Abstract
Between August 1994 and September 1996, 28 glycopeptide-resistant enterococci (GRE) were isolated from 8 infected patients and 11 intestinal carriers hospitalized at the University Hospital of Geneva. Identification to the species was made by both phenotypic (API 20 STREP and Rapid ID 32 STREP systems, and Vitek Gram Positive Identification Card) and genotypic methods using a multiplex PCR assay developed also for the determination of the genotype of glycopeptide resistance (vanA, vanB, vanC1, and vanC2-C3 genes). Fifteen isolates were identified as Enterococcus faecium, 8 as E. gallinarum, 4 as E. faecalis, and 1 as E. hirae. All of the phenotypic identification methods failed to differentiate some isolates of E. gallinarum from E. faecium, or vice versa. Both vanA (n = 18) and vanB (n = 4) glycopeptide resistance genotypes were found. For the first time, the vanB determinant was found in two isolates of E. gallinarum. Two patients were colonized by two different species containing the vanA gene and one by two different species containing the vanB gene. All vanA isolates were highly resistant to both vancomycin and teicoplanin except for three isolates which were susceptible to teicoplanin. Molecular typing by pulsed-field gel electrophoresis showed identical or similar patterns among E. faecium isolates with the vanA gene in five patients for whom the epidemiological link could not be always elucidated. This study emphasizes the necessity of utilizing both phenotypic and genotypic methods to characterize GRE.
Enterococci now represent the second leading cause of nosocomial urinary tract infections and the third leading cause of nosocomial bacteremia (4, 22). Enterococci can survive for prolonged periods on environmental surfaces and on the hands of health care workers. In humans, the major reservoir of enterococci is the gastrointestinal and genitourinary tracts (4, 22).
The genus Enterococcus includes 20 species, but most human enterococcal infections are caused by E. faecalis (12). E. faecium is the second most commonly identified species (12). A few cases of infections caused by E. durans, E. gallinarum, and E. casseliflavus have been reported (18, 26). Identification to the species level of enterococci is not routinely performed except for E. faecalis and E. faecium (23), which may explain a possible underestimation of the frequency of occurrence of the other species.
The emergence of enterococci during the last decade has resulted mostly from their antimicrobial resistance and less from their virulence factors (17). The antimicrobial resistance spans different antimicrobial groups, including β-lactam antibiotics, macrolides, aminoglycosides, and glycopeptides. Glycopeptide-resistant enterococci (GRE), first described in 1988 (19), have been reported in North America and several European countries (2, 5, 14–16). In certain U.S. hospitals the incidence reached 14% in 1995 (24). Three types of acquired glycopeptide resistance are known: VanA and VanB are the most predominant (21, 34), whereas vanD has been reported only in one strain of E. faecium (29). VanC glycopeptide resistance is intrinsic in E. gallinarum, E. casseliflavus, and E. flavescens (20, 27). The resistance is due to the synthesis of modified peptidoglycan precursors with reduced affinity to glycopeptides (21, 34).
The difficulty of treating infections due to GRE (10, 21), which might be resistant to all antimicrobial agents used for treatment of systemic infections (16, 28), emphasizes the need for detection of acquired GRE rapidly and accurately (31, 34). This also help to limit the intrahospital dissemination of GRE.
From August 1994 to September 1996, 28 GRE were isolated in our laboratory. The aim of the present study was to determine the correlation between phenotypic and genotypic identification methods, to characterize the phenotypes and genotypes of the glycopeptide-resistant isolates, and to explore the genetic relationship between the isolates.
(This study was presented in part at the 8th European Congress of Clinical Microbiology and Infectious Diseases, Lausanne, Switzerland, 25 to 28 May 1997.)
MATERIALS AND METHODS
Patients and bacterial isolates.
The University Hospital of Geneva is a 1,300-bed health care center providing primary and tertiary care for the city and the surrounding area. GRE were isolated from infected patients by classical microbiological techniques (12). Whenever possible, patients infected by GRE were subsequently screened for intestinal carriage. From stools, GRE were isolated with selective bile-esculin-azide agar (Difco, Detroit, Mich.) supplemented with 8 mg of vancomycin (Eli Lily) per liter (1). A second source of GRE were stools from immunocompromised patients hospitalized in the hematology-oncology and bone marrow transplant units which were controlled by surveillance cultures. If a predominant organism was found, it was further analyzed for identification. In total, 28 isolates of GRE were collected from nineteen hospitalized patients between August 1994 and September 1996. The origins of the isolates are summarized in Table 1.
TABLE 1.
Characteristics of 28 GRE isolated at the University Hospital of Geneva
| Patient | Isolate | Site | Date (mo/yr) | Warda | Genotypic identificationb | MIC (mg/liter)c
|
Glycopeptide genotype | PFGE pattern | |
|---|---|---|---|---|---|---|---|---|---|
| VA | TEI | ||||||||
| 1 | 1 | Stool | 8/94 | Medecine | E. faecium | ≥256 | ≥256 | vanA | A1 |
| 2 | 2 | Prosthesis | 2/95 | Orthopedics | E. faecium | ≥256 | 64 | vanA | B1 |
| 3 | Stool | 3/95 | Orthopedics | E. faecium | ≥256 | 64 | vanA | B2 | |
| 3 | 4 | Ulcer | 3/95 | Orthopedics | E. faecium | ≥256 | 64 | vanA | B1 |
| 5 | Stool | 3/95 | Orthopedics | E. faecium | ≥256 | 64 | vanA | B1 | |
| 4 | 6 | Stool | 3/95 | Hemat/onco | E. faecium | ≥256 | ≥256 | vanA | B3 |
| 5 | 7 | Stool | 6/95 | Medecine | E. gallinarum | 8 | 0.5 | vanC1 | C1 |
| 6 | 8 | Stool | 6/95 | Surg/ICU | E. gallinarum | 8 | 0.12 | vanC1 | D |
| 7 | 9 | Ascites | 6/95 | Surg/ICU | E. gallinarum | 4 | 0.25 | vanC1 | E |
| 8 | 10 | Wound | 9/95 | Med/ICU | E. faecalis | 64 | 4 | vanA | F |
| 9 | 11 | Stool | 9/95 | Medecine | NR | ≥256 | 0.25 | vanA | Not done |
| 10 | 12 | Stool | 10/95 | Long-term H | E. faecium | ≥256 | 4 | vanA | A2 |
| 11 | 13 | Stool | 12/95 | Surgery | E. faecium | ≥256 | 64 | vanA | G |
| 12 | 14 | Stool | 12/95 | Medecine | E. gallinarum | 16 | 2 | vanC1 | H |
| 13 | 15 | Stool | 1/96 | Medecine | E. gallinarum | ≥256 | 3 | vanC1, vanB | I |
| 14 | 16 | Stool | 1/96 | Hemat/onco | E. gallinarum | 16 | 0.2 | vanC1 | C1 |
| 15 | 17 | Stool | 2/96 | Surg/ICU | E. gallinarum | 8 | 0.25 | vanC1 | C2 |
| 16 | 18 | Peritoneum | 4/96 | Surg/ICU | E. faecium | ≥256 | 64 | vanA | K |
| 17 | 19 | Peritoneum | 5/96 | Surg/ICU | E. faecium | ≥256 | 64 | vanA | B4 |
| 20 | Stool | 7/96 | Surg/ICU | E. faecium | ≥256 | 64 | vanA | B5 | |
| 21 | Stool | 7/96 | Surg/ICU | E. faecalis | ≥256 | 64 | vanA | L | |
| 18 | 22 | Urethra | 5/96 | Surg/ICU | E. faecium | ≥256 | 64 | vanA | B4 |
| 23 | Stool | 6/96 | Surg/ICU | E. faecalis | ≥256 | 64 | vanA | L | |
| 24 | Wound | 7/96 | Surg/ICU | E. faecalis | ≥256 | 64 | vanA | L | |
| 25 | Stool | 7/96 | Surg/ICU | E. faecium | ≥256 | 64 | vanA | B6 | |
| 19 | 26 | Stool | 7/96 | Hemat/onco | E. faecium | 32 | 0.5 | vanB | M1 |
| 27 | Stool | 7/96 | Hemat/onco | E. gallinarum | 64 | 2 | vanC1, vanB | N | |
| 28 | Gallbladder | 9/96 | Med/ICU | E. faecium | 32 | 0.5 | vanB | M2 | |
Hemat/onco, hematology-oncology; Med/ICU, medical intensive care unit; Surg/ICU, surgical intensive care unit; long-term H, long-term hospitalization unit.
NR, no result obtained.
VA, vancomycin; TEI, teicoplanin.
Phenotypic identification.
Identification of the isolates to the genus level was performed by Gram staining, catalase reaction, growth and blackening of bile-esculin agar, and growth in the presence of 6.5% NaCl. Identification to species level was performed by using API 20 STREP and rapid ID 32 STREP (bioMérieux, Marcy l’Etoile, France) and the Vitek Gram Positive Identification Card (GPI) (bioMérieux Vitek Inc., Hazelwood, Mich.), according to the recommendations of the manufacturers. Three categories of scores for species level identification were used: (i) excellent or very good, (ii) good, and (iii) uncertain.
Antimicrobial susceptibility testing.
Susceptibility tests were performed and interpreted according to guidelines from the National Committee for Clinical Laboratory Standards (NCCLS) (25). GRE were detected by their growth on brain heart infusion agar (BBL Microbiology Systems, Cockeysville, Md.) containing 6 mg of vancomycin per liter, as recommended by NCCLS (25). MICs were determined by the E-test method (AB Biodisk, Solna, Sweden) on Mueller-Hinton agar plates (Oxoid Ltd., Basingstoke, England). The following antibiotics were tested: penicillin, ampicillin, erythromycin, vancomycin, teicoplanin, gentamicin, and streptomycin. High-level resistance to aminoglycosides was determined by use of the E-test with high drug concentrations (range, 0.064 to 1,024 mg/liter). The presence of β-lactamase was determined with cefinase disks (BBL Microbiology Systems). E. faecalis ATCC 29212 and Staphylococcus aureus ATCC 29213 were used as quality control strains.
Genotypic identification and determination of glycopeptide resistance genotype.
The genes encoding d-alanine–d-alanine ligases specific for E. faecium (ddlE. faecium) and for E. faecalis (ddlE. faecalis) and the glycopeptide resistance determinants vanA, vanB, vanC1, and vanC2-C3 were detected by a multiplex PCR assay, as described by Dutka-Malen et al. (9). The following well-characterized GRE strains belonging to genotypes vanA, vanB, and vanC were used as quality control strains: E. faecium BM4147 (vanA), E. faecalis V583 (vanB), E. gallinarum BM4174 (vanC1), and E. casseliflavus ATCC 25788 (vanC2).
Genotyping.
Molecular typing was performed by pulsed-field gel electrophoresis (PFGE) with a temperature-controlled CHEF DR III System (Bio-Rad Laboratories, Hercules, Calif.). Genomic DNA was digested by the restriction endonuclease SmaI (New England Biolabs, Beverly, Mass.) and additionally by EagI for E. gallinarum. The molecular size standard (S. aureus NCTC 8325 DNA digested with SmaI) was run in three lanes per gel. Following staining with ethidium bromide, restriction fragments were visualized by a UV transilluminator and documented by use of a video gel documentation system (MWG-BIOTECH, Ebersberg, Germany). PFGE pattern analysis was performed with GelCompar 4.0 software (Applied Maths, Kortrijk, Belgium). The dendrograms were calculated by the unweighted pair group method using arithmetic averages. The restriction patterns were interpreted according to the method of Tenover et al. (32). PFGE types were designated by letters; subtypes (two to six band differences) were designated by numerals.
RESULTS
GRE isolates.
From August 1994 to September 1996, a total of 28 GRE were collected from 19 patients hospitalized in various units, including 4 isolates from the orthopedic unit, 11 isolates from the surgical intensive care unit, 4 isolates from the hematology-oncology unit, and nine isolates from various other units of the medical or surgery departments (Table 1). Three patients were infected by GRE (patients 7, 8, and 16), 5 were simultaneously infected and colonized (patients 2, 3, 17, 18, and 19), and 11 were colonized (patients 1, 4 through 6, and 9 through 15). Three infected patients were colonized by more than one species of GRE (patients 17, 18, and 19).
Identification.
Molecular identification showed that 15 isolates were E. faecium, 4 were E. faecalis, and 8 were E. gallinarum (see Table 2). One isolate (isolate 11) which could not be identified by the PCR multiplex assay was identified as E. durans by API 20 STREP and as E. hirae by rapid ID 32 STREP and Vitek GPI. The strain was considered to be E. hirae because of its capacity to metabolize sucrose and raffinose (13). The four isolates identified as E. faecalis by PCR were confirmed by API 20 STREP, rapid ID 32 STREP, and Vitek GPI. Discrepancies in the species identification of E. faecium and E. gallinarum isolates were observed between the genotypic and phenotypic methods. Moreover, differences in identification were observed between API 20 STREP, rapid ID 32 STREP, and Vitek GPI. Four of the eight isolates identified as E. gallinarum by PCR assay did not ferment raffinose. They were identified as E. faecium by API 20 STREP with a very good identification score, and the other four isolates were identified as E. casseliflavus (isolates 14, 15, 17, and 27). In contrast, all eight of these isolates were identified as E. gallinarum by the rapid ID 32 STREP method, five with an excellent score (99.9%; T = 0.85), one with a very good score (77.3%; T = 0.56), one with a doubtful score (99.9%; T = 0.69), and one with good identification to the genus (57.2%; T = 0.49). The Vitek GPI method identified two isolates as E. gallinarum (one with a good identification score and one with a presumptive identification score), two as E. casseliflavus/gallinarum, and four as E. faecium, one of which had a good identification score. Of the 15 isolates identified as E. faecium by PCR, all were identified as such by API 20 STREP and Vitek GPI, but four isolates were identified as E. gallinarum by the Rapid ID 32 STREP assay (isolates 2, 3, 5, and 12).
TABLE 2.
Comparative identification and susceptibility to antimicrobial agents of 28 GRE isolated at the University Hospital of Geneva
| Patient | Isolate | Organism identified (identification score)a
|
Genotypic identificationb | MIC (mg/liter)c
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| API 20 STREP | Rapid ID 32 STREP | Vitek GPI | P | AM | E | GM | SM | VA | TEI | |||
| 1 | 1 | E. faecium (2) | E. faecium (2) | E. faecium (1) | E. faecium | 8 | 1 | ≥256 | 16 | 96 | ≥256 | ≥256 |
| 2 | 2 | E. faecium (2) | E. gallinarum (3) | E. faecium (1) | E. faecium | ≥256 | 16 | ≥256 | 24 | ≥1,024 | ≥256 | 64 |
| 3 | E. faecium (2) | E. gallinarum (3) | E. faecium (1) | E. faecium | ≥256 | 16 | ≥256 | 24 | ≥1,024 | ≥256 | 64 | |
| 3 | 4 | E. faecium (2) | E. faecium (3) | E. faecium (1) | E. faecium | ≥256 | 16 | ≥256 | 24 | ≥1,024 | ≥256 | 64 |
| 5 | E. faecium (2) | E. gallinarum (1) | E. faecium (1) | E. faecium | ≥256 | 16 | ≥256 | 24 | ≥1,024 | ≥256 | 64 | |
| 4 | 6 | E. faecium (2) | E. faecium (2) | E. faecium (1) | E. faecium | ≥256 | ≥256 | ≥256 | 24 | ≥1,024 | ≥256 | ≥256 |
| 5 | 7 | E. faecium (2) | E. gallinarum (3) | E. faecium (3) | E. gallinarum | 0.5 | 0.5 | 0.38 | 8 | 32 | 8 | 0.5 |
| 6 | 8 | E. faecium (2) | E. gallinarum (1) | E. gallinarum (1) | E. gallinarum | 1 | 0.5 | 0.38 | 12 | 48 | 8 | 0.12 |
| 7 | 9 | E. faecium (2) | E. gallinarum (1) | E. faecium (1) | E. gallinarum | 1 | 0.75 | 2 | 12 | 32 | 4 | 0.25 |
| 8 | 10 | E. faecalis (1) | E. faecalis (1) | E. faecalis (1) | E. faecalis | 1.5 | 0.25 | ≥256 | ≥1,024 | ≥1,024 | 64 | 4 |
| 9 | 11 | E. durans (2) | E. hirae (1) | E. hirae (1) | NR | 0.75 | 0.5 | 0.5 | 16 | ≥256 | ≥256 | 0.25 |
| 10 | 12 | E. faecium (2) | E. gallinarum (3) | E. faecium (1) | E. faecium | 8 | 1.5 | 8 | 24 | 128 | ≥256 | 4 |
| 11 | 13 | E. faecium (3) | E. faecium (1) | E. faecium (1) | E. faecium | 0.125 | 0.125 | ≥256 | 6 | 48 | ≥256 | 64 |
| 12 | 14 | E. casseliflavus (3) | E. gallinarum (3) | E. faecium (3) | E. gallinarum | 1 | 0.75 | 1 | 6 | 24 | 16 | 2 |
| 13 | 15 | E. casseliflavus (3) | E. gallinarum (1) | E. gallinarum (3) | E. gallinarum | 4 | 1.5 | ≥256 | 6 | 16 | ≥256 | 3 |
| 14 | 16 | E. faecium (2) | E. gallinarum (3) | E. faecium (3) | E. gallinarum | 0.5 | 0.5 | 0.25 | 8 | 32 | 16 | 0.2 |
| 15 | 17 | E. casseliflavus (1) | E. gallinarum (1) | E. cass/gall (1) | E. gallinarum | 0.75 | 0.75 | 2 | 12 | 32 | 8 | 0.25 |
| 16 | 18 | E. faecium (2) | E. faecium (1) | E. faecium (1) | E. faecium | 16 | 2 | ≥256 | 24 | 128 | ≥256 | 64 |
| 17 | 19 | E. faecium (2) | E. faecium (3) | E. faecium (1) | E. faecium | ≥256 | 24 | ≥256 | 24 | ≥1,024 | ≥256 | 64 |
| 20 | E. faecium (2) | E. faecium (1) | E. faecium (1) | E. faecium | ≥256 | 24 | ≥256 | 24 | ≥1,024 | ≥256 | 64 | |
| 21 | E. faecalis (1) | E. faecalis (1) | E. faecalis (1) | E. faecalis | 1.5 | 0.38 | ≥256 | 8 | ≥1,024 | ≥256 | 64 | |
| 18 | 22 | E. faecium (2) | E. faecium (3) | E. faecium (1) | E. faecium | ≥256 | 24 | ≥256 | 24 | ≥1,024 | ≥256 | 64 |
| 23 | E. faecalis (1) | E. faecalis (1) | E. faecalis (1) | E. faecalis | 1.5 | 0.25 | ≥256 | 24 | ≥1,024 | ≥256 | 64 | |
| 24 | E. faecalis (1) | E. faecalis (1) | E. faecalis (1) | E. faecalis | 1.5 | 0.25 | ≥256 | 24 | ≥1,024 | ≥256 | 64 | |
| 25 | E. faecium (2) | E. faecium (1) | E. faecium (1) | E. faecium | ≥256 | 4 | ≥256 | 24 | ≥1,024 | ≥256 | 64 | |
| 19 | 26 | E. faecium (2) | E. faecium (1) | E. faecium (1) | E. faecium | ≥256 | 24 | ≥256 | 16 | ≥1,024 | 32 | 0.5 |
| 27 | E. casseliflavus (3) | E. gallinarum (1) | E. cass/gall (3) | E. gallinarum | 2 | 1 | ≥256 | 16 | ≥1,024 | 64 | 2 | |
| 28 | E. faecium (2) | E. faecium (1) | E. faecium (1) | E. faecium | ≥256 | 24 | ≥256 | 16 | ≥1,024 | 32 | 0.5 | |
Identification scores of phenotypic identifications: 1, excellent or very good identification; 2, good identification; 3, uncertain identification to the species level. E. cass/gall, E. casseliflavus/gallinarum.
NR, no result obtained.
P, penicillin; AM, ampicillin; E, erythromycin; GM, gentamicin; SM, streptomycin; VA, vancomycin; TEI, teicoplanin.
Antimicrobial susceptibility testing.
Table 2 presents MICs of vancomycin for the 28 GRE isolates. Six had intermediate resistance (MIC, >4 to <32 mg/liter), and 22 were resistant (MIC ≥ 32 mg/liter). Thirteen of these were susceptible to teicoplanin (MIC ≤ 8 mg/liter). All E. faecalis and E. gallinarum isolates were susceptible to penicillin (MIC ≤ 8 mg/liter) and ampicillin (MIC ≤ 8 mg/liter). In contrast, 11 of 15 E. faecium isolates were highly resistant to penicillin (MIC ≥ 256 mg/liter) and 1 of them was resistant to ampicillin (MIC ≥ 256 mg/liter). None of the strains showed β-lactamase activity. Only 1 isolate was highly resistant to gentamicin, whereas 16 of 28 isolates were highly resistant to streptomycin. Erythromycin resistance was present in 20 of 28 isolates of GRE. One isolate of E. faecium recovered from a stool sample (isolate 6) was highly resistant to all antimicrobial agents tested except gentamicin.
Genotype of glycopeptide resistance.
The vanA gene was detected in 18 of 28 isolates (Table 1) including 14 E. faecium, 3 E. faecalis, and 1 E. hirae. These 18 vanA isolates were all highly resistant to vancomycin (MIC ≥ 64 mg/liter). Fifteen of these were highly resistant to teicoplanin (MIC ≥ 64 mg/liter), whereas the other three (isolates 10, 11, and 12) were susceptible to this compound (MIC ≤ 4 mg/liter). The vanB gene was detected in 4 of 28 isolates, including two E. faecium isolates (isolates 26 and 28) and two E. gallinarum isolates (isolates 15 and 27). MICs of vancomycin for vanB strains ranged from 32 to 256 mg/liter, whereas those of teicoplanin remained in the susceptibility range (MIC ≤ 4 mg/liter). All eight E. gallinarum isolates contained the vanC1 gene. The six isolates of E. gallinarum containing the vanC1 gene without the additional glycopeptide resistance gene (isolates 7 through 9, 14, 16, and 17) showed intermediate resistance to vancomycin (MIC = 8 to 16 mg/liter) and susceptibility to teicoplanin (MIC ≤ 2 mg/liter). When patients were colonized by different species of enterococci, similar genotypes were observed for the different species of a single patient (vanA for patients 17 and 18 and vanB for patient 19).
Genotyping.
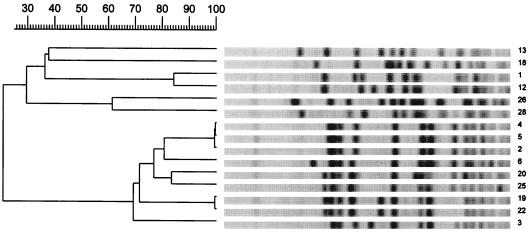
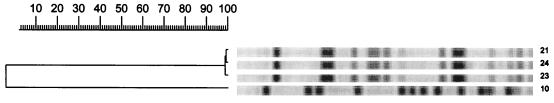
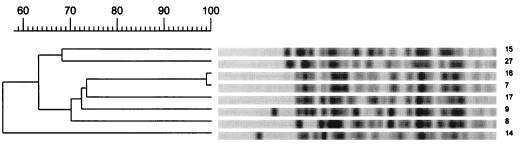
The results of genotyping are presented in Table 1 and Fig. 1, 2, and 3. Analysis of PFGE patterns obtained with the 15 E. faecium isolates showed a cluster of 9 isolates of E. faecium vanA (isolates 2 through 6, 19, 20, 22, and 25) with identical or similar banding patterns (PFGE pattern B, zero to six fragment differences) (Fig. 1). These isolates came from five patients (patients 2 through 4, 17, and 18) who were hospitalized in three different wards. In addition, the E. faecium stool isolates (isolates 1 and 12) from two other patients (patients 1 and 10) differed by only three fragments and may be considered probably genetically related (PFGE pattern A) (Fig. 1). The two isolates of E. faecium vanB (isolates 26 and 27) from the same patient (patient 19) present differences in six bands and were interpreted as possibly related (PFGE pattern M) (Fig. 1). The other E. faecium isolates (isolates 13 and 18), from patients 11 and 16, were not related. The E. faecalis isolates (isolates 21, 23, and 24) from patients 17 and 18, who were hospitalized in the surgical intensive care unit in the same time period, appeared to be identical (PFGE pattern L) (Fig. 2). Among the E. gallinarum isolates, two isolates (isolates 7 and 16) from two different patients (5 and 14) showed identical banding patterns (PFGE pattern C1) (Fig. 3). The six other isolates (isolates 8, 9, 14, 15, 17, and 27), from patients 6, 7, 12, 13, 15, and 19, including the two E. gallinarum isolates with the vanB gene, were not related (Fig. 3).
FIG. 1.
PFGE restriction fragment patterns of SmaI-digested genomic DNA obtained from glycopeptide-resistant E. faecium isolates and a dendrogram showing similarities. Numbering of isolates corresponds to the numbering in Tables 1 and 2.
FIG. 2.
PFGE restriction fragment patterns of SmaI-digested genomic DNA obtained from glycopeptide-resistant E. faecalis isolates and a dendrogram showing similarities. Numbering of isolates corresponds to the numbering in Tables 1 and 2.
FIG. 3.
PFGE restriction fragment patterns of EagI-digested genomic DNA obtained from glycopeptide-resistant E. gallinarum isolates and a dendrogram showing similarities. Numbering of isolates corresponds to the numbering in Tables 1 and 2.
DISCUSSION
The present study emphasizes the difficulties of phenotypical characterization of clinical isolates of GRE. Characterizing GRE allows the distinction between acquired GRE and intrinsic GRE to be made. This is important for clinicians and for implementation of infection control measures (21, 31, 34). Acquired GRE are more difficult to treat than intrinsic GRE due to their broad-spectrum antimicrobial resistance. Moreover, only acquired resistance is transferable to other enterococci and is associated with nosocomial epidemics (21, 34). In the present study, three limitations of the phenotypic methods used for both the identification of enterococci and the determination of the type of glycopeptide resistance are highlighted. First, enterococci are not correctly identified to the species level by phenotypic methods. Second, because intrinsic GRE can acquire additional genes of vancomycin resistance, the identification of enterococci to the species level does not predict the glycopeptide resistance type. Third, discrepancies between the VanA and VanB phenotypes and their correspondent vanA and vanB genotypes may exist.
Our results showed that three commercially available kits frequently used in microbiology laboratories for identification of enterococci (API 20 STREP, Rapid ID 32 STREP, and Vitek GPI), failed to differentiate some E. gallinarum isolates from E. faecium, or vice versa: E. gallinarum isolates that did not ferment raffinose were misidentified as E. faecium by API 20 STREP and Vitek GPI. Rapid ID 32 STREP, which performed well for the identification of E. gallinarum, failed to identify some E. faecium isolates, which it misidentified as E. gallinarum. These findings, previously reported for API 20 STREP (15) and Rapid ID 32 STREP (33), emphasize that based on biochemical reactions, it is not possible to differentiate between E. faecium and E. gallinarum, which belong to the same biochemical group, group II (12). With regard to these results, the characterization of GRE based on phenotypic identification (31) of enterococci cannot be recommended.
For the first time, as far as we are aware, two E. gallinarum isolates were found harboring the vanB gene. The two strains were isolated from two different patients 6 months apart and were not related by PFGE. Dutka-Malen et al. have previously reported the transfer of the vanA gene to E. gallinarum and E. casseliflavus (8). The acquisition of additional glycopeptide resistance genes by intrinsic GRE emphasizes that the characterization of intrinsic GRE should be based on genotypic analysis of the van type. Using phenotypic methods, VanB resistance is difficult to detect because MICs of vancomycin may be only moderately increased, comparable to those of the VanC type (11, 21).
Of the 18 GRE with the vanA genotype analyzed in the present study, three isolates were susceptible to teicoplanin, corresponding to a VanB phenotype. This finding was previously reported by others (34). Conversely, it has been reported that some strains of the vanB genotype can have a VanA phenotype, with coresistance to both vancomycin and teicoplanin (33). Thus, for unusual isolates the phenotype cannot be inferred from the genotype, and vice versa.
The multiplex PCR assay proposed by Dutka-Malen et al. (9) and used in the present study presents a clear advantage over phenotypic methods with regard to specificity and rapidity. Identification of the most frequently occurring species of enterococci and determination of vanA and vanB genotypes were performed in a single reaction. In conjunction with the determination of MICs of vancomycin and teicoplanin, this approach is useful for microbiology laboratories facing the need to characterize GRE. In the present study only one isolate, a strain of E. hirae, could not be identified by the multiplex PCR assay, because the primers of the d-alanine–d-alanine ligase gene specific to this species were not included in the assay.
No epidemiological conclusion can be drawn from this study concerning extent of colonization, because GRE were not looked for prospectively in the stools. However, of the five infected patients analyzed for stool carriage of GRE, all were found to be positive with a similar or related strain, confirming the role of the gastrointestinal tract as a reservoir of GRE. In addition, three patients were colonized by several GRE belonging to different species, two with E. faecium and E. faecalis containing the vanA gene and one with E. faecium and E. gallinarum containing the vanB gene. This suggests an in vivo transfer of glycopeptide resistance in the intestinal tract, as previously reported for the vanA determinant (8). It also emphasizes that, with regard to the recent report of a vanB transferable determinant in Streptococcus bovis in France (30), the dissemination of vanB resistance to enterococcal species other than E. faecium and E. faecalis or to other genera should be closely monitored.
The results of the present study showed a predominance of E. faecium with the vanA gene among acquired GRE with both sporadic cases and clusters of cases. The major cluster of five patients included four of the five patients infected with E. faecium vanA. The dissemination of the same strain of E. faecium vanA within a single unit may be easily explained by cross-contamination. However, we have no explanation for the recovery of a similar strain of E. faecium vanA after an interval of 1 year in two different units. It emphasizes that the mechanisms by which resistance is disseminated within hospitals have not been fully elucidated (21) and are more complex than initially thought. In Europe, the food chain has been suspected to be a source of GRE (3, 21) in relation to the use of avoparcin as a food additive for animals (21). The isolation in the present study of an E. hirae strain supports this hypothesis, as this species is predominant in the digestive tracts of poultry and cattle (6, 7).
In conclusion, this study shows the importance of characterizing GRE by both phenotypic and genotypic methods. With the rapid increase in the occurrence of such isolates, the use of both types of methods will provide useful information for clinicians and will implement infection control measures.
ACKNOWLEDGMENTS
We thank P. Courvalin for providing reference strains and P. Majcherczyk and P. Moreillon for kindly reviewing the manuscript.
REFERENCES
- 1.Barton A L, Doern G V. Selective media for detecting gastrointestinal carriage of vancomycin-resistant enterococci. Diagn Microbiol Infect Dis. 1995;23:119–122. doi: 10.1016/0732-8893(95)00174-3. [DOI] [PubMed] [Google Scholar]
- 2.Boyce J M, Opal S M, Chow J W, Zervos M J, Potter-Bynoe G, Sherman C B, Romulo R L C, Fortna S, Medeiros A A. Outbreak of multidrug-resistant Enterococcus faecium with transferable vanB class vancomycin resistance. J Clin Microbiol. 1994;32:1148–1153. doi: 10.1128/jcm.32.5.1148-1153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chadwick P R, Woodford N, Kaczmarski E B, Gray S, Barrell R A, Oppenheim B A. Glycopeptide-resistant enterococci isolated from uncooked meat. J Antimicrob Chemother. 1996;38:908–909. doi: 10.1093/jac/38.5.908. [DOI] [PubMed] [Google Scholar]
- 4.Chenoweth C, Shaberg D. The epidemiology of Enterococci. Eur J Clin Microbiol Infect Dis. 1990;9:80–89. doi: 10.1007/BF01963631. [DOI] [PubMed] [Google Scholar]
- 5.Clark N C, Cooksey R C, Hill B C, Swenson J M, Tenover F C. Characterization of glycopeptide-resistant enterococci from U.S. hospitals. Antimicrob Agents Chemother. 1993;37:2311–2317. doi: 10.1128/aac.37.11.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devriese L A, Hommez J, Wijfels R, Haesebrouck F. Composition of the enterococcal and streptococcal intestinal flora of poultry. J Appl Bacteriol. 1991;71:46–50. [PubMed] [Google Scholar]
- 7.Devriese L A, Laurier L, De Herdt P, Haesebrouck F. Enterococcal and streptococcal species isolated from faeces of calves, young cattle and diary cows. J Appl Bacteriol. 1992;72:29–31. doi: 10.1111/j.1365-2672.1992.tb04877.x. [DOI] [PubMed] [Google Scholar]
- 8.Dutka-Malen S, Blaimont B, Wauters G, Courvalin P. Emergence of high-level resistance to glycopeptides in Enterococcus gallinarum and Enterococcus casseliflavus. Antimicrob Agents Chemother. 1994;38:1675–1677. doi: 10.1128/aac.38.7.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutka-Malen S, Evers S, Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol. 1995;33:24–27. doi: 10.1128/jcm.33.1.24-27.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eliopoulos G M. Increasing problems in the therapy of enterococcal infections. Eur J Clin Microbiol Infect Dis. 1993;12:409–412. doi: 10.1007/BF01967433. [DOI] [PubMed] [Google Scholar]
- 11.Endtz H P, Van Den Braak N, Van Belkum A, Goessens W H, Kreft D, Stroebel A B, Verbrugh H A. Comparison of eight methods to detect vancomycin resistance in enterococci. J Clin Microbiol. 1998;36:592–594. doi: 10.1128/jcm.36.2.592-594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Facklam R R, Sahm D F. Enterococcus. In: Murray P R, Barron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. pp. 308–314. [Google Scholar]
- 13.Farrow J A E, Collins M D. Enterococcus hirae, a new species that includes amino acid assay strain NCDO 1258 and strains causing growth depression in young chickens. Int J Syst Bacteriol. 1985;35:73–75. [Google Scholar]
- 14.Frieden T R, Munsiff S S, Low D E. Emergence of vancomycin-resistant enterococci in New York City. Lancet. 1993;342:76–79. doi: 10.1016/0140-6736(93)91285-t. [DOI] [PubMed] [Google Scholar]
- 15.Gordts B, Van Landuyt H, Ieven M, Vandame P, Goossens H. Vancomycin-resistant enterococci colonizing the intestinal tracts of hospitalized patients. J Clin Microbiol. 1995;33:2842–2846. doi: 10.1128/jcm.33.11.2842-2846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handwerger S, Raucher B, Altarac D, Monka J, Marchione S, Singh K V, Murray B E, Wolff J, Walters B. Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin, and gentamicin. Clin Infect Dis. 1993;16:750–755. doi: 10.1093/clind/16.6.750. [DOI] [PubMed] [Google Scholar]
- 17.Jett B D, Huycke M M, Gilmore M S. Virulence of enterococci. Clin Microbiol Rev. 1994;7:462–478. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan A H, Gilligan P H, Facklam R R. Recovery of resistant enterococci during vancomycin prophylaxis. J Clin Microbiol. 1988;26:1216–1218. doi: 10.1128/jcm.26.6.1216-1218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leclercq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988;319:157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- 20.Leclercq R, Dutka-Malen S, Duval J, Courvalin P. Vancomycin resistance gene vanC is specific to Enterococcus gallinarum. Antimicrob Agents Chemother. 1992;36:2005–2008. doi: 10.1128/aac.36.9.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leclercq R, Courvalin P. Resistance to glycopeptides in enterococci. Clin Infect Dis. 1997;24:545–556. doi: 10.1093/clind/24.4.545. [DOI] [PubMed] [Google Scholar]
- 22.Moellering R C. Emergence of Enterococcus as a significant pathogen. Clin Infect Dis. 1992;14:1173–1178. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- 23.Murray B E. The life and times of the enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray B E. What can we do about vancomycin-resistant enterococci? Clin Infect Dis. 1995;20:1134–1136. doi: 10.1093/clinids/20.5.1134. [DOI] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. Approved standard M100-S7. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 26.Nauschvetz W F, Trevino S B, Harrison L S, Longfield R N, Fletcher L, Wartham W G. Enterococcus casseliflavus as an agent of nosocomial bloodstream infections. Med Microbiol Lett. 1993;2:102–108. [Google Scholar]
- 27.Navarro F, Courvalin P. Analysis of genes encoding d-alanine–d-alanine ligase-related enzymes in Enterococcus casseliflavus and Enterococcus flavescens. Antimicrob Agents Chemother. 1994;38:1788–1793. doi: 10.1128/aac.38.8.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pegues D A, Pegues C F, Hibberd P L, Ford D S, Hooper D C. Emergence and dissemination of a highly vancomycin-resistant vanA strain of Enterococcus faecium at a large teaching hospital. J Clin Microbiol. 1997;35:1565–1570. doi: 10.1128/jcm.35.6.1565-1570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perichon B, Reynolds P, Courvalin P. VanD-type glycopeptide-resistant Enterococcus faecium BM4339. Antimicrob Agents Chemother. 1997;41:2016–2018. doi: 10.1128/aac.41.9.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poyart C, Pierre C, Quesne G, Pron B, Berche P, Trieu-Cuot P. Emergence of vancomycin resistance in the genus Streptococcus: characterization of a vanB transferable determinant in Streptococcus bovis. Antimicrob Agents Chemother. 1997;41:24–29. doi: 10.1128/aac.41.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahm D F, Free L, Smith C, Eveland M, Mundy L M. Rapid characterization schemes for surveillance isolates of vancomycin-resistant enterococci. J Clin Microbiol. 1997;35:2026–2030. doi: 10.1128/jcm.35.8.2026-2030.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandamme P, Vercauteren E, Lammens C, Pensart N, Ieven M, Pot B, Leclercq R, Goossens H. Survey of enterococcal susceptibility patterns in Belgium. J Clin Microbiol. 1996;34:2572–2576. doi: 10.1128/jcm.34.10.2572-2576.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodford N, Johnson A P, Morrison D, Speller D C E. Current perspectives on glycopeptide resistance. Clin Microbiol Rev. 1995;8:585–615. doi: 10.1128/cmr.8.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]