Abstract
Recently, extensions of the range of Echinococcus multilocularis in Europe and North America and drastic increases in fox populations in Europe put an increasing proportion of the human population at risk of alveolar echinococcosis. To obtain data on the local infection pressure, studies of the prevalence of the parasite in the animals that transmit the parasite, foxes, dogs, and cats, are urgently required. Such investigations, however, have been hampered by the need for necropsy of the host animal to specifically diagnose infection with the parasite. In this study, a nested PCR and an improved method for DNA extraction were developed to allow the sensitive and specific diagnosis of E. multilocularis infections directly from diluted fecal samples from foxes. The target sequence for amplification is part of the E. multilocularis mitochondrial 12S rRNA gene. The specificity of the method was 100% when it was tested against 18 isolates (metacestodes and adult worms) of 11 cestode species, including E. granulosus. The sensitivity of the method was evaluated by adding egg suspensions and individual eggs to samples of diluted feces from uninfected foxes. The presence of one egg was sufficient to give a specific signal. To confirm the PCR results, an internal probe which hybridized only with E. multilocularis amplification products but not with the DNA of other cestodes was constructed. In order to investigate the applicability of this method for epidemiological studies, 250 wild foxes from a area in southern Germany where echinococcosis is highly endemic were examined by both necropsy and PCR of rectal contents. The sensitivity correlated with the parasites’ number and stage of maturity. It ranged from 100% (>1,000 gravid worms) to 70% (<10 nongravid worms). On the basis of positive PCR results for 165 foxes, the sensitivity of the traditional and widely used necropsy method was found to be not higher than 76%. We therefore present this PCR system as an alternative method for the routine diagnosis of E. multilocularis in carnivores.
Alveolar echinococcosis (AE), caused by the metacestode of the fox tapeworm Echinococcus multilocularis, is a potentially lethal human disease. In its natural wildlife hosts (canines and rodents) the parasite is present across most regions of the northern hemisphere, whereas transmission to humans seems to occur predominantly focally. In Alaska and parts of northern China, where AE poses one of the most serious health problems, the disease seems to be transmitted mainly by domestic dogs. In central Europe and North America, AE in humans is a comparatively rare disease; wild foxes (Vulpes vulpes) are thought to be the principal transmitters, although the role of domestic dogs and cats has not been satisfactorily evaluated. There is, however, concern due to extensions of the parasite’s range in Europe and North America, the drastic increases in wild fox populations in most parts of Europe, the increasingly close association of wild foxes with human habitations, and in some regions, sharply increasing rates of prevalence of E. multiocularis in foxes which locally may exceed 70% (14, 21). The exact route of transmission to humans is unknown. Ingestion of contaminated berries or herbs from forests were thought to be potential sources of infection; in some studies, farming was found to be a risk factor (11, 18). For several European regions, detailed information on the prevalence rates in foxes is available; on the contrary, few data on the role of domestic dogs and cats, which may carry the parasite as a spillover of the wildlife cycle in areas where echinococcosis is endemic, exist. The reason for the paucity of data on these hosts, which may be of prime importance in carrying the disease to humans, is the difficult diagnostic procedure, which largely relies on inspection of the dead animal’s intestine and visual identification of the worms. This technique, although sensitive, extremely specific, and applicable to the examination of species of wildlife, is also expensive and hazardous and largely prevents the examination of owned domestic animals. Detection of eggs in feces is not a method for specific diagnosis, since the eggs of all taeniid cestodes of foxes and dogs are morphologically indistinguishable.
In the search for alternative methods for the diagnosis of infections with Echinococcus spp. in definitive hosts (hosts containing the adult tapeworms), antibody serology has up to now proved to be unsatisfactory. With varying levels of success, several approaches have been used to develop test systems for coprodiagnosis. The methods include the use of monoclonal antibodies to label artificially hatched eggs (5) and the immunological detection of coproantigens (1, 6). Recently, PCR was introduced as a tool for the coprodiagnosis of E. multilocularis infection (4). In the following sections we present a novel approach to coprodiagnosis by PCR with unprocessed fecal samples.
MATERIALS AND METHODS
Collection of samples. (i) E. multilocularis metacestodes.
Seventeen E. multilocularis isolates were used to evaluate the PCR method: five isolates originated from common voles (Microtus arvalis) and eight originated from water voles (Arvicola terrestris) trapped in an area of the Swabian Jura in southern Germany of high endemicity, one isolate was from Clethrionomys rufocanus from eastern Hokkaido, Japan, and three isolates were from Microtus oeconomus from Alaska. The diagnosis was based on parasitological examination. The parasite material was removed within 24 h after the death of the host, cleaned from the host tissue as much as possible, and stored in 70% ethanol.
(ii) E. multilocularis adult worms and eggs.
Two isolates of adult worms with mature eggs and two isolates of immature worms originated from the intestines of wild foxes (V. vulpes) shot by hunters in southern Germany. After removal from the intestinal mucosa, the worms were rinsed in water, frozen for 5 days at −80°C for safety reasons, and stored in 70% alcohol. Eggs were isolated as described by Müller (17); in short, a suspension containing gravid proglottids underwent digestion with proteinase K for 1 h followed by purification on 60% Percoll. The purified egg suspension was then either stored at 4°C in phosphate-buffered saline or frozen at −80°C.
(iii) Fecal samples.
A total of 250 fecal samples originated from wild foxes shot by hunters in an area of southern Germany of high endemicity (Baden-Württemberg) and 42 samples from an area of northeastern Germany of low endemicity (Brandenburg). In addition, four samples from captive foxes and four samples from dogs were tested. All fecal samples were removed from the rectum, avoiding contamination, frozen at −80°C for 5 days for safety reasons, and subsequently stored at −20°C.
(iv) Other helminths.
For specificity screening, the following samples of cestodes other than E. multilocularis were used: Echinococcus granulosus (three isolates, metacestodes, from Kenya and Germany), Taenia crassiceps (one isolate, a metacestode, from Germany), Taenia hydatigena (three isolates, a metacestode and adults, from Kenya and Switzerland), Taenia martis (two isolates, metacestodes, from Germany), Taenia mustelae (one isolate, a metacestode, from Germany), Taenia ovis (one isolate, an adult, from Australia), Taenia pisiformis (one isolate, an adult, from Australia), Taenia polyacantha (two isolates, metacestodes, from Germany), Taenia serialis (one isolate, an adult, from Australia), Taenia taeniaeformis (two isolates, a metacestode and an adult, from Germany and Switzerland), and Mesocestoides leptothylacus (one isolate, an adult, from Germany). In addition, several adult nematodes of fox origin (Toxocara sp. and Uncinaria sp.) were tested.
Examination of fox intestines.
Small intestines were opened with gut scissors and were visually inspected for the presence of E. multilocularis and other helminths. After removal of coarse intestinal contents, smear samples were taken from locations at 10-cm intervals by scraping the mucosa with microscopic glass strips which were pressed on square polystyrene petri dishes (8). The samples were examined with a stereomicroscope at ×8 to ×50 magnification. All procedures were performed under appropriate safety conditions. The numbers and developmental stages of the parasites seen were recorded.
Extraction of DNA. (i) Cestode tissue.
Samples of parasite tissue were digested as described elsewhere (3), with the following modifications. Samples (up to 0.3 g) were cut into small pieces and were digested in the presence of 900 μg of proteinase K (Boehringer GmbH, Mannheim, Germany) at 56°C for 6 to 12 h in 0.5 ml of 10 mM Tris-HCl (pH 7.5), 10 mM EDTA, 50 mM NaCl, 2% sodium dodecyl sulfate, and 20 mM dithiothreitol. DNA was extracted as described before (20) with phenol-chloroform-isoamyl alcohol (25:24:1) and chloroform-isoamyl alcohol. The DNA was precipitated with 3 M sodium acetate (pH 4.8) (1:10) and ethanol (2:1) at −20°C (3). After vacuum drying the precipitate was suspended in 100 μl of double-distilled H2O.
(ii) Fecal samples.
Fecal samples (minimum amount, 0.5 g) were diluted 1:2 (vol/vol) with distilled water. A total of 1,500 μl of the resulting suspension was used to extract the DNA. Since proteinase K proved to be ineffective in digesting the embryophore of cestode eggs, a DNA extraction method based on alkaline hydrolysis as described previously (4) was modified as follows. To each 1,500 μl of the fecal suspension, 108 μl of 1 M KOH and 30 μl of 1 M dithiothreitol were added. After vortexing, the sample was incubated at 65°C for 30 min and neutralized with 270 μl of 2 M Tris-HCl (pH 8.3) and 40.5 μl of 25% HCl. The DNA was extracted with 1,950 μl of phenol-chloroform-isoamyl alcohol (25:24:1), and the aqueous phase was transferred to a 12-ml tube. The DNA was purified and concentrated with the Prep-A-Gene purification kit (Bio-Rad Laboratories GmbH, Munich, Germany). A total of 5,400 μl of binding buffer was added to 1,800 μl of the aqueous phase and mixed briefly. A total of 30 μl of the Prep-A-Gene matrix was added, and the samples were incubated at 37°C for 60 min with frequent agitation. After centrifugation, the pellet was washed once with 1,000 μl of binding buffer and three times with 1,000 μl of washing buffer. To remove the ethanol the pellet was vacuum dried. The DNA was eluted by resuspending the matrix in 100 μl of double-distilled H2O and incubating the mixture for 15 min at 50°C. After centrifugation, the supernatant containing the DNA was ready to be used in the PCR.
PCR.
The target sequence for amplification is part of the E. multilocularis mitochondrial 12S rRNA gene, which has been used in phylogenetic studies (24). The PCR was conducted in two steps. For the first PCR, the primer pair P60.for. and P375.rev. amplified a 373-bp fragment (Fig. 1). A total of 10 μl of DNA was added to a 90-μl reaction mixture containing 20 mM Tris-HCl (pH 8.5), 16 mM (NH4)2SO4 0.2 mM MgCl2, 50 mM KCl, each deoxynucleoside triphosphate at a concentration of 0.2 mM, 40 pmol of each primer, and 2 U of Taq polymerase (AGS GmbH, Heidelberg, Germany). The sample fluid was covered with 55 μl of mineral oil to prevent evaporation. Thermal cycling of the amplification mixture was performed in a DNA Thermal Cycler 480 (Perkin-Elmer) for 50 cycles. A cycle represents denaturation for 60 s at 93°C, annealing for 90 s at 55°C, and elongation for 120 s at 73°C. In a second step, the primer pair Pnest.for. and Pnest.rev. (Fig. 1) was used for a nested PCR. It is located downstream of the first primer pair and amplifies a 250-bp fragment. The reaction mixture consisted of 3 μl of amplification product, 20 mM Tris-HCl (pH 8.5), 16 mM (NH4)2SO4, 1.5 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 0.2 mM, 50 pmol of each primer, and 2 U of Taq polymerase (AGS GmbH). The nested PCR was performed for 40 cycles, with each cycle consisting of denaturation for 60 s at 93°C, annealing for 60 s at 59°C, and elongation for 120 s at 73°C. After amplification, 10 μl of the PCR products was visualized on a 1.5% agarose gel containing 1 μg of ethidium bromide per ml.
FIG. 1.
Sequence of part of the mitochondrial 12S rRNA gene from E. multilocularis. Primers and probe are underlined.
Control for contamination.
To exclude the possibility of contamination with specific DNA, in each test run (usually done with 16 samples) 2 negative controls were included and underwent the entire procedure starting with DNA extraction.
Control for inhibition.
Due to unknown factors present in some fecal samples, PCRs may occasionally be inhibited and may therefore give false-negative results (25). To control for such inhibitions, 100 ng of E. multilocularis DNA was added to each negative sample and the first PCR was repeated. The test sample was recorded as negative only if a signal was obtained; if not, the result was considered inconclusive.
Hybridization of PCR products.
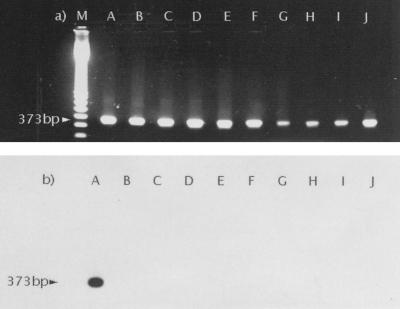
To control the specificity of the PCR, an internal oligonucleotide, E.multi.1. (Fig. 1), was constructed. Agarose gels were blotted onto nylon membranes (Quiagen GmbH, Hilden, Germany) and, after prehybridization at 68°C for 1 h, were probed with E.multi.1., which was 5′ end labeled with digoxigenin. Hybridization was performed at 48°C for 2 h. For detection the DIG Luminescent Detection Kit (Boehringer Mannheim) was used. The hybridization signal was visualized with E. multilocularis DNA after amplification with P60.for.-P375.rev. and Pnest.for.-Pnest.rev. but not with P60.for.-P375.rev. amplification products of other cestode DNAs (Fig. 2).
FIG. 2.
PCR amplification of DNA from 10 different tapeworm species with P60.for.-P375.rev. A total of 10 μl of the PCR products was separated on a 1.5% agarose gel and stained with ethidium bromide (a). PCR products were analyzed by Southern transfer and hybridized with internal oligonucleotide E.multi.1. labeled at the 5′ end with digoxigenin (b). Lanes A, E. multilocularis; lanes B, E. granulosus; lanes C, T. taeniaeformis; lanes D, T. hydatigena; lanes E, T. pisiformis; lanes F, T. serialis; lanes G, T. martis; lanes H, T. ovis; lanes I, T. mustelae; lanes J, T. polyacantha; lanes M, size marker.
RESULTS
PCR of metacestode tissue.
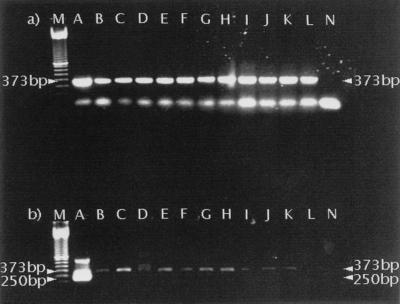
The primer pair P60.for.-P375.rev. amplified the target sequences of all 12 cestode species which were tested (Fig. 3). The second (nested) PCR with Pnest.for.-Pnest.rev. was found to selectively amplify E. multilocularis DNA. All 17 isolates of E. multilocularis metacestodes yielded the same characteristic band of 250 bp. In contrast, this band was never visualized after amplification of other cestode DNAs with Pnest.for.-Pnest.rev. (Fig. 3), including three isolates of E. granulosus.
FIG. 3.
PCR amplification with P60.for.-P375.rev. (a) followed by amplification with Pnest.for.-Pnest.rev. (b) of DNA from 12 different tapeworm species. A total of 10 μl of PCR products was separated on a 1.5% agarose gel and stained with ethidium bromide. Lanes A, E. multilocularis; lanes B, E. granulosus; lanes C, T. hydatigena; lanes D, T. martis; lanes E, T. taeniaeformis; lanes F, T. crassiceps; lanes G, T. mustelae; lanes H, T. ovis; lanes I, T. pisiformis; lanes J, T. polyacantha; lanes K, T. serialis; lanes L, M. leptothylacus; lanes N, negative control; lanes M, size marker.
PCR of fecal samples. (i) Sensitivity.
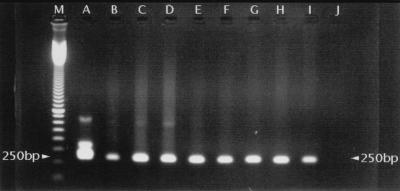
To determine the number of E. multilocularis eggs necessary to give a positive PCR result, an egg suspension was diluted to obtain 100-μl batches with mean egg contents of from 200 eggs to 1 egg. These batches were added to 1,500 μl of diluted feces from captive foxes free of E. multilocularis. To be certain that a signal could be obtained from suspensions with only one egg, single eggs were also added to diluted feces. One egg was found to be sufficient to give a specific signal (Fig. 4). The same result was obtained by adding 10 pg of E. multilocularis DNA (one egg contains approximately 8 pg of DNA [19]) to 1,500 μl of diluted fox feces free of E. multilocularis.
FIG. 4.
Nested PCR amplification of DNA from eggs added to 1,500 μl of diluted fox feces free of E. multilocularis. A total of 10 μl of PCR products was separated on a 1.5% agarose gel and stained with ethidium bromide. Lane A, positive control; lane B, one egg; lanes C, D, E, F, G, H, and I, egg suspensions; lane C, 200 eggs; lane D, 100 eggs; lane E, 50 eggs; lane F, 20 eggs; lane G, 10 eggs; lane H, 2 eggs; lane I, 1 egg; lane J, negative control; lane M, size marker.
Even in the case of immature infections prior to the production of eggs, whole E. multilocularis worms may be present in the feces, and therefore, DNA may be detectable. To test whether the DNA extraction method used for eggs would also be suitable for somatic cestode tissue, six immature E. multilocularis worms (without visible eggs) were subjected to the DNA extraction by using alkaline lysis. The subsequent PCR gave positive results in all cases.
(ii) Specificity.
Four fecal samples from captive foxes and four fecal samples from dogs free of E. multilocularis gave negative PCR results. To exclude the possibility that some organism other than E. multilocularis present in the intestine or food of wild foxes may give a positive signal, fecal samples from 42 foxes which were from the area of Brandenburg, which is of low endemicity, and which were negative by intestinal inspection (22) were tested by PCR. All gave negative results.
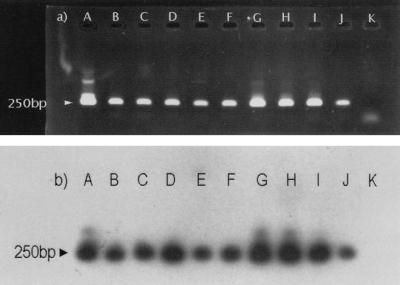
As an additional control for specificity, nested PCR products from 60 positive fecal samples were randomly selected and underwent hybridization with the specific probe E.multi.1. With all samples a hybridization signal was obtained (Fig. 5).
FIG. 5.
Nested PCR amplification of DNA from nine positive fox fecal samples; ethidium bromide staining of 10 μl of PCR products after 1.5% agarose gel electrophoresis showed the specific 250-bp band (a). The reaction products were analyzed by Southern transfer and hybridized with internal oligonucleotide E.multi.1. labeled at the 5′ end with digoxigenin (b). Lanes A, positive control; lanes B, C, D, E, F, G, H, I, and J, positive fox fecal samples; lanes K, negative control.
(iii) Comparison of methods.
A total of 250 wild foxes from an area of high endemicity were examined for E. multilocularis by both necropsy (intestinal inspection) and PCR of fecal samples (rectal content). Nine of the 250 fecal samples (3.6%) were found to contain factors inhibiting the PCR and therefore gave no result; data for these nine foxes (seven positive and two negative at postmortem examination) were excluded from the following calculations. The E. multilocularis prevalence based on necropsy results was 59% (142 of 241), the prevalence based on PCR results was 68% (165 of 241), and the overall prevalence (foxes positive by at least one method) was 75% (181 of 241).
The overall sensitivity of PCR, based on the 142 positive results at necropsy, was 89%. Sensitivity was influenced by the worm burden (Table 1); it ranged from 100% (foxes with >1,000 worms seen at necropsy) to 78% (foxes with <10 worms). Infections with worms containing mature eggs were more reliably detected by PCR (97%) than infections with immature worms (78%) (Table 1).
TABLE 1.
PCR results for fecal samples (rectal contents) from wild foxes positive for E. multilocularis by necropsy
| No. of worms seen at necropsy | Mature eggsa
|
No mature eggsb
|
Total
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. examinedc | No. positived | Sensitivity (%)e | No. examined | No. positive | Sensitivity (%) | No. examined | No. positive | Sensitivity (%) | |
| >1,000 | 14 | 14 | 100 | 5 | 5 | 100 | 19 | 19 | 100 |
| 101–1,000 | 30 | 29 | 97 | 13 | 12 | 92 | 43 | 41 | 95 |
| 11–100 | 22 | 22 | 100 | 22 | 16 | 73 | 44 | 38 | 86 |
| 1–10 | 13 | 12 | 92 | 23 | 16 | 70 | 36 | 28 | 78 |
| Total | 79 | 77 | 97 | 63 | 49 | 78 | 142 | 126 | 89 |
Worms containing eggs which appeared mature at microscopic examination (the presence of eggs in feces is likely).
Worms containing no eggs or eggs appearing immature (the presence of eggs in feces is unlikely).
Total number of fecal samples (from individual foxes) examined by PCR.
Number of fecal samples positive by PCR.
Sensitivity of the PCR method with fecal samples from foxes with different worm counts at necropsy.
By reciprocal calculation, the sensitivity of the necropsy method, based on 165 PCR-positive specimens, was 76%.
Within the group of foxes negative for E. multilocularis at necropsy, the presence of other cestode species (Taenia spp. and Mesocestoides sp.) did not influence the PCR results: of 39 PCR-positive foxes, 54% harbored other cestodes, whereas 58% of 62 PCR-negative foxes harbored other cestodes.
(iv) Cost and processing capacity.
The approximate cost for processing one fecal sample by PCR (consumables only, not counting equipment and labor) was approximately US$10. The processing capacity for one person was some 70 samples per week, approximately equal to the capacity for postmortem examinations.
DISCUSSION
At present, routine diagnosis of E. multilocularis infections in definitive hosts largely depends on necropsy and the visual detection of the worms in the intestines. Serological screening is generally considered unsuitable for the reliable diagnosis of infections with Echinococcus spp. in definitive hosts because of a poor correlation between antibody titers and the presence of worms in the intestine (7, 12). Recent approaches to the detection of coproantigen by enzyme-linked immunosorbent assay showed high sensitivities with heavy infections only (6, 7), while the diagnostic sensitivity for the detection of E. multilocularis in foxes with individual worm burdens of less than 20 worms may be as low as 38% (7). A method based on PCR for the detection of E. multilocularis DNA directly from fecal samples, tested with only 29 fecal samples of foxes (4), proved to be difficult to reproduce because of false-negative results due to the presence of PCR-inhibitory substances (16). In order to overcome such problems the investigators (16) improved this technique at the cost of a very time consuming DNA extraction protocol. Therefore, the applicability of this method for epidemiological purposes is only limited. As an alternative approach, Mathis et al. (15) have published a method of PCR identification of E. multilocularis eggs after isolation of the eggs from fecal samples. Although sensitive and specific, this approach is suitable for the diagnosis of gravid infections only (with eggs present in the feces). Coproantigen enzyme-linked immunosorbent assays, although not suitable for the detection of light infections, are still considered suitable for epidemiological purposes since they will reliably detect heavy infections, which are responsible for the bulk of environmental contamination. However, there are situations in which the detection of light infections is equally important (e.g., surveillance of chemotherapy studies and diagnosis of infections in domestic animals with contact with humans). A sensitive test is even more important, since in our representative sample of foxes, 25% were in the category of foxes containing 1 to 10 worms.
For the first time, we developed a method that was evaluated against the traditional postmortem examination using a large number of foxes in the routine laboratory and that compared favorably with the traditional method concerning specificity, sensitivity, cost, and the processing time needed.
The specificity of our test system was evaluated against a variety of cestode species including E. granulosus and other helminths regularly found in the intestines of foxes in the study area. Nevertheless, 39 (16%) of 241 foxes were negative at necropsy and reacted positively by the PCR. We therefore had to exclude the possibility that amplification of non-E. multilocularis DNA present in the intestinal contents of wild foxes may give an amplification product of a similar size. This was done by testing fecal samples from foxes from areas of very low endemicity (none of which gave a signal) and by hybridizing the nested PCR product with an E. multilocularis-specific probe, which succeeded in all cases. We are therefore certain that the DNA of E. multilocularis was amplified. The probability of accidental contamination was minimal since negative control samples were included in each test run (36 negative control samples in 18 test runs), none of which ever gave a signal. Theoretically, positive PCR results can also be obtained by amplifying DNA from immature E. multilocularis metacestodes which have been ingested by the fox together with the intermediate host (voles). However, calculations that consider the rate of mature and immature infections in voles and the prevalence and life span of the adult worm in foxes indicate that voles with immature metacestodes cannot be present in the intestines of more than 2% of foxes at a given time. Therefore, positive PCR results which are not confirmed by necropsy in most cases cannot be considered false-positive results but must be attributed to low-intensity infections overlooked during the visual examination of the intestines.
PCR test systems for viral, bacterial, and protozoan organisms are known to detect extremely small quantities of DNA (2, 9, 10, 13). In our system, signals were obtained from single E. multilocularis eggs, which have a DNA content of approximately 8 ng (19). However, even in foxes with mature infections, eggs or gravid proglottids are not shed continuously and are not homogeneously distributed within the feces. This explains the moderate decrease in sensitivity from 100% for animals with heavy infections to 92% for animals with very light infections (10 or fewer worms seen at necropsy). A surprisingly high PCR sensitivity was found with fecal samples from foxes with immature infections (100% with heavy infections; it was still 70% for animals with 10 or fewer worms). This may be attributable to the detection of tissue fragments or whole worms shed with the feces, although the presence of undetected mature, egg-producing worms (in addition to the immature stages seen at necropsy) can in no case entirely be excluded. For 3.6% of all samples the PCR result was inconclusive due to inhibition. Fecal samples are known to occasionally contain factors, which are as yet unidentified, which interfere with the amplification process (25), rendering these samples unsuitable for PCR testing. Although the percentage of such samples in our study was acceptably small, further efforts are necessary to overcome this obstacle.
To date, routine examination of definitive hosts for the presence of E. multilocularis is limited to a very few parasitology or veterinary laboratories. Safety precautions taken to exclude accidental infection of personnel and contamination of the environment require laboratories with high levels of safety and with specialized facilities for heat decontamination, since the infectious eggs are largely resistant to chemical disinfectants (23). The high cost required to construct and maintain such laboratories prevents the routine monitoring of E. multilocularis in wild and domestic animals, as is done with rabies, for example. Freezing of the carcasses at −80°C for 4 days is also a suitable means of destroying cestode eggs before performing postmortem examination in a routine laboratory. However, the large freezer capacity needed also confines this approach to a few institutions. Compared with the facilities necessary for necropsy, PCR equipment is cheap and is usually present in every routine laboratory. Fecal samples can easily be rendered noninfectious by freezing them for some days at −80°C before entering the laboratory. Since in our system DNA extraction is done directly from feces without preprocessing, the workload for one person (using one set of PCR equipment) would be some 70 samples per week, which also compares favorably with the workload for postmortem examination. A total of 3.6% of fecal samples are unsuitable for PCR due to inhibition factors. However, not all fox carcasses delivered by hunters or from other sources are suitable for postmortem examination due to decomposition of the intestine; in our laboratory, the rate of occurrence of such specimens ranges from 4 to 10%.
Domestic dogs and cats are suitable hosts for E. multilocularis and may be important transmitters of echinococcosis to humans. The evaluation of their epidemiological role has until now been impossible because, for obvious reasons, representative samples for necropsy could not be obtained. Coprodiagnosis by PCR with fresh fecal samples will overcome this problem in the near future, although the test system will have to be evaluated separately for each host species.
PCR of fox feces for the detection of E. multilocularis is an important step in simplifying the routine diagnosis of infection with the parasite. In our study this technique was evaluated with fresh samples removed from the rectums of foxes that had been shot. To exploit the full potential of the technique, it should in future be evaluated with deposited fox feces from the environment. However, the influences of various factors (e.g., age, temperature, and desiccation) on the reliability of the test system are unknown, and to the authors’ knowledge, no diagnostic PCR system that uses fecal samples from the environment has been developed. Therefore, the practicality of the method needs to be determined by testing large numbers of fecal samples randomly collected from areas where prevalence rates (and their temporal variations) have previously been established by necropsy of adequate numbers of animals.
Our sample of 241 foxes showed a rate of E. multilocularis prevalence of 59% by necropsy examination which, by adding PCR as a second method, increased to 75% (that is, samples positive by at least one method). Since some infections may fail to be detected by both methods, the real prevalence may be even slightly higher. With 165 PCR-positive foxes, it was for the first time possible to determine the sensitivity rate of the necropsy method, which has been in use (with modifications) since the 1970s. Our set of data showed a sensitivity of 76%. Since most of the recently published rates of E. multilocularis prevalence in definitive hosts were established by using necropsy with smear samples as the only method of detection (14), this sensitivity rate is of prime importance for interpretation of these data.
ACKNOWLEDGMENTS
This work was financially supported by the local government of Baden-Württemberg (ministries of agriculture, research, and social affairs) and the German Federal Ministry of Health.
We thank Kirsten Tackmann (Wusterhausen, Germany) for kindly providing fox fecal samples. We also thank Peter Deplazes (Zürich, Switzerland), Marshall Lightowlers (Werribee, Australia), Kenishi Takahashi (Sapporo, Japan) and Eberhard Zeyhle (Nairobi, Kenya) for supplying cestode material from various species. We thank Ute Mackenstedt and Brigitte Frank (Hohenheim, Germany) for general support and advice.
REFERENCES
- 1.Allan J C, Craig P S, Garcia Noval J, Mencos F, Liu D, Wang Y, Wen H, Zhou P, Stringer R, Rogan M, Zeyhle E. Coproantigen detection for immunodiagnosis of echinococcosis and taeniasis in dogs and humans. Parasitology. 1992;104:347–355. doi: 10.1017/s0031182000061801. [DOI] [PubMed] [Google Scholar]
- 2.Allard A, Girones R, Juto P, Wadell G. Polymerase chain reaction for detection of adenoviruses in stool samples. J Clin Microbiol. 1990;28:2659–2667. doi: 10.1128/jcm.28.12.2659-2667.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1987. pp. 2.1.1–2.1.2. [Google Scholar]
- 4.Bretagne S, Guillou J-P, Morand M, Houin R. Detection of Echinococcus multilocularis DNA in fox faeces using DNA amplification. Parasitology. 1993;106:193–199. doi: 10.1017/s0031182000074990. [DOI] [PubMed] [Google Scholar]
- 5.Craig P S, Macpherson C N L, Watson-Jones D L, Nelson G S. Immunodetection of Echinococcus eggs from naturally infected dogs and from environmental contamination sites in settlements in Turkana, Kenya. Trans R Soc Trop Med Hyg. 1988;82:268–274. doi: 10.1016/0035-9203(88)90445-2. [DOI] [PubMed] [Google Scholar]
- 6.Deplazes P, Gottstein B, Eckert J, Jenkins D J, Ewald D, Jimenez-Palacios S. Detection of Echinococcus coproantigens by enzyme-linked immunosorbent assay in dogs, dingoes and foxes. Parasitol Res. 1992;78:303–308. doi: 10.1007/BF00937088. [DOI] [PubMed] [Google Scholar]
- 7.Deplazes P, Eckert J. Diagnosis of the Echinococcus multilocularis infection in final hosts. Appl Parasitol. 1996;37:245–252. [PubMed] [Google Scholar]
- 8.Eckert J, Deplazes P, Ewald D, Gottstein B. Parasitologische und immunologische Methoden zum Nachweis von Echinococcus multilocularis bei Füchsen. Mitt Österr Ges Tropenmed Parasitol. 1991;13:25–30. [Google Scholar]
- 9.Eiden J J, Wilde J, Firoozmand F, Yolken R. Detection of animal and human group B rotaviruses in fecal specimens by polymerase chain reaction. J Clin Microbiol. 1991;29:539–543. doi: 10.1128/jcm.29.3.539-543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frankel G, Riley L, Giron J A, Valmassoi J, Friedmann A, Strockbine N, Falkow S, Schoolnik G K. Detection of Shigella in feces using DNA amplification. J Infect Dis. 1990;161:1252–1256. doi: 10.1093/infdis/161.6.1252. [DOI] [PubMed] [Google Scholar]
- 11.Gloor B. Echinokokkose beim Menschen in der Schweiz 1970–1983. Ph.D. dissertation. Zurich, Switzerland: University of Zurich; 1988. [Google Scholar]
- 12.Jenkins D J, Gasser R B, Zeyhle E, Romig T, Macpherson C N L. Assessment of a serological test for the detection of Echinococcus granulosus infection in dogs in Kenya. Acta Trop. 1990;47:245–248. doi: 10.1016/0001-706x(90)90016-s. [DOI] [PubMed] [Google Scholar]
- 13.Kratzwinkel-Wladarsch S, Löscher T, Rinder H. Direct amplification and differentiation of pathogenic and nonpathogenic Entamoeba histolytica DNA from stool specimens. Am J Trop Med Hyg. 1994;51:115–118. doi: 10.4269/ajtmh.1994.51.115. [DOI] [PubMed] [Google Scholar]
- 14.Lucius R, Bilger B. Echinococcus multilocularis in Germany: increased awareness or spreading of a parasite? Parasitol Today. 1995;11:430–434. doi: 10.1016/0169-4758(95)80030-1. [DOI] [PubMed] [Google Scholar]
- 15.Mathis A, Deplazes P, Eckert J. Meeting of the World Health Organization Informal Working Group on Echinococcosis, Limassol, Cyprus. 1995. Improved test system for PCR-based detection of E. multilocularis eggs in faeces of final hosts. Unpublished. [Google Scholar]
- 16.Monnier P, Cliquet F, Aubert M, Bretagne S. Improvement of a polymerase chain reaction assay for the detection of Echinococcus multilocularis DNA in faecal samples of foxes. Vet Parasitol. 1996;67:185–195. doi: 10.1016/s0304-4017(96)01039-4. [DOI] [PubMed] [Google Scholar]
- 17.Müller V. Studien zu einer rekombinanten Vakzine gegen Echinococcus multilocularis im Mausmodell. Ph.D. thesis. Stuttgart, Germany: University of Hohenheim; 1995. [Google Scholar]
- 18.Nothdurft H D, Jelinek T, Mai A, Sigl B, von Sonnenburg F, Löscher T. Epidemiology of alveolar echinococcosis in southern Germany (Bavaria) Infection. 1995;23:85–88. doi: 10.1007/BF01833871. [DOI] [PubMed] [Google Scholar]
- 19.Rishi A K, McManus D P. Genomic cloning of human Echinococcus granulosus DNA: isolation of recombinant plasmids and their use as genetic markers in strain characterization. Parasitology. 1987;94:369–383. doi: 10.1017/s0031182000054020. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Schantz P M, Chai J, Craig P S, Jenkins D J, Macpherson C N L, Thakur A. Epidemiology and control of hydatid disease. In: Thompson R C A, Lymbery A J, editors. Echinococcus and hydatid disease. Wallingford, United Kingdom: CAB International; 1995. pp. 233–331. [Google Scholar]
- 22.Tackmann, K. Personal communication.
- 23.Veit P, Bilger B, Schad V, Schäfer J, Frank W, Lucius R. Influence of environmental factors on the infectivity of Echinococcus multilocularis eggs. Parasitology. 1995;110:79–86. doi: 10.1017/s0031182000081075. [DOI] [PubMed] [Google Scholar]
- 24.von Nickisch-Rosenegk, M., R. Lucius, and B. Frank. Contributions to the phylogeny of the Cyclophyllidea (Cestoda) inferred from mitochondrial 12S rDNA. J. Mol. Evol., in press. [DOI] [PubMed]
- 25.Wilde J, Eiden J, Yolken R. Removal of inhibitory substances from human fecal specimens for detection of group A rotaviruses by reverse transcriptase and polymerase chain reaction. J Clin Microbiol. 1990;28:1300–1307. doi: 10.1128/jcm.28.6.1300-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]