Summary
Bladder cancer (BLCA) is more common in men but more aggressive in women. Sex-based differences in cancer biology are commonly studied using a murine model with BLCA generated by N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN). While tumors in the BBN model have been profiled, these profiles provide limited information on the tumor microenvironment. Here, we applied single-cell RNA sequencing to characterize cell-type specific transcriptional differences between male and female BBN-induced tumors. We found proportional and gene expression differences in epithelial and non-epithelial subpopulations between male and female tumors. Expression of several genes predicted sex-specific survival in several human BLCA datasets. We identified novel and clinically relevant sex-specific transcriptional signatures including immune cells in the tumor microenvironment and it validated the relevance of the BBN model for studying sex differences in human BLCA. This work highlights the importance of considering sex as a biological variable in the development of new and accurate cancer markers.
Subject areas: Cancer, Cancer systems biology, Immunology
Graphical abstract

Highlights
-
•
Single-cell RNA sequencing highlights the role of sex in bladder cancer
-
•
Identification of sex- specific transcription factors that regulate immune cells
-
•
Macrophage migration inhibitory factor (MIF) signaling is male-specific
-
•
Sex-specific signatures may help tailor cancer therapy in bladder cancer
Cancer; Cancer systems biology; Immunology
Introduction
Bladder cancer (BLCA) is the 4th most common cancer in men and the 17th most common in women in the US.1,2,3 Interestingly, progression of BLCA is more rapid in females than males.4 Sex chromosomes and their corresponding gene products play an important role in the incidence and aggressiveness of BLCA by influencing cancer initiation and progression.5,6,7,8 Furthermore, gene copy number changes and somatic mutations also differ by sex.9,10 However, a full understanding of sex-associated molecular differences is still missing for BLCA.
Most cases of BLCA are due to carcinogen exposure, primarily as a result of smoking.11 Currently, the most common autochthonous model of BLCA involves feeding mice N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN), which results in muscle-invasive bladder cancer (MIBC) similar to the human disease, based on bulk RNA and DNA evaluation.12,13 In this model, as in humans, male mice develop more tumors than female mice when exposed to BBN.14 Furthermore, BBN and its metabolites are carcinogens related to the chemicals found in cigarette smoke.15,16
In addition to being a tool for studying BLCA development and tumor progression, the BBN murine model has also been used in the preclinical evaluation of treatments17 and it is especially useful in studying immunotherapy. While immune checkpoint inhibitors (ICIs) targeting the PD-1/PD-L1 axis have shown promising results in BLCA patients, only 20% of patients respond to these treatments.18,19,20 Thus, autochthonous, immunocompetent murine BLCA models are critical for preclinical evaluation of therapies that could increase response to ICI.21,22 The BBN murine model is particularly important for this since the efficacy of ICI appears to be partially dependent on sex with better outcome in males.23
Given the widespread use of the BBN model, we sought to fully characterize the tumor cellular heterogeneity in a sex-dependent setting using single-cell RNA sequencing (scRNA-seq).24,25,26 Our data revealed differences in the epithelial subtype composition of BBN tumors as a function of sex, as well as significant gene expression differences between malignant cells in male and female tumors. Importantly, a subset of differentially expressed genes can predict sex-specific outcomes following surgery in multiple independent clinical datasets. Our findings provide the first evidence for sex-specific transcriptional signatures in BLCA cells that are also clinically relevant candidate prognostic biomarkers in BLCA patients. This study also demonstrates the relevance of the BBN model for studying sex differences in human BLCA.
Results
Single-cell RNA sequencing of murine bladder cancer
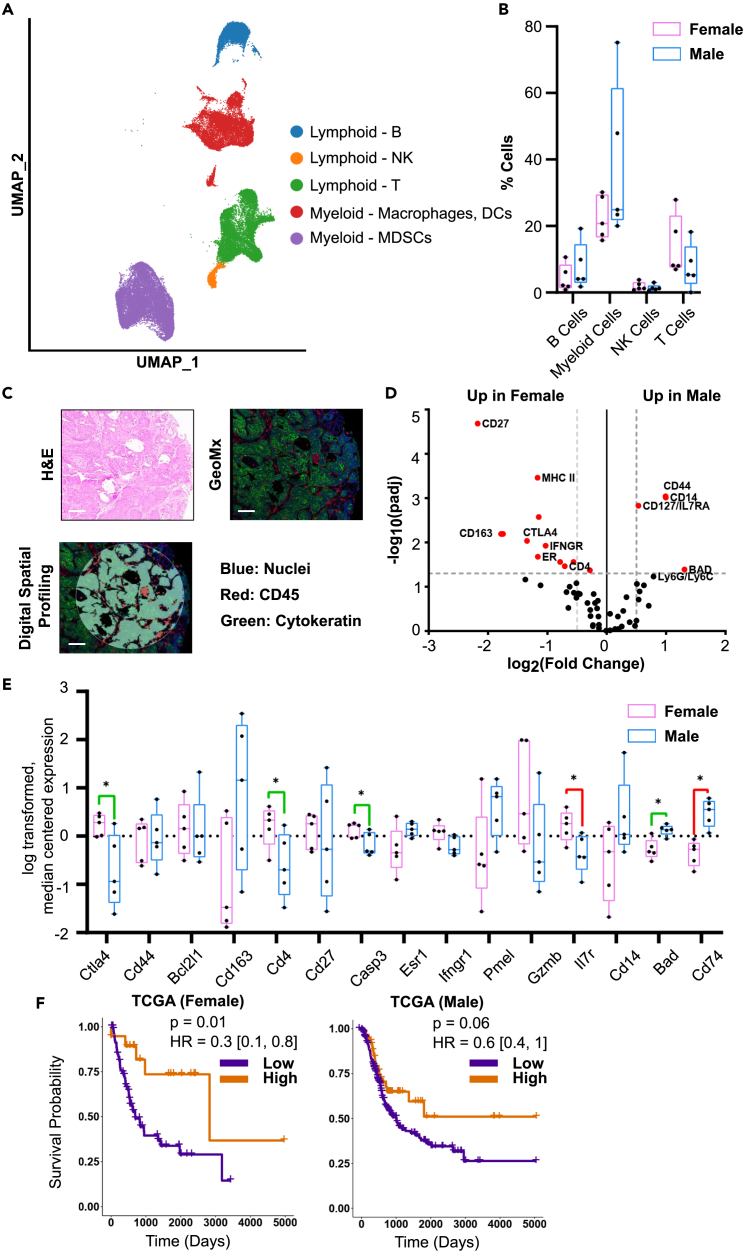
Male and female C57BL/6 mice were treated with 0.05% BBN water for 180 days to induce BLCA and then sacrificed based on the standard criteria (experimental model and subject details, Figure 1A; Figure S1A). Consistent with earlier studies, male mice developed tumors faster than females (Figure S1B). H&E staining was characteristic of what we and others had previously observed in BBN-generated tumors (Figure S1C).27,28 scRNA-seq was performed on male and female tumors, and 89,728 single cells were clustered into 11 major cell types: epithelial cells, fibroblasts, myeloid cells (macrophages, MDSCs), lymphoid cells (T cells, NK cells, B cells), endothelial cells, lymphatic endothelial cells, muscle cells, and neurons (Figure 1B; Figure S1D). Cellular composition of the major cell types varied greatly between the samples analyzed (each sample was comprised of a pool of tumors) (Figure 1C).
Figure 1.
Single cell transcriptome profiling of BBN-induced mouse bladder tumors
(A) Overview of BBN treatment regime to induce bladder cancer in C57BL/6 mice.
(B) UMAP showing 11 clusters of cells identified in BBN-induced bladder tumors from 10 male and 10 female mice.
(C) Cellular composition of BBN-induced mice bladders. Bladders from 10 male and 10 female BBN treated mice were pooled into 5 samples each (female: FB1-FB5, male: MB1-MB5).
Malignant epithelial cell subtype composition and transcriptional profiles reveal differences in male and female tumors
First, differences in malignant epithelial cells as a function of sex were determined. We re-clustered cells from malignant bladder epithelium to identify two major epithelial subtypes: basal and luminal cells along with four minor subtypes: epithelial mesenchymal transition (EMT) like, umbrella cells, inflammatory cells, and proliferating cells (Figure 2A; Figure S2A).
Figure 2.
Malignant epithelial cell subtype composition and transcriptional profiles reveal differences in male and female tumors
(A) UMAP showing bladder epithelial subtypes in male and female mice (n = 10 each).
(B) Quantification of epithelial subtypes in male and female BBN-treated mice.
(C) Heatmap of male vs. female differentially expressed genes (padj <0.05) in epithelial cells.
(D) Canonical pathway analysis of epithelial DEGs (padj <0.05) using Qiagen IPA. Only the top significant canonical pathways based on |Z score| are shown.
(E) Heatmap showing activity scores of indicated transcription factors (TFs) calculated using SCENIC package in BBN treated mice and human patients from Chen dataset. Only TFs detected in both mice and human datasets are shown.
(F) Heatmap of ligand-receptor (LR) interactions between epithelial subtypes (source) and fibroblasts, lymphoid, myeloid subtypes (target). Each row represents an LR pair while each column corresponds to source and target cells involved in the interaction.
As reported earlier,27 most of the epithelial cells were of the basal subtypes following BBN treatment. While no significant enrichment of basal or luminal epithelial cells were observed in male compared to female mice (Figure 2B), differential expression analysis found 1,125 differentially expressed genes (DEGs) as a function of sex (padj <0.05) (Figure 2C; Table S1). Ingenuity pathway analysis (IPA) revealed metabolic pathways such as glycolysis and gluconeogenesis as well as signaling pathways such as protein kinase A (PKA) signaling, and the tumor microenvironment (TME) are more active in males. Thrombin, endocannabinoid cancer inhibition, and oxytocin signaling pathways were found to be more active in females (Figure 2D). These differences suggest male-specific pathways of tumor progression could include inhibition of the endocannabinoid pathway coupled with an active TME and elevated metabolic activity.
IPA upstream regulator analysis also revealed several classes of proteins (cytokines, growth factors, receptors, enzymes, transcriptional and translational regulators) were differentially activated between sexes (Figure S2B). Interestingly, most regulators have similar expression levels in both sexes, except for TGFB1, MDK, FN1, THBS2, and CCL2 (circled in Figure S2B).
Due to high tumor heterogeneity in human samples combined with dropout effects seen in single cell RNA sequencing data technology, we were unable to observe similarity between mouse and human datasets at a genetic level. To validate our findings in mice, we performed regulatory network (regulon) analysis using SCENIC.29 Although regulons were not enriched as a function of sex in human patients, we identified several regulons whose activities were enriched in both mice and human datasets (Figure 2E). We also analyzed sex-specific interactions between epithelial cells and other cell types. We found increased signaling via macrophage migration inhibitory factor (MIF) from epithelial to myeloid and lymphoid cells in males compared to females. This finding was validated by similar enhanced MIF signaling in human male patients compared to female patients (Figure 2F) indicating immune inhibition.
Evaluation of infiltrating immune cells in BBN-induced bladder cancer
Within the immune compartment, we identified B cells, T cells, NK cells, and myeloid cells (Figure 3A). Although no proportional, sex-stratified differences in the total immune infiltrate (CD45-positive) or in individual immune cell types were found between male and female tumors (Figure 3B), there were DEGs identified as a function of sex (Figures S3A–S3C). We next applied NanoString GeoMx Digital Spatial Profiler (DSP) technology to study the expression patterns of 62 immune-related proteins in CD45+ cells contained in formalin-fixed paraffin embedded tumor microarray (FFPE TMA) blocks made from the male and female murine bladder tumors (Figures 3C; Figure S3D). 15 of 62 proteins were differentially expressed, with 11 upregulated in females (CD27, MHC II, Cleaved Caspase, CD163, CTLA4, IFNGR1/2, ER, BCLXL, Pmel17, CD4, and GZMB) and 4 upregulated in males (CD44, CD14, CD127/IL7RA, and BAD, Figure 3D). We compared these differentially expressed proteins to the scRNA-seq data and only four genes (CTLA4, CD4, CASP3, and BAD) were significantly regulated (Figure 3E). We next investigated the association between expression of the 15 genes above and overall survival (OS) in BLCA within data from The Cancer Genome Atlas Program (TCGA) (Figure S3E). Interestingly, high levels of CTLA4 (HR = 0.3, p = 0.01) were associated with statistically significant better prognosis in females but not in males (Figure 3F). Similar findings were reported by Saad et al. where female melanoma patients showed improved survival with adjuvant CTLA4 blockade compared to males.30
Figure 3.
Evaluation of infiltrating immune cells in BBN-induced bladder cancer
(A) UMAP showing bladder immune cells in male and female mice (n = 10 each).
(B) Quantification of immune cell types in male and female BBN-treated mice.
(C) Representative H&E image, immunofluorescence detection of nuclei (blue), cytokeratin (green), and CD45+ immune cells (red) and associated computational digital profiling. Scale bar 125 μm.
(D) Volcano plot of GeoMx protein markers between female and male BBN-induced tumors. Statistically significant proteins (padj <0.05, |log2FC| > 0.58) are shown in red.
(E) Expression of genes encoding statistically significant GeoMx marker proteins in CD45+ cells from scRNA sequenced BBN-induced bladder tumors. Genes that do not correlate with GeoMx are shown in red while genes that correlate with GeoMx are shown in green. All significant comparisons are shown. ∗p ≤ 0.05.
(F) Overall survival curves stratified by CTLA4 expression levels in TCGA BLCA.
Distinct transcription factors regulate immune cells in males and females
Within the immune cell clusters, the most noteworthy differences between the sexes were observed in myeloid cells. Subclustering of the 26,747 captured myeloid cells revealed the presence of macrophages, conventional dendritic cells (cDCs), plasmacytoid dendritic cells (pDCs), and myeloid-derived suppressor cells (MDSCs) (Figures 4A and 4B; Figure S4A). During tumor progression, myeloid cells can develop into MDSCs, which play an important role in the suppression of anti-tumor immunity.31 Accumulation of MDSCs has been correlated with tumor progression, metastasis, and recurrence of several types of cancers.32 The efficacy of immunotherapy was negatively correlated with an increased MDSC frequency.33
Figure 4.
Single-cell transcriptome profiling of myeloid cells in BBN-induced bladder tumors
(A) UMAP showing bladder myeloid subtypes in male and female mice (n = 10 each).
(B) Quantification of myeloid subtypes in male and female BBN-treated mice.
(C) Heatmap of differentially expressed genes (padj <0.05) between male and female myeloid cells.
(D) Canonical pathway analysis of myeloid DEGs (padj <0.05) using Qiagen IPA. Only the top 10 significant canonical pathways based on |Z score| are shown.
(E) Heatmap of 20 transcription factors differentially activated in males and females (only TFS common among mice and human datasets are shown).
Transcriptional profiling of the myeloid population identified 661 DEGs with padj <0.05 (Figures 4C; Table S2). IPA analysis revealed metabolic (glycolysis and gluconeogenesis) as well as inflammation-related pathways (STAT3, IL17, and macrophage MIF) as being upregulated in males (Figure 4D). Thus, prolonged upregulation of these pathways could result in inflammation, thereby creating an environment conducive to the development of cancer.34
We used the Dorothea and Viper packages35 to identify key transcription factors (TFs) that are differentially regulated in either sex. In this context, TF activities were computed based on the mRNA expression levels of corresponding targets. We found several TFs that were differentially active between male and female mice (Figure 4E). Among them, 16 TFs were also highly active in human male patients including the AR signaling pathway. Four TFs were active in female patients, which confirms the relevance of these TFs in BLCA.
Males have a CD8 T cell exhaustion program that correlates with androgen receptor activity
We found significant male bias in expression of multiple androgen-response genes across T cell subsets using mouse HALLMARK_ANDROGEN_RESPONSE signature from the GSEA MSigDB (Figure S5A). This finding correlates with our recently published discovery that the AR-driven exhaustion program in CD8+ tumor-infiltrating T lymphocytes mediates sex disparity in murine bladder tumor growth.36 With these findings in mind, we focused our investigations on the CD8+ T cell exhaustion program in our dataset. First, we clustered the 2,464 CD8+ T cells from both sexes into eight subclusters based on their transcriptome (Figure S5B). Subsequently, a publicly available naïve-like T cell gene signature and a terminal exhaustion gene signature37 were used to determine the cell states of CD8+ T cells, as performed previously.36 Through gene signature analysis (Figures S5C and S5D), cluster 6 (C6) showed a higher naïve-like gene signature score, while C3 and C4 were more enriched for the terminally exhausted CD8+ T cell gene signature. To assess the association between androgen responsiveness and exhaustion state, we also performed gene signature enrichment analysis using the same HALLMARK_ANDROGEN_RESPONSE gene signatures (Figure S5E). Notably, terminally exhausted CD8+ T cells displayed a more enriched activity of the androgen-response gene signature. Through DEG analysis in all CD8+ T cell subsets between males and females, we found seven androgen-response genes to be male-biasedly expressed in clusters within C0∼C3 and one gene to be female-biasedly expressed in C4 (Figure S5F). Several of these genes have been reported to play a role in T cell exhaustion (e.g., Maf)38 and the differentiation of tissue-resident CD8+ T cells (e.g., Pmepa1),39 while the role(s) of the remaining genes require further studies. To infer CD8+ T cell ontogeny, we used the Slingshot algorithm for trajectory analysis and found C0, C3, C6, and C7 to be involved in the exhaustion lineage (Figures S5G and S5H). To compare how fast CD8+ T cells become exhausted in male versus female mice, we implemented the Kolmogorov-Smirnov test (K-S) to calculate exhaustion velocity, which showed accelerated development of exhaustion in CD8+ T cells in male mice compared to female mice (Figure S5I). We conclude that there is a male-biased T cell exhaustion program in the BBN-induced BLCA model that correlated significantly with the activity of androgen.
Cancer-associated fibroblasts (CAFs) express distinctive pathways as a function of sex
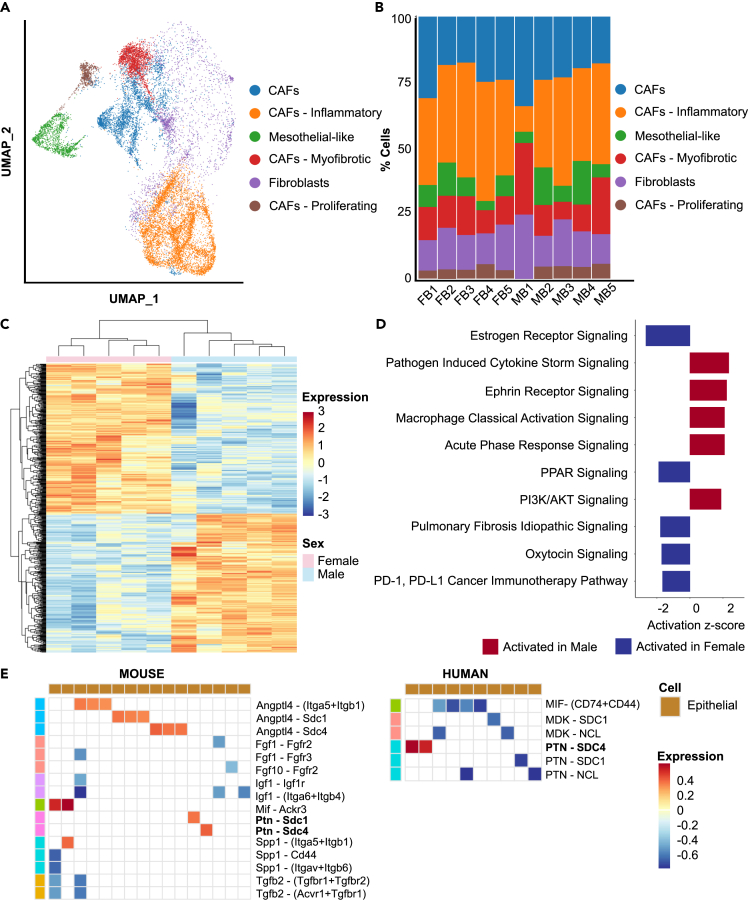
To investigate gender-specific differences between cancer-associated fibroblasts (CAFs) that could affect the TME and in turn, tumor progression, we subclustered the 15,306 captured mesenchymal cells and then used established markers to classify the resultant clusters into CAFs, fibroblasts, and mesothelial-like fibroblasts (Figure 5A; Figure S6A). No significant sex-related differences were observed between subtype compositions (Figure 5B).
Figure 5.
Cancer-Associated Fibroblasts (CAFs) express distinctive pathways as a function of sex in BBN-induced bladder tumors
(A) UMAP showing bladder fibroblast subtypes in male and female mice (n = 10 each).
(B) Quantification of fibroblast subtypes in male and female BBN-treated mice.
(C) Heatmap of differentially expressed genes (padj <0.05) between male and female fibroblasts.
(D) Canonical pathway analysis of fibroblast DEGs (padj <0.05) using Qiagen IPA. Only the top 10 significant canonical pathways based on |Z score| are shown.
(E) Heatmap of ligand-receptor (LR) interactions between fibroblast subtypes (source) and epithelial subtypes (target). Each row represents an LR pair while each column corresponds to source and target cells involved in the interaction.
Pathway analysis on genes differentially expressed in this compartment suggests males show increased cytokine signaling, macrophage activation, and acute phase response indicative of elevated immune response compared to females (Figures 5C and 5D; Table S3). Hormonal signaling via estrogen and oxytocin were downregulated in males, while ephrin receptor and PI3K/AKT signaling were upregulated in males. Further support for the upregulation of PI3K/AKT signaling in male CAFs also came from an identified downregulation of PPARγ signaling and its target genes.40
To identify clinically relevant ligand receptor interactions, we performed CellChat analysis on both mouse and human datasets. Ligand-receptor (LR) pairs enriched in either sex were identified (Figure 5E). Among all differential LR pairs between fibroblasts and other cell types, we observed that only signaling via Ptn expressed on fibroblasts was enriched in males both in mice and human bladder tumors. Since Ptn is known to be involved in immune regulation,41 its role in BLCA could be investigated further.
In terms of upstream regulators, Toll-like receptor 4 (TLR4), which promotes immune evasion by inducing inflammatory signaling, was more active in male CAFs than female CAFs42 (Figure S6B). In contrast, the proposed tumor suppressors EGR2 and EGR3 were predicted to be more active in females, which could explain their lower cancer incidence compared to males.43,44 Finally, the Polo-like kinases PLK2 and PLK4 were found to be more active in females, which is potentially of interest as several Plk inhibitors are currently in development.45
Novel sex-specific tumor tissue gene signatures can stratify patient survival
Based on the analyses above in, the most significant sex-specific differences at the transcriptional level were in the epithelial compartment, indicating this compartment could be responsible for tumor growth and progression in both sexes (Figure 2). To test this hypothesis and to help focus future investigations on genes that are valid biomarkers and therapeutic targets in humans, we constructed sex-specific prognostic signatures from malignant epithelial and non-epithelial cells. These were then tested in multiple patient cohorts with Muscle Invasive BLCA (MIBC) that had been treated by surgery. This approach also offers the opportunity to use the BBN model to mechanistically examine the role of markers that are associated with outcomes.
First, we identified differentially upregulated genes in male (286 genes) and female (398 genes) mice in our BBN dataset and obtained their human homologs (Table S1). We analyzed each gene for their ability to stratify overall survival (OS) in male BLCA patients as well as female BLCA patients from TCGA and classified them into 4 groups: Male HR > 1, Male HR < 1, Female HR > 1, Female HR < 1 (Figure 6A). Of the 286 genes upregulated in male BBN-treated mice, only 64 genes showed poor OS upon high expression (HR > 1) specifically in male BLCA patients in TCGA. Similarly, of the 398 genes upregulated in female BBN-treated mice, only 33 genes showed poor OS upon high expression (HR > 1) specifically in female BLCA patients in TCGA (Figure 6B; Table S4). Next, we constructed sex-specific expression signatures from these two gene sets and evaluated their ability to stratify male and female OS in 3 additional microarray studies focusing on primary BLCA or MIBC. These studies were: MSKCC,46 Blaveri,47 and GSE32894.48 Importantly, despite the microarray studies lacking data for some signature genes, both male and female signatures stratified OS in all cohorts (Figures 6C; Table S5).
Figure 6.
Novel sex-specific tumor tissue gene signatures can stratify patient survival
(A) Significant highly upregulated genes (FC>=1.5, padj<0.05) in male and female epithelial cells were identified. Human homologs of these genes were obtained using gProfiler (https://biit.cs.ut.ee/gprofiler/convert). Survival curves for each upregulated gene was plotted (ACOT7 shown as example) using survival and survminer packages to assess their prognostic significance (p < 0.05).
(B) Venn diagram representation of upregulated genes in male epithelial and female epithelial cells based on gender specific prognosis (HR < 1 indicates good prognosis, HR > 1 indicates bad prognosis).
(C) Validation of gene signatures using survival analysis in indicated studies (Ratio within brackets indicate number of signature genes present in the study/number of signature genes).
Similarly, we analyzed the non-epithelial cells from the BBN tumors (Figure 7A; Table S6) and identified a 37 and a 11 gene signature for male and female tumors, respectively (Figure 7B; Table S4). The male signature stratified overall survival in the Blaveri cohort but because none of the 11 female signature genes were present in the microarray data this signature could not be evaluated. Genes measured in the GSE13507 cohort did allow the female signature to be evaluated for overall survival, and it did successfully stratify for female patients. However, the male signature was not successful in stratifying for overall survival49 (Figures 7C; Table S5). Importantly, cross-sex evaluations of overall survival for epithelial (Figures S7A and S7B) and non-epithelial (Figures S7C and S7D) signatures were also performed. The epithelial signatures were highly sex-specific (only 1 of 6 cross-sex comparisons was significant) as compared to non-epithelial genes signatures (3 of 5 cross-sex comparisons were significant).
Figure 7.
Development and validation of non-epithelial gene signature
(A) Significant highly upregulated genes (FC>=1.5, padj<0.05) in male and female non-epithelial cells were identified. Human homologs of these genes were obtained using gProfiler (https://biit.cs.ut.ee/gprofiler/convert). Survival curves for each upregulated gene was plotted (CEACAM16 shown as example) using survival and survminer packages to assess their prognostic significance (p < 0.05).
(B) Venn diagram representation of upregulated genes in male non-epithelial and female non-epithelial cells based on gender specific prognosis (HR < 1 indicates good prognosis, HR > 1 indicates bad prognosis).
(C) Validation of gene signatures using survival analysis in indicated studies (Ratio within brackets indicate number of signature genes present in the study/number of signature genes).
Discussion
There are well-documented differences in the incidence, progression, therapy response, and outcomes of male and female BLCA patients,50,51 but the mechanisms behind these differences are not understood. Sex hormone receptors have been suggested to be involved in modulating the TME and sensitivity to conventional non-surgical therapy.52 Similarly, activation of AR signaling is associated with resistance to chemotherapy, radiotherapy, and BCG immunotherapy.51 This highlights the need for studies focusing on the impact of biological sex on cancer initiation, progression, and therapy response.53
Animal models that recapitulate the key features of the human disease are critical to understanding BLCA. The BBN-induced BLCA model produces tumors that are histologically and genetically similar to human BLCA54 including an immune infiltration pattern that is consistent with human basal tumors.55 This model is used for therapeutic studies that are translated into human clinical trials,54 and a deeper understanding of the relevance of biologic sex in this model is warranted.
We isolated single-cells from BBN-induced BLCA from male and female C57BL/6 mice and performed scRNA-seq. This revealed that male cells have higher metabolic and glycolytic capacity. It has been reported that the metabolism of tumor cells shifts from oxidative phosphorylation to glycolysis in the presence of oxygen (Warburg effect) and this is essential for tumor growth and proliferation.56 This enhanced metabolic activity may be the cause for the faster tumor growth observed in males. Additionally, as cancer cells upregulate their metabolism and bioenergetic pathways to meet the energetic and nutrient demands of their high proliferation rate, they create an environment that alters immune cells and promotes resistance to immunotherapy.57 Thus, targeting tumor metabolism along with immunotherapy while accounting for sex-specific differences may improve the therapeutic efficacy of ICIs.58
Apart from metabolic pathways, certain signaling pathways are also preferentially deregulated in a sex-specific manner. We found that male epithelial cells have elevated expression of TGFB1, MDK, CCL2, TBHS2, and FN1. The TGFB1 signaling pathway is deregulated in cancers and is associated with poor prognosis.59,60 TGFB1 is also thought to promote metastasis, angiogenesis, immunosuppression, fibroblast activation, and EMT.60,61 Increased CCL2 expression promotes metastasis by recruitment of macrophages.62,63 It has been shown that high expression of FN1 was associated with advanced stage and predicted an unfavorable outcome in BLCA patients. Also, FN1 expression was positively associated with immune cells infiltration.64 Hence, elevation of these signaling pathways may lead to the faster proliferation and poorer prognosis in male patients.
MDSCs play an important role in the suppression of anti-tumor immunity.31 MDSCs are potent immune suppressors associated with negative clinical outcomes and immune therapy resistance in several cancers.65 MDSCs can suppress T cell, NK cell, and B cell functions through the expression of arginase 1 (ARG1), indolamine 2,3-dioxyenase (IDO), and inducible nitric oxide synthase (iNOS).66 In BLCA patients, high levels of MDSCs were observed in tumor tissues.67 Our findings in this study showed increased expression of THBS2 by male epithelial cells, and this could induce infiltration of MDSCs which in turn could suppress anti-tumor effects. Similarly, increased activity of IL17 is known to stimulate infiltration of MDSCs.68,69 On the other hand, PPARγ pathway activation can suppress cytokine secretion leading to partial resistance to immunotherapy70,71 with better outcomes in males explained in part by the inhibition of PPARγ pathway.
Our study also identified that within the CD45+ population, female mice exhibit higher levels of MHC II expression. MHC molecules present antigens to T cells and generate immune response.72 A recent study in BLCA patients found that MHC II is associated with anti-tumor immunity of BLCA.73 Estrogen has been shown to increase MHC II expression while androgen decreases MHC II expression on dendritic cells (DCs).74 DCs play an important role in anti-tumor immune responses and T cell differentiation in a sex-specific manner.72 Thus, our findings on higher MHC II expression in females correlates with previous studies and clinical data, further supporting the clinical relevance of the BBN murine tumor model as a means of investigating sex differences.
By analysis of tumor-infiltrating lymphocytes, we found significant male biases in the enrichment of androgen-responsive genes across T cell subsets, especially in terminally exhausted CD8+ T cells. This may in part explain the accelerated development of T cell exhaustion in tumors from male mice. This observation is in line with a previous study from our group36 that identifies similar roles played by T cell intrinsic androgens in promoting CD8+ T cell exhaustion and mediating male-biased growth of another murine bladder tumor model:MB49, given subcutaneously. These two different models of murine BLCA complement each other and underscore the importance of androgen signaling in regulating anti-tumor immune response and contributing to male-biased high incidence and mortality of BLCA. The extent to which CD8+ T cell-intrinsic androgen signaling mediates sex disparity in BBN-induced tumor growth remains to be further investigated.
In conclusion, through scRNA-seq of a popular murine model of BLCA, we have identified candidate sex-relevant regulators in epithelial, fibroblast, and myeloid populations. These regulators may explain how BLCA progression, immune response, and the TME vary in men and women. Future elucidation of the mechanistic roles of these regulators will likely enable us to develop therapies tailored specifically for men or women. Of additional translational significance, our study is the first to report that sex-specific gene signatures are effective in stratifying survival outcomes in several human datasets. These sex-based markers have potential in improving prognostication in BLCA patients. The unique transcriptional data generated in this study will be a valuable resource for the entire BLCA research field.
Limitations of the study
We validate the findings of our mouse single cell data using human single cell, human bulk RNA-seq and human microarray data. Unfortunately, the human datasets have significantly more male samples as compared to females hence affecting the robustness of our female gene signatures. Moreover, in order to identify prognostic genes, we used human bulk RNA-seq and microarray data which represent the transcriptional profile of multiple cell types in the tumor (epithelial cells, fibroblasts, immune cells) unlike single cell data which represent transcriptional profiles of a single cell type (epithelial cells).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| BBN | TCI | B0938 |
| Gibco® ACK Lysing Buffer | Thermo Fisher Scientific | A1049201 |
| Corning® DMEM | Fisher Scientific | MT10017CV |
| PBS | Thermo Fisher Scientific | 10010023 |
| Critical commercial assays | ||
| Chromium Next GEM Single Cell 3′ GEM, Library & Gel Bead Kit v3.1, 16 rxns | 10X genomics | 1000121 |
| Chromium Next GEM Chip G Single Cell Kit, 48 rxns | 10X genomics | 1000120 |
| Chromium Single Cell 3′ Feature Barcode Library Kit, 16 rxns | 10X genomics | 1000079 |
| gentleMACS C Tubes | Miltenyi Biotec | 130-093-237 |
| Tumor Dissociation Kit, mouse | Miltenyi Biotec | 130-096-730 |
| Dead Cell Removal Kit | Miltenyi Biotec | 130-090-101 |
| GeoMx® Immune Cell Profiling Panel Mouse Protein Core for nCounter | Nanostring | GMX-PROCO-NCT-MICP-12 |
| GeoMx IO Drug Target Panel Mouse Protein Module for nCounter |
Nanostring | GMX-PROMODNCT-MIODT-12 |
| GeoMx Immune Activation Status Panel Mouse Protein Module for nCounter | Nanostring | GMX-PROMODNCT-MIAS-12 |
| GeoMx Immune Cell Typing Panel Mouse Protein Module for nCounter | Nanostring | GMX-PROMODNCT-MICT-12 |
| GeoMx Pan-Tumor Panel Mouse Protein Module for nCounter |
Nanostring | GMX-PROMODNCT-MPT-12 |
| GeoMx Cell Death Panel Mouse Protein Module for nCounter |
Nanostring | GMX-PROMODNCT-MCD-12 |
| GeoMx Protein Slide Prep Kit for FFPE | Nanostring | GMX-PREP-PRO-FFPE-12 |
| GeoMx Hyb Code Pack Protein | Nanostring | GMX-PRO-HYB-96 |
| GeoMx DSP Collection Plate | Nanostring | GMX-DSP-COLL-PLT-4 |
| GeoMx DSP Instrument Buffer Kit | Nanostring | GMX-DSP-BUFF-KIT |
| nCounter Analysis System Master Kit Reagents and Cartridges | Nanostring | NAA-AKIT-012 |
| nCounter SPRINT Cartridge | Nanostring | SPRINT-CAR-1.0 |
| nCounter SPRINT Reagent Pack | Nanostring | SPRINT-REAG-KIT |
| Deposited data | ||
| scRNA-seq data | This paper | GSE229168 |
| Experimental models: Organisms/strains | ||
| C57BL/6 mice | Taconic | RRID: MGI:2159769 |
| Software and algorithms | ||
| R (v4.2.2) | https://www.r-project.org/ | RRID: SCR_001905 |
| DESeq2 (v1.38.3) | https://www.bioconductor.org/ | RRID: SCR_015687 |
| Seurat (v4.3.0.1) | https://cran.r-project.org/web/packages/ | RRID: SCR_007322 |
| CellRanger (v7.1.0) | https://www.10xgenomics.com/ | RRID: SCR_023221 |
| SCENIC (v1.3.1) | https://github.com/aertslab/SCENIC | RRID: SCR_017247 |
| AUCell (v1.20.2) | https://www.bioconductor.org/ | RRID: SCR_021327 |
| viper (v1.32.0) | https://www.bioconductor.org/ | RRID: SCR_006442 |
| dorothea (v1.10.0) | https://www.bioconductor.org/ | RRID: SCR_006442 |
| CellChat (v1.6.1) | https://github.com/sqjin/CellChat | RRID: SCR_021946 |
| survival (v0.4.9) | https://cran.r-project.org/web/packages/ | RRID: SCR_021137 |
| survminer (v3.5-5) | https://cran.r-project.org/web/packages/ | RRID: SCR_021094 |
| Slingshot (v2.2.1) | https://www.bioconductor.org/ | RRID: SCR_017012 |
| Qiagen IPA | https://www.qiagen.com/us | RRID: SCR_008653 |
| GraphPad Prism (v9.3.1) | https://www.graphpad.com/ | RRID: SCR_002798 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact Dan Theodorescu (dan.theodorescu@cshs.org).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
BBN-induced mouse model
C57BL/6 male and female mice were housed under standard laboratory conditions in the Laboratory Animal Care facility at Cedars-Sinai Medical Center. All animal procedures were in accordance with institutional guidelines, and all animal treatments were done under an institutional protocol that was approved by Institutional Animal Care and Use Committee (IACUC 8253). Mice were housed under standard conditions in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-approved vivarium including HEPA filtration, ad libitum supply of food and water, and daily health monitoring.
Bladder cancer was induced in 6–8 weeks of age mice by administration of 0.05% BBN (TCI, Cat. #B0938) in the drinking water for 26 weeks and afterward changed to tap water until the end of the experiment. The drinking water containing BBN was changed twice a week. All mice were monitored for any abnormal characteristics such as hematuria, hunched back, body weight loss, and palpable tumor. The primary tumors were resected upon detection of palpable big masses, loss of body weight, or hematuria. The tumors were cut sagittal into three parts. The first part was designated for single-cell preparation, the second part was stored in 10% neutral formalin for paraffin embedding and histological examination, and the third part was stored in liquid nitrogen. Once the bladders were fixed, they were processed by routine tissue processing methods, and embedded in paraffin. Serial tissue sections (5 μm) were cut, processed, and stained with H&E for histopathologic evaluation.
Method details
Single-cell preparation
Tumors were cut into approximately 0.5–2 mm3 samples, transferred to a gentleMACS C-tube (Miltenyi Biotec) containing 2.3 mL of cold DMEM media. Once all the tumors had been processed, 100 μL of Enzyme D, 50 μL of Enzyme R, and 12.5 μL of Enzyme A were added to each gentleMACS C-tube. The C-tubes were attached to the gentleMACS Dissociator with heater and incubated for 30 min at 37°C. Once the dissociation program completed, the cell suspension was passed through a pre-wetted 40 μm cell strainer (Falcon), washed with cold PBS, and centrifuged for 5 min at 500 g at 4°C. Samples were depleted of red blood cells using ACK Lysing buffer (Gibco). Cells were centrifuged and resuspend with 5 mL FACS buffer. Dead Cell Removal Kit (Miltenyi Biotec) was further used to remove dead cells. We obtained the single-cell suspension and detected live cells using a hemocytometer with trypan blue.
Sex-biased gene expression analysis for T cell subtypes
Signature genes of AR-response (HALLMARK_ANDROGEN_RESPONSE) were obtained from GSEA MSigDB. Each AR-response gene was compared between males and females using the Findmarker function regarding each T cell subtype. Genes with Bonferroni-adjusted p values <0.05 were selected as sex-biased genes, and the heatmap visualized sex-specific directions according to the log fold changes.
Single-cell data processing
CellRanger (v 7.1.0) was used to perform alignment, barcode counting, and UMI counting to generate feature-barcode matrices. Unfiltered feature-barcode matrices were used as input for further analysis using Seurat (v4.2.2). Filtering was performed to remove poor quality cells (i.e., cells with nUMIs <500, nGenes <250, mitochondrial ratio >20% and novelty score <0.8). Novelty score [ = log10(nGenes)/log10(nUMIs)] is a measure of diversity of mRNAs captured per cell relative to sequencing depth. Following the initial filtering, 103,576 cells remained, and their counts were normalized using Seurat’s SCTransform approach while regressing for cell cycle score and mitochondrial ratio. Ribosomal genes were removed from variable features prior to running PCA. Batch effects between samples were corrected by integration. No reference samples were used during integration and clustering was performed at a resolution of 1.4. The resulting clusters were annotated as 11 cell types: epithelial cells, fibroblasts, myeloid cells (macrophages, MDSCs), lymphoid cells (T cells, NK cells, B cells), endothelial cells, lymphatic endothelial cells, muscle cells, and neurons based on expression of published markers in each cluster. Clusters expressing markers for multiple cell types were removed.
Subtype analysis was performed on fibroblasts, epithelial cells, and myeloid cells. For each cell type, all cells belonging to a particular cell type were subset and Seurat’s SCTransform approach was applied again while regressing for cell cycle score and mitochondrial ratio. Integration was performed and clustered using a resolution of 1.4. Batch specific clusters and clusters expressing markers for multiple cell types were removed from analysis. To get accurate markers for subtypes, SCTransform was performed again, and clusters were annotated based on markers, receptors, or function (based on GSEA).
To identify differential expressed genes between male and female samples in fibroblasts, epithelial cells, and myeloid cells, pseudo bulk RNA Seq approach was used. For each cell type, the reads of all genes belonging to male samples were added and the reads of all genes belonging to female samples were added. The resulting read count matrix which is similar to bulk RNA Seq read count matrix was analyzed using DESeq2 with ∼ sex as the design variable.
Identification of CD8 T cell subtypes and sex-biased gene expression analysis
Subtype analysis was performed on 2,464 CD8 T cells. The SCTransform method was used for normalization. Integration was performed using female samples as a reference and clustered using a resolution of 0.9. For CD8 T cells, AR-response genes were tested for sex-biased gene expression analysis. Based on the same method as the previous section, sex-biased genes were calculated and visualized.
Calculation of CD8 T cell signature gene scores
The signature genes of exhausted CD8 T cell (GSE41867_NAIVE_VS._DAY30_LCMV_ CLONE13_EXHAUSTED_CD8_T cell_DN), naive CD8 T cells (GSE40666_NAIVE_VS._ EFFECTOR_CD8_T cell_UP) were obtained from GSEA MSigDB. As previously described,36 the expression submatrix was constructed by matching signature genes for three gene lists. PCA was implemented to decompose the submatrix, producing the loading matrix (PC by gene) and the score matrix (cell by PC). PC1 of the score matrix was used as the signature score and visualized on UMAP.
Trajectory inference using exhaustion signature gene score
Slingshot (v.2.2.1) was used to conduct trajectory analysis as previously described.36 Based on the naive and exhaustion signature gene score, the “C6” and “C3” clusters were selected as the start cluster and the end cluster for the slingshot function, respectively. For exhaustion lineage, K-S non-parametric tests were conducted to test the velocity of female and male CD8 T cell exhaustion.
Ingenuity pathway analysis
We performed upstream regulator and canonical pathway analyses using Qiagen IPA. All significant differentially expressed genes without any cutoff on expression (padj <0.05) for each cell type were mapped to their corresponding entry in the Qiagen knowledge base. The significance values (p value of overlap) for the canonical pathways are calculated by the right-tailed Fisher’s Exact Test. The significance indicates the probability of association of molecules from the dataset with the canonical pathway by random chance alone. The overall activation/inhibition states of canonical pathways are predicted based on a Z score algorithm. Pathways with Z score >2 are considered to be activated and those with Z score <2 to be inhibited.
Upstream Regulator Analysis identifies upstream regulators that may be responsible for gene expression changes observed in the dataset. The analysis examines the known targets of each upstream regulator in the dataset, compares the target’s actual direction of change to expectations derived from the literature, then issues a prediction for each upstream regulator using a Z score algorithm. The Z score algorithm is designed to reduce the chance that random data will generate significant predictions and the direction of change is the gene expression in the experimental samples relative to a control. An upstream regulator is activated if the Z score is ≥2 and inhibited if the Z score ≤ −2.
Survival analysis
Bulk RNA seq data for bladder cancer in the Pan-Cancer Atlas was downloaded from GDC portal using project id (TCGA-BLCA). Normalized counts were calculated using DESeq2 package and patients without survival data were excluded from the analysis. Microarray data for GSE13507 and MKSCC cohort were provided by Dr. Garrett M. Dancik, Blaveri cohort was obtained from Blaveri et al.,47 GSE32894 was obtained from GEO. Normalized counts of TCGA bulk RNA Seq data or microarray data were log transformed (log2(1+exp)) and median centered. Based on the previous study method,75 we first performed survival analysis independently (one gene at a time) for each of the upregulated genes using male TCGA patients. Briefly, surv_cutpoint function from survminer package was used to find optimum expression cutoff for each gene to stratify male patients into high and low expression groups. Univariate cox regression analysis was used to determine statistical significance (p values) and hazard ratio (HR) for each gene. Genes with p value >0.05 were excluded from further analysis. Genes with p value <0.05 were classified into two groups: Male (HR > 1) and Male (HR < 1). Similarly, we performed survival analysis for each of the upregulated gene using female TCGA patients and classified the genes with p value <0.05 into two groups: Female (HR > 1) and Female (HR < 1). Venn diagram was used to identify sex specific prognostic genes whose high expression correlated to poor survival in either male or female patients. Next, we verified if the gene signatures stratified survival in microarray datasets. Since the microarray datasets didn’t contain expression data for all the signature genes, we computed aggregate expression of only those signature genes that were represented in each microarray dataset and performed survival analysis. Aggregate expression of signature genes were calculated for each sample similar to the “Z score activation metric” described in Levine et al.76 Next, surv_cutpoint function from survminer package was used to stratify patients into high and low expression groups. survival and survminer packages were used to perform survival analysis and univariate cox regression analysis was used to determine statistical significance and hazard ratio.
Nanostring GeoMx Digital Spatial Profiling (DSP)
Tumor microarray (TMA) was generated by a clinical pathologist (Cedars-Sinai Biobank Core) from male and female BBN-induced tumors. The TMA block were sectioned 5μm-thick, stained, and analyzed on the Nanostring GeoMx Digital Spatial Profiling (DSP) platform at the Cedars-Sinai Medical Center biobank as per manufacturer’s instructions.77 Briefly, the TMA slides were stained with a mixture of detection and morphological markers. The detection antibodies include six modules of the GeoMx assay (mouse immune cell profiling, immuno-oncology (IO) drug targets, immune activation, immune cell typing, pan-tumor, and cell death markers). S6 ribosomal protein and histone 3 were included in the panel as housekeeping proteins.
One region of interest (ROI) per core was selected by aligning fluorescent images with H&E images predetermined by a pathologist. Region with blood vessels and necrosis were kept to a minimum. ROI selection on each TMA core was performed such that 660 μm circles were segmented into cytokeratin+ and CD45+ regions. Barcodes from these regions were collected to generate measurements per compartment. Barcodes were sequenced, mapped, and counted by next-generation sequencing (NGS) readouts as per manufacturer’s instructions. Antibody barcodes were counted on an nCounter platform as per manufacturer’s instructions allowing quantitative comparisons of antibodies between ROIs in male and female BBN tumors. The GeoMx DSP analysis suite (GEOMX-0069) allowed inspection, quality control (QC), normalization, and differential expression to be performed. Briefly, normalization using Histone H3 and S6 proteins was performed. Differential expression was performed by a Mann–Whitney test with Benjamini–Hochberg correction.
Estimation of transcription factor activity
We used SCENIC-AUCell (SCENIC v.1.3.1 and AUCell v.1.20.2) and VIPER-DOROTHEA (viper v.1.32.0 and dorothea v.1.10.0) for inferring TF activity in the samples. We used ABC categories of DOROTHEA TFs in our analysis. The viper scores were incorporated into Seurat object and differential regulons between male and female samples were identified using FindMarkers() from Seurat. The average TF activity for each sample was calculated and plotted as heatmaps.
For SCENIC analysis, we followed the standard workflow and calculated TF activity in each cell using AUCell. The AUCell scores were incorporated into Seurat object and differential regulons between male and female samples were identified using FindMarkers() from Seurat. The average TF activity for each sample was calculated and plotted as heatmaps.
Identification of ligand-receptor pairs
We used CellChat (v.1.6.1) to identify ligand-receptor pairs that were differentially expressed. Briefly, we identified LR pairs where both the ligand as well as the receptor are overexpressed (log2FC ≥ 0.2) in male samples and expressed in at least 20% of cells. The average expression of each LR pair was plotted as heatmap.
Quantification and statistical analysis
All statistical testing was performed using either R (version 4.2.2) or GraphPad Prism software (version 8). A p value cutoff of less than 0.05 was considered significant. p values in survival analysis was calculated using surv_pvalue() of survminer package while HR values and confidence intervals were determined using coxph() of survival package. p values in boxplots (Figures 3B and 3E) were calculated using two-tailed t-tests in GraphPad Prism. Differential expression in GeoMX was performed by Mann–Whitney test with Benjamini–Hochberg correction.
Acknowledgments
This work was supported by NIH CA075115 to D.T., NIH P01CA278732 to D.T, H.A.A., Z.L. and R01CA262069 to Z.L.. Vanessa Neang, and Domenico Viola assisted in sample preparation for single cell analysis. Microarray data for GSE13507 and MKSCC cohort were provided by Dr. Garrett M. Dancik. We thank Krishnan Ramanujan, Xiaopu Yuan and Mingtian Che (Cedars Sinai Biobank and Translational Core).
Author contributions
The study was conceptualized by H.A.A. and D.T. H.A.A. and D.T. designed the experiments and provided reagents; H.A.A. and K.H.G. conducted experiments and acquired data; H.A.A., S.K.M., K.H.G., W.H., Y.C., T.X., Q.M., and S.K. analyzed data, discussed, and interpreted results; H.A.A., S.K.M., Y.C., and Q.M. generated the figures; H.A.A., S.K.M., T.X., and D.T. wrote the manuscript. H.A.A., S.K.M., S.K., D.T., and Z.L. reviewed the manuscript. All authors reviewed and approved the final manuscript.
Declaration of interests
Z.L. reports personal consultation fees from Alphamab, HanchorBio, Henlius, Heat Biologic, and Ikonisys outside the submitted work. All other authors declare no competing interests.
Published: August 23, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107703.
Contributor Information
Simon R.V. Knott, Email: simon.knott@cshs.org.
Dan Theodorescu, Email: dan.theodorescu@cshs.org.
Supplemental information
Data and code availability
-
•
Single-cell RNA-seq data have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Anwar M., Ejaz M., Ijaz M., Ahmad G., Ayub H., Ali S.H.B. Association of IL-17A promoter region SNP-rs2275913 with urinary bladder cancer. Int. J. Health Sci. 2023;17:33–38. [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Lam C.M., Li Z., Theodorescu D., Li X. Mechanism of Sex Differences in Bladder Cancer: Evident and Elusive Sex-biasing Factors. Bladder Cancer. 2022;8:241–254. doi: 10.3233/BLC-211658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks P., Soave A., Shariat S.F., Fajkovic H., Fisch M., Rink M. Female with bladder cancer: what and why is there a difference? Transl. Androl. Urol. 2016;5:668–682. doi: 10.21037/tau.2016.03.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theodorescu D., Li Z., Li X. Sex differences in bladder cancer: emerging data and call to action. Nat. Rev. Urol. 2022;19:447–449. doi: 10.1038/s41585-022-00591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaneko S., Li X. X chromosome protects against bladder cancer in females via a KDM6A-dependent epigenetic mechanism. Sci. Adv. 2018;4:eaar5598. doi: 10.1126/sciadv.aar5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran L., Xiao J.F., Agarwal N., Duex J.E., Theodorescu D. Advances in bladder cancer biology and therapy. Nat. Rev. Cancer. 2021;21:104–121. doi: 10.1038/s41568-020-00313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koti M., Ingersoll M.A., Gupta S., Lam C.M., Li X., Kamat A.M., Black P.C., Siemens D.R. Sex Differences in Bladder Cancer Immunobiology and Outcomes: A Collaborative Review with Implications for Treatment. Eur. Urol. Oncol. 2020;3:622–630. doi: 10.1016/j.euo.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Li C.H., Prokopec S.D., Sun R.X., Yousif F., Schmitz N., Boutros P.C. Sex differences in oncogenic mutational processes. Nat. Commun. 2020;11:4330. doi: 10.1038/s41467-020-17359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopes-Ramos C.M., Quackenbush J., DeMeo D.L. Genome-Wide Sex and Gender Differences in Cancer. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.597788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellamri M., Walmsley S.J., Brown C., Brandt K., Konorev D., Day A., Wu C.F., Wu M.T., Turesky R.J. DNA Damage and Oxidative Stress of Tobacco Smoke Condensate in Human Bladder Epithelial Cells. Chem. Res. Toxicol. 2022;35:1863–1880. doi: 10.1021/acs.chemrestox.2c00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fantini D., Glaser A.P., Rimar K.J., Wang Y., Schipma M., Varghese N., Rademaker A., Behdad A., Yellapa A., Yu Y., et al. A Carcinogen-induced mouse model recapitulates the molecular alterations of human muscle invasive bladder cancer. Oncogene. 2018;37:1911–1925. doi: 10.1038/s41388-017-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams P.D., Lee J.K., Theodorescu D. Molecular credentialing of rodent bladder carcinogenesis models. Neoplasia. 2008;10:838–846. doi: 10.1593/neo.08432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertram J.S., Craig A.W. Specific induction of bladder cancer in mice by butyl-(4-hydroxybutyl)-nitrosamine and the effects of hormonal modifications on the sex difference in response. Eur. J. Cancer. 1972;8:587–594. doi: 10.1016/0014-2964(72)90137-5. [DOI] [PubMed] [Google Scholar]
- 15.Becci P.J., Thompson H.J., Strum J.M., Brown C.C., Sporn M.B., Moon R.C. N-butyl-N-(4-hydroxybutyl)nitrosamine-induced urinary bladder cancer in C57BL/6 X DBA/2 F1 mice as a useful model for study of chemoprevention of cancer with retinoids. Cancer Res. 1981;41:927–932. [PubMed] [Google Scholar]
- 16.Dietrich K., Demidenko E., Schned A., Zens M.S., Heaney J., Karagas M.R. Parity, early menopause and the incidence of bladder cancer in women: a case-control study and meta-analysis. Eur. J. Cancer. 2011;47:592–599. doi: 10.1016/j.ejca.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lombardo K.A., Obradovic A., Singh A.K., Liu J.L., Joice G., Kates M., Bishai W., McConkey D., Chaux A., Eich M.L., et al. BCG invokes superior STING-mediated innate immune response over radiotherapy in a carcinogen murine model of urothelial cancer. J. Pathol. 2022;256:223–234. doi: 10.1002/path.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellmunt J., de Wit R., Vaughn D.J., Fradet Y., Lee J.L., Fong L., Vogelzang N.J., Climent M.A., Petrylak D.P., Choueiri T.K., et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg J.E., Hoffman-Censits J., Powles T., van der Heijden M.S., Balar A.V., Necchi A., Dawson N., O'Donnell P.H., Balmanoukian A., Loriot Y., et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma P., Callahan M.K., Bono P., Kim J., Spiliopoulou P., Calvo E., Pillai R.N., Ott P.A., de Braud F., Morse M., et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17:1590–1598. doi: 10.1016/S1470-2045(16)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glaser A.P., Procissi D., Yu Y., Meeks J.J. Magnetic Resonance Imaging Assessment of Carcinogen-induced Murine Bladder Tumors. J. Vis. Exp. 2019 doi: 10.3791/59101. [DOI] [PubMed] [Google Scholar]
- 22.Tu M.M., Lee F.Y.F., Jones R.T., Kimball A.K., Saravia E., Graziano R.F., Coleman B., Menard K., Yan J., Michaud E., et al. Targeting DDR2 enhances tumor response to anti-PD-1 immunotherapy. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aav2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conforti F., Pala L., Bagnardi V., De Pas T., Marco M., Viale G., Gelber R., Goldhirsch A. Sex as a predictor of response to cancer immunotherapy - Authors' reply. Lancet Oncol. 2018;19:e380–e381. doi: 10.1016/S1470-2045(18)30535-7. [DOI] [PubMed] [Google Scholar]
- 24.Lee H.W., Chung W., Lee H.O., Jeong D.E., Jo A., Lim J.E., Hong J.H., Nam D.H., Jeong B.C., Park S.H., et al. Single-cell RNA sequencing reveals the tumor microenvironment and facilitates strategic choices to circumvent treatment failure in a chemorefractory bladder cancer patient. Genome Med. 2020;12:47. doi: 10.1186/s13073-020-00741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gouin K.H., 3rd, Ing N., Plummer J.T., Rosser C.J., Ben Cheikh B., Oh C., Chen S.S., Chan K.S., Furuya H., Tourtellotte W.G., et al. An N-Cadherin 2 expressing epithelial cell subpopulation predicts response to surgery, chemotherapy and immunotherapy in bladder cancer. Nat. Commun. 2021;12:4906. doi: 10.1038/s41467-021-25103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z., Zhou L., Liu L., Hou Y., Xiong M., Yang Y., Hu J., Chen K. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Commun. 2020;11:5077. doi: 10.1038/s41467-020-18916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Degoricija M., Korac-Prlic J., Vilovic K., Ivanisevic T., Haupt B., Palada V., Petkovic M., Karaman I., Terzic J. The dynamics of the inflammatory response during BBN-induced bladder carcinogenesis in mice. J. Transl. Med. 2019;17:394. doi: 10.1186/s12967-019-02146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overdevest J.B., Knubel K.H., Duex J.E., Thomas S., Nitz M.D., Harding M.A., Smith S.C., Frierson H.F., Conaway M., Theodorescu D. CD24 expression is important in male urothelial tumorigenesis and metastasis in mice and is androgen regulated. Proc. Natl. Acad. Sci. USA. 2012;109:E3588–E3596. doi: 10.1073/pnas.1113960109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aibar S., González-Blas C.B., Moerman T., Huynh-Thu V.A., Imrichova H., Hulselmans G., Rambow F., Marine J.C., Geurts P., Aerts J., et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods. 2017;14:1083–1086. doi: 10.1038/nmeth.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saad M., Lee S.J., Tan A.C., El Naqa I.M., Hodi F.S., Butterfield L.H., LaFramboise W.A., Storkus W., Karunamurthy A.D., Conejo-Garcia J., et al. Enhanced immune activation within the tumor microenvironment and circulation of female high-risk melanoma patients and improved survival with adjuvant CTLA4 blockade compared to males. J. Transl. Med. 2022;20:253. doi: 10.1186/s12967-022-03450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang H., Li H., Sun Z. Targeting myeloid-derived suppressor cells for cancer therapy. Cancer Biol. Med. 2021;18:992–1009. doi: 10.20892/j.issn.2095-3941.2020.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leblond M.M., Zdimerova H., Desponds E., Verdeil G. Tumor-Associated Macrophages in Bladder Cancer: Biological Role, Impact on Therapeutic Response and Perspectives for Immunotherapy. Cancers. 2021;13 doi: 10.3390/cancers13184712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh N., Baby D., Rajguru J.P., Patil P.B., Thakkannavar S.S., Pujari V.B. Inflammation and cancer. Ann. Afr. Med. 2019;18:121–126. doi: 10.4103/aam.aam_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Alonso L., Holland C.H., Ibrahim M.M., Turei D., Saez-Rodriguez J. Benchmark and integration of resources for the estimation of human transcription factor activities. Genome Res. 2019;29:1363–1375. doi: 10.1101/gr.240663.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon H., Schafer J.M., Song N.J., Kaneko S., Li A., Xiao T., Ma A., Allen C., Das K., Zhou L., et al. Androgen conspires with the CD8(+) T cell exhaustion program and contributes to sex bias in cancer. Sci. Immunol. 2022;7 doi: 10.1126/sciimmunol.abq2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doering T.A., Crawford A., Angelosanto J.M., Paley M.A., Ziegler C.G., Wherry E.J. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giordano M., Henin C., Maurizio J., Imbratta C., Bourdely P., Buferne M., Baitsch L., Vanhille L., Sieweke M.H., Speiser D.E., et al. Molecular profiling of CD8 T cells in autochthonous melanoma identifies Maf as driver of exhaustion. EMBO J. 2015;34:2042–2058. doi: 10.15252/embj.201490786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crowl J.T., Heeg M., Ferry A., Milner J.J., Omilusik K.D., Toma C., He Z., Chang J.T., Goldrath A.W. Tissue-resident memory CD8(+) T cells possess unique transcriptional, epigenetic and functional adaptations to different tissue environments. Nat. Immunol. 2022;23:1121–1131. doi: 10.1038/s41590-022-01229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lv S., Wang W., Wang H., Zhu Y., Lei C. PPARgamma activation serves as therapeutic strategy against bladder cancer via inhibiting PI3K-Akt signaling pathway. BMC Cancer. 2019;19:204. doi: 10.1186/s12885-019-5426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorrelle N., Dominguez A.T.A., Brekken R.A. From top to bottom: midkine and pleiotrophin as emerging players in immune regulation. J. Leukoc. Biol. 2017;102:277–286. doi: 10.1189/jlb.3MR1116-475R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Domenis R., Cifù A., Marinò D., Fabris M., Niazi K.R., Soon-Shiong P., Curcio F. Toll-like Receptor-4 Activation Boosts the Immunosuppressive Properties of Tumor Cells-derived Exosomes. Sci. Rep. 2019;9:8457. doi: 10.1038/s41598-019-44949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unoki M., Nakamura Y. EGR2 induces apoptosis in various cancer cell lines by direct transactivation of BNIP3L and BAK. Oncogene. 2003;22:2172–2185. doi: 10.1038/sj.onc.1206222. [DOI] [PubMed] [Google Scholar]
- 44.Shin S.H., Kim I., Lee J.E., Lee M., Park J.W. Loss of EGR3 is an independent risk factor for metastatic progression in prostate cancer. Oncogene. 2020;39:5839–5854. doi: 10.1038/s41388-020-01418-5. [DOI] [PubMed] [Google Scholar]
- 45.Liu X. Targeting Polo-Like Kinases: A Promising Therapeutic Approach for Cancer Treatment. Transl. Oncol. 2015;8:185–195. doi: 10.1016/j.tranon.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez-Carbayo M., Socci N.D., Lozano J., Saint F., Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J. Clin. Oncol. 2006;24:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 47.Blaveri E., Simko J.P., Korkola J.E., Brewer J.L., Baehner F., Mehta K., Devries S., Koppie T., Pejavar S., Carroll P., Waldman F.M. Bladder cancer outcome and subtype classification by gene expression. Clin. Cancer Res. 2005;11:4044–4055. doi: 10.1158/1078-0432.CCR-04-2409. [DOI] [PubMed] [Google Scholar]
- 48.Sjödahl G., Lauss M., Lövgren K., Chebil G., Gudjonsson S., Veerla S., Patschan O., Aine M., Fernö M., Ringnér M., et al. A molecular taxonomy for urothelial carcinoma. Clin. Cancer Res. 2012;18:3377–3386. doi: 10.1158/1078-0432.CCR-12-0077-T. [DOI] [PubMed] [Google Scholar]
- 49.Kim W.J., Kim E.J., Kim S.K., Kim Y.J., Ha Y.S., Jeong P., Kim M.J., Yun S.J., Lee K.M., Moon S.K., et al. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol. Cancer. 2010;9:3. doi: 10.1186/1476-4598-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mancini M., Righetto M., Baggio G. Spotlight on gender-specific disparities in bladder cancer. Urologia. 2020;87:103–114. doi: 10.1177/0391560319887327. [DOI] [PubMed] [Google Scholar]
- 51.Ide H., Miyamoto H. Sex Hormone Receptor Signaling in Bladder Cancer: A Potential Target for Enhancing the Efficacy of Conventional Non-Surgical Therapy. Cells. 2021;10 doi: 10.3390/cells10051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han J., Yang Y., Li X., Wu J., Sheng Y., Qiu J., Wang Q., Li J., He Y., Cheng L., Zhang Y. Pan-cancer analysis reveals sex-specific signatures in the tumor microenvironment. Mol. Oncol. 2022;16:2153–2173. doi: 10.1002/1878-0261.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wuidar V., Gillot L., Dias Da Silva I., Lebeau A., Gallez A., Pequeux C. Sex-Based Differences in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2021;1329:499–533. doi: 10.1007/978-3-030-73119-9_23. [DOI] [PubMed] [Google Scholar]
- 54.Constantin T., Păvălean M., Bucur Ș., Constantin M.M., Nicolescu A.C., Pacu I., Mădan V. Animal Models in Bladder Cancer. Biomedicines. 2021;9 doi: 10.3390/biomedicines9121762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Saito R., Smith C.C., Utsumi T., Bixby L.M., Kardos J., Wobker S.E., Stewart K.G., Chai S., Manocha U., Byrd K.M., et al. Molecular Subtype-Specific Immunocompetent Models of High-Grade Urothelial Carcinoma Reveal Differential Neoantigen Expression and Response to Immunotherapy. Cancer Res. 2018;78:3954–3968. doi: 10.1158/0008-5472.CAN-18-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu Z., Wen Z., Li Z., Yu M., Ye G. Identification and prognostic value of a glycolysis-related gene signature in patients with bladder cancer. Medicine (Baltim.) 2021;100 doi: 10.1097/MD.0000000000023836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scholtes M.P., de Jong F.C., Zuiverloon T.C.M., Theodorescu D. Role of Bladder Cancer Metabolic Reprogramming in the Effectiveness of Immunotherapy. Cancers. 2021;13 doi: 10.3390/cancers13020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Afonso J., Santos L.L., Longatto-Filho A., Baltazar F. Competitive glucose metabolism as a target to boost bladder cancer immunotherapy. Nat. Rev. Urol. 2020;17:77–106. doi: 10.1038/s41585-019-0263-6. [DOI] [PubMed] [Google Scholar]
- 59.Colak S., Ten Dijke P. Targeting TGF-beta Signaling in Cancer. Trends Cancer. 2017;3:56–71. doi: 10.1016/j.trecan.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Mariathasan S., Turley S.J., Nickles D., Castiglioni A., Yuen K., Wang Y., Kadel E.E., III, Koeppen H., Astarita J.L., Cubas R., et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhuang J., Lu Q., Shen B., Huang X., Shen L., Zheng X., Huang R., Yan J., Guo H. TGFbeta1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci. Rep. 2015;5 doi: 10.1038/srep11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kadomoto S., Izumi K., Mizokami A. Roles of CCL2-CCR2 Axis in the Tumor Microenvironment. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22168530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tu M.M., Abdel-Hafiz H.A., Jones R.T., Jean A., Hoff K.J., Duex J.E., Chauca-Diaz A., Costello J.C., Dancik G.M., Tamburini B.A.J., et al. Inhibition of the CCL2 receptor, CCR2, enhances tumor response to immune checkpoint therapy. Commun. Biol. 2020;3:720. doi: 10.1038/s42003-020-01441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang L., Wang Y., Song M., Chang A., Zhuo W., Zhu Y. Fibronectin 1 as a Key Gene in the Genesis and Progression of Cadmium-Related Bladder Cancer. Biol. Trace Elem. Res. 2022;201:4349–4359. doi: 10.1007/s12011-022-03510-1. [DOI] [PubMed] [Google Scholar]
- 65.Gabrilovich D.I. All Myeloid-Derived Suppressor Cells Are Not Created Equal: How Gender Inequality Influences These Cells and Affects Cancer Therapy. Cancer Discov. 2020;10:1100–1102. doi: 10.1158/2159-8290.CD-20-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Veglia F., Perego M., Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018;19:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schneider A.K., Chevalier M.F., Derré L. The multifaceted immune regulation of bladder cancer. Nat. Rev. Urol. 2019;16:613–630. doi: 10.1038/s41585-019-0226-y. [DOI] [PubMed] [Google Scholar]
- 68.Zhao J., Chen X., Herjan T., Li X. The role of interleukin-17 in tumor development and progression. J. Exp. Med. 2020;217 doi: 10.1084/jem.20190297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song X., Wei C., Li X. The potential role and status of IL-17 family cytokines in breast cancer. Int. Immunopharm. 2021;95 doi: 10.1016/j.intimp.2021.107544. [DOI] [PubMed] [Google Scholar]
- 70.Korpal M., Puyang X., Jeremy Wu Z., Seiler R., Furman C., Oo H.Z., Seiler M., Irwin S., Subramanian V., Julie Joshi J., et al. Evasion of immunosurveillance by genomic alterations of PPARgamma/RXRalpha in bladder cancer. Nat. Commun. 2017;8:103. doi: 10.1038/s41467-017-00147-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tate T., Xiang T., Wobker S.E., Zhou M., Chen X., Kim H., Batourina E., Lin C.S., Kim W.Y., Lu C., et al. Pparg signaling controls bladder cancer subtype and immune exclusion. Nat. Commun. 2021;12:6160. doi: 10.1038/s41467-021-26421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taneja V. Sex Hormones Determine Immune Response. Front. Immunol. 2018;9:1931. doi: 10.3389/fimmu.2018.01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yi R., Hong S., Zhang Y., Lin A., Ying H., Zou W., Wang Q., Wei T., Cheng Q., Zhu W., et al. MHC-II Signature Correlates With Anti-Tumor Immunity and Predicts anti-PD-L1 Response of Bladder Cancer. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.757137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kovats S., Carreras E. Regulation of dendritic cell differentiation and function by estrogen receptor ligands. Cell. Immunol. 2008;252:81–90. doi: 10.1016/j.cellimm.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu G.M., Zeng H.D., Zhang C.Y., Xu J.W. Identification of a six-gene signature predicting overall survival for hepatocellular carcinoma. Cancer Cell Int. 2019;19:138. doi: 10.1186/s12935-019-0858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levine D.M., Haynor D.R., Castle J.C., Stepaniants S.B., Pellegrini M., Mao M., Johnson J.M. Pathway and gene-set activation measurement from mRNA expression data: the tissue distribution of human pathways. Genome Biol. 2006;7:R93. doi: 10.1186/gb-2006-7-10-r93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hernandez S., Lazcano R., Serrano A., Powell S., Kostousov L., Mehta J., Khan K., Lu W., Solis L.M. Challenges and Opportunities for Immunoprofile Using a Spatial High-Plex Technology: The NanoString GeoMx((R)) Digital Spatial Profiler. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.890410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Single-cell RNA-seq data have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.