Abstract
Biomaterials, when implanted in the human body, can induce a series of cell- and cytokine-related reactions termed foreign body reactions (FBRs). In the progression of FBRs, macrophages regulate inflammation and healing by polarizing to either a pro-inflammatory or pro-healing phenotype and recruit fibroblasts by secreting cytokines. Stimulated by the biomaterials, fibrotic capsule is formed eventually. The implant, along with its newly formed capsule, introduces various mechanical cues that influence cellular functions. Mechanosensing proteins, such as integrins or ion channels, transduce extracellular mechanical signals into cytoplasm biochemical signals in response to mechanical stimuli. Consequently, the morphology, migration mode, function, and polarization state of the cells are affected. Modulated by different intracellular signaling pathways and their crosstalk, the expression of fibrotic genes increases with fibroblast activation and fibroblast to myofibroblast transition under stiff or force stimuli. However, summarized in most current studies, the outcomes of macrophage polarization in the effect of different mechanical cues are inconsistent. The underlying mechanisms should be investigated with more advanced technology and considering more interfering aspects. Further research is needed to determine how to modulate the progression of fibrotic capsule formation in FBR artificially.
Keywords: Biomaterials, Mechanical cues, Foreign body reaction, Fibrosis, Macrophages, Fibroblasts
Graphical abstract
1. Introduction
As biomaterial engineering advances, an increasing number of biocompatible materials are being incorporated into the human body for therapeutic purposes, such as breast prostheses [1], joint repair [2], and vascular valve replacement [3]. However, any biomaterials implanted in the human body can set off a cascade reaction of inflammatory cells, creating a fibrotic capsule as the immune barrier against the implanted devices finally. Foreign body reaction (FBR) is the common term used to characterize this phenomenon [4].
The progression of FBR can be roughly divided into five phases: the interaction of hematological components and the synthesis of temporary extracellular matrix; acute inflammation; chronic inflammation; activation of macrophages and transformation of foreign giant cells; and activation of fibroblasts and fibrotic capsules formation [[4], [5], [6]]. Implant material allergies [7], bacterial infections [8], and fibrotic capsular contracture are the most common complications of FBR, which can develop into implant loss. Along with the severe complications of FBR, the application of medical biomaterial devices is hampered. As one of the foremost significant complications of FBR, the formation of a contracted fibrotic capsule can cause function loss of the biomaterial devices [4,5].
Cells can detect various extracellular environmental signals, including chemical signals and mechanical signals. Mechanical cues that can be sensed by cells from their surroundings include physical forces, such as tension, compression, and shear [9,10]. Tension and compression may be generated between neighboring cells, ECM, and extracellular materials. Under some situations, tension (or stretch) and compression on an in vivo tissue may transfer the stress on each cell and affect their behaviors. Blood or other flowing fluid exerts shear stress on epithelial cells. Stiffness is also a kind of mechanical cue that can be sensed by cells after focal adhesion (Fig. 1). Stiffness is a metric of the rigidity property of a matrix sensed by cells via the application of cell-generated forces. The stiffness of matrices is often determined under the assumption of elasticity. Methods such as atomic force microscopy measure the elastic modulus (E; Young's modulus/kPa) to represent the stiffness of the material. Young's modulus is measured through the longitudinal stress divided by the strain, describing the deformity of material undergoing tension or compression in one direction [11]. The stiffness of the extracellular environment provides external resistance or compliance to conduct force on the cells. Cells also exert intrinsic forces against the environment and neighboring cells [10].
Fig. 1.
Following cell junction and focal adhesion, compression and tension could exist between cells and the surrounding ECM. Shear of fluid exerts on the cell surface of a lumen space. Between the surface of ECM or biomaterial with a certain stiffness and the cells, an interaction force consisting of an external resistance and an intrinsic force is generated. Created with BioRender.com.
During the process of FBR, mechanical cues generated from implants and the newly formed ECM are induced into the environment generated from implants and the newly formed ECM. Implants with varying stiffness, stretching from biofilm architecture, and force formed by tightening after capsule contraction could all influence cell behavior [12,13], especially for fibroblasts and macrophages during FBR. Fibroblasts and macrophages exist in FBR persistently. How they function directs the result of the fibrotic capsule. However, most studies revealed this progression through the inflammatory aspect rather than the mechanical aspect. The study about the role of different mechanical cues playing in this scenario is still lacking, especially the interaction between the cells under mechanical stimuli.
In this review, we focus on the effects of mechanical cues on macrophages and fibroblasts during FBR. We summarized the most current understandings and controversies of how macrophages and fibroblasts interact with different mechanical cues and the way they drive the fibrotic progression during FBR. These summaries may provide us with a deeper understanding of how fibrotic capsule formation under mechanical stimuli. Further research on biomaterial development and regulation of fibrotic progression of FBR could be inspired.
2. The bio function of macrophages and fibroblasts in FBR
With the implantation of biomaterials, hemorrhage-evoked platelets migrate to the cavity. Proteins such as albumins from plasma are absorbed in the biomaterials and are replaced gradually by less motile proteins such as high molecular weight kininogen(HMWK), fibrinogen, fibronectin, and vitronectins [14]. This absorption and desorption process of proteins is named the Vroman effect [15]. On the material surface, the integrin-mediated formation of a fibrin-dominated thrombus provisional matrix is significant for monocyte adhesion and further differentiation [[16], [17], [18], [19]]. Cytokines released by polymorphonuclear leukocytes (PMNs) and mast cells have an impact on the subsequent recruitment and activation of inflammatory cells [[20], [21], [22]]. The injury caused by implantation induces PMNs infiltration and degranulation of neutrophils [21]. Tissue-resident macrophages generated from embryonic progenitors and macrophages derived from monocytes are two types of macrophages inhabited [23]. Cytokines secreted by mast cells, such as histamine, IL-4, and IL-13 attract monocytes and macrophages that play a key role in the chronic inflammation phase [22,24,25].
2.1. Macrophages
Macrophages are a type of innate immune cells that engulf and digest pathogens, such as cancer cells, microbes, cellular debris, and foreign substances. They play a key role in tissue repair, regeneration, and fibrosis. They are very plastic cells with different phenotypes and functions, which are impacted both by their origin and resident tissue microenvironment [26]. In the presence of foreign implants, macrophages differentiate into classically activated pro-inflammatory macrophages(M1s) and alternatively activated pro-healing macrophages(M2s). Macrophages could be activated classically by binding the absorbed proteins on the implant surface. Lipopolysaccharide (LPS) and interferon-gamma (IFN γ) could also induce the differentiation of monocytes into M1s [27]. Secretion of IL-1β, IL-6, reactive oxygen species (ROS), inducible nitric oxide synthase (iNOS) and tumor necrosis factor-α (TNF-α), which promote inflammatory responses are hallmarks of M1s [28]. M1s degrade materials through phagocytosis and release reactive oxygen species (ROS) and lysosomal enzymes. M2s are induced by IL-4 and IL-13 from mast cells or T helper 2 cells [29]. To balance the inflammatory response, M2s secrete the anti-inflammatory cytokines IL-10 and transforming growth factor-β (TGF-β) and induce tissue remodeling by matrix-metalloproteinases (MMP) [30]. Multinucleated foreign body giant cells (FBGCs) are formed by the fusion of macrophages that are frustrated to degrade materials through phagocytosis and secrete enzymes. Doloff et al. found that colony-stimulating factor 1 receptor (CSF1R) plays a crucial role in the formation of FBGCs and promotes ROS production and phagocytosis [31]. Meanwhile, macrophages transmit signals to recruit supporting cells, including fibroblasts, to participate in tissue healing [32,33]. Fibroblasts are recruited prominently by the ECM‐stored latent TGF‐β activated by macrophages [34].
2.2. Fibroblasts
Fibroblasts are common throughout the connective tissue of the human body and constitute the ECM from collagen, elastin, laminin, and proteoglycan [35]. Fibroblasts have pivotal roles in ECM generation [36], angiogenesis [37], cancer progression [38] et al. Activated Fibroblasts could convert into primitive myofibroblasts, myofibroblasts, and fibrocytes, demonstrating remarkable plasticity with specific phenotypes for specific functions [39].
Fibroblasts migrate to the surface of the biomaterial and form granulation tissue composed of loose collagen fibers and other extracellular matrix proteins. The granulation tissue will mature into a fibrous capsule with fewer cells and denser collagen fibers. The progression of ECM remodeling is influenced by profibrotic growth factors such as TGF-β and platelet-derived growth factor (PDGF) [40,41]. Proteolytic enzymes such as MMPs are involved in counteracting further fibrosis [42].
As the expected outcome of fibrosis progresses, fibrinolytic macrophages reduce the deposition of collagen. At the same time, fibroblasts undergo apoptosis and senescence, and some are converted to fibrocytes after the maturation and remodeling of the newly formed ECM around the biomaterial [43,44]. However, these reactions rarely occur because biomaterials are present as initiators of proinflammatory and profibrotic cells. The deposition of fibrotic proteins is exacerbated by fibroblast to myofibroblast transition (FMT) under the influence of TGF-β mainly [45], and the programmed apoptosis of myofibroblasts is lost [46]. The degree of FMT can represent the degree of fibrotic progression. As a special phenotype of fibroblast, myofibroblast expresses α-SMA and represents contracted fibrosis of ECM. With the contraction of myofibroblasts, the density and stiffness of the ECM increase [4,47]. With the deterioration of excessively fibrotic capsules formation, the application of many biomaterials failed [48,49].
2.3. Interactions between macrophages and fibroblasts
Macrophages are known to influence the function of fibroblasts in both health and disease conditions. Fibroblast-macrophage interactions are centered on the colony-stimulating factor 1 and colony-stimulating factor 1 receptor (CSF1-CSF1R) axis in healthy tissue [50]. Fibroblasts are a dominant source of CSF1 for macrophages to maintain basic functions [51]. Following biomaterial implantation, CSF1R in macrophages is significantly increased [31]. Meanwhile, macrophages provide PDGF ligands required for fibroblast survival in vivo [52]. Activated macrophages can also be a source of TGF-β which drives fibroblasts to activate fibrosis actions through paracrine secretion [53]. Using decellularized macrophage medium to culture fibroblasts, M2s induced by IL4+IL13 or platelet lysate increased collagen production and angiogenesis gene expression of fibroblasts [54,55]. In the coculture system consisting of fibroblasts and M1s or M2s, fibrogenic activities of fibroblasts are promoted by M2s [56,57]. Mechanical cues surely influence macrophages and fibroblasts in FBR, but the influence of mechanical cues has been merely investigated as an intervening condition between these two kinds of cells.
3. Mechanosensing proteins of the cells in FBR
Living cells are constantly exposed to mechanical stimuli arising from the surrounding extracellular matrix or neighboring cells. Mechanosensing refers to the sense and response to mechanical cues of cells [58]. After the sensing of mechanical cues, cells could transform the extracellular mechanical signals into intracellular biological responses, which can be defined as mechanotransduction [59]. Mechanosensing and mechanotransduction are associated with the progression of cell division, adhesion, migration, motivation, differentiation, and gene expression. It is essential for tissue development, and physiological and pathological progression [13,60,61].
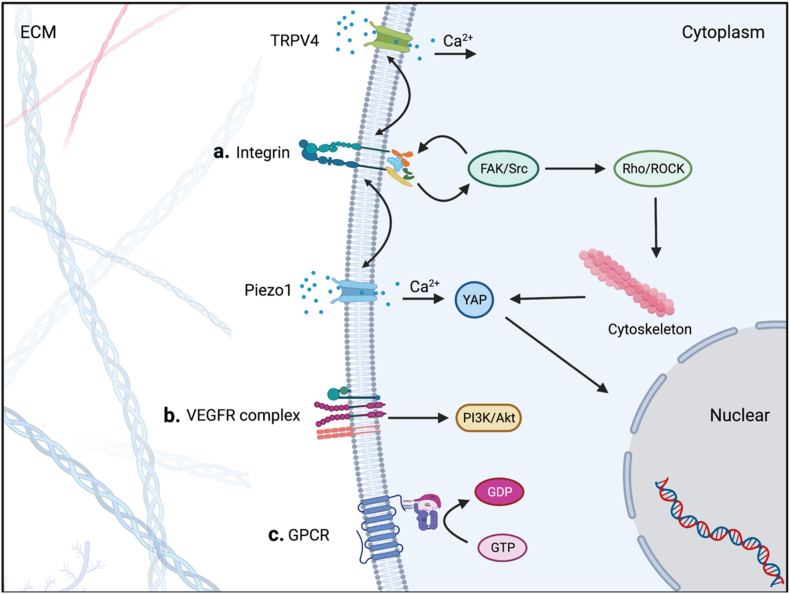
Mechanosensing initiates from the conformational change caused by mechanical cues with mechanosensitive transmembrane proteins on the cell membrane being activated. Various kinds of mechanosensing proteins on cell membranes can sense mechanical cues, including integrins, mechanosensitive ion channels, G protein-coupled receptor (GPCR) superfamily, and vascular endothelial growth factor receptors (VEGFRs) complex (Fig. 2).
Fig. 2.
a. After the extracellular part of Integrins is activated, the intracellular part is assembled with integrin-associated proteins such as talin and vinculin. Meanwhile, FAK/Src signaling is phosphorylated. FAK activates downstream signaling while also recruiting more integrins to form a denser arrangement. As the downstream, Rho and ROCK change actin polymerization by modulating myosin. Piezo1 and TRPV4 participate in mehcanosensing and coordinate with integrins.YAP's nuclear translocations is activated by Ca2+ influx and cytoskeleton change. b. Shear exerts on VEGFR2/3 in a VE-cadherin-dependent manner and activates downstream PI3K/Akt signaling. c. The structural change of the lipid bilayer cell membrane by extracellular force or traction on the N-terminus causes GPCR activation. Created with BioRender.com.
3.1. Integins
Integrins, which act as mechanosensor and mechanotransducer by adhesion to ECM proteins or substrates, are heterodimers composed of α and β subunits. As a dominant mechanosensitive receptor, the type of integrins expressed on the cell surface regulate cells' reaction to mechanical cues [62,63]. It is known that 18 α subunits and 8 β subunits are assembled into 24 distinct integrins [64]. Integrins can be defined as laminin-binding integrins, collagen-binding integrins, and FN/vitronectin (RGD; Arg-Gly-Asp-motif)-binding integrins by binding proteins [65]. In FBR, integrins dominate the mechanosensing proteins of fibroblasts and macrophages. The αvβ1, α5β1, and αvβ3 integrin on fibroblasts bind to biomaterials surface for mechanosensing mainly [[66], [67], [68]], while αMβ2 integrin plays a major role in the mechanosensing of macrophages [69]. How they function will be described in the latter sections.
3.2. Ion channels
Mechanosensitive Ca2+ permeating channels Piezo1 and transient receptor potential vanilloid 4 (TRPV4) are verified as mechanosensing proteins. They can be activated by the changes the dynamic balance of hydrophobic and lipid–lipid interactions of the lipid bilayer membranes [70,71]. Piezo1 is a trimeric structure located in the plasma membrane that opens in response to mechanical stimuli. Ca2+, K+ and Na + flow through the channel when it opens [72]. It plays a vital role in macrophages' mechanical sensing. In an in vivo study, Atcha H et al. implanted polyethylene glycol diacrylate-400 (PEGDA-400) hydrogels subcutaneously into Piezo1 depleted mice, the thickness of the collagen capsule and macrophages accumulation caused by stiff ones (140 kPa) was reduced [73].
TRPV4 always mediates the activation of fibroblasts and FBGCs in response to stiffness. A subcutaneous implant mice model was used in the study of Goswami R et al. The rigid collagen-coated PA hydrogel discs (50 kPa) increased the accumulation of macrophages, FBGCs and myofibroblasts in WT compared to Trpv4−/− mice. But soft discs(1 kPa) induced no difference results with the regulation of TRPV4 independently [74]. They also observed TRPV4 promoted BMDMs stiffness. Further research revealed that TRPV4 interacts with Rac1 and regulates cytoskeletal remodeling and intracellular stiffness. This TRPV4-Rac1 signaling axis modulates the formation of FBGCs [75].
They also work in collaboration with integrins synergistically. In a study of cardiac fibroblasts, a positive feedback loop between β1 integrin and Piezo1 activation exists with stiffness increased [76]. When the stretch is applied to macrophages, an underlining relationship between the expression of Piezo1 and αM integrin is also observed [76,77]. TRPV4 expression is associated with the decreased cellular abundance of the β1 integrin in fibroblasts [78]. Between the ion channels, Piezo1 distinct regulated TRPV4 under mechanical activation [79].
3.3. Others
There are other types of proteins that have mechanosensing roles in addition to the proteins stated above. Platelet endothelial cell adhesion molecule-1(PECAM-1), VE-cadherin (VEcad) and vascular endothelial growth factor receptors (VEGFRs) compose a protein complex as a fluid shear sensor directing vascular development, homeostasis and atherogenesis [80]. Mechanosensitive GPCRs can be activated by shear stress and cell swelling/stretch and mediate a wide range of biological processes [81]. However, they have limited function in FBR and therefore will not be described further.
Based on integrins, the dominant mechanosensing proteins in FBR, the bio functions of cells are regulated through mechanotransduction by cytoskeleton contraction or intracellular signaling pathways. In other words, the effects of mechanical signals' transduction could be summarized into morphological effects and biochemical effects.
4. The morphological changes under mechanical stimuli in FBR
A specific adhesion between cells and their surroundings named integrin adhesion complexes (IACs) is formed after integrins sense the mechanical cues. IACs are composed of integrin receptors that bind to ECM ligands and recruit a large, heterogeneous set of cytoplasmic proteins, including talin, vinculin, paxillin, and FAK [82]. Focal adhesions (FAs), a type of IACs, are assembled and form mechanical links between intracellular actin bundles and the extracellular substrate, as well as focal adhesion kinases (FAKs) are activated after adhesion [83]. The dynamic molecular bonds formed by IACs drive cell morphogenesis, movement, and ECM remodeling [62,[84], [85], [86]].
4.1. Shape of cells
A mathematical model based on thermodynamics was developed and demonstrated that cells spread more on stiffer substrates [87]. Once applied to integrins' extracellular structure, a mechanical force will transmit to the actin cytoskeleton with IACs, and the force will be transmitted back to the ECM, reciprocally [88,89]. The force could degenerate from pulling a mature focal adhesion as an anchor when cells contact with a stiff substrate [90]. Fibroblasts increase their spreading area with a protruding shape on stiff substrates [91,92]. By blocking αv integrins, fibroblasts' mechanosensitivity and activation are hampered [68]. In a study by Ariel Wang et al. focal adhesion structures of fibroblasts were distributed throughout the cell membrane, presenting a large “supernature” pattern on stiff substrates [63]. The rheology transition leading to higher order and higher tension of actin cytoskeleton on stiffer substrates also determined the shape of fibroblasts [93].
4.2. Migration models
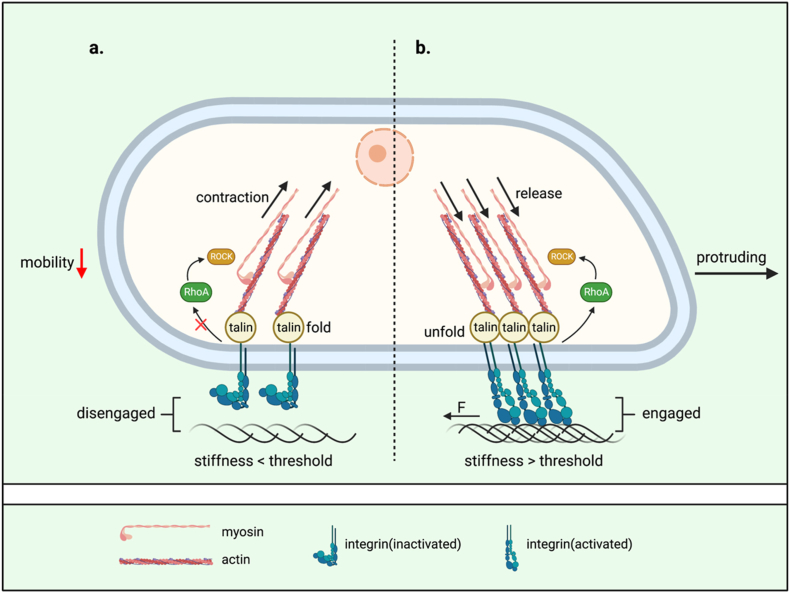
Increasing the stiffness also has been shown to increase cell migration [87]. The motility of fibroblasts is increased depending on the contraction and release of integrin/talin/actomyosin cytoskeleton in the amoeboid migration pattern relies on high Rho-associated protein kinase (ROCK)-myosin II activity (Fig. 3). With inactivating the myosin light chain 2 (MLC2) phosphatase and phosphorylated myosin light chain (pMLC) increased, ROCK increases the activity of the motor protein Myosin II [68,94]. In this machinery, a force threshold for talin unfolding transduced from integrins may mediate the stiffness sensation. If talin unfolds, vinculin binding leads to adhesion reinforcement and integrins recruitment [95]. Meanwhile, recruitment of FAK to integrins has been suggested to precede talin recruitment [96].
Fig. 3.
An ECM-integrin-talin-actin clutch orchestrates cell morphology and mobility. a. When the stiffness of the ECM does not reach the required threshold, the extracellular part of integrins does not engage with the ECM. Myosin maintains the pulling on actin while talin folds. With the cytoskeleton contracting, the mobility of the cell is reduced. b. When the stiffness of the ECM reaches the threshold required for integrins engagement, talin unfolds and myosin discharges actin depending on RhoA/ROCK. With the engagement of integrins and ECM, a force aiding cell protrusion is generated by cell-ECM adhesion. The cell presents a spreading shape with increased mobility. Created with BioRender.com.
Different from fibroblasts, macrophages present either amoeboid or mesenchymal migration modes, depending on their underlying substrate's physical and chemical properties. Macrophages move in a ROCK-independent mesenchymal migration pattern with podosome formation in dense matrices, while ROCK-dependent amoeboid migration mode in loose, fibrillar matrices [97]. It is consistent with the observation of macrophages on stiff or soft substrates [98]. In a mesenchymal migration, podosomes of macrophages exert proteolytic activity to degrade the underlying matrix, but stiffer substrates also impaired macrophage phagocytosis remarkably [98,99]. On the soft substrates, macrophages reduce their adhesion area and migrate at a higher speed [97,98].
It's inescapable to notice that Hsieh JY et al. cultured macrophages on a crosslinked fibrin gel with higher rigidity and found macrophages exhibit greater cell spreading and expression of αM integrin. Even less αM integrin was expressed than on glass, macrophages were the most motile on crosslinked fibrin surfaces, in terms of both velocity and maximum displacement. This phenomenon promotes the migration of macrophages, which exacerbates fibrosis [100]. With various culturing conditions in different studies, the migration of macrophages is influenced not only by matrix surface architectures but also by the polarization directions, which we will discuss in detail later.
Except for morphological deformity of cells, mechanical cues in FBR cause also conduct intracellular biochemical signals to manipulate fibroblasts and macrophages following cellular reactions.
5. Mechanical cues regulate fibroblasts by various signal pathways
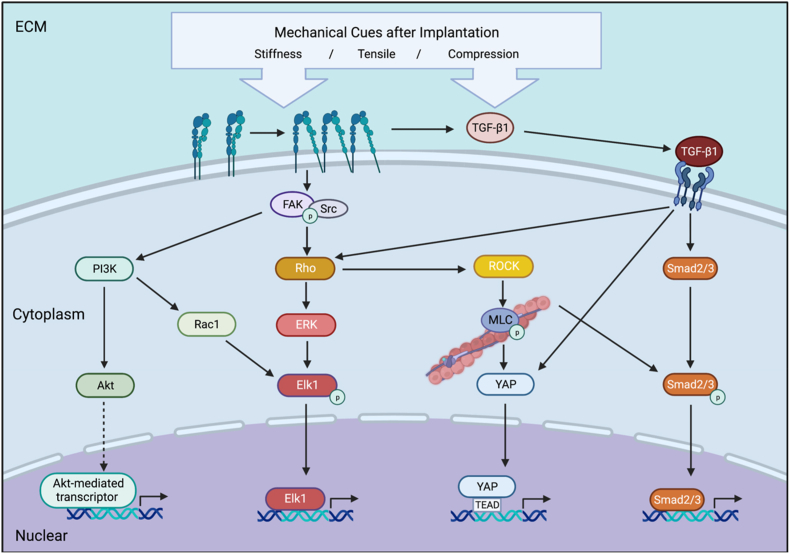
Mechanical cues in FBR are verified as one of the initiators of these actions. During the process of fibrous capsule formation, the stiffness of the biomaterials could be sensed by cells and influence their behaviors. On stiff substrates, fibroblasts are induced to a synthetic phenotype with elevated ECM formation [91,101]. The observation of increased αSMA in the stiffer matrix also indicates that the stiff substrates promote FMT [92]. Stretch and stiff substrates could induce high expression of six fibrotic genes in fibroblasts, including Col1a1, Col3a1, Eln, Fn1, Loxl1, and Acta2 [63]. Collagen I is the most abundant among the components of ECM, the long and compact collagen I fibers could increase the stiffness of the fibronectin network in the ECM apparently [102]. The ECM surrounding biomaterial forms a fibrous capsule with different stiffness gradually, which also functions as a mechanical cue on cells. The activation of fibroblasts could be reversed on more compliant substrates [103,104]. These reactions of fibroblasts are realized through different intracellular signal pathways through mechanotransduction. Meanwhile, crosstalks exist between these signal pathways and integrins (Fig. 4).
Fig. 4.
A. Under the stimulation of mechanical cues in FBR, integrins are recruited and activated on the cell membrane. FAK is recruited to the integrin clustering and interacts with integrin-associated proteins. Integrin stimulates FAK phosphorylation and binds with Src to form a FAK/Src complex. FAK/Rho/ROCK signaling also regulates the cytoskeleton by phosphorylating MLC. FAK also activates PI3K/Akt signal pathway and regulates Akt-mediated gene transcription. Rac-1, as a downstream of PI3K, activate Elk-1 which also is regulated from Rho to ERK and regulates related transcription in nuclear after phosphorylation. Latent TGF-β1 is stored in the ECM bounding latency-associated peptide (LAP) non-covalently. Mechanical cues cause the activation of TGF-β1 by an integrin-mediated conformational change in the LAP-TGF-β1 straitjacket configuration. The TGF-β1/Smad2/3 signaling is activated. TGF-β1 also induced Rho activation that changes the cytoskeleton which further enhanced the Smad2-phosphorylation and nuclear translocation. The cytoskeleton alignment changes lead to YAP activation and accumulation in the nuclei for TEAD-dependent gene expression. Created with BioRender.com.
5.1. YAP
By transmitting mechanical cues into biochemical signals, cells respond through transcriptional regulation in the nucleus [93,105]. Gałdyszyńska M et al. found α2β1 integrin and its downstream FAK/Src kinase signaling regulated collagen metabolism in cardiac fibroblasts cultured on the stiffer substrate, collagen content was elevated and regulated by MMP-1 and MMP-9 on soft substrates [106]. Yes-associated protein/transcriptional co-activator with PDZ-binding motifs (YAP/TAZ) are transcriptional co-activators that play a key role in the Hippo signaling pathway and are involved in various cellular processes. YAP/TAZ can also be regulated by the Hippo-independent pathway in response to mechanical cues [107]. Niu L et al. demonstrated YAP plays an important role in cardiac fibroblast activation in the stiff matrix [108]. YAP was a core mediator of the pro-fibrotic integrin pathway in the study of Martin K et al., which perpetuated integrin β1 expression in myofibroblasts proliferation, migration, contraction, and production of fibrotic ECM components [109]. Myocardin-related transcription factor-A (MRTF-A), similar to YAP, was found indispensable for the spread, stress fibers formation, and cell growth rates of fibroblasts under cyclic stretching [110].
Depending on integrins, the formation of IACs and cytoskeleton fibers deform the nuclear shape of fibroblasts. The nuclear deformity and focal adhesion cause YAP/TAZ and MRTF-A, as transcription regulators, to accumulate in the nucleus of fibroblasts [95,107,111,112]. FAK and Src-family kinases as the common intermediator between integrin and YAP, complete the signaling pathways [113]. Gene expression altered by transcriptional enhanced association domain (TEAD) transcription factors overlapping within the promoter region, α-smooth muscle actin (α-SMA) expression launched with fibroblasts activation and ECM deposition [68,114,115].
Ion channels also coordinate with integrin-based transcription regulations. Piezo1 ion channel, acting as an upstream mechanical response to YAP, participates in the mechanosensitive stretch and stiff matrix in human atrial fibroblasts [116]. TRPV4 also modulates the Rho/MRTF-A pathway of fibroblasts whose differentiation and function were regulated [117].
5.2. TGF-β1
Transforming growth factor-beta 1 (TGF-β1) is a key activator of fibroblasts and a central mediator in FMT [118]. TGF-β1 is stored in the ECM as a latent complex with its prodomain, and its activation depends on the binding of the RGD motif with αv integrin. The release of TGF-β1 needs conformational changes of the "straitjacket" structure of latency-associated peptide (LAP)-TGF-β1 structure on the cell membrane induced by extracellular force transduction [119]. Bound with its receptor, TGF-β1 induced Rho activation that changes the cytoskeleton by actin stress fiber enhancement and enhanced Smad2-phosphorylation and nuclear translocation [120]. In this way, TGF-β1 in the ECM activates fibroblasts and myofibroblasts, and further fibrotic proteins production continues [121,122].
Stiffer substrates enhanced FMT, αv and β1 integrins on the membrane mediate the recruitment of TGF-β1. Using a pump releasing the inhibitor of αv integrins (CWHM-12) beneath mice's skin, myofibroblast activation and capsule thickness were reduced in an in vivo study [68]. Besides, tensile forces could override the effect of TGF-β1 on the level of α-SMA expression [123]. In FBR, stimulating by TGF-β1 [124], ROS [125], and mechanical force [123], αSMA fiber across myofibroblast enhanced intracellular stress and cell-cell adhesion force, leading to contraction of the fibrotic capsule.
5.3. PI3K and MAPK
Studies demonstrated that mechanical cues, such as increased stiffness, could lead to fibroblast activation, depending on β1 integrin on cell membrane and its intracellular signaling pathways that include FAK, phosphoinositide 3-kinase (PI3K), and PI3K/Akt pathway [[126], [127], [128]]. RhoA and Rac-1 participate in cell sensitivity to mechanical strain and modulate Erk pathway. PI3K, as an effector of Rac-1, regulated the MEK-Erk pathway downstream Elk-1 activation by mechanical forces [129].
MAP kinases Erk and p38 are activated by mechanical strain, activation of Elk-1 by mechanical strain, mainly through a MEK-Erk pathway. RhoA and Rac-1 participate in cell sensitivity to mechanical strain and lead to the modulation of the Erk pathway. inhibition of PI3K, an effector of Rac-1, efficiently prevented Elk-1 activation by mechanical forces. These results point out that the two small G proteins RhoA and Rac-1 participate in cell sensitivity to mechanical strain and lead to the modulation of the Erk pathway [129].
6. Macrophages respond inconsistently to mechanical cues
Macrophages are one of the earliest responders following biomaterial implantation. There is mounting evidence that the migration pattern, function, and polarization status of macrophages are affected by the material's chemistry [130], mechanical cues [131], or porosity [132]. In the process of FBR and healing, the role of macrophages depends on the functions of the different phenotypes. In the early phase of injury, classically activated M1s become dominant. When it transfers into the healing phase, the number of polarized alternatively activated M2s increases, which is associated with pro-fibrotic responses and facilitates the fibrotic process by activating signaling such as TGF-β/Smad [27]. Mechanical cues certainly impact the polarization direction of macrophages and the combination of M1s/M2s. By secreting cytokines and growth factors, macrophage polarization can affect the degree of fibroblast activation, which contribute to fibrous capsule formation. Regarding the macrophage's dominating involvement in the immune response, it could be viewed as an important point for regulating FBR [133]. However, there are some controversies about the effect of mechanical cues on macrophages between the results of different studies.
6.1. Conflicting results from similar stimuli
In some studies, macrophages were induced to M1s on soft substrates and to M2s under stiffer stimuli. In these studies, the pro-inflammatory cytokines secretion of macrophages on the soft substrate was enhanced [134,135]. A in vivo study also observed that soft scaffolds result in a pro-inflammatory phenotype of macrophages [136].In addition, the stiffer substrate promoted macrophages polarization to M2s in the studies of Xiaoxia Xing et al. [137] and Rebecca A Scott et al. [138]. Exploring the underlying mechanisms revealed that soft substrate promoted the expression of NF-κB-inducing kinase (NIK), phosphorylated p65 (pi-p65) and phosphorylated IκB (pi-IκB), dictating that the polarization of M1 phenotype may be modulated by the ROS-initiated NF-κB pathway [135]. Pro-Inflammatory behavior enhanced by NLRP3 inflammasome formation increased on soft substrates and was modulated by actomyosin contractility [139]. In the stiff matrix, M2 macrophage polarization was remarkably strengthened and HIF-1α and LOXL2 expression was upregulated by activation of the integrin β5-FAK-MEK1/2-ERK1/2 pathway [137].
In contrast, other studies observed that stiff substrate prime macrophages toward pro-inflammatory(M1) phenotype. The in vivo study of Taufalele PV et al. applied single-cell RNA sequencing to investigate the differences in the transcriptional landscapes between stiff and compliant tumor enviroments and revealed a higher M2 phenotype expression in stiffer matrix [140]. In a model of mice wound, M2s were much more upregulated after being treated by soft zwitterionic poly(sulfobetaine methacrylate)(polySBMA) hydrogels compare to stiff ones [141]. In studies that came to conflicting previous conclusions, toll-like receptor 4 (TLR4) signaling is activated in macrophages on stiff substrates, promoting the release of TNF-α, IL-1β, NO, and other inflammatory cytokines [142]. Atcha H et al. found that in response to IFN γ/LPS stimulation, murine BMDMs enhanced the inflammatory reaction by the activation of NFκB on stiff substrates. It was regulated by Piezo1 expression of macrophages under stiff stimuli, and Ca2+ influx was intensified, which enhances inflammation [73]. Meli VS et al. implant stiff materials into murine, and higher percent YAP+ and iNOS+(M1 marker) macrophages were presented. Compared to soft implants, increased collagen was deposited in response to stiff implants [143]. In stiffer skin tissue, macrophages polarized to M1 phenotype depending on TRPV4, which is not the case in soft tissue [144].
6.2. Summary of the studies’ controversy
We are curious about the reasons for this controversy and summarize a table (Table 1) from different classifications. From the species level, bone marrow-derived macrophages or RAW 264.7 cells from murine and human myeloid leukemia mononuclear cell (THP-1) derived macrophages homo sapiens were chosen mostly. Bone marrow-derived macrophages cultured on polyacrylamide hydrogels with similar stiffness range presented opposite results [135,139,142]. With stiffness increase, the concentration of TNF-α increased, however, the change of the concentration of IL-1β separated between these studies. Mimi Chen et al. found more surface protein CD68(M1 marker) expressed by the BMDMs on softer substrates, meanwhile, with IL-1β mRNA, iNOS mRNA expression increased [135]. A difference in expression level between 3d and 5d was found and may be a factor influencing the opposite results because Previtera ML et al. cultured the BMDMs for 6–7 days. The expression of these cytokines enhanced significantly after LPS priming [142].
Table 1.
Studies about the effects of materials with different stiffness on macrophage polarization.
| Author | Year | Macrophage origins | Material and stiffness (Young's modulus) | Polarization to M1 | Polarization to M2 | |
|---|---|---|---|---|---|---|
| Murine | Michelle L. Previtera et al. [142] | 2015 | Murine BMDMs | Polyacrylamide hydrogels (0.3, 1, 6, 27, 47, 120, and 230 kPa) | stiff | – |
| Mimi Chen et al. [135] | 2020 | Murine BMDMs | Polyacrylamide hydrogels (3 kPa,40 kPa,70 kPa) | soft | stiff | |
| Hamza Atcha et al. [73] | 2021 | Murine BMDMs | Fibronectin-conjugated polyacrylamide substrates (1 kPa, 20 kPa, 40 kPa, 280 kPa) | stiff | stiff | |
| Joan-Carles Escolano et al. [139] | 2021 | Murine BMDMs | Polyacrylamide hydrogels (0.2 kPa, 14.3 kPa, 33.1 kPa) | soft | – | |
| Martin Haschak et al. [145] | 2021 | Murine BMDMs | Poly-dimethyl-siloxane hydrogels coated cardiac ECM (2 kPa, 8 kPa, 16 kPa, 32 kPa, 64 kPa) | stiff | – | |
| Nina Noskovicova et al. [68] | 2021 | Murine BMDMs | PDMS silicone elastomer (2.3 kPa, 2151 kPa) | irrelevant | irrelevant | |
| Erika Gruber et al. [146] | 2018 | Murine BMDMs | Fibronectin-coated polyacrylamide gels (1 kPa, 20 kPa, 150 kPa) | soft | soft | |
| RAW 264.7 cells | irregular | irregular | ||||
| Anna K. Blakney et al. [134] | 2012 | RAW 264.7 cells | PEG-RGD hydrogels (130 kPa, 240 kPa, 840 kPa) | soft | stiff | |
| Xiaotao He [147]. | 2018 | RAW 264.7 cells | 3D transglutaminase cross-linked gelatin (1.5 kPa, 60 kPa) | stiff | soft | |
| Minchae Kim [148]. | 2019 | RAW264.7 cells | 3D poly (ethylene glycol)-based hydrogel (1 kPa,4.5 kPa) | stiff | stiff | |
| Xiqiu Liu et al. [149] | 2020 | RAW 264.7 cells | (PLL/HA) Multilayer films (100 kPa, 200 kPa, 300 kPa, 400 kPa, 500 kPa) | stiff | none | |
| Zhuqing li et al. [99] | 2021 | RAW 264.7 cells | Gellan gum hydrogels (5 kPa, 30 kPa) | stiff | soft | |
| Zhen tang et al. [150] | 2021 | RAW 264.7 cells | Porous and solid titanium alloy scaffolds (1.92 Gpa, 16.1Gpa) | stiff | soft | |
| Huacheng He et al. [141] | 2019 | Macrophages in tissue of healing model of mice | Zwitterionic sulfated poly (sulfobetaine methacrylate) hydrogels (6.7 kPa, 50.4 kPa) | stiff | soft | |
| Bidisha Dutta et al. [144] | 2020 | Thioglycolate-induced macrophages from mice | Collagen-coated polyacrylamide hydrogels (1 kPa, 50 kPa) | stiff | – | |
| Sandra Camarero-Espinosa et al. [136] | 2022 | NR8383 cells | Poly(lactide-co-caprolactone) 3D scaffolds (4 kPa, 400 kPa) | soft | stiff | |
| Homo sapiens | Rukmani Sridharan et al. [98] | 2019 | THP-1 derived macrophages | Polyacrylamide gels (11 kPa, 88 kPa, 323 kPa) | stiff | soft |
| Vijaykumar S. Meli et al. [143] | 2020 | THP-1 derived macrophages | Collagen gel(1 kPa), Matrigel(20 kPa), poly (ethylene glycol) (280 kPa) | stiff | none | |
| Xiaoxia xing et al. [137] | 2021 | THP-1 derived macrophages | Phorbol 12-myristate 13-acetate (6 kPa, 10 kPa, 16 kPa) | – | stiff | |
| Rebecca A Scott et al. [138] | 2020 | Macrophage derived from Cord blood CD14+ monocytes | Poly (ethylene glycol)-based hydrogels (0.1 kPa, 3.4 kPa, 10.3 kPa) | – | stiff | |
| Markus Friedemann et al. [151] | 2017 | Freshly isolated monocytes | 3D: Coll (27.1 ± 9.8 Pa), EDC (57.5 ± 25.9Pa), HA (73.6 ± 27.5Pa), sHA (118.5 ± 34Pa) | soft | stiff | |
| 2D: Coll (27.1 ± 9.8 Pa), EDC (57.5 ± 25.9Pa), HA (73.6 ± 27.5Pa), sHA (118.5 ± 34Pa) | soft | stiff |
Comparing BMDMs culturing on fibronectin-coated polyacrylamide gels, Hamza Atcha et al. found IFNγ/LPS-induced iNOS expression and IL4/IL13-induced ARG1 expression of BMDMs increased significantly on stiff substrates (280 kPa) [73]. However, when Erika Gruber et al. treated BMDMs on soft substrates (1 kPa) with LPS and CpG DNA induction, cytokine secretion of TNF-α and IL10 also increased [146]. These two studies revealed that with opposite stiffness cues, BMDMs polarization to M1s and M2s were both promoted intriguingly. However, when changed the macrophages to RAW 264.7 cells, different adherence resulted in a biphasic response with LPS and CpG DNA-induced cytokines secretion, and no liner results were obtained [146].
6.3. Possible explains
The stiffness of substrates is certainly not the only indicator of macrophage polarization. The controversy of these results may be caused by the presence of too many interference factors. In aspects of different culture conditions, such as encapsulated cells in methacrylate gelatine (GelMA) or polyethylene glycol diacrylate (PEGDA), macrophages showed anti- or pro-inflammatory reactions variously [152].
In FBR, the Vroman effect results in fibrotic proteins coated biomaterials preliminarily, studies simulating this condition were established. Jessica Y Hsieh et al. suggested that the presentation of fibrin(ogen) may be a key switch in regulating macrophage phenotype behavior [153]. In a study by Rukmani Sridharan et al. different crosslinking methods used on collagen scaffolds of similar bulk modulus altered the pro- and anti-inflammatory responses from macrophages [154]. Li Z et al. found that hydrogel surface with RGD peptides enhanced the adhesion and migration of macrophages even less the population of macrophages on the softer gel and increased pro-inflammatory macrophages. Macrophages bind to the RGD binding integrins directly influencing the function of phagocytosis and cell migration and can be regulated by ROCK inhibitor [99]. These findings may correlate with the migration model induced by the matrix we've mentioned before. With a different sensation of the matrix cues, macrophages may choose their polarization direction and migration model simultaneously. To address this point, Trel'ová D et al. have coated polyimide, which is usually used in biomedical applications, with a soft zwitterionic hydrogel, both macrophages and fibroblasts presented the minimum adhesion of the softest surface [155]. The studies of Chu C, et al., explored modulating the FBR by recruiting M2s. With the application of Epigallocatechin-3-gallate (EGCG) modified collagen membranes, the recruitment of M2s was enhanced [156,157]. The in vivo study suggested the regulation may be involved with CCR2 signaling which plays a crucial role in the recruitment and activation of macrophages [157,158].
The porosity of the material also influences the polarization of macrophages initiated by cell adhesion. Yanwei Zhang et al. seeded alveolar macrophages (AMs) on decellularized ECM derived from early radiation-induced pulmonary fibrosis (dECM-RIPF) and normal lung tissue with a significant difference in surface roughness, but no difference in stiffness, and found AMs on dECM-RIPF induced a higher M1 activation [159]. Camarero-Espinosa S et al. fabricated a series of 3D printed dual-porosity scaffolds with different stiffness and porosity using stiffer P(l)LCL and softer P(l,d)LCL [136]. Scaffolds fabricated from stiffer P(l)LCL showed a dramatic increase in the number of adherent cells as the porosity of the scaffolds was increased. The release profile of IL-10 had a positive correlation with pore diameter. With IL-10 and TGF-β release increased, stiffer P(l)LCL induced M2s phenotype. These observations on the cell attachment and secretion of the two scaffolds suggested that there is a balance point that can be reached between the mechanical cues and porosity of the material.
7. Conclusions and outlook
It is a complex process after biomaterials are implanted into the body in which multifarious cells and cytokines are involved. The mechanical cues of biomaterials could profoundly influence cells from morphology to functions after implantation. The stiffness of the newly formed ECM surrounding the implants could sequentially affect the fibrotic process. Macrophages and fibroblasts, the protagonists in this process, both present different phenotypes in response to different mechanical stimuli. The exploration of current studies discovered many details about the mechanosensing of cells and their intracellular pro-fibrotic pathways. Understanding the biological behaviors of cells during tissue healing and foreign body responses under different mechanical cues is crucial for the application of biomaterials.
From the articles we have summarized, we discovered that the effect of mechanical cues on fibroblasts is relatively stable. With a mature adhesion formed induced by stiffer substrates or forces, fibroblasts mobilize IACs-based signaling transduction and enhanced related fibrotic gene transcription. Different from fibroblasts, macrophages are kind of versatile immunity cells with various phenotypes. The conflicting results of the studies validate this characteristic of macrophages. Analyzing the underlying reasons, macrophages may appear to secrete different levels of cytokines depending on the assay times; macrophages from different species exhibit various responses to the same material with various degrees of adhesion; and for materials with similar stiffness, different surface properties may also influence the direction of macrophage polarization.
Further study is needed to give more elucidate answers about the mechanisms from two perspectives. The first one is about the reactions of macrophages to different mechanical cues exerted from both materials and newly formed ECMs. These mechanical cues are derived from various materials with their characteristics and may cause fibroblast fabricating various collagen arrangements in ECM. Macrophages migrating in these ECM respond differently in either migration model or polarization to function in both phagocytosis and modulation of fibroblasts with different cytokines released. Secondly, studies should also focus on at different points of time how different cells interact with each other and modulate fibrotic capsule formation in response to the various mechanical cues during FBR. With the widespread use of single-cell RNA sequencing technology, elucidating the interactions between cells, proteins, and signal pathways becomes possible. Using more in vivo models to simulate clinical scenarios or to test innovative materials, the limits of using only in vitro experiments with limited cells, such as co-culture systems, could be broken.
Even though current studies have roughly revealed the mechanisms of mechanical cues effects in FBR and used antagonists to verify the role of proteins or pathways. But few of them have been able to translate findings into medical materials or drugs. But two directions to manipulate the progress of FBR could be concluded. One direction is to use a drug that is programmed to be released to modulate a target that plays a central role in the process and to terminate the formation of excessive fibrous envelopes in a timely manner, without affecting normal biological activities of the body. So, the crosstalk of different signal pathways beneath cell interactions also needs persistent attention.
Another research direction is to rely on the development of materials science, by creating material surfaces with special mechanical characteristics or by making specific changes in the material surface over time phases. The mechanical cues of the material surface affect the mechanosensing and adhesion of cells to it, which in turn changes the cell phenotype and thus controls the cell function in FBR. Of course, some attempts have been made by regulating material surfaces with special coatings. It is promising to see great progress in this area in the near future.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank every colleague who contributed to the proofing and revising of the manuscript. Y.Y.F. and F.J. take equal responsibility for this work.
Contributor Information
Yuanyuan Fu, Email: yyfu@cmu.edu.cn.
Feng Jin, Email: jinfeng@cmu.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Kessler D.A. The basis of the FDA's decision on breast implants. N. Engl. J. Med. 1992;326(25):1713–1715. doi: 10.1056/NEJM199206183262525. [DOI] [PubMed] [Google Scholar]
- 2.Milner P.E., Parkes M., Puetzer J.L., Chapman R., Stevens M.M., Cann P., Jeffers J.R.T. A low friction, biphasic and boundary lubricating hydrogel for cartilage replacement. Acta Biomater. 2018;65:102–111. doi: 10.1016/j.actbio.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Landes U., Sathananthan J., Witberg G., De Backer O., Sondergaard L., Abdel-Wahab M., Holzhey D., Kim W.K., Hamm C., Buzzatti N., Montorfano M., Ludwig S., Conradi L., Seiffert M., Guerrero M., El Sabbagh A., Rodés-Cabau J., Guimaraes L., Codner P., Okuno T., Pilgrim T., Fiorina C., Colombo A., Mangieri A., Eltchaninoff H., Nombela-Franco L., Van Wiechen M.P.H., Van Mieghem N.M., Tchétché D., Schoels W.H., Kullmer M., Tamburino C., Sinning J.M., Al-Kassou B., Perlman G.Y., Danenberg H., Ielasi A., Fraccaro C., Tarantini G., De Marco F., Redwood S.R., Lisko J.C., Babaliaros V.C., Laine M., Nerla R., Castriota F., Finkelstein A., Loewenstein I., Eitan A., Jaffe R., Ruile P., Neumann F.J., Piazza N., Alosaimi H., Sievert H., Sievert K., Russo M., Andreas M., Bunc M., Latib A., Godfrey R., Hildick-Smith D., Chuang M.A., Blanke P., Leipsic J., Wood D.A., Nazif T.M., Kodali S., Barbanti M., Kornowski R., Leon M.B., Webb J.G. Transcatheter replacement of transcatheter versus surgically implanted aortic valve bioprostheses. J. Am. Coll. Cardiol. 2021;77(1):1–14. doi: 10.1016/j.jacc.2020.10.053. [DOI] [PubMed] [Google Scholar]
- 4.Anderson J.M., Rodriguez A., Chang D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noskovicova N., Hinz B., Pakshir P. Implant fibrosis and the underappreciated role of myofibroblasts in the foreign body reaction. Cells. 2021;10(7) doi: 10.3390/cells10071794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klopfleisch R., Jung F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. 2017;105(3):927–940. doi: 10.1002/jbm.a.35958. [DOI] [PubMed] [Google Scholar]
- 7.Rosner G.A., Fonacier L.S. Hypersensitivity to biomedical implants: prevention and diagnosis. Allergy Asthma Proc. 2017;38(3):177–183. doi: 10.2500/aap.2017.38.4052. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J.B., Carroll C., Tenenbaum M.M., Myckatyn T.M. Breast implant-associated infections: the role of the national surgical quality improvement program and the local microbiome. Plast. Reconstr. Surg. 2015;136(5):921–929. doi: 10.1097/PRS.0000000000001682. [DOI] [PubMed] [Google Scholar]
- 9.Butcher D.T., Alliston T., Weaver V.M. A tense situation: forcing tumour progression. Nat. Rev. Cancer. 2009;9(2):108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vining K.H., Mooney D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017;18(12):728–742. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.T.E.o. Encyclopaedia, Young's modulus. https://www.britannica.com/science/Youngs-modulus. (Accessed 5 April 2023)..
- 12.Yeung T., Georges P.C., Flanagan L.A., Marg B., Ortiz M., Funaki M., Zahir N., Ming W., Weaver V., Janmey P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60(1):24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 13.Discher D.E., Janmey P., Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 14.Wilson C.J., Clegg R.E., Leavesley D.I., Pearcy M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11(1–2):1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 15.Vroman L., Adams A.L., Fischer G.C., Munoz P.C. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood. 1980;55(1):156–159. [PubMed] [Google Scholar]
- 16.Williams D.F. On the nature of biomaterials. Biomaterials. 2009;30(30):5897–5909. doi: 10.1016/j.biomaterials.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Rivera-Chacon D.M., Alvarado-Velez M., Acevedo-Morantes C.Y., Singh S.P., Gultepe E., Nagesha D., Sridhar S., Ramirez-Vick J.E. Fibronectin and vitronectin promote human fetal osteoblast cell attachment and proliferation on nanoporous titanium surfaces. J. Biomed. Nanotechnol. 2013;9(6):1092–1097. doi: 10.1166/jbn.2013.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenney C.R., Anderson J.M. Adsorbed serum proteins responsible for surface dependent human macrophage behavior. J. Biomed. Mater. Res. 2000;49(4):435–447. doi: 10.1002/(sici)1097-4636(20000315)49:4<435::aid-jbm2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.McNally A.K., Jones J.A., Macewan S.R., Colton E., Anderson J.M. Vitronectin is a critical protein adhesion substrate for IL-4-induced foreign body giant cell formation. J. Biomed. Mater. Res. 2008;86(2):535–543. doi: 10.1002/jbm.a.31658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scapini P., Lapinet-Vera J.A., Gasperini S., Calzetti F., Bazzoni F., Cassatella M.A. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi S.D., Voyich J.M., Whitney A.R., DeLeo F.R. Spontaneous neutrophil apoptosis and regulation of cell survival by granulocyte macrophage-colony stimulating factor. J. Leukoc. Biol. 2005;78(6):1408–1418. doi: 10.1189/jlb.0605289. [DOI] [PubMed] [Google Scholar]
- 22.Bryers J.D., Giachelli C.M., Ratner B.D. Engineering biomaterials to integrate and heal: the biocompatibility paradigm shifts. Biotechnol. Bioeng. 2012;109(8):1898–1911. doi: 10.1002/bit.24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak M.L., Koh T.J. Phenotypic transitions of macrophages orchestrate tissue repair. Am. J. Pathol. 2013;183(5):1352–1363. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witherel C.E., Abebayehu D., Barker T.H., Spiller K.L. Macrophage and fibroblast interactions in biomaterial-mediated fibrosis. Adv. Healthcare Mater. 2019;8(4) doi: 10.1002/adhm.201801451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auffray C., Sieweke M.H., Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 26.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., Locati M., Mantovani A., Martinez F.O., Mege J.L., Mosser D.M., Natoli G., Saeij J.P., Schultze J.L., Shirey K.A., Sica A., Suttles J., Udalova I., van Ginderachter J.A., Vogel S.N., Wynn T.A. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown B.N., Ratner B.D., Goodman S.B., Amar S., Badylak S.F. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33(15):3792–3802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer A., Roch T., Kratz K., Lendlein A., Jung F. Pro-angiogenic CD14(++) CD16(+) CD163(+) monocytes accelerate the in vitro endothelialization of soft hydrophobic poly (n-butyl acrylate) networks. Acta Biomater. 2012;8(12):4253–4259. doi: 10.1016/j.actbio.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Doloff J.C., Veiseh O., Vegas A.J., Tam H.H., Farah S., Ma M., Li J., Bader A., Chiu A., Sadraei A., Aresta-Dasilva S., Griffin M., Jhunjhunwala S., Webber M., Siebert S., Tang K., Chen M., Langan E., Dholokia N., Thakrar R., Qi M., Oberholzer J., Greiner D.L., Langer R., Anderson D.G. Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates. Nat. Mater. 2017;16(6):671–680. doi: 10.1038/nmat4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasse G.F., Nizamoglu M., Heijink I.H., Schlepütz M., van Rijn P., Thomas M.J., Burgess J.K., Melgert B.N. Macrophage-stroma interactions in fibrosis: biochemical, biophysical, and cellular perspectives. J. Pathol. 2021;254(4):344–357. doi: 10.1002/path.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacLauchlan S., Skokos E.A., Meznarich N., Zhu D.H., Raoof S., Shipley J.M., Senior R.M., Bornstein P., Kyriakides T.R. Macrophage fusion, giant cell formation, and the foreign body response require matrix metalloproteinase 9. J. Leukoc. Biol. 2009;85(4):617–626. doi: 10.1189/jlb.1008588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly A., Gunaltay S., McEntee C.P., Shuttleworth E.E., Smedley C., Houston S.A., Fenton T.M., Levison S., Mann E.R., Travis M.A. Human monocytes and macrophages regulate immune tolerance via integrin αvβ8-mediated TGFβ activation. J. Exp. Med. 2018;215(11):2725–2736. doi: 10.1084/jem.20171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kendall R.T., Feghali-Bostwick C.A. Fibroblasts in fibrosis: novel roles and mediators. Front. Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jordana M., Särnstrand B., Sime P.J., Ramis I. Immune-inflammatory functions of fibroblasts. Eur. Respir. J. 1994;7(12):2212–2222. doi: 10.1183/09031936.94.07122212. [DOI] [PubMed] [Google Scholar]
- 37.Newman A.C., Nakatsu M.N., Chou W., Gershon P.D., Hughes C.C. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol. Biol. Cell. 2011;22(20):3791–3800. doi: 10.1091/mbc.E11-05-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tripathi M., Billet S., Bhowmick N.A. Understanding the role of stromal fibroblasts in cancer progression. Cell Adhes. Migrat. 2012;6(3):231–235. doi: 10.4161/cam.20419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reilkoff R.A., Bucala R., Herzog E.L. Fibrocytes: emerging effector cells in chronic inflammation. Nat. Rev. Immunol. 2011;11(6):427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koh T.J., DiPietro L.A. Inflammation and wound healing: the role of the macrophage. Expet Rev. Mol. Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun M., Deng J., Tang Z., Wu J., Li D., Chen H., Gao C. A correlation study of protein adsorption and cell behaviors on substrates with different densities of PEG chains. Colloids Surf. B Biointerfaces. 2014;122:134–142. doi: 10.1016/j.colsurfb.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 42.Moretti A.I., Pinto F.J., Cury V., Jurado M.C., Marcondes W., Velasco I.T., Souza H.P. Nitric oxide modulates metalloproteinase-2, collagen deposition and adhesion rate after polypropylene mesh implantation in the intra-abdominal wall. Acta Biomater. 2012;8(1):108–115. doi: 10.1016/j.actbio.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Jun J.I., Lau L.F. Cellular senescence controls fibrosis in wound healing. Aging (Albany NY) 2010;2(9):627–631. doi: 10.18632/aging.100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wermuth P.J., Jimenez S.A. The significance of macrophage polarization subtypes for animal models of tissue fibrosis and human fibrotic diseases. Clin. Transl. Med. 2015;4:2. doi: 10.1186/s40169-015-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doolin M.T., Smith I.M., Stroka K.M. Fibroblast to myofibroblast transition is enhanced by increased cell density. Mol. Biol. Cell. 2021;32(22):ar41. doi: 10.1091/mbc.E20-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desmoulière A., Redard M., Darby I., Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am. J. Pathol. 1995;146(1):56–66. [PMC free article] [PubMed] [Google Scholar]
- 47.LeBleu V.S., Neilson E.G. Origin and functional heterogeneity of fibroblasts. Faseb. J. 2020;34(3):3519–3536. doi: 10.1096/fj.201903188R. [DOI] [PubMed] [Google Scholar]
- 48.Bachour Y. Capsular contracture in breast implant surgery: where are we now and where are we going? Aesthetic Plast. Surg. 2021;45(3):1328–1337. doi: 10.1007/s00266-021-02141-6. [DOI] [PubMed] [Google Scholar]
- 49.An V.V.G., Scholes C.J., Fritsch B.A. Factors affecting the incidence and management of fixed flexion deformity in total knee arthroplasty: a systematic review. Knee. 2018;25(3):352–359. doi: 10.1016/j.knee.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Buechler M.B., Fu W., Turley S.J. Fibroblast-macrophage reciprocal interactions in health, fibrosis, and cancer. Immunity. 2021;54(5):903–915. doi: 10.1016/j.immuni.2021.04.021. [DOI] [PubMed] [Google Scholar]
- 51.Roth P., Stanley E.R. The biology of CSF-1 and its receptor. Curr. Top. Microbiol. Immunol. 1992;181:141–167. doi: 10.1007/978-3-642-77377-8_5. [DOI] [PubMed] [Google Scholar]
- 52.Ivey M.J., Kuwabara J.T., Riggsbee K.L., Tallquist M.D. Platelet-derived growth factor receptor-α is essential for cardiac fibroblast survival. Am. J. Physiol. Heart Circ. Physiol. 2019;317(2):H330–h344. doi: 10.1152/ajpheart.00054.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ueshima E., Fujimori M., Kodama H., Felsen D., Chen J., Durack J.C., Solomon S.B., Coleman J.A., Srimathveeravalli G. Macrophage-secreted TGF-β(1) contributes to fibroblast activation and ureteral stricture after ablation injury. Am. J. Physiol. Ren. Physiol. 2019;317(7):F52–f64. doi: 10.1152/ajprenal.00260.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witherel C.E., Sao K., Brisson B.K., Han B., Volk S.W., Petrie R.J., Han L., Spiller K.L. Regulation of extracellular matrix assembly and structure by hybrid M1/M2 macrophages. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2021.120667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scopelliti F., Caterina C., Valentina D., Gianfranco C., Concetta M., Andrea C. Platelet lysate converts M (IFNγ+LPS) macrophages in CD206(+) TGF-β(+) arginase(+) M2-like macrophages that affect fibroblast activity and T lymphocyte migration. J. Tissue Eng. Regen. Med. 2021;15(9):788–797. doi: 10.1002/term.3229. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Z., Ding J., Ma Z., Iwashina T., Tredget E.E. Alternatively activated macrophages derived from THP-1 cells promote the fibrogenic activities of human dermal fibroblasts. Wound Repair Regen. 2017;25(3):377–388. doi: 10.1111/wrr.12532. [DOI] [PubMed] [Google Scholar]
- 57.Gao Q., Li Z., Rhee C., Xiang S., Maruyama M., Huang E.E., Yao Z., Bunnell B.A., Tuan R.S., Lin H., Gold M.S., Goodman S.B. Macrophages modulate the function of MSC- and iPSC-derived fibroblasts in the presence of polyethylene particles. Int. J. Mol. Sci. 2021;22(23) doi: 10.3390/ijms222312837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Y., Ju L., Rushdi M., Ge C., Zhu C. Receptor-mediated cell mechanosensing. Mol. Biol. Cell. 2017;28(23):3134–3155. doi: 10.1091/mbc.E17-04-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burridge K., Monaghan-Benson E., Graham D.M. Mechanotransduction: from the cell surface to the nucleus via RhoA. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019;374(1779) doi: 10.1098/rstb.2018.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jaalouk D.E., Lammerding J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009;10(1):63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chaudhuri O., Cooper-White J., Janmey P.A., Mooney D.J., Shenoy V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature. 2020;584(7822):535–546. doi: 10.1038/s41586-020-2612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elosegui-Artola A., Bazellières E., Allen M.D., Andreu I., Oria R., Sunyer R., Gomm J.J., Marshall J.F., Jones J.L., Trepat X., Roca-Cusachs P. Rigidity sensing and adaptation through regulation of integrin types. Nat. Mater. 2014;13(6):631–637. doi: 10.1038/nmat3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang A., Cao S., Stowe J.C., Valdez-Jasso D. Substrate stiffness and stretch regulate profibrotic mechanosignaling in pulmonary arterial adventitial fibroblasts. Cells. 2021;10(5) doi: 10.3390/cells10051000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hynes R.O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 65.Green H.J., Brown N.H. Integrin intracellular machinery in action. Exp. Cell Res. 2019;378(2):226–231. doi: 10.1016/j.yexcr.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 66.Wei L., Chen Q., Zheng Y., Nan L., Liao N., Mo S. Potential role of integrin α₅β₁/focal adhesion kinase (FAK) and actin cytoskeleton in the mechanotransduction and response of human gingival fibroblasts cultured on a 3-dimension lactide-Co-glycolide (3D PLGA) scaffold. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.921626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keselowsky B.G., García A.J. Quantitative methods for analysis of integrin binding and focal adhesion formation on biomaterial surfaces. Biomaterials. 2005;26(4):413–418. doi: 10.1016/j.biomaterials.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 68.Noskovicova N., Schuster R., van Putten S., Ezzo M., Koehler A., Boo S., Coelho N.M., Griggs D., Ruminski P., McCulloch C.A., Hinz B. Suppression of the fibrotic encapsulation of silicone implants by inhibiting the mechanical activation of pro-fibrotic TGF-β. Nat. Biomed. Eng. 2021;5(12):1437–1456. doi: 10.1038/s41551-021-00722-z. [DOI] [PubMed] [Google Scholar]
- 69.Zaveri T.D., Lewis J.S., Dolgova N.V., Clare-Salzler M.J., Keselowsky B.G. Integrin-directed modulation of macrophage responses to biomaterials. Biomaterials. 2014;35(11):3504–3515. doi: 10.1016/j.biomaterials.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He J., Fang B., Shan S., Xie Y., Wang C., Zhang Y., Zhang X., Li Q. Mechanical stretch promotes hypertrophic scar formation through mechanically activated cation channel Piezo1. Cell Death Dis. 2021;12(3):226. doi: 10.1038/s41419-021-03481-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin P., Jan L.Y., Jan Y.N. Mechanosensitive ion channels: structural features relevant to mechanotransduction mechanisms. Annu. Rev. Neurosci. 2020;43:207–229. doi: 10.1146/annurev-neuro-070918-050509. [DOI] [PubMed] [Google Scholar]
- 72.Ridone P., Vassalli M., Martinac B. Piezo1 mechanosensitive channels: what are they and why are they important. Biophys. Rev. 2019;11(5):795–805. doi: 10.1007/s12551-019-00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Atcha H., Jairaman A., Holt J.R., Meli V.S., Nagalla R.R., Veerasubramanian P.K., Brumm K.T., Lim H.E., Othy S., Cahalan M.D., Pathak M.M., Liu W.F. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun. 2021;12(1):3256. doi: 10.1038/s41467-021-23482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goswami R., Arya R.K., Sharma S., Dutta B., Stamov D.R., Zhu X., Rahaman S.O. Mechanosensing by TRPV4 mediates stiffness-induced foreign body response and giant cell formation. Sci. Signal. 2021;14(707) doi: 10.1126/scisignal.abd4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arya R.K., Goswami R., Rahaman S.O. Mechanotransduction via a TRPV4-Rac1 signaling axis plays a role in multinucleated giant cell formation. J. Biol. Chem. 2021;296 doi: 10.1074/jbc.RA120.014597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Forget A., Gianni-Barrera R., Uccelli A., Sarem M., Kohler E., Fogli B., Muraro M.G., Bichet S., Aumann K., Banfi A., Shastri V.P. Mechanically defined microenvironment promotes stabilization of microvasculature, which correlates with the enrichment of a novel piezo-1(+) population of circulating CD11b(+)/CD115(+) monocytes. Adv. Mater. 2019;31(21) doi: 10.1002/adma.201808050. [DOI] [PubMed] [Google Scholar]
- 77.Atcha H., Meli V.S., Davis C.T., Brumm K.T., Anis S., Chin J., Jiang K., Pathak M.M., Liu W.F. Crosstalk between CD11b and Piezo1 mediates macrophage responses to mechanical cues. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.689397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ji C., Wang Y., Wang Q., Wang A., Ali A., McCulloch C.A. TRPV4 regulates β1 integrin-mediated cell-matrix adhesions and collagen remodeling. Faseb. J. 2023;37(6) doi: 10.1096/fj.202300222R. [DOI] [PubMed] [Google Scholar]
- 79.Sianati S., Schroeter L., Richardson J., Tay A., Lamandé S.R., Poole K. Modulating the mechanical activation of TRPV4 at the cell-substrate interface. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.608951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tzima E., Irani-Tehrani M., Kiosses W.B., Dejana E., Schultz D.A., Engelhardt B., Cao G., DeLisser H., Schwartz M.A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 81.Wilde C., Mitgau J., Suchý T., Schöneberg T., Liebscher I. Translating the force-mechano-sensing GPCRs. Am. J. Physiol. Cell Physiol. 2022;322(6):C1047–c1060. doi: 10.1152/ajpcell.00465.2021. [DOI] [PubMed] [Google Scholar]
- 82.Chastney M.R., Conway J.R.W., Ivaska J. Integrin adhesion complexes. Curr. Biol. 2021;31(10):R536–R542. doi: 10.1016/j.cub.2021.01.038. [DOI] [PubMed] [Google Scholar]
- 83.Nader G.P., Ezratty E.J., Gundersen G.G. FAK, talin and PIPKIγ regulate endocytosed integrin activation to polarize focal adhesion assembly. Nat. Cell Biol. 2016;18(5):491–503. doi: 10.1038/ncb3333. [DOI] [PubMed] [Google Scholar]
- 84.Case L.B., Waterman C.M. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. Cell Biol. 2015;17(8):955–963. doi: 10.1038/ncb3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Case L.B., De Pasquale M., Henry L., Rosen M.K. Synergistic phase separation of two pathways promotes integrin clustering and nascent adhesion formation. Elife. 2022;11 doi: 10.7554/eLife.72588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun Z., Guo S.S., Fässler R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016;215(4):445–456. doi: 10.1083/jcb.201609037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ni Y., Chiang M.Y.M. Cell morphology and migration linked to substrate rigidity. Soft Matter. 2007;3(10):1285–1292. doi: 10.1039/b703376a. [DOI] [PubMed] [Google Scholar]
- 88.Kechagia J.Z., Ivaska J., Roca-Cusachs P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019;20(8):457–473. doi: 10.1038/s41580-019-0134-2. [DOI] [PubMed] [Google Scholar]
- 89.Elosegui-Artola A., Trepat X., Roca-Cusachs P. Control of mechanotransduction by molecular clutch dynamics. Trends Cell Biol. 2018;28(5):356–367. doi: 10.1016/j.tcb.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 90.Lawson C., Schlaepfer D.D. Integrin adhesions: who's on first? What's on second? Connections between FAK and talin. Cell Adhes. Migrat. 2012;6(4):302–306. doi: 10.4161/cam.20488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Herum K.M., Choppe J., Kumar A., Engler A.J., McCulloch A.D. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol. Biol. Cell. 2017;28(14):1871–1882. doi: 10.1091/mbc.E17-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schwager S.C., Bordeleau F., Zhang J., Antonyak M.A., Cerione R.A., Reinhart-King C.A. Matrix stiffness regulates microvesicle-induced fibroblast activation. Am. J. Physiol. Cell Physiol. 2019;317(1):C82–c92. doi: 10.1152/ajpcell.00418.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gupta M., Sarangi B.R., Deschamps J., Nematbakhsh Y., Callan-Jones A., Margadant F., Mège R.M., Lim C.T., Voituriez R., Ladoux B. Adaptive rheology and ordering of cell cytoskeleton govern matrix rigidity sensing. Nat. Commun. 2015;6:7525. doi: 10.1038/ncomms8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Totsukawa G., Yamakita Y., Yamashiro S., Hartshorne D.J., Sasaki Y., Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 2000;150(4):797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Elosegui-Artola A., Oria R., Chen Y., Kosmalska A., Pérez-González C., Castro N., Zhu C., Trepat X., Roca-Cusachs P. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol. 2016;18(5):540–548. doi: 10.1038/ncb3336. [DOI] [PubMed] [Google Scholar]
- 96.Lawson C., Lim S.T., Uryu S., Chen X.L., Calderwood D.A., Schlaepfer D.D. FAK promotes recruitment of talin to nascent adhesions to control cell motility. J. Cell Biol. 2012;196(2):223–232. doi: 10.1083/jcb.201108078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van Goethem E., Poincloux R., Gauffre F., Maridonneau-Parini I., Le Cabec V. Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J. Immunol. 2010;184(2):1049–1061. doi: 10.4049/jimmunol.0902223. [DOI] [PubMed] [Google Scholar]
- 98.Sridharan R., Cavanagh B., Cameron A.R., Kelly D.J., O'Brien F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019;89:47–59. doi: 10.1016/j.actbio.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 99.Li Z., Bratlie K.M. Effect of RGD functionalization and stiffness of gellan gum hydrogels on macrophage polarization and function. Mater. Sci. Eng., C. 2021;128 doi: 10.1016/j.msec.2021.112303. [DOI] [PubMed] [Google Scholar]
- 100.Hsieh J.Y., Keating M.T., Smith T.D., Meli V.S., Botvinick E.L., Liu W.F. Matrix crosslinking enhances macrophage adhesion, migration, and inflammatory activation. APL Bioeng. 2019;3(1) doi: 10.1063/1.5067301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miller C.J., Davidson L.A. The interplay between cell signalling and mechanics in developmental processes. Nat. Rev. Genet. 2013;14(10):733–744. doi: 10.1038/nrg3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang V.W. Collagen, stiffness, and adhesion: the evolutionary basis of vertebrate mechanobiology. Mol. Biol. Cell. 2020;31(17):1823–1834. doi: 10.1091/mbc.E19-12-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Parker M.W., Rossi D., Peterson M., Smith K., Sikström K., White E.S., Connett J.E., Henke C.A., Larsson O., Bitterman P.B. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J. Clin. Invest. 2014;124(4):1622–1635. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carloni A., Poletti V., Fermo L., Bellomo N., Chilosi M. Heterogeneous distribution of mechanical stress in human lung: a mathematical approach to evaluate abnormal remodeling in IPF. J. Theor. Biol. 2013;332:136–140. doi: 10.1016/j.jtbi.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 105.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 106.Gałdyszyńska M., Radwańska P., Szymański J., Drobnik J. The stiffness of cardiac fibroblast substrates exerts a regulatory influence on collagen metabolism via α2β1 integrin, FAK and Src kinases. Cells. 2021;10(12) doi: 10.3390/cells10123506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dasgupta I., McCollum D. Control of cellular responses to mechanical cues through YAP/TAZ regulation. J. Biol. Chem. 2019;294(46):17693–17706. doi: 10.1074/jbc.REV119.007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Niu L., Jia Y., Wu M., Liu H., Feng Y., Hu Y., Zhang X., Gao D., Xu F., Huang G. Matrix stiffness controls cardiac fibroblast activation through regulating YAP via AT(1) R. J. Cell. Physiol. 2020;235(11):8345–8357. doi: 10.1002/jcp.29678. [DOI] [PubMed] [Google Scholar]
- 109.Martin K., Pritchett J., Llewellyn J., Mullan A.F., Athwal V.S., Dobie R., Harvey E., Zeef L., Farrow S., Streuli C., Henderson N.C., Friedman S.L., Hanley N.A., Piper Hanley K. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nat. Commun. 2016;7 doi: 10.1038/ncomms12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cui Y., Hameed F.M., Yang B., Lee K., Pan C.Q., Park S., Sheetz M. Cyclic stretching of soft substrates induces spreading and growth. Nat. Commun. 2015;6:6333. doi: 10.1038/ncomms7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roca-Cusachs P., Gauthier N.C., Del Rio A., Sheetz M.P. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc. Natl. Acad. Sci. U. S. A. 2009;106(38):16245–16250. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Elosegui-Artola A., Andreu I., Beedle A.E.M., Lezamiz A., Uroz M., Kosmalska A.J., Oria R., Kechagia J.Z., Rico-Lastres P., Le Roux A.L., Shanahan C.M., Trepat X., Navajas D., Garcia-Manyes S., Roca-Cusachs P. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell. 2017;171(6):1397–1410.e14. doi: 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 113.Zhu L., Liu L., Wang A., Liu J., Huang X., Zan T. Positive feedback loops between fibroblasts and the mechanical environment contribute to dermal fibrosis. Matrix Biol. 2023;121:1–21. doi: 10.1016/j.matbio.2023.05.001. [DOI] [PubMed] [Google Scholar]
- 114.Angelini A., Trial J., Ortiz-Urbina J., Cieslik K.A. Mechanosensing dysregulation in the fibroblast: a hallmark of the aging heart. Ageing Res. Rev. 2020;63 doi: 10.1016/j.arr.2020.101150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu F., Lagares D., Choi K.M., Stopfer L., Marinkovic A., Vrbanac V., Probst C.K., Hiemer S.E., Sisson T.H., Horowitz J.C., Rosas I.O., Fredenburgh L.E., Feghali-Bostwick C., Varelas X., Tager A.M., Tschumperlin D.J. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308(4):L344–L357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Emig R., Knodt W., Krussig M.J., Zgierski-Johnston C.M., Gorka O., Groß O., Kohl P., Ravens U., Peyronnet R. Piezo1 channels contribute to the regulation of human atrial fibroblast mechanical properties and matrix stiffness sensing. Cells. 2021;10(3) doi: 10.3390/cells10030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Adapala R.K., Kanugula A.K., Paruchuri S., Chilian W.M., Thodeti C.K. TRPV4 deletion protects heart from myocardial infarction-induced adverse remodeling via modulation of cardiac fibroblast differentiation. Basic Res. Cardiol. 2020;115(2):14. doi: 10.1007/s00395-020-0775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Frangogiannis N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020;217(3) doi: 10.1084/jem.20190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shi M., Zhu J., Wang R., Chen X., Mi L., Walz T., Springer T.A. Latent TGF-β structure and activation. Nature. 2011;474(7351):343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meyer-ter-Vehn T., Sieprath S., Katzenberger B., Gebhardt S., Grehn F., Schlunck G. Contractility as a prerequisite for TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest. Ophthalmol. Vis. Sci. 2006;47(11):4895–4904. doi: 10.1167/iovs.06-0118. [DOI] [PubMed] [Google Scholar]
- 121.Dong X., Zhao B., Iacob R.E., Zhu J., Koksal A.C., Lu C., Engen J.R., Springer T.A. Force interacts with macromolecular structure in activation of TGF-β. Nature. 2017;542(7639):55–59. doi: 10.1038/nature21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Buscemi L., Ramonet D., Klingberg F., Formey A., Smith-Clerc J., Meister J.J., Hinz B. The single-molecule mechanics of the latent TGF-β1 complex. Curr. Biol. 2011;21(24):2046–2054. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 123.Kollmannsberger P., Bidan C.M., Dunlop J.W.C., Fratzl P., Vogel V. Tensile forces drive a reversible fibroblast-to-myofibroblast transition during tissue growth in engineered clefts. Sci. Adv. 2018;4(1) doi: 10.1126/sciadv.aao4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alili L., Sack M., Puschmann K., Brenneisen P. Fibroblast-to-myofibroblast switch is mediated by NAD(P)H oxidase generated reactive oxygen species. Biosci. Rep. 2014;34(1) doi: 10.1042/BSR20130091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bocchino M., Agnese S., Fagone E., Svegliati S., Grieco D., Vancheri C., Gabrielli A., Sanduzzi A., Avvedimento E.V. Reactive oxygen species are required for maintenance and differentiation of primary lung fibroblasts in idiopathic pulmonary fibrosis. PLoS One. 2010;5(11) doi: 10.1371/journal.pone.0014003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McLane J.S., Ligon L.A. Palladin mediates stiffness-induced fibroblast activation in the tumor microenvironment. Biophys. J. 2015;109(2):249–264. doi: 10.1016/j.bpj.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xia H., Nho R.S., Kahm J., Kleidon J., Henke C.A. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J. Biol. Chem. 2004;279(31):33024–33034. doi: 10.1074/jbc.M313265200. [DOI] [PubMed] [Google Scholar]
- 128.Wang H., Tibbitt M.W., Langer S.J., Leinwand L.A., Anseth K.S. Hydrogels preserve native phenotypes of valvular fibroblasts through an elasticity-regulated PI3K/AKT pathway. Proc. Natl. Acad. Sci. U. S. A. 2013;110(48):19336–19341. doi: 10.1073/pnas.1306369110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Laboureau J., Dubertret L., Lebreton-De Coster C., Coulomb B. ERK activation by mechanical strain is regulated by the small G proteins rac-1 and rhoA. Exp. Dermatol. 2004;13(2):70–77. doi: 10.1111/j.0906-6705.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- 130.Qian Y., Li L., Song Y., Dong L., Chen P., Li X., Cai K., Germershaus O., Yang L., Fan Y. Surface modification of nanofibrous matrices via layer-by-layer functionalized silk assembly for mitigating the foreign body reaction. Biomaterials. 2018;164:22–37. doi: 10.1016/j.biomaterials.2018.02.038. [DOI] [PubMed] [Google Scholar]
- 131.Luu T.U., Gott S.C., Woo B.W., Rao M.P., Liu W.F. Micro- and nanopatterned topographical cues for regulating macrophage cell shape and phenotype. ACS Appl. Mater. Interfaces. 2015;7(51):28665–28672. doi: 10.1021/acsami.5b10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garg K., Pullen N.A., Oskeritzian C.A., Ryan J.J., Bowlin G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials. 2013;34(18):4439–4451. doi: 10.1016/j.biomaterials.2013.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chu C., Liu L., Rung S., Wang Y., Ma Y., Hu C., Zhao X., Man Y., Qu Y. Modulation of foreign body reaction and macrophage phenotypes concerning microenvironment. J. Biomed. Mater. Res. 2020;108(1):127–135. doi: 10.1002/jbm.a.36798. [DOI] [PubMed] [Google Scholar]
- 134.Blakney A.K., Swartzlander M.D., Bryant S.J. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. 2012;100(6):1375–1386. doi: 10.1002/jbm.a.34104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen M., Zhang Y., Zhou P., Liu X., Zhao H., Zhou X., Gu Q., Li B., Zhu X., Shi Q. Substrate stiffness modulates bone marrow-derived macrophage polarization through NF-κB signaling pathway. Bioact. Mater. 2020;5(4):880–890. doi: 10.1016/j.bioactmat.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Camarero-Espinosa S., Carlos-Oliveira M., Liu H., Mano J.F., Bouvy N., Moroni L. 3D printed dual-porosity scaffolds: the combined effect of stiffness and porosity in the modulation of macrophage polarization. Adv. Healthcare Mater. 2022;11(1) doi: 10.1002/adhm.202101415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xing X., Wang Y., Zhang X., Gao X., Li M., Wu S., Zhao Y., Chen J., Gao D., Chen R., Ren Z., Zhang K., Cui J. Matrix stiffness-mediated effects on macrophages polarization and their LOXL2 expression. FEBS J. 2021;288(11):3465–3477. doi: 10.1111/febs.15566. [DOI] [PubMed] [Google Scholar]
- 138.Scott R.A., Kiick K.L., Akins R.E. Substrate stiffness directs the phenotype and polarization state of cord blood derived macrophages. Acta Biomater. 2021;122:220–235. doi: 10.1016/j.actbio.2020.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Escolano J.C., Taubenberger A.V., Abuhattum S., Schweitzer C., Farrukh A., Del Campo A., Bryant C.E., Guck J. Compliant substrates enhance macrophage cytokine release and NLRP3 inflammasome formation during their pro-inflammatory response. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.639815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taufalele P.V., Wang W., Simmons A.J., Southard-Smith A.N., Chen B., Greenlee J.D., King M.R., Lau K.S., Hassane D.C., Bordeleau F., Reinhart-King C.A. Matrix stiffness enhances cancer-macrophage interactions and M2-like macrophage accumulation in the breast tumor microenvironment. Acta Biomater. 2022;163:365–377. doi: 10.1016/j.actbio.2022.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.He H., Xiao Z., Zhou Y., Chen A., Xuan X., Li Y., Guo X., Zheng J., Xiao J., Wu J. Zwitterionic poly(sulfobetaine methacrylate) hydrogels with optimal mechanical properties for improving wound healing in vivo. J. Mater. Chem. B. 2019;7(10):1697–1707. doi: 10.1039/c8tb02590h. [DOI] [PubMed] [Google Scholar]
- 142.Previtera M.L., Sengupta A. Substrate stiffness regulates proinflammatory mediator production through TLR4 activity in macrophages. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meli V.S., Atcha H., Veerasubramanian P.K., Nagalla R.R., Luu T.U., Chen E.Y., Guerrero-Juarez C.F., Yamaga K., Pandori W., Hsieh J.Y., Downing T.L., Fruman D.A., Lodoen M.B., Plikus M.V., Wang W., Liu W.F. YAP-mediated mechanotransduction tunes the macrophage inflammatory response. Sci. Adv. 2020;6(49) doi: 10.1126/sciadv.abb8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dutta B., Goswami R., Rahaman S.O. TRPV4 plays a role in matrix stiffness-induced macrophage polarization. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.570195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Haschak M., LoPresti S., Stahl E., Dash S., Popovich B., Brown B.N. Macrophage phenotype and function are dependent upon the composition and biomechanics of the local cardiac tissue microenvironment. Aging (Albany NY) 2021;13(13):16938–16956. doi: 10.18632/aging.203054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gruber E., Heyward C., Cameron J., Leifer C. Toll-like receptor signaling in macrophages is regulated by extracellular substrate stiffness and Rho-associated coiled-coil kinase (ROCK1/2) Int. Immunol. 2018;30(6):267–278. doi: 10.1093/intimm/dxy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.He X.T., Wu R.X., Xu X.Y., Wang J., Yin Y., Chen F.M. Macrophage involvement affects matrix stiffness-related influences on cell osteogenesis under three-dimensional culture conditions. Acta Biomater. 2018;71:132–147. doi: 10.1016/j.actbio.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 148.Kim M., Lee S., Ki C.S. Cellular behavior of RAW264.7 cells in 3D poly(ethylene glycol) hydrogel niches. ACS Biomater. Sci. Eng. 2019;5(2):922–932. doi: 10.1021/acsbiomaterials.8b01150. [DOI] [PubMed] [Google Scholar]
- 149.Liu X.Q., Chen X.T., Liu Z.Z., Gu S.S., He L.J., Wang K.P., Tang R.Z. Biomimetic matrix stiffness modulates hepatocellular carcinoma malignant phenotypes and macrophage polarization through multiple modes of mechanical feedbacks. ACS Biomater. Sci. Eng. 2020;6(7):3994–4004. doi: 10.1021/acsbiomaterials.0c00669. [DOI] [PubMed] [Google Scholar]
- 150.Tang Z., Wei X., Li T., Wu H., Xiao X., Hao Y., Li S., Hou W., Shi L., Li X., Guo Z. Three-dimensionally printed Ti2448 with low stiffness enhanced angiogenesis and osteogenesis by regulating macrophage polarization via Piezo1/YAP signaling Axis. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.750948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Friedemann M., Kalbitzer L., Franz S., Moeller S., Schnabelrauch M., Simon J.C., Pompe T., Franke K. Instructing human macrophage polarization by stiffness and glycosaminoglycan functionalization in 3D collagen networks. Adv. Healthcare Mater. 2017;6(7) doi: 10.1002/adhm.201600967. [DOI] [PubMed] [Google Scholar]
- 152.Cha B.H., Shin S.R., Leijten J., Li Y.C., Singh S., Liu J.C., Annabi N., Abdi R., Dokmeci M.R., Vrana N.E., Ghaemmaghami A.M., Khademhosseini A. Integrin-mediated interactions control macrophage polarization in 3D hydrogels. Adv. Healthcare Mater. 2017;6(21) doi: 10.1002/adhm.201700289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hsieh J.Y., Smith T.D., Meli V.S., Tran T.N., Botvinick E.L., Liu W.F. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomater. 2017;47:14–24. doi: 10.1016/j.actbio.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sridharan R., Ryan E.J., Kearney C.J., Kelly D.J., O'Brien F.J. Macrophage polarization in response to collagen scaffold stiffness is dependent on cross-linking agent used to modulate the stiffness. ACS Biomater. Sci. Eng. 2019;5(2):544–552. doi: 10.1021/acsbiomaterials.8b00910. [DOI] [PubMed] [Google Scholar]
- 155.Trel'ová D., Salgarella A.R., Ricotti L., Giudetti G., Cutrone A., Šrámková P., Zahoranová A., Chorvát D., Jr., Haško D., Canale C., Micera S., Kronek J., Menciassi A., Lacík I. Soft hydrogel zwitterionic coatings minimize fibroblast and macrophage adhesion on polyimide substrates. Langmuir. 2019;35(5):1085–1099. doi: 10.1021/acs.langmuir.8b00765. [DOI] [PubMed] [Google Scholar]
- 156.Chu C., Wang Y., Wang Y., Yang R., Liu L., Rung S., Xiang L., Wu Y., Du S., Man Y., Qu Y. Evaluation of epigallocatechin-3-gallate (EGCG) modified collagen in guided bone regeneration (GBR) surgery and modulation of macrophage phenotype. Mater. Sci. Eng., C. 2019;99:73–82. doi: 10.1016/j.msec.2019.01.083. [DOI] [PubMed] [Google Scholar]
- 157.Chu C., Liu L., Wang Y., Yang R., Hu C., Rung S., Man Y., Qu Y. Evaluation of epigallocatechin-3-gallate (EGCG)-modified scaffold determines macrophage recruitment. Mater. Sci. Eng., C. 2019;100:505–513. doi: 10.1016/j.msec.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 158.Boniakowski A.E., Kimball A.S., Joshi A., Schaller M., Davis F.M., denDekker A., Obi A.T., Moore B.B., Kunkel S.L., Gallagher K.A. Murine macrophage chemokine receptor CCR2 plays a crucial role in macrophage recruitment and regulated inflammation in wound healing. Eur. J. Immunol. 2018;48(9):1445–1455. doi: 10.1002/eji.201747400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang Y., Zhu L., Hong J., Chen C. Extracellular matrix of early pulmonary fibrosis modifies the polarization of alveolar macrophage. Int. Immunopharm. 2022;111 doi: 10.1016/j.intimp.2022.109179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.