Abstract
Background & Aims
HBV infection is a global health burden. Covalently closed circular DNA (cccDNA) transcriptional regulation is a major cause of poor cure rates of chronic hepatitis B (CHB) infection. Herein, we evaluated whether targeting host factors to achieve functional silencing of cccDNA may represent a novel strategy for the treatment of HBV infection.
Methods
To evaluate the effects of Jumonji C domain-containing (JMJD2) protein subfamily JMJD2A-2D proteins on HBV replication, we used lentivirus-based RNA interference to suppress the expression of isoforms JMJD2A-2D in HBV-infected cells. JMJD2D-knockout mice were generated to obtain an HBV-injected model for in vivo experiments. Co-immunoprecipitation and ubiquitylation assays were used to detect JMJD2D-HBx interactions and HBx stability modulated by JMJD2D. Chromatin immunoprecipitation assays were performed to investigate JMJD2D-cccDNA and HBx-cccDNA interactions.
Results
Among the JMJD2 family members, JMJD2D was significantly upregulated in mouse livers and human hepatoma cells. Downregulation of JMJD2D inhibited cccDNA transcription and HBV replication. Molecularly, JMJD2D sustained HBx stability by suppressing the TRIM14-mediated ubiquitin-proteasome degradation pathway and acted as a key co-activator of HBx to augment HBV replication. The JMJD2D-targeting inhibitor, 5C-8-HQ, suppressed cccDNA transcription and HBV replication.
Conclusion
Our study clarified the mechanism by which JMJD2D regulates HBV transcription and replication and identified JMJD2D as a potential diagnostic biomarker and promising drug target against CHB, and HBV-associated hepatocarcinoma.
Impact and implications
HBV cccDNA is central to persistent infection and is a major obstacle to healing CHB. In this study, using cellular and animal HBV models, JMJD2D was found to stabilise and cooperate with HBx to augment HBV transcription and replication. This study reveals a potential novel translational target for intervention in the treatment of chronic hepatitis B infection.
Keywords: Chronic hepatitis B, HBV replication, cccDNA transcription, JMJD2D, HBx, Intervention target
Graphical abstract

Highlights
-
•
JMJD2D promotes HBV transcription and replication by stabilising HBx protein.
-
•
JMJD2D sustains HBx protein stability by suppressing the TRIM14-modulated ubiquitin-proteasome degradation pathway.
-
•
JMJD2D cooperates with HBx to drive cccDNA transcription by demethylating H3K9me3 and antagonising the repressive activity of p53.
-
•
Knockdown of JMJD2D or 5C-8-HQ-mediated-JMJD2D inhibition effectively suppresses HBV replication in vitro and in vivo.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common and deadliest types of cancer globally.1,2 HBV infection, which can lead to the development of chronic liver diseases such as cirrhosis,1,3 is the most important risk factor for HCC. HBV infection remains a global health threat, despite the availability of prophylactic vaccines. The current treatment of chronic hepatitis B (CHB) infection is not optimistic.
HBV is a DNA virus belonging to the Hepadnaviridae family and retains a distinctive strategy for replication.4 HBV enters hepatocytes by binding to the sodium taurocholate cotransporting polypeptide (NTCP) receptor on the hepatocyte membrane.5 After the coat protein of HBV is removed, the viral capsids are translocated to the nucleus. Then, the viral relaxed circular DNA (rcDNA) is converted into covalently closed circular DNA (cccDNA).6 cccDNA serves as a template for the transcription of all HBV RNAs, including pregenomic RNA (pgRNA) and subgenomic RNAs for antigen production and viral replication in host cells.7 HBV pgRNA serves as the template for the translation of the core protein and polymerase. In the cytoplasm, pgRNA is encapsulated with polymerase by HBcAg and is reverse-transcribed into rcDNA. HBsAg then coats the nucleocapsid containing rcDNA to produce more viruses.7,8
Currently, two classes of drugs are approved for the treatment of CHB infection, including interferons and nucleoside analogues. Both treatments have some limitations, more side effects, and little or no effect on cccDNA; thus, neither can eliminate HBV infection.9 However, the why CHB is so difficult to cure is actively being researched. Current studies have found that the major obstacles to antiviral therapies are viral cccDNA persistence and transcription.[10], [11], [12] Therefore, it is essential to develop a treatment strategy for CHB infection that either eliminates or continuously suppresses the transcriptional activity of viral cccDNA.10,13,14
In the hepatocyte nucleus, HBV cccDNA can recruit histones or other non-histone proteins to organise into a minichromosome with a chromatin-like structure. Even after long-term antiviral therapy, this minichromosome can stably exist in liver cells for a prolonged period. Increasing evidence has shown that, similar to host genes, HBV cccDNA undergoes epigenetic modifications and that cccDNA transcription is regulated by many transcriptional regulators.3,15
Among the proteins that regulate cccDNA transcription is HBx, a critical viral regulatory protein, which plays a dominant role in stimulating HBV transcription and replication.16,17 The HBV X gene encodes HBx, a nonstructural protein consisting of 154 amino acids, which has an extensive transactivation function and can promote the development of HCC.[17], [18], [19] Although HBx interacts with the proteins involved in epigenetic regulation in host cells and participates in the regulation of HBV transcription and replication, the precise mechanism by which HBx interacts with host factors to coordinate the regulation of HBV transcription and replication remains unclear.
JMJD2D belongs to the Jumonji C domain-containing (JMJD2) protein subfamily that includes four members (JMJD2A-2D). All four JMJD2 members can catalyse the demethylation of lysine to regulate gene expression.20 Furthermore, upregulation of JMJD2D promotes hepatic fibrogenesis and the development of liver cancer.21,22 Our previous studies systematically elucidated the regulatory roles of JMJD2D in colon cancer and liver cancer development.[22], [23], [24] Previous studies have demonstrated that JMJD2D can promote HCC initiation and progression by simultaneously antagonising the tumour suppressor protein p53 and activating the oncogenic Wnt/β-catenin signalling pathway.[22], [23], [24] Since HBV infection is the most important risk factor for HCC, it is important to understand the role of JMJD2D in HBV transcription and replication.
In the current study, we established HBV-infected hepatocytes and an HBV-expressing mouse model and used these tools to demonstrate that JMJD2D is a critical host factor that influences HBV replication by enhancing HBV cccDNA transcription in an HBx-dependent manner.
Materials and methods
Cell culture
HepG2, Huh-7, 293T, and HepAD38 cells were maintained in DMEM (Yuanpei Technologies) containing 10% foetal bovine serum (PAN). HepAD38 cells were supplemented with 400 μg/ml G418 (Solarbio). HepaRG cells were cultured in William’s E medium supplemented with 10% FCS, 7 × 10−2 mM hydrocortisone hemisuccinate, and 5 μg/ml insulin. HepaRG cell differentiation was induced as described previously.25,26 All cells were cultured in a humidified incubator at 37 °C with 5% CO2. The cell lines used are listed in Table S1.
Plasmids and transfection
The pHBV1.3 plasmid, containing 1.3 units of the HBV genome was purchased from Addgene. HBx and JMJD2D expression plasmids were generated and stored in our laboratory. The HBx expression plasmid was constructed by cloning the HBx-encoding fragment from the pHBV1.3 plasmid and inserting it into pCMV plasmids tagged HA or Flag by expression under the CMV promoter. The different HBx domains were cloned from the HBx expression plasmid and individually inserted into the pcDNA3.1-GFP plasmid. The plasmids were transfected into HepG2 cells using Lipofectamine 2000 (Invitrogen). The GFP-tagged HBx and its truncated proteins were pulled down using magnetic protein A/G bead-conjugated GFP antibody (MedChemExpress). Western blotting was performed to detect interactions between truncated HBx proteins and JMJD2D.
HBV infection
The supernatants from cultured HepAD38 cells were harvested and concentrated. After the collected medium was filtered, PEG8000 was added to the final concentration of 10%, and the virus pellet was dissolved in a serum-free medium after centrifugation as described previously.27 For HBV infection, HepG2-NTCP, Huh-7-NTCP, and differentiated HepaRG cells were cultured in a medium supplemented with 4% PEG8000 and incubated with concentrated HBV particles.27
HBV cccDNA extraction and quantitation
HBV cccDNA was extracted from infected cells using the Hirt method with minor modifications.28,29 Quantification of cccDNA was performed by quantitative PCR. cccDNA levels were normalised to mitochondria DNA (mtDNA). The specific primers of cccDNA and mtDNA are listed in Table S2.
RNA extraction and real-time PCR
Cellular RNA and HBV RNA was extracted using TRIzol (Life Technologies) according to the manufacturer’s protocol. Reverse transcription was performed by using RT Master Mix (Abm). Real-time quantitative PCR was performed using universal SYBR green Master Mix (Abclonal) to quantitate the levels of HBV RNA. Primers used for Real-time PCR are listed in Table S2.
Chromatin immunoprecipitation assay
HBV-infected cells were fixed with formaldehyde and de-crosslinked with glycine. Then, JMJD2D (Proteintech, 22591-1-AP) and HBx (Abcam, ab2741) antibodies were used to pull down the protein-DNA complexes as previously described.26 Immunoprecipitated cccDNA was quantified by quantitative PCR (qPCR) using specific primers. The primers used for the ChIP assay are listed in Table S2.
Enzyme-linked immunosorbent assays
The levels of HBsAg and HBeAg in the supernatants of HBV-infected hepatocytes were examined using ELISA kits (Kehua Biotech) according to the manufacturer's protocol. The ELISA data were obtained from four independent experiments.
Co-immunoprecipitation and western blotting
The interaction between JMJD2D and HBx proteins was validated by co-immunoprecipitation (Co-IP) using magnetic protein A/G beads (MedChemExpress) conjugated to JMJD2D (Abcam, ab93694) and HBx (BioVendor, RD981038100) antibodies. Proteins in pull-down samples were examined by western blotting. Flag- or HA-tagged HBx together with JMJD2D were overexpressed in 293T cells, and cell lysate was harvested and subjected to immunoprecipitation using Flag or HA magnetic beads (MedChemExpress). Western blotting was performed to examine JMJD2D (Abcam, ab93694) and HBx (BioVendor, RD981038100) expression and the interaction between HBx and JMJD2D using anti-Flag (Sigma, F3165), anti-HA (Abclonal, AE008) and JMJD2D antibodies. The antibodies used in this study are listed in Table S3.
HBx protein stability and ubiquitylation assays
Cycloheximide (CHX) chase assay was used to determine the stability of HBx protein. HBx-expressing cells were transfected with shRNA to knockdown JMJD2D or transfected with JMJD2D plasmids, and were treated with CHX for the indicated time periods. Then, cell lysates were collected and analyzed for HBx stability by western blotting. For HBx protein stability assay, MG132 was used to treat HBx-expressing Control and shJMJD2D cells for 6 hours, and samples were collected for western blotting.
Huh-7 or 293T cells expressing HA-HBx (or Flag-HBx) and Ub were transfected with control vector or JMJD2D plasmid for ubiquitylation assays. The immune complexes were pulled down using protein A/G beads conjugated to IgG and HA (or Flag) antibodies, and the ubiquitylated HBx protein ladder was then detected by western blotting.
Murine model
All animal experiments were approved by the Institutional Animal Committee of Xiamen University and were performed according to the specified guidelines. All mice were bred and housed at the Laboratory Animal Center of Xiamen University. Mice were housed under specific pathogen-free conditions with a 12-h/12-h light-dark cycle, and free access to food and water.
Generation of the HBV injection mouse model
The HBV-injected mouse model was generated by hydrodynamic injection of pHBV1.3 plasmids which encoded 1.3 units of the HBV genome. In brief, 15 μg of pHBV1.3 plasmids were diluted in 1 ml of saline, and within 6 to 8 s were injected into the tail veins of the male C57BL/6 mice (6 to 8 weeks old).30 The control group received an equivalent volume of saline without any plasmids.31
In vivo inhibitor intervention
To generate a hydrodynamic HBV-injected mouse model, male C57BL/6 mice (6 to 8 weeks old) were injected with 15 μg of pHBV1.3 plasmids and were then randomly divided into two groups: one group was treated with 10 mg/kg 5C-8-HQ (MedChemExpress), dissolved in 1% sodium carboxymethyl cellulose, and the control group was treated with an equivalent volume of vehicle, respectively. All mouse experiments were conducted in accordance with guidelines established by the Institutional Animal Committee of Xiamen University.
Measurement of serum HBsAg and HBeAg in HBV-injected mice
Male JMJD2D knockout mice were injected with HBV DNA by hydrodynamic injection. Mice were euthanised at the indicated time, and blood was immediately collected. The levels of HBsAg and HBeAg in the sera of mice were examined on day 7 after hydrodynamic injection using ELISA kits (Meike Biotech). The concentration was calculated according to the manufacturer's protocol.
Human specimens
Paraffin-embedded tissue sections of 11 cases of non-HBV-infected liver specimens and 18 cases of HBV-infected liver specimens were obtained from the Chenggong Hospital of Xiamen University, with patient consent and institutional review board approval. The study protocol conformed to the ethical guidelines and was approved by the Institute Research Ethics Committee at Xiamen University. More detailed information on information collected from the patient records is listed in Table S4.
Immunohistochemistry
Liver tissue specimens were fixed in 4% formalin, embedded in paraffin, and then cut into slices of 5-μm in thickness. Immunohistochemistry was performed to stain for JMJD2D using specific antibodies and using an immunohistochemistry (IHC) kit (ZSGB-BIO) according to the manufacturer's protocol. Quantification of positive staining was achieved using ImageJ software.
Gene expression omnibus data analysis
Gene expression data of HBV-infected patients have been deposited in the NCBI Gene Expression Omnibus database under accession number GSE83148 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE83148).
Statistical analysis
Statistical analyses were performed using GraphPad Prism v.7 software. Student’s t test was used to compare the differences between two groups. One-way ANOVA analysis was used to compare three or more groups. All data are expressed as the mean ± standard deviation (SD). Differences were considered statistically significant at p <0.05.
Results
HBV infection enhanced JMJD2D expression
HBV infection is the most prominent risk factor for developing HCC. Our previous study found that JMJD2D is a critical epigenetic factor in HCC progression;22,32 however, whether JMJD2D plays a facilitator role in HBV infection is unclear. Therefore, a dataset consisting of HBV-infected patients (GSE83148) was employed to explore the potential connection between JMJD2D and HBV infection. JMJD2D mRNA levels in the liver of patients infected with HBV were higher than those of healthy individuals, whereas the mRNA levels of other JMJD2 family members (JMJD2A-2C) did not differ significantly (Fig. 1A). Consistently, IHC staining of liver biopsy sections from healthy and HBV-infected individuals showed that the JMJD2D protein levels were higher in HBV-infected liver tissues than in non-infected liver tissues (Fig. 1B and Table. S4). These findings indicate that HBV infection may facilitate JMJD2D upregulation in the liver.
Fig. 1.
HBV infection enhances JMJD2D expression.
(A) The mRNA levels JMJD2D in the liver of HBV-infected patients were higher than those of healthy individuals. JMJD2 protein family (JMJD2A–2D) RNA levels in the GSE83148 database containing gene expression data of 122 HBV-infected liver tissues and six healthy samples were analysed with GEO2R. (B) Immunohistochemistry assay revealed that JMJD2D was frequently increased in liver biopsies from HBV-infected patients. Scale bar: 50 μm. (C) mRNA and protein levels of liver JMJD2D were upregulated in mice tail vein hydrodynamic-injected liver tissues, as measured by qPCR and western blotting, n = 5 in each group. (D) The expression of JMJD2D and p-p65 was increased after HBV infection and was reduced when HBV-infected HepG2-NTCP cells were exposed to 3.0 μM aspirin, as detected by western blotting (left panel). Knockdown of p65 attenuates the expression of JMJD2D induced by HBV infection as detected by western blotting (right panel). For B and C, data are presented as the mean ± SD of multiple samples. Unpaired two-tailed Student’s t test for A-C, n.s., not significant, ∗∗p <0.01, ∗∗∗p <0.001. qPCR, quantitative PCR.
To verify this association, we mimicked HBV infection in vivo by hydrodynamically injecting the tail vein of mice with plasmids carrying HBV DNA; the mice were then euthanised after 7 days for liver mRNA and protein extraction. JMJD2D mRNA and protein levels were higher in the livers of HBV mimic-infected mice than those in the control group (Fig. 1C). To further examine whether HBV infection can upregulate JMJD2D expression autonomously in cells, conditioned medium, containing HBV particles produced by HepAD38 cells was used to culture HepG2-NTCP cells. As shown in Fig. 1D and Fig. S1, the protein levels of JMJD2D were higher in HBV-infected cells than in uninfected cells. Previously, we showed that JMJD2D expression could be upregulated by activated p65 and NF-κB signaling.[22], [23], [24] NF-κB signalling has been reported to be activated during HBV infection.[33], [34], [35] Consistent with this finding, experimental HBV infection activated NF-κB signalling in HepG2-NTCP cells as demonstrated by the increased phosphorylation of p65 (Fig. 1D). Similarly, an increased phosphorylation of p65 in the liver was observed in our hydrodynamic HBV-injected mouse model (Fig. 1C, right panel). Aspirin can inhibit NF-κB signaling;24,36,37 thus, we treated HBV-infected HepG2-NTCP cells with aspirin and then examined protein levels of phosphorylated p65 and JMJD2D. As expected, exposure to aspirin significantly reduced the levels of phosphorylated p65 and JMJD2D which had increased subsequent to HBV infection (Fig. 1D). Furthermore, knockdown of p65 in HBV-infected cells significantly downregulated JMJD2D expression (Fig. 1D). Taken together, these results suggest that HBV infection enhances JMJD2D expression, possibly through activation of p65.
JMJD2D enhanced cccDNA transcription and HBV replication
To identify whether and which JMJD2A-2D isoform could regulate HBV expression, we used lentivirus-based RNA interference (shRNA) to suppress the expression of JMJD2A-2D in HBV-infected Huh-7-NTCP cells (Fig. S2) and then performed ELISA and western blotting to investigate the expression of HBV antigens. The production of HBV antigens was suppressed in JMJD2D-deficient cells as demonstrated by the attenuated levels of HBeAg, HBsAg, and HBx antigen (Fig. 2A and B). In cells deficient in JMJD2A and JMJD2B, viral antigens remained generally unchanged (Fig. 2A and B). In the JMJD2C-knockdown cells, the observed reduction in HBV antigens was inconsistent; statistically significant decreases in HBeAg and HBx were observed, but there was no significant decrease in HBsAg (Fig. 2A and B). Taken together, these results indicate that the JMJD2D isoform plays a more critical role in HBV replication among the JMJD2 family members.
Fig. 2.
Knockdown of JMJD2D attenuates HBV transcription and replication.
(A, B) Knockdown of JMJD2A-2D was used to measure the production of different HBV antigens. Knockdown of JMJD2D but not of the other JMJD2 family members (JMJD2A-2C) attenuated the protein levels of HBeAg (A), HBsAg (A), and HBx (B). (C) JMJD2D expression was reduced in JMJD2D-knockdown HepG2 cells which were transfected with HBV 1.3-fold genomic plasmid (pHBV1.3). (D) Knockdown of JMJD2D dramatically reduced the ratios of pgRNA/cccDNA and total RNA/cccDNA. HBV cccDNA, pgRNA, and total RNA were quantified by RT-qPCR. (E) Knockdown of JMJD2D significantly decreased the levels of extracellular HBV DNA. (F) The production of HBsAg and HBeAg were significantly decreased in JMJD2D-knockdown cells. (G) JMJD2D expression was reduced in JMJD2D-knockdown HBV-infected Huh-7-NTCP cells. (H) Knockdown of JMJD2D dramatically reduced the ratios of pgRNA/cccDNA and total RNA/cccDNA in HBV-infected Huh-7-NTCP cells. HBV cccDNA, pgRNA, and total RNA were quantified by RT-qPCR. The ratios of pgRNA to cccDNA and total RNA to cccDNA were calculated. (I) Knockdown of JMJD2D significantly decreased the level of extracellular HBV DNA HBV-infected Huh-7-NTCP cells. (J) Knockdown of JMJD2D reduced the production of HBsAg and HBeAg in HBV-infected Huh-7-NTCP cells. (K) JMJD2D knockout dramatically reduced the ratio of HBV pgRNA to cccDNA in mice livers that injected HBV DNA (Left), the production of HBsAg (middle) and HBeAg (Right) in the serum of mice, as measured by real-time qPCR and ELISA, respectively. For (A, D, F) and (H–K), data are from at least three independent experiments, presented as the mean ± SD of multiple samples. Ordinary one-way ANOVA analysis for D, F, and H–J, unpaired two-tailed Student’s t test for K, ∗∗p <0.01, ∗∗∗p <0.001, ∗∗∗∗p <0.0001. cccDNA, covalently closed circular DNA; HBx, Hepatitis B virus X protein; pgRNA, pregenomic RNA.
Considering that HBV expresses the viral protein in host cells through its cccDNA, we wondered whether JMJD2D upregulated HBV gene expression by enhancing HBV cccDNA transcription. We transfected HBV DNA into HepG2 cells, and then knocked down of JMJD2D expression using the shJMJD2D lentivirus (Fig. 2C). The transcriptional activity of HBV cccDNA was significantly attenuated in JMJD2D-deficient cells as demonstrated by reduced ratios of pgRNA to cccDNA and total RNA to cccDNA (Fig. 2D), suggesting that JMJD2D can upregulate HBV cccDNA transcription. Moreover, quantification of extracellular antigens and viral particle DNA levels revealed that knockdown of JMJD2D significantly decreased the production of HBV DNA, HBsAg, and HBeAg (Fig. 2E and F). Next, HepAD38 cell-produced HBV was used to infect Huh-7-NTCP cells, and shJMJD2D lentivirus was used to reduce JMJD2D expression, the intracellular and extracellular profiles of viral particles assembled in these cells were then measured (Fig. 2G). Consistently, HBV replication was suppressed in JMJD2D-deficient cells as demonstrated by attenuated ratios of pgRNA/cccDNA and total RNA/cccDNA (Fig. 2H), extracellular viral particle DNA levels (Fig. 2I), and HBsAg and HBeAg levels (Fig. 2J). To determine whether JMJD2D could enhance HBV replication in vivo, the levels of liver HBV pgRNA and serum HBsAg in HBV DNA-injected wild-type and JMJD2D-knockout mice were measured. As shown in Fig. 2K, the levels of liver HBV pgRNA, serum HBsAg and HBeAg were significantly lower in JMJD2D-knockout mice than those in wild-type mice. Collectively, these results demonstrate that JMJD2D enhances HBV transcription and replication both in vitro and in vivo.
JMJD2D interacted with HBx protein
HBx is a regulatory protein that can elevate HBV transcription and replication.16,38 To investigate whether JMJD2D promotes HBV replication by interacting with HBx to regulate its function, HA-tagged HBx plasmids and Flag-tagged JMJD2D plasmids were co-transfected into 293T cells. Co-IP analysis confirmed an interaction between HBx and JMJD2D (Fig. 3A and B). Furthermore, HBV-positive HepAD38 cells were employed to investigate interactions between endogenous JMJD2D and HBx. Using a Co-IP assay (Fig. 3C and D) confirmed that endogenous JMJD2D interacts with HBV-produced HBx.
Fig. 3.
JMJD2D is a novel HBx-interacting protein.
(A, B) Exogenous HBx interacted with JMJD2D in 293T cells, as determined by the Co-IP assay. (C, D) Endogenous HBx interacted with JMJD2D in HepAD38 cells, as determined by the Co-IP assay. (E) GST pull-down analysis of the interaction of HBx with different JMJD2D domains shown in the schematic diagram of the JMJD2D protein structure. (F) Schematic diagram showing the construction of GFP-tagged HBx truncation protein and the Co-IP assays were employed to validate the interaction of JMJD2D with truncated proteins of HBx. Co-IP, co-immunoprecipitation assay; HBx, Hepatitis B virus X protein.
To determine which domain of JMJD2D interacts with HBx, glutathione-S-transferase (GST) pulldown assays were performed. The results showed that the C terminal domain (CTD) of JMJD2D could interact with the HBx protein (Fig. 3E). Previously published studies reported that HBx protein domains (51–154) and (1–100) containing signal transactivation sequences are required for HBV cccDNA transcription and HBV replication.39,40 To examine which domain of the HBx protein is required for the interaction between HBx and JMJD2D proteins, different GFP-tagged HBx truncation proteins (Fig. 3F) were transfected into HepG2 cells, and then Co-IP assays were performed using GFP antibody. The Co-IP assays showed that full-length HBx (GFP-HBx-FL), truncated HBx proteins GFP-HBx-51-154 and GFP-HBx-1-100 could interact with JMJD2D protein (Fig. 3F), whereas GFP-HBx-101-154 could not, indicating that the N-terminal domain (1–50) of HBx is responsible for the interaction between HBx and JMJD2D proteins.
JMJD2D modulated HBx protein expression by increasing its stability
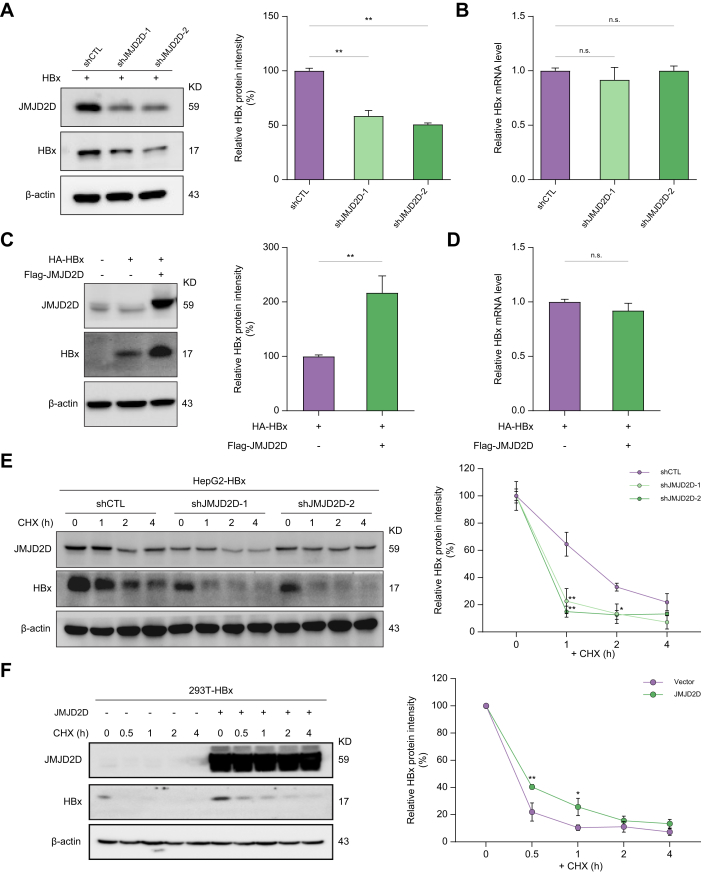
To determine whether the interaction between JMJD2D and HBx affects HBx protein levels or its function, we knocked down JMJD2D expression in HBx-expressing HepG2 cells and examined the protein and mRNA levels of HBx. JMJD2D knockdown decreased HBx protein levels (Fig. 4A) but did not affect HBx mRNA levels (Fig. 4B). In contrast, overexpression of JMJD2D in HBx-expressing cells increased HBx protein levels (Fig. 4C and Fig. S3), but not its mRNA levels (Fig. 4D). These results suggest that JMJD2D can increase HBx protein levels, which prompted us to examine whether JMJD2D could influence the stability of HBx protein.
Fig. 4.
JMJD2D enhances HBx protein expression by modulating its stability.
(A) Knockdown of JMJD2D significantly decreased HBx protein levels in HBx-expressing HepG2 cells, as measured by western blotting. Quantification of HBx band intensity and normalisation with β-Actin as the loading control. (B) Knockdown of JMJD2D was unable to downregulate the mRNA levels of HBx, as quantified by Real-time qPCR. (C, D) Ectopic expression of JMJD2D significantly upregulated the protein level of HBx in 293T cells but failed to upregulate the mRNA levels of HBx. (E) Knockdown of JMJD2D dramatically reduced the protein stability of HBx in HepG2 cells after incubation with CHX at the indicated times. (F) Overexpression of JMJD2D sustains the protein stability of HBx in 293T cells after CHX treatment for the indicated time. A representative experiment is shown. The intensity of HBx protein was analysed and normalised with β-Actin. Data are from four independent experiments and one representative experiment is shown. For A–F, data are shown as mean ± SD. Ordinary one-way ANOVA analysis for A, B, E, and F, unpaired two-tailed Student’s t test for C, D, n.s., not significant, ∗p <0.05, ∗∗p <0.01. CHX, cycloheximide; HBx, Hepatitis B virus X protein; qPCR, quantitative PCR.
Therefore, the cycloheximide (CHX) chase assay was used to block cellular protein synthesis in HBx-expressing HepG2 cells with or without JMJD2D knockdown and examined the protein levels of HBx at different time points after CHX treatment, As shown in Fig. 4E, knockdown of JMJD2D accelerated the degradation of HBx protein. In contrast, overexpression of JMJD2D in HBx-transfected 293T cells delayed HBx protein degradation (Fig. 4F). These results suggest that JMJD2D could enhance the stability of HBx protein.
JMJD2D restricted TRIM14-modulated ubiquitin-proteasome degradation of HBx protein
To determine whether JMJD2D enhances HBx protein stability by preventing proteasomal degradation of HBx protein, MG132, a proteasome inhibitor, was used to treat HBx-expressing Huh-7-shCTL and Huh-7-shJMJD2D cells, and then western blotting was performed to examine HBx protein levels. We confirmed that JMJD2D knockdown reduced HBx protein levels, whereas MG132 treatment reversed the decrease caused by JMJD2D knockdown (Fig. 5A).
Fig. 5.
JMJD2D inhibits TRIM14-modulated ubiquitin-proteasome degradation of HBx protein.
(A) MG132, a proteasome inhibitor, prevented the reduction of HBx protein levels caused by JMJD2D knockdown. Cells were treated with MG132 (20 μM) for 6 h and then protein lysates were used for western blotting to examine HBx protein levels. (B) Overexpression of JMJD2D prevented endogenous ubiquitin binding to HBx protein in 293T cells, the polyubiquitin ladder was detected by Co-IP coupled western blotting analysis. (C) Overexpression of JMJD2D prevented ectopic ubiquitin binding to HBx protein in 293T cells, the polyubiquitin ladder was detected by Co-IP coupled western blotting analysis. (D) TRIM14 interacted with HBx (Left) and JMJD2D (Right) in Huh-7 cells by Co-IP coupled western blotting assay. (E) Overexpression of JMJD2D prevented TRIM14-mediated reduction of HBx protein in Huh-7 cells as measured by western blotting. (F) Overexpression of JMJD2D blocked TRIM14-mediated ubiquitylation of HBx protein in Huh-7 cells, as examined by IP-coupled western blotting assay. Data were obtained from at least three independent experiments. For (A), data are presented as the mean ± SD of multiple samples and subjected to ordinary one-way ANOVA analysis, ∗∗p <0.01, ∗∗∗p <0.001. Co-IP, co-immunoprecipitation assay; HBx, Hepatitis B virus X protein; IP, immunoprecipitation.
To investigate whether JMJD2D promotes HBx protein stability by suppressing HBx polyubiquitylation, we first analysed the effects of JMJD2D on HBx protein ubiquitylation. HBx and JMJD2D plasmids were co-transfected into 293T cells, and then HBx protein was pulled down and detected with anti-ubiquitin antibody. HBx protein generated a weaker polyubiquitin ladder in the presence of exogenously expressed JMJD2D (Fig. 5B and C), indicating that JMJD2D inhibits HBx ubiquitylation. Taken together, these results suggest that JMJD2D stabilises HBx protein by inhibiting ubiquitin-proteasome mediated degradation of HBx.
Given that the E3 ubiquitin ligase tripartite motif-containing protein 14 (TRIM14) promotes the degradation of NS5A, a nonstructural protein of HCV, by the ubiquitin-proteasome system,41 interacts with HBx protein during HBV infection,42 and interacts with JMJD2D to inhibit its autophagic degradation,43 we hypothesised that JMJD2D may prevent TRIM14-modulated ubiquitin-proteasome degradation of HBx. To test this hypothesis, we first performed Co-IP assays to validate interactions between TRIM14, HBx, and JMJD2D in our experimental systems (Fig. 5D). In this study, we confirmed that TRIM14, an E3 ubiquitin ligase interacts with the HBx protein and targets HBx for ubiquitination and proteasomal degradation (Fig. 5E and F, and Fig. S4).
We next examined the effects of JMJD2D on TRIM14-mediated HBx degradation and found that JMJD2D could prevent TRIM14-mediated HBx degradation (Fig. 5E). Finally, we examined the effects of JMJD2D on TRIM14-modulated HBx ubiquitylation and found that JMJD2D could prevent TRIM14-modulated HBx ubiquitylation (Fig. 5F). Taken together, these results demonstrate that JMJD2D can prevent TRIM14-modulated ubiquitin-proteasome degradation of HBx. From the results of the Co-IP assays using anti-HA antibody to pull down HA-HBx, we found that HBx interacted with less TRIM14 protein in the presence of JMJD2D (Fig. 5F), suggesting that JMJD2D prevents interaction between HBx and TRIM14 to restrict TRIM14-modulated ubiquitin-proteasome degradation of HBx.
JMJD2D interacted with HBx protein to drive cccDNA transcription
Previous studies have shown that HBx can be recruited to HBV cccDNA to activate cccDNA transcription and stimulate HBV replication,16,17 As a histone demethylase, JMJD2D transcriptionally regulates gene expression by demethylating H3K9me3.20 Since demethylation of H3K9me3 is essential for HBV cccDNA transcription,3,16,17 and JMJD2D interacts with HBx, we hypothesised that HBx may cooperate with JMJD2D to promote HBV cccDNA transcription by recruiting JMJD2D to HBV cccDNA to demethylate H3K9me3. To test this hypothesis, we first examined whether JMJD2D and HBx cooperatively promote HBV cccDNA transcription by measuring pgRNA/cccDNA levels in HBV-infected Huh-7 cells transfected with expression plasmids of JMJD2D and HBx, and both JMJD2D and HBx expression plasmids. As shown in Fig. 6A, the pgRNA/cccDNA levels in JMJD2D and HBx co-transfected cells were significantly higher than those in cells transfected with either JMJD2D or HBx alone. Furthermore, Huh-7-shCTL or Huh-7-shJMJD2D cells infected with wild-type HBV or the HBV mutant with defective HBx expression (HBV-ΔX) (Fig. 6B) were employed to examine the effect of deficiency in JMJD2D, HBx, or JMJD2D and HBx on HBV cccDNA transcription (Fig. 6C). HBV-ΔX-infected Huh-7-shJMJD2D cells exhibited the lowest levels of viral antigen compared with HBV-infected Huh-7-shCTL cells, HBV-infected Huh-7-shJMJD2D cells, and HBV-ΔX-infected Huh-7-shCTL cells (Fig. 6D). Taken together, these results indicate that JMJD2D can cooperate with HBx to enhance the transcription of HBV cccDNA.
Fig. 6.
JMJD2D and HBx cooperatively promote HBV cccDNA transcription.
(A) The ratios of pgRNA/cccDNA in JMJD2D and HBx co-transfected HBV-infected Huh-7 cells was significantly higher than those in cells transfected with JMJD2D or HBx alone, as measured by Real-time qPCR assay. HBV-infected Huh-7 cells were transfected with JMJD2D and gradient concentrations of HBx plasmids and the ratio of pgRNA/cccDNA was calculated. (B) Schematic illustration of the construction of HBx-deleted HBV DNA plasmid. HBV-ΔX represents HBx deleted HBV. (C) Western blotting failed to detect HBx protein expression in Huh-7-shJMJD2D cells that were transfected with HBV-ΔX DNA. (D) HBV-ΔX-infected Huh-7-shJMJD2D cells exhibited the lowest levels of viral antigen compared with HBV-infected Huh-7-shCTL cells, HBV-infected Huh-7-shJMJD2D cells, and HBV-ΔX-infected Huh-7-shCTL cells, as measured by ELISA assay. (E) The recruitment of JMJD2D and HBx on HBV cccDNA was detected by ChIP assay in HBV-infected Huh-7-NTCP cells. (F) HBx deficiency remarkably reduced JMJD2D recruitment of HBV cccDNA, as examined by ChIP-qPCR assay. (G) The enrichment of H3K9me3 on HBV cccDNA was significantly increased after knockdown of JMJD2D, HBx absence, or both, as measured by ChIP-qPCR using H3K9me3 antibodies. Representative data are from at least three independent experiments. Data are presented as the mean ± SD of multiple samples. Unpaired two-tailed Student’s t test for E, and ordinary one-way ANOVA analysis for A, D, F–H and J, ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001, n.s., not significant. ChIP, chromatin immunoprecipitation; cccDNA, covalently closed circular DNA; HBx, Hepatitis B virus X protein.
We next employed ChIP assays to investigate whether JMJD2D and HBx can be recruited to HBV cccDNA. As shown in Fig. 6E, the recruitment of JMJD2D and HBx to HBV cccDNA was detected in HBV-infected Huh-7-NTCP cells (Fig. 6E). However, the recruitment of JMJD2D on HBV cccDNA was absent when HBx was defective (Fig. 6F), indicating that the recruitment of JMJD2D on HBV cccDNA is dependent on HBx.
We finally employed the ChIP assays using the H3K9me3 antibody to examine the effects of JMJD2D and HBx deficiency on H3K9me3 levels in HBV cccDNA. H3K9me3 levels of HBV cccDNA were significantly increased in HBV-infected Huh-7-shJMJD2D cells and HBV-ΔX-infected Huh-7-shCTL cells, whereas H3K9me3 levels were further elevated in HBV-ΔX-infected Huh-7-shJMJD2D cells (Fig. 6G), suggesting that JMJD2D demethylates H3K9me3 on HBV cccDNA, and its demethylation activity is enhanced by HBx.
p53 plays a defensive role against cccDNA transcriptional activity and HBV replication by binding to HBV cccDNA and inducing HBx protein degradation.44,45 We previously showed that JMJD2D could antagonise p53 transactivational function by preventing p53 from binding to its response elements on the promoters of its target genes.22 Therefore, we hypothesised that JMJD2D may prevent p53 from binding to HBV cccDNA and antagonise p53-induced HBx protein degradation to promote cccDNA transcription and HBV replication. We first performed the ChIP assays and showed that the recruitment of p53 to HBV cccDNA was dramatically increased in the absence of either JMJD2D, HBx, or both (Fig. 6H). Next, we investigated whether JMJD2D can antagonise p53-mediated HBx reduction by ectopically expressing JMJD2D and p53 in HBx-expressing HepG2 cells. Ectopic p53 expression decreased HBx protein, whereas ectopic JMJD2D expression significantly antagonised p53-mediated HBx reduction (Fig. 6I). To investigate whether JMJD2D antagonises p53-mediated inhibition of HBV replication in an HBx-dependent manner, we employed ELISA assay to detect extracellular HBV antigen production. As shown in Fig. 6H, ectopic JMJD2D expression dramatically restricted p53-mediated reduction of HBeAg and HBsAg when HBx was present; however, this effect was missing when HBx was absent, suggesting that JMJD2D enhances cccDNA transcription and HBV replication by antagonising the repressive activity of p53 in an HBx-dependent manner.
In summary, these results suggest that HBx cooperates with JMJD2D to promote HBV cccDNA transcription and HBV replication by recruiting JMJD2D to demethylate H3K9me3 on HBV cccDNA and antagonise the repressive activity of p53.
The JMJD2D inhibitor 5C-8-HQ effectively intervened in hepatitis B virus replication
We previously reported that the JMJD2D inhibitor 5C-8-HQ suppressed the progression of colorectal cancer by targeting JMJD2D.24 This prompted us to investigate whether 5C-8-HQ could suppress HBV replication by targeting JMJD2D in this study. We treated HBV-infected HepG2-NTCP cells and differentiated HepaRG cells with 5C-8-HQ and IFNα, respectively. In these treated cells, we found that the protein levels of HBx were dramatically attenuated (Fig. 7A), whereas the cell viability was not affected during our observation time (Fig. S5). Consistently, the ability of the intervention groups to replicate the virus was also dramatically attenuated (Fig. 7B). Of note, the antiviral effect of 5C-8-HQ was similar to that obtained on exposure to IFNα (Fig. 7B), indicating that JMJD2D is a novel antiviral target. Furthermore, we proceeded to evaluate the interference effect of 5C-8-HQ on HBV replication in HBV-infected differentiated HepaRG cells. These results confirmed that 5C-8-HQ can effectively interfere with the transcription of HBV cccDNA (Fig. 7C).
Fig. 7.
JMJD2D-specific inhibitor 5C-8-HQ effectively interferes with HBV transcription and replication.
(A) The protein levels of HBx were reduced in HBV-infected HepG2-NTCP cells after 5C-8-HQ (20 μM) and IFNα (20 ng/ml) treatment, respectively, as measured by western blotting. (B) The ratios of pgRNA/cccDNA and HBV total RNA/cccDNA were significantly decreased in HBV-infected HepG2 cells after 5C-8-HQ and IFNα treatment, respectively. (C) Schematic illustration of the experimental strategy of HBV-infected differentiated HepaRG cells (Left). The ratios of pgRNA/cccDNA and HBV total RNA/cccDNA were also significantly decreased in HBV-infected differentiated HepaRG cells (Right). (D) Schematic overview of the experimental strategy: C57BL/6 mice were hydrodynamically injected with HBV DNA and treated with 5C-8-HQ or IFNα. (E) HBV pgRNA levels were reduced in HBV-injected mice after 5C-8-HQ and IFNα treatment, respectively, as analysed by qPCR (n = 7). (F) Levels of HBV antigens including HBeAg (Left) and HBsAg (Right) in the mouse serum were decreased after 5C-8-HQ and IFNα treatment, respectively, as measured by ELISA (n = 8). Data are presented as the mean ± SD of multiple samples. Ordinary one-way ANOVA analysis for B, C, unpaired two-tailed Student’s t test for E, F, ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001. (G) Schematic presentation of the mechanisms by which JMJD2D promotes HBV transcription and replication. HBV infection activates phosphorylation of p65 in NF-κB signalling to induce JMJD2D expression, then JMJD2D stabilises HBx protein by preventing TRIM14-mediated ubiquitin-proteasome degradation of HBx and cooperates with HBx to promote cccDNA transcription by demethylating H3K9me3 on cccDNA and preventing p53 binding to cccDNA. cccDNA, covalently closed circular DNA; HBx, Hepatitis B virus X protein; pgRNA, pregenomic RNA.
To evaluate the antiviral effects of 5C-8-HQ in vivo, mice given hydrodynamic HBV injection and with various interventions were employed (Fig. 7D). 5C-8-HQ had a significant inhibitory effect on the level of HBV pgRNA (Fig. 7E) and the expression of antigens including HBeAg and HBsAg (Fig. 7F), demonstrating that 5C-8-HQ achieved an antiviral function that could inhibit HBV cccDNA transcription and replication in vivo.
Discussion
Our current study identifies JMJD2D as a novel host factor that promotes HBV transcription and replication. In this study, we screened the GEO database including CHB patients infection (GSE83148) and found that of all JMJD2 family members, only JMJD2D expression was significantly upregulated in HBV-infected human liver tissues compared with healthy samples. Additionally, JMJD2D expression was also significantly upregulated in HBV-infected mouse liver tissues and in human liver cells.
Wang et al. reported that transfection of the HBx-expressing vector in HCC cells downregulates the RNA and protein levels of JMJD2B. We used the same approach to ectopically express HBx in HepG2 cells to detect JMJD2B RNA and protein levels. Accordingly, HBx downregulated the expression of JMJD2B (Fig. S6A and B). However, JMJD2B expression may not be consistent after hepatitis B virus infection. We individually infected HepG2-NTCP cells with wild-type HBV and HBV-ΔX, HBV mutant with defective HBx expression. Consistent with the GEO data (GSE83148), infection with HBV did not alter JMJD2B expression, but infection with HBx-deficient HBV upregulated JMJD2B expression (Fig. S6C and D). These results suggest that HBx protein suppresses JMJD2B expression, but the expression level of HBx in cells or liver tissues infected with HBV is not sufficient to reduce JMJD2B expression.
Our previous study showed that JMJD2D expression could be induced by p65, a key regulator of NF-κB signalling in colorectal cancer cells.24 In this study, we showed that the phosphorylated p65 (p-p65) expression was increased in the hydrodynamic HBV-injected mouse model and the HBV-infected cell model, suggesting that HBV infection can activate p65 phosphorylation to induce JMJD2D expression. Knockdown of JMJD2D in HBV-infected cells resulted in significant reductions in the ratio of pgRNA/cccDNA and total RNA/cccDNA as well as reductions in the production of HBeAg and HBsAg; similar results were observed in a hydrodynamic HBV-injected JMJD2D-knockout mouse model, suggesting that JMJD2D can promote HBV replication. Thus, HBV infection induces JMJD2D expression by activating the p65 signalling pathway and then upregulated JMJD2D, which in turn, promoted HBV replication, forming a positive-feedback regulatory loop to propagate HBV infection.
Previous studies have reported that JMJD2D serves as a coactivator to transcriptionally upregulate oncogenic gene expression by demethylating H3K9me2/me3 on promoter sequences, consequently promoting the proliferation, migration, and invasion of cancer cells.[22], [23], [24] Based on the gene regulation function of JMJD2D, we hypothesised that JMJD2D may promote HBV replication by transcriptionally regulating host factors known to modulate HBV replication. To investigate this possibility, we conducted RNA-seq of JMJD2D-knockdown HepG2 cells to identify the host factors transcriptionally regulated by JMJD2D. Approximately, 1070 genes exhibited upregulated expression, whereas, 1103 genes exhibited downregulated expression (Fig. S7A–C). To identify cellular or host genes with altered expression that can regulate HBV replication, we grouped JMJD2D-regulated genes using the KEGG pathway and disease analyses. As shown in Fig. S7D and S7E, genes with downregulated expression were mainly enriched in adherens junction, PPAR signalling pathway, and calcium signalling pathways. Restrictive cardiomyopathy and acrodysostosis were the mainly enriched KEGG diseases. Furthermore, upregulated genes were mainly enriched in ECM-receptor interaction, small cell lung cancer, glycogen storage disease, and laryngeal cancer. Unfortunately, the KEGG pathway and disease analysis of the significantly changed genes including host factors presented no enrichment in cases of HBV infection and CHB (Figs. S7 and S8). Thus, we examined whether JMJD2D can modulate HBx protein expression and function to promote HBV replication.
HBx is a known regulatory viral protein essential for HBV replication that interacts with several cellular proteins to release HBV minichromosome-mediated repression involving histone methyltransferases and histone deacetylases.46,47 In addition, HBx enhances HBV replication by promoting SMC5/6 degradation.27,48 Kouwaki et al. reported that JMJD5 interacts with HBx to promote the transcription of key hepatocyte host factors such as HNF4A, CEBPA, and FOXA3, enhancing HBV replication through JMJD5 hydroxylase activity but not demethylase activity.49 This interesting finding suggests that the JmjC domain-containing family may play an important role in HBV replication and may be a new target for antiviral drug intervention. Similar to JMJD5, we discovered that JMJD2D interacts with HBx to promote HBV replication. Remarkably, unlike JMJD5, JMJD2D is a novel interactor of HBx that enhances HBx protein stability and cooperates with HBx to promote cccDNA transcription. HBx is an unstable viral protein due to its rapid ubiquitin‒proteasome-mediated degradation in the host cell.50,51 TRIM14 is a key interferon-stimulated gene that reduces HBx protein levels during HBV infection.42 and promotes degradation of HCV non-structural protein NS5A in a ubiquitination-dependent way.41 In this study, TRIM14 acted as a key E3 ligase to promote the polyubiquitination and degradation of HBx. Recently, TRIM14 was reported to interact with JMJD2D, prompting us to examine whether JMJD2D interferes with the interaction between TRIM14 and HBx to prevent TRIM14-mediated polyubiquitination and degradation of HBx. Indeed, in the presence of JMJD2D, the interaction between TRIM14 and HBx as well as TRIM14-mediated polyubiquitination and degradation of HBx were attenuated. These findings indicate that JMJD2D restricts the TRIM14-modulated ubiquitin-proteasome degradation of HBx protein by interacting with both HBx and TRIM14 to directly repress the binding of TRIM14 to HBx.
Molecularly, HBV cccDNA is assembled into a minichromosome, and its transcriptional activity is modulated by epigenetic modifications such as methylation and acetylation of histones binding to HBV cccDNA. H3K9me3 is a key repressive histone modification that needs to be reduced during HBV cccDNA transcription. In the present study, both JMJD2D and HBx could be recruited to HBV cccDNA and the H3K9me3 levels of HBV cccDNA were significantly elevated in the absence of JMJD2D, HBx, or both, suggesting that JMJD2D and HBx play essential roles in the demethylation of H3K9me3 on the HBV cccDNA. The absence of HBx markedly abolished the recruitment of JMJD2D on HBV cccDNA, indicating that HBx is responsible for JMJD2D recruitment. Thus, JMJD2D and HBx cooperatively promote HBV cccDNA transcription by demethylating H3K9me3 on the HBV cccDNA. In this study, we observed that the recruitment of p53 to HBV cccDNA was dramatically increased after knockdown of JMJD2D expression, the absence of HBx, or both. Moreover, we observed that JMJD2D restricted p53-modulated HBx protein downregulation to attenuate p53 suppression of HBV antigen production. Together, these results suggest that JMJD2D may cooperate with HBx protein to enhance cccDNA transcription and HBV replication by antagonising the repressive activity of p53.
Our study showed that the JMJD2D inhibitor 5C-8-HQ interfered with HBV cccDNA transcription and the production of antigens as effectively as IFNα, suggesting that JMJD2D is an attractive target for the treatment of CHB. CHB may require a long-term drug application. Since JMJD2D-knockout mice are healthy with no significant defects, prolonged treatment of CHB targeting JMJD2D may not cause significant side effects. In addition, we recently showed that JMJD2D deficiency can prevent HCC initiation and progression;22 therefore, a long-term drug application targeting JMJD2D may not only treat CHB but may also prevent HCC initiation and progression related to HBV infection.
Based on the findings of this and previous studies, we propose a working model for the role of JMJD2D in promoting HBV transcription and replication (Fig. 7G), in which HBV infection activates NF-κB signalling to induce JMJD2D expression. In this model, JMJD2D stabilises HBx protein by direct interaction with HBx and TRIM14 to prevent TRIM14-mediated polyubiquitination and degradation of HBx while simultaneously cooperating JMJD2D cooperates with HBx to promote HBV cccDNA transcription by demethylating H3K9me3 on HBV cccDNA and preventing p53 from binding to HBV cccDNA. Targeting JMJD2D by shRNA or pharmacological inhibitors represses HBV replication and may be used to treat both CHB and HBV-related hepatocarcinogenesis.
Financial support
This work was supported by National Science and Technology Major Project of China (no. 2017ZX10203206 to WGL), National Natural Science Foundation of China (no. 81972223 to WGL, no. 81970485 and no. 82173086 to CDY), Scientific Research Foundation for Advanced Talents of Xiang’an Hospital of Xiamen University (no. PM20180917008 to WGL), Postdoctoral Research Foundation of China (no. 2019M662246 to XK), The Special Fund for Public Welfare Research Institutes of Fujian Province (no. 2023R1036 to XK), Youth Foundation of Xiang’an Hospital of Xiamen University (no. XM01030001 to XK), Natural Science Foundation of Fujian Province (no. 2020J011175 to XHL).
Authors’ contributions
Designed the experiments: XK, CDY, WGL. Performed the experiments: XK, ZFL, FAX, RYZ, RBL, YZ, BL, YLH, QC. Analysed the data: XK, ZFL, RYZ, FAX, RBL, LLY, LJF, CDY, WGL. Collected and analysed clinical data: ZFL, WSY, XGX. Interpreted the data: XK, ZFL, LLY, XL, TW, CDY, WGL. Wrote the manuscript: XK, TW, CDY, WGL. Obtained funding: XK, XHL, YS, CDY, WGL.
Data availability statement
The data analysed in Fig. 1A were obtained from the Gene Expression Omnibus (GEO) at GSE83148. All original data are available from the corresponding author upon reasonable request.
Conflicts of interest
The authors declare no competing interests.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The authors thank Prof. Jiahuai Han (Xiamen University) for providing the HepaRG cell. Prof. Quan Yuan (Xiamen University) for providing the HepAD38 cell.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100849.
Contributor Information
Ye Shen, Email: jerry.shen@delpstroke.com.
Ting Wu, Email: wuting78@189.cn.
Chundong Yu, Email: cdyu@xmu.edu.cn.
Wengang Li, Email: lwgang@xmu.edu.cn.
Supplementary data
The following are the supplementary data to this article.
References
- 1.Zapatka M., Borozan I., Brewer D.S., Iskar M., Grundhoff A., Alawi M., et al. The landscape of viral associations in human cancers. Nat Genet. 2020;52:320–330. doi: 10.1038/s41588-019-0558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong X., Kim E.S., Guo H. Epigenetic regulation of hepatitis B virus covalently closed circular DNA: implications for epigenetic therapy against chronic hepatitis B. Hepatology. 2017;66:2066–2077. doi: 10.1002/hep.29479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeger C., Mason W.S. Molecular biology of hepatitis B virus infection. Virology. 2015;479-480:672–686. doi: 10.1016/j.virol.2015.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan H., Zhong G.C., Xu G.W., He W.H., Jing Z.Y., Gao Z.C., et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1 doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwamoto M., Soso W., Sugiyama R., Ishii K., Ohki M., Nagamori S., et al. Epidermal growth factor receptor is a host-entry cofactor triggering hepatitis B virus internalization. P Natl Acad Sci USA. 2019;116:8487–8492. doi: 10.1073/pnas.1811064116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J., Shen T., Huang X.B., Kumar G.R., Chen X.M., Zeng Z.Z., et al. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J Hepatol. 2016;65:700–710. doi: 10.1016/j.jhep.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y., Wen J., Xiao W., Zhang B. Pregenomic RNA: how to assist the management of chronic hepatitis B? Rev Med Virol. 2019;29 doi: 10.1002/rmv.2051. [DOI] [PubMed] [Google Scholar]
- 9.Xia Y., Guo H. Hepatitis B virus cccDNA: formation, regulation and therapeutic potential. Antivir Res. 2020;180 doi: 10.1016/j.antiviral.2020.104824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L.P., Huang W.F., Wang L., Zhang Z.L., Zhang F., Zheng S.Z., et al. The effects of epigenetic modification on the occurrence and progression of liver diseases and the involved mechanism. Expert Rev Gastroent. 2020;14:259–270. doi: 10.1080/17474124.2020.1736042. [DOI] [PubMed] [Google Scholar]
- 11.Tang L.S.Y., Covert E., Wilson E., Kottilil S. Chronic hepatitis B infection: a review. JAMA. 2018;319:1802–1813. doi: 10.1001/jama.2018.3795. [DOI] [PubMed] [Google Scholar]
- 12.Villeret F., Zoulim F. New therapeutic targets for a chronic hepatitis B cure. Bull Acad Natl Med. 2020;204:890–899. [Google Scholar]
- 13.Yardeni D., Ghany M.G. Review article: hepatitis B-current and emerging therapies. Aliment Pharm Ther. 2022;55:805–819. doi: 10.1111/apt.16828. [DOI] [PubMed] [Google Scholar]
- 14.Seeger C. Control of viral transcripts as a concept for future HBV therapies. Curr Opin Virol. 2018;30:18–23. doi: 10.1016/j.coviro.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Gao W.W., Jia Z.C., Tian Y., Yang P.H., Sun H., Wang C.H., et al. HBx protein contributes to liver carcinogenesis by H3K4me3 modification through stabilizing WD repeat domain 5 protein. Hepatology. 2020;71:1678–1695. doi: 10.1002/hep.30947. [DOI] [PubMed] [Google Scholar]
- 16.Belloni L., Pollicino T., De Nicola F., Guerrieri F., Raffa G., Fanciulli M., et al. Nuclear HBx binds the HBV minichromosome and modifies the epigenetic regulation of cccDNA function. P Natl Acad Sci USA. 2009;106:19975–19979. doi: 10.1073/pnas.0908365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riviere L., Gerossier L., Ducroux A., Dion S., Deng Q., Michel M.L., et al. HBx relieves chromatin-mediated transcriptional repression of hepatitis B viral cccDNA involving SETDB1 histone methyltransferase. J Hepatol. 2015;63:1093–1102. doi: 10.1016/j.jhep.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Zhang T.Y., Chen H.Y., Cao J.L., Xiong H.L., Mo X.B., Li T.L., et al. Structural and functional analyses of hepatitis B virus X protein BH3-like domain and Bcl-xL interaction. Nat Commun. 2019;10:3192. doi: 10.1038/s41467-019-11173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sartorius K., An P., Winkler C., Chuturgoon A., Li X.D., Makarova J., et al. The epigenetic modulation of cancer and immune pathways in hepatitis B virus-associated hepatocellular carcinoma: the influence of HBx and miRNA dysregulation. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.661204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin S., Janknecht R. Diversity within the JMJD2 histone demethylase family. Biochem Bioph Res Commun. 2007;353:973–977. doi: 10.1016/j.bbrc.2006.12.147. [DOI] [PubMed] [Google Scholar]
- 21.Dong F.Y., Jiang S.H., Li J., Wang Y.H., Zhu L.L., Huang Y.Q., et al. The histone demethylase KDM4D promotes hepatic fibrogenesis by modulating Toll-like receptor 4 signaling pathway. Ebiomedicine. 2019;39:472–483. doi: 10.1016/j.ebiom.2018.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M., Deng Y., Zhuo M.H., Zhou H., Kong X., Xia X.G., et al. Demethylase-independent function of JMJD2D as a novel antagonist of p53 to promote liver cancer initiation and progression. Theranostics. 2020;10:8863–8879. doi: 10.7150/thno.45581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng K.S., Kou L.L., Yu L., Bai C.N., Li M., Mo P.L., et al. Histone demethylase JMJD2D interacts with beta-catenin to induce transcription and activate colorectal cancer cell proliferation and tumour growth in mice. Gastroenterology. 2019;156:1112–1126. doi: 10.1053/j.gastro.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 24.Zhuo M.H., Chen W.B., Shang S.H., Guo P., Peng K.S., Li M., et al. Inflammation-induced JMJD2D promotes colitis recovery and colon tumourigenesis by activating Hedgehog signaling. Oncogene. 2020;39:3336–3353. doi: 10.1038/s41388-020-1219-2. [DOI] [PubMed] [Google Scholar]
- 25.Gripon P., Rumin S., Urban S., Le Seyec J., Glaise D., Cannie I., et al. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci U S A. 2002;99:15655–15660. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang G., Feng J.Y., Liu Y.X., Zhao M., Yuan Y., Yuan H.F., et al. HAT1 signaling confers to assembly and epigenetic regulation of HBV cccDNA minichromosome. Theranostics. 2019;9:7345–7358. doi: 10.7150/thno.37173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy C.M., Xu Y.P., Li F., Nio K., ReszkA, Blanco N., et al. Hepatitis B virus X protein promotes degradation of SMC5/6 to enhance HBV replication. Cell Rep. 2016;16:2846–2854. doi: 10.1016/j.celrep.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arad U. Modified Hirt procedure for rapid purification of extrachromosomal DNA from mammalian cells. Biotechniques. 1998;24:760–762. doi: 10.2144/98245bm14. [DOI] [PubMed] [Google Scholar]
- 29.Wang S.J., Chen Z.M., Wei M., Liu J.Q., Li Z.L., Shi T.S., et al. Specific determination of hepatitis B e antigen by antibodies targeting precore unique epitope facilitates clinical diagnosis and drug evaluation against hepatitis B virus infection. Emerg Microbes Infec. 2021;10:37–50. doi: 10.1080/22221751.2020.1862631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang P.L., Althage A., Chung J., Chisari F.V. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc Natl Acad Sci U S A. 2002;99:13825–13830. doi: 10.1073/pnas.202398599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen K., Liu J., Liu S.X., Xia M., Zhang X.M., Han D., et al. Methyltransferase SETD2-mediated methylation of STAT1 Is critical for interferon antiviral activity. Cell. 2017;170:492–506. doi: 10.1016/j.cell.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 32.Deng Y., Li M., Zhuo M.H., Guo P., Chen Q., Mo P.L., et al. Histone demethylase JMJD2D promotes the self-renewal of liver cancer stem-like cells by enhancing EpCAM and Sox9 expression. J Biol Chem. 2021;296 doi: 10.1074/jbc.RA120.015335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yun C., Um H.R., Jin Y.H., Wang J.H., Lee M.O., Park S., et al. NF-kappa B activation by hepatitis B virus X (HBx) protein shifts the cellular fate toward survival. Cancer Lett. 2002;184:97–104. doi: 10.1016/s0304-3835(02)00187-8. [DOI] [PubMed] [Google Scholar]
- 34.Cooper A., Tal G., Lider O., Shaul Y. Cytokine induction by the hepatitis B virus capsid in macrophages is facilitated by membrane heparan sulfate and involves TLR2. J Immunol. 2005;175:3165–3176. doi: 10.4049/jimmunol.175.5.3165. [DOI] [PubMed] [Google Scholar]
- 35.Namineni S., O'Connor T., Faure-Dupuy S., Johansen P., Riedl T., Liu K.J., et al. A dual role for hepatocyte-intrinsic canonical NF-kappa B signaling in virus control. J Hepatol. 2020;72:960–975. doi: 10.1016/j.jhep.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Kopp E., Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 37.Yin M.J., Yamamoto Y., Gaynor R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 38.Decorsiere A., Mueller H., Van Breugel P.C., Abdul F., Gerossier L., Beran R.K., et al. Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor. Nature. 2016;531:386–389. doi: 10.1038/nature17170. [DOI] [PubMed] [Google Scholar]
- 39.Murakami S., Cheong J., Kaneko S. Human hepatitis-virus X-gene encodes a regulatory domain that represses transactivation of X-protein. J Biol Chem. 1994;269:15118–15123. [PubMed] [Google Scholar]
- 40.Kim G.W., Siddiqui A. Hepatitis B virus X protein recruits methyltransferases to affect cotranscriptional N6-methyladenosine modification of viral/host RNAs. P Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2019455118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S., Chen Y., Li C., Wu Y., Guo L., Peng C., et al. TRIM14 inhibits hepatitis C virus infection by SPRY domain-dependent targeted degradation of the viral NS5A protein. Sci Rep. 2016;6 doi: 10.1038/srep32336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan G.Y., Xu F.C., Song H.X., Yuan Y., Xiao Q.F., Ma F., et al. Identification of TRIM14 as a type I IFN-stimulated gene controlling hepatitis B virus replication by targeting HBx. Front Immunol. 2018;9:1872. doi: 10.3389/fimmu.2018.01872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu D., Zhao Z.Y., She Y.C., Zhang L., Chen X.T., Ma L., et al. TRIM14 inhibits OPTN-mediated autophagic degradation of KDM4D to epigenetically regulate inflammation. P Natl Acad Sci USA. 2022;119 doi: 10.1073/pnas.2113454119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ori A., Zauberman A., Doitsh G., Paran N., Oren M., Shaul Y. p53 binds and represses the HBV enhancer: an adjacent enhancer element can reverse the transcription effect of p53. EMBO J. 1998;17:544–553. doi: 10.1093/emboj/17.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim H.Y., Han J., Yoon H., Jang K.L. Tumor suppressor p53 inhibits hepatitis B virus replication by downregulating HBx via E6AP-mediated proteasomal degradation in human hepatocellular carcinoma cell lines. Viruses. 2022;14:2313. doi: 10.3390/v14102313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keasler V.V., Hodgson A.J., Madden C.R., Slagle B.L. Enhancement of hepatitis B virus replication by the regulatory X protein in vitro and in vivo. J Virol. 2007;81:2656–2662. doi: 10.1128/JVI.02020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang H., Delgermaa L., Huang F.J., Oishi N., Liu L., He F., et al. The transcriptional transactivation function of HBx protein is important for its augmentation role in hepatitis B virus replication. J Virol. 2005;79:5548–5556. doi: 10.1128/JVI.79.9.5548-5556.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdul F., Filleton F., Gerossier L., Paturel A., Hall J., Strubin M., et al. Smc5/6 antagonism by HBx is an evolutionarily conserved function of hepatitis B virus infection in mammals. J Virol. 2018;92 doi: 10.1128/JVI.00769-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kouwaki T., Okamoto T., Ito A., Sugiyama Y., Yamashita K., Suzuki T., et al. Hepatocyte Factor JMJD5 regulates hepatitis B virus replication through interaction with HBx. J Virol. 2016;90:3530–3542. doi: 10.1128/JVI.02776-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xian L.L., Zhao J., Wang J., Fang Z., Peng B., Wang W.Z., et al. p53 Promotes proteasome-dependent degradation of oncogenic protein HBx by transcription of MDM2. Mol Biol Rep. 2010;37:2935–2940. doi: 10.1007/s11033-009-9855-1. [DOI] [PubMed] [Google Scholar]
- 51.Kim J.H., Sohn S.Y., Yen T.S.B., Ahn B.Y. Ubiquitin-dependent and -independent proteasomal degradation of hepatitis B virus X protein. Biochem Bioph Res Co. 2008;366:1036–1042. doi: 10.1016/j.bbrc.2007.12.070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analysed in Fig. 1A were obtained from the Gene Expression Omnibus (GEO) at GSE83148. All original data are available from the corresponding author upon reasonable request.