Abstract
Background & Aims
The vast majority of studies evaluating differences in on-treatment risks of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B (CHB) have been conducted in Asia. Data on the course of CHB on antiviral therapy among predominantly non-Asian populations is less well described. We aimed to evaluate overall risks of cirrhosis and HCC and the influence of baseline factors on this risk among a predominantly non-Asian cohort of patients with CHB in the US.
Methods
Using longitudinal data from the national Veterans Affairs database, we evaluated the incidence of cirrhosis or HCC among adults with non-cirrhotic CHB on continuous antiviral therapy. Cumulative incidence functions and adjusted Cox proportional hazards models employed competing risks methods and evaluated overall risk and predictors of developing cirrhosis or HCC while on treatment.
Results
Among 2,496 patients with non-cirrhotic CHB (39.1% African American, 38.4% non-Hispanic White, 18.8% Asian, mean age 58.0 ± 13.4 years), the overall incidences of cirrhosis and HCC were 3.99 per 100 person-years (95% CI 3.66-4.35) and 0.43 per 100 person-years (95% CI 0.33-0.54), respectively. The highest incidences of cirrhosis and HCC were observed in non-Hispanic White patients (5.74 and 0.52 per 100 person-years, respectively), which were significantly higher than in Asian patients (1.93 and 0.17 per 100 person-years, respectively, p <0.0001). On multivariate regression, only baseline FIB-4 score was consistently associated with long-term risk of cirrhosis or HCC.
Conclusions
Using a longitudinal cohort of predominantly non-Asian Veterans with non-cirrhotic CHB on antiviral therapy (an understudied population), we provide important epidemiological data to describe long-term risks of cirrhosis and HCC.
Impact and implications
In one of the largest studies to date of a predominantly non-Asian cohort of patients with non-cirrhotic chronic hepatitis B, we provide important epidemiological data describing the long-term risks of cirrhosis and hepatocellular carcinoma among patients on antiviral therapies. Among this understudied population, the overall incidence of cirrhosis was 3.99 per 100-person-years (95% CI 3.66-4.35) and of HCC was 0.43 per 100-person-years (95% CI 0.33-0.54). These data also emphasize the importance of continued monitoring and HCC surveillance among CHB patients who are maintained on antiviral therapies.
Keywords: HBV, cirrhosis, hepatocellular carcinoma, veterans, antivirals
Graphical abstract
Highlights
-
•
We studied 2,496 patients with non-cirrhotic CHB (39.1% African American, 38.4% non-Hispanic White, 18.8% Asian) among a national cohort of US Veterans.
-
•
Overall incidences of cirrhosis and HCC were 3.99 per 100 person-years and 0.43 per 100 person-years, respectively.
-
•
The incidences of cirrhosis and HCC were highest in non-Hispanic white patients with CHB and lowest in Asian patients with CHB.
Introduction
Chronic hepatitis B (CHB) remains a leading cause of liver-related morbidity and mortality worldwide, with nearly 300 million individuals affected globally.1,2 Existing studies have reported on disparities in the CHB cascade of care, with gaps in timely diagnosis and linkage to care, as well as delays in timely initiation of antiviral therapy. In 2016, it was estimated that approximately 10% of all individuals with CHB worldwide were diagnosed, and only 5% of individuals with CHB eligible for treatment actually received antiviral therapy.2 In the US, the prevalence of CHB may be as high as 2.4 million, and similar disparities in the CHB cascade of care have been reported.[3], [4], [5], [6], [7]
Antiviral therapy for CHB is associated with reduced risks of progression to cirrhosis and HCC.[8], [9], [10] However, the overall disease course in patients with CHB on antiviral therapy, particularly among a predominantly non-Asian cohort, is not well described. In particular, it is not clear whether there exist differences in on-treatment risks of cirrhosis or HCC based on baseline factors. Recent data have explored differences in long-term risk of HCC between different types of antiviral therapy with mixed conclusions.[11], [12], [13], [14], [15] Additional studies have also implicated concurrent diabetes, obesity, and alcohol use in increasing the risk of HCC among individuals with CHB.[16], [17], [18] However, the vast majority of these studies were from Asia and have mostly focused on long-term HCC risk. Data among predominantly non-Asian populations, and data evaluating baseline factors affecting risk of cirrhosis are scarce. The current study utilizes a large longitudinal cohort of predominantly non-Asian US Veterans with CHB to evaluate on-treatment risks of cirrhosis and HCC as well as the influence of baseline factors on the risk of cirrhosis or HCC while on antiviral therapy.
Patients and methods
We utilized data from the 2010-2022 Veterans Affairs (VA) Corporate Data Warehouse (CDW), which is a US national database that captures data on all Veterans utilizing healthcare services among VA facilities across the US. The VA health system is the largest integrated health system in the US, caring for more than 9 million individuals. The VA CDW allows for longitudinal assessment of patient outcomes, captures important demographic data, comorbidities, and risk behaviors such as alcohol use, incorporating longitudinal laboratory data in addition to clinical encounters, and clinical outcomes for data analyses.
Adults with CHB were identified by at least two positive results for hepatitis B surface antigen (HBsAg), hepatitis B virus (HBV) DNA, or hepatitis B e antigen (HBeAg) at least 6 months apart, or at least one positive result for HBsAg, HBV DNA, or HBeAg, and one ICD-9/10 code for chronic HBV. Patients with CHB were excluded if there was evidence of concurrent HIV, hepatitis C, or hepatitis delta infections. The current study focused on differences in on-treatment risks of cirrhosis or HCC; thus, we retrospectively evaluated each patient’s electronic medical records to determine the first start date of CHB antiviral treatment documented in the medical record, which was set as the index date for assessing outcomes in the follow-up period. Comprehensive review of pharmacy data ensured continuous antiviral therapy prescription, and patients were followed until the last date of antiviral therapy prescribed, development of study outcomes (e.g. cirrhosis, HCC), death, or until the end of the study period. Patients with cirrhosis or HCC at index date or within 12 months of starting antiviral therapy were excluded to ensure that incident events were captured. Cirrhosis and HCC were identified using established definitions based on ICD-9/10 codes.19 Race/ethnicity in the VA CDW was based on self-reporting and included non-Hispanic White (NHW), black or African American, Asian or Pacific Islander (API), Hispanic, and American Indian or Alaska Native. American Indian or Alaska Natives were excluded from our analyses given the small sample size (n = 6). Alcohol use was assessed based on documented AUDIT-C scores20 closest to the time of first start of antiviral therapy and were categorized as 1) no alcohol use (AUDIT-C = 0), 2) mild levels of alcohol use (AUDIT-C 1-2 for women and 1-3 for men), and 3) moderate-high levels of alcohol use (AUDIT-C ≥3 for women and ≥4 for men). Baseline fibrosis-4 (FIB-4) scores at index date were calculated and grouped into three categories based on established criteria: FIB-4 <1.45, FIB-4 1.45–3.25, and FIB-4 >3.25.21 HBV DNA levels were evaluated at baseline prior to start of antiviral therapy and longitudinal repeated measures of HBV DNA on treatment were evaluated. As a surrogate for adherence to treatment, we determined whether patients were able to achieve HBV DNA <2,000 IU/ml, and outcomes were stratified by patients who achieved HBV DNA <2,000 IU/ml vs. those who did not.
Baseline demographics and disease characteristics are presented as frequencies and proportions for categorical variables, and mean and SD or median and IQR for continuous variables. Incidence of cirrhosis or HCC was presented as incidence per 100 person-years. Comparisons of univariate unadjusted outcomes were compared between groups using chi-square testing as well as the z-statistic using standard equations. The overall incidence of cirrhosis or HCC was also evaluated using the Nelson-Aalen methods for estimating cumulative hazards rate, and competing risks methods were applied with death as a censoring event. Comparisons of cumulative incidence curves between groups utilized log-rank testing. Multivariable competing risks Cox proportional hazards models were utilized to evaluate for independent predictors of cirrhosis or HCC. Variables that demonstrated significance in the univariate model (p < 0.10) or those with biological significance determined a priori were selected for inclusion in the final multivariate model. Statistical analyses were performed using SQL and SAS® Studio 3.6 on SAS® 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical significance was defined as a two-tailed p value <0.05. This study was approved by the Stanford University Institutional Review Board and the VA Palo Alto Healthcare System Research and Development Committee. All research was conducted in accordance with both the Declarations of Helsinki and Istanbul. A waiver of informed consent was granted by the aforementioned institutional review board.
Results
A total of 2,496 patients with non-cirrhotic CHB were identified (median follow-up of 11,856 person-years, IQR 6,032–26,023), among whom 94.4% were male, 39.1% were African American, 38.4% were NHW, 18.8% were API, and 3.5% were Hispanic (Table 1). Mean age was 58.0 ± 13.4 years and mean BMI was 27.8 ± 5.6 kg/m2. Mean baseline HBV DNA prior to the start of antiviral therapy was 1.52 × 108 IU/ml (±2.66 × 108 IU/ml SD). Overall, 28.6% had concurrent diabetes mellitus and 62.1% had concurrent hypertension. When assessing alcohol use based on self-reported AUDIT-C scores, 46.0% reported mild alcohol use and 16.6% reported moderate-heavy alcohol use. Type of CHB antiviral therapy utilized included entecavir in 59.7%, tenofovir disoproxil fumarate in 32.7%, and tenofovir alafenamide in 7.6%. At the time of antiviral therapy initiation, 44.3%, 36.0% and 19.7% had FIB-4 scores <1.45, 1.45-3.25, and >3.25, respectively.
Table 1.
Characteristics of the study cohort.
| Variables | Proportion (%) | Frequency (N) |
|---|---|---|
| Total | 100 | 2,496 |
| Antiviral treatment | ||
| Entecavir | 59.70 | 1,490 |
| Tenofovir alafenamide | 7.61 | 190 |
| Tenofovir disoproxil fumarate | 32.69 | 816 |
| Sex | ||
| Female | 5.57 | 139 |
| Male | 94.43 | 2,357 |
| Race/ethnicity | ||
| Asian or Pacific Islander | 18.78 | 450 |
| Black or African American | 39.11 | 937 |
| Hispanic | 3.51 | 84 |
| Non-Hispanic White | 38.36 | 919 |
| Age (mean ± SD) | ||
| 18-39 years | (58.00 ± 13.40) | |
| 40-59 years | 12.86 | 321 |
| 60 years and over | 42.03 | 1,049 |
| BMI (mean ± SD kg/m2) | (27.77 ± 5.64) | |
| 18.0–24.9 | 27.98 | 672 |
| 25.0–29.9 | 36.97 | 888 |
| 30.0–34.9 | 22.52 | 541 |
| 35.0 and over | 12.53 | 301 |
| Comorbidities | ||
| Diabetes mellitus | 28.57 | 713 |
| Hypertension | 62.14 | 1,551 |
| Alcohol use | ||
| No alcohol | 37.48 | 808 |
| Mild alcohol | 45.96 | 991 |
| Moderate to heavy alcohol | 16.56 | 357 |
| Tobacco use | ||
| Never | 39.27 | 792 |
| Past history of tobacco | 22.76 | 459 |
| Active current tobacco | 37.98 | 766 |
| FIB-4 categories | ||
| FIB-4 Score <1.45 | 44.32 | 994 |
| FIB-4 Score 1.45-3.25 | 36.02 | 808 |
| FIB-4 Score >3.25 | 19.66 | 441 |
Alcohol use categories based on AUDIT-C scores - No alcohol (AUDIT-C = 0), mild alcohol use (AUDIT-C 1-2 for women and 1-3 for men), and moderate-heavy (AUDIT-C >3 for women and >4 for men). FIB-4, fibrosis-4.
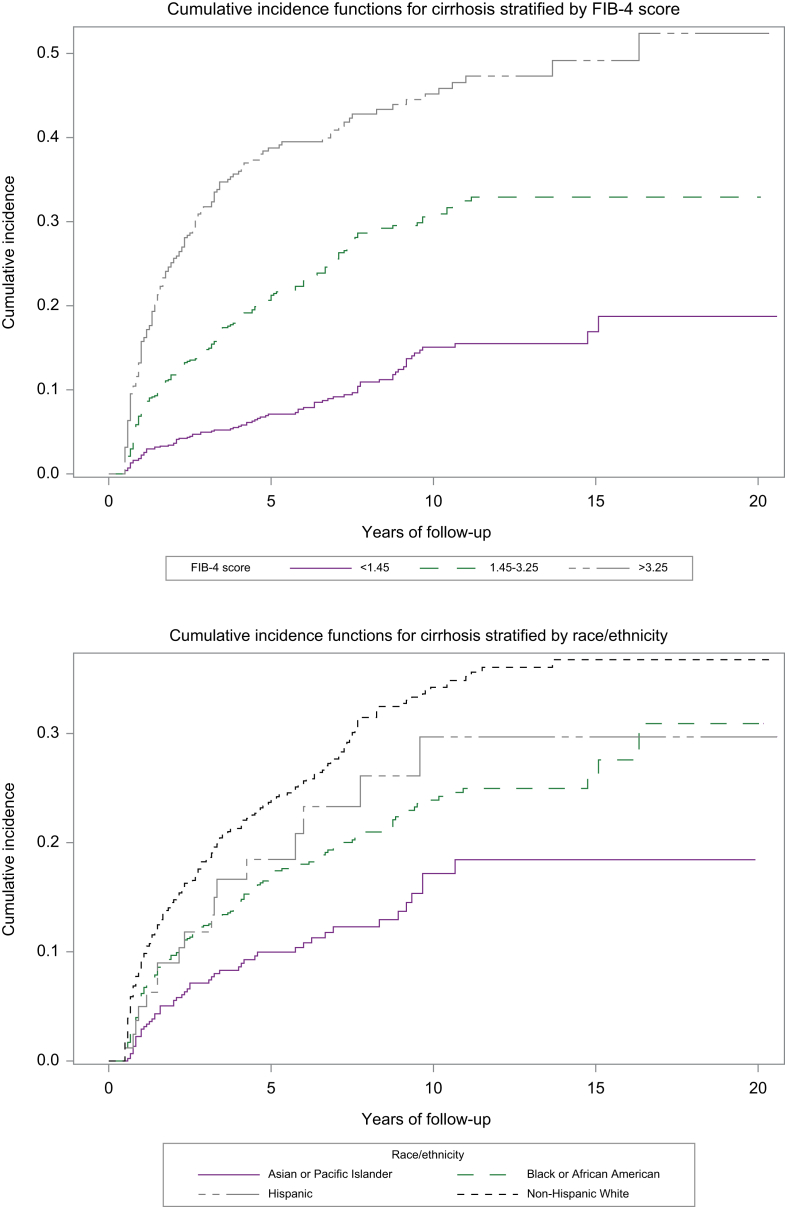
The overall incidence of cirrhosis was 3.99 per 100-person-years (95% CI 3.66-4.35) (Table 2). No significant difference in risk of cirrhosis by antiviral treatment type was observed. We performed sub-analyses to evaluate whether treatment-specific differences in risk of cirrhosis were observed in Asians and separately in non-Asians. Among both Asians and non-Asians, there was no significant difference in risk of cirrhosis based on type of antiviral therapy. Compared to females, males had a significantly higher incidence of cirrhosis (4.19 vs. 0.87 per 100 person-years, p < 0.0001). Patients who were HBeAg positive at index date had a higher incidence of cirrhosis than those who were HBeAg negative (4.93 vs. 3.32 per 100 person-years, p = 0.0001). No significant difference in cirrhosis was observed between patients who had HBV DNA <2,000 IU/ml vs. those who had HBV DNA ≥2,000 IU/ml during follow-up. When stratified by race/ethnicity, the highest incidence of cirrhosis was observed in the NHW group (5.74 per 100 person-years), which was significantly higher than the incidence seen in the API group (1.93 per 100 person-years, p < 0.0001) (Fig. 1). Increasing age was associated with increasing incidence of cirrhosis and there was a trend towards increasing cirrhosis incidence associated with increasing BMI (Table 2). A significantly higher incidence of cirrhosis was observed in patients with CHB and concurrent diabetes (5.61 vs. 3.46 per 100 person-years in those without diabetes, p < 0.0001) and those with concurrent hypertension (4.86 vs. 2.76 per 100 person-years in those without hypertension, p < 0.0001). Active tobacco use was associated with a higher incidence of cirrhosis, but active alcohol use was not. Compared to patients with FIB-4 <1.45, those with FIB-4 >3.25 had a significantly higher incidence of cirrhosis (11.00 vs. 1.58 per 100 person-years, p < 0.0001) (Fig. 1). On multivariate competing risks Cox regression analysis, a significantly higher risk of cirrhosis was observed in males vs. females (hazard ratio [HR] 8.58, 95% CI 1.19-61.60, p = 0.03) (Table 3). There was a trend towards lower risk of cirrhosis in patients who serocleared to HBsAg negative (HR 0.59, 95% CI 0.32-1.07, p = 0.08). Compared to the API group, there was a trend towards higher risk of cirrhosis among NHW patients (HR 1.65, 95% CI 0.97-2.83, p = 0.07). There was also a trend towards higher risk of cirrhosis in patients with diabetes vs. those without diabetes (HR 1.32, 95% CI 0.96-1.80, p = 0.08). Compared to patients with CHB reporting no history of tobacco use, those with active tobacco use had significantly higher risk of cirrhosis (HR 1.41, 95% CI 1.02-1.93, p = 0.04). Compared to patients with CHB and FIB-4 <1.45 at baseline, those with FIB-4 >3.25 had a significantly higher risk of cirrhosis (HR 5.55, 95% CI 3.66-8.41, p < 0.0001).
Table 2.
Incidence of cirrhosis or HCC among patients with chronic HBV presented as incidence per 100 person-years.
| Incidence of cirrhosis per 100 person-years | 95% CI | p value | Incidence of HCC per 100 person-years | 95% CI | p value | |
|---|---|---|---|---|---|---|
| Total | 3.99 | 3.66, 4.35 | 0.43 | 0.33, 0.54 | ||
| Antiviral treatment | 0.3784 | 0.0262 | ||||
| Entecavir | 4.09 | 3.66, 4.57 | 0.50 | 0.37, 0.67 | ||
| Tenofovir alafenamide | 2.93 | 1.79, 4.78 | 0.17 | 0.02, 1.23 | ||
| Tenofovir disoproxil fumarate | 3.94 | 3.41, 4.55 | 0.33 | 0.21, 0.52 | ||
| HBeAg status | 0.0001 | 0.9338 | ||||
| HBeAg negative | 3.32 | 2.84, 3.89 | 0.47 | 0.32, 0.70 | ||
| HBeAg positive | 4.93 | 4.28, 5.67 | 0.47 | 0.31, 0.71 | ||
| HBsAg during follow-up | 0.2202 | 0.6218 | ||||
| HBsAg negative | 3.73 | 3.31, 4.19 | 0.39 | 0.27, 0.55 | ||
| HBsAg positive | 4.27 | 3.14, 5.79 | 0.36 | 0.13, 0.95 | ||
| HBV DNA during follow-up | 0.8143 | 0.8264 | ||||
| HBV DNA <2,000 IU/ml | 3.96 | 3.60, 4.36 | 0.31 | 0.10, 0.96 | ||
| HBV DNA ≥2,000 IU/ml | 4.40 | 3.20, 6.05 | 0.43 | 0.33, 0.56 | ||
| Sex | <0.0001 | 0.087 | ||||
| Female | 0.87 | 0.42, 1.83 | 0.12 | 0.02, 0.85 | ||
| Male | 4.19 | 3.84, 4.58 | 0.44 | 0.35, 0.57 | ||
| Race/ethnicity | <0.0001 | <0.0001 | ||||
| Asian or Pacific Islander | 1.93 | 1.47, 2.54 | 0.17 | 0.07, 0.42 | ||
| Black or African American | 3.51 | 3.02, 4.07 | 0.45 | 0.31, 0.67 | ||
| Hispanic | 4.02 | 2.50, 6.46 | 0.39 | 0.10, 1.58 | ||
| Non-Hispanic White | 5.74 | 5.07, 6.49 | 0.52 | 0.36, 0.76 | ||
| Age | <0.0001 | 0.0165 | ||||
| 18-39 years | 1.02 | 0.67, 1.55 | 0.13 | 0.04, 0.41 | ||
| 40-59 years | 3.77 | 3.30, 4.30 | 0.29 | 0.19, 0.45 | ||
| 60 years and over | 5.57 | 4.95, 6.28 | 0.70 | 0.51, 0.95 | ||
| BMI categories | 0.0539 | 0.7546 | ||||
| 18.0–24.9 | 3.61 | 3.04, 4.30 | 0.32 | 0.19, 0.56 | ||
| 25.0–29.9 | 3.83 | 3.30, 4.44 | 0.50 | 0.34, 0.73 | ||
| 30.0–34.9 | 3.98 | 3.30, 4.79 | 0.43 | 0.26, 0.73 | ||
| 35.0 and over | 5.34 | 4.28, 6.67 | 0.28 | 0.12, 0.68 | ||
| Comorbidities | ||||||
| No diabetes | 3.46 | 3.10, 3.85 | <0.0001 | 0.40 | 0.30, 0.53 | 0.3904 |
| Yes diabetes | 5.61 | 4.84, 6.50 | 0.51 | 0.32, 0.80 | ||
| No hypertension | 2.76 | 2.35, 3.25 | <0.0001 | 0.45 | 0.31, 0.66 | 0.5906 |
| Yes hypertension | 4.86 | 4.39, 5.39 | 0.41 | 0.29, 0.57 | ||
| Alcohol use categories | 0.0179 | 0.9692 | ||||
| No alcohol | 5.06 | 4.43, 5.77 | 0.40 | 0.26, 0.61 | ||
| Mild alcohol | 3.82 | 3.24, 4.50 | 0.35 | 0.21, 0.59 | ||
| Moderate to heavy alcohol | 3.90 | 3.04, 4.99 | 0.66 | 0.38, 1.17 | ||
| Tobacco use categories | <0.0001 | 0.5068 | ||||
| Never | 3.06 | 2.58, 3.64 | 0.30 | 0.18, 0.50 | ||
| Past history of tobacco | 3.90 | 3.17, 4.79 | 0.52 | 0.31, 0.89 | ||
| Active current tobacco | 5.41 | 4.69, 6.25 | 0.65 | 0.45, 0.95 | ||
| FIB-4 categories | <0.0001 | <0.0001 | ||||
| FIB-4 score <1.45 | 1.58 | 1.29, 1.93 | 0.14 | 0.07, 0.28 | ||
| FIB-4 score 1.45-3.25 | 4.76 | 4.13, 5.49 | 0.35 | 0.22, 0.57 | ||
| FIB-4 score >3.25 | 11.00 | 9.47, 12.78 | 1.60 | 1.14, 2.24 | ||
Alcohol use categories based on AUDIT-C scores - No alcohol (AUDIT-C = 0), mild alcohol use (AUDIT-C 1-2 for women and 1-3 for men), and moderate-heavy (AUDIT-C >3 for women and >4 for men). Comparisons between groups utilized chi-square testing and the z-statistic, and p <0.05 was used to determine significant differences. FIB-4, fibrosis-4; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma.
Fig. 1.
Incidence of cirrhosis stratified by FIB-4 score and by race/ethnicity.
FIB-4, fibrosis-4.
Table 3.
Multivariable competing risks Cox proportional hazards regression evaluating risk of cirrhosis.
| HR | 95% CI | p value | |
|---|---|---|---|
| HBeAg status | |||
| HBeAg negative | Reference | ||
| HBeAg positive | 0.91 | (0.69, 1.21) | 0.5228 |
| HBsAg during follow-up | |||
| HBsAg positive | Reference | ||
| HBsAg negative | 0.59 | (0.32, 1.07) | 0.0831 |
| HBV DNA during follow-up | |||
| HBV DNA <2,000 IU/ml | Reference | ||
| HBV DNA ≥2,000 IU/ml | 1.13 | (0.71, 1.80) | 0.6066 |
| Sex | |||
| Female | Reference | ||
| Male | 8.58 | (1.19, 61.60) | 0.0326 |
| Race/ethnicity | |||
| Asian or Pacific Islander | Reference | ||
| Black or African American | 1.12 | (0.65, 1.92) | 0.6824 |
| Hispanic | 1.71 | (0.4, 3.97) | 0.2136 |
| Non-Hispanic White | 1.65 | (0.97, 2.83) | 0.0663 |
| Age | |||
| 18-39 years | Reference | ||
| 40-59 years | 1.26 | (0.64, 2.51) | 0.5016 |
| 60 years and over | 1.13 | (0.55, 2.36) | 0.7367 |
| BMI categories | |||
| 18.0–24.9 | Reference | ||
| 25.0–29.9 | 0.99 | (0.69, 1.43) | 0.9714 |
| 30.0–34.9 | 1.14 | (0.77, 1.70) | 0.5120 |
| 35.0 and over | 1.43 | (0.93, 2.20) | 0.1024 |
| Comorbidities | |||
| No diabetes | Reference | ||
| Yes diabetes | 1.32 | (0.96, 1.80) | 0.0854 |
| No hypertension | Reference | ||
| Yes hypertension | 1.21 | (0.85, 1.73) | 0.2891 |
| Alcohol use categories | |||
| No alcohol | Reference | ||
| Mild alcohol | 0.83 | (0.61, 1.12) | 0.2266 |
| Moderate to heavy alcohol | 0.79 | (0.54, 1.16) | 0.2330 |
| Tobacco use categories | |||
| Never | Reference | ||
| Past history of tobacco | 1.12 | (0.78, 1.61) | 0.5500 |
| Active current tobacco | 1.41 | (1.02, 1.93) | 0.0352 |
| FIB-4 categories | |||
| FIB-4 score <1.45 | Reference | ||
| FIB-4 score 1.45-3.25 | 2.61 | (1.77, 3.87) | <0.0001 |
| FIB-4 score >3.25 | 5.55 | (3.66, 8.41) | <0.0001 |
Multivariable competing risks Cox proportional hazards regression model was used to evaluate for predictors of cirrhosis risk. Multivariable model adjusted for HBeAg status, age, sex, race/ethnicity, BMI, diabetes, hypertension, alcohol use, tobacco use, and FIB-4 score. P < 0.05 was used to evaluate significant differences between groups. FIB-4, fibrosis-4; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma.
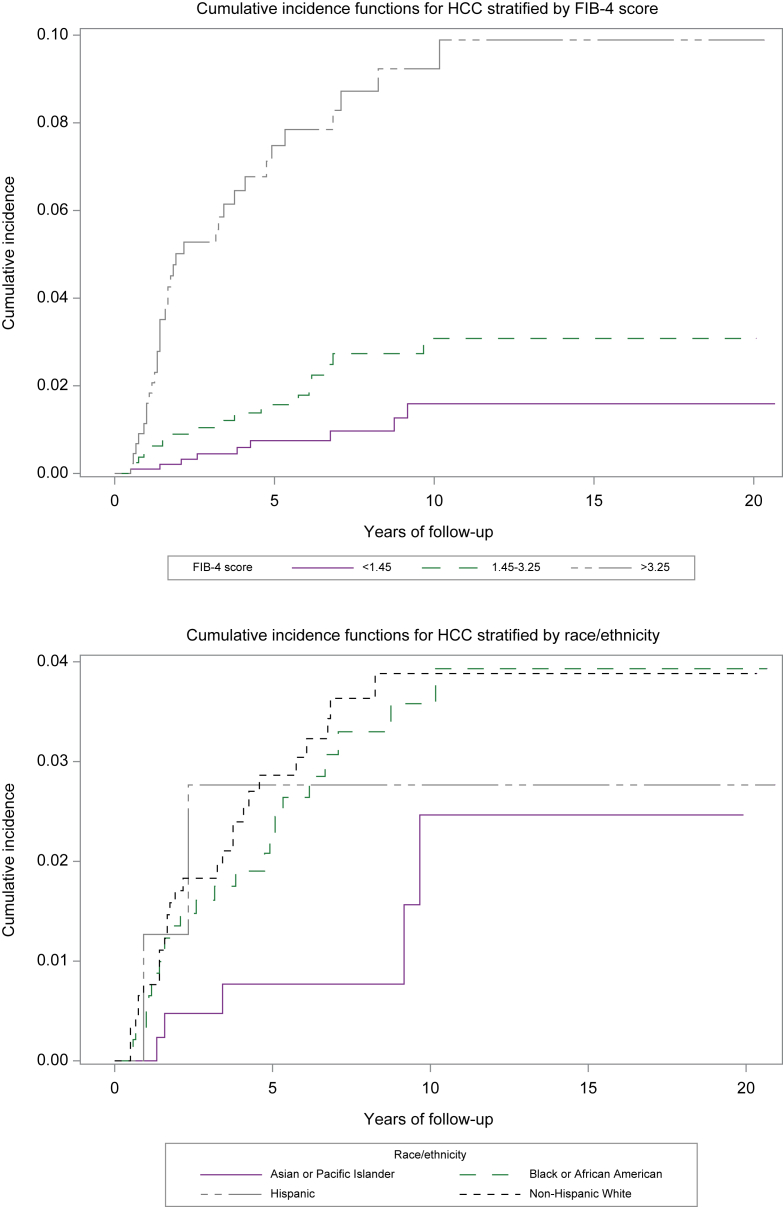
The overall incidence of HCC was 0.43 per 100 person-years (95% CI 0.33-0.54) (Table 2). Compared to patients on entecavir at baseline, lower incidence of HCC was observed in those who were treated with tenofovir alafenamide or those treated with tenofovir disoproxil fumarate. We performed sub-analyses to evaluate whether treatment-specific differences in risk of HCC were observed in Asians and separately in non-Asians. In Asians, HCC risk was similar between patients treated with entecavir vs. tenofovir disoproxil fumarate (0.17 vs. 0.21 per 100-person-years, p = 0.91). Similarly, among non-Asians, risk of HCC was lower in patients treated with tenofovir disoproxil fumarate or tenofovir alafenamide when compared to entecavir, but these differences did not reach statistical significance (0.23 in tenofovir alafenamide vs. 0.58 in entecavir per 100 person-years, p = 0.62; 0.35 in tenofovir disoproxil fumarate vs. 0.58 in entecavir per 100 person-years, p = 0.15). No significant difference in cirrhosis was observed between patients who had HBV DNA <2,000 IU/ml vs. those who had HBV DNA ≥2,000 IU/ml during follow-up. When stratified by race/ethnicity, the highest incidence of HCC was observed in NHW patients (0.52 per 100-person-years), which was significantly higher than the incidence of HCC observed in the API group (0.17 per 100 person-years) (Fig. 2). Increasing age was associated with higher incidence of cirrhosis. Compared to patients with FIB-4 <1.45, those with FIB-4 >3.25 had a significantly higher incidence of HCC (1.60 vs. 0.14 per 100 person-years, p < 0.0001) (Fig. 2). On multivariate competing risks Cox regression, no significant differences were observed in risk of HCC by type of antiviral therapy, sex, race/ethnicity, or whether patients achieved HBsAg seroclearance (Table 4). Compared to patients with baseline FIB-4 <1.45, a significantly higher risk of HCC was observed in those with FIB-4 >3.25 (HR 7.80, 95% CI 3.44-17.67, p < 0.0001).
Fig. 2.
Incidence of HCC stratified by FIB-4 score and by race/ethnicity.
FIB-4, fibrosis-4; HCC, hepatocellular carcinoma.
Table 4.
Multivariable competing risks Cox proportional hazards regression evaluating risk of HCC.
| HR | 95% CI | p value | |
|---|---|---|---|
| Antiviral treatment | |||
| Entecavir | Reference | ||
| Tenofovir alafenamide | 0.35 | (0.05, 2.62) | 0.3088 |
| Tenofovir disoproxil fumarate | 0.68 | (0.38, 1.22) | 0.1934 |
| HBsAg during follow-up | |||
| HBsAg positive | Reference | ||
| HBsAg negative | 0.79 | (0.27, 2.52) | 0.6527 |
| HBV DNA during follow-up | |||
| HBV DNA <2,000 IU/ml | Reference | ||
| HBV DNA ≥2,000 IU/ml | 1.34 | (0.56, 1.59) | 0.5100 |
| Sex | |||
| Female | Reference | ||
| Male | 1.71 | (0.23, 12.68) | 0.6015 |
| Race/ethnicity | |||
| Asian or Pacific Islander | Reference | ||
| Black or African American | 1.44 | (0.51, 4.05) | 0.4899 |
| Hispanic | 1.32 | (0.24, 7.16) | 0.7459 |
| Non-Hispanic White | 1.24 | (0.44, 3.55) | 0.6840 |
| Age | |||
| 18-39 years | Reference | ||
| 40-59 years | 1.12 | (0.30, 4.09) | 0.8694 |
| 60 years and over | 1.63 | (0.44, 6.12) | 0.4699 |
| FIB-4 categories | |||
| FIB-4 score <1.45 | Reference | ||
| FIB-4 score 1.45-3.25 | 1.88 | (0.78, 4.51) | 0.1587 |
| FIB-4 score >3.25 | 7.80 | (3.44, 17.67) | <0.0001 |
Multivariable competing risks Cox proportional hazards regression model was used to evaluate for predictors of HCC risk. Multivariable model adjusted for antiviral therapy, age, sex, race/ethnicity, and fibrosis-4 score. P < 0.05 was used to evaluate significant differences between groups. FIB-4, fibrosis-4; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma.
Discussion
Among a national cohort of predominantly non-Asian US Veterans with non-cirrhotic CHB on antiviral therapy, the overall incidence of cirrhosis was 3.99 per 100 person-years and the overall incidence of HCC was 0.43 per 100 person-years. Our study provides important data describing the on-treatment risks of cirrhosis and HCC among an understudied population and evaluates baseline factors that may influence long-term outcomes.
Race/ethnicity-specific differences in on-treatment CHB outcomes observed in our study are particularly important to highlight given our predominantly non-Asian cohort, a population that has not been well investigated. We observed the highest incidence of cirrhosis among the NHW population which was significantly higher than in the API population, but after adjusting for baseline comorbidities and potential confounders, these differences no longer met statistical significance at the p < 0.05 level. However, there still seems to be a trend towards higher risk in the NHW population. No significant race/ethnicity-specific differences in HCC risk were observed. Existing population-based data in the US have reported higher incidence of HCC among non-white ethnic minorities, but these data do not specifically focus on patients with CHB, and it has been speculated that the race/ethnicity-specific differences in HCC incidence observed are directly correlated with underlying liver disease etiology.22 Hence, it is possible that when specifically focusing on a CHB cohort who are all on treatment, antiviral therapy is equally effective at lowering HCC risk across race/ethnic groups. However, the explanation for the observed racial/ethnic disparities in cirrhosis incidence in our cohort is not clear. It is possible that concurrent risk factors and comorbidities such as obesity, diabetes, or alcohol use may be contributing to the higher observed cirrhosis incidence in NHW patients with CHB. Nevertheless, we acknowledge there may remain unmeasured confounders that could have influenced our observations. When evaluating the impact of alcohol use on risk of cirrhosis or HCC, no significant influence on risk of cirrhosis was observed on multivariate regression. Furthermore, even in the univariate model, baseline alcohol use did not seem to be correlated with long-term risks of HCC. However, the lack of association may be due to our focus on baseline alcohol use using AUDIT-C scores, and patients’ alcohol use patterns may have changed significantly over the follow-up period. Thus, while our study specifically focuses on the impact of baseline factors, for alcohol use in particular, future studies may need to incorporate repeated measures or changes in alcohol use patterns over time to accurately assess the association between an individual’s alcohol use and clinical outcomes.
The increased risk of HCC associated with higher baseline FIB-4 scores in patients with CHB has previously been reported, albeit mostly in Asian populations. Tada et al. evaluated 539 patients with CHB on antiviral therapy in Japan over a median follow-up of 5.9 years. The authors observed that FIB-4 >2.65 at 24 weeks after starting antiviral therapy was associated with a 5-fold higher risk of HCC than a FIB-4 <2.65.23 Suh et al. also demonstrated that a high FIB-4 score was associated with higher risk of HCC among a Korean cohort of 986 patients with CHB, but these patients did not have active disease and were not on antiviral therapy.24 In contrast, Demir et al. evaluated 373 predominantly non-Asian patients with CHB in Germany and did not observe a significant association between elevated FIB-4 score and higher risk of HCC.25 However, in this cohort, the vast majority of patients with CHB were not on antiviral therapy and the authors evaluated FIB-4 score as a bivariate predictor using a threshold of 1.25. Our current study is one of the largest non-Asian cohorts evaluating baseline characteristics predictive of on-treatment CHB outcomes. Compared to patients with CHB and FIB-4 <1.45 at the time of antiviral treatment initiation, we observed that patients with high FIB-4 scores >3.25 had a >7-fold higher risk of HCC. We also found increasing FIB-4 scores at baseline to be significantly associated with higher risk of cirrhosis. This observation is aligned with existing studies in predominantly Asian CHB populations.26 The increased risk of HCC associated with higher FIB-4 scores, especially FIB-4 >3.25, is not surprising given that FIB-4 >3.25 is associated with advanced fibrosis, and hence would be expected to be associated with a higher risk of HCC.
The strengths of this study include the utilization of a large, established, longitudinal cohort, which allows for accurate assessment of long-term CHB outcomes. In particular, our cohort is one of the largest evaluating on-treatment outcomes among a predominantly non-Asian population of patients with CHB. Veterans receive most if not all of their healthcare within VA health systems, and given the integrated nature of the national VA healthcare system, accurate health outcomes data are captured. However, certain limitations should be acknowledged. Given the nature of the study population, patients were predominantly men, older, and non-Asian (which is also a strength given the lack of data in non-Asian populations), which may limit the generalizability to women and younger populations. While we utilized established definitions and previously validated algorithms to identify our study population and to define our study outcomes, there is a possibility of misclassification bias, although we would not necessarily expect any bias to be differential in nature. For example, some patients with FIB-4 >3.25 may have undiagnosed cirrhosis. However, we used established definitions and algorithms that have been previously used with the current dataset to accurately identify cirrhosis and thus we believe any misclassification was likely minimal. In addition, while we used repeated assessments of prescription data to ensure patients were on continuous treatment, there is the possibility that not all patients were completely adherent to antiviral therapies. To address this concern, we incorporated repeated longitudinal measures of HBV DNA during follow-up, with achievement of HBV DNA <2,000 IU/ml used as an additional surrogate of adherence to antiviral therapies. Our study aim was to evaluate the influence of baseline factors at the time of antiviral therapy on long-term risks of cirrhosis and HCC. However, we acknowledge that some of these variables (e.g. alcohol use, BMI, tobacco use) can change over time, and thus future studies may be conducted to understand how longitudinal changes in these baseline factors mediate CHB outcomes. As previously noted, while we attempted to adjust for important baseline confounders, we acknowledge that unmeasured confounders may have affected the study outcomes as with all observational studies. HBV genotype may influence long-term patient outcomes. However, HBV genotype is not routinely performed in this population as part of standard care and thus genotype data were not available for evaluation. Finally, one common limitation of observational studies is loss to follow-up or challenges in ascertaining outcome assessment for patients that utilize different healthcare systems. However, we believe the integrated nature of the VA health system is a strength in this regard, given that most Veterans will continue longitudinal care within VA healthcare systems.
In conclusion, among a large longitudinal cohort of predominantly non-Asian Veterans with non-cirrhotic CHB on antiviral therapy, we provide important epidemiological data to describe long-term risks of cirrhosis and HCC among this understudied population. While race/ethnicity-specific differences in cirrhosis and HCC were observed, these differences did not meet statistical significance in competing risks adjusted regression models. As expected, higher FIB-4 scores at baseline were consistently associated with higher risks of cirrhosis and HCC. Ensuring timely diagnosis of CHB, linkage to care, and consistent antiviral therapy is critical to improve long-term CHB outcomes.
Financial support
This study was supported by an investigator-initiated research study grant from Gilead Sciences (Grant number: IN-US-988-6569).
Authors’ contributions
Study concept and design: all authors. Acquisition of data: Yang, Wong. Analysis and interpretation of the data: all authors. Statistical analyses: Yang, Wong. Drafting of the manuscript: Yang, Wong. Critical revision of the manuscript for important intellectual content: all authors. Study supervision: Wong. All authors had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Conflicts of interests
ZY, AC, and WZ report no disclosures. RGG reports grants received from Gilead Sciences; has served as an advisor or consultant to Abbot, AbbVie, Altimunne, Antios, Arrowhead, Dynavax, Eiger, Eisai, Enyo, Genentech, Genlantis, Gerson Lehrman Group, Gilead Sciences, Helios, HepaTX, HepQuant, Intercept, Janssen, Merck, Pfizer, Topography Health, Venatorx, Prodigy, Fibronostics, Fujifilm/Wako, Perspectum, Quest, Sonic Incytes; has served on the data safety monitoring board for Altimmune, Arrowhead, CymaBay Therapeutics, Durect; has served on the speaker’s bureau for AbbVie, BMS, Eisai, Genentech, Gilead Sciences Inc., Intercept; is a minor stock shareholder of RiboSciences, CoCrystal; has received stock options from Eiger, Genlantis, HepQuant, AngioCrine. RJW has received funding (to his institution) from Gilead Sciences and Exact Sciences, and has served as a consultant for Gilead Sciences.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100852.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Wong R.J., Brosgart C.L., Welch S., Block T., Chen M., Cohen C., et al. An updated assessment of chronic hepatitis B prevalence among foreign-born persons living in the United States. Hepatology (Baltimore, Md) 2021;74:607–626. doi: 10.1002/hep.31782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polaris Observatory C. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 3.Wong R.J., Jain M.K., Therapondos G., Niu B., Kshirsagar O., Thamer M. Low rates of hepatitis B virus treatment among treatment-eligible patients in safety-net health systems. J Clin Gastroenterol. 2022;56:360–368. doi: 10.1097/MCG.0000000000001530. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa E., Yeo Y.H., Dang N., Le M.H., Jeong D., Tran S., et al. Diagnosis rates of chronic hepatitis B in privately insured patients in the United States. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang E., Torres S., Liu B., Baden R., Bhuket T., Wong R.J. High prevalence of cirrhosis at initial presentation among safety-net adults with chronic hepatitis B virus infection. J Clin Exp Hepatol. 2018;8:235–240. doi: 10.1016/j.jceh.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang E., Liu B., Bhuket T., Wong R.J. Low rates of linkage and retention into care among patients with chronic HBV infection. Clin Gastroenterol Hepatol. 2019;17(9):1909–1911. doi: 10.1016/j.cgh.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Hu D.J., Xing J., Tohme R.A., Liao Y., Pollack H., Ward J.W., et al. Hepatitis B testing and access to care among racial and ethnic minorities in selected communities across the United States, 2009-2010. Hepatology (Baltimore, Md) 2013;58:856–862. doi: 10.1002/hep.26286. [DOI] [PubMed] [Google Scholar]
- 8.Marcellin P., Gane E., Buti M., Afdhal N., Sievert W., Jacobson I.M., et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 9.Wong R.J., Jain M.K., Therapondos G., Niu B., Kshirsagar O., Thamer M. Antiviral therapy reduces risk of cirrhosis in noncirrhotic HBV patients among 4 urban safety-net health systems. The Am J Gastroenterol. 2021;116:1465–1475. doi: 10.14309/ajg.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 10.Wong G.L., Chan H.L., Mak C.W., Lee S.K., Ip Z.M., Lam A.T., et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology (Baltimore, Md) 2013;58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 11.Kim W.R., Telep L.E., Jump B., Lu M., Ramroth H., Flaherty J., et al. Risk of hepatocellular carcinoma in treatment-naive chronic hepatitis B patients receiving tenofovir disoproxil fumarate versus entecavir in the United States. Aliment Pharmacol Ther. 2022;55:828–835. doi: 10.1111/apt.16786. [DOI] [PubMed] [Google Scholar]
- 12.Lee H.W., Cho Y.Y., Lee H., Lee J.S., Kim S.U., Park J.Y., et al. Effect of tenofovir alafenamide vs. tenofovir disoproxil fumarate on hepatocellular carcinoma risk in chronic hepatitis B. J Viral Hepat. 2021;28:1570–1578. doi: 10.1111/jvh.13601. [DOI] [PubMed] [Google Scholar]
- 13.Choi J., Kim H.J., Lee J., Cho S., Ko M.J., Lim Y.S. Risk of hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: a Korean nationwide cohort study. JAMA Oncol. 2019;5:30–36. doi: 10.1001/jamaoncol.2018.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan D.J.H., Ng C.H., Tay P.W.L., Syn N., Muthiah M.D., Lim W.H., et al. Risk of hepatocellular carcinoma with tenofovir vs entecavir treatment for chronic hepatitis B virus: a reconstructed individual patient data meta-analysis. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.19407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng C.H., Hsu Y.C., Chen T.H., Ji F., Chen I.S., Tsai Y.N., et al. Hepatocellular carcinoma incidence with tenofovir versus entecavir in chronic hepatitis B: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:1039–1052. doi: 10.1016/S2468-1253(20)30249-1. [DOI] [PubMed] [Google Scholar]
- 16.Chen C.L., Yang H.I., Yang W.S., Liu C.J., Chen P.J., You S.L., et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 17.Hassan M.M., Hwang L.Y., Hatten C.J., Swaim M., Li D., Abbruzzese J.L., et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology (Baltimore, Md) 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 18.Shin H.S., Jun B.G., Yi S.W. Impact of diabetes, obesity, and dyslipidemia on the risk of hepatocellular carcinoma in patients with chronic liver diseases. Clin Mol Hepatol. 2022;28:773–789. doi: 10.3350/cmh.2021.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanwal F., Kramer J.R., Mapakshi S., Natarajan Y., Chayanupatkul M., Richardson P.A., et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155:1828–1837 e1822. doi: 10.1053/j.gastro.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brummer J., Bloomfield K., Karriker-Jaffe K.J., Pedersen M.M., Hesse M. Using the alcohol use disorders identification test to predict hospital admission for alcohol-related conditions in the Danish general population: a record-linkage study. Addiction. 2023;118:86–94. doi: 10.1111/add.16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao G., Yang J., Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology (Baltimore, Md) 2015;61:292–302. doi: 10.1002/hep.27382. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez C.S., Petrick J.L., Parisi D., McMahon B.J., Graubard B.I., McGlynn K.A. Racial/ethnic disparities in hepatocellular carcinoma incidence and mortality rates in the United States, 1992-2018. Hepatology (Baltimore, Md) 2022;76:589–598. doi: 10.1002/hep.32394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tada T., Kumada T., Toyoda H., Tsuji K., Hiraoka A., Tanaka J. Impact of FIB-4 index on hepatocellular carcinoma incidence during nucleos(t)ide analogue therapy in patients with chronic hepatitis B: an analysis using time-dependent receiver operating characteristic. J Gastroenterol Hepatol. 2017;32:451–458. doi: 10.1111/jgh.13473. [DOI] [PubMed] [Google Scholar]
- 24.Suh B., Park S., Shin D.W., Yun J.M., Yang H.K., Yu S.J., et al. High liver fibrosis index FIB-4 is highly predictive of hepatocellular carcinoma in chronic hepatitis B carriers. Hepatology (Baltimore, Md) 2015;61:1261–1268. doi: 10.1002/hep.27654. [DOI] [PubMed] [Google Scholar]
- 25.Demir M., Grunewald F., Lang S., Schramm C., Bowe A., Muck V., et al. Elevated liver fibrosis index FIB-4 is not reliable for HCC risk stratification in predominantly non-Asian CHB patients. Medicine. 2016;95 doi: 10.1097/MD.0000000000004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y., Chen Y., Zhao Y. The diagnostic value of the FIB-4 index for staging hepatitis B-related fibrosis: a meta-analysis. PloS one. 2014;9 doi: 10.1371/journal.pone.0105728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.