Summary
Background
Thrombosis with thrombocytopenia syndrome (TTS) associated with viral vector COVID-19 vaccines, including ChAdOx1-S (AstraZeneca AZD1222) vaccine, can result in significant morbidity and mortality. We report the clinicopathological features of TTS following ChAdOx1-S vaccination and summarise the case outcomes in Australia.
Methods
In this cohort study, patients diagnosed with TTS in Australia between 23 March and 31 December 2021 were identified according to predefined criteria. Cases were included if they met the Therapeutic Goods Administration (TGA) probable and confirmed case definitions and were reclassified using Centres for Disease Control and Prevention (CDC) definition for analysis. Data were collected on patient baseline characteristics, clinicopathological features, risk factors, treatment and outcomes.
Findings
A total of 170 TTS cases were identified, with most occurring after the first dose (87%) of ChAdOx1-S. The median time to symptom onset after vaccination and symptom onset to admission was 11 and 2 days respectively. The median age of cases was 66 years (interquartile range 55–74). All except two patients received therapeutic anticoagulation and 66% received intravenous immunoglobulin. Overall, 85.3% of cases were discharged home after a median hospitalisation of 6 days, 9.4% required ongoing rehabilitation and 5.3% died. Eight deaths were related to TTS, with another dying from an unrelated condition while receiving treatment for TTS. Deaths occurred more commonly in those classified as Tier 1 according to the CDC definition and were associated with more severe thrombocytopenia and disease-related haemorrhage.
Interpretation
TTS, while rare, can be severe and have catastrophic outcomes in some individuals. In Australia, the mortality rate was low compared to that reported in other high-income countries. Almost all received therapeutic anticoagulation with no bleeding complications and were successfully discharged. This emphasises the importance of community education and an established pathway for early recognition, diagnosis and treatment of TTS.
Funding
Australian Commonwealth Department of Health and Aged Care. H.A Tran, N. Wood, J. Buttery, N.W. Crawford, S.D. Chunilal, V.M. Chen are supported by Medical Research Future Funds (MRFF) grant ID 2015305.
Keywords: Thrombosis, Thrombocytopenia, Adverse events, Vaccination, COVID-19
Research in context.
Evidence before this study
Thrombosis and thrombocytopenia Syndrome (TTS) is a rare but serious adverse event that emerged following large-scale community vaccination with adenoviral vectored COVID-19 vaccines. Detailed clinicopathological features, risk factors, response to treatment and outcomes of TTS cases is limited.
Added value of this study
This large national cohort study of cases identified by the national regulatory agency adverse vaccine safety reporting system reports the characteristics and outcomes of individual diagnosed with TTS in Australia in the period in which ChAdOx1 COVID-19 vaccine was utilised. Lower platelet count and intracranial haemorrhage are associated with an increased risk of death. Treatment was predominantly with non-heparin-based anticoagulants. 92% of CDC Tier 1 cases also received intravenous-gammaglobulin. We report a higher survival rate compared with other national cohorts (95%), most likely due to widespread clinician education, awareness for early presentation coupled with a streamlined pathway for diagnosis and immediate treatment.
Implications of all the available evidence
This case series supports use of the rapidly implemented guidance for recognition and management of COVID-19 vaccines related TTS. Time appropriate therapy is important to avoid thrombus progression and could potentially reduce mortality. This is of importance as ChAdOx1 COVID-19 vaccine continues to be used in some parts of the world for protection against COVID-19.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for the COVID-19 pandemic, had infected over 250 million individuals worldwide by July 2022 with almost 5 million reported deaths.1 The pandemic has resulted in significant disruptions to social and healthcare systems globally. With contemporary science and multiagency developmental partnerships, the unprecedented speed in the development of effective vaccines against COVID-19 using novel vaccine platforms has led to a similarly impressive and successful implementation of vaccination programs in high income countries. A rare but serious adverse event emerged following large-scale community vaccination that was not identified in clinical trials that was characterised by rapidly progressive thrombosis involving cerebral venous sinuses, splanchnic veins, pulmonary embolism (PE)/deep vein thrombosis (DVT) and/or arteries accompanied by thrombocytopenia, variably termed “Thrombosis with Thrombocytopenia Syndrome (TTS)” or “Vaccine-induced Immune Thrombotic Thrombocytopenia” (VITT).
Epidemiological definitions for TTS developed for vaccine pharmacovigilance (Supplementary Table S1) were utilised by regulatory authorities including the Therapeutic Goods Administration (TGA) of Australia, with inclusion criteria that closely aligned with the United Kingdom's Medicines and Healthcare products Regulatory Agency (MHRA). VITT defined a more specific clinic-pathological entity with criteria incorporating a time window after adenovirus vectored vaccine exposure (4–24 days), marked elevation of D-Dimer and anti-platelet factor 4 (PF4) antibodies with evidence of PF4-dependent platelet activation. VITT was first described in March 2021 where affected individuals were healthy with no risk factors for thrombosis and occasionally had low fibrinogen levels.2, 3, 4 VITT involves specific immunological drivers of thrombosis via platelet activation resulting in a spectrum disorder with variable clinical severity. Anti-platelet factor 4 (PF4) antibodies with evidence of PF4-dependent platelet activation are the hallmark of this condition, suggesting a mechanism similar to heparin-induced thrombocytopenia (HIT) but without prior heparin exposure.5 An immunological basis for this syndrome is further supported by the ability of both pooled human intravenous immunoglobulins (IVIg) and a monoclonal antibody called “IV.3” (used in vitro) which blocks the immune complex receptors on platelets (FcgRIIa), to abrogate platelet activation leading to a rapid normalisation of platelet count and clinical recovery.6,7 Regulators in several countries have concluded a causal link between the ChAdOx1-S (Vaxzevria [AstraZeneca]) and Ad26.COV2.S (Janssen [J&J]) COVID-19 vaccines and VITT with both vaccines using a non-replicating adenoviral vector platform.8, 9, 10 VITT has not been causally linked with other COVID-19 vaccine platforms, such as the mRNA vaccines (Comirnaty [Pfizer] and Spikevax [Moderna]).
ChAdOx1-S, the only viral-vector vaccine used in Australia, was rolled out from 8 March 2021 following initial approval for all individuals aged 18 years and older. With real time review of data of TGA reported Australian TTS cases showing mortality and morbidity skewed towards younger individuals both locally and internationally, the Australian Technical Advisory Group for Immunisation (ATAGI) adjusted clinical recommendations for the preferential use of mRNA vaccines in individuals aged <50 years on 8 April 2021 and then <60 years on 17 June 2021 for first dose COVID-19 vaccine. ChAdOx1-S continued to be used in Australia as primary vaccination against COVID-19 with 13.7 million doses of ChAdOx1-S administered by 31 December 2021.
We report here the clinicopathological features, risk factors, treatment and outcomes of 170 adenoviral-vaccine related TTS cases identified in reports of adverse events submitted to the national regulatory agency in Australia, to add to the evidence on the identification and management of this rare but serious condition. This is of importance as ChAdOx1 COVID-19 vaccine continues to be used in some parts of the world for protection against COVID-19, including in Australia.
Methods
Study design & participants
Adverse events following immunisation (AEFI) are reported to Australia's national regulatory agency, the Therapeutic Goods Administration (TGA) by healthcare providers, patients, state/territory health departments and pharmaceutical companies are entered into the Adverse Event Management System (AEMS) database. Reporting of some AEFI to state/territory health departments by healthcare providers is mandated in some states and territories in Australia. Pharmaceutical companies are also required to report serious adverse events to the TGA. 22 suspected cases met the criteria for additional evaluation by the TGA's national Vaccine Safety Investigation Group (VSIG). These included the first Australian cases, complex and/or serious cases where the diagnosis did not readily meet pre-specified criteria and were therefore reviewed by the national panel of expert medical advisors to the TGA including vaccinologists, haematologists, and related sub-specialists for consideration of classification as TTS.
All confirmed and probable cases of TTS as defined by TGA criteria (Supplementary Table S1) reported between 8 March 2021 and 31 December 2021 entered into AEMS are included in this analysis. Cases satisfying TGA criteria were subsequently sub-classified by researchers into US CDC Thrombosis with thrombocytopenia syndrome (TTS) Tier 1 and Tier 2 definition criteria and neither Tier 1 or Tier 2 (Supplementary Table S2).11 CDC classification was used as a surrogate for severity (Tier 1 cases contains all cases of thrombosis in unusual sites) and PF4 detection: “common site” thrombosis with anti-PF4 antibodies (Tier 2) or “common site” thrombosis without PF4 antibodies (neither Tier 1 or Tier 2).
Data collection
Following case notification to state/territory health authorities and/or the TGA, data were collated from medical records by study investigators including relevant pathology results and radiology reports. Patient demographics, history and potential risk factors for thrombosis as well as thrombocytopenia, presenting clinical features, laboratory results, radiological findings, treatment, hospital management intensive care unit (ICU) admission, surgical intervention, complications (further haemorrhage or thromboses), and discharge outcomes were collected. Vaccination date and thus time to onset of symptoms and vaccine brand was confirmed on the Australian Immunisation Register (AIR). As the TGA criteria12 did not include functional tests, these are not taken into consideration for this case series.
Procedures & outcomes
Diagnostic imaging
Imaging results from studies performed on patients according to locally developed guidelines to diagnose thromboses were collated.13 In particular, brain CT-venogram and/or magnetic resonance imaging (MRI)-venogram was used to diagnose cerebral venous sinus thrombosis (CVST).
Laboratory testing
Platelet count, fibrinogen, and D-dimer levels were performed in local accredited laboratories and compared to the laboratory normal ranges.
Anti-PF4 antibody: immunoassay
Enzyme Linked ImmunoSorbent Assay (Asserachrom HPIA IgG Diagnostica Stago, Australia) recognising adsorbed PF4/heparin was performed in reference laboratories according to manufacturer instructions. Individuals from one of eight states (Victoria) underwent anti-PF4 ELISA testing by Zymutest HIA IgG, Hyphen, recognising PF4 released from a platelet lysate complexed with heparin.14 The magnitude of positive results was expressed in optical density (OD) units.
Statistical analysis
Variables are described as numbers and percentages or as medians, ranges, and interquartile ranges. A logistic regression model was used to investigate the association between the variables (presence of CVST and/or splanchnic thrombus, haemorrhage at presentation, platelet count at presentation and nadir, use of anticoagulation and further haemorrhage following presentation) and mortality; platelet count was log-transformed (to log base 2) to remove positive skewness for this analysis. All analyses were conducted in R version 4.1.0.
Ethics approval
This study was conducted as part of the AusVaxSafety Adverse Event of Special Interest Program of Research, approved by The Sydney Children's Hospital Network Human Research Ethics Committee (2021/ETH11149).
Role of the funding source
Funding from the Australian Commonwealth Department of Health and Aged Care and the Medical Research Future Funds (MRFF) grant ID 2015305 was used to support data collection. The funders had no role in analysis, interpretation and writing of the report.
Results
From 8 March 2021 to 31 December 2021, there were 174 TTS cases identified from the TGA Adverse Events Management System (AEMS) database including 10 VSIG adjudication (Supplementary Figure S4). Medical record information was unavailable for 4 cases which were therefore excluded from this analysis, leaving 170 cases. All VSIG included cases met criteria for definite or probable TTS. Of these, 147 (87%) occurred after dose 1. Sub-classification of the 170 cases based on CDC classification showed 51 (30%) Tier 1 cases, 53 (31%) Tier 2 cases and 66 (39%) that were neither Tier 1 nor 2. By definition, all unusual site thrombosis were classified in Tier 1 and “typical site” thrombosis with PF4 antibodies in Tier 2. The “neither” group consisted of TTS cases with “typical site thrombosis” that were anti-PF4 negative by ELISA (n = 57) or not done (n = 9) (Table 1). Overall, the median age [interquartile range, IQR] was 66 years [55–74], and 48% were female. CDC Tier 1 cases were of a similar age compared to Tier 2 cases (median [IQR] 59 [50–68] years vs 64 [55–75]) and more likely to be females (69% vs 40%). One quarter (24%) reported a prior history of thrombosis and 15% had prior history of thrombocytopenia with no differences by vaccine dose (1 vs 2) or CDC classification.
Table 1.
Patient baseline characteristics and past medical history by vaccine dose and CDC case classification.
| All cases N = 170 | Vaccine |
CDC classification |
||||
|---|---|---|---|---|---|---|
| ChAdOx1-S dose 1 N = 147 | ChAdOx1-S dose 2 N = 23 | Tier 1 N = 51 | Tier 2 N = 53 | Neithera N = 66 | ||
| Vaccine dose | ||||||
| ChAdOx1-S Dose 1 | 147 (87%) | 49 (96%) | 46 (87%) | 52 (79%) | ||
| ChAdOx1-S Dose 2 | 23 (13%) | 2 (3.9%) | 7 (13%) | 14 (21%) | ||
| TGA classification | ||||||
| Confirmed | 88 (52%) | 82 (56%) | 6 (26%) | 35 (69%) | 50 (94%) | 3 (4.5%) |
| Probable | 82 (48%) | 65 (44%) | 17 (74%) | 16 (31%) | 3 (5.7%) | 63 (95%) |
| Age | ||||||
| Median [IQR] | 66 [55–74] | 64 [53–74] | 72 [64–79] | 59 [50–68] | 64 [55–75] | 71 [62–76] |
| Range | 18–96 | 18–96 | 21–92 | 22–96 | 18–92 | 21–96 |
| Sex | ||||||
| Male | 89 (52%) | 74 (50%) | 15 (65%) | 16 (31%) | 32 (60%) | 41 (62%) |
| Female | 81 (48%) | 73 (50%) | 8 (35%) | 35 (69%) | 21 (40%) | 25 (38%) |
| Aboriginal and/or Torres Strait Islander | 1 (0.6%) | 1 (0.7%) | 0 (0%) | 1 (2.0%) | 0 (0%) | 0 (0%) |
| BMI | ||||||
| Median [IQR] | 27 [24–32] | 27 [24–31] | 30 [27–34] | 28 [25–30] | 27 [24–32] | 27 [22–33] |
| Underweight (<18.5) | 5 (4.2%) | 5 (4.9%) | 0 (0%) | 0 (0%) | 2 (5.3%) | 3 (6.5%) |
| Normal (18.5–24.9) | 30 (25%) | 27 (26%) | 3 (17%) | 10 (28%) | 10 (26%) | 10 (22%) |
| Overweight (25.0–29.9) | 44 (37%) | 39 (38%) | 5 (28%) | 15 (42%) | 12 (32%) | 17 (37%) |
| Obese (≥30) | 41 (34%) | 31 (30%) | 10 (56%) | 11 (31%) | 14 (37%) | 16 (35%) |
| Unknown | 50 | 45 | 5 | 15 | 15 | 20 |
| History of thrombosis | 24 (14%) | 18 (12%) | 6 (26%) | 6 (12%) | 8 (15%) | 10 (15%) |
| Risk factors for thrombosisb | 133 (78%) | 112 (76%) | 21 (91%) | 37 (73%) | 42 (79%) | 54 (82%) |
| History of/prior thrombocytopenia | 26 (15%) | 23 (16%) | 3 (13%) | 5 (9.8%) | 8 (15%) | 13 (20%) |
IQR = interquartile range.
“Neither” includes the 66 cases did not fulfill CDC Tier 1 nor Tier 2 case classification.
Risk factors for thrombosis including thrombophilia, family history of thrombosis, hospitalisation in last 3 months, long-term bed rest, paralysis, long-term sitting (e.g. travel), major injury in last 3 months, major surgery in last 3 months, central venous catheterisation, active cancer (including chemotherapy or radiotherapy in last 12 months), heart failure/disease, hormone replacement therapy or oral contraceptive pill, obesity, smoking, pregnancy (incl. 6 weeks post-partum) and varicose veins.
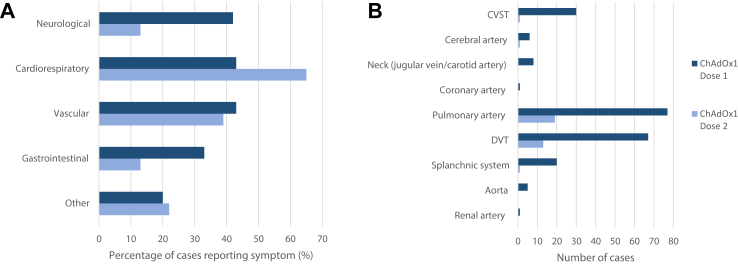
The time to onset of symptoms from vaccination, median [IQR] was 11 [7–20] days; 7% (n = 10) were asymptomatic at time of diagnosis (Table 2). Neurological symptoms were more common than symptoms of other organ manifestations following dose 1, while cardiorespiratory and vascular/limb symptoms were more common following dose 2 (Fig. 1A, Supplementary Table S3).
Table 2.
Radiological, diagnostics, clinical findings and outcomes by vaccine dose and CDC case classification.
| Characteristic | All N = 170 | Vaccine |
CDC classification |
|||
|---|---|---|---|---|---|---|
| ChAdOx1-S dose 1 N = 147 | ChAdOx1-S dose 2 N = 23 | Tier 1 N = 51 | Tier 2 N = 53 | Neither N = 66 | ||
| Radiological findings (thrombus details on initial imaging) | ||||||
| Type | ||||||
| Arterial only | 7 (4.1%) | 7 (4.8%) | 0 (0%) | 0 (0%) | 4 (7.5%) | 3 (4.5%) |
| Venous only | 154 (91%) | 132 (90%) | 22 (96%) | 46 (90%) | 46 (87%) | 62 (94%) |
| Both | 9 (5.3%) | 8 (5.4%) | 1 (4.3%) | 5 (9.8%) | 3 (5.7%) | 1 (1.5%) |
| Haemorrhagic | 23 (15%) | 23 (18%) | 0 (0%) | 16 (35%) | 5 (11%) | 2 (3.4%) |
| Number | ||||||
| Single | 101 (59%) | 90 (61%) | 11 (48%) | 33 (65%) | 33 (62%) | 35 (53%) |
| Multiple | 69 (41%) | 57 (39%) | 12 (52%) | 18 (35%) | 20 (38%) | 31 (47%) |
| Location | ||||||
| CVST | 31 (18%) | 30 (20%) | 1 (4.3%) | 31 (61%) | 0 (0%) | 0 (0%) |
| Cerebral artery | 7 (4.1%) | 6 (4.1%) | 1 (4.3%) | 2 (3.9%) | 4 (7.5%) | 1 (1.5%) |
| Neck vein (jugular) | 4 (2.4%) | 4 (2.7%) | 0 (0%) | 4 (7.8%) | 0 (0%) | 0 (0%) |
| Neck artery (carotid) | 4 (2.4%) | 4 (2.7%) | 0 (0%) | 2 (3.9%) | 2 (3.8%) | 0 (0%) |
| Coronary artery | 1 (0.6%) | 1 (0.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.5%) |
| Pulmonary artery | 96 (56%) | 77 (52%) | 19 (83%) | 13 (25%) | 35 (66%) | 48 (73%) |
| Splanchnic system | 21 (12%) | 20 (14%) | 1 (4.3%) | 21 (41%) | 0 (0%) | 0 (0%) |
| Renal artery | 1 (0.6%) | 1 (0.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.5%) |
| Aorta | 5 (2.9%) | 5 (3.4%) | 0 (0%) | 1 (2.0%) | 2 (3.8%) | 2 (3.0%) |
| DVT—any LL | 80 (47%) | 67 (46%) | 13 (57%) | 7 (14%) | 30 (57%) | 43 (65%) |
| DVT—proximal LL | 54 (32%) | 47 (32%) | 7 (30%) | 4 (7.8%) | 19 (36%) | 31 (47%) |
| DVT—distal LL | 26 (15%) | 20 (14%) | 6 (26%) | 3 (5.9%) | 11 (21%) | 12 (18%) |
| DVT—UL | 2 (1.2%) | 1 (0.7%) | 1 (4.3%) | 0 (0%) | 0 (0%) | 2 (3.0%) |
| Diagnostic findings | ||||||
| Platelets | ||||||
| <150 on initial | 160 (95%) | 137 (94%) | 23 (100%) | 49 (98%) | 49 (94%) | 62 (94%) |
| Initial, median (range) | 104 (7–296) | 97 (7–296) | 121 (34–261) | 90 (11–222) | 86 (7–296) | 124 (30–229) |
| Nadir, median (range) | 83 (6–150) | 76 (6–150) | 101 (34–146) | 50 (6–150) | 62 (7–150) | 111 (24–150) |
| >50% drop | 43 (29%) | 41 (32%) | 2 (9.5%) | 18 (38%) | 18 (41%) | 7 (12%) |
| Recovery, median (range) | 166 (86–373) | 168 (86–373) | 165 (151–232) | 166 (86–373) | 172 (123–296) | 165 (150–284) |
| Fibrinogen, median (range) | 3.80 (1.00–9.70) | 3.75 (1.00–9.70) | 4.80 (2.40–7.20) | 4.00 (1.00–7.90) | 3.40 (1.05–9.70) | 3.70 (1.40–7.50) |
| D-dimer (mg/L FEU), median (range) | 16 (2–137) | 17 (2–137) | 10 (2–125) | 20 (2–137) | 19 (2–128) | 12 (2–125) |
| D-dimer x ULN, median (range) | 33 (4–274) | 36 (4–274) | 26 (5–250) | 40 (4–274) | 38 (4–256) | 25 (4–250) |
| HIT ELISA | ||||||
| Positive | 87 (51%) | 80 (54%) | 7 (30%) | 34 (67%) | 53 (100%) | 0 (0%) |
| Negative | 74 (44%) | 60 (41%) | 14 (61%) | 17 (33%) | 0 (0%) | 57 (86%) |
| Not done | 9 (5.3%) | 7 (4.8%) | 2 (8.7%) | 0 (0%) | 0 (0%) | 9 (14%) |
| Positive OD readings, median (range) | 1.21 (0.20–3.25) | 1.32 (0.20–3.25) | 0.41 (0.34–2.28) | 1.08 (0.20–2.94) | 1.30 (0.20–3.25) | N/A |
| Clinical findings, management and outcomes | ||||||
| Timing | ||||||
| Asymptomatic | 10 (5.9%) | 9 (6.1%) | 1 (4.3%) | 2 (3.9%) | 5 (9.4%) | 3 (4.5%) |
| Time to symptom onset, median [IQR] | 11 [7–20] | 10 [7–18] | 15 [8–32] | 9 [7–16] | 10 [8–15] | 15 [8–29] |
| Symptom onset to admission, median [IQR] | 2.0 [0.0–7.0] | 2.0 [0.0–6.8] | 3.0 [1.0–7.5] | 2.0 [0.0–5.5] | 2.0 [0.0–6.0] | 2 [0.0–8.0] |
| Level of care | ||||||
| Hospital admission | 164 (96%) | 142 (97%) | 22 (96%) | 50 (98%) | 52 (98%) | 62 (94%) |
| Hospital length of stay, median [IQR] | 6 [3–13] | 6 [3–13] | 5 [4–10] | 11 [5–17] | 6 [3–14] | 4 [3–8] |
| ICU admission | 67 (39%) | 62 (42%) | 5 (22%) | 31 (61%) | 16 (30%) | 20 (30%) |
| Surgical intervention | 28 (16%) | 27 (18%) | 1 (4.3%) | 17 (33%) | 6 (11%) | 5 (7.6%) |
| Inpatient treatment | ||||||
| Anticoagulation (any) | 168 (99%) | 145 (99%) | 23 (100%) | 49 (96%) | 53 (100%) | 66 (100%) |
| Apixaban | 92 (54%) | 80 (54%) | 12 (52%) | 21 (41%) | 31 (58%) | 40 (61%) |
| Argatroban | 20 (12%) | 17 (12%) | 3 (13%) | 10 (20%) | 5 (9.4%) | 5 (7.6%) |
| Bivalirudin | 37 (22%) | 34 (23%) | 3 (13%) | 22 (43%) | 8 (15%) | 7 (11%) |
| Dabigatrin | 8 (4.7%) | 7 (4.8%) | 1 (4.3%) | 4 (7.8%) | 3 (5.7%) | 1 (1.5%) |
| Danaparoid | 7 (4.1%) | 6 (4.1%) | 1 (4.3%) | 3 (5.9%) | 2 (3.8%) | 2 (3%) |
| Enoxaparin | 30 (18%) | 23 (16%) | 7 (30%) | 0 (0%) | 13 (25%) | 17 (26%) |
| Fondaparinux | 44 (26%) | 40 (27%) | 4 (17%) | 14 (27%) | 17 (32%) | 13 (20%) |
| Heparin | 8 (4.7%) | 6 (4.1%) | 2 (8.7%) | 1 (2.0%) | 4 (7.5%) | 3 (4.5%) |
| Rivaroxaban | 41 (24%) | 35 (24%) | 6 (26%) | 12 (24%) | 12 (23%) | 17 (26%) |
| Warfarin | 15 (8.8%) | 9 (6.1%) | 6 (26%) | 7 (14%) | 4 (7.5%) | 4 (6.1%) |
| Blood products | ||||||
| IVIg | 113 (66%) | 101 (69%) | 12 (52%) | 47 (92%) | 35 (66%) | 31 (47%) |
| Platelet | 7 (4.1%) | 7 (4.8%) | 0 (0%) | 6 (12%) | 1 (1.9%) | 0 (0%) |
| FFP | 3 (1.8%) | 2 (1.4%) | 1 (4.3%) | 2 (3.9%) | 1 (1.9%) | 0 (0%) |
| Cryoprecipitate | 9 (5.3%) | 8 (5.4%) | 1 (4.3%) | 6 (12%) | 3 (5.7%) | 0 (0%) |
| PRBC | 9 (5.3%) | 8 (5.4%) | 1 (4.3%) | 4 (7.8%) | 2 (3.8%) | 3 (4.5%) |
| Plasmapheresis | 3 (1.8%) | 3 (2.0%) | 0 (0%) | 3 (5.9%) | 0 (0%) | 0 (0%) |
| Thrombolysis | 10 (5.9%) | 9 (6.1%) | 1 (4.3%) | 2 (3.9%) | 3 (5.7%) | 5 (7.6%) |
| Steroids | 35 (21%) | 33 (22%) | 2 (8.7%) | 13 (25%) | 13 (25%) | 9 (14%) |
| Inpatient complications | ||||||
| Haemorrhage | 22 (13%) | 19 (13%) | 3 (13%) | 11 (22%) | 5 (9.4%) | 6 (9.1%) |
| Further thromboses | 13 (7.6%) | 13 (8.8%) | 0 (0%) | 7 (14%) | 5 (9.4%) | 1 (1.5%) |
| Discharge destination/outcome | ||||||
| Dieda | 9 (5.3%) | 9 (6.1%) | 0 (0%) | 6 (12%) | 1 (1.9%) | 2 (3.0%) |
| Rehabilitation facility/long term care | 16 (9.4%) | 16 (11%) | 0 (0%) | 8 (16%) | 4 (7.5%) | 4 (6.1%) |
| Home | 145 (86%) | 122 (83%) | 23 (100%) | 37 (73%) | 48 (91%) | 60 (91%) |
| Outpatient therapy | ||||||
| Occupational therapy | 6/161 (3.7%) | 5/138 (3.6%) | 1/23 (4.3%) | 3/45 (6.7%) | 0/52 (0%) | 3/64 (4.7%) |
| Physiotherapy | 7/161 (4.3%) | 6/138 (4.3%) | 1/23 (4.3%) | 4/45 (8.9%) | 1/52 (1.9%) | 2/64 (3.1%) |
| Rehabilitation | 7/161 (4.3%) | 7/138 (5.1%) | 0/23 (0%) | 4/45 (8.9%) | 1/52 (1.9%) | 2/64 (3.1%) |
| Ongoing symptoms on discharge | 77/161 (48%) | 68/138 (49%) | 9/23 (39%) | 28/45 (62%) | 24/52 (46%) | 25/64 (39%) |
| Neurological | 20/161 (12%) | 18/138 (13%) | 2/23 (8.7%) | 13/45 (29%) | 4/52 (7.7%) | 2/64 (3.1%) |
| Cardiorespiratory | 27/161 (17%) | 22/138 (16%) | 5/23 (22%) | 2/45 (4.4%) | 12/52 (23%) | 13/64 (20%) |
| Gastrointestinal | 6/161 (3.7%) | 6/138 (4.3%) | 0/23 (0%) | 6/45 (13.3%) | 0/52 (0%) | 0/64 (0%) |
| Vascular | 20/161 (11%) | 17/138 (12%) | 3/23 (13%) | 1/45 (2.2%) | 5/52 (24%) | 14/64 (22%) |
| Discharge medications | ||||||
| Anticoagulation | 161/161 (100%) | 138/138 (100%) | 23/23 (100%) | 45/45 (100%) | 52/52 (100%) | 64/64 (100%) |
| Apixaban | 93/161 (58%) | 84/138 (61%) | 10/23 (4.3%) | 21/45 (47%) | 29/52 (56%) | 44/64 (69%) |
| Dabigatran | 7/161 (4.3%) | 6/138 (4.3%) | 1/23 (4.3%) | 3/45 (6.7%) | 3/52 (5.8%) | 1/64 (1.6%) |
| Enoxaparin | 1/161 (0.6%) | 0/138 (0%) | 1/23 (4.3%) | 0/45 (0%) | 0/52 (0%) | 1/64 (1.6%) |
| Fondaparinux | 7/161 (4.3%) | 7/138 (5.1%) | 0/23 (0%) | 5/45 (11%) | 2/52 (3.8%) | 0/64 (0%) |
| Rivaroxaban | 36/161 (22%) | 30/138 (22%) | 6/23 (26%) | 10/45 (22%) | 11/52 (21%) | 15/64 (23%) |
| Warfarin | 16/161 (10%) | 11/138 (8.0%) | 5/23 (22%) | 6/45 (13%) | 6/52 (12%) | 4/64 (6.3%) |
| Prednisone | 13/161 (8.1%) | 11/138 (8.0%) | 2/23 (8.7%) | 4/45 (8.9%) | 6/52 (11.5%) | 3/64 (4.7%) |
| Anti-seizure medication | 6/161 (3.7%) | 4/138 (2.9%) | 2/23 (8.7%) | 1/45 (2.2%) | 2/52 (3.8%) | 3/64 (4.7%) |
CVST = cerebral venous sinus thrombosis, DVT = deep vein thrombosis, FEU = fibrinogen equivalent units, FFP = fresh frozen plasma, HIT ELISA = heparin induced thrombocytopenia enzyme-linked immunosorbent assay, IQR = interquartile range, ICU = intensive care unit, IVIg = intravenous immunoglobulin, LL = lower limb, OD = optical density, PRBC = packed red blood cells, UL = upper limb, ULN = upper limit of normal.
This includes the 8 deaths assessed by the Therapeutic Goods Administration as being related to TTS, and one additional case where information from the coroner indicates that the death was caused by other health conditions and was not related to TTS. This case is included here as a death as the patient was not discharged as they died during their admission to hospital with probable TTS.
Fig. 1.
A. Percentage∗ of cases reporting symptoms by vaccine dose. B. Number of cases by sites and vaccine dose.
Thrombosis
The majority of thromboses were venous in origin (91%, n = 154). PE was most common (56%, n = 96); followed by lower limb deep vein thrombosis (DVT) (47%, n = 80), CVST (18%, n = 31) and splanchnic vein thrombosis (12%, n = 21) (Table 2). There were 7 (4%) arterial only thromboses, 9 (5%) involving both venous and arterial vasculature and 69 (41%) with thromboses involving more than one site. Almost all CVST cases (97%, n = 30/31) occurred after dose 1. Following dose 2, PE was the most common type of thrombosis (83%, n = 19/23) (Table 2, Fig. 1B). Radiologically confirmed haemorrhage related to the presenting thrombosis was present in 22 (13%) cases, of which 13 involved a CVST, 3 were in the splanchnic system and 6 were PE.
Laboratory features and anti-PF4 antibodies
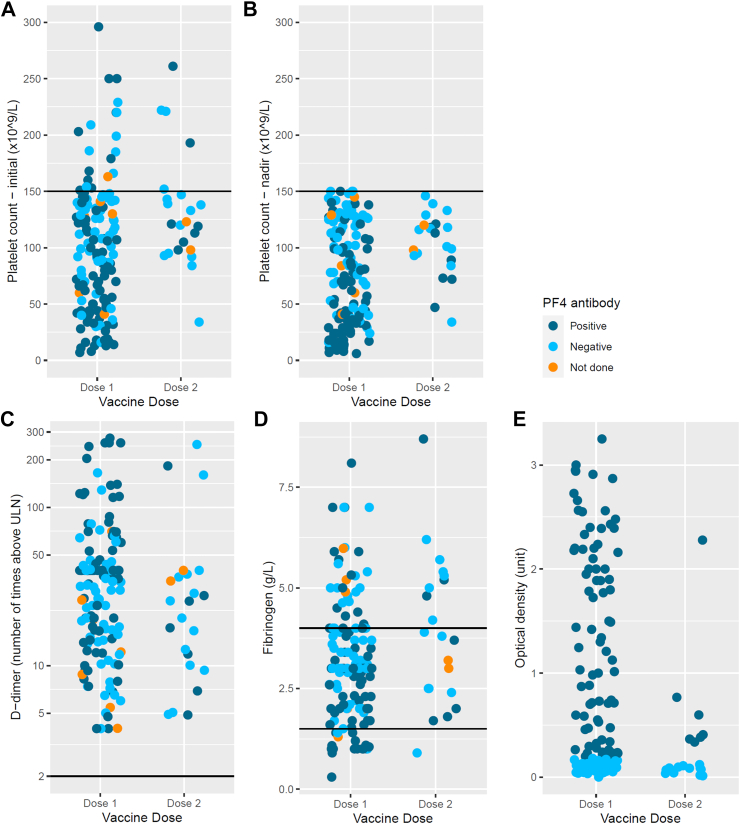
Among the 170 cases, 160 (95%) had an initial platelet count <150 × 109/L at presentation, with median (range) of 104 × 109/L (7–296), and 83 × 109/L (6–150) for initial and nadir platelet counts respectively. The median (range) was 3.80 g/L (1.00–9.70) for fibrinogen and 33 (4–274) times the upper limit of normal for D-dimer (Table 2, Fig. 2). There was no difference by vaccine dose (1 vs 2) or CDC classification.
Fig. 2.
Platelet, d-dimer and fibrinogen levels by vaccine dose and optical density of anti-PF4 antibodies for cases. A. Initial platelet count of TTS cases. B. Platelet count nadir of TTS cases. C. D-dimer, number of fold above upper limit of normal of TTS cases at presentation. D. Fibrinogen of TTS cases at presentation. E. Optical density of anti-platelet factor 4 antibody of TTS cases.
Of the 170 TTS cases, 161 underwent anti-PF4 antibody ELISA testing and 87 were positive (51% of all TTS cases). The median optical density (OD) for anti-PF4 ELISA positive cases was 1.32 (range 0.2–3.25) (Table 2).
Treatment
Nearly all (96%, n = 164) patients with TTS were hospitalised for treatment with median length of stay [IQR] of 6 days [3–13]; 39% (n = 67) were admitted to ICU, and 16% (n = 28) required surgical intervention (Table 2). Surgical intervention included embolectomy or thrombectomy (n = 14), craniectomy (n = 5), laparotomy (n = 5), extra-ventricular drain insertion (n = 2), fasciotomy (n = 1) and insertion of inferior vena cava filter (n = 1). Nearly all patients (99%, n = 168) received anticoagulation with apixaban (54%, n = 92), fondaparinux (26%, n = 44) and rivaroxaban (24%, n = 41) being the most common. Heparin or low molecular weight heparin (LMWH, enoxaparin) was administered in 8 (5%) and 30 (18%) patients respectively. Intravenous immunoglobulin (IVIg) was administered in 113 (66%) patients; ten patients (6%) received thrombolysis, seven (4%) platelet transfusions and 35 (21%) received steroids. Three patients underwent plasma exchange (2 CVST and 1 splanchnic vein thrombosis) one of whom (with CVST) died.
161 patients were discharged from hospital on anticoagulation; apixaban (58%, n = 94), rivaroxaban (22%, n = 36), warfarin (10%, n = 16), dabigatran (4%, n = 7), fondaparinux (4%, n = 7) and enoxaparin (n = 1).
Outcomes
Of the 170 cases, 145 (85.3%) were discharged home, 16 (9.4%) required inpatient rehabilitation or long-term care and 9 cases (5.3%) died in hospital. Of the 9 deaths, there were 8 deaths assessed by the TGA as being related to TTS and one death where information from the coroner indicated that the death was caused by other health conditions and was not related to TTS.15 Of the 9 deaths, all were following dose 1, with their major thrombosis location being CVST (n = 4), cerebral arterial thromboses with haemorrhage (n = 2), splanchnic thromboses (n = 2) and massive PE (n = 1). Their time to onset was similar to those who survived (median [IQR] 12 [9–14] days vs 15 [11–27] days, P = 0.2). Among these 9 deaths, 6 had haemorrhage at presentation, 2 were deemed beyond resuscitation at presentation thus did not receive anticoagulation or IVIg; 6 (67%) deaths occurred within 48 h of admission. Those who died were younger (median [IQR] 53 [48–60] vs 66 [56–75] years, P = 0.02). Factors associated with higher risk of death included a lower presenting platelet count (OR 1.82 (1.09–3.07), lower platelet nadir (OR 2.30 (1.34–3.95), having a Tier 1 classification (CVST or splanchnic thrombus) (OR 5.16 (1.23–21.50), presence of haemorrhage on presentation (OR 14.82 (3.39–64.83) and further haemorrhage during admission (OR 6.36 (1.56–25.86) (Table 3).
Table 3.
Clinical and laboratory features at initial presentation with respect to hospitalisation outcomes in patients with TTS.
| Variable | All N = 170 | Alive N = 161 | Died N = 9a | Univariate OR (95% CI) |
|---|---|---|---|---|
| Age median [IQR] | 66 [55–74] | 66 [56–75] | 53 [48–60] | 0.96 (0.93–1.00) |
| Sex | ||||
| Male | 89 (52%) | 86 (53%) | 3 (33%) | |
| Female | 81 (48%) | 75 (47%) | 6 (67%) | |
| Vaccine dose | ||||
| 1 | 147 (86%) | 138 (86%) | 9 (100%) | |
| 2 | 23 (14%) | 23 (14%) | 0 (0%) | |
| Time from vaccination to presentation, median [IQR] | 16 [11–27] | 16 [11–28] | 11 [9–13] | 0.94 (0.86–1.02) |
| CVST or splanchnic | 51 (30%) | 45 (28%) | 6 (67%) | 5.16 (1.23–21.5) |
| Haemorrhage at presentation | 23 (15%) | 17 (12%) | 6 (67%) | 14.82 (3.39–64.83) |
| Investigation | ||||
| Platelets—initial, median [IQR] | 104 [57–138] | 106 [62–139] | 44 [28–127] | 1.82 (1.09–3.07)b |
| Platelets—nadir, median [IQR] | 83 [40–120] | 85 [41–121] | 37 [15–43] | 2.30 (1.34–3.95)b |
| D-dimer (mg/L FEU), median [IQR] | 16 [7–21] | 15 [7–21] | 20 [18–23] | |
| Fibrinogen, median [IQR] | 3.00 [2.00–4.00] | 3.00 [2.02–4.00] | 2.80 [1.09–3.40] | |
| OD reading, median [IQR] | 1.21 [0.46–2.18] | 1.21 [0.41–2.19] | 1.44 [0.77–2.09] | |
| Level of care | ||||
| Hospital length of stay, median [IQR] | 6 [3–13] | 7 [4–13] | 2 [2–4] | |
| ICU admission | 67 (39%) | 59 (37%) | 8 (89%) | |
| Surgical intervention | 28 (16%) | 23 (14%) | 5 (56%) | |
| Inpatient treatment | ||||
| Anticoagulation | 168 (99%) | 161 (100%) | 7 (78%) | |
| Blood products | ||||
| IVIG | 113 (66%) | 106 (66%) | 7 (78%) | |
| Platelet | 7 (4.1%) | 7 (4.3%) | 0 (0%) | |
| FFP | 3 (1.8%) | 3 (1.9%) | 0 (0%) | |
| Cryoprecipitate | 9 (5.3%) | 8 (5.0%) | 1 (11%) | |
| PRBC | 9 (5.3%) | 9 (5.6%) | 0 (0%) | |
| Plasmapheresis | 3 (1.8%) | 2 (1.2%) | 1 (11%) | |
| Thrombolysis | 10 (5.9%) | 9 (5.6%) | 1 (11%) | |
| Steroids | 35 (21%) | 31 (19%) | 4 (44%) | |
| Inpatient complications | ||||
| Haemorrhage | 22 (13%) | 18 (11%) | 4 (44%) | 6.36 (1.56–25.86) |
| Further thromboses | 13 (7.6%) | 12 (7.5%) | 1 (11%) |
This includes the 8 deaths assessed by the Therapeutic Goods Administration as being related to TTS, and one additional case where information from the coroner indicates that the death was caused by other health conditions and was not related to TTS. This case is included here as a death as the patient was not discharged as they died during their admission to hospital with probable TTS.
For every 50% decrease in platelet count; IQR = interquartile range, CI = confidence interval, FEU = fibrinogen equivalent units, OD = optical density, ICU = intensive care unit, IVIg = intravenous immunoglobulin, FFP = fresh frozen plasma, PRBC = packed red blood cells.
Secondary haemorrhage was reported in 22 (13%) patients following initial diagnosis; 8 cases of intracranial bleed, 6 haematuria, 1 haemoptysis and 1 gastrointestinal bleed were deemed related to the presenting thrombosis rather than anticoagulation. Epistaxis (n = 1) and menorrhagia (n = 2) were also reported. Anticoagulation was continued without worsening of reported site bleeding.
Discussion
In Australia, 13.7 million doses of ChAdOx1-S vaccine were administered as part of a national mass vaccination program in 2021 (6.9 million dose 1 and 6.8 million dose 2). Similar to other countries where ChAdOx1-S vaccine was used, in our cohort of TTS cases, most occurred after dose 1 (87%).2,16,17 International reported cases satisfying TTS criteria post dose 2 ChAdOx1-S vaccination are much rarer compared with dose 1.3,18, 19, 20 In this cohort, TTS rates following ChAdOx1-S dose 1 and 2 were 2.1 and 0.34 cases per 100,000 doses respectively, dose 1 rates which are comparable with UK incidence of 1:100,000 in age >50 and 1:50,000 in age <50 years.3,18,19
The TGA definition used to define this cohort was based on case definitions initially established by the UK MHRA (Supplementary Table S1). The CDC criteria were also applied by the TGA as a surrogate for severity as “unusual site thrombosis” such as CVST or splanchnic vein thrombosis are classified as Tier 1. Given the rapidly evolving evidence, the TGA case definition was intended to be as sensitive as possible.21 We believe inclusion rates are high given high levels of syndrome awareness and reporting to TGA with subsequent case ascertainment. It is worth noting that the CDC definition for TTS is based on data from the Janssen Ad26. COV2 COVID-19 vaccine and intended principally for the evaluation of case severity.11 Given the decision to capture cases using a TTS rather than VITT definition, it is possible that this case series contains non-VITT cases. The “Pavord criteria” are most widely accepted for VITT.4 Overall, 73% (124/170) of the Australian TTS cases would have been classified “definite” (all 5 criteria) or “probable” (four of five criteria) VITT using criteria proposed (Supplementary Table S6) and 24% (41/170) falling into the “possible” category (three of five criteria).
While only 51% of these cases were anti-PF4 antibody positive by ELISA, the remainder (classified as “probable” by TGA) behaved in a similar manner to the ELISA positive cohort with improvement or resolution of symptoms and normalisation of the thrombocytopenia with therapeutic anticoagulation (Supplementary Table S5). Cases meeting CDC Tier 1 criteria had features of increased severity including longer length of stay, higher proportion of ICU admission and surgical intervention. However, TTS cases presenting with DVT/PE rather than unusual site thrombosis had similar clinical characteristics and outcomes whether PF4 positive (CDC Tier 2 cases) or negative (CDC neither Tier 1 nor Tier 2).
There is no gold standard for anti-PF4 testing in the diagnosis of VITT or TTS. Commercial ELISAs used to detect anti-PF4 antibody have different sensitivities and specificities for VITT, ranging from 71% to 100% and 56%–100% respectively.22 In addition, approximately 5% of individuals post vaccination yield a low-titre “false positive” ELISA anti-PF4 antibody with no evidence of syndrome.23,24 Discordance in positivity exists between different anti-PF4 ELISA tests. Asserachrom HPIA IgG assay was the primary ELISA used in Australia and we have previously reported up to one-third of cases demonstrating detectable the full clinical TTS syndrome with PF4 dependent platelet activating antibody on functional testing were negative using the IgG Asserochrom ELISA.22,25 Systematic inclusion of a second PF4 ELISA platform and functional testing to confirm pathological VITT would add to the certainty of classification of TTS cases as VITT, analogous to functional confirmation of pathological HIT.6,22 It is also possible that platelet activation-associated thrombosis in VITT is not only mediated via PF4, but by the involvement of related platelet-specific chemokine NAP2 using in vitro experiments.26 Conversely, without correlating functional test results for platelet activation, it is possible that patients with a negative ELISA were not VITT.25
This heterogeneity in confirmatory testing indicates that in resource constrained countries where anti-PF4 antibody testing is not available, the use of clinical criteria to guide time appropriate therapy is important to avoid thrombus progression and could potentially reduce mortality.
Unlike earlier published case series, TTS affected older people in our cohort with a median age of 66 years with no differences between gender in the overall confirmed and probable cases of TTS. However, there was a higher proportion of females that satisfied the CDC Tier 1 classification compared to Tier 2, suggesting females are more likely to have the more serious phenotype of TTS. The age of cases was likely impacted by the evolution of age criteria in COVID-19 vaccination policy in Australia, where ATAGI recommended preferential use of mRNA vaccines for <50 years of age, and <60 years of age, in early April and early June 2021 respectively, due to increasing reports of more severe Tier 1 VITT cases affecting younger individuals overseas.2 The preferential age recommendations may have resulted in another difference in this cohort, that PE with or without DVT was the most common thrombosis site, followed by CVST, splanchnic vein thrombosis and arterial thrombosis. In 41% of cases, thrombosis occurred at multiple sites. The presence of a prior history of thrombosis and thrombocytopenia in 24% and 15% of patients respectively in this TTS cohort is higher than previously reported. This might reflect the older median age with greater likelihood for pre-existing commodities but similarly not all were able to be verified.
There were differences between TTS dose 1 and dose 2 cases in this cohort. Dose 2 cases were older, with the most common site being PE with or without DVT and less likely to have thrombosis extension. There were no deaths associated with TTS post dose 2 in this cohort.
Virtually all patients in this cohort received therapeutic anticoagulation at presentation, most with a non-heparin drug. Eight patients (5%) received heparin at presentation but were switched to a non-heparin anticoagulant when TTS or VITT was considered as a diagnosis. None of these patients developed an extension of thrombosis that is seen in HIT. Heparin therapy may be less problematic in VITT than HIT, since it has been shown that the VITT antibody may displace heparin from PF4 since they share a binding site epitope27; however, a small subset of VITT cases demonstrated a heparin enhancing effect on platelet activation by function testing, either by flow cytometry or serotonin release assay (SRA).6 Hence where available, a non-heparin anticoagulant remains preferred over heparin to treat VITT, although heparin is preferred over no anticoagulation in this very prothrombotic condition.28
IVIg was made available specifically for TTS management in Australia, soon after the first cases were identified.29 While it is now more apparent that IVIg is beneficial in the treatment of VITT, it may not be warranted for all cases.7 In our cohort, 92% of the CDC Tier 1 cases received IVIg but only 66% of CDC Tier 2 patients (DVT/PE with positive PF4 ELISA) received IVIg in addition to anticoagulation with no difference in frequency of use between those who died and those who did not. Therefore, it is possible to infer that IVIg should be preferentially administered to more severe CDC Tier 1 cases (e.g., CVST and splanchnic vein thrombosis) cases, or where there is progression of thrombosis despite therapeutic anticoagulation.
Surgical intervention was needed in 16% of patients, mostly in those with CVST and splanchnic vein thrombosis, and thrombolytic therapy was used in 6% of patients, all for massive PE. Haemorrhage was reported in 22% of patients, attributed to venous hypertension and infarction rather than therapeutic anticoagulation. This reflects the intensely prothrombotic nature of VITT and necessitates early presentation following symptom onset and recognition with immediate therapeutic intervention when clinically suspected.
We report a high survival rate of 95%; 85.3% of the cohort were discharged home after a median hospital length of stay of 6 days and 9.4% required ongoing rehabilitation. The mortality rate of TTS in this cohort was 5.3% and contrasts with the reported mortality rate of 22% in the largest published case series from the United Kingdom (UK), or higher mortality rates in earlier and smaller series when the entity was not well established.2,4 While this may be due to differences in case definition, the reported mortality for our cases would remain 5.6% (7 of 124) if only cases that meet Pavord et al., criteria for definite or probable VITT, are included.4 The widespread clinician education, awareness for early presentation coupled with a streamlined pathway for diagnosis and immediate treatment that includes therapeutic anticoagulation preferentially with non-heparin drugs and IVIg in Australia may have reduced morbidity and mortality.14 The older median age in our cohort could have also contributed to differences given younger age is a predictor of poorer outcomes. We speculate that the high proportion of patients with PE/DVT reflects early intervention with IVIg therapy and anticoagulation, prior to established serious site thrombosis.30
While there were no clinical features associated with the risk for TTS, similar to the UK VITT series, we report that the presence of intracranial haemorrhage and a lower nadir platelet count was associated with an increased risk of death (OR (95% CI) 14.82 (3.39–64.83) and OR 2.30 (1.34–3.95) respectively.31 One of the three patients in our cohort who underwent plasma exchange died and this small number of cases does not further clarify for the role of plasma exchange in the treatment of this syndrome.32
A potential weakness of this study is case ascertainment bias.14,25 TTS cases may have not been identified if they presented with a normal platelet count without follow-up testing to detect a nadir platelet count of <150 × 109/L, if no thrombosis was present at first presentation (pre-VITT) or if the case was not reported to the TGA.30,33 However, Australia had system of systematic review of patients undergoing ELISA testing and a high level of health provider and community awareness of VITT/TTS with high AEFI reporting rates making under reporting less likely.34 Over-inclusion is also possible given results of anti-PF4 antibody-dependent platelet activation assays were not utilised. Using alternative PF4 ELISA platforms, PF4-enhanced platelet activation assays, platelet-specific chemokine NAP2 assays would likely would have improved confidence in case classification.26
In summary, we report on a large TTS cohort post-ChAdOx1-S COVID-19 vaccination in Australia, where TTS predominantly occurred after dose 1, with lower severity in post dose 2 cases. It is likely that, together with changes in the national vaccine program, clinician awareness and recognition of TTS, and efficient diagnostic pathway and prompt therapy have contributed to a higher recovery rate and one of the lowest mortality rates in the world. However, a significant level of morbidity remains within this cohort, and we aim to report on long-term outcomes in the future.
Contributors
HT, LD, NW, VC, MN: conceptualisation, data curation, formal analysis, investigation, methodology, supervision, validation, writing—original draft, and writing—review & editing.
PC, NCr, EK: writing—review & editing.
SS: investigation and writing—review & editing.
SK, IMS: data curation, investigation, methodology, supervision, and writing—review & editing.
RB, PE, CLa: data curation and writing—review & editing.
SB: funding acquisition and writing—review & editing.
BM: conceptualisation, data curation, funding acquisition, investigation, project administration, resources, software, supervision, writing—original draft, and writing—review & editing.
CWT: data curation, formal analysis, investigation, methodology, validation, visualisation, and writing—review & editing.
MG: validation, writing—original draft, and writing—review & editing.
PH: investigation, methodology, project administration, resources, validation, writing—original draft, and writing—review & editing.
SM: data curation, investigation, methodology, project administration, resources, validation, writing—original draft, and writing—review & editing.
LCl, SC: data curation, investigation, methodology and writing—review & editing.
JB: supervision, validation, and writing—review & editing.
HC: data curation, formal analysis, investigation, methodology, supervision, validation, visualisation, and writing—review & editing.
LP, DP: data curation, investigation, and writing—review & editing.
CP: data curation, methodology, supervision and writing—review & editing.
NCa: project administration.
KM: conceptualisation, funding acquisition, investigation, methodology, resources, software, supervision, and writing—review & editing.
MM: conceptualisation, data curation, formal analysis, methodology, and writing—review & editing.
TM: conceptualisation, data curation, investigation, and writing—review & editing.
Data sharing statement
Individual participant data that underlie the results reported in this article are not available.
Declaration of interests
HT acknowledged grants/contracts with the National Health and Medical Research Council (NHMRC) MRFF; NCr with Serious Adverse Events Following Vaccination in the Community (SAEFVIC); KM, NW with the Australian Government Department of Health, Australian Government Department of Foreign Affairs and Trade, NSW Department of Health, NHMRC, WHO and Gavi the Vaccine Alliance.
CP disclosed support for the present manuscript through funding from the National Centre for Immunisation Research and Surveillance; KM, NW through the Australian Government Department of Health and NHMRC.
KM declared payment for expert testimony and participation on a Data Safety Monitoring Board or Advisory Board.
Acknowledgements
We thank all the surveillance nurses, research and TGA staff involved in the data collection for this study: Michelle Daly, Annette Alafaci, Sarah Naseem, Karen Moore, Daniel Wise, Cathy Pienaar, Fiona Steele, Kathryn Tapper and Narelle Duncan.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100894.
Appendix A. Supplementary data
References
- 1.Coronavirus resource centre. https://coronavirus.jhu.edu/map.html
- 2.Schultz N.H., Sørvoll I.H., Michelsen A.E., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavord S., Scully M., Lester W., Makris M., Hunt B.J. Just how common is TTS after a second dose of the ChAdOx1 nCov-19 vaccine? Lancet. 2021;398:1801. doi: 10.1016/S0140-6736(21)02285-6. [DOI] [PubMed] [Google Scholar]
- 4.Pavord S., Scully M., Hunt B.J., et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385:1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scully M., Singh D., Lown R., et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee C.S.M., Liang H.P.H., Connor D.E., et al. A novel flow cytometry procoagulant assay for diagnosis of vaccine-induced immune thrombotic thrombocytopenia. Blood Adv. 2022;6:3494–3506. doi: 10.1182/bloodadvances.2021006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McFadyen J.D., Sharma P., Moon M.J., et al. Activation of circulating platelets in vaccine-induced thrombotic thrombocytopenia and its reversal by intravenous immunoglobulin. Br J Haematol. 2022;196:234–237. doi: 10.1111/bjh.17750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Medicines Agency Vaxzevria (previously COVID-19 Vaccine AstraZeneca): link between the vaccine and the occurrence of thrombosis in combination with thrombocytopenia. https://www.ema.europa.eu/en/medicines/dhpc/vaxzevria-previously-covid-19-vaccine-astrazeneca-link-between-vaccine-occurrence-thrombosis
- 9.Schneider J., Sottmann L., Greinacher A., et al. Postmortem investigation of fatalities following vaccination with COVID-19 vaccines. Int J Legal Med. 2021;135:2335–2345. doi: 10.1007/s00414-021-02706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry R.J., Tamborska A., Singh B., et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. Lancet. 2021;398:1147–1156. doi: 10.1016/S0140-6736(21)01608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimabukuro T. Update: thrombosis with thrombocytopenia syndrome (TTS) following COVID-19 vaccination. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/07-COVID-Shimabukuro-508.pdf
- 12.Gollamudi J., Sartain S.E., Navaei A.H., et al. Thrombosis and thromboembolism: brighton collaboration case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40:6431–6444. doi: 10.1016/j.vaccine.2022.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Tran H.A., Gibbs H., Merriman E., et al. New guidelines from the Thrombosis and Haemostasis Society of Australia and New Zealand for the diagnosis and management of venous thromboembolism. Med J Aust. 2019;210:227–235. doi: 10.5694/mja2.50004. [DOI] [PubMed] [Google Scholar]
- 14.Chen V.M., Curnow J.L., Tran H.A., Choi P.Y. Australian and New Zealand approach to diagnosis and management of vaccine-induced immune thrombosis and thrombocytopenia. Med J Aust. 2021;215:245–249.e1. doi: 10.5694/mja2.51229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therapeutic Goods Administration COVID-19 vaccine weekly safety report - 27-01-2022. https://www.tga.gov.au/news/covid-19-vaccine-safety-reports/covid-19-vaccine-weekly-safety-report-27-01-2022
- 16.Wise J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021;372:n699. doi: 10.1136/bmj.n699. [DOI] [PubMed] [Google Scholar]
- 17.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavord S., Makris M. Second-dose VITT: rare but real. Blood. 2022;139:2581–2583. doi: 10.1182/blood.2022016118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke L., Brighton T., Chunilal S.D., et al. Vaccine-induced immune thrombotic thrombocytopenia post dose 2 ChAdOx1 nCoV19 vaccination: less severe but remains a problem. Vaccine. 2023;41:3285–3291. doi: 10.1016/j.vaccine.2023.03.071. [DOI] [PubMed] [Google Scholar]
- 20.Krzywicka K., van de Munckhof A., Zimmermann J., et al. Cerebral venous thrombosis due to vaccine-induced immune thrombotic thrombocytopenia after a second ChAdOx1 nCoV-19 dose. Blood. 2022;139:2720–2724. doi: 10.1182/blood.2021015329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavord S.L.W., Makris M., Scully M., Hunt B. Guidance from the expert haematology panel (EHP) on covid-19 vaccine-induced immune thrombocytopenia and thrombosis (VITT) https://media.b-s-h.org.uk/20499/guidance-version-22-20210903.pdf [DOI] [PMC free article] [PubMed]
- 22.Platton S., Bartlett A., MacCallum P., et al. Evaluation of laboratory assays for anti-platelet factor 4 antibodies after ChAdOx1 nCOV-19 vaccination. J Thromb Haemost. 2021;19:2007–2013. doi: 10.1111/jth.15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sørvoll I.H., Horvei K.D., Ernstsen S.L., et al. An observational study to identify the prevalence of thrombocytopenia and anti-PF4/polyanion antibodies in Norwegian health care workers after COVID-19 vaccination. J Thromb Haemost. 2021;19:1813–1818. doi: 10.1111/jth.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiele T., Ulm L., Holtfreter S., et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 2021;138:299–303. doi: 10.1182/blood.2021012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Favaloro E.J., Clifford J., Leitinger E., et al. Assessment of immunological anti-platelet factor 4 antibodies for vaccine-induced thrombotic thrombocytopenia (VITT) in a large Australian cohort: a multicenter study comprising 1284 patients. J Thromb Haemost. 2022;20:2896–2908. doi: 10.1111/jth.15881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rauova L., Poncz M. Understanding VITT(ual) reality. Blood. 2021;138:285–286. doi: 10.1182/blood.2021012524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huynh A., Kelton J.G., Arnold D.M., Daka M., Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature. 2021;596:565–569. doi: 10.1038/s41586-021-03744-4. [DOI] [PubMed] [Google Scholar]
- 28.Greinacher A., Langer F., Makris M., et al. Vaccine-induced immune thrombotic thrombocytopenia (VITT): update on diagnosis and management considering different resources. J Thromb Haemost. 2022;20:149–156. doi: 10.1111/jth.15572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thrombosis and Haemostasis society of Australia and New Zealand IVIg available under national blood arrangements for VITT. https://www.thanz.org.au/news/ivig-available-under-national-blood-arrangements-for-vitt
- 30.Salih F., Schönborn L., Kohler S., et al. Vaccine-induced thrombocytopenia with severe headache. N Engl J Med. 2021;385:2103–2105. doi: 10.1056/NEJMc2112974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavord S., Hunt B.J., Horner D., Bewley S., Karpusheff J., Guideline Committee Vaccine induced immune thrombocytopenia and thrombosis: summary of NICE guidance. BMJ. 2021;375:n2195. doi: 10.1136/bmj.n2195. [DOI] [PubMed] [Google Scholar]
- 32.Patriquin C.J., Laroche V., Selby R., et al. Therapeutic plasma exchange in vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;385:857–859. doi: 10.1056/NEJMc2109465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19) https://apps.who.int/iris/bitstream/handle/10665/342999/WHO-2019-nCoV-TTS-2021.1-eng.pdf?sequence=1&isAllowed=y
- 34.Thrombosis and Haemostasis society of Australia and New Zealand THANZ multidisciplinary VITT guidelines for doctors. https://www.thanz.org.au/documents/item/590
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.