48% were of Hispanic or Latino ethnicity. At the time of randomization, 48% of participants had a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of greater than 30 and 40% had type 2 diabetes. Three or more antihypertensive medications were taken by 42% of the study population. Cohort 1 participants had a mean baseline systolic AOBP of 142.2 (SD, 12.5) mm Hg and a mean diastolic AOBP of 81.5 (SD, 9.7) mm Hg, whereas cohort 2 participants had a mean baseline systolic BP of 139.1 (SD, 8.7) mm Hg and diastolic BP of 79.1 (SD, 9.7) mm Hg.
Figure 1. Flow of Participants Through Target-HTN.
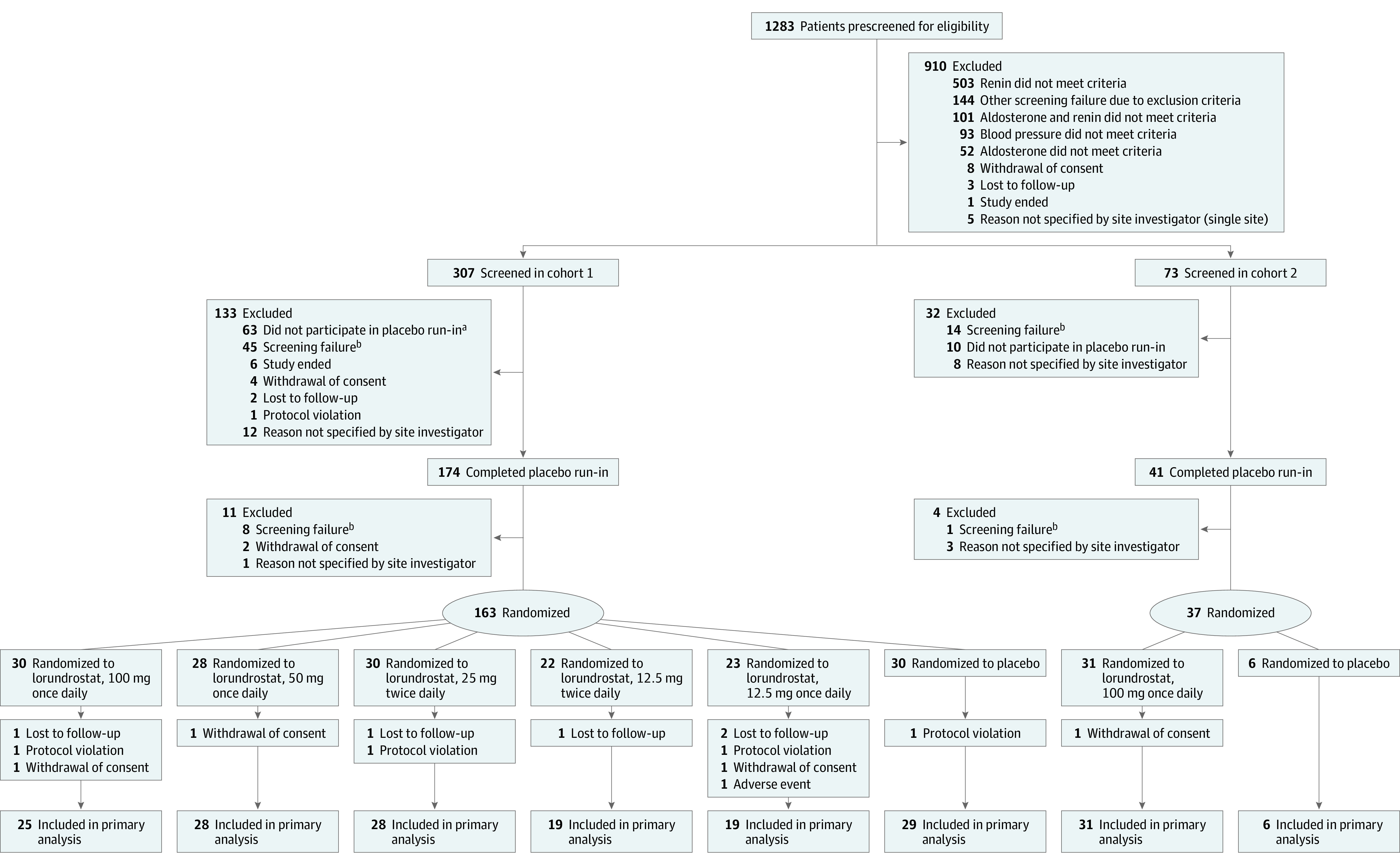
Cohort 1: plasma renin activity (PRA) ≤1.0 ng/mL/h; cohort 2: PRA >1.0 ng/mL/h. See Statistical Analysis for definitions of intercurrent event types and Results for a summary of participants in each group with each type.
aSeven participants were inadvertently screened following prescreening failure and did not participate in run-in.
bOther reasons included not meeting blood pressure criteria at screening visit; not meeting laboratory entry criteria, such as patients with hyperkalemia, decreased estimated glomerular filtration rate, or low serum cortisol; exclusionary medical conditions; or current use of exclusionary medications.
Table 1. Demographics and Baseline Participant Characteristics.
| Characteristics | Cohort 1 (PRA ≤1.0 ng/mL/h) | Exploratory cohort 2 (PRA >1.0 ng/mL/h) | ||||||
|---|---|---|---|---|---|---|---|---|
| Lorundrostat | Placebo (n = 30) | Lorundrostat, 100 mg once daily (n = 31) | Placebo (n = 6) | |||||
| 100 mg once daily (n = 30) | 50 mg once daily (n = 28) | 25 mg twice daily (n = 30) | 12.5 mg twice daily (n = 22)a | 12.5 mg once daily (n = 23)a | ||||
| Age, mean (SD), y | 68.7 (8.9) | 64.7 (9.5) | 64.8 (9.7) | 68.1 (10.1) | 65.2 (11.3) | 62.6 (10.7) | 66.6 (10.6) | 62.7 (12.3) |
| Sex, No. (%) | ||||||||
| Female | 18 (60.0) | 15 (53.6) | 19 (63.3) | 14 (63.6) | 12 (52.2) | 17 (56.7) | 21 (67.7) | 4 (66.7) |
| Male | 12 (40.0) | 13 (46.4) | 11 (36.7) | 8 (36.4) | 11 (47.8) | 13 (43.3) | 10 (32.3) | 2 (33.3) |
| Race, No. (%)b | ||||||||
| Asian | 1 (3.3) | 0 | 0 | 0 | 1 (4.3) | 1 (3.3) | 0 | 0 |
| Black or African American | 15 (50.0) | 8 (28.6) | 7 (23.3) | 7 (31.8) | 11 (47.8) | 16 (53.3) | 6 (19.4) | 2 (33.3) |
| White | 14 (46.7) | 19 (67.9) | 23 (76.7) | 15 (68.2) | 11 (47.8) | 13 (43.3) | 25 (80.6) | 4 (66.7) |
| Hispanic or Latino ethnicity, No. (%)b | 16 (53.3) | 14 (50.0) | 17 (56.7) | 7 (31.8) | 10 (43.5) | 12 (40.0) | 17 (54.8) | 2 (33.3) |
| Body mass index, mean (SD)c | 30.4 (5.5) | 32.0 (5.0) | 30.6 (5.5) | 32.0 (5.2) | 30.6 (4.9) | 31.9 (5.0) | 30.5 (4.4) | 32.0 (3.9) |
| Automated office BP at randomization, mean (SD), mm Hg | ||||||||
| Systolic | 142.2 (13.4) | 140.0 (12.1) | 142.8 (13.1) | 142.6 (13.3) | 142.9 (13.7) | 142.9 (10.7) | 139.8 (9.1) | 135.3 (5.5) |
| Diastolic | 78.5 (10.0) | 84.7 (7.0) | 80.1 (9.3) | 81.6 (9.4) | 80.3 (12.0) | 83.8 (9.5) | 78.6 (10.0) | 81.5 (7.9) |
| eGFR, mean (SD), mL/min/1.73 m2 | 77.4 (14.0) | 77.2 (14.1) | 80.9 (12.4) | 81.7 (16.3) | 77.9 (18.7) | 81.6 (17.3) | 79.9 (13.1) | 83.9 (18.6) |
| Comorbidities, No. (%) | ||||||||
| Diabetes | 8 (26.7) | 8 (28.6) | 11 (36.7) | 9 (40.9) | 11 (47.8) | 14 (46.7) | 16 (51.6) | 2 (33.3) |
| Heart failure | 1 (3.3) | 1 (3.6) | 1 (3.3) | 1 (4.5) | 1 (4.3) | 0 | 0 | 0 |
| Medications, No. (%) | ||||||||
| 2 Background BP medications | 14 (46.7) | 20 (71.4) | 14 (46.7) | 7 (31.8) | 14 (60.9) | 17 (56.7) | 20 (64.5) | 4 (66.7) |
| ≥3 Background BP medications | 16 (53.3) | 8 (28.6) | 16 (53.3) | 15 (68.2) | 9 (39.1) | 13 (43.3) | 11 (35.5) | 2 (33.3) |
| Thiazide-type diuretic use | 17 (56.7) | 16 (57.1) | 18 (60.0) | 13 (59.1) | 12 (52.2) | 16 (53.3) | 19 (61.3) | 5 (83.3) |
| ACE inhibitor or ARB use | 23 (76.7) | 20 (71.4) | 27 (90.0) | 17 (77.3) | 18 (78.3) | 22 (73.3) | 30 (96.8) | 5 (83.3) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; eGFR, estimated glomerular filtration rate; PRA, plasma renin activity.
Following an interim analysis, randomization to the lowest doses was discontinued.
Self-reported race and ethnicity. There was 1 participant in the 50-mg once-daily lorundrostat group whose race was not reported.
Calculated as weight in kilograms divided by height in meters squared.
In the primary analysis population of cohort 1, participants receiving lorundrostat had ICE as follows: 100 mg once daily: 1 had ICE type 1 (initiation of rescue medication; systolic BP that triggered rescue medication is imputed), 4 had ICE type 3 (withdrawal from treatment for treatment-related reason or worsening; baseline systolic BP is imputed), and 3 had ICE type 4 (withdrawal from treatment for non–treatment-related reasons; mixed-model repeated-measures estimate); 50 mg once daily: 1 had ICE type 1; 25 mg twice daily: 1 had ICE type 3 and 2 had ICE type 4; 12.5 mg twice daily: 1 had ICE type 3; and 12.5 mg once daily: 1 had ICE type 1, 4 had ICE type 3, and 4 had ICE type 4. In the placebo group, 1 had ICE type 1 and 1 had ICE type 4. In the primary analysis population of cohort 2 receiving lorundrostat, 2 had ICE type 1, 2 had ICE type 3, and 1 had ICE type 4.
Primary End Point
Cohort 1 demonstrated a decrease in least-squares mean systolic AOBP with lorundrostat compared with placebo at the 50-mg and 100-mg once-daily doses (Table 2). At week 8, the placebo group demonstrated a change of −4.1 (SE, 2.6) mm Hg in systolic AOBP. Mixed-model repeated-measures systolic AOBP changes were −5.6 (SE, 3.2) mm Hg, −13.7 (SE, 2.7) mm Hg, and −11.9 (SE, 2.8) mm Hg at the 12.5-mg, 50-mg, and 100-mg once-daily doses of lorundrostat, respectively. Systolic AOBP changes were −11.3 (SE, 3.2) mm Hg and −11.1 (SE, 2.7) mm Hg for the 12.5-mg and 25-mg twice-daily doses. In the once-daily dose cohorts, the placebo-subtracted change in systolic AOBP was −1.5 mm Hg (90% CI, −8.3 to 5.3 mm Hg; P = .71) for 12.5 mg, −9.6 mm Hg (90% CI, −15.8 to −3.4 mm Hg; P = .01) for 50 mg, and −7.8 mm Hg (90% CI, −14.1 to −1.5 mm Hg; P = .04) for 100 mg. The change in BP demonstrated with the twice-daily doses, compared with placebo, showed similar BP reduction. Individual participant BP changes at week 8 are shown in Figure 2. Weekly changes in systolic AOBP are shown in eFigure 2 in Supplement 2.
Table 2. Primary and Secondary Efficacy End Pointsa.
| End points | Cohort 1 (PRA ≤1.0 ng/mL/h) | Exploratory cohort 2 (PRA >1.0 ng/mL/h)b | ||||||
|---|---|---|---|---|---|---|---|---|
| Lorundrostat | Placebo (n = 29) | Lorundrostat, 100 mg once daily (n = 31) | Placebo (n = 6) | |||||
| 100 mg once daily (n = 25) | 50 mg once daily (n = 28) | 25 mg twice daily (n = 28) | 12.5 mg twice daily (n = 19) | 12.5 mg once daily (n = 19) | ||||
| Primary end point | ||||||||
| Automated office SBP, baseline to wk 8 | ||||||||
| Baseline SBP, mean (SD), mm Hg | 143.8 (12.0) | 141.8 (10.9) | 140.9 (9.6) | 144.6 (10.5) | 147.1 (11.6) | 142.7 (10.2) | 140.4 (8.6) | 132.9 (8.4) |
| Wk 8 SBP, mean (SD), mm Hg | 131.3 (14.1) | 128.6 (13.7) | 131.3 (16.6) | 131.9 (12.9) | 140.8 (22.0) | 139.1 (14.2) | 129.3 (15.4) | 128.3 (18.9) |
| Change in SBP, least-squares mean (SE), mm Hg | −11.9 (2.8) | −13.7 (2.7) | −11.1 (2.7) | −11.3 (3.2) | −5.6 (3.2) | −4.1 (2.6) | −11.4 (2.5) | |
| Difference from placebo in SBP change, least-squares mean, (90% CI), mm Hg | −7.8 (−14.1 to −1.5) | −9.6 (−15.8 to −3.4) | −7.0 (−13.1 to −0.8) | −7.2 (−14.0 to −0.4) | −1.5 (−8.3 to 5.3) | |||
| P value | .04 | .01 | .06 | .08 | .71 | |||
| Secondary end point | ||||||||
| Automated office DBP, baseline to wk 8 | ||||||||
| Baseline DBP, mean (SD), mm Hg | 78.9 (9.6) | 84.9 (6.4) | 78.9 (8.0) | 81.6 (7.6) | 82.4 (10.7) | 83.4 (8.8) | 78.1 (7.6) | 78.3 (7.6) |
| Wk 8 DBP, mean (SD), mm Hg | 73.0 (9.4) | 76.8 (9.8) | 75.4 (13.1) | 75.2 (10.1) | 81.4 (17.2) | 81.3 (9.4) | 72.2 (8.4) | 76.2 (11.0) |
| Change in DBP, least-squares mean (SE), mm Hg | −5.8 (1.8) | −7.1 (1.7) | −4.1 (1.7) | −5.5 (2.0) | −3.8 (2.0) | −1.6 (1.7) | −5.6 (1.4) | |
| Difference from placebo in DBP change, least-squares mean, (90% CI), mm Hg | −4.1 (−8.1 to −0.1) | −5.5 (−9.4 to −1.5) | −2.5 (−6.4 to 1.4) | −3.8 (−8.2 to 0.5) | −2.1 (−6.5 to 2.2) | |||
| P value | .09 | .02 | .30 | .14 | .42 | |||
Abbreviations: DBP, diastolic blood pressure; PRA, plasma renin activity; SBP, systolic blood pressure.
Analysis used a mixed-model repeated-measures approach with fixed effects for categorical terms for treatment and baseline SBP as a fixed continuous covariate.
Because cohort 2 was an exploratory end point, no formal statistical testing was performed comparing lorundrostat with placebo.
Figure 2. Observed Systolic Automated Office Blood Pressure Changes From Baseline to Week 8 Among Trial Participants.
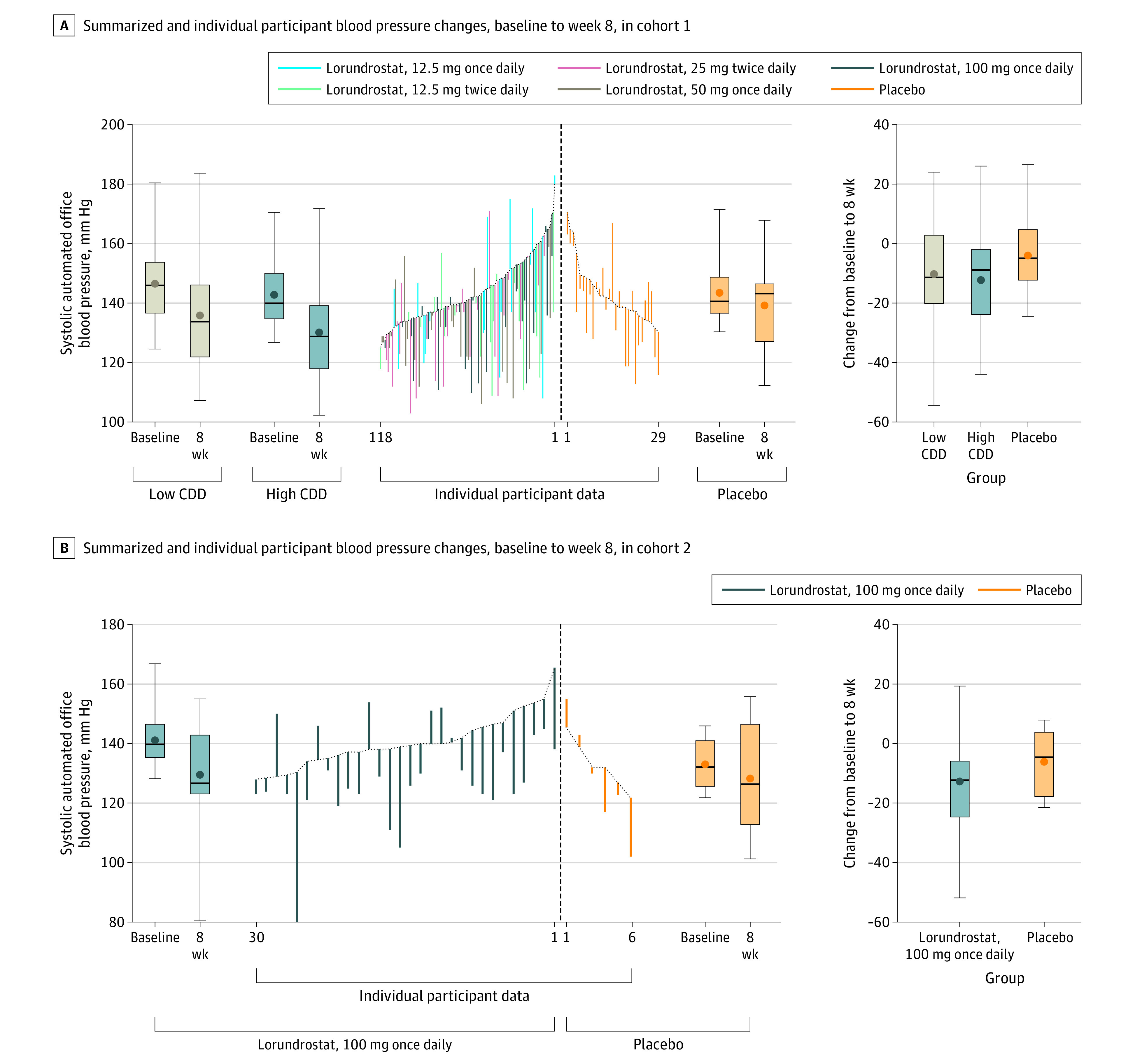
CDD indicates cumulative daily dose. A, The central data show individual participant blood pressure changes. Box plots demonstrate median (thick horizontal line), mean (circle), IQR (box top and bottom), and maximum and minimum blood pressure changes (whiskers). The tan plot shows pooled low CDD changes (≤25 mg/d total). The blue plot shows pooled high CDD changes (≥50 mg/d total). The orange plot shows changes in the placebo group. The far-right graph shows blood pressure change during the trial for each group. B, The central data show individual participant blood pressure changes. Box plots demonstrate median (thick horizontal line), mean (circle), IQR (box top and bottom), and maximum and minimum blood pressure changes (whiskers). The blue plot shows changes in the lorundrostat, 100-mg once-daily group; the orange plot shows changes in the placebo group. The far-right graph shows blood pressure change during the trial for each group.
Secondary End Points
The 50-mg once-daily dose of lorundrostat decreased diastolic AOBP by 5.5 mm Hg compared with placebo (90% CI, −9.4 to −1.5 mm Hg; P = .02). Other doses did not demonstrate statistically significant lowering of diastolic AOBP compared with placebo. Twenty-four-hour ambulatory BP monitoring results including mean overnight and central systolic BP as assessed by pulse-wave velocity are reported in eTables 3 and 4 in Supplement 2. The proportion of participants with AOBP of less than 130/80 mm Hg at trial conclusion did not statistically differ between treatment groups and placebo (eFigure 3 in Supplement 2).
Prespecified Exploratory End Points
Prespecified subgroups of interest examined the impact of BMI and concomitant administration of a thiazide-type diuretic on the degree of BP lowering. Most notably, participants with obesity demonstrated greater BP lowering in response to lorundrostat. Individuals with a BMI greater than 30 randomized to 50 mg once daily of lorundrostat demonstrated a −16.7 mm Hg (90% CI, −25.5 to −7.9 mm Hg) change in systolic AOBP compared with placebo. With 100 mg once daily of lorundrostat, participants with obesity saw a −12.3 mm Hg (90% CI, −21.6 to −3.1 mm Hg) change in systolic AOBP compared with placebo. Among individuals with a BMI of 25 to 30, the BP changes were 2.2 mm Hg (90% CI, −7.4 to 11.8 mm Hg) and −4.5 (90% CI, −14.5 to 5.5 mm Hg) for the 50-mg and 100-mg once-daily doses, respectively. Similarly, participants taking a thiazide-type diuretic randomized to 50 mg or 100 mg once daily of lorundrostat demonstrated changes of −12.9 mm Hg (90% CI, −21.2 to −4.7 mm Hg) and −10.0 mm Hg (90% CI, −21.2 to −1.6 mm Hg) in systolic AOBP compared with placebo. Blood pressure decreases were attenuated among participants not taking a diuretic (eTable 1 in Supplement 2).
Participants with PRA of greater than 1.0 ng/mL/h (cohort 2) randomized to 100 mg once daily of lorundrostat demonstrated a similar change in mean systolic AOBP of −11.4 (SD, 2.5) mm Hg compared with those randomized to 100 mg once daily with suppressed plasma renin in cohort 1. The least-squares mean systolic AOBP difference between the 100-mg once-daily cohorts was 0.74 mm Hg (90% CI, −5.4 to 6.9 mm Hg).
Pharmacodynamic Biomarker End Points
Changes in serum aldosterone, cortisol, PRA, and eGFR during the study are reported in eTable 2 in Supplement 2. Lorundrostat resulted in reductions in serum aldosterone at all doses, whereas the cohort 1 placebo group saw an increase in mean serum aldosterone level of 0.14 (SD, 3.68) ng/dL. The 12.5-mg, 50-mg, and 100-mg once-daily doses in cohort 1 reduced mean serum aldosterone by 1.05 (SD, 4.33) ng/dL, 2.56 (SD, 3.72) ng/dL, and 2.88 (SD, 4.44) ng/dL, respectively. The twice-daily doses in cohort 1 reduced mean serum aldosterone by 2.42 (SD, 2.78) ng/dL in the 12.5-mg group and 3.39 (SD, 4.22) ng/dL in the 25-mg group. In cohort 2, 100 mg daily of lorundrostat decreased mean serum aldosterone by 1.19 (SD, 5.38) ng/dL. Anticipated increases in PRA were observed ranging between 0.78 (SD, 3.44) ng/mL/h to 4.65 (SD, 11.27) ng/mL/h in cohort 1, and there were small increases in serum cortisol (eTable 2 in Supplement 2). Declines in eGFR paralleled the reduction in systolic AOBP and were consistent with expected lowering of intraglomerular pressures (eTable 2 in Supplement 2).
Safety End Points
No participant deaths occurred during the trial. Three serious adverse events occurred; only 1 was deemed treatment related. A participant randomized to 100 mg once daily of lorundrostat in cohort 2 had worsening of hyponatremia necessitating stopping the drug. A total of 110 participants (55%) experienced any adverse event during the trial (Table 3). Most adverse events were classified as mild by investigators. No adrenocortical insufficiency occurred during the trial. Cosyntropin stimulation testing in participants randomized to 100 mg once daily in both cohort 1 and cohort 2 was normal, with stimulated values of cortisol greater than 18 µg/dL in all individuals receiving cosyntropin (eFigure 4 in Supplement 2). Prespecified adverse events of special interest included 3 participants (2%) with hypotension. Mean serum potassium increases were similar across all lorundrostat doses, including increases of 0.25 mmol/L in the 50-mg and 100-mg once-daily groups. Six participants (3.6%) had serum potassium levels above 6.0 mmol/L during the trial (Table 3). No instances of hyperkalemia required intervention beyond discontinuation or reduction in the dose of lorundrostat.
Table 3. Adverse Events and Serum Potassium Changes.
| Measures | Cohort 1 (PRA ≤1.0 ng/mL/h) | Exploratory cohort 2 (PRA >1.0 ng/mL/h) | ||||||
|---|---|---|---|---|---|---|---|---|
| Lorundrostat | Placebo (n = 30) | Lorundrostat, 100 mg once daily (n = 31) | Placebo (n = 6) | |||||
| 100 mg once daily (n = 30) | 50 mg once daily (n = 28) | 25 mg twice daily (n = 30) | 12.5 mg twice daily (n = 22) | 12.5 mg once daily (n = 23) | ||||
| Participants with any serious adverse event, No. (%) | 0 | 0 | 0 | 0 | 2 (9)a | 0 | 1 (3)b | 0 |
| Participants with any adverse event, No. (%)c | 17 (57) | 12 (43) | 20 (67) | 13 (59) | 16 (70) | 12 (40) | 19 (61) | 1 (17) |
| Participants with hypotension, No. (%) | 1 (3) | 0 | 0 | 1 (5) | 0 | 0 | 1 (3) | 0 |
| Potassium-related eventsd | ||||||||
| Change from baseline in serum potassium level, mean (SD), mmol/L | 0.29 (0.59) | 0.25 (0.36) | 0.34 (0.46) | 0.32 (0.53) | 0.31 (0.44) | 0.03 (0.37) | 0.21 (0.54) | −0.05 (0.28) |
| Participants with serum potassium level 5.6-6.0 mmol/L, No. (%) | 5 (16) | 1 (4) | 2 (7) | 2 (9) | 3 (13) | 0 | 2 (6) | 0 |
| Participants with serum potassium level 6.1-6.5 mmol/L, No. (%) | 0 | 0 | 1 (3) | 1 (5) | 1 (4) | 0 | 1 (3) | 0 |
| Participants with serum potassium level >6.5 mmol/L, No. (%) | 1 (3) | 1 (4) | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviation: PRA, plasma renin activity.
One participant was identified with worsening of preexisting coronary artery disease and 1 participant was found to have metastatic cancer in their peritoneum.
Participant had hyponatremia that resolved on drug discontinuation.
Site investigators were requested to assess the relationship between study intervention and each occurrence of each adverse event. Investigators were directed to use clinical judgment to determine the relationship as either unlikely related, unrelated, possibly related, or definitely related. Criteria for deeming possibly or definitely related (and thus treatment related) include that there is a reasonable possibility that the adverse event may have been caused by the study drug. Examples of more prevalent adverse events included hyperkalemia and decline in estimated glomerular filtration rate.
Affected individuals were counted once at highest-grade abnormality. Hemolyzed blood samples with serum potassium levels that were not reproducible on repeat testing were not included.
Discussion
In this multicenter, prospective, randomized, placebo-controlled, dose-ranging, double-blind clinical trial among adults with uncontrolled hypertension, aldosterone synthase inhibition with lorundrostat was well tolerated and reduced AOBP at doses of 50 and 100 mg once daily.
Contemporary US BP guidelines recommend similar medication combinations for most patients, regardless of underlying comorbidities or the dominant pathophysiologic process for increased BP. However, new approaches to treating hypertension are needed because management of hypertension in the United States is relatively poor and hypertension remains a major cause of excess morbidity and mortality.3,15 A targeted approach to hypertension management has the potential to improve rates of BP control, particularly in patients for whom elevated aldosterone is a driving factor for BP increases.6,7 Accumulating data suggest that obesity-related excess aldosterone production represents a unique endotype characterized by dysregulation of normal homeostatic mechanisms due to abnormalities in adipokine production.16,17 Other pathological mechanisms, including genetic and epigenetic differences, may also contribute to dysregulation of aldosterone production among African American persons and other aldosterone-sensitive individuals.18
The adverse effects of excess aldosterone on the cardiovascular system are well established and the prevalence of aldosterone excess is underdiagnosed among individuals with hypertension.5,19 Furthermore, patients with common cardiovascular comorbidities such as obstructive sleep apnea demonstrate excess circulating aldosterone levels, which contribute to increased cardiovascular risk.20,21 Current paradigms for treating hypertension in patients in whom aldosterone excess plays a major role include prescribing MRAs such as spironolactone, which effectively lower BP, particularly among individuals with resistant hypertension.12,22,23 However, MRA use and efficacy may be limited due to hormonal adverse effects as well as enhancement of the nongenomic effects of aldosterone, which include increasing vascular stiffness and sympathetic nervous system activation.24 Concerns regarding nongenomic effects of aldosterone are highlighted in cases of primary aldosteronism where, when feasible, surgical treatment that eliminates excess aldosterone is preferred to long-term MRA use, which increases circulating aldosterone and is associated with less favorable cardiometabolic outcomes.25,26,27
In the current trial, aldosterone synthase inhibition with lorundrostat lowered both serum aldosterone and BP. The 50-mg once-daily dose of lorundrostat lowered office BP to a similar degree as the 100-mg once daily dose, but resulted in fewer adverse events, including fewer episodes of hyperkalemia. Lorundrostat has a relatively short half-life, and once-daily dosing resulted in a 25% lower mean change in serum potassium when 50 mg once daily was compared with 25 mg twice daily. This suggests that allowing an overnight period of escape from aldosterone suppression may have safety benefits when compared with aldosterone synthase inhibitors or aldosterone receptor blockers with longer half-lives, including spironolactone, which has active metabolites with half-lives exceeding 24 hours. Although the relatively short half-life of lorundrostat limited the 24-hour efficacy of the 12. 5-mg once-daily dose, effective once-daily dosing was observed with higher lorundrostat doses.
Inhibition of aldosterone synthase is not a new concept. Osilodrostat, the first aldosterone synthase inhibitor, lowered aldosterone and BP compared with placebo in phase 2 studies but was not pursued for a BP-lowering indication.28 This is primarily due to its lack of specificity to exclusively decrease aldosterone production via aldosterone synthase inhibition. CYP11B2 encodes aldosterone synthase and has greater than 93% homology to CYP11B1, which encodes 11-β-hydroxylase, the rate-limiting step in cortisol synthesis. Given this substantial homology, inhibitors of aldosterone synthase such as osilodrostat that are not highly selective also inhibit cortisol synthesis, and 20% of osilodrostat trial participants demonstrated inadequate cortisol production following adrenocorticotropic hormone stimulation. Problematically, osilodrostat also substantially increased production of 11-deoxycorticosterone, which is a mineralocorticoid receptor agonist.28,29 Lorundrostat appears to overcome this issue given its high selectivity for inhibition of human CYP11B2 vs CYP11B1 (374:1) in a preclinical cell-based activity assay (Mitsubishi-Tanabe Pharmaceuticals). Other highly specific aldosterone synthase inhibitors are under ongoing investigation. Baxdrostat, with a selectivity ratio of 100:1, demonstrated similar placebo-subtracted BP lowering in a dose-ranging phase 2 trial.30 Lorundrostat and baxdrostat result in similar rates of hyperkalemia and expected modest initial declines in eGFR. Glomerular filtration rate decreases can be seen following adrenalectomy in patients with aldosterone producing adenomas, likely reflecting hyperfiltration induced by aldosterone, and are also seen when initiating an MRA, angiotensin-converting enzyme inhibitor, or angiotensin receptor blocker, and are related to beneficial lowering of intraglomerular pressure.31
Limitations
This trial has limitations. First, multiple dosing regimens were tested for efficacy, and each studied dose had a limited sample size. Second, 24-hour ambulatory BP monitoring was not used to confirm sustained hypertension before randomization, and this resulted in a disproportionate percentage of participants with a prominent white-coat effect in the 50-mg once-daily lorundrostat group. Third, cohort 1 of this trial selected participants with suppressed renin at baseline. Suppressed renin is common among patients with hypertension; however, theoretically these patients stand to benefit most from drugs that decrease aldosterone production. Nevertheless, cohort 2 demonstrated similar BP-lowering efficacy in participants with nonsuppressed plasma renin, and a trial of spironolactone demonstrated BP reduction across a wide range of plasma renin levels.22 Fourth, 2 interim analyses were performed; multiple interim analyses would not be appropriate in subsequent confirmatory trials.
Conclusions
Lorundrostat at doses of 50 mg and 100 mg once daily decreased AOBP significantly more than placebo. Blood pressure reduction was particularly evident among participants with hypertension and concomitant obesity. Lorundrostat was well tolerated, and small expected increases in serum potassium and declines in eGFR suggest a favorable safety profile, particularly with a 50-mg once-daily dose. The trial results support further study of lorundrostat as a treatment for uncontrolled hypertension.
Trial Protocol
Target-HTN Sites
eTable 1. Select pre-specified analysis of factors anticipated to impact blood pressure lowering response to lorundrostat in cohort 1
eTable 2. Change in pharmacodynamic biomarkers compared with baseline
eTable 3. Observed 24-hour ambulatory blood pressure changes among all cohort 1 participants
eTable 4. Observed 24-hour ambulatory blood pressure changes among cohort 1 participants with baseline 24-hour mean systolic blood pressure >130 mm Hg
eFigure 1. Study design schema
eFigure 2. Weekly automated office systolic blood pressure changes with lorundrostat
eFigure 3. Proportion of participants with automated office blood pressure <130/80 mmHg at week 8 compared with baseline among cohort 1 participants
eFigure 4. Cosyntropin-stimulated cortisol production after 8 weeks of lorundrostat treatment
Statistical Analysis Plan
Nonauthor Collaborators. Target-HTN Investigators
Data Sharing Statement
References
- 1.Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80(25):2361-2371. doi: 10.1016/j.jacc.2022.11.005 [DOI] [PubMed] [Google Scholar]
- 2.Muntner P, Hardy ST, Fine LJ, et al. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324(12):1190-1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsao CW, Aday AW, Almarzooq ZI, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circulation. 2023;147(8):e93-e621. doi: 10.1161/CIR.0000000000001123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leggio M, Lombardi M, Caldarone E, et al. The relationship between obesity and hypertension: an updated comprehensive overview on vicious twins. Hypertens Res. 2017;40(12):947-963. doi: 10.1038/hr.2017.75 [DOI] [PubMed] [Google Scholar]
- 5.Brown JM, Siddiqui M, Calhoun DA, et al. the unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med. 2020;173(1):10-20. doi: 10.7326/M20-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funder JW, Carey RM. Primary aldosteronism: where are we now? where to from here? Hypertension. 2022;79(4):726-735. doi: 10.1161/HYPERTENSIONAHA.121.18761 [DOI] [PubMed] [Google Scholar]
- 7.Kawarazaki W, Fujita T. The role of aldosterone in obesity-related hypertension. Am J Hypertens. 2016;29(4):415-423. doi: 10.1093/ajh/hpw003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Heizhati M, Li N, et al. Association of objective and subjective parameters of obstructive sleep apnea with plasma aldosterone concentration in 2,066 hypertensive and 25,368 general population. Front Endocrinol (Lausanne). 2023;13:1016804. doi: 10.3389/fendo.2022.1016804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens. 2012;26(5):281-287. doi: 10.1038/jhh.2011.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzaga CC, Gaddam KK, Ahmed MI, et al. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med. 2010;6(4):363-368. doi: 10.5664/jcsm.27878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koo P, Muntner P, Hall ME, Gjelsvik A, McCool FD, Eaton CB. Relationship between risks for obstructive sleep apnea, resistant hypertension, and aldosterone among African American adults in the Jackson Heart Study. Am J Hypertens. 2022;35(10):875-883. doi: 10.1093/ajh/hpac091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carey RM, Calhoun DA, Bakris GL, et al. ; American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Genomic and Precision Medicine, Council on Peripheral Vascular Disease, Council on Quality of Care and Outcomes Research, and Stroke Council . Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72(5):e53-e90. doi: 10.1161/HYP.0000000000000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funder JW. Minireview: aldosterone and the cardiovascular system: genomic and nongenomic effects. Endocrinology. 2006;147(12):5564-5567. doi: 10.1210/en.2006-0826 [DOI] [PubMed] [Google Scholar]
- 14.Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47(3):312-318. doi: 10.1161/01.HYP.0000201443.63240.a7 [DOI] [PubMed] [Google Scholar]
- 15.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324. doi: 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 16.Packer M. Leptin-aldosterone-neprilysin axis: identification of its distinctive role in the pathogenesis of the three phenotypes of heart failure in people with obesity. Circulation. 2018;137(15):1614-1631. doi: 10.1161/CIRCULATIONAHA.117.032474 [DOI] [PubMed] [Google Scholar]
- 17.Huby AC, Otvos L Jr, Belin de Chantemèle EJ. Leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in obese female mice. Hypertension. 2016;67(5):1020-1028. doi: 10.1161/HYPERTENSIONAHA.115.06642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zilbermint M, Hannah-Shmouni F, Stratakis CA. Genetics of hypertension in African Americans and others of African descent. Int J Mol Sci. 2019;20(5):1081. doi: 10.3390/ijms20051081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen JB, Cohen DL, Herman DS, Leppert JT, Byrd JB, Bhalla V. Testing for primary aldosteronism and mineralocorticoid receptor antagonist use among U.S. veterans: a retrospective cohort study. Ann Intern Med. 2021;174(3):289-297. doi: 10.7326/M20-4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krug AW, Ehrhart-Bornstein M. Aldosterone and metabolic syndrome: is increased aldosterone in metabolic syndrome patients an additional risk factor? Hypertension. 2008;51(5):1252-1258. doi: 10.1161/HYPERTENSIONAHA.107.109439 [DOI] [PubMed] [Google Scholar]
- 21.Calhoun DA, Sharma K. The role of aldosteronism in causing obesity-related cardiovascular risk. Cardiol Clin. 2010;28(3):517-527. doi: 10.1016/j.ccl.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams B, MacDonald TM, Morant S, et al. ; British Hypertension Society’s PATHWAY Studies Group . Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386(10008):2059-2068. doi: 10.1016/S0140-6736(15)00257-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudenbostel T, Calhoun DA. Use of aldosterone antagonists for treatment of uncontrolled resistant hypertension. Am J Hypertens. 2017;30(2):103-109. doi: 10.1093/ajh/hpw105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funder JW. Aldosterone and mineralocorticoid receptors—physiology and pathophysiology. Int J Mol Sci. 2017;18(5):1032. doi: 10.3390/ijms18051032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(1):51-59. doi: 10.1016/S2213-8587(17)30367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed S, Hundemer GL. Benefits of surgical over medical treatment for unilateral primary aldosteronism. Front Endocrinol (Lausanne). 2022;13:861581. doi: 10.3389/fendo.2022.861581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi GP, Maiolino G, Flego A, et al. ; PAPY Study Investigators . Adrenalectomy lowers incident atrial fibrillation in primary aldosteronism patients at long term. Hypertension. 2018;71(4):585-591. doi: 10.1161/HYPERTENSIONAHA.117.10596 [DOI] [PubMed] [Google Scholar]
- 28.Calhoun DA, White WB, Krum H, et al. Effects of a novel aldosterone synthase inhibitor for treatment of primary hypertension: results of a randomized, double-blind, placebo- and active-controlled phase 2 trial. Circulation. 2011;124(18):1945-1955. doi: 10.1161/CIRCULATIONAHA.111.029892 [DOI] [PubMed] [Google Scholar]
- 29.Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16(11 Pt 1):925-930. doi: 10.1016/S0895-7061(03)01032-X [DOI] [PubMed] [Google Scholar]
- 30.Freeman MW, Halvorsen YD, Marshall W, et al. ; BrigHTN Investigators . Phase 2 trial of baxdrostat for treatment-resistant hypertension. N Engl J Med. 2023;388(5):395-405. doi: 10.1056/NEJMoa2213169 [DOI] [PubMed] [Google Scholar]
- 31.Palmer BF. Renal dysfunction complicating the treatment of hypertension. N Engl J Med. 2002;347(16):1256-1261. doi: 10.1056/NEJMra020676 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Target-HTN Sites
eTable 1. Select pre-specified analysis of factors anticipated to impact blood pressure lowering response to lorundrostat in cohort 1
eTable 2. Change in pharmacodynamic biomarkers compared with baseline
eTable 3. Observed 24-hour ambulatory blood pressure changes among all cohort 1 participants
eTable 4. Observed 24-hour ambulatory blood pressure changes among cohort 1 participants with baseline 24-hour mean systolic blood pressure >130 mm Hg
eFigure 1. Study design schema
eFigure 2. Weekly automated office systolic blood pressure changes with lorundrostat
eFigure 3. Proportion of participants with automated office blood pressure <130/80 mmHg at week 8 compared with baseline among cohort 1 participants
eFigure 4. Cosyntropin-stimulated cortisol production after 8 weeks of lorundrostat treatment
Statistical Analysis Plan
Nonauthor Collaborators. Target-HTN Investigators
Data Sharing Statement


