Abstract
We present a case report of a 20-year-old male who suffered a stab injury to the left supraclavicular region, resulting in the formation of a pseudoaneurysm of the left subclavian artery. Initial endovascular management with a self-expandable covered stent graft showed promising results, but recurrence with proximal and distal end leaks necessitated further intervention. The patient's financial constraints delayed subsequent treatment, leading to worsening symptoms, including left upper limb paraparesis. Facing technical challenges due to the large size of the aneurysm and proximity to the vertebral artery, a vertebral artery confluence was performed, followed by a longer stent-graft placement to address the pseudoaneurysm successfully. This case highlights the potential advantages of endovascular approaches in complex subclavian artery injuries and emphasizes the importance of timely intervention to avoid complications and improve patient outcomes.
Keywords: Pseudoaneurysm, Subclavian artery, Endovascular management, Endovascular trauma
Introduction
Traumatic subclavian artery injury can result in pseudoaneurysm, a life-threatening condition characterized by transgression of the arterial wall leading to the formation of pulsatile hematoma [1]. Open surgical repair is traditionally the preferred method, but the artery's deep cervical location could be hazardous and cause complications, including massive blood loss and nerve injury. Surgical repairs in complex injuries may require bypass grafts, necessitating extensive dissection [2,3]. However, endovascular management has emerged as a promising alternative in recent years with potential advantages such as reduced morbidity and improved outcomes [3,4]. The most common endovascular approach involves accessing the injured vessel percutaneously and placing a stent in the vessel to displace and restore the injured segment while maintaining blood flow [5].
Case presentation
A 20-year-old male presented with a painless swelling in the left supraclavicular region following a stab injury with a knife. The injury was treated with analgesic and local antiseptic since the wound was not deep. The swelling had been progressively increasing in size for the last 2 weeks. There was no history of breathlessness or chest pain, and he had no significant past medical or family history. On examination, he was hemodynamically stable. There was no jugular venous distension. Local examination revealed a 1.0 cm × 1.0 cm (approximately) pulsatile swelling in the left supraclavicular region with a healed wound from the injury. It was soft in consistency with a palpable thrill, and no tenderness or subcutaneous crepitations were noted. Auscultation of the swelling revealed a bruit. Respiratory system examination was normal, with the trachea in the midline and normal vesicular breathing. The rest of the systemic examination was unremarkable.
The basic metabolic profile was within normal limits. A plain chest radiograph showed no abnormalities. Color Doppler ultrasonography (USG) of the swelling showed a large hypoechoic cystic structure measuring 2.5 cm × 3.0 cm in relation to the left subclavian artery, having a swirling flow motion within it, suggesting a pseudoaneurysm. Left upper limb arteries had relatively normal color flow and spectral waveforms. Left vertebral artery also showed normal flow and waveform. Computed tomography (CT) angiography (Fig. 1) showed a contrast-filled outpouching arising from the anterior aspect of the left subclavian, measuring 2.2 cm × 1.8 cm × 3.6 cm, with no fistula formation.
Fig. 1.
CT angiography showing contrast filled outpouching arising from the anterior aspect of left subclavian measuring 2.2 × 1.8 × 3.6 cm.
Under fluoroscopic guidance, an 8 mm × 40 mm self-expandable covered stent graft, with a proximal landing zone just distal to the origin of branches of the first part of the subclavian artery, was placed in the arterial wall defect via the right femoral artery using a 6 French vascular access sheath. Selective catheterization of the left subclavian artery using an H1 catheter was done, and a guidewire was passed across the aneurysm. Before deploying the stent, the vascular access sheath was exchanged with a 9 French sheath over an ultra-stiff guidewire (0.035 inches) (Fig. 2). Postprocedure CT angiogram showed no outpouching, and the stent could be visualized in place. There was no bleeding or fistulas (Fig. 3). He was started on aspirin 325 mg once daily and was advised to review after 1 week.
Fig. 2.
Selective catheterization of left subclavian artery using H1 catheter (A), followed by passing of guidewire across the aneurysm (B), stent placed at the site of aneurysm (C).
Fig. 3.
Post procedure CT angiogram showed no outpouching and the stent visualized in place.
He presented again in 1 week with increased size in swelling associated with severe pain in the left supraclavicular region. The swelling appeared to be 0.5 × 0.5 cm on examination and was pulsatile, associated with reduced power in the upper limb. A repeat CT angiogram showed proximal and distal endoleaks (Fig. 4). The possible causes could be sustained trauma, poor compliance, or shorter stent. Re-stenting with a longer stent was advised to the patient. The procedure was deferred by a month due to financial constraints.
Fig. 4.
One-week follow-up CT angiogram showing proximal and distal endoleaks.
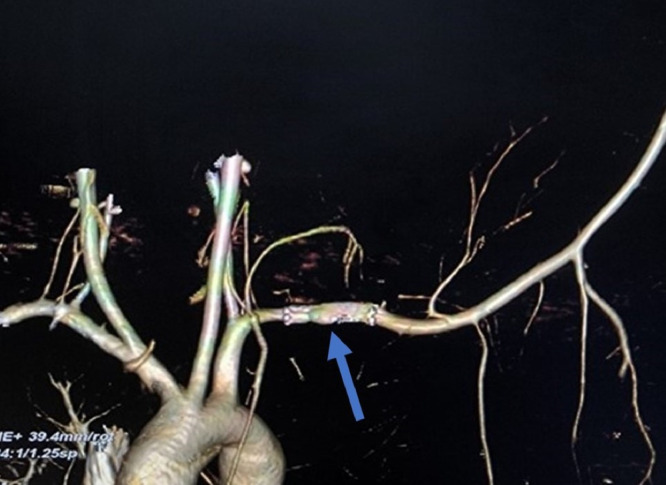
After a month, he presented with increased swelling, excruciating pain, and left upper limb paraparesis. The swelling was around 1 cm × 1 cm on examination, with a feeble pulse. The left upper extremity was relatively warm, with no evidence of hypoxic injury. Muscle power was severely diminished (MRC grade 2). USG of the swelling showed an increased leak size compared to the previous CT angiogram and an aneurysm. Some of the technical challenges faced at this point were the large size of the aneurysm, the requirement for a longer stent and an elevated risk of thrombosis. The major challenge was the proximity of the proximal leakage site to the origin of the vertebral artery. A vertebral artery confluence was done at an adequate distance from the proximal edge of the aneurysm on the left side (Fig. 5). The right vertebral artery was capable of supplying both posterior cerebral arteries. This was followed by a longer stent graft placement across the aneurysm. Postprocedure CT angiogram and USG Doppler showed normal flow and wavefront (Fig. 6). He was discharged with aspirin 75 mg/day and clopidogrel 75 mg/day to prevent thrombosis and analgesics for pain. On follow-up, the organized hematoma was shown to be compressing on the brachial plexus causing plexopathy. He was managed conservatively with opioid analgesics for pain and physiotherapy for weakness, as progressive liquefaction of the hematoma was noted on follow-up USG scans.
Fig. 5.
Vertebral artery confluence with adequate distance from proximal edge of aneurysm on left side with the right vertebral artery supplying both posterior cerebral arteries.
Fig. 6.
Post procedure CT angiogram showing normal flow across the stent.
Discussion
Pseudoaneurysm is formed by a break in the arterial wall leading to hematoma formation, contained within adventitia or surrounding tissue through the patent lumen connecting the artery and pseudoaneurysm [1]. This breach in the wall occurs due to trauma, infection, iatrogenic procedures, intravenous drug abuse or atherosclerosis. Trauma could be blunt or penetrating injuries, infections like mycobacterial or syphilitic, and iatrogenic causes during central line insertion. Although females carry a higher risk, pseudoaneurysms are more commonly seen in males (60%-80%) than females [1], [2], [3]. Other risk factors include calcified arteries, old age (>70 years), low platelet counts and high body-mass index [2]. They commonly involve femoral artery (47%), and subclavian artery pseudoaneurysm represents about 3% of the cases with peripheral pseudoaneurysm [3]. The majority of subclavian artery pseudoaneurysms occur due to sharp injuries, and 25% are due to blunt trauma [4].
The history of trauma is almost always present preceding the development of subclavian artery aneurysms. Pain, swelling, and hemorrhage are common clinical features, as with other pseudoaneurysms. Depending on the site and size, several presenting features may be noted. For subclavian artery pseudoaneurysm, a tender pulsatile mass in the supraclavicular fossa is noted. Due to its proximity to several vital structures, complications commonly occur with subclavian artery pseudoaneurysm. A deeply seated aneurysm within the thoracic cavity may present with chest and shoulder pain, pain while swallowing, hoarseness in voice, Horner's syndrome, or venous congestion [5]. Sudden hemorrhage due to rupture, thromboembolism and death are some concerning complications. Estimated mortality of 60% among patients who do not make it to the hospital and 5 to 30% among those who reach the hospital following the triggering event suggest that prompt diagnosis and immediate treatment are vital [6]. Another important complication is brachial plexus compression. Patients present with claudication, sensory or motor weakness or paralysis of the limbs on the involved side [7]. Our patient was presented initially with pulsatile swelling and later developed limb weakness due to brachial plexus compression.
Conventional angiography is the investigation of choice to diagnose pseudoaneurysm. Other modalities like duplex Doppler USG, CT angiography, and magnetic resonance angiography can also be utilized. CT angiography provides immediate results and aids in diagnosis of the disease quickly [8], [9], [10]. They appear as an outpouching of the artery on hemodynamic evaluation of a specific artery and help identify the collateral vessels to determine donor artery [9], [10], [11].
Initial treatment includes ultrasound-guided thrombin injection. Endovascular treatment includes embolization of the pseudoaneurysm sac with coils or covered stent grafting across the defect [10]. For subclavian pseudoaneurysm, the recommended treatment is endovascular stent graft, as it is safe and effective. Several complications are associated with the procedure, including endoleak leading to recurrence of the pseudoaneurysm, thrombosis and migration of the stent. Patients are advised to take aspirin 100 mg/day and clopidogrel 75 mg/day to prevent thrombosis [10], [11], [12], [13]. We observed a recurrence of the pseudoaneurysm in our patient due to smaller stent graft leading to endoleak at both ends of the stent placed. Re-stenting with a longer graft stopped the leak through it. Another problem encountered in our case was the proximity of the proximal leakage site to the origin of the vertebral artery. A vertebral artery confluence was done at an adequate distance from the proximal edge of the pseudoaneurysm on left side. The right vertebral artery was capable of supplying both posterior cerebral arteries. Another complication noted in our patient was brachial plexopathy due to compression of the brachial plexus by the organized hematoma.
Conclusion
Our case highlights the successful management of a complex subclavian artery injury resulting in a pseudoaneurysm through an endovascular approach. Timely intervention is crucial in such cases to avoid complications and improve patient outcomes. Endovascular techniques offer potential advantages, including reduced morbidity and improved outcomes compared to traditional open surgical repair. Regular follow-up and compliance with recommended treatment plans are important to prevent recurrence and ensure optimal patient care. This case report highlights the significance of considering endovascular approaches in complex subclavian artery injuries. It emphasizes the need for timely and comprehensive management to achieve favorable outcomes in patients with pseudoaneurysms. Further research and experience in endovascular interventions are warranted to expand our understanding of their benefits and limitations in managing such cases.
Patient consent
Signed consent for a case report was obtained from the patient's legally authorized representative. The IRB approval was taken from Regency Hospital Ethics Committee.
Footnotes
All authors have seen and approved the case report submitted.
No part of the submitted work has been published or is under consideration for publication elsewhere.
Competing Interests: None.
Contributor Information
Deepa Francis, Email: francisdeepa04@gmail.com.
Mahendra Kumar, Email: mkumar.md7@gmail.com.
Mansi Singh, Email: singhmansi57@gmail.com.
Toochukwu Lilian Okafor, Email: drlilianokafor@gmail.com.
Murali Mohan Rama Krishna Reddy, Email: muralimohan9797@gmail.com.
Pugazhendi Inban, Email: inban.pugaz@gmail.com.
Prabhparmeet Singh, Email: prabh2611.ps@gmail.com.
Vaishnavi Sirekulam, Email: Vaishnavisirekulam@gmail.com.
Ogbonnaya Akuma, Email: akuma4christ@gmail.com.
Chinaza Mercy Akuma, Email: chinazaakuma20@gmail.com.
Keval Thakkar, Email: kevalvthakkar@gmail.com.
References
- 1.Akuma O, Akuma C, Addi Palle LR, Carredo CKC, Savul S, Khawer S, et al. Subclavian artery pseudoaneurysm in an eight-year-old boy: a rare case report and review of literature. Cureus. 2023;15(7) doi: 10.7759/cureus.41488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mudoni A, Cornacchiari M, Gallieni M, Guastoni C, McGrogan D, Logias F, et al. Aneurysms and pseudoaneurysms in dialysis access. Clin Kidney J. 2015;8(4):363–367. doi: 10.1093/ckj/sfv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Dong X, Liang H, Mkangala A, Su Y, Liu D. Endovascular treatment of subclavian artery pseudoaneurysm. Ann Vasc Surg. 2020;65:284.e1–284.e6. doi: 10.1016/j.avsg.2019.10.096. [DOI] [PubMed] [Google Scholar]
- 4.Quinones-Baldrich WJ. Right subclavian pseudoaneurysm secondary to blunt trauma in an arteriovenous malformation. J Vasc Surg. 2010;51(1):228–229. doi: 10.1016/j.jvs.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 5.van der Weijde E, Vos JA, Heijmen RH. Hybrid repair of a large pseudoaneurysm of the proximal right subclavian artery in a Marfan patient. J Vasc Surg Cases Innov Tech. 2017;3(4):215–217. doi: 10.1016/j.jvscit.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luther A, Kumar A, Negi KN. Peripheral arterial pseudoaneurysms-a 10-year clinical study. Indian J Surg. 2015;77:603–607. doi: 10.1007/s12262-013-0939-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry JC, Franz RW. Pseudoaneurysms of the peripheral arteries. Int J Angiol. 2019;28(1):20–24. doi: 10.1055/s-0039-1677676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaiswal LS, Pandit N, Prasad JN, Adhikary S. Pseudoaneurysm of peripheral arteries: our experience in a community-based hospital. Indian J Vasc Endovasc Surg. 2019;6(2):102. [Google Scholar]
- 9.Enamorado-Enamorado J, Egea-Guerrero JJ, Revuelto-Rey J, Gordillo-Escobar E, Herrera-Melero C. Left subclavian artery pseudoaneurysm after a traffic accident: a case report. Case Rep Crit Care. 2011;2011:451819. doi:10.1155/2011/451819 [DOI] [PMC free article] [PubMed]
- 10.Davidovic LB, Markovic DM, Pejkic SD, Kovacevic NS, Colic MM, Doric PM. Subclavian artery aneurysms. Asian J Surg. 2003;26:7–11. doi: 10.1016/S1015-9584(09)60206-2. [DOI] [PubMed] [Google Scholar]
- 11.Cheema M, Kirton OC, Lukose B, Gallagher J. Ligation of the subclavian artery after blunt trauma presenting as massive hemothorax. J Trauma. 2008;64(4):1126–1130. doi: 10.1097/01.ta.0000195726.14064.0f. [DOI] [PubMed] [Google Scholar]
- 12.Huang T, Armstrong CW, Panjeton GD. Delayed onset of subclavian artery pseudoaneurysm with brachial plexus compression following gunshot wound injury. Cureus. 2022;14(2):e22457. doi: 10.7759/cureus.22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinley AG, Carrim AT, Robbs JV. Management of proximal axillary and subclavian artery injuries. Br J Surg. 2000;87(1):79–85. doi: 10.1046/j.1365-2168.2000.01303.x. [DOI] [PubMed] [Google Scholar]