Abstract
Intramuscular fat (IMF) is an important factor affecting chicken quality. However, the age-related mechanism of IMF deposition has not yet been elucidated. In this study, the IMF, phospholipids (PL), triglycerides (TG), and fatty acid (FA) content in the breast muscle of Beijing-You chicken (BJY) at 1, 56, 98, and 120 d of age was measured, and mRNA and miRNA sequencing was integrated to explore the regulatory genes of IMF deposition. The results showed that the IMF content of BJY at 1 d of age was significantly higher than that at later stage of birth (P < 0.05). The transcriptome sequencing results showed that 7, 225 differentially expressed genes (DEGs) and 243 differentially expressed miRNAs (DE-miRNAs) were identified. The cluster analysis showed that the expression of DEGs and DE-miRNAs at 1 d of age was significantly different from that at later stages of birth. Furthermore, a potential mRNA-miRNA regulatory network related to IMF deposition was established by weighted gene co-expression network analysis (WGCNA); gga-miR-29c-3p-PIK3R1, gga-miR-6701-3p-PTEN, gga-miR-363-3p-PTEN, gga-miR-1563-WWP1, gga-miR-449c/d-5p-TRAF6, and gga-miR-6701-3p-BMPR1B were identified as key mRNA-miRNA pairs for the regulation of IMF deposition. These results will help elucidate the mechanism of IMF formation mediated by miRNAs in chickens, and provide a theoretical foundation for the genetic improvement of broiler meat quality.
Key words: intramuscular fat, Beijing-You chicken, transcriptome, miRNA
INTRODUCTION
With improvements in living standards, consumers are paying more attention to the choice of meat quality and flavor characteristics. Therefore, improving chicken quality is an important research topic in broiler breeding. Intramuscular fat (IMF) refers to the fat deposited between and within muscle fibers, which has an important effect on the sensory quality, juiciness, tenderness, flavor, and processing value of chickens (Hocquette et al., 2010). The IMF consists of phospholipids (PL), triglycerides (TG) and cholesterol (TC). TG has little effect on the characteristic flavor and aroma of meat (Mottram and Edwards, 1983). PL is an important precursor substance that affects the volatile flavor components of meat because it has a much higher unsaturated fatty acid (UFA) content than TG (Mottram, 1998).
Fatty acids (FA) are important chemicals that constitute TG and PL and are aromatic substances or precursors of aromatic substances. Oxidative degradation products of intramuscular free fatty acids can impart meat flavor (Ping et al., 2008; Wood et al., 2008). Meat with low content of saturated fatty acids (SFA) and high content of monounsaturated fatty acids (MUFA) is fresh, tender and juicy, with high flavor score (Cameron and Enser, 1991). Polyunsaturated fatty acids (PUFA) are easily oxidized and their oxidation products directly affect the composition of meat flavor components (Nestel, 1987). When the distribution of SFA, MUFA, and PUFA in meat is balanced, the nutritional and taste needs of humans can be met. Therefore, the type and content of FA are important factors affecting the nutritional value and quality of chicken meat.
MicroRNAs (miRNAs) are small noncoding RNAs with a length of 19 to 23 nucleotides, that mainly inhibit the translation or degrade messenger RNAs (mRNAs) by targeted binding to mRNAs, thereby regulating the expression of target genes after transcription and inhibiting protein synthesis (Ling and Kebin, 2021). Studies have shown that miRNA play key roles in regulating IMF deposition in chickens. miR-18b-3p and miR-223 inhibit the differentiation of chicken intramuscular adipocytes and participate in IMF deposition (Guirong et al., 2019; Li et al., 2019a). miR-122 is involved in cholesterol synthesis and lipid metabolism (Hicks et al., 2010). However, the mechanisms by which miRNA regulate IMF deposition have not been fully elucidated.
Age affects IMF deposition in chickens. Studies have shown that the IMF content of Anka and Arbor Acres (AA) chickens increases from 28 to 140 d of age (Chen et al., 2005a; Chang et al., 2010), and the flavor and taste of the meat become more intense at a later stage (Sun et al., 2006). Cui et al. (2018) found that the IMF content in Beijing-You chicken (BJY) was highest at 90 d of age and slightly decreased at 120 d of age. The fatty acid composition of chicken meat is also affected by age. Aging chickens have higher levels of long-chain and total PUFA, and the ratio of n-6/n-3 decreases significantly (Bosco et al., 2014; Popova et al., 2016). However, the mechanism of IMF deposition during development has not yet been thoroughly clarified.
The BJY is a distinctive native chicken breed that is well-known for its meat quality and flavor in China. The IMF content of breast muscle in BJY is higher than that in AA chickens (Popova et al., 2016). In this study, we collected the breast muscles at 4 developmental stages (d 1, 56, 98, and 120) of BJY, and identified the key genes related to IMF deposition by mRNA and miRNA sequencing, which will help elucidate the mechanism of IMF formation mediated by miRNAs in chickens and provide a theoretical foundation for the genetic improvement of broiler meat quality.
MATERIALS AND METHODS
Ethics Approval
All animal welfare practices and experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (Ministry of Science and Technology of China, 2006). All procedures were approved by the Animal Ethics Committee of the Beijing University of Agriculture.
Animal and Sample Collection
Hundred 1 d of age BJY were used in this experiment. They were raised on a farm in a suburb of Beijing. All chickens were raised under standard management conditions, fed the same diet, and had free access to water. The ingredients and compositions of the BJY diets at different feeding stages are shown in Table S1. The immune procedures at different developmental stages are shown in Table S2. 10 healthy chickens were randomly selected at 1 (birth stage), 56 (rapid growth stage), 98 (stage with high IMF deposition), and 120 (marketing stage) days of age, respectively. The weight of the chickens at each stage was in the normal range and basically the same. Euthanasia was performed by cutting the carotid artery under carbon dioxide anesthesia, the breast muscle without skin was then collected from similar sampling sites and stored at -80 °C.
Determination of the IMF and FA Content
The contents of IMF, TG, TC, and FA in breast muscle of 40 BJY at 4 developmental stages (10 chickens per stage) were determined. The IMF content in breast muscle tissue was determined by Soxhlet extraction according to the GB/T 5009.6–2016 National Standard for Food Safety determination of fat in food. Each sample was weighed (0.1 g), and 0.9 mL PBS was added for homogenization. The supernatant was used for the determination of TG and TC content using ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Fatty acids in homogenate were extracted with chloroform and methanol. After filtration, the extracted fatty acids were methylated with tri-boron trichloride-methanol, and then the types and contents of fatty acids were detected by Gas Chromatography-Mass Spectrometer (GC-MS).
RNA Extraction and Library Preparation
A total of 40 samples from 4 developmental stages were used for transcriptomic analysis. Total RNA was extracted from breast muscle samples using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The ARNA 6000 Nano LabChip Kit for Bioanalyzer 2100 (Agilent, Santa Clara, CA) was used to determine the purity and concentration of the RNA. Forty cDNA libraries were created by reverse transcription using an mRNA-Seq Sample Preparation Kit (Illumina, San Diego, CA). Forty small RNA libraries were prepared using a TruSeq Small RNA Sample Preparation Kit.
Data Analysis of Gene Expression
A HiSeq 2500 (Illumina) was used for paired-end sequencing. Raw data (raw reads) in fastq format were filtered first. The reads with an adapters and low-quality reads were excluded. All reads containing a base and those with an N ratio higher than 10% were eliminated to ensure quality. Using Hisat2 with the default settings, clean paired-end reads were aligned with the reference genome (version: GCF_016699485.2). Stringtie (http://ccb.jhu.edu/software/stringtie/) was used to assemble transcripts. Gene expression levels were estimated as counts per million cells (CPM). The DEGs were calculated using the edger R package (V 3.34.1), which were defined as genes with false discovery rate (FDR) ≤ 0.05 and |log2 (fold change) | ≥ 1.
Functional Analysis of DEGs and WGCNA Analysis
Hierarchical cluster analysis of DEGs was performed by the ggplot2 package (V 3.3.6). Gene Set Enrichment Analysis (GSEA) was performed by the R package clusterProfiler (V 4.0.5). The co-expression network modules of all DEGs were constructed by the WGCNA R package (V 1.70-3). The parameters used were minModuleSize = 30, mergeCutHeight = 0.25, and soft threshold power = 10. The module-trait relationship was obtained after correlation analysis between the expression levels of DEGs and phenotypes by the labeled heatmap function. The key modules were selected according to the screening condition P < 0.05, and the genes in the modules were screened for subsequent analysis according to the criteria of gene significance |GS| ≥ 0.7 and module membership |MM| ≥ 0.7.
Data Analysis of miRNA Expression
Forty small RNA libraries were sequenced on a HiSeq 2500 platform (Illumina), and single-end reads were obtained. Fastx-tool was used for quality control to remove adapters and low-quality reads. Clean and high-quality reads 18 to 30 nt in length were used for subsequent analyses. Bowtie2 (https://sourceforge.net/projects/bowtie-bio/files/) was used to map all the clean sequencing reads to the chicken genome. Known miRNAs were quantitated by Hetseq-count. The edger R package was used to identify differentially expressed miRNA (DE-miRNA) with the screening criteria of the FDR ≤ 0.05 and |log2 (fold change) | ≥ 1.
Prediction and Functional Analysis of DE-miRNAs Target Genes
To understand the molecular function of DE-miRNAs in breast muscle, we used TargetScan (http://www.targetscan.org/vert_72/) and miRDB (http://www.mirdb.org/) to predict their target mRNAs. The results were combined with the DEGs data obtained by RNA-Seq to predict important candidate target genes. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses of the target genes were performed by the omicshare (http://www.omicshare.com).
Construction of a Protein-Protein Interaction Network and mRNA-miRNA Network
Protein-protein interaction (PPI) pairs between genes obtained by WGCNA analysis were constructed using the String database (http://string-ab.org/), and disconnected genes in the network were deleted. The Spearman's method was used to calculate the correlation between the genes in the PPI network and their targeted DE-miRNAs. DE mRNA-miRNA pairs with r < -0.6, P < 0.05 were selected. The DE mRNA-miRNA network was constructed by Cytoscape (V 3.8.2). A hub network was constructed using the degree algorithm in CytoHubba.
RESULTS
IMF and FA Content Analysis of Breast Muscle
The IMF content of the BJY breast muscle was the highest on d 1 and was significantly higher than that at later stages of birth (P < 0.05). The IMF content gradually increased to its peak from d 56 to 98, and then significantly declined at d 120 (P < 0.05). The changing trend in the TG content was consistent with that of the IMF content. The PL content in the breast muscle gradually increased to a maximum from d 1 to 98 and decreased slightly at d 120. The TC content was the highest on d 1, gradually decreased to its lowest on d 98, and slightly increased on d 120. The MUFA to PUFA ratio was the highest a d 1 and increased significantly with an increase in age from d 56 to 120 (Table 1).
Table 1.
Intramuscular fat (IMF), phospholipid (PL), triglyceride (TG), cholesterol (TC), and fatty acid (FA) content in the breast muscle of Beijing-You (BJY) chickens at different developmental stages.1
| Ages (d) | IMF (%) | PL (mg/100g) | TG (mg/100g) | TC (mg/100g) | MUFA/PUFA (%) |
|---|---|---|---|---|---|
| 1 | 1.90 ± 0.33a | 10.36 ± 1.39c | 1997.38 ± 78.93a | 463.86 ± 6.68a | 38.64 ± 1.42a |
| 56 | 0.62 ± 0.23bc | 20.31 ± 0.73a | 1621.64 ± 47.83c | 339.30 ± 4.13b | 26.74 ± 1.60d |
| 98 | 0.73 ± 0.11b | 20.37 ± 0.16a | 1770.42 ± 128.96b | 321.42 ± 6.95c | 32.80 ± 0.82c |
| 120 | 0.55 ± 0.10c | 19.06 ± 0.50b | 854.67 ± 21.14d | 325.48 ± 7.96c | 35.43 ± 0.71b |
Data are means ± SD. n = 10.
Means within a column with different superscripts differ significantly (P < 0.05).
Transcriptome and DEGs Analysis
RNA-seq was conducted to examine the gene expression profiles of breast muscles in BJY at different developmental stages. A total of 7,225 DEGs were identified based on FDR ≤ 0.05 and |log2(fold change)| ≤ 1, including 6,521 at d 1 vs. 56, 819 at d 56 vs. 98, and 747 at d 98 vs. 120 (Figure 1A). Cluster analysis revealed differential gene expression at different stages. The expression of DEGs at d 1 was significantly different from that at the other 3 developmental stages (Figure 1B).
Figure 1.
The differential expression analysis of mRNAs. (A) Volcano plot of differentially expressed genes (DEGs) between different stages; significantly downregulated genes are represented as blue dots and significantly upregulated genes are represented as red dots. (B) Heatmap of DEGs by cluster analysis. n = 10.
GSEA Analysis
GSEA was performed to understand the role of the DEG in the development of BJY. The pentose phosphate pathway, glycolysis/gluconeogenesis and histidine metabolism pathways were activated at 1 to 56 d of age, whereas phenylalanine metabolism was inhibited at this stage (Figure 2A, Table S3). The DEGs at d 56 vs. 98 were significantly enriched in the cytokine-cytokine receptor interaction, PPAR signaling pathway, and glycine, serine, and threonine metabolism pathway (Figure 2B, Table S4). The cell adhesion molecules, mucin type O-glycan biosynthesis, and ECM-receptor interaction pathways were activated from 98 to 120 d of age, while fatty acid biosynthesis, protein export and the cysteine and methionine metabolism pathways were inhibited at this stage (Figure 2C, Table S5).
Figure 2.
Functional analysis of differentially expressed genes (DEGs) at d 1 vs. 56, 56 vs. 98 and 98 vs. 120 by Gene Set Enrichment Analysis (GSEA). The ordinate represented the enrichment score value, and the abscissa represented a ranked DEGs list correlation profile. n = 10.
WGCNA Analysis
To understand the gene regulation of IMF deposition during BJY development, we used the WGCNA method to obtain a co-expression network of 7,225 DEGs and identified 9 co-expression modules (Figure 3A, Table S6). The blue and cyan modules had the largest number of genes (3,529 and 2,727 genes respectively), and totally 3,024 DEGs were identified with |GS| ≥ 0.7 and |MM| ≥ 0.7 as screening criteria. The DEGs of BJY at d 1 vs. 56 were mainly gathered in the blue module, whereas the DEGs at d 56 vs. 98 and d 98 vs. 120 were mostly located in the cyan module. The correlation between DEGs and the IMF, PL, TG, TC, and MUFA/PUFA content was analyzed by WGCNA. According to the heatmap of the module-trait relationships (Figure 3B), the IMF, TC, and MUFA/PUFA content had significantly positive correlations with the blue module, whereas PL had a significantly negative correlation with the blue module. The cyan module had a significantly negative correlation with IMF, TG, and TC, and a significant positive correlation with PL.
Figure 3.
Weighted gene co-expression network analysis of differentially expressed genes and traits in breast muscle of Beijing-You chicken (BJY). (A) Nine modules obtained by weighted gene co-expression network analysis (WGCNA). The dendrogram showed the co-expression clusters, the different colors indicated the co-expression modules. (B) Heat map showed module-trait relationships. Each row represented a module. Each column represented a trait. The red color indicated that there was a significantly positive correlation between this module and the trait, and the green color indicated a significantly negative correlation (P < 0.05). n = 10.
DE-miRNAs Analysis
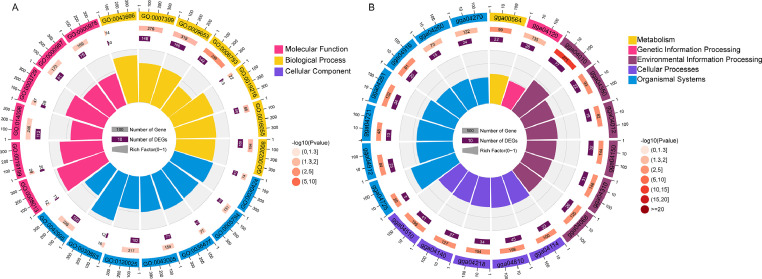
A Small RNA library of the breast muscles at different developmental stages of BJY was sequenced. A total of 877 miRNAs were identified, of which 243 were identified as DE-miRNAs using the edgeR package, including 214 on d 1 vs. 56, 55 on d 56 vs. 98, and 10 on d 98 vs. 120 (Figure 4). To better understand the function of DE-miRNAs, we identified a total of 7, 309 target genes in TargetScan and MiRDB databases, and 2,084 target genes were obtained after intersection with DEGs. We investigated the function of 2,084 target genes by GO analysis. The pathways that were significantly enriched in biological processes included the Wnt signaling pathway, phosphorus metabolic process, regulation of phosphoprotein phosphatase activity, and regulation of lipid metabolic process. Cellular components included plasma membrane bounded cell projection, cortical cytoskeleton, and cell projection. Molecular function involved 7 significant terms, such as β-catenin binding, transmembrane receptor protein kinase activity, and catalytic activity (Figure 5A). The KEGG enrichment analysis identified 35 significant KEGG pathway terms, including the MAPK signaling pathway, Wnt signaling pathway, focal adhesion, and TGF-β signaling pathway (Figure 5B).
Figure 4.
Volcano plot of differentially expressed mi-RNAs (DE-miRNAs) between different stages; significantly downregulated genes are represented as blue dots and significantly upregulated genes are represented as red dots. n = 10.
Figure 5.
Functional analysis of miRNA target genes. (A) The top 200 significant Gene Ontology (GO) enrichment terms. (B) The top 20 significant terms by Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. n = 10.
Protein-Protein Interaction and miRNA-mRNA Network
Correlation analysis was performed between 3,024 DEGs and 243 DE-miRNAs with targeting relationships. According to the screening conditions r < -0.6 and P < 0.05, a total of 126 miRNA-mRNA pairs were obtained, of which 76 DEGs were used for further analysis (Table S7).
To further explore the interactions of the 76 DEGs selected from the WGCNA analysis, a PPI network was established with the connected nodes (genes) (Figure 6A). The correlation between the genes in the PPI network and their target DE-miRNAs was calculated using the Spearman method. DE-miRNA-mRNA pairs with r < -0.6, P < 0.05 were selected. An mRNA-miRNA network was constructed by Cytoscape (Figure 6B). Five hub genes and corresponding mRNA-miRNA pairs were obtained using the degree algorithm in CytoHubba, including gga-miR-29c-3p-PIK3R1 (Phosphoinositide-3-kinase regulatory subunit 1), gga-miR-6701-3p-PTEN (Phosphatase and tensin homolog), gga-miR-363-3p-PTEN, gga-miR-1563-WWP1 (WW domain containing E3 ubiquitin protein ligase 1), gga-miR-449c/d-5p-TRAF6 (TNF receptor associated factor 6), and gga-miR-6701-3p-BMPR1B (Bone morphogenetic protein receptor type 1B) (Figure 6C, Table S8).
Figure 6.
Analysis of the interaction between mRNA and miRNA. (A) Protein-protein interaction (PPI) network of significant genes in weighted gene co-expression network analysis (WGCNA). (B) mRNA-miRNA regulatory network. (C) A core network identified by the Degree algorithm in Cytoscape. The cyan circle represented the differentially expressed genes (DEGs) in the cyan module, the blue circle represented the DEGs in the blue module, and the pink box represented the DE-miRNAs. n = 10.
DISCUSSION
The IMF content is closely related to the flavor and quality of meat. Therefore, it is significant to clarify the regulatory mechanism of IMF synthesis and accumulation to improve chicken quality. MiRNAs are crucial molecules that regulatory transcription. However, the mechanism through which miRNAs regulate IMF deposition during chicken development have not yet been elucidated. BJY is a distinctive native chicken breed in China that is well-known for its meat quality and flavor. In this study, RNA-seq was used to conduct a transcriptome analysis of BJY breast muscle at 4 stages, and the key genes that regulate IMF were identified to provide a theoretical basis for genetic improvement and breeding related to IMF deposition.
The IMF content of BJY at 1 d of age was significantly higher than that at the other 3 growth stages (P < 0.05), which was consistent with the results of Liu et al. (2017). This phenomenon was attributed to the fact that a large amount of cholesterol-rich lipoproteins was taken up from the yolk sac membrane by apolipoprotein when chickens hatched (Liu et al., 2016). The IMF content was highest 98 d after birth, which was consistent with the fundings of Cui Huanxian (Cui et al., 2018). In our study, the changing trends in TG and IMF content were consistent. Previous studies have shown that the increase in the IMF content is mainly influenced by the TG content (Zhang et al., 2015). Zhao et al. (2011) studied the lipid characteristics of breast muscle of 120 d of age BJY and AA chickens. The results showed that the IMF, arachidonic acid, essential FA, and total unsaturated FA contents of BJY were significantly higher than those in AA chickens. These results indicate that BJY have advantages in term of IMF content and FA composition and are excellent research subjects.
The expression profiles of mRNAs in the breast muscle of BJY at the 4 developmental stages showed that there were significant differences between the 1 d of age and later stages of birth. GSEA revealed that pentose phosphate pathway and glycolysis/gluconeogenesis pathway were activated from 1 to 56 d of age. The direct energy sources for fatty acid biosynthesis are ATP and NAPDH from the pentose phosphate pathway, and the direct carbon source is acetyl-CoA from the glycolysis pathway (Getachew et al., 2019), which is the main pathway for fat deposition. However, genes related to fat transport, such as apolipoprotein family A1 (APOA1, APOA4, and APOA5) and apolipoprotein A-I binding protein (APOA1BP), were down-regulated at this stage, which may explain the decrease in IMF content at 56 d of age. On d 98, the IMF content increased significantly and reached its highest level after birth. At this stage, the PPAR signaling pathway is activated. PPARγ enriched in this pathway is necessary for fat formation (Stephen, 2006). The cell adhesion molecule pathway was activated during 98 to 120 d of age. This pathway plays an important role in the formation and maintenance of skeletal muscle cells and inhibits the biosynthesis of fatty acids (Taylor et al., 2022). This indicates a high capacity for muscle development and reduced capacity for fat synthesis at this stage.
MiRNAs are involved in IMF deposition by mediating gene silencing. In this study, we identified 243 DE-miRNAs during the development of BJY. A total of 2,084 target genes were obtained by taking the intersection of target genes and DEGs. Based on the KEGG enrichment analysis, 2,084 target genes were enriched in the MAPK, Wnt, TGF-β signaling pathways, and focal adhesion. The MAPK signaling pathway regulates the proliferation and differentiation of intramuscular and subcutaneous adipocytes (Wu et al., 2017). After 3 wk of adipocyte development, the number of adipocytes could be reduced by adding transforming growth factor-Beta TGF-β in human bone marrow stromal fibroblasts (Locklin et al., 1999). The Wnt signaling pathway inhibits adipogenesis in mice preadipocytes (Ross et al., 2000). In combination with the above studies, these target genes may be involved in IMF metabolism in BJY.
We constructed mRNA-miRNA networks by WGCNA and PPI analyses, in which gga-miR-29c-3p-PIK3R1, gga-miR-6701-3p-PTEN, gga-miR-363-3p-PTEN, gga-miR-1563-WWP1, gga-miR-449c/d-5p-TRAF6, and gga-miR-6701-3p-BMPR1B were identified as key mRNA-miRNA pairs affecting IMF deposition in BJY. PIK3R1 encodes a phosphatidylinositol 3-kinase that plays an important role in insulin metabolism (Terauchi et al., 1999; Cantley, 2002). Under a high-fat diet, the body weight and white adipose tissue content of mice with PIK3R1 knockout increased significantly (Terauchi et al., 2004). MiR-29c-3p potentially regulates the expression of genes involved in several pathways associated with obesity, metabolism, and inflammation (Assmann et al., 2020). In ovarian cancer cells, the loss of PIK3R1 reduces the PTEN protein levels (Li et al., 2019b). PTEN contributes to the regulation of adipocytes size and fat distribution in transgenic mouse models (Garcia et al., 2012; Joan et al., 2012; Ortega et al., 2012). The overexpression of PTEN in adipocytes inhibits AKT phosphorylation, which in turn enhances lipolysis and reduces the adipocytes size and fat content in mice (Huang et al., 2020). MiR-363-3p is up-regulated by resveratrol and participates in fat metabolism by improving insulin resistance in mice (Shu et al., 2020). WWP1 is present in all eukaryotes and regulates various cellular functions. The substrates of WWP1 include spastic paraplegia 20 (SPG20) (Eastman et al., 2009), Krupp-like factor 2 (KLF2) (Conkright et al., 2001), and KLF5 (Chen et al., 2005b), SPG20 regulates the size and number of lipid droplets (Eastman et al., 2009), while KLF2 and KLF5 participate in adipocyte differentiation (Zhang et al., 2004; Oishi et al., 2008). Therefore, WWP1 may play key roles in fat formation. Zhang et al. (2021) found that the TRAF6 inhibits the expression of adipogenic genes in porcine intramuscular preadipocytes through the AKT/mTORC1 signaling pathway, thus inhibiting lipogenesis. The expression of BMPR1B increased with age in the subsequent days after 1 d of age. In addition, there was a significantly negative correlation between BMPR1B and PL (r = -0.74, P < 0.05). Recent studies have shown that BMPR1B is associated with fat deposition in children (Gagné et al., 2020). To date, no studies have directly shown that miR-6701-3p, miR-449c/d-5p, and miR-1563 are associated with adipogenesis. Therefore, the regulatory mechanisms of these genes during adipose tissue deposition should be studied further. In summary, these genes can be regarded as key candidates for future studies on IMF deposition in broiler chickens.
CONCLUSIONS
In conclusion, this study clarified the mechanism of the miRNA-mediated regulation of IMF deposition during BJY development. The content of IMF was the highest at the age of 1 d. The results of transcriptome analysis showed that the expression of PIK3R1 was the highest at 1 d of age, and then decreased significantly. PTEN, WWP1, and TRAF6 were up-regulated with age. In addition, the expression of BMPR1B increased with age in the subsequent days after 1 d of age. Combined analysis of mRNA-miRNA showed that gga-miR-29c-3p-PIK3R1, gga-miR-6701-3p-PTEN, gga-miR-363-3p-PTEN, gga-miR-1563-WWP1, gga-miR-449c/d-5p-TRAF6, and gga-miR-6701-3p-BMPR1B were the key genes and miRNAs involved in regulating IMF deposition in BJY. However, the biological function and molecular mechanism of these key genes need to be further verified. The functional genetic variants can be applied to the molecular breeding of broilers meat quality. In brief, these results will help elucidate the molecular mechanism of IMF formation in chickens, and provide important candidate genes for the molecular breeding of broiler meat quality.
ACKNOWLEDGMENTS
This work was supported by the Beijing Innovation Consortium of Agriculture Research System (BAIC06-2023) and Beijing Municipal Bureau of Agriculture and Rural Affairs.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.103035.
Appendix. Supplementary materials
REFERENCES
- Assmann T.S., Riezu-Boj J.I., Milagro F.I., Martínez J.A. Circulating adiposity-related microRNAs as predictors of the response to a low-fat diet in subjects with obesity. J. Cell Mol. Med. 2020;24:2956–2967. doi: 10.1111/jcmm.14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A.D., Mattioli S., Ruggeri S., Mugnai C., Castellini C. Effect of slaughtering age in different commercial chicken genotypes reared according to the organic system: 2. Fatty acid and oxidative status of meat. Italian J. Anim. Sci. 2014;13:462–466. [Google Scholar]
- Cameron N.D., Enser M.B. Fatty acid composition of lipid in Longissimus dorsi muscle of Duroc and British Landrace pigs and its relationship with eating quality. Meat. Sci. 1991;29:295–307. doi: 10.1016/0309-1740(91)90009-F. [DOI] [PubMed] [Google Scholar]
- Cantley L.C. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Chang G.B., Lei L.L., Zhang X.Y., Wang K.H., Chen R., Luan D.Q., Chen G.H. Development rule of intramuscular fat content in chicken. J. Anim. Vet. Adv. 2010;9:297–298. [Google Scholar]
- Chen C., Sun X., Guo P., Dong X.Y., Sethi P., Cheng X., Zhou J., Ling J., Simons J.W., Lingrel J.B. Human Krüppel-like factor 5 is a target of the E3 ubiquitin ligase WWP1 for proteolysis in epithelial cells. J. Biol. Chem. 2005;280:41553–41561. doi: 10.1074/jbc.M506183200. [DOI] [PubMed] [Google Scholar]
- Chen J.L., Wen J., Wang S.B., Zhao G.P., Zheng M.Q. Studies on the characteristics of deposition of chicken IMP and IMF. Acta Vet. Zootech Sin. (Chinese) 2005;36:843–845. [Google Scholar]
- Conkright M.D., Wani M.A., Lingrel J.B. Lung Krüppel-like factor contains an autoinhibitory domain that regulates its transcriptional activation by binding WWP1, an E3 ubiquitin ligase. J. Biol. Chem. 2001;276:29299–29306. doi: 10.1074/jbc.M103670200. [DOI] [PubMed] [Google Scholar]
- Cui H.X., Liu R.R., Zhao G.P. Identification of differentially expressed genes and pathways for intramuscular fat deposition in pectoralis major tissues of fast-and slow-growing chickens. BMC Genomics. 2018;19:55. doi: 10.1186/1471-2164-13-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman S.W., Yassaee M., Bieniasz P.D. A role for ubiquitin ligases and Spartin/SPG20 in lipid droplet turnover. J. Cell Biol. 2009;184:881–894. doi: 10.1083/jcb.200808041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagné O.V., Breton E., Thibeault K., Fortin C.A., Desgagne V., Tremblay E.G., Cardenas A., Guerin R., Perron P., Hivert M.F. Placental epigenome-wide association study identified loci associated with childhood adiposity at 3 years of age. Int. J. Mol. Sci. 2020;21:7201. doi: 10.3390/ijms21197201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C.I., Song M.S., Hobbs R.M., Laurent G., Giorgi C., DeBoer V., Anastasiou D., Ito K., Sasaki A.T., Rameh L. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell. 2012;149:49–62. doi: 10.1016/j.cell.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew D.B., Joao S., Joao R., Ludgero T., Ivan V., Jose T., Paulo J.O., Maria P.M., John G.J. Transfer of glucose hydrogens via acetyl-CoA, malonyl-CoA, and NADPH to fatty acids during de novo lipogenesis. J. Lipid Res. 2019;60:2050–2056. doi: 10.1194/jlr.RA119000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirong S., Li F., Ma X.F., Sun J.W., Jiang R.R., Tian Y.D., Han R.L., Li G.X., Li Y.B.Z.J., Kang X.T., Li W.T. gga-miRNA-18b-3p Inhibits intramuscular adipocytes differentiation in chicken by targeting the ACOT13 gene. Cells. 2019;8:556. doi: 10.3390/cells8060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J.A., Trakooljul N., Liu H.C. Discovery of chicken microRNAs associated with lipogenesis and cell proliferation. Physiol. Genomics. 2010;41:185–193. doi: 10.1152/physiolgenomics.00156.2009. [DOI] [PubMed] [Google Scholar]
- Hocquette J.F., Gondret F., Baéza E., Médale F., Jurie C., Pethick D.W. Intramuscular fat content in meat-producing animals: development, genetic and nutritional control, and identification of putative markers. Animal. 2010;4:303–319. doi: 10.1017/S1751731109991091. [DOI] [PubMed] [Google Scholar]
- Huang W., Nicholas J.Q., Travis B.M., Seemaab A., Ryan K.W., Bhavya A., Lei C. Adipose PTEN acts as a downstream mediator of a brain-fat axis in environmental enrichment. Compr. Psychoneuroendocrinol. 2020;4 doi: 10.1016/j.cpnec.2020.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joan S.G., Hung C.M., Sparks C.A., Tang Y., Li H., Guertin D.A. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 2012;16:348–362. doi: 10.1016/j.cmet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Li D.H., Zhang M., Sun J.W., Li W.T., Jiang R.R., Han R.L., Wang Y.B., Tian Y.D., Kang X.T., Sun G.R. miRNA-223 targets the GPAM gene and regulates the differentiation of intramuscular adipocytes. Gene. 2019;685:106–113. doi: 10.1016/j.gene.2018.10.054. [DOI] [PubMed] [Google Scholar]
- Li X.R., Mak V., Zhou Y., Wang C., Wong E., Rakesh S., Lu Y.L., Cheung A., Mills G.B., Cheung L. Deregulated Gab2 phosphorylation mediates aberrant AKT and STAT3 signaling upon PIK3R1 loss in ovarian cancer. Nat. Commun. 2019;10:716. doi: 10.1038/s41467-019-08574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L., Kebin H. MiR-147: functions and implications in inflammation and diseases. Microrna. 2021;10:91–96. doi: 10.2174/2211536610666210707113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Fu R.Q., Liu R.R., Zhao G.P., Zheng M.Q., X.Cui H., Li Q.H., Song J., Wang J., Wen J. Protein profiles for muscle development and intramuscular fat accumulation at different post-hatching ages in chickens. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.R., Wang H.Y., Liu J., Wang J., Zhang M.Q., Tan X.D., Xing S.Y., Cui H.X., Li Q.H., Zhao G.P., Wen J. Uncovering the embryonic development related proteome and metabolome signatures in breast muscle and intramuscular fat of fast-and slow-growing chickens. BMC Genomics. 2017;18:816–830. doi: 10.1186/s12864-017-4150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locklin R.M., Oreffo R.O., Triffitt J.T. Effects of TGFβ and bFGF on the differentiation of human bone marrow stromal fibroblasts. Cell Biol. Int. 1999;23:185–194. doi: 10.1006/cbir.1998.0338. [DOI] [PubMed] [Google Scholar]
- Mottram D.S. Flavour formation in meat and meat products: a review. Food Chem. 1998;62:415–424. [Google Scholar]
- Mottram D.S., Edwards R.A. The role of triglycerides and phospholipids in the aroma of cooked beef. J. Sci. Food Agric. 1983;34:517–522. [Google Scholar]
- Nestel P.J. Polyunsaturated fatty acids (n-3, n-6) Am. J. Clin. Nutr. 1987;45:1161–1167. doi: 10.1093/ajcn/45.5.1161. [DOI] [PubMed] [Google Scholar]
- Oishi Y., Manabe I., Tobe K., Ohsugi M., Kubota T., Fujiu K., Maemura K., Kubota N., Kadowaki T., Nagai R. SUMOylation of Krüppel-like transcription factor 5 acts as a molecular switch in transcriptional programs of lipid metabolism involving PPAR-delta. Nat. Med. 2008;14:656–666. doi: 10.1038/nm1756. [DOI] [PubMed] [Google Scholar]
- Ortega M.A., Efeyan A., Lopez G.E., Munoz M.M., Gomez L.G., Canamero M., Mulero F., Pastor J., Martinez S., Romanos E., Gonzalez B.M., Rial E., Valverde A.M., Bischoff J.R., Serrano M. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab. 2012;15:382–394. doi: 10.1016/j.cmet.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Ping L., Defa L., Jingdong Y., Liying Z., Zongyi W. Flavour differences of cooked longissimus muscle from Chinese indigenous pig breeds and hybrid pig breed (Duroc×Landrace×Large White) Food Chem. 2008;107:1529–1537. [Google Scholar]
- Popova T., Ignatova M., Petkov E., Stanišić N. Difference in fatty acid composition and related nutritional indices of meat between two lines of slow-growing chickens slaughtered at different ages. Arch. Anim. Breed. 2016;59:319–327. [Google Scholar]
- Ross S.E., Hemati N., Longo K.A., Bennett C.N., Lucas P.C., Erickson R.L., MacDougald O.A. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Shu L., Zhao H., Huang W., Hou G., Song G., Ma H. Resveratrol upregulates mmu-miR-363-3p via the PI3K-Akt pathway to improve insulin resistance induced by a high-fat diet in mice. Diabetes Metab. Syndr. Obes. 2020;13:391–403. doi: 10.2147/DMSO.S240956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen R.F. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.X, Tian Y., He H.X., Wang J.D. Review of the factors and genes on intramuscular fatty acid. Prog. Vet. Med. 2006;27:49–53. [Google Scholar]
- Taylor L., Wankell M., Saxena P., McFarlane C., Hebbard L. Cell adhesion an important determinant of myogenesis and satellite cell activity. Biochim. Biophys. Acta Mol. Cell Res. 2022;1869 doi: 10.1016/j.bbamcr.2021.119170. [DOI] [PubMed] [Google Scholar]
- Terauchi Y., Matsui J., Kamon J., Yamauchi T., Kubota N., Komeda K., Aizawa S., Akanuma Y., Tomita M., Kadowaki T. Increased serum leptin protects from adiposity despite the increased glucose uptake in white adipose tissue in mice lacking p85alpha phosphoinositide 3-kinase. Diabetes. 2004;53:2261–2270. doi: 10.2337/diabetes.53.9.2261. [DOI] [PubMed] [Google Scholar]
- Terauchi Y., Tsuji Y., Satoh S., Minoura H., Murakami K., Okuno A., Inukai K., Asano T., Kaburagi Y., Ueki K. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase. Nat. Genet. 1999;21:230–235. doi: 10.1038/6023. [DOI] [PubMed] [Google Scholar]
- Wood J.D., Enser M., Fisher A.V., Nute G.R., Sheard P.R., Richardson R.I., Hughes S.I., Whittington F.M. Fat deposition, fatty acid composition and meat quality: a review. Meat. Sci. 2008;78:343–358. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Wu W., Zhang J., Zhao C., Sun Y., Pang W., Yang G. CTRP6 Regulates porcine adipocyte proliferation and differentiation by the AdipoR1/MAPK signaling pathway. Food Chem. 2017;65:5512–5522. doi: 10.1021/acs.jafc.7b00594. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Cai R., Tang G., Zhang W., Pang W. MiR-146a-5p targeting SMAD4 and TRAF6 inhibits adipogenensis through TGF-β and AKT/mTORC1 signal pathways in porcine intramuscular preadipocytes. J. Anim. Sci. Biotechnol. 2021;12:12. doi: 10.1186/s40104-020-00525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Luo J.Q., Zheng P., Yu B., Huang Z.Q., Mao X.B., He J., Yu J., Chen J.L., Chen D.W. Differential expression of lipid metabolism-related genes and myosin heavy chain isoform genes in pig muscle tissue leading to different meat quality. Animal. 2015;9:1073–1080. doi: 10.1017/S1751731115000324. [DOI] [PubMed] [Google Scholar]
- Zhang X., Srinivasan S.V., Lingrel J.B. WWP1-dependent ubiquitination and degradation of the lung Kruppel-like factor KLF2. J. Biol. Chem. 2004;316:139–148. doi: 10.1016/j.bbrc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Zhao G.P., Cui H.X., Liu R.R., Zheng M.Q., Chen J.L., Wen J. Comparison of breast muscle meat quality in 2 broiler breeds. Poult. Sci. 2011;90:2355–2359. doi: 10.3382/ps.2011-01432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.