Abstract
Sleep deprivation in humans is associated with both cognitive impairment and immune dysregulation. An animal model of neuropathogenesis may provide insight to understand the effects of sleep deprivation on the brain. Human neurocognition is more closely mirrored by nonhuman primates (NHP) than other animals. As such, we developed an NHP model to assess the impact of sleep deprivation on neurocognition and markers of systemic immune activation. Six male rhesus macaques underwent three rounds of sleep deprivation (48 h without sleep) at days 0, 14, and 28. We performed domain specific cognitive assessments using the Cambridge Neuropsychological Test Automated Battery (CANTAB) via a touch screen before and after 24 and 48 h of sleep deprivation. Immune activation markers were measured in the blood by multiplex assay and flow cytometry. Although we observed variability in cognitive performance between the three rounds of sleep deprivation, cognitive impairments were identified in all six animals. We noted more cognitive impairments after 48 h than after 24 h of sleep deprivation. Following 48 h of sleep deprivation, elevations in markers of immune activation in the blood were observed in most animals. The observed impairments largely normalized after sleep. The co-occurrence of systemic immune alterations and cognitive impairment establishes this model as useful for studying the impact of sleep deprivation on neurobehavior and immune perturbations in rhesus macaques.
Keywords: Sleep deprivation, Neurocognition, Immune activation, Non-human primates, Rhesus macaques
Highlights
-
•
The impacts of sleep deprivation on neurocognition and immune activation are assessed in a rhesus macaque model.
-
•
Markers of immune activation are elevated in peripheral blood following sleep deprivation.
-
•
Neurocognitive impairments are observed following sleep deprivation.
-
•
Neurocognitive impairments and immune activation largely return to normal after sleep.
1. Introduction
Significant associations between sleep deprivation, neurocognitive functions, and immune activation are documented in humans. Several meta-analyses have been conducted on the effects of sleep deprivation on neurocognitive performance in humans. Koslowsky and Babkoff (1992) summarized 27 studies and reported that total sleep deprivation (i.e., the lack of sleep for at least 24 h) showed greater correlations with performance decrement as the duration of deprivation increased, and that speed or latency variables were generally affected more than accuracy measures (Koslowsky and Harvey, 1992). Pilcher and Huffcutt (1996) analyzed 19 studies of the effects of sleep deprivation on cognitive task performance, motor task performance, and mood. They concluded that sleep deprivation strongly impairs cognitive functions and mood. “Complex and long” tasks were more affected than “simple” tasks after short periods of sleep deprivation, but the reverse is true for extended periods (>45 h) of sleep deprivation (Pilcher and Huffcutt, 1996). Philibert (2005) conducted a meta-analysis of 60 studies to assess the effects of acute and partial chronic sleep deprivation (sleep duration of <5–6 h for several consecutive nights) on cognitive and clinical task performance in physicians and nonphysicians (Philibert, 2005). Across all studies in the analysis, sleep loss reduced cognitive performance by nearly one standard deviation. Sleep loss was associated with a more significant decrement in vigilance and clinical performance than in memory and cognitive function. Lim and Dinges (2010) conducted a meta-analysis of 70 studies to discover the effects of short-term (<48 h) total sleep deprivation on both speed and accuracy measures in six cognitive categories: simple attention, complex attention, working memory, processing speed, short-term memory, and reasoning (Lim and Dinges, 2010). A total sleep deprivation period of 24–48 h significantly reduced performance in all cognitive domains, except for accuracy measures for tasks of processing speed and accuracy measures on tests of reasoning and crystallized intelligence, as there were relatively few studies in each of these categories. Sleep deprivation of 24–48 h had the most significant effects on tests of vigilance, or simple attention, moderate effects for complex attention and working memory, and a small but significant impact on tests of processing speed (Lim and Dinges, 2010). Therefore, sleep deprivation has a significant deleterious impact across most cognitive domains.
Sleep deprivation can also modulate immune parameters critical to host defense against microorganisms, decreasing T cell proliferation (Bollinger et al., 2009) and natural killer (NK) cell cytotoxicity (Irwin et al., 1996). Shorter sleep duration (<7 h per night) has been associated with increased susceptibility to acute infectious illness (Prather et al., 2015). Furthermore, sleep deprivation has also been associated with increases in markers of systemic inflammation that are also related to the development of cardiovascular disease (Mullington et al., 2010; Irwin et al., 2016; Irwin, 2019), such as inflammatory cytokines, cortisol, and C-reactive protein.
A suitable and representative animal model of neuropathogenesis is essential to understand the effects of disease processes and potential therapeutic interventions on the brain, which is usually difficult and complicated to feasibly sample in the living human. Neurocognition of nonhuman primates (NHP) mirrors humans more closely than other animals. Thus, NHP are ideal for correlating neurocognitive performance changes with brain pathologies. The Cambridge Neurological Test Automated Battery (CANTAB) is a tool that contains a series of computerized tests of memory, attention, and executive function, administered through a touch-sensitive screen (Sahakian and Owen, 1992). This tool has been validated for the assessment of neurocognitive deficits associated with neurodegenerative diseases, brain damage, and infectious diseases in humans and animal models (Aslam et al., 2018; Fray and Robbins, 1996; Luciana, 2003; Weed et al., 2004; Hsu et al., 2022). Although NHP models of sleep deprivation have been developed previously to evaluate the effectiveness of drug interventions in improving cognitive deficits resulting from loss of sleep (Porrino et al., 2005; Deadwyler et al., 2007), the current study developed an NHP model to investigate the impacts of sleep deprivation on neurocognition (using the Monkey CANTAB Intellistation) and immune dysfunction.
2. Methods
2.1. Animal selection
Animals were housed at the AAALAC International-accredited Armed Forces Research Institute of Medical Science (AFRIMS) in Bangkok, Thailand. The protocol was approved by the AFRIMS Institutional Animal Care and Use Committee. The research was conducted in compliance with Thailand laws, the Animal Welfare Act, and other U.S. federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals (2011) edition (National Research Council, 2011).
2.2. Study design
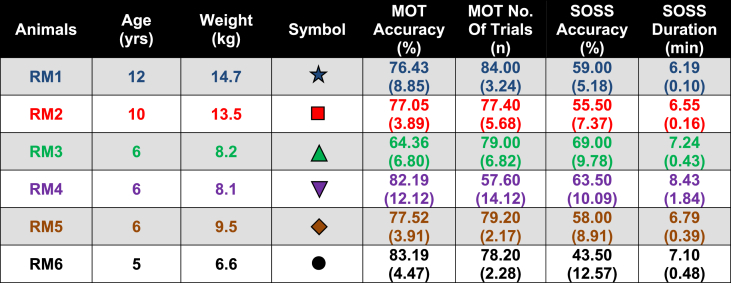
Six male Indian-origin rhesus macaques (Macaca mulatta), with a median age of 6 (range 5–12) years and a median weight of 8.85 (range 6.6–14.7) kg were used in the study. Macaques within this age range are in early to middle adulthood. Before entry into the study, animals were screened and demonstrated normal physical condition, complete blood count, and blood chemistry. As shown in Fig. 1, animals were trained to undergo CANTAB assessment for 20 weeks before the first round of sleep deprivation (total of 48 h without sleep) on day 0. At wk-2, baseline blood was collected. Pre-sleep deprivation neurocognitive performance was established using results from CANTAB assessments at wks −2 and −1. Rounds 1, 2 and, 3 of sleep deprivation occurred on days 0, 14, and 28, respectively. Animals underwent CANTAB assessment after 24 and 48 h of sleep deprivation, and blood was collected after 48 h of sleep deprivation. Animals were allowed to sleep after blood collection. Animals also underwent post-sleep CANTAB assessments and blood collection on days 8, 5, and 2 after completion of the first, second, and third rounds of 48 h of sleep deprivation, respectively. Blood sampling was performed on awake animals using pole collar and primate chair restraint. Characteristics of the animals, including age and weight, are listed in Table 1.
Fig. 1.
Study Schema. Six adult, Indian-origin rhesus macaques were trained to undergo CANTAB assessments, three times per week, for 20 weeks. At week-2, baseline blood was collected. Pre-sleep deprivation neurocognitive performance was established using results from CANTAB assessments at weeks −2 and −1. Round 1, 2 and 3 of sleep deprivation (48 h without sleep) took place on days 0, 14, and 28, respectively. Animals underwent CANTAB assessment after 24 and 48 h of sleep deprivation, followed by blood collection after 48 h of each round of sleep deprivation. Animals were allowed to sleep after blood collection. Animals also underwent post-sleep CANTAB assessment.
Table 1.
Characteristics of the animals used in the study including age and weight, as well as the mean and standard deviation (SD) of pre-sleep deprivation neurocognitive assessment scores by the Cambridge Neuropsychological Test Automated Battery (CANTAB) that was calculated from the last five assessment sessions before the first round of sleep deprivation. Each animal is represented by different symbols and colors.
2.3. CANTAB training and assessment
Animals were trained to undergo CANTAB assessments 2–3 times a week in 5–25 min sessions in the afternoon for 20 weeks, as described previously (Hsu et al., 2022). Step 1: To familiarize the animal, CANTAB equipment was brought in front of the animal's cage. The animal was allowed to explore it and take rewards from the dispenser in response to an auditory stimulus from a manual clicker. Step 2: A colored stimulus, filling the whole screen, would appear, and the animal would receive a reward after touching the stimulus. Step 3: A colored stimulus (that reduced in size iteratively after each set of ten consecutive correct responses) would appear, and the animal would receive a reward after touching the stimulus. Step 4 (Motor Screening Task, MOT): A colored stimulus of fixed-size would appear on the screen in random locations, and the animal would receive a reward after touching the stimulus. Animals were given 10 min to complete as many motor tasks as possible. MOT assesses sensorimotor deficits and reaction speed. Accuracy was reflected by the percentage of correct responses over total responses, and speed was reflected by the number of tasks the animal completed within 10 min. Step 5 (Self-ordered spatial search, SOSS): two colored stimuli of fixed size would appear on the screen in random locations. After the animal touched a stimulus, the screen would blank transiently. The same two stimuli would re-appear, and the animal will only receive a reward after touching the previously untouched stimulus. Animals were required to complete 40 SOSS tasks. SOSS is a measure of working memory and executive function, which are fundamental processes involved in many cognitive tasks. Deficits in these processes can have broad impacts on cognition, such as cognitive deficits resulting from loss of sleep. Accuracy on SOSS was reflected by the percentage of correct responses over total responses, and speed was reflected by the time taken to complete 40 tasks.
2.4. Sleep deprivation procedures
Animals were kept awake for 48 h starting from 7 a.m. when the facility's lights were turned on. Lights in the room remained switched on during the 48 h of sleep deprivation. One or two staff (working in 3–4 h shifts) were always in the room with the animals, ensuring that they remained awake. Methods to keep animals awake included music, video, food enrichment, toys, and human interactions, including talking to, reading to, playing with the animals, blowing soap bubbles, ringing a bell, and gently pulling the squeeze bar.
2.5. Measurement of cellular immune activation
Whole-blood samples were obtained from animals at various times pre- and post-sleep deprivation, as indicated in Fig. 1. The whole-blood was directly stained with fluorescently conjugated antibodies against CD3, CD4, CD8, CD20, CD159a, CD14, CD16, HLA-DR, and CD69 (BD Biosciences, eBioscience, Biolegend). After incubation with the antibodies, the blood was then lysed with RBC lysing buffer (BD Biosciences) and run on an LSRII flow cytometer (BD Biosciences, San Jose, California, USA) to assess the distribution and activation status of peripheral immune cells at different times during the study. Data were analyzed using FlowJo software v.9.9.6 (BD Biosciences).
2.6. Measurement of plasma cytokine levels
Plasma soluble markers of immune activation (including G-CSF, GM-CSF, IFNγ, IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12/23(p40), IL-13, IL-15, IL-17A, MCP-1, MIP-1β, MIP-1α, sD40L, TGF-α, TNF-α, VEGF, and IL-18) were quantified using the MILLIPLEX MAP NHP Cytokine Magnetic Bead Panel (EMD Millipore Corporation, Billerica, Massachusetts, USA) per manufacturer instructions, as previously described (Hsu et al., 2018).
2.7. Measurement of plasma cortisol and C-reactive protein levels
Plasma levels of cortisol (R&D Systems, Inc., Minneapolis, MN, USA) and C-reactive protein (Life Diagnostics, Inc., West Chester, PA) were measured by enzyme-linked immunosorbent assays (ELISA) according to the manufacturer's instructions.
2.8. Statistical analyses
Analyses of CANTAB and immunologic data were performed using GraphPad Prism v8.4.3 (GraphPad Software). We used the Wilcoxon matched-pairs signed rank test to compare between different time points in the same animals. Pre-sleep deprivation CANTAB performance was established by generating mean and standard deviation (SD) using the last five assessment sessions before round 1 of sleep deprivation. To quantify changes post-sleep deprivation, individualized Z-scores (number of SD below or above the pre-sleep deprivation mean) were generated using data from each animal at each assessment post 24 and 48 h of sleep deprivation. Impairment was defined as >2.5 SD reduction in accuracy for both MOT and SOSS, >2.5 SD reduction in the number of MOT performed within 10 min or >2.5 SD increase in time to complete 40 SOSS tasks. This method of quantification of neurocognitive Impairment was based on the work of Weed et al. (2004) and is advantageous as it accounts for individual variability in performance in each animal pre- and post-sleep deprivation.
3. Results
3.1. Neurocognitive impairments post sleep deprivation
Six rhesus macaques underwent three rounds of sleep deprivation (48 h without sleep) at days 0, 14, and 28. Pre-sleep deprivation CANTAB performance for each animal was established based on the mean and standard deviation (SD) of the scores from the last five assessment sessions before the first sleep deprivation round (Table 1).
To assess changes post-sleep deprivation, individualized Z-scores (number of SD below or above the pre-sleep deprivation mean) were generated for each animal at each assessment post 24 and 48 h of sleep deprivation (Fig. 2A and B). Though there were variabilities in neurocognitive performance between the three rounds of sleep deprivation, importantly, impairments defined as >2.5 SD change were identified in all six animals (Fig. 2C). Reductions in accuracy and performance speed were seen in both MOT and SOSS with performance speed appearing to be more affected than accuracy, especially for MOT. More impairments were identified after 48 h than after 24 h of sleep deprivation. The observed impairments largely normalized after sleep.
Fig. 2.
Neurocognitive outcome as assessed by the Cambridge Neuropsychological Test Automated Battery (CANTAB). Individualized Z-scores (number of SD below or above the pre-sleep deprivation mean) were generated for each animal after 24 and 48 h of sleep deprivation. Impairment (shaded in orange) is defined as > 2.5 SD reduction in accuracy for both Motor Screening Task (MOT) (A) and Self-Ordered Spatial Search (SOSS) (B), > 2.5 SD reduction in the number of MOT performed within 10 min or > 2.5 SD increase in time to complete 40 SOSS tasks. Each color represents data from individual animals. (C) Timing of occurrence of impairments (as defined in the figure legend of 2A and 2b), (at 24 and/or 48 h post sleep deprivation) are listed for each individual animal.
3.2. Transient immune activation in the peripheral blood
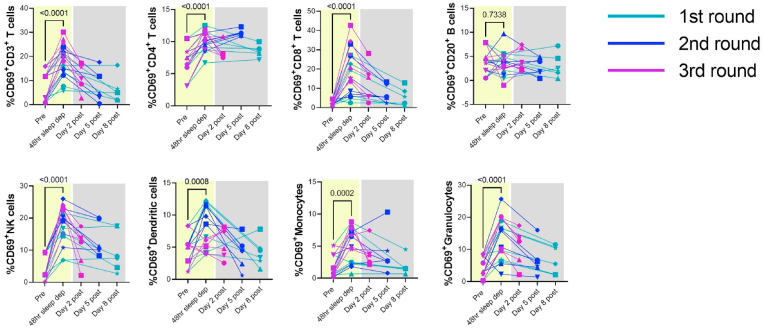
Significant increases in cellular markers of immune activation after 48 h of sleep deprivation, as evidenced by increased CD69 expression, were observed in total T-cells and both CD4+ and CD8+ T cells (p < 0.0001) in the peripheral blood (Fig. 3) when we combined data from animals from the first, second, and third rounds (n = 6 per round) of sleep deprivation. Total T-cell and CD8+ T cell activation mostly resolved by 2 days after the completion of the third round of sleep deprivation and sleep. On the other hand, CD4+ T cell activation was slower to resolve and remained elevated in a subset of animals (RM3, RM4, RM5, and RM6) even eight days following the completion of the first round of sleep deprivation. Significant increases in immune activation after 48 h of sleep deprivation were also observed in innate immune cells, including NK cells (p < 0.001), dendritic cells (p = 0.008), monocytes (p = 0.0002), and granulocytes (p < 0.0001). The cellular immune activation observed in these cells was also transient and resolved after sleep. Thus, 48 h of sleep deprivation was associated with transient cellular immune activation in the peripheral blood, which largely normalized following sleep, except for residual CD4 T cell activation in a subset of animals.
Fig. 3.
Cellular markers of immune activation increased post-sleep deprivation in multiple subsets of immune cells in the peripheral blood. Each color represents data from each round of sleep deprivation, and different symbols represent individual animals as listed Table 1. P values were derived from Wilcoxon matched-pairs signed rank test (n = 18).
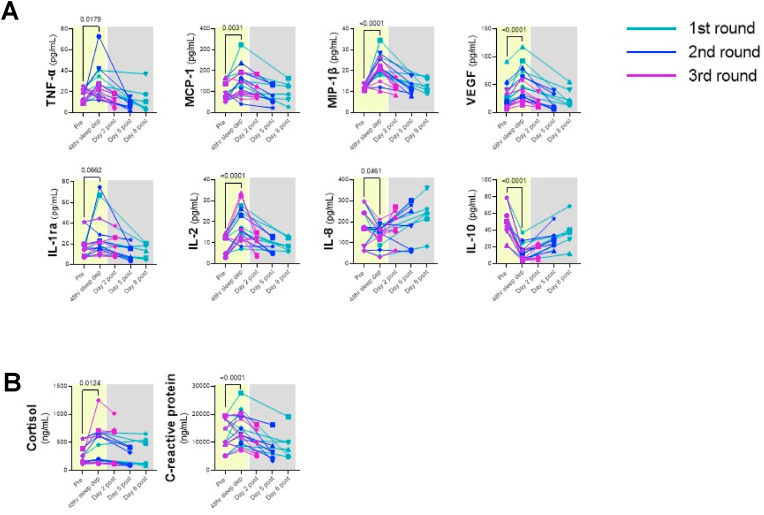
We also observed significant increases in several soluble markers of immune activation, including TNF-α (p = 0.0179), MCP-1 (p = 0.0031), MIP-1β (p < 0.0001), VEGF (p < 0.001), and IL-2 (p < 0.001) in plasma after 48 h of sleep deprivation (Fig. 4A). The levels of these soluble markers reduced and normalized following sleep. We also noted significant decreases in the levels of IL-8 (p = 0.0461), and IL-10 (p < 0.001) (Fig. 4A) following sleep deprivation. Finally, we measured plasma levels of cortisol and C-reactive protein (CRP), which were both significantly elevated [i.e., cortisol (p = 0.0124) and CRP (p < 0.0001)] after 48 h of sleep deprivation, when we combined results from animals after sleep deprivation rounds 1 (n = 6), 2 (n = 6), and 3 (n = 6), (Fig. 4B). These changes normalized following sleep. Interestingly, the observed transient increases in immune activation were concurrent with neurocognitive impairment. This observation suggests a link between inflammation and neurocognitive impairment.
Fig. 4.
Effects of sleep deprivation on plasma soluble markers of immune activation. Plasma levels of TNF-α, MCP-1, MIP-1β, VEGF, IL-1ra, and IL-2 increased, while IL-8 and IL-10 decreased after 48 h of sleep deprivation (A). Plasma cortisol and C-reactive protein also increased post sleep deprivation (B). Each color represents data from each round of sleep deprivation, and different symbols represent individual animals as listed in Table 1. P values were derived from Wilcoxon matched-pairs signed rank test (n = 18).
4. Discussion
To our knowledge, this is the first report demonstrating the associations between sleep deprivation, neurocognitive impairments, and concurrent immune activation in rhesus macaques. Our NHP model of sleep deprivation closely mirrored findings from studies on the impact of sleep deprivation on neurocognition and immune activation in humans, thus validating this model.
Research studies suggest that chronic sleep disturbance or deprivation in humans can lead to neurocognitive dysfunction, sustained immune activation, and alterations of circulating levels of inflammatory markers (Irwin, 2002, 2019; Garbarino et al., 2021; Westermann et al., 2015; Born et al., 1997a). Our NHP results support the above findings. These phenomena could subsequently contribute to the pathogenesis of multiple conditions, including cardiovascular disease, infectious disease, and Alzheimer's disease-associated dementia.
In this study, neurocognition was assessed using MOT (that assesses sensorimotor deficits and reaction speed) and SOSS (that assesses frontal lobe working memory functions) on the Monkey CANTAB, which was adapted from a human neuropsychological testing battery (Roberts et al., 1993). Both of these tests have been widely used in humans and monkeys, across a range of ages (Nagahara et al., 2010; Luciana and Nelson, 1998; Strike et al., 2016; Abbott et al., 2018; Weed et al., 1999) and pathological conditions, including neurodegenerative (Lawrence et al., 1996; Owen et al., 1992), neuropsychiatric (Stip et al., 2005), infectious (Weed et al., 2004; Gold et al., 1998) and to assess the effects of psychotropic agents (D'SouzaDeepak Cyril et al., 2008; Mehta et al., 2001; Taffe et al., 2002; Weed and Gold, 1998). We observed sleep deprivation-related neurocognitive impairments (>2.5 SD change) across six animals. Reductions in accuracy and performance speed were seen in both MOT and SOSS with performance speed appearing to be more affected than accuracy, especially for MOT. More impairments were identified after 48 h than after 24 h of sleep deprivation. A variety of cognitive performance tests have been used in humans to assess the effects of sleep deprivation on neurocognition. Meta-analyses of these studies found that 24–48 h of sleep deprivation were associated with reduction in reaction times, processing speed and accuracy with average effect sizes for speed and accuracy being similar within each cognitive domain (Philibert, 2005; Lim and Dinges, 2010). Thus, findings from our NHP model closely mirrored findings in humans.
Importantly, we found that cellular and soluble immune activation markers increased after 48 h of sleep deprivation. Specifically, we observed significant increase in immune activation in adaptive (i.e., total T-cells and both CD4+ and CD8+ T cells) as well as in innate immune cells (i.e., NK cells, dendritic cells, monocytes, and granulocytes). Though activation in most immune cells normalized following sleep, a subset of animals had increased CD4+ activation eight days after the completion of the third round of sleep deprivation and sleep.
Sleep deprivation's effect on circulating inflammatory cytokine levels has been examined in several studies with inconsistent findings. Some studies showed increases in cytokines, such as IL-6, IL-10, and TNF-α (Vgontzas et al., 2004; Shearer et al., 2001), while others showed decreases (Haack et al., 2002) or no change (Born et al., 1997b; Dinges et al., 1995) during 15–88 h of total sleep deprivation. In our NHP model of sleep deprivation, we observed increases in cellular and soluble markers of immune activation after 48 h of sleep deprivation. Further, consistent with prior studies, we also found higher levels of cortisol and CRP, significant predictors of cardiovascular disease, after 48 h of sleep deprivation that returned to baseline level after sleep.
Our study showed an inherent variability in the parameters studied. Increasing the sample size and increasing the number of rounds of sleep deprivation would likely mitigate this caveat. Furthermore, the three rounds of sleep deprivation used in this study cannot accurately reflect the impact of recurrent episodes of sleep deprivation over a lifetime. Nevertheless, investigators can utilize this model in combination with the sampling of blood and CSF, neurocognitive assessments and brain imaging to dissect the pathogenesis of sleep deprivation. The model also has utility for evaluations of the impact of sleep deprivation on susceptibility to infection and adverse neurologic outcomes. Finally, investigators can implement the NHP model to evaluate the safety and impact of interventions that enhance performance during sleep deprivation.
Funding
This work was supported by a cooperative agreement (W81XWH-18-2-0040) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., (HJF) and the U.S. Department of Defense (DoD). MSP is supported by a K01 award from the National Institutes of Health's Office of the Director (K01OD031900).
Disclaimer
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
Research was conducted under an IACUC-approved animal use protocol in an AAALAC International - accredited facility with a Public Health Services Animal Welfare Assurance and in compliance with the Animal Welfare Act and other federal statutes and regulations relating to laboratory animals.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2023.100683.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- Abbott Rosemary A., et al. Vol. 11. 2018. pp. 36–44. (Normative Data from Linear and Nonlinear Quantile Regression in CANTAB: Cognition in Mid-to-late Life in an Epidemiological Sample). Alzheimer's & dementia (Amsterdam, Netherlands) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam Rabeea'h W., et al. A systematic review of the diagnostic accuracy of automated tests for cognitive impairment. Int. J. Geriatr. Psychiatr. 2018;33(4):561–575. doi: 10.1002/gps.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger T., et al. Sleep-dependent activity of T cells and regulatory T cells. Clin. Exp. Immunol. 2009;155(2):231–238. doi: 10.1111/j.1365-2249.2008.03822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J., et al. Effects of sleep and circadian rhythm on human circulating immune cells. J. Immunol. 1997;158(9):4454–4464. [PubMed] [Google Scholar]
- Born J., et al. Effects of sleep and circadian rhythm on human circulating immune cells. J. Immunol. 1997;158(9):4454–4464. [PubMed] [Google Scholar]
- D'Souza, Deepak Cyril, et al. Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Delta-9-tetrahydrocannabinol in humans. Psychopharmacology. 2008;198(4):587–603. doi: 10.1007/s00213-007-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler Sam A., et al. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J. Neurosci. : the official journal of the Society for Neuroscience. 2007;27(52):14239–14247. doi: 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges D.F., et al. Sleep deprivation and human immune function. Adv. Neuroimmunol. 1995;5(2):97–110. doi: 10.1016/0960-5428(95)00002-j. [DOI] [PubMed] [Google Scholar]
- Fray P.J., Robbins T.W. CANTAB battery: proposed utility in neurotoxicology. Neurotoxicol. Teratol. 1996;18(4):499–504. doi: 10.1016/0892-0362(96)00027-x. [DOI] [PubMed] [Google Scholar]
- Garbarino Sergio, et al. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 2021;4(1):1304. doi: 10.1038/s42003-021-02825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L.H., et al. Longitudinal analysis of behavioral, neurophysiological, viral and immunological effects of SIV infection in rhesus monkeys. J. Med. Primatol. 1998;27 doi: 10.1111/j.1600-0684.1998.tb00234.x. 2-3. 104-12. [DOI] [PubMed] [Google Scholar]
- Haack Monika, et al. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinology. 2002;27(8):921–931. doi: 10.1016/s0306-4530(02)00006-9. [DOI] [PubMed] [Google Scholar]
- Hsu Denise C., et al. Central nervous system inflammation and infection during early, nonaccelerated simian-human immunodeficiency virus infection in rhesus macaques. J. Virol. 2018;92(11) doi: 10.1128/JVI.00222-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Denise C., et al. Neurocognitive impact of Zika virus infection in adult rhesus macaques. J. Neuroinflammation. 2022;19(1):40. doi: 10.1186/s12974-022-02402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin Michael. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav. Immun. 2002;16(5):503–512. doi: 10.1016/s0889-1591(02)00003-x. [DOI] [PubMed] [Google Scholar]
- Irwin Michael R. Sleep and inflammation: partners in sickness and in health. Nat. Rev. Immunol. 2019;19(11):702–715. doi: 10.1038/s41577-019-0190-z. [DOI] [PubMed] [Google Scholar]
- Irwin M., et al. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. Faseb. J. : official publication of the Federation of American Societies for Experimental Biology. 1996;10(5):643–653. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- Irwin Michael R., et al. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatr. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslowsky Meni, Harvey Babkoff. Meta-analysis of the relationship between total sleep deprivation and performance. Chronobiol. Int. 1992;9(2):132–136. doi: 10.3109/07420529209064524. [DOI] [PubMed] [Google Scholar]
- Lawrence A.D., et al. Executive and mnemonic functions in early Huntington's disease. Brain : J. Neurol. 1996;119(Pt 5):1633–1645. doi: 10.1093/brain/119.5.1633. [DOI] [PubMed] [Google Scholar]
- Lim Julian, Dinges David F. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol. Bull. 2010;136(3):375–389. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana Monica. Practitioner review: computerized assessment of neuropsychological function in children: clinical and research applications of the Cambridge Neuropsychological Testing Automated Battery (CANTAB) J. Child Psychol. Psychiatry Allied Discip. 2003;44(5):649–663. doi: 10.1111/1469-7610.00152. [DOI] [PubMed] [Google Scholar]
- Luciana M., Nelson C.A. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36(3):273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Mehta Mitul A., et al. In: Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. Solanto M.V., Arnsten A.F.T., Castellanos F.X., editors. Oxford University Press; 2001. Comparative psychopharmacology of methylphenidate and related drugs in human volunteers, patients with ADHD, and experimental animals; pp. 303–331. [Google Scholar]
- Mullington Janet M., et al. Sleep loss and inflammation. Best Pract. Res. Clin. Endocrinol. Metabol. 2010;24(5):775–784. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara Alan H., et al. Age-related cognitive deficits in rhesus monkeys mirror human deficits on an automated test battery. Neurobiol. Aging. 2010;31(6):1020–1031. doi: 10.1016/j.neurobiolaging.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- Owen A.M., et al. Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain : J. Neurol. 1992;115(Pt 6):1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Philibert Ingrid. Sleep loss and performance in residents and nonphysicians: a meta-analytic examination. Sleep. 2005;28(11):1392–1402. doi: 10.1093/sleep/28.11.1392. [DOI] [PubMed] [Google Scholar]
- Pilcher J.J., Huffcutt A.I. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19(4):318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- Porrino Linda J., et al. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3(9):e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather Aric A., et al. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. 2015;38(9):1353–1359. doi: 10.5665/sleep.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A.C., Sahakain B.J. In: Comparable Tests of Cognitive Function in Monkey and Man. Behavioural Neuroscience: A Practical Approach. Rickwood D., Hames B.D., editors. 1993. pp. 164–184. Oxford University Press. [Google Scholar]
- Sahakian B.J., Owen A.M. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J. R. Soc. Med. 1992;85(7):399–402. [PMC free article] [PubMed] [Google Scholar]
- Shearer W.T., et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J. Allergy Clin. Immunol. 2001;107(1):165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- Stip Emmanuel, et al. Cognitive discernible factors between schizophrenia and schizoaffective disorder. Brain Cognit. 2005;59(3):292–295. doi: 10.1016/j.bandc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Strike Siobhán C., et al. A high omega-3 fatty acid multinutrient supplement benefits cognition and mobility in older women: a randomized, double-blind, placebo-controlled pilot study. The journals of gerontology. Series A, Biological sciences and medical sciences. 2016;71(2):236–242. doi: 10.1093/gerona/glv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe Michael A., et al. Ketamine impairs multiple cognitive domains in rhesus monkeys. Drug Alcohol Depend. 2002;68(2):175–187. doi: 10.1016/s0376-8716(02)00194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas A.N., et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J. Clin. Endocrinol. Metabol. 2004;89(5):2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Weed M.R., Gold L.H. The effects of dopaminergic agents on reaction time in rhesus monkeys. Psychopharmacology. 1998;137(1):33–42. doi: 10.1007/s002130050590. [DOI] [PubMed] [Google Scholar]
- Weed M.R., et al. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain research. Cognitive brain research. 1999;8(3):185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Weed Michael R., et al. Impaired performance on a rhesus monkey neuropsychological testing battery following simian immunodeficiency virus infection. AIDS Res. Hum. Retrovir. 2004;20(1):77–89. doi: 10.1089/088922204322749521. [DOI] [PubMed] [Google Scholar]
- Westermann Jürgen, et al. System consolidation during sleep - a common principle underlying psychological and immunological memory formation. Trends Neurosci. 2015;38(10):585–597. doi: 10.1016/j.tins.2015.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.