Abstract
The uncontrolled discharge of industrial wastewater leads to a significant cadmium (Cd) accumulation in waste activated sludge (WAS), posing a serious threat to the steady operation of the anaerobic digestion (AD) system in wastewater treatment plants (WWTPs). Therefore, developing a viable approach to cope with the adverse effects of high-concentration Cd on the AD system is urgently required. This study aims to investigate the potential of using anionic polyacrylamide (APAM), a commonly used agent in WWTPs, to mitigate the adverse effects of Cd in a toxic amount (i.e., 5.0 mg per g total suspended solids (TSS)) on AD of WAS. The results showed that the effectiveness of higher APAM on Cd toxicity alleviation was less than that of lower APAM at the studied level (i.e., the effectiveness order was 1.5 mg APAM per g TSS > 3.0 mg APAM per g TSS > 6.0 mg APAM per g TSS). The moderate supplement of APAM (i.e., 1.5 mg per g TSS) recovered the accumulative methane yield from 190.5 ± 3.6 to 228.9 ± 4.1 mL per g volatile solids by promoting solubilization, hydrolysis, and acidification processes related to methane production. The application of APAM also increased the abundance of key microbes in the AD system, especially Methanolinea among methanogens and Caldilineaceae among hydrolyzers. Furthermore, APAM facilitated the key enzyme activities involved in AD processes and reduced reactive oxygen species (induced by Cd) production via adsorption/enmeshment of Cd by APAM. These findings demonstrate the feasibility of using moderate APAM to mitigate Cd toxicity during AD, providing a promising solution for controlling Cd or other heavy metal toxicity in WWTPs.
Keywords: Waste activated sludge, Anaerobic digestion, Cadmium toxicity, Anionic polyacrylamide
Graphical abstract
Highlights
-
•
Anionic polyacrylamide (APAM) can weaken Cd toxicity in anaerobic digestion.
-
•
The adsorption/enmeshment of Cd by APAM plays a key role.
-
•
Added APAM promotes solubilization, acidification, and acidification processes.
-
•
Added APAM increases the abundance of hydrolyzer and acidogens.
-
•
Added APAM decreases reactive oxygen species generation and promotes key enzyme activities (e.g., F420).
1. Introduction
Cadmium (Cd) is widely applied to batteries, pigments, metal coatings, and chemicals. The large-scale use and improper disposal of Cd products have caused anthropogenic emissions of Cd, ultimately ending up in wastewater treatment plants (WWTPs) [1]. The poorly constructed wastewater diversion collection systems in developing countries have also resulted in around 35% of industrial wastewater containing Cd entering WWTPs [2]. It is reported that Cd2+ concentration in WWTPs in Central China ranges from 0.54 to 1.39 mg L−1 [3]. Cd entering WWTPs is eventually adsorbed into waste activated sludge (WAS), the major by-product of WWTPs [4]. It has been stated Cd concentration in WAS ranges from 2.26 to 19.72 mg per kg dry sludge [5]. As a well-known biotechnology, anaerobic digestion (AD), which can reduce and stabilize volatile solids while recovering energy, such as methane, has been used to treat WAS on a large scale [6,7]. However, the negative impacts of Cd on AD inevitably occur in WWTPs, which has drawn the close interest of investigators. For example, Altas et al. [8] found Cd at 36 mg L−1 can reduce methane production by half compared with a pristine sludge reactor without Cd addition. Hence it is necessary to limit this undesirable influence, and researchers have made various attempts. The most common approaches are complexation (and precipitation) [9], alkaline treatment [3], chemical neutralization, and electrochemical treatment [10]. However, these methods are not economical as they require large quantities of reagents, such as HCl, NaOH, and sulfide, and also require restrictive operating conditions. For instance, when using sulfide to chelate/precipitate heavy metals, too little sulfide is ineffective, and too much can seriously inhibit methanogens [11]. A simpler and more efficient strategy is needed to address the adverse effects of Cd on AD systems.
In general, Cd is toxic to microorganisms in two ways. First, Cd can disrupt enzymatic structure and function by combining with or substituting naturally occurring substances in the enzyme's prosthetic group [12]. Second, Cd can produce reactive oxygen species (ROS), leading to oxidative damage to the membrane and eventual cell death [13]. Therefore, reducing Cd exposure to cells is key to mitigating Cd toxicity. Polyacrylamide (PAM), one of the most popular flocculants/coagulant aids, is widely used for sludge dewatering in WWTPs [14,15]. Adsorption/charge neutralization and interparticle adsorption bridging are generally considered the most important mechanisms for separating and removing particulate matter (including Cd/Cd-containing particles) [16,17]. Our previous work found that cationic PAM can trap zinc oxide nanoparticles and mitigate their adverse effects by reducing ROS induced in the AD process, which largely implies PAM has a potential function in trapping Cd/Cd-containing particulate matter and reducing oxidative stress [18,19]. In particular, anionic polyacrylamide (APAM) is negatively charged and can bind positively charged Cd ions (Cd2+) by electrostatic adsorption [20]. Given the above information, APAM may be a candidate for mitigating Cd toxicity in AD systems. However, little is known regarding the feasibility of APAM in alleviating the Cd inhibition on methane yield.
In this study, we test the impact of APAM in alleviating the negative effects of Cd on AD of WAS. The influence of APAM addition on methane yield from AD of WAS containing Cd was estimated by biochemical methane potential (BMP) tests. Furtherly, we explored the potential impact of APAM on each stage of AD for Cd-containing sludge and revealed the mechanisms underlying how APAM alters the toxicity of Cd via flocculation tests in both sludge and pure water. The findings obtained in this work shed light on the possibility of using APAM to mitigate Cd toxicity during AD, providing knowledge about Cd treatment and important guidance for regulating heavy metal toxicity.
2. Materials and methods
2.1. WAS collection and chemicals preparation
The WAS used in the following AD was from a secondary sedimentation tank of a municipal wastewater treatment plant in Changsha, China, where no PAM-based wastewater treatment was carried out. The background Cd concentration detected in untreated sludge was 0.11 ± 0.02 mg L−1. The collected fresh sludge was filtered through a 0.45-mm stainless steel screen to remove impurities and concentrated at 4 °C for 24 h. The supernatant liquid was removed, and the remainder was the sludge used in the experiment. The major characteristic of sludge included the following: total suspended solids (TSS) 29101 ± 345 mg L−1, volatile suspended solids (VSS) 16033 ± 203 mg L−1, and pH 6.8 ± 0.1.
The inoculum (sludge retention time: 20 d; TSS: 23532 ± 302 mg L−1; total chemical oxygen demand (COD): 18061 ± 223 mg L−1) was attained from a long-term anaerobic digester in the laboratory. APAM was purchased from Meiyuan Water Purification Agents company (Gongyi, China) with a molecular weight of 10 million Da and a charge density of 30%. As a heavy metal salt solution, cadmium nitrate tetrahydrate [Cd(NO3)2·4H2O] with a purity of 99.0% was purchased from Sigma Aldrich and used in this study. Cd stock solution (6.0 g L−1) was prepared with deionized water.
2.2. Batch experiment for methane production from AD of WAS containing Cd in different APAM
This batched experiment was conducted in five triplicate serum bottles (working volume of 500 mL each). First, 1000 mL of WAS containing 5 mg Cd per g TSS was added into four serum bottles in equal portions. The Cd content (i.e., 5 mg per g TSS) was based on the previous study [3]. Different amounts of APAM were then dosed separately into these reactors to achieve the target levels (0, 1.5, 3.0, 6.0 mg per g TSS), followed by 200 rpm of stirring for 10 min. Later, 25 mL of laboratory-cultured inoculum was added, and each serum bottle was diluted to 300 mL with deionized water. After exhausting the air in the reactors with nitrogen, all bottles were immediately capped with a butyl rubber stopper, tightly closed, and placed in an air-constant temperature incubator at 35 ± 1 °C. Besides the control (only Cd addition) and experimental group (1.5, 3.0, or 6.0 mg APAM per g TSS), a blank without any addition (with the same operation as the control and experimental group) was set up to evaluate the BMP of the pristine WAS (Table S1). The total gas volume from each reactor was measured by releasing the pressure in the reactors using a glass syringe [21]. The BMP tests modeling and methane detection are presented in Supporting Information.
2.3. Experimental setup to evaluate the roles of APAM on solubilization, hydrolysis, and acidification of AD
To investigate the impact of APAM on the related processes in AD of WAS containing Cd, batch tests were carried out following the same experimental protocol as the BMP tests, with the addition of 2-bromoethanesulphonate (SBES) to each reactor [22]. All reactors prepared in triplicate were spiked with 50 mM SBES to inhibit methanogens. After three days, soluble chemical oxygen demand (SCOD), soluble proteins, soluble polysaccharides, and short-chain fatty acids (SCFAs) were detected in all digestors. The activity of enzymes (i.e., protease, acetate kinase (AK), F420) involved in AD was also determined. The details are outlined in Supporting Information.
2.4. Semi-continuous operation from anaerobic reactors
To evaluate the long-term impacts of APAM on the microbial community, three continuous bench-scale reactors were operated. All reactors were identical with 900 mL working volume and placed in a water constant temperature incubator at 35 ± 1 °C. The sludge retention time for each digestor was maintained for 15 days by removing 60 mL of digested sludge and adding the same volume of fresh WAS daily. Sludge volatile solids (VS) destruction rates and daily methane production in each reactor were determined every three days throughout the two-stage experiment, which lasted for a total of 130 days (Fig. S3). Three continuous anaerobic reactors were operated for 130 days with two stages.
In Stage I (days 0–30), all reactors were operated identically to establish convergence. After the reactors reached similar VS destruction rate and daily methane yield, Stage II started (days 30–130). In this stage, the three reactors were blank, control, and experimental reactor, respectively. For the blank, 60 mL of digested sludge was removed, and the same volume of fresh WAS was added daily. For the control, 60 mL of digested sludge was removed, and the same volume of WAS containing 5 mg Cd per g TSS was added daily. For the experimental reactor, 60 mL of digested sludge was removed, and the same volume of WAS containing 5 mg Cd per g TSS and 1.5 mg APAM per g TSS was added daily. Throughout Stage II, the daily feeding differed in the three reactors, but the other experimental arrangements remained the same as in Stage I. Stage II ended (day 130) when the VS destruction rate and daily methane yield were both within a 3% difference in continuous intervals. At this time, the ROS and lactate dehydrogenase (LDH) content in each reactor were detected to assess the degree of oxidative stress and cell breakage.
2.5. Microbial community analysis
Sludge samples were taken from each reactor on day 130 of semi-continuous operation and then stored at −80 °C until analysis. The high-throughput 16S rRNA gene sequencing (Majorbio Bio-Pharm Technology Co. Ltd., Shanghai, China) was used to analyze the microbial community. The DNA of samples was extracted by FastDNA® Spin Kit for Soil (MP Biomedicals, USA). The quantity and concentration of DNA were detected with a Nanodrop2000 spectrophotometer (Thermo Scientific, USA). The primers 515FmodF (5′-GTGYCAGCMGCCGCGGTAA-3′) and 806RmodR (5′-GGACTACNVGGGTWTCTAAT-3′) targeting the V4 region of bacterial and archaeal 16S rDNA [23] were selected. Sequencing was performed on the Illumina PE300 platform (Illumina, San Diego, USA). Bioinformatic analysis of raw data was carried out using the Majorbio Cloud platform (https://www.majorbio.com).
2.6. The flocculation test to adsorption/enmeshment of Cd by APAM
To further investigate the enmeshment and adsorption effect of APAM on Cd that could attenuate the adverse effect of Cd on the AD system, two experiments were conducted in triplicate. The first was performed in a sludge system (a “sludge flocculation test”). Five reactors were equipped in triplicate as detailed in Section 2.2, differing in that the APAM content was adjusted to 0.3, 0.6, 0.9, 1.2, and 1.5 mg per g TSS, respectively. The second one was based on the pure water system (a “pure water flocculation test”), using a Color Display Coagulation Test Stirrer (Wuhan Meiyu Instruments Co., Ltd, China, MY3000-6 N). Five beakers (1 L working volume) were spiked with 600 mL of Cd solution at a concentration of 145.5 mg L−1, equal to the Cd concentration in sludge. Then different volumes of APAM solutions were spiked to make the APAM concentrations in the beakers 8.73, 17.46, 26.19, 34.92, and 43.65 mg L−1 to simulate the APAM levels in sludge (0.3, 0.6, 0.9, 1.2, and 1.5 mg APAM per g TSS). The mixture was stirred for 25 min at 20 r min−1 and left to settle for 30 min. The supernatant was filtered through a 0.22-μm mixed cellulose ester membrane, acidified with 2% nitric acid, and the residual amount of Cd in the supernatant was determined. The detailed setup for the pure water flocculation test was shown in Table S2.
2.7. Analytical methods
SCFAs were detected by gas chromatography coupled with a flame ionization detector (GC-FID, Agilent 7890B) and expressed as the sum of the respective constituents. Methane was monitored with a gas chromatograph coupled with a thermal conductivity detector (GC-TCD, Agilent 7890B), where nitrogen was the carrier gas [19]. Detailed working requirements for GC-TCD and GC-FID are given in Supporting Information. Cd was measured by atomic absorption spectroscopy (PinAAcle900F). TCOD, TSS, and VSS were measured according to standard approaches [24]. Sample pre-treatment required for the measurement of soluble protein and soluble polysaccharide was as specified previously [23], with the bicinchoninic acid (BCA) protein technique and the colorimetry of phenol-sulfuric acid applied for their detection, respectively [25,26]. LDH content was detected by the Lactate Dehydrogenase Assay Kit (Njjcbio, Nanjing, China), and the procedure for ROS detection is detailed in Supporting Information. The significance of the experimental data was assessed using the least significant difference test, and P < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Effects of APAM on methane production from WAS containing Cd
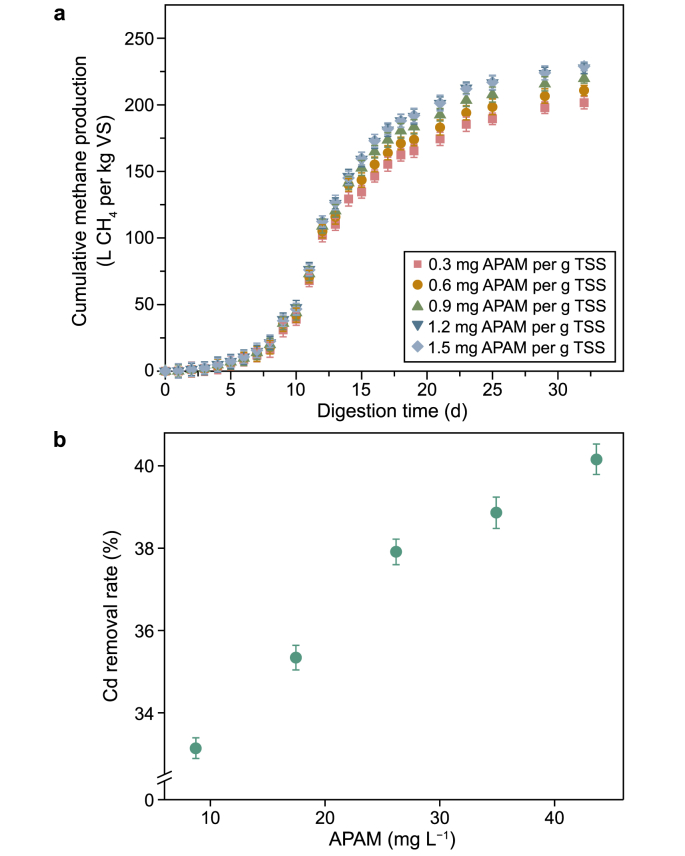
The accumulative methane production during AD of WAS containing Cd at different levels of APAM is presented in Fig. 1. It can be seen that 273.3 ± 3.4 mL per g VS of methane was generated in the blank, while only 190.5 ± 3.6 mL per g VS (69.7% of blank) in the control, which was consistent with previous reports of heavy metal toxicity [3,27,28]. However, with APAM addition (1.5, 3.0, and 6.0 mg per g TSS), methane production increased to 228.9 ± 4.1, 211.5 ± 3.7, and 204.1 ± 3.3 mL per g VS, respectively, compared with the control, which was 83.8%, 77.4%, and 74.7% of blank, respectively. Obviously, the addition of 1.5 mg APAM per g TSS exhibited the maximized methane recovery of 14.1% (P < 0.01) in the experimental group, but the methane yield decreased as APAM further increased (to 3.0 and then to 6.0 mg APAM per g TSS), which could be attributed to two aspects. For one thing, sludge floc was enlarged due to the aggregation of sludge particles when dosing excessive APAM [29,30], which not only led to an enhanced mass transfer resistance [31], reducing the efficiency of soluble organic polymers entering microbial cells, but also restricted sludge disintegration, ultimately weakening the methane production of AD [32]. For another, the excessive addition of APAM caused the accumulation of toxic degradation metabolites (such as acrylamide, acrylic acid, and polyacrylic acid) in microbial cells in the AD system, resulting in the decrease in methane yield [33]. It should be emphasized that the presence of only 3.0 mg APAM per g TSS had no effect (P > 0.05) on methane yield during WAS (i.e., pristine sludge) AD (Fig. S1), but compared with WAS containing Cd, the simultaneous presence of Cd and APAM (3.0 mg per g TSS) inhibited the methane production, which can be explained by the toxicity combination effect [34]. When Cd was available, it has been already caused toxic effects on microbial cells, and APAM (3.0 mg per g TSS) as an exogenous contaminant, further damages microbial cells. In this case, the toxic dosage of APAM differed from that in the original sludge. Taken together, although both higher concentrations of Cd (5 mg per g TSS) and high-dose APAM (6 mg per g TSS) adversely affected AD, respectively, all available results still implied that APAM exerted a facilitative effect on methane production from AD of WAS containing Cd at the levels studied. Additionally, methane production increased by 1.0 ± 3.0, 1.1 ± 2.8, and −13.2 ± 2.3 mL per g VS at 1.5, 3.0, and 6.0 mg APAM per g TSS compared with pristine sludge, which showed APAM had no effect (1.5 and 3.0 mg APAM per g TSS, P > 0.05) or a negative effect (6.0 mg APAM per g TSS) on methane content. This further indicated that the increased methane production was due to the mitigation of Cd toxicity by APAM rather than a contribution to methane production by APAM itself (Fig. S1).
Fig. 1.
Cumulative methane yield from WAS containing a Cd concentration of 5 mg per g TSS at different APAM levels. Error bars are the standard deviations of triplicate tests.
3.2. Effects of APAM on solubilization, hydrolysis, and acidification of AD
In general, AD undergoes three successive processes before methanogenesis: solubilization, hydrolysis, and acidification [35]. All three processes are linked to methanogenesis (Fig. S2); thus, monitoring the soluble organic matter associated with the processes can better understand the impacts of exogenous contaminants on the AD processes. AD was performed under prohibiting methanogens, and no methane production was detected in all reactors after three days of digestion. Sludge solubilization involves converting sludge particulate matter into soluble macromolecules, implying that the released SCOD can also represent the extent of sludge disintegration. The content of soluble organic substrates (expressed as SCOD) after three days of AD is shown in Fig. 2. The decline for released SCOD from 6672 ± 71 mg L−1 in the blank to 5602 ± 83 mg L−1 in the control (P < 0.001), verifying the adverse influences of Cd on the sludge solubilization process. Surprisingly, 1.5 mg APAM per g TSS facilitated the dissolution of Cd-containing sludge, resulting in a discharge of 5977 ± 123 mg SCOD L−1 (P < 0.01). However, the released SCOD declined gradually in the digestors as increasing amounts of APAM (3.0–6.0 mg per g TSS), reaching 5619 ± 103 (P < 0.01) and 5280 ± 92 mg L−1 (P < 0.01), respectively. These could be accounted for by the formation of sludge aggregates (1.5 mg APAM per g TSS addition), where the expansion of specific microorganisms was further triggered [36], which increased the consumption of organic substrates and allowed the transfer of macromolecular polymers from the solid sludge floc to the liquid phase. Overdose of APAM rendered the sludge flocculated, thereby hindering the release of SCOD.
Fig. 2.
Distribution of soluble organic substrates at different APAM levels after three days. Error bars are the standard deviations of triplicate tests.
The hydrolysis process is associated with the transformation and consumption of soluble proteins and polysaccharides in AD. The presence of Cd hindered the consumption of soluble proteins and soluble polysaccharides, causing them to accumulate compared with the blank (1109 ± 31 to 1391 ± 35 mg L−1 and 189 ± 31 to 390 ± 23 mg L−1, respectively) (Fig. 2). Whereas the addition of 1.5 mg APAM per g TSS accelerated the hydrolysis of soluble proteins and soluble polysaccharides to 81.1% and 56.5% of the control (P < 0.01), respectively. However, when the APAM dose was increased to 3.0 and 6.0 mg APAM per g TSS, soluble proteins and soluble polysaccharides began to accumulate gradually again (P < 0.01) (from 86.4% to 94.4% of control for soluble proteins and from 76.9% to 87.7% of control for soluble polysaccharides). This finding demonstrated low levels of APAM (i.e., 1.5 mg APAM per g TSS) promoted the hydrolysis of WAS containing Cd, while high levels (i.e., 3.0 and 6.0 mg APAM per g TSS) restrained it.
SCFAs, including acetate, propionate, butyrate, and valerate, are produced during the acidification of WAS [37]. Inhibition of methanogens led to a substantial accumulation of SCFAs (Fig. 2). Similar to the response to the solubilization and hydrolysis process, Cd addition impeded the acidification of the WAS, resulting in a decrease in SCFAs production to 86.2% (of the blank) in the control reactor (P < 0.001), which further caused a reduction in methane production. In contrast, the levels of SCFAs in the APAM-added reactors were all higher than those in the control, with the maximum SCFAs (92.8% of blank) observed at 1.5 mg APAM per g TSS (P < 0.01). However, with an increase in APAM from 3.0 to 6.0 mg per g TSS, the SCFAs decreased from 90.5% to 88.6% of blank (P < 0.01), possibly due to the excess APAM restricting SCOD release. In summary, this pattern suggests that the increased methane yield of Cd-added WAS exposed to APAM at the studied levels may be due to the promotion of SCFAs production.
3.3. Effects of APAM on the microbial community in WAS containing Cd anaerobic reactors
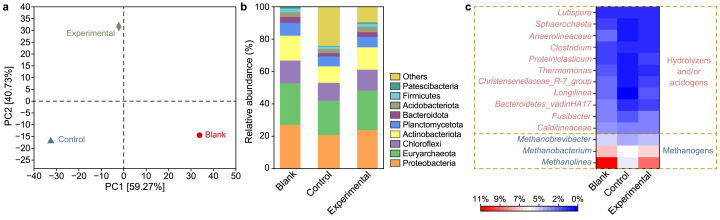
To explain the effects of APAM on AD of WAS containing Cd from the perspective of microbial mechanisms, the microbial communities in three semi-continuous operation reactors were analyzed (i.e., blank, control, and 1.5 mg APAM per g TSS added reactors). A total of 55499, 53781, and 55373 sequences were obtained from blank, control, and experimental digestors. There is a significant difference in the number of operational taxonomic units (OTUs) in the blank, control, and experimental reactor (1578, 1572, and 1645, respectively), with a shared OTUs number of 1192 (Fig. S4). The microbial community compositions differed in all reactors by the principal component analysis (PCA) (Fig. 3a). The eigenvalues of the two principal components (PC1: 59.27%; PC2: 40.73%) accounted for 100% of the observations. This indicated that PC1 and PC2 could represent all of the information of the original variable [38]. It can be seen PC2 failed to distinguish between blank and control, which implied that PC1 was explained by APAM. However, PC1 differentiated well among the three data groups, which indicated that PC1 (where the experimental group was in the middle of blank and control) may be interpreted by Cd toxicity. Assessment of α diversity (Table S3) at the OTU level showed the development of community richness, diversity, and phylogenetic diversity was prevented by Cd, while APAM facilitated that development. These findings were also supported by the accumulative methane production in BMP tests.
Fig. 3.
Microbial community composition in the blank (pristine WAS), the control (with 5 mg Cd per g TSS), and the experimental (with 5 mg Cd per g TSS and 1.5 mg APAM per g TSS) reactor. a, PCA on OTU level. b, Distributions of the microbial population at the phylum level. c, Heatmap at the genus level.
The microbial community structure of three reactors at the phylum level is depicted in Fig. 3b. The dominant phyla in the pristine WAS, Cd-added, and APAM-added groups were Proteobacteria, Euryarchaeota, Chloroflexi, and Actinobacteriota, representing 82.1%, 63.2%, and 74.9% of all sequences, respectively. Many of the microorganisms in these phyla were considered to degrade complex organic substances such as proteins, polysaccharides, and volatile SCFAs [39,40]. However, the long-term exposure to Cd decreased the abundance of methanogens in these phyla, while the APAM addition increased their abundance. For instance, the relative abundance of phyla Euryarchaeota decreased from 25.7% to 21.3% upon Cd addition, while the experimental reactors exhibited a rebound to 24.4%, consistent with the positive impacts of APAM on methane production from AD of WAS containing Cd. In addition, the long-term exposure to Cd negatively affected the abundances of other predominant bacterial phyla, e.g., Proteobacteria and Chloroflexi (Fig. 3b), whereas continuous addition of APAM restored them. The heatmap (Fig. 3c) revealed substantial differences in microbial populations at the genus level among the three reactors. Fusibacter, which is known to degrade complex organic substances and generate acetic acid, was widely present in three reactors, and its abundance decreased from 3.0% to 2.3% upon exposure to 5 mg Cd per g TSS, while the value increased to 2.8% with the addition of 1.5 mg APAM per g TSS [40]. Caldilineaceae, another key anaerobe during hydrolysis, showed a similar trend as Fusibacter. As for methanogenic archaea, they were broadly distributed in all AD systems. The population of Methanolinea reduced from the blank (10.8%) to the control (5.1%) group, rebounding to 8.6% in APAM-added reactors. A similar tendency was also observed in Methanobacterium and Methanobrevibacter related to the methanogenesis process. It can be concluded that 1.5 mg APAM per g TSS addition attenuated the negative effects of Cd on key hydrolytic microbes, acidogens, and methanogens, resulting in improved AD performance, which deepens our understanding of enhanced methane yield in BMP tests.
3.4. How APAM mitigates the Cd inhibition
The results above prove that APAM at low levels (especially at 1.5 mg per g TSS) will benefit AD of WAS containing Cd. To further reveal the adsorption/enmeshment impacts of APAM, a sludge flocculation test and pure water flocculation test were conducted. The adsorption/enmeshment effect of APAM on Cd in the sludge system was expressed indirectly through cumulative methane production, while in the pure water system was represented directly through the removal rate of Cd. As is shown in Fig. 4, a dramatic increase in accumulative methane yield (Fig. 4a) was observed from 0.3 to 1.5 mg APAM per g TSS, with methane yield of 201.6 ± 2.8, 210.8 ± 3.0, 219.8 ± 2.8, 227.6 ± 3.2, and 228.9 ± 1.9 mL per g VS, respectively. This may be because the adsorption and enmeshment of Cd by APAM slowed down the mobility of Cd and thus weakened its toxic effect on microbial cells related to AD, leading to an increase in methane production. However, there was only a slight increase (1.3 mL per g VS, P > 0.05) in methane production at 1.5 mg APAM per g TSS compared with reactors with the addition of 1.2 mg APAM per g TSS, suggesting that adsorption saturation may have occurred at this point [41]. The effectiveness of APAM also can be partially proved by its ability to remove Cd in pure water (rather than a sludge system). The results are presented in Fig. 4b. It was shown the removal rate increased as APAM increased from 8.73 to 43.65 mg L−1. APAM at 43.65 mg L−1 achieved the highest Cd removal rate of 40.2% (P < 0.001). This has also been evidenced in prior studies [[42], [43], [44]]. From the above analysis, it can be indirectly proved the improved performance of the AD system was mainly due to the adsorption/enmeshment of Cd by APAM, although the process was not visualized.
Fig. 4.
Removal of Cd by APAM adsorption/enmeshment to enhance methane production. a, Cumulative methane yield from WAS containing a Cd concentration of 5 mg per g TSS at levels of 0.3, 0.6, 0.9, 1.2, and 1.5 mg APAM per g TSS. b, Cd removal by APAM in pure water. The concentrations of APAM in pure water from low to high correspond to 0.3, 0.6, 0.9, 1.2, and 1.5 mg APAM per g TSS in sludge, respectively. Error bars are the standard deviations of triplicate tests.
In general, there are two major flocculation mechanisms of PAM in sludge (or wastewater): (1) Charge neutralization: PAM dissolves in an aqueous solution and the active functional groups on its long chains (positively and negatively charged) and the colloidal matters (or organic matters and adsorbed metal elements, microorganisms, pathogens, and insect eggs, etc.) combine by electrostatic bonding, causing the particles to aggregate [14,15]; and (2) Adsorption bridging: PAM molecular chains are fixed to the different particles by adsorption and hydrogen bonding, where they form polymeric ‘bridges’ between the individual particulates, making the flocculated particles re-flocculate and settle [45]. It is likely via the above interaction that APAM limits the flow of Cd and retards its negative effect on AD, which is the primary contributor to increased methane production. The immobilization of (heavy) metals by the PAM has also been reported in the literature. For example, cationic PAM was found to be beneficial for chromium (Ⅵ) ion immobilization on the surface of kaolinite [46]. Song et al. [47] also found that PAM reduced the ecotoxicity of bioavailable Cd, Co, and Zn in dredged sediment by 27.49%, 16.10%, and 20.89%, respectively.
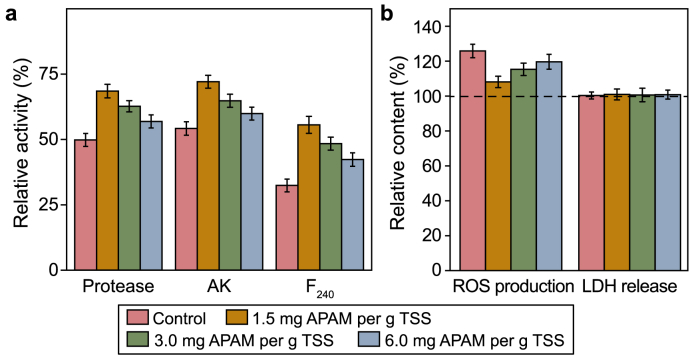
Typically, there are two major toxic effects of heavy metals on microorganisms. First, heavy metals can disrupt enzyme function and structure by binding to thiols and other groups on protein molecules, or by substituting naturally occurring metals in enzyme prosthetic groups [48]. Protease, one of the key hydrolysis enzymes, is responsible for the hydrolysis of protein [49,50]. AK and F420 are the key enzymes relevant to acetic and methane production, respectively [51,52]. Fig. 5a shows the activities of these enzymes in all tested reactors. The presence of Cd can significantly reduce protease, AK, and F420 to 49.8%, 54.2%, and 32.4% (P < 0.001), respectively, while the values of those three picked up to 68.5%, 72.1%, and 55.6%, respectively, with the addition of 1.5 mg APAM per g TSS (P < 0.01), confirming the ability of APAM to diminish the negative effects of heavy metals on microbial enzymes. This trend can also be supported by the soluble protein and SCFAs analysis in Section 3.2 above. The second toxic effect of heavy metals is ROS production induced by themselves and further oxidative damage to the cell membrane or even release of intracellular substances, such as LDH [53,54]. As is shown in Fig. 5b, Cd made ROS content increased by 25.8% compared with blank (the untreated WAS), whereas APAM (1.5, 3.0, and 6.0 mg per g TSS) made the ROS production reduced by 17.7%, 10.5%, and 6.2% compared with control (P < 0.01), respectively, indicating alleviation of APAM for ROS induction. LDH content did not alter dramatically at all tested APAM concentrations (0, 1.5, 3.0, and 6.0 mg per g TSS), implying the addition of Cd and APAM was not sufficient to provoke cell rupture and death (P > 0.05). A similar phenomenon has also been discovered by previous researchers [3]. This also reinforced the fact that the discharge of SCOD with the APAM addition (Section 3.2) was attributed to the disintegration of particulate matter in sludge rather than the overflow of intracellular matters. In fact, Cd itself does not generate ROS directly [55], but rather depletes the cells' major antioxidants (especially those involving thiols), disrupting the inherent oxidation-antioxidation system of microbial cells and enabling ROS to accumulate [56]. The enhanced production of ROS further causes cellular dysfunction and even death (hence the term “oxidative challenge” for the cell) [57]. The adsorption and neutralization of APAM may reduce the Cd bound to the antioxidant and restore the performance of the cellular oxidative-oxidative system, thereby enhancing methane production.
Fig. 5.
Effects of APAM on the activities of key enzymes (a) and ROS/LDH content (b) from AD of WAS containing a Cd concentration of 5 mg per g TSS. Error bars are the standard deviations of triplicate tests.
3.5. Implications to AD of WAS containing heavy metals
Using APAM to alleviate the negative impact of Cd during AD was economically viable on a laboratory basis after calculating the benefits (see Supporting Information). However, further confirmation should be carried out in actual operation. In general, PAM (both cationic PAM and APAM) is mainly applied in the back-end treatment of the wastewater treatment chain (i.e., sludge treatment and disposal). The recommended technical route for sludge treatment and disposal is thickening–anaerobic digestion–dewatering–land use [58]. It is mentioned that the majority of PAM (both cationic PAM and APAM) in WWTPs is applied to the sludge dewatering process after AD [59], and cationic PAM is more effective than APAM. However, the dosage of APAM is one to two times lower than that of cationic PAM, and it is relatively inexpensive, so it should be used as the preferred agent for sludge dewatering [60]. It is advised that the PAM used to regulate the toxicity of heavy metals should be determined based on the actual wastewater treatment situation, such as heavy metals concentration, sludge properties, and wastewater treatment processes. Furthermore, the actual sewage/sludge involves a variety of heavy metals, and the combination of coagulants/flocculants is commonly applied in wastewater treatment plants, where the role of coagulants/flocculants in controlling the toxicity of heavy metals needs further investigation. In addition, although APAM is low-cost and readily available in WWTPs, its degradation metabolites, such as acrylamide, acrylic acid, and polyacrylic acid, also can restrain the AD process of WAS [33]. Therefore, adjusting the dosage of APAM to meet requirements for heavy metal toxicity control is essential.
4. Conclusions
This study uncovered the APAM effect on alleviating Cd toxicity and potential mechanism during AD of WAS, and the main conclusions are as follows:
-
(1)
The addition of APAM promoted methane production from AD of Cd-added WAS. The methane production increased by 14.1% at 1.5 mg APAM per g TSS compared with the control (Cd-added WAS).
-
(2)
The enhanced methane yield was primarily due to the solubilization, hydrolysis, and acidification processes related to AD facilitated by APAM.
-
(3)
The presence of APAM at 1.5 mg per g TSS increased the abundance of hydrolytic microbes, acidogens, and methanogens related to AD.
-
(4)
The supplement of APAM promoted the enzyme activities associated with AD processes and decreased ROS content induced by Cd.
-
(5)
The results of the flocculation test in sludge and pure water provided additional evidence that the attenuated Cd toxicity was primarily attributed to the adsorption/enmeshment for Cd by APAM.
CRediT authorship contribution statement
Baowei Zhang: Conceptualization, Methodology, Writing - Original Draft. Xiang Tang: Writing - Review & Editing. Qiuxiang Xu: Conceptualization. Changzheng Fan: Writing - Review & Editing, Funding acquisition. Yuying Gao: Data Curation. Shuang Li: Investigation. Mier Wang: Investigation. Chao Li: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was financially supported by the project of the National Natural Science Foundation of China (NSFC): 52070075; and the Natural Science Foundation of Hunan Province: 2020JJ4187.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ese.2023.100306.
Contributor Information
Changzheng Fan, Email: fancz@hnu.edu.cn.
Chao Li, Email: harveylee@hnu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Olabarrieta I., L'Azou B., Yuric S., Cambar J., Cajaraville M.P. In vitro effects of cadmium on two different animal cell models. Toxicol. Vitro. 2001;15(4):511–517. doi: 10.1016/s0887-2333(01)00056-x. [DOI] [PubMed] [Google Scholar]
- 2.Luo J., Chen Y., Feng L. Polycyclic aromatic hydrocarbon affects acetic acid production during anaerobic fermentation of waste activated sludge by altering activity and viability of acetogen. Environ. Sci. Technol. 2016;50(13):6921–6929. doi: 10.1021/acs.est.6b00003. [DOI] [PubMed] [Google Scholar]
- 3.Xu Q., Li X., Ding R., Wang D., Liu Y., Wang Q., Zhao J., Chen F., Zeng G., Yang Q., Li H. Understanding and mitigating the toxicity of cadmium to the anaerobic fermentation of waste activated sludge. Water Res. 2017;124:269–279. doi: 10.1016/j.watres.2017.07.067. [DOI] [PubMed] [Google Scholar]
- 4.Luo J., Chen Y., Feng L. Polycyclic aromatic hydrocarbon affects acetic acid production during anaerobic fermentation of waste activated sludge by altering activity and viability of acetogen. Environ. Sci. Technol. 2016;50(13):6921–6929. doi: 10.1021/acs.est.6b00003. [DOI] [PubMed] [Google Scholar]
- 5.Xie Y., Zhang T., Xu C., Hui Y., Ouyang W. Study on variation trends of heavy metals contents in process of sludge energy utilization. Adv. Mater. Res. 2012;610–613:198–202. [Google Scholar]
- 6.Odnell A., Recktenwald M., Stensen K., Jonsson B.H., Karlsson M. Activity, life time and effect of hydrolytic enzymes for enhanced biogas production from sludge anaerobic digestion. Water Res. 2016;103:462–471. doi: 10.1016/j.watres.2016.07.064. [DOI] [PubMed] [Google Scholar]
- 7.Ge H., Jensen P.D., Batstone D.J. Pre-treatment mechanisms during thermophilic–mesophilic temperature phased anaerobic digestion of primary sludge. Water Res. 2010;44(1):123–130. doi: 10.1016/j.watres.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Altas L. Inhibitory effect of heavy metals on methane-producing anaerobic granular sludge. J. Hazard Mater. 2009;162(2–3):1551–1556. doi: 10.1016/j.jhazmat.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 9.Kieu H.T., Muller E., Horn H. Heavy metal removal in anaerobic semi-continuous stirred tank reactors by a consortium of sulfate-reducing bacteria. Water Res. 2011;45(13):3863–3870. doi: 10.1016/j.watres.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y., Lu Y., Dai X., Dai L. Enhancing anaerobic digestion of waste activated sludge by solid–liquid separation via isoelectric point pretreatment. ACS Sustainable Chem. Eng. 2018;6(11):14774–14784. [Google Scholar]
- 11.Chen Y., Cheng J.J., Creamer K.S. Inhibition of anaerobic digestion process: a review. Bioresour. Technol. 2008;99(10):4044–4064. doi: 10.1016/j.biortech.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 12.Sengul A.B., Asmatulu E. Toxicity of metal and metal oxide nanoparticles: a review. Environ. Chem. Lett. 2020;18(5):1659–1683. [Google Scholar]
- 13.Sun J., Yang Q., Wang D., Wang S., Chen F., Zhong Y., Yi K., Yao F., Jiang C., Li S., Li X., Zeng G. Nickel toxicity to the performance and microbial community of enhanced biological phosphorus removal system. Chem. Eng. J. 2017;313:415–423. [Google Scholar]
- 14.Moussas P.A., Zouboulis A.I. A new inorganic–organic composite coagulant, consisting of Polyferric Sulphate (PFS) and Polyacrylamide (PAA) Water Res. 2009;43(14):3511–3524. doi: 10.1016/j.watres.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Aguilar M.I., Sáez J., Lloréns M., Soler A., Ortuño J.F., Meseguer V., Fuentes A. Improvement of coagulation–flocculation process using anionic polyacrylamide as coagulant aid. Chemosphere. 2005;58(1):47–56. doi: 10.1016/j.chemosphere.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W., Xiao P., Liu Y., Xu S., Xiao F., Wang D., Chow C.W.K. Understanding the impact of chemical conditioning with inorganic polymer flocculants on soluble extracellular polymeric substances in relation to the sludge dewaterability. Sep. Purif. Technol. 2014;132:430–437. [Google Scholar]
- 17.Yang R., Li H., Huang M., Yang H., Li A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016;95:59–89. doi: 10.1016/j.watres.2016.02.068. [DOI] [PubMed] [Google Scholar]
- 18.Vaiopoulou E., Gikas P. Effects of chromium on activated sludge and on the performance of wastewater treatment plants: a review. Water Res. 2012;46(3):549–570. doi: 10.1016/j.watres.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B., Tang X., Fan C., Hao W., Zhao Y., Zeng Y. Cationic polyacrylamide alleviated the inhibitory impact of ZnO nanoparticles on anaerobic digestion of waste activated sludge through reducing reactive oxygen species induced. Water Res. 2021;205 doi: 10.1016/j.watres.2021.117651. [DOI] [PubMed] [Google Scholar]
- 20.Wei H., Gao B., Ren J., Li A., Yang H. Coagulation/flocculation in dewatering of sludge: a review. Water Res. 2018;143:608–631. doi: 10.1016/j.watres.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 21.Owen W.F., Stuckey D.C., Healy J.B., Young L.Y., McCarty P.L. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979;13(6):485–492. [Google Scholar]
- 22.Zeng Y., Tang X., Fan C., Tang L., Zhou M., Zhang B., Wang R., Li G. Evaluating the effects of different pretreatments on anaerobic digestion of waste activated sludge containing polystyrene microplastics. ACS ES&T Water. 2022;2(1):117–127. [Google Scholar]
- 23.Fan C., Zhou M., Tang X., Zeng G., Xu Q., Song B., Gong R., Zhang B., Xiong W., Lu Y., Dong H., Ding N., Luo Z., Wang L., Wei J. Triclosan enhances short-chain fatty acid production from sludge fermentation by elevating transcriptional activity of acidogenesis bacteria. Chem. Eng. J. 2020;384 [Google Scholar]
- 24.Rice E.W., Baird R.B., Eaton A.D. American Public Health Association; 2017. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- 25.Smith P.e., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M., Fujimoto E.K., Goeke N.M., Olson B.J., Klenk D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 26.Raunkjær K., Hvitved-Jacobsen T., Nielsen P.H. Measurement of pools of protein, carbohydrate and lipid in domestic wastewater. Water Res. 1994;28(2):251–262. [Google Scholar]
- 27.Abdel-Shafy H.I., Mansour M.S.M. Biogas production as affected by heavy metals in the anaerobic digestion of sludge, Egypt. J. Petrol. 2014;23(4):409–417. [Google Scholar]
- 28.Zhao J., Gui L., Wang Q., Liu Y., Wang D., Ni B., Li X., Xu R., Zeng G., Yang Q. Aged refuse enhances anaerobic digestion of waste activated sludge. Water Res. 2017;123:724–733. doi: 10.1016/j.watres.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Dentel S.K., Gossett J.M. Effect of chemical coagulation on anaerobic digestibility of organic materials. Water Res. 1982;16(5):707–718. [Google Scholar]
- 30.Campos E., Almirall M., Mtnez-Almela J., Palatsi J., Flotats X. Feasibility study of the anaerobic digestion of dewatered pig slurry by means of polyacrylamide. Bioresour. Technol. 2008;99(2):387–395. doi: 10.1016/j.biortech.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Chu C.P., Tsai D.G., Lee D.J., Tay J.H. Size-dependent anaerobic digestion rates of flocculated activated sludge: role of intrafloc mass transfer resistance. J. Environ. Manag. 2005;76(3):239–244. doi: 10.1016/j.jenvman.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Dai X., Luo F., Zhang D., Dai L., Chen Y., Dong B. Waste-activated sludge fermentation for polyacrylamide biodegradation improved by anaerobic hydrolysis and key microorganisms involved in biological polyacrylamide removal. Sci. Rep. 2015;5(1) doi: 10.1038/srep11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D., Liu X., Zeng G., Zhao J., Liu Y., Wang Q., Chen F., Li X., Yang Q. Understanding the impact of cationic polyacrylamide on anaerobic digestion of waste activated sludge. Water Res. 2018;130:281–290. doi: 10.1016/j.watres.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Wang R., Zhang J., Liu J., Yu D., Zhong H., Wang Y., Chen M., Tong J., Wei Y. Effects of chlortetracycline, Cu and their combination on the performance and microbial community dynamics in swine manure anaerobic digestion. J. Environ. Sci. 2018;67:206–215. doi: 10.1016/j.jes.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q., Ye L., Jiang G., Jensen P.D., Batstone D.J., Yuan Z. Free nitrous acid (FNA)-based pretreatment enhances methane production from waste activated sludge. Environ. Sci. Technol. 2013;47(20):11897–11904. doi: 10.1021/es402933b. [DOI] [PubMed] [Google Scholar]
- 36.Tang X., Zhang B., Fan C., Zeng G., Xiong W., Zhou C., Yang Y., Ren X., Li X., Luo K. The presence of cationic polyacrylamide attenuated the toxicity of polyvinyl chloride microplastics to anaerobic digestion of waste activated sludge. Chem. Eng. J. 2022;427 [Google Scholar]
- 37.Mu H., Chen Y. Long-term effect of ZnO nanoparticles on waste activated sludge anaerobic digestion. Water Res. 2011;45(17):5612–5620. doi: 10.1016/j.watres.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Q., Li X., Lin Y., Yang C., Tang W., Wu S., Li D., Lou W. Effects of copper ions on removal of nutrients from swine wastewater and on release of dissolved organic matter in duckweed systems. Water Res. 2019;158:171–181. doi: 10.1016/j.watres.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 39.Ariesyady H.D., Ito T., Okabe S. Functional bacterial and archaeal community structures of major trophic groups in a full-scale anaerobic sludge digester. Water Res. 2007;41(7):1554–1568. doi: 10.1016/j.watres.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 40.Nelson M.C., Morrison M., Yu Z. A meta-analysis of the microbial diversity observed in anaerobic digesters. Bioresour. Technol. 2011;102(4):3730–3739. doi: 10.1016/j.biortech.2010.11.119. [DOI] [PubMed] [Google Scholar]
- 41.Kulal P., Badalamoole V. Efficient removal of dyes and heavy metal ions from waste water using Gum ghatti – graft – poly (4-acryloylmorpholine) hydrogel incorporated with magnetite nanoparticles. J. Environ. Chem. Eng. 2020;8(5) [Google Scholar]
- 42.López-Maldonado E.A., Oropeza-Guzman M.T., Jurado-Baizaval J.L., Ochoa-Terán A. Coagulation–flocculation mechanisms in wastewater treatment plants through zeta potential measurements. J. Hazard Mater. 2014;279:1–10. doi: 10.1016/j.jhazmat.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 43.Xu D., Zhou B., Yuan R. Optimization of coagulation-flocculation treatment of wastewater containing Zn(II) and Cr(VI) IOP Conf. Ser. Earth Environ. Sci. 2019;227 [Google Scholar]
- 44.Nyström F., Nordqvist K., Herrmann I., Hedström A., Viklander M. Removal of metals and hydrocarbons from stormwater using coagulation and flocculation. Water Res. 2020;182 doi: 10.1016/j.watres.2020.115919. [DOI] [PubMed] [Google Scholar]
- 45.Wei W., Li A., Yang J., Ma F., Wu D., Xing J., Zhou X., Zhao D. Synergetic effects and flocculation behavior of anionic polyacrylamide and extracellular polymeric substrates extracted from Klebsiella sp. J1 on improving soluble cadmium removal. Bioresour. Technol. 2015;175:34–41. doi: 10.1016/j.biortech.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 46.Szewczuk-Karpisz K., Fijałkowska G., Wiśniewska M., Wójcik G. Chromium(VI) reduction and accumulation on the kaolinite surface in the presence of cationic soil flocculant. J. Soils Sediments. 2020;20(10):3688–3697. [Google Scholar]
- 47.Song Z., Gao H., Zhang W., Wang D. Influence of flocculation conditioning on environmental risk of heavy metals in dredged sediment. J. Environ. Manag. 2021;297 doi: 10.1016/j.jenvman.2021.113313. [DOI] [PubMed] [Google Scholar]
- 48.Vallee B.L., Ulmer D.D. Biochemical effects of mercury, cadmium, and lead. Annu. Rev. Biochem. 1972;41:91–128. doi: 10.1146/annurev.bi.41.070172.000515. [DOI] [PubMed] [Google Scholar]
- 49.Feng L., Chen Y., Zheng X. Enhancement of waste activated sludge protein conversion and volatile fatty acids accumulation during waste activated sludge anaerobic fermentation by carbohydrate substrate addition: the effect of pH. Environ. Sci. Technol. 2009;43(12):4373–4380. doi: 10.1021/es8037142. [DOI] [PubMed] [Google Scholar]
- 50.Goel R., Mino T., Satoh H., Matsuo T. Enzyme activities under anaerobic and aerobic conditions in activated sludge sequencing batch reactor. Water Res. 1998;32(7):2081–2088. [Google Scholar]
- 51.Zhao J., Zhang C., Wang D., Li X., An H., Xie T., Chen F., Xu Q., Sun Y., Zeng G., Yang Q. Revealing the underlying mechanisms of how sodium chloride affects short-chain fatty acid production from the cofermentation of waste activated sludge and food waste. ACS Sustainable Chem. Eng. 2016;4(9):4675–4684. [Google Scholar]
- 52.Zayed G., Winter J. Inhibition of methane production from whey by heavy metals – protective effect of sulfide. Appl. Microbiol. Biotechnol. 2000;53(6):726–731. doi: 10.1007/s002530000336. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y., Wang D., Zhu X., Zheng X., Feng L. Long-term effects of copper nanoparticles on wastewater biological nutrient removal and N2O generation in the activated sludge process. Environ. Sci. Technol. 2012;46(22):12452–12458. doi: 10.1021/es302646q. [DOI] [PubMed] [Google Scholar]
- 54.Xia T., Kovochich M., Liong M., Mädler L., Gilbert B., Shi H., Yeh J.I., Zink J.I., Nel A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2(10):2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valko M., Morris H., Cronin M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 56.Ercal N., Gurer-Orhan H., Aykin-Burns N. Toxic metals and oxidative stress part I mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 57.Jozefczak M., Remans T., Vangronsveld J., Cuypers A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012;13(3):3145–3175. doi: 10.3390/ijms13033145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang G., Zhang G., Wang H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015;78:60–73. doi: 10.1016/j.watres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Cydzik-Kwiatkowska A., Nosek D., Wojnowska-Baryła I., Mikulski A. Efficient dewatering of polymer-rich aerobic granular sludge with cationic polymer containing hydrocarbons. Int. J. Environ. Sci. Technol. 2019;17(1):361–370. [Google Scholar]
- 60.Ma J., Zheng H., Tan M., Liu L., Chen W., Guan Q., Zheng X. Synthesis, characterization, and flocculation performance of anionic polyacrylamide P (AM-AA-AMPS) J. Appl. Polym. Sci. 2013;129(4):1984–1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.