Abstract
Chronic pancreatitis is a chronic fibro-inflammatory disorder of the pancreas, resulting in recurrent abdominal pain, diabetes mellitus, and malnutrition. It may lead to various other complications such as pseudocyst formation, benign biliary stricture, gastric outlet obstruction; and vascular complications like venous thrombosis, variceal and pseudoaneurysmal bleed. Development of varices is usually due to chronic venous thrombosis with collateral formation and variceal bleeding can easily be tackled by endoscopic therapy. Pseudoaneurysmal bleed can be catastrophic and requires radiological interventions including digital subtraction angiography followed by endovascular obliteration, or sometimes with a percutaneous or an endoscopic ultrasound-guided approach in technically difficult situations. Procedure-related bleed is usually venous and mostly managed conservatively. Procedure-related arterial bleed, however, may require radiological interventions.
Keywords: Chronic pancreatitis, Pseudoaneurysm, Vascular complications, Varices, Venous thrombosis
Core Tip: Patients with chronic pancreatitis (CP) are prone to various venous and arterial complications that may sometimes lead to life-threatening bleed. A prompt approach to such vascular complications includes early identification and appropriate management by various modalities including endovascular, percutaneous, endoscopic ultrasound-guided, or surgical in certain cases. A knowledge of these complications, their presentation, and management are important for improving outcomes in patients with CP.
INTRODUCTION
Chronic pancreatitis (CP) is a progressive fibro-inflammatory disorder of the pancreas, with irreversible loss of the exocrine and endocrine function[1]. Pain is one of the predominant symptoms of CP[2], however, it may be associated with other local and systemic complications. Local complications include development of pseudocysts (8%-40%)[3-5], benign biliary strictures (3%-23%)[3], pancreatic fistulas (1.5%-3.5%)[3], gastric outlet obstruction (0.5%-13%)[3,5], and inflammatory head mass (1.5%-5.2%)[4,6]. Systemic complications include diabetes mellitus (21%-47%)[4,7], steatorrhea (20%-48%)[4,7], sarcopenia (17%-62%)[8,9], malnutrition (46.4%)[10] and metabolic bone diseases (17%-52%)[5,7]. The prevalence of CP is 36-125 per 100000 population and higher in eastern part of the globe with India having a major fraction of the disease burden[7,11]. The etiologic factors include environmental and genetic. The most common aetiology of CP are alcohol (42%-77%) followed by idiopathic (28%-80%)[7,12].
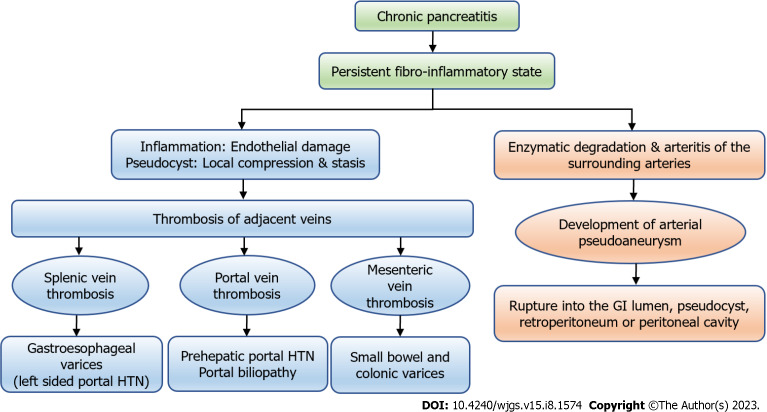
Vascular complications are associated with both acute pancreatitis (AP) and CP. CP has persistent ongoing inflammation, which leads to diverse vascular complications (Figure 1). It may sometimes present with torrential life-threatening bleed, and hence requires an early recognition and prompt intervention, which is the key for favourable outcomes. In this review, we will summarize the available literature regarding the etiopathogenesis, diagnosis and management of vascular complications in CP and will be broadly divided into venous and arterial sections. This review will mainly focus on venous thrombosis and arterial pseudoaneurysm (PsA), and rare vascular events in CP will not be discussed in detail. We will also outline the approach and management of gastrointestinal (GI) bleed in CP.
Figure 1.
The types of vascular complications in chronic pancreatitis and their consequences. GI: Gastrointestinal; HTN: Hypertension.
TYPES OF VASCULAR COMPLICATIONS IN CP
Different types of vascular complications are described in patients with CP. They are broadly divided into arterial and venous. Venous complications include splanchnic venous thrombosis i.e., thrombosis of splenic, portal and mesenteric veins, either alone or in various combinations and rarely inferior vena cava and renal vein thrombosis[13]. Arterial complications include arterial PsAs, percutaneous drain-related arterial bleeding, rarely aortic PsA[14,15] and arterial thrombosis[16] (Figure 1).
The type and distribution of vascular complications in patients with CP has been described in different studies and a summary of which along with distribution of vascular lesions has been shown in Table 1. The cumulative incidence of vascular events was 3.2% at 5 years and increased to 24.5% at 15 years in a study of 394 patients with CP[17]. The pooled prevalence of splanchnic vein thrombosis was found to be 11.6% in a meta-analysis of 44 studies[18]. Different studies have identified the prevalence of splanchnic vein thrombosis to 3%-41% among patients with CP[17]. The pooled incidence rate of PsA in CP was 0.03% in a meta-analysis of 29 studies[19]. There was heterogeneity among the studies included and differences in results could be explained by small sample sizes, retrospective nature and selection bias (Figure 1).
Table 1.
Frequency and risk factors of vascular complications in patients with chronic pancreatitis in different studies
|
Ref.
|
Number of patients (vascular complications/total CP patients)
|
Vascular complications
|
Risk factors
|
| Udd et al[81], 2007 | 33/745 (4.4%) | PsA: 33 (4.4%) | - |
| Agarwal et al[20], 2008 | 34/157 (21.6%) | Splenic vein thrombosis: 34 (21.6%) | Pseudocyst (OR = 4.01) |
| Pandey et al[23], 2019 | 37/187 (19.7%) | Splenic vein thrombosis: 37 (19.7%) | Smoking (OR = 3.021). Pseudocyst (OR = 3.743) |
| Anand et al[21], 2020 | 166/1363 (12.2%) | Venous thrombosis: 132 (9.6%). PsA: 17/166 (1.24%). Both: 17 (1.24%) | Venous thrombosis: Alcohol (OR = 2.1); pseudocyst (OR = 4.6); inflammatory head mass (OR = 3.1). Pseudoaneurysms: Alcohol (OR = 3.49); pseudocyst (OR = 3.2) |
| Ru et al[22], 2020 | 89/3358 (2.6%) | Splenic vein thrombosis: 89 (2.6%) | Alcohol (OR = 1.28). History of AP (OR = 2.56). Diabetes mellitus (OR = 3.82). Pseudocyst (OR = 8.54) |
| Vujasinovic et al[17], 2021 | 33/394 (8.37%) | Venous thrombosis: 30 (7.6%). PsA: 3 (0.8%) | Alcohol (HR = 3.56). Pseudocyst (HR = 8.66) |
AP: Acute pancreatitis; HR: Hazard ratio; OR: Odds ratio; CP: Chronic pancreatitis; PsA: Pseudoaneurysm.
RISK FACTORS FOR VASCULAR COMPLICATIONS IN CP
Identification of risk factors (Table 1) would allow to risk stratify the patients and take appropriate measures to identify and manage vascular complications. The presence of pseudocyst significantly increases the risk of splanchnic vein thrombosis[17,20-22]. A plausible explanation is that pseudocyst present in the vicinity of splanchnic veins causes local compression and stasis in splanchnic veins that predisposes to thrombosis. Other risk factors identified in different studies are alcohol as the aetiology of CP, history of acute pancreatitis (AP) and diabetes mellitus[17,21,23]. Smoking has been found to be associated with a higher risk of splanchnic vein thrombosis (odds ratio: 3.02) in a study probably due to the endothelial damage and oxidative stress incurred by its metabolites on endothelium[23].
Anand et al[21] observed that the risk of venous thrombosis increased with the presence of inflammatory head mass that might cause local compression and inflammation around the splanchnic veins. This study also identified alcohol and pseudocyst as a risk factor for PsA formation[21]. Most of the studies suggested that alcohol and pseudocyst are the common risk factors for the development of vascular complications in a patient with CP. Most of these studies reported risk factors related to the venous thrombosis but failed to identify risk factors for PsA formation.
VENOUS COMPLICATIONS
As discussed above, venous thrombosis is one of the major vascular complications in CP. Thrombosis predominantly affects the splanchnic veins with variable involvement of splenic, portal, and mesenteric veins.
Pathogenesis
Various proposed mechanisms for splanchnic vein thrombosis include pancreatic inflammation in the vicinity of splanchnic veins causing direct vascular endothelial damage, compression, or pressure from oedematous pancreas or pseudocyst causing venous stasis and hence thrombosis[24]. The persistent release of the inflammatory mediators can activate the coagulation system and may stimulate the formation of platelets and fibrin-rich thrombin. Lastly, certain factors like interleukin-1 (IL-1), IL-6 and tumor necrosis factor-alfa released from the damaged pancreatic tissue into the blood may also trigger a coagulation cascade, leading to endothelial damage and venous thrombosis[25].
Distribution
The extent of thrombosis in splanchnic veins is variable. A meta-analysis including 44 studies showed that the pooled prevalence of splanchnic venous thrombosis was 11.6% [95% confidence interval (CI): 8.5%-15.1%], splenic vein thrombosis was 12.8% (95%CI: 8.7%-17.6%), portal vein thrombosis was 3.5% (95%CI: 2.3%-4.8%), and mesenteric vein thrombosis was 1.2% (95%CI: 0.4%-2.5%) in patients with CP[18]. Another meta-analysis estimated the incidence of splenic vein thrombosis to be 12.4% among patients with CP[26]. Various studies have shown that splenic vein is most commonly involved due to its anatomical proximity along the posterior surface of pancreas. This is followed by involvement of portal and mesenteric veins to variable extent as depicted in Table 2.
Table 2.
Studies showing the frequency and site of venous thrombosis in chronic pancreatitis
|
Ref.
|
Venous thrombosis
|
Distribution
|
| Bernades et al[85], 1992 | 35/266 (13%) | SVT: 22 (8%) |
| PVT: 4% (10%) | ||
| SMVT: 3(1%) | ||
| Heider et al[28], 2004 | Venous thrombosis with chronic pancreatitis: 53 | SVT: 34 (64.3%) |
| SVT and SMVT: 10 (18.7%) | ||
| SVT and PVT: 3 (5.7%) | ||
| SVT, SMVT and PVT: 6 (11.3%) | ||
| Anand et al[21], 2020 | 149/1363 (10.9%) | SVT: 95 (63.8%) |
| PVT: 29 (19.4%) | ||
| SVT and PVT: 25 (16.8%) | ||
| Vujasinovic et al[17], 2021 | 30/394 (7.6%) | PVT: 2/30 (6.7%) |
| SVT: 16/30 (53.3%) | ||
| MVT: 1/30 (3.3%) | ||
| PVT and SVT: 3/30 (10.0%) | ||
| PVT and MVT: 2/30 (6.7%) | ||
| MVT and SVT: 4/30 (13.3%) | ||
| SVT, PVT, and MVT: 2/30 (6.7%) |
MVT: Mesenteric vein thrombosis; PVT: Portal vein thrombosis; SVT: Splenic vein thrombosis; SMVT: Superior mesenteric vein thrombosis.
Clinical presentation
Venous thrombosis in the setting of underlying CP may be either asymptomatic and detected incidentally or present as various clinical manifestations as shown in Table 3. Venous thrombosis can be acute or chronic. Acute venous thrombosis is uncommon in CP as compared to AP.
Table 3.
Clinical consequences of venous thrombosis in patients with chronic pancreatitis in different studies
|
Ref.
|
Varices
|
Splenomegaly
|
Clinical presentation
|
| Bernades et al[85], 1992 (n = 266) | Esophageal: 2 (5%). Gastric: 4 (10%) | - | Hematemesis: 1. Melena: 1 |
| Sakorafas et al[29], 2000 (n = 34) | Gastroesophageal: 12 (35%) | - | Variceal bleed: 6/34 (17.6%) |
| Heider et al[28], 2004 (n = 53) | Overall gastroesophageal varices: 41/53 (77%). On CT: 40/53 (75.4%). On EGD: 11/36 (30.5%). Both CT and EGD: 10/36 (27.7%) | - | Gastric variceal bleed: 2 (4%) |
| Agarwal et al[20], 2008 (n = 34) | Varices: 11 /34. Gastric: 7/11 (64%). Esophageal: 4/11 (36%) | 13/34 (38%) | Variceal bleed: 5/34 (15%). Gastric variceal bleed: 3/5 (60%). PHG bleed: 2/5 (40%) |
| Pandey et al[23], 2019 (n = 157) | IGV: 7 (18.9%). GOV: 1 (2.7%) | - | Upper GI bleed: 7 (18.9%). Gastric variceal bleed: 3 (8.1%). Nonvariceal: 4 (10.8%) |
| Ru et al[22], 2020 (n = 3358) | Gastric: 45/89 (50.6%) | 50/3358 (1.5%) | Variceal bleed: 17/89 (19.1%). Melena: 13 (76.5%). Hematemesis: 10 (58.8%). Both: 8 (47%) |
| Anand et al[21], 2020 (n = 1363) | 43/149 (28.9%) | 27/149 (18.1%) | GI bleed: 21/149 (14.1%) |
| Vujasinovic et al[17], 2021 (n = 394) | 3/30 (10%) | 6/30 (20%) | GI bleed: 0/30. Intraabdominal bleed: 0/30 |
CT: Computed tomography; EGD: Esophagogastroduodenoscopy; GI: Gastrointestinal; GOV: Gastro-esophageal varices; IGV: Isolated gastric varices; PHG: Portal hypertensive gastropathy.
Chronic splanchnic venous thrombosis in CP is associated with formation of collaterals along the splenoportal and gastroepiploic venous systems that may give rise to gastro-esophageal varices. These varices develop due to dilation of the short gastric, gastroepiploic and coronary veins[26]. This phenomenon is referred to as “left sided”, “compartmental”, “lineal”, “splenoportal” or “sinistral’’ portal hypertension. The estimated prevalence of gastro-esophageal varices on esophagogastroduodenoscopy (EGD) ranges from 10%-77%[17,21,23,26-28]. The pooled prevalence of gastro-esophageal varices in CP on EGD in a meta-analysis was 53%, 77.3% of which were gastric, however all these studies had significant heterogeneity[26]. Table 3 shows the relative distribution of varices on EGD. Incidentally detected abdominal collaterals on imaging without definitive varices may be present in 30%-83% patients of CP[17,21] (Figure 2).
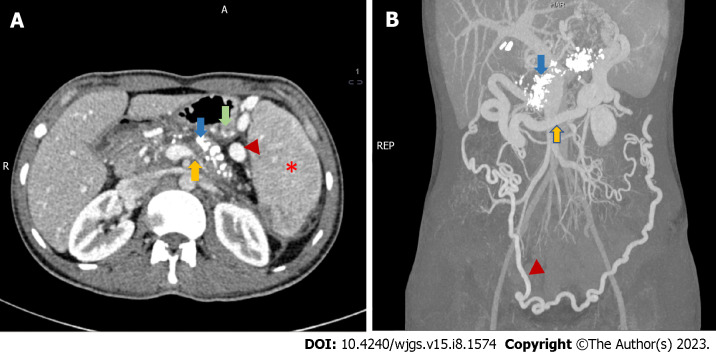
Figure 2.
Radiological features of chronic calcific pancreatitis and its complications including venous thrombosis and collaterals. A: An axial section of contrast-enhanced computed tomography (CECT) showing chronic pancreatitis with calcifications (blue arrow), attenuated splenic vein (yellow arrow), multiple perigastric collaterals (green arrow), gastrosplenic collaterals (red arrowhead) and splenomegaly (red asterisk); B: Coronal section of CECT of the same patient showing extensive pancreatic calcification (blue arrow) with dilated gastroepiploic vein (yellow arrow) and omental collaterals (red arrowhead). Courtesy: Dr Madhusudhan KS, Department of Radiodiagnosis.
The clinical presentation as variceal bleed in patients with CP ranges from 3%-20% in different studies[17,20-22]. Bleeding is less frequent in chronic splanchnic venous thrombosis related varices due to development of abundant collaterals with a pooled prevalence of 12.3% in a meta-analysis[20-22,25,26,28-31]. The common presentation of variceal bleed is hematemesis or melena in variable frequency as per different studies[21,23]. Various studies highlighting the prevalence of varices and clinical presentation as bleed have been elaborated in Table 3. Similarly, splenomegaly has been noted in a variable frequency (20%-54%)[17,21,26] in studies as shown in Table 3.
Portal vein thrombosis leads to development of collaterals around the common bile duct (epicholedochal and paracholedochal), which may give rise to benign biliary strictures, predispose to choledocholithiasis and even acute cholangitis[32]. Long standing strictures may eventually lead to secondary sclerosing cholangitis and biliary cirrhosis. The data on the prevalence of portal cavernoma in CP is, however, lacking.
There is wide discrepancy regarding the detection of varices and bleeding manifestations due to variable sample size, retrospective nature of most studies, difference in detection strategies of varices (screening vs EGD when symptomatic), and lastly due to the differences in surgical vs medical series as methods of detection and management were different among them. Although the presence of varices is higher, but risk of variceal bleed is lower in patients with CP likely due to decompression by the presence of intrabdominal collaterals.
Diagnosis
Venous thrombosis is usually detected on imaging, done for either symptomatic patients or asymptomatic patients undergoing imaging for non-bleed related indications. In the past, angiography was used for diagnosis of splanchnic venous thrombosis, but currently it has limited diagnostic role in this setting[33]. Ultrasound colour doppler is the initial screening modality for detection of splanchnic venous thrombosis[25]. It shows echogenic contents within the vessel lumen along with reduced or absent flow on colour doppler, depending on the degree of occlusion[34]. The visualisation of portal vein is better than splenic and mesenteric veins on ultrasonography. Ultrasound might also show the presence of splenomegaly, collaterals, or portal cavernoma. Ultrasound doppler has a sensitivity of 93% and a specificity of 83% for splenic vein thrombosis[35] and sensitivity and specificity is more than 95% for portal vein thrombosis[34,36]. Endoscopic ultrasound (EUS) has been shown to have a sensitivity of 81%, specificity of 93% and accuracy of 89% for detecting thrombosis in the porto-splanchnic venous system[37].
Contrast-enhanced computed tomography (CECT) (Figure 2) is the investigation of choice for evaluation of extent of splanchnic vein thrombosis and collateral formation, and also gives additional information regarding pancreatic parenchymal or ductal changes, calcification, local complications such as pseudocyst, benign biliary stricture, and to rule out an associated mass, either inflammatory or neoplastic[34,38]. It provides information regarding the degree of occlusion, extent of involvement and ischemic changes in the bowel wall, if any. Venous thrombosis is visible as hypodense non-enhancing contents within the vessels on the portal venous phase with a sensitivity and specificity of more than 90%[39,40] (Figure 2).
Contrast-enhanced magnetic resonance angiography (MRA) may also be used to evaluate the extent of thrombosis with a sensitivity of 100% and specificity of 98%[41]. It shows the clot in the lumen of the veins as isointense material on T1-weighted images and more intense on T2-weighted images[34]. However, its use is limited to indications like evaluation of head mass or biliary obstruction, since CECT has similar sensitivity and specificity for splanchnic venous thrombosis with a short study period, lower cost and easy availability.
Management
Management includes acute management of GI bleed and long-term definitive management. Acute management of GI bleed includes resuscitation by intravenous crystalloids and airway protection, followed by EGD and endotherapy for the variceal and non-variceal sources of bleed. In case of variceal bleed, the endotherapy depends on the source of bleed and involves variceal band ligation or sclerotherapy for esophageal varices or cyanoacrylate glue injection into the gastric varices[42]. EUS-guided coiling with or without glue injection can be used in selected patients where bleeding cannot be controlled by endotherapy (Figure 3B). A recent study showed 100% technical success rate in six patients of CP with splenic vein thrombosis who presented with upper GI bleed from gastric varices using EUS-guided combined coiling and cyanoacrylate glue injection[43].
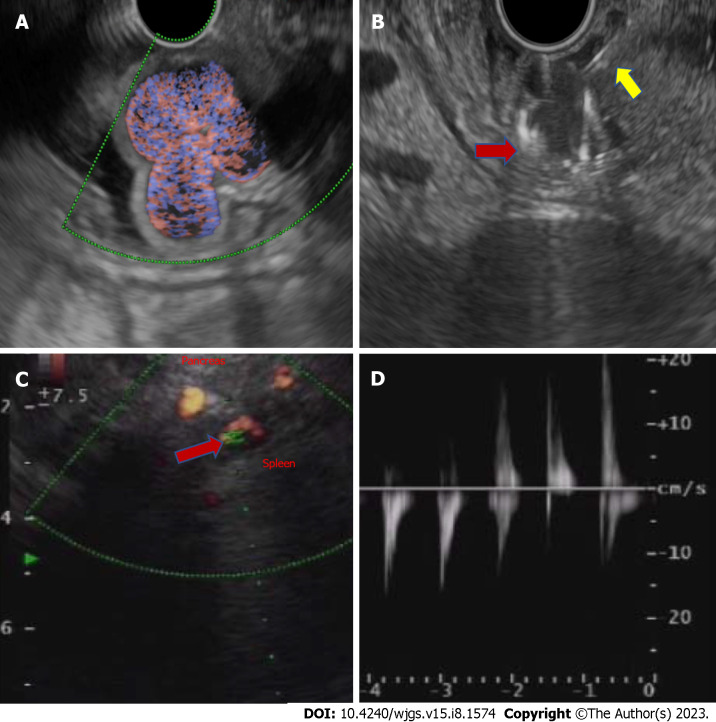
Figure 3.
Endoscopic ultrasound-guided fundal variceal obliteration, and pseudoaneurysm on endoscopic ultrasound. A: Linear endoscopic ultrasound showing the fundal varices on doppler study; B: Linear endoscopic ultrasound guided metal coil (red arrow, hyperechoic curved structure) being pushed into the varices for obliteration after the endoscopic ultrasound needle puncture (yellow arrow, hyperechoic linear structure); C: Linear endoscopic ultrasound showing an arterial pseudoaneurysm (red arrow) on doppler study; D: On power doppler mode Doppler showing an arterial waveform with bidirectional flow, classically labelled as “yin-yang” sign.
The definitive management of CP-related venous thrombosis is still a matter of debate. Since most of these patients are asymptomatic and up to 30% have spontaneous venous recanalization[44], the definite role of anticoagulation is not established. There are no well-defined risk factors to predict the group of patients who will have spontaneous recanalization. All patients with CP with venous thrombosis, particularly splenic vein thrombosis, should undergo an EGD to identify the presence of varices. The data on the role of anticoagulation including newer oral anticoagulants (NOACs) in venous thrombosis in the setting of CP is scarce as compared to AP, hence, no definite recommendation can be made[24]. Indications of anticoagulation in CP include, the extension of acute thrombus to portal and mesenteric vein, and the development of mesenteric ischemia.
Splenectomy can be considered in some cases with recurrent variceal bleed[29,45]. Some studies suggest that in patients with splenic vein thrombosis, who are undergoing pancreatic surgery, prophylactic splenectomy can be performed simultaneously to decrease the risk of variceal bleed, however it is still a matter of debate[25]. In patients who are not suitable for splenectomy, splenic artery embolization remains an alternate option[46,47]. Therefore, the treatment must be individualised keeping the patient’s characteristics and available resources in mind.
For prophylaxis of variceal bleed, beta blockers have been recommended for secondary prophylaxis in extrahepatic portal venous obstruction as extrapolated from data of cirrhotic portal hypertension with no first hand data on their role in CP-related varices[48]. Data on the role of beta blockers for primary prophylaxis is lacking. So, a definite recommendation for beta blockers cannot be made based on available evidence.
ARTERIAL COMPLICATIONS
Arterial complications include formation of PsAs, which may lead to life threating consequences. It may be seen in both AP and CP due to ongoing inflammation. PsA is defined as an encapsulated hematoma communicating with the lumen of the vessel and the outer wall consists of perivascular tissue, fibrosis, or clot making it prone to rupture[24,49].
Pathogenesis
PsA is usually formed as a local complication due to surrounding pancreatic inflammation which causes necrotising arteritis with subsequent erosion of the vessel wall due to enzymatic degradation[49,50]. This leads to weakening and ballooning of the vessel wall resulting in communication with the surrounding fluid collection[51,52]. PsA may also develop in relation to percutaneously placed drains or plastic stents placed for pseudocyst/walled off necrosis drainage due to local irritation and ongoing inflammation due to collection[50,53].
Location
As understood from the pathophysiology, PsA are usually formed adjacent to the site of significant inflammation, in relation to necrotizing pancreatitis in patients with AP and adjacent to pseudocysts in those with CP[54]. A meta-analysis by Sagar et al[19] including 29 studies (840 cases of pancreatitis) estimated the pooled incidence of PsA to be 0.05% and 0.03% in AP and CP, respectively. The most commonly involved artery in this meta-analysis was splenic artery (37.7%) followed by gastroduodenal artery (23.6%), pancreaticoduodenal artery (10.6%), hepatic artery (8.3%), left gastric artery (3.3%), superior mesenteric artery (3.3%), colic artery (2%), jejunal arteries (1.1%), coeliac artery (0.6%), and inferior mesenteric arteries (0.2%). The size can be variable and can reach up to 85 mm[55]. The relative distribution of PsA in various studies is elaborated in Table 4.
Table 4.
Studies showing sites and clinical manifestations of arterial pseudoaneurysms in patients with chronic pancreatitis
|
Ref.
|
Vascular complications
|
PsAs
|
Site- artery involved
|
Clinical presentation
|
| Bergert et al[86], 2004 | 36/541 | 25/36 | Splenic: 8/25 (32%). Pancreaticoduodenal (superior or inferior): 7/25 (28%). Gastroduodenal: 4/25 (16%). Superior mesenteric: 2/25 (8%). Jejunal branches: 2/25 (8%). Left gastric: 1/25 (4%). Right colic: 1/25 (4%) | Acute abdominal pain: 12 (48%). Haemorrhagic shock: 10 (40%). Acute upper GI bleed: 9 (36%). Acute lower GI bleed: 3 (12%). Chronic anaemia: 3 (12%). Acute on chronic pancreatitis: 5 (20%) |
| Udd et al[81], 2007 | 33/745 | 33 | Gastroduodenal/pancreaticoduodenal: 19 (58%). Splenic or its branches: 14 (42%) | Abdominal pain: 22 (66.6%). GI bleed: 17 (51%) |
| Sethi et al[87], 2010 | Chronic pancreatitis with PsA: 16 | 16 | Splenic: 7 (43.7%). Hepatic: 3 (18.75%). Gastroduodenal: 2 (12.5%). Right gastric: 2 (12.5%). Left gastric: 1 (6.25%). Pancreaticoduodenal: 1 (6.25%) | Intraabdominal bleed: 2 (13%). GI bleed: 8 (50%). Occult bleed: 10 (63). Pain: 14 (88) |
| Mallick et al[88], 2019 | 27/380 | 27 | Gastroduodenal: 13 (48.2%). Splenic: 10 (37.1%). Superior mesenteric: 2 (7.4%). Left gastric: 1 (3.7%). Inferior pancreaticoduodenal: 2 (7.4%) | Hematemesis: 6/27 (22.2%). Melena: 17/27 (63.0%). PCD bleed: 1/27 (3.7%) |
| Zabicki et al[55], 2018 | Chronic pancreatitis with PsA: 15 | 15 | Splenic: 7/15 (46.7%). Common hepatic: 2/15 (13.3%). Right gastroepiploic: 2/15 (13.3%) | - |
| Anand et al[21], 2020 | 166/1363 (12.2%) | PsA: 34/1363 (2.5%). PsA alone: 17 (50%). PsA with VT: 17 (50%) | Splenic: 25/33 (75.7%). Gastroduodenal: 6/33 (18.2%). Inferior pancreatico-duodenal: 1/33 (3.0%). Left gastric: 1/33 (3.0%) | GI bleed: 22/34 (64.7%) |
| Vujasinovic et al[17], 2021 | 33/394 | 3/394 (0.8%) | Splenic: 2/3 (66.7). Left gastric: 1/3 (33.3) | Incidental finding: 3/3 (100%). Intraabdominal bleeding: 0/3 (0%) |
| Madhusudhan et al[68], 2021 | 56 patients of chronic pancreatitis | PsA: 61 | Splenic: 31/56 (55.3%). Gastroduodenal: 18/56 (32.1%). Inferior pancreaticoduodenal: 1/56 (1.7%). Colic: 1/56 (1.7%). Hepatic: 4/56 (7.1%). Left gastric: 5/56 (8.9%) | Upper GI bleed: 40/56 (71.4%). PCD bleed: 1/56 (1.7%). Pain: 4/56 (7.14%). Incidentally detected on imaging: 11/56 (19.6%) |
GI: Gastrointestinal; PCD: Percutaneous drain; VT: Venous thrombosis; PsA: Pseudoaneurysm.
Clinical presentation
PsAs can be either asymptomatic and detected incidentally while imaging for other indications or may present with abdominal pain or overt GI bleed. The reported incidence of PsA-related bleed is around 4%-10%[52] with the risk of rupture in up to 50% and mortality after rupture as high as 15%-40% in old surgical series[56-58]. Various studies with reported frequency of clinical presentations with PsAs have been enumerated in Table 4.
Rupture of PsA may be into the GI tract presenting as hematemesis or melena, into abdominal cavity presenting as pain, or features of peritonitis, or retroperitoneum where the main presentation is pain with or without hemodynamic worsening. Less commonly, it may rupture into the pancreatic duct (hemosuccus pancreaticus)[59] or bile duct (hemobilia) resulting in hematemesis or melena[24,60]. Sometimes, the PsA may rupture into a pseudocyst cavity and if the pseudocyst is being drained percutaneously, it may present as bleed in the drainage tube[60].
Most of the studies have investigated the symptomatic luminal gastrointestinal bleed and data on intraabdominal and retroperitoneal bleed is still scant which usually present as worsening abdominal pain with or without hemodynamic instability and hemoglobin drop[24,61]. Retroperitoneal bleed was seen in 2 (5%) of 39 cases presenting with pseudoaneurysmal bleed in a study[62]. Clinical manifestations of a ruptured PsA apart from overt luminal or intra-abdominal bleed include acute worsening of abdominal pain, abdominal distension, or unexplained sudden hemodynamic worsening. The reported frequency of these symptoms as per different studies has been mentioned in Table 4. The bleed resulting due to rupture of PsA can be life threatening if not identified and managed promptly.
Diagnosis
The diagnosis is established by demonstrating a PsA on imaging modalities such as ultrasound doppler, EUS, multidetector CT angiography (CTA), MRA or conventional angiography [digital subtraction angiography (DSA)]. Among these modalities, EUS and DSA are both diagnostic as well as therapeutic, as definitive obliteration can be done in the same setting.
On ultrasonography, a hypoechoic cystic structure may be seen adjacent to a vessel. On doppler imaging, blood flow within that cystic structure has typical turbulent swirling motion known as the “yin-yang sign”[63]. The diagnosis is confirmed by demonstration of a communicating channel (neck) between the cystic structure and the feeding artery with a “to-and-fro” waveform[63]. Thus, doppler increases the detection rate and should always be used to evaluate cystic lesions to avoid confusing PsA with a complex cyst[64]. Doppler can miss small and partially or completely thrombosed PsAs since it may not show characteristic flow patterns[24]. Endosonography (Figures 3C and D) may show similar findings with hypoechoic area adjacent to feeding artery with swirling turbulent blood flow on doppler, the “yin-yang” sign as discussed above[65].
CTA is currently the most widely used modality because of its high sensitivity and specificity, easy availability, low cost, user independence and short procedure time (Figures 4A and B). On non-contrast CT, the presence of hyperdense content adjacent to or within a pseudocyst cavity might indicate rupture of PsA[63]. After contrast injection, contrast leak in the collection, or presence of contrast in the cystic structure or communication with the adjacent feeding artery suggests PsA (Figure 4A)[63]. The lumen of PsA may not be completely opacified with contrast owing to thrombosis. CTA has been shown to have a sensitivity and specificity of more than 95% in a study[66]. Three-dimensional reconstruction of CT imaging gives a better idea of anatomy of the PsA and helps in providing a roadmap for further therapeutic interventions (Figure 4B).
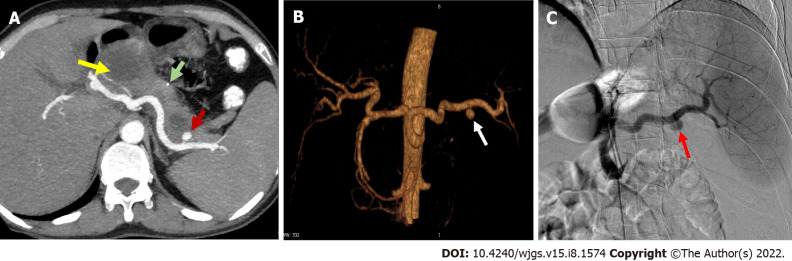
Figure 4.
Arterial pseudoaneurysm in chronic pancreatitis. A: Axial contrast-enhanced computed tomography in a patient of chronic pancreatitis showing a pseudoaneurysm (PsA) (red arrow) arising from splenic artery along with a specks of parenchymal calcification (green arrow) and a pseudocyst (yellow arrow) in the head of pancreas; B: Reconstructed angiographic image of the same patient showing splenic artery PsA (white arrow); C: Digital subtraction angiography of the same patient showing contrast outpouching from the splenic artery suggestive of splenic artery PsA (red arrow) with contrast opacification before endovascular therapy. Courtesy: Dr Madhusudhan KS, Department of Radiodiagnosis.
MRA is a valuable tool for detection of PsA especially where CT is contraindicated like in contrast allergy, with no radiation risk. It has high sensitivity and specificity for identifying the size, location and feeding artery of PsA, but clinical utility is limited owing to several disadvantages like prolonged procedure time (not feasible in actively bleeding patient), high cost, metal artifacts, patient mobility and limited availability[63].
DSA is the gold standard for diagnosis and treatment (Figure 4C). Major advantages include real time assessment of the PsA, its feeding artery, presence of collateral supply, any active extravasation, determination of neck size, detection of small lesions missed by CT and the potential for therapeutic intervention in the same setting[63]. However, it has some limitations including inaccurate assessment of size of PsA in presence of thrombus, exposure to ionizing radiation, and iodinated contrast-related complications (allergy or nephrotoxicity). DSA may be associated with various procedure-related complications namely development of PsA, hematoma or arteriovenous fistula at the site of puncture, distal embolization and ischemia, arterial spasm, intimal dissection, and vessel thrombosis[67].
Henceforth, approach to diagnosis include assessment of patient related factors, available modalities, and clinical presentation like active bleed with hemodynamic instability which requires an early intervention. The accepted approach for diagnosis of pseudoaneurysmal bleed is CTA followed by DSA and its obliteration.
Pang et al[60] described a classification system for peripancreatic PsA from a study which included both pancreatitis-related and postoperative PsAs. The classification system is based on the type of artery involved, its communication with the GI tract, and exposure to pancreatic juice. This proposed classification system may help in therapeutic decision making, approach to a particular lesion and outcome assessments. However, its external validity is lacking.
Management
As a dictum, all PsAs, including those detected incidentally on imaging done for other indications in CP have to be treated owing to the risk of potential life-threatening bleeding. Various treatment strategies and modalities are available which are employed depending on the mode of presentation. These include endovascular, percutaneous, EUS-guided and surgical treatment[50]. Studies showing various interventions and their outcomes in patients with PsA associated with CP have been enumerated below in Table 5.
Table 5.
Studies showing types of intervention and its outcomes in patients with chronic pancreatitis with pseudoaneurysms
|
Ref.
|
Patients
|
Presentation
|
Intervention for PsA
|
Outcomes
|
Complications
|
| Bergert et al[86], 2004 | Chronic pancreatitis: 541. Bleed: 36 | Acute bleed with haemorrhagic shock: 10/36 (27.7%). GI bleed: 12 (33.3%). Acute severe abdominal pain: 12 (33.3%) | PsA: 25/36 (69.4%). Angioembolization: 9(47%). Surgery: 16 (53%) | Higher rebleeding rate after surgery (25% vs 11% after embolization) | Deaths after surgery: 2. Deaths after embolization: 1 |
| On follow-up, one patient presented with a left hepatic artery PsA 18 mo post embolization of the gastroduodenal artery | Hospital mortality determinants: Haemorrhagic shock and amount of blood transfusion required | ||||
| Balachandra et al[89], 2005 | Total PsA: 214. Spontaneous: 160. Postoperative: 40. CP: 40. Pseudocyst: 135. AP: 39 | GI bleed: 147 (69%). Intra-abdominal bleed: 30 (14%) | Angiographic embolization attempted: 115 (66%). Successful: 85 (74%). Surgery: 62 (30%) | Among angiography group: 55 (37%) had subsequent surgery; 94 (63%) underwent embolization. In 30 (48%) of the 62 patients undergoing surgery as first intervention require: Angiography: 21/30; re-operation: 9/30 | - |
| Hsu et al[90], 2006 | CP with PsA-9 | - | Arterial embolization: 5. Surgical intervention: 9 | Success rates: Embolization: 20% (1/5). Surgery: 88.9% (8/9) | Mortality: Surgery (0). Post embolization [1 (sepsis)] |
| Zyromski et al[61], 2007 | PsA: 24 in pancreatitis. Acute on chronic pancreatitis: 22 (91.6%). Acute pancreatitis: 2 (8.6%). Most common etiology: Alcohol (79%) | GI bleed: 7 (29%). Increasing abdominal pain: 15 (62%) | Coil embolization: 23. Covered stent: 1 | Repeat embolization: 1 | - |
| Udd et al[81], 2007 | Chronic pancreatitis: 745. PsA: 33 | GI bleed: 17. Abdominal pain: 22. Bleeding confined to the pseudocyst: 9 (27%). Peritoneal bleed: 5 (15%). Retroperitoneal bleed: 3 (9%) | Angioembolization attempted: 23/33 (70%). Technical failure: 7 cases. Vessel not visualized: 3. Surgery: 4/5 cases with bleeding into the peritoneal cavity | Angioembolization success rate: 22/33 (67%). Re-embolization for recurrent bleed: 3. Success rate: 16/20 (80%) when the pseudocyst in head region and 50% when splenic artery was the source of bleed. Follow-up of surgical cases (14 mo): no rebleed or surgical intervention | 4 complications in the embolization procedure: Coil pushed to the MPD: 1 (endoscopically removed); dissection of the bleeding artery: 1; coil pushed into the iliac artery: 1; PsA at inguinal puncture: 1; mortality: 1 |
| Tulsyan et al[91], 2007 | Visceral aneurysms: 90. PsA: 28 | - | Coil embolization: 96%. N-butyl-cyanoacrylate (glue): 19% | Endovascular treatment technically successful: 98%. Secondary interventions for persistent flow: 1. Recurrent bleeding from previously embolized aneurysms: 2 | Postembolization syndrome developed: 3 (6%). 30-d mortality: 4 (8.3%) |
| Kim et al[92], 2015 | Total cases: 37. Chronic pancreatitis: 31 | - | 41 procedures. Transcatheter embolization: 39 (95.1%). Stent-grafts: 2 | Successful haemostasis: 34 (91.9%). Rebleed: 2 (treated by reintervention) | Focal splenic infarction: 8. Splenic abscess: 3 (2/3 died from sepsis) |
| Zabicki et al[55], 2018 | Chronic pancreatitis with PsA: 15 | - | Microcoils: 5. Bovine thrombin: 5. Squid embolization: 1. Stent graft: 1. Coil + vascular plug: 1. Thrombin and coil embolization with splenectomy: 1. Squid embolization with splenectomy: 1 | Complete exclusion of PsA from systemic circulation: 14/15(93.3%). Reintervention: 1. No recanalization at the follow-up CT after 1 to 3 wk | Splenic ischemia requiring splenectomy: 2 cases. No mortality at 30 d |
| Mallick et al[88], 2019 | Chronic pancreatitis: 380 | PsA: 27 | Endovascular coiling: 13 (48.2%). Endovascular glue: 3 (11.1%). Endovascular coiling + glue: 1 (3.7%). Percutaneous thrombin injection: 8 (29.6%). Conservative management: 1 (3.7%). Surgery: 1 (3.7%) | Technical success of embolization: 17/21 (80.9%). Clinical success of embolization: 16/17 (94.1%). Rebleed: 4 (14.8%) | Major complications of embolization: 1 (3.7%). Death: 1 (3.7%) |
| Madhusudhan et al[68], 2021 | 56 patients of chronic pancreatitis with 61 PsAs | Upper GI bleed: 40/56 (71.4%) | Embolization: 59/61 lesions. Technical success rate: 96.7%. Clinical success rate: 83.9% | Recurrent bleed: 9 (16.1%) (stopped spontaneously in 6/7, one expired). 49 patients followed up for a mean duration of 24.1 mo. Late recurrence of bleeding from a different artery- 4 patients (mean duration of 5.4 mo) | Major complications: 6 (10.7%). Splenic infarcts: 2/6. Splenic abscesses: 4/6 |
| Agents used for obliteration: Coils: 24; glue: 15; coils + glue: 15; gel foam: 2; others: 3 | Minor complications: Abdominal pain: 5 (8.9%); mortality rate: 1/56 (1.8%) | ||||
| Dhali et al[93], 2022 | 26 patients with CP with PsA | Upper GI bleed: 25 (96%). Incidental detection: 1 (4%) | Embolization: 11 (42%). Coil embolization: 10 (91%), followed by injection of glue in one patient (9%). Surgery: 20 (77%) including 5 patients after failed embolization | Embolization failed: 3 (27%). Rebleed from embolised PsA: 2 (18%). Over a median follow-up of 24 (6-122) mo, none had rebleed | Embolization-induced complications: 4/11 (36%). Colonic ischemia: 1. Splenic infarct: 1. Splenic abscess: 1. Acute renal failure: 1. The most common postoperative complication was wound infection followed by pancreatic fistula. No procedure-related death |
AP: Acute pancreatitis; CP: Chronic pancreatitis; CT: Computed tomography; GI: Gastrointestinal; MPD: Main pancreatic duct; PsA: Pseudoaneurysm.
Endovascular interventions
It is the mainstay of management of PsAs with high technical (89%-99%) and clinical success rates (74%-88%)[19,68]. The main aim is to exclude the PsA from systemic circulation. This can be achieved either by slowing down the flow of blood within the PsA (using coils, stent grafts), by inducing thrombosis (coils and liquid embolic agents) or by stimulating local inflammation (coils and liquid agents)[69]. Various types of embolic agents available are coils (stainless steel or platinum), n-butyl cyanoacrylate glue, stents (covered or uncovered), gelfoam, thrombin or vascular plugs. They can be used either alone or in combination[63,70,71] (Figure 5).
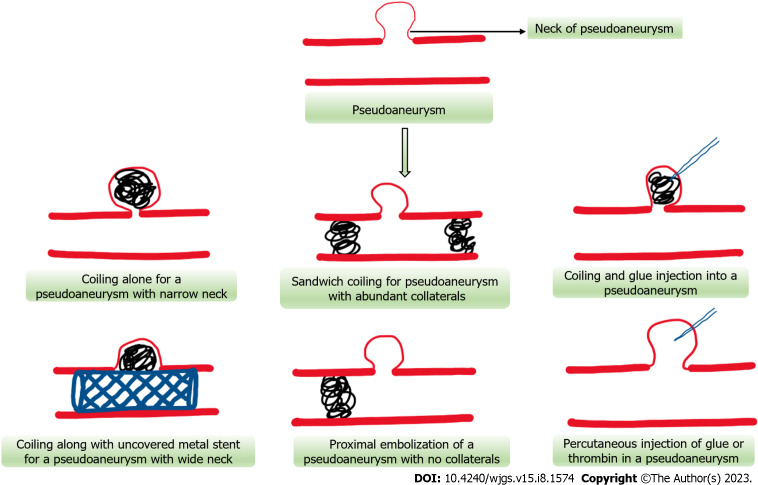
Figure 5.
Schematic representation of various endovascular and percutaneous approaches for pseudoaneurysm obliteration.
Metal coils (micro coils) act by slowing down the blood flow by causing mechanical obstruction and inducing thrombosis by their thrombogenic fibres and eliciting local inflammatory reaction. Once inside the PsA, they assume their spiral shape and cause occlusion of the PsA and its neck from main circulation and induce thrombosis. Various coil embolization techniques have been described namely, “sandwich technique”[71] (occlusion of artery proximal and distal to PsA- most commonly used), “sack packing”[72] (occluding the lumen of PsA with microcoils- when the neck is narrow) or proximal embolization[73] (where distal end cannot be cannulated or in case of end arteries). The indications of each technique depend on several factors including the size, location of PsA, nature of feeding artery, collaterals, and size of the neck. A simplified approach to the management of pseudoaneurysmal bleed has been illustrated in Figures 5 and 6. Complications include splenic infarction, splenic abscess formation, coil migration (wide neck), intestinal ischemia and vascular dissection[49] (Figure 5).
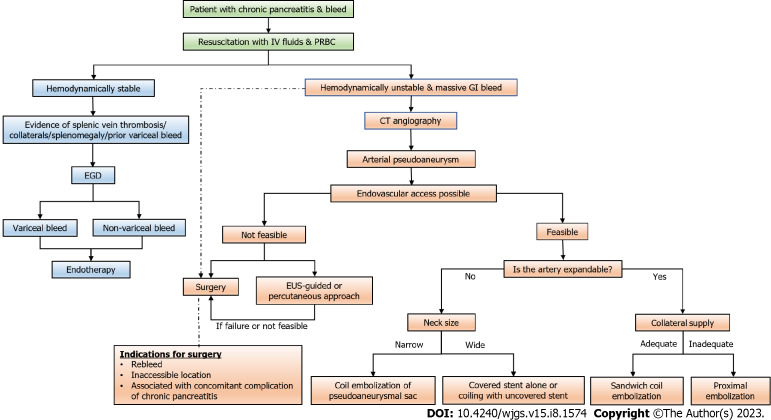
Figure 6.
Flow diagram depicting the approach to a case of gastrointestinal bleeding in patients with chronic pancreatitis (dashed arrow suggests an alternative management strategy). CT: Computed tomography; EGD: Esophagogastroduodenoscopy; GI: Gastrointestinal; PRBC: Packed red blood cell; EUS: Endoscopic ultrasound.
N-butyl cyanoacrylate glue is a liquid embolic agent which polymerises to form a hard cast after coming in contact with anions (blood)[69]. Glue is used when the target site is difficult to reach due to a tortuous arterial course, revascularization of PsA post coil embolization, and in patients with coagulation abnormalities, as coils need normal coagulation parameters for thrombosis[70]. It may also be used in combination with coils to provide a scaffolding and to prevent dislodgement of the coils (Figure 5).
Endovascular stent placement with or without coiling may be used in certain situations like PsA with wide neck to prevent coil migration and to preserve the patency of a parent artery[63,74]. Either covered stents alone or uncovered stents along with coiling of sac may be used to obliterate and exclude the PsA, while maintaining adequate flow in the parent artery[75]. This approach is useful where the PsA is located proximally in a large artery like hepatic, proximal splenic or superior mesenteric artery to prevent embolism or ischemia to the major organs[76]. Stent patency rates of about 82% has been reported in a study[76] (Figure 5).
Percutaneous interventions
Percutaneous obliteration of PsA is usually done under ultrasonography or CT guidance (Figure 5). This approach is usually adopted in cases where the PsA cannot be approached endovascularly and is surrounded by a solid organ which provides scaffolding[74]. It involves puncturing the PsA under imaging guidance and injection of various embolic agents including thrombin, glue or coils. Various studies have showed technical success rates of 92%-100%[75,77,78]. It may be associated with complications like rupture of PsA, cellulitis, embolic events and incomplete occlusion[63,74] (Figure 5).
EUS-guided interventions
EUS-guided obliteration is used in cases where endovascular approach fails or PsA is not visible on angiography but detected on EUS. EUS-guided obliteration using thrombin, glue or coils can be done to achieve haemostasis[79]. EUS-guided thrombin injection was successful in controlling bleed from splenic and gastroduodenal PsAs in three patients using 300-500 IU with no major complications in a study where endovascular approach was not feasible[56]. EUS-guided glue injection was successful in obliterating a large PsA in left inferior phrenic artery in a case of alcoholic CP after failed embolization and revascularization of PsA after percutaneous thrombin injection[80]. A study of six patients showed complete occlusion of splenic artery PsA using EUS-guided coil and glue injection at 12 wk with no complications[79]. Complications of this approach include bleed from puncture site, embolization to non-target organs or perforation peritonitis[56,79].
Overall, the choice of embolization and the approach depends on a variety of factors[63,74] - the size of PsA and its neck- [choice of coil or glue (narrow neck) or stent (wide neck)]; the nature of feeding artery- end artery or not, abundance of collaterals, accessibility, expandability; location of PsA- landing zone of coils and ease of cannulation; and coagulation parameters of patients- glue is preferable in deranged coagulation parameters.
Surgical management
It is indicated in cases with massive GI bleed and hemodynamic instability or if there is failure of endovascular interventions. The surgical options include direct vessel ligation to pancreatectomy, gastrectomy, or small intestinal resection, depending on the affected vessel and ischemia to the adjacent organs[54,81]. Surgery can be also considered, if a patient has concomitant complications of CP such as pseudocyst, benign biliary strictures, gastric outlet obstruction, painful inflammatory head mass or for pain relief[24,82]. In a nutshell, the optimal management of PsA is by the endovascular approach, however, a different alternative approach can be utilised on an individual patient basis.
Procedure-related bleeding
Patients with CP usually undergo various diagnostic and therapeutic interventions and some of these patients may develop bleeding as a procedure-related complication. The procedures commonly performed in CP include EUS-guided sampling, cyst aspiration, pseudocyst or ductal drainage, endoscopic retrograde cholangiopancreatography for various indications and also by percutaneous approach. These procedures may be associated with variable frequencies of risk of bleed[83,84]. Most of these bleeds are self-limiting and controlled endoscopically at the time of the endoscopic procedures, while some patients may require endovascular or surgical interventions, particularly in hemodynamically unstable patients or in cases of failure of endoscopic methods to control the bleed.
If bleed happens after percutaneous intervention, then patient should be observed carefully for haemoglobin drop and hemodynamic instability. If any of these events happen, patient should undergo CTA, followed by approach similar to as discussed earlier.
APPROACH TO GI BLEED IN PATIENT WITH CP
Whenever a patient with known or suspected CP presents with overt GI bleed or acute worsening of abdominal pain with hemodynamic worsening or bleed from percutaneous drain site, the patient should be immediately evaluated for the source and cause of the bleed. Initial management is like any other cause of GI bleed and includes securing two wide bore cannulas, resuscitation with intravenous fluids, airway protection and blood investigations including cross-matching for anticipated requirement of blood transfusion[24,38,50,63,71,74] (Figure 6).
The initial investigation of choice is multidetector CTA owing to its high sensitivity and low acquisition time. If an arterial lesion in the form of PsA is found, further management depends on hemodynamic stability. If a patient is hemodynamically unstable with ongoing bleed and other associated complications, the patient might be considered for upfront surgical intervention. In a hemodynamically stable patient, approach to lesion depends on the factors enumerated above along with the availability of interventional radiological services. The optimal management includes endovascular embolization and obliteration of the lesion. Other options include percutaneous and EUS-guided occlusion which might be considered on a case-to-case basis. In case of high suspicion of arterial bleed and non-identification of definite source of bleed on CTA, a patient can be directly considered for DSA both as a diagnostic and therapeutic option[24,38,50,63,71,74] (Figure 6).
If the imaging shows venous thrombosis with or without splenomegaly or abdominal collaterals, the patient should undergo an EGD for both diagnostic as well as therapeutic options for variceal bleed as described in venous thrombosis section (see above).
In cases of hemosucuss pancreaticus and hemobilia, CTA is used to identify the culprit vessels and further management is similar to what has been described earlier[50,59]. A simplified algorithm to approach a case of GI bleed in CP patient has been shown in Figure 6.
FUTURE DIRECTIONS AND UNRESOLVED ISSUES
So far, we have discussed about what is known about the epidemiology, presentation, and management of vascular complications in patients with CP. There are still many lacunae in the knowledge of these manifestations. Most of these studies are retrospective in nature, so knowing the denominator is difficult. We need a prediction model of factors that might predispose to such complications and in whom a possible role of proactive screening for such vascular lesions might be warranted before they land in life threatening conditions. Another potential area of investigation could be the role of anticoagulation in pancreatitis-related splanchnic venous or arterial thrombosis including NOACs. Another aspect of further investigation could be prospective studies in evaluating the role of endovascular and endosonography-related techniques of managing the PsA exploring the technical and clinical success rates and complications, and in which subgroup of patients which techniques have to be used. Therefore, further investigation into various aspects of pancreatitis-related vascular complications and their management must be explored to optimize patient care.
CONCLUSION
The patients with CP are prone to development of several vascular complications such as vascular thrombosis and PsAs, which sometimes might lead to life threatening consequences. Early identification with a high degree of suspicion and prompt management of these complications has a significant impact on patient outcomes. The management options have evolved over the years from a predominant surgical to a endovascular approach with high technical and clinical success rates. Optimal utilization of these resources can prevent catastrophes and optimise the management of patients with CP.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 28, 2023
First decision: May 4, 2023
Article in press: June 6, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ma L, China; Shiryajev YN, Russia S-Editor: Wang JJ L-Editor: A P-Editor: Ji MX
Contributor Information
Dinesh Walia, Gastroenterology and Human Nutrition Unit, All India Institute of Medical Sciences, New Delhi 110029, India.
Anoop Saraya, Gastroenterology and Human Nutrition Unit, All India Institute of Medical Sciences, New Delhi 110029, India.
Deepak Gunjan, Gastroenterology and Human Nutrition Unit, All India Institute of Medical Sciences, New Delhi 110029, India. drdg_01@rediffmail.com.
References
- 1.Vege SS, Chari ST. Chronic Pancreatitis. N Engl J Med. 2022;386:869–878. doi: 10.1056/NEJMcp1809396. [DOI] [PubMed] [Google Scholar]
- 2.Olesen SS, Krauss T, Demir IE, Wilder-Smith OH, Ceyhan GO, Pasricha PJ, Drewes AM. Towards a neurobiological understanding of pain in chronic pancreatitis: mechanisms and implications for treatment. Pain Rep. 2017;2:e625. doi: 10.1097/PR9.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murruste M, Kirsimägi Ü, Kase K, Veršinina T, Talving P, Lepner U. Complications of chronic pancreatitis prior to and following surgical treatment: A proposal for classification. World J Clin Cases. 2022;10:7808–7824. doi: 10.12998/wjcc.v10.i22.7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal S, Sharma S, Gunjan D, Singh N, Kaushal K, Poudel S, Anand A, Gopi S, Mohta S, Sonika U, Saraya A. Natural course of chronic pancreatitis and predictors of its progression. Pancreatology. 2020;20:347–355. doi: 10.1016/j.pan.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey ML, Conwell DL, Hart PA. Complications of Chronic Pancreatitis. Dig Dis Sci. 2017;62:1745–1750. doi: 10.1007/s10620-017-4518-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta AK, Chacko A. Head mass in chronic pancreatitis: Inflammatory or malignant. World J Gastrointest Endosc. 2015;7:258–264. doi: 10.4253/wjge.v7.i3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh VK, Yadav D, Garg PK. Diagnosis and Management of Chronic Pancreatitis: A Review. JAMA. 2019;322:2422–2434. doi: 10.1001/jama.2019.19411. [DOI] [PubMed] [Google Scholar]
- 8.Bundred J, Thakkar RG, Pandanaboyana S. Systematic review of sarcopenia in chronic pancreatitis: prevalence, impact on surgical outcomes, and survival. Expert Rev Gastroenterol Hepatol. 2022;16:665–672. doi: 10.1080/17474124.2022.2091544. [DOI] [PubMed] [Google Scholar]
- 9.Kuan LL, Dennison AR, Garcea G. Prevalence and Impact of Sarcopenia in Chronic Pancreatitis: A Review of the Literature. World J Surg. 2021;45:590–597. doi: 10.1007/s00268-020-05828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gopi S, Qamar S, Singh N, Agarwal S, Yegurla J, Rana A, Gunjan D, Saraya A. Malnutrition by GLIM criteria in chronic pancreatitis: Prevalence, predictors, and its impact on quality of life. Pancreatology. 2022;22:367–373. doi: 10.1016/j.pan.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Garg PK. Chronic pancreatitis in India and Asia. Curr Gastroenterol Rep. 2012;14:118–124. doi: 10.1007/s11894-012-0241-0. [DOI] [PubMed] [Google Scholar]
- 12.Yadav D, Hawes RH, Brand RE, Anderson MA, Money ME, Banks PA, Bishop MD, Baillie J, Sherman S, DiSario J, Burton FR, Gardner TB, Amann ST, Gelrud A, Lawrence C, Elinoff B, Greer JB, O'Connell M, Barmada MM, Slivka A, Whitcomb DC North American Pancreatic Study Group. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169:1035–1045. doi: 10.1001/archinternmed.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukund A, Gamanagatti S, Saraya A. Chronic pancreatitis causing thrombotic occlusion of IVC and renal veins. Trop Gastroenterol. 2013;34:39–41. doi: 10.7869/tg.2012.91. [DOI] [PubMed] [Google Scholar]
- 14.Rana SS, Sharma V, Reddy S, Bhasin DK. Combined endovascular and endoscopic management of thoracic aortic pseudoaneurysm, mediastinal pseudocyst, and pancreatic pleural effusion due to chronic pancreatitis. Gastrointest Endosc. 2015;81:1501–1502. doi: 10.1016/j.gie.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 15.Takagi H, Manabe H, Sekino S, Kato T, Matsuno Y, Umemoto T. Abdominal Aortic Pseudoaneurysm Associated with Chronic Pancreatitis. EJVES Extra. 2005;9:46–48. [Google Scholar]
- 16.Garcia-Rodriguez V, Jacob R, daSilva-deAbreu A. A Rare Case of Pancreatitis-Induced Thrombosis of the Aorta and Superior Mesenteric Artery. Methodist Debakey Cardiovasc J. 2019;15:220–222. doi: 10.14797/mdcj-15-3-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vujasinovic M, Dugic A, Nouri A, Brismar TB, Baldaque-Silva F, Asplund E, Rutkowski W, Ghorbani P, Sparrelid E, Hagström H, Löhr JM. Vascular Complications in Patients with Chronic Pancreatitis. J Clin Med. 2021;10 doi: 10.3390/jcm10163720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu W, Qi X, Chen J, Su C, Guo X. Prevalence of Splanchnic Vein Thrombosis in Pancreatitis: A Systematic Review and Meta-Analysis of Observational Studies. Gastroenterol Res Pract. 2015;2015:245460. doi: 10.1155/2015/245460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sagar S, Soundarajan R, Gupta P, Praveen Kumar M, Samanta J, Sharma V, Kochhar R. Efficacy of endovascular embolization of arterial pseudoaneurysms in pancreatitis: A systematic review and meta-analysis. Pancreatology. 2021;21:46–58. doi: 10.1016/j.pan.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal AK, Raj Kumar K, Agarwal S, Singh S. Significance of splenic vein thrombosis in chronic pancreatitis. Am J Surg. 2008;196:149–154. doi: 10.1016/j.amjsurg.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Anand A, Gunjan D, Agarwal S, Kaushal K, Sharma S, Gopi S, Mohta S, Madhusudhan KS, Singh N, Saraya A. Vascular complications of chronic pancreatitis: A tertiary center experience. Pancreatology. 2020;20:1085–1091. doi: 10.1016/j.pan.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Ru N, He CH, Ren XL, Chen JY, Yu FF, Yan ZJ, Guo JY, Zhu JH, Wang YC, Qian YY, Pan J, Hu LH, Li ZS, Zou WB, Liao Z. Risk factors for sinistral portal hypertension and related variceal bleeding in patients with chronic pancreatitis. J Dig Dis. 2020;21:468–474. doi: 10.1111/1751-2980.12916. [DOI] [PubMed] [Google Scholar]
- 23.Pandey V, Patil M, Patel R, Chaubal A, Ingle M, Shukla A. Prevalence of splenic vein thrombosis and risk of gastrointestinal bleeding in chronic pancreatitis patients attending a tertiary hospital in western India. J Family Med Prim Care. 2019;8:818–822. doi: 10.4103/jfmpc.jfmpc_414_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalas MA, Leon M, Chavez LO, Canalizo E, Surani S. Vascular complications of pancreatitis. World J Clin Cases. 2022;10:7665–7673. doi: 10.12998/wjcc.v10.i22.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pancreas Study Group; Chinese Society of Gastroenterology Chinese Medical Association. Practice guidance for diagnosis and treatment of pancreatitis-related splanchnic vein thrombosis (Shenyang, 2020) J Dig Dis. 2021;22:2–8. doi: 10.1111/1751-2980.12962. [DOI] [PubMed] [Google Scholar]
- 26.Butler JR, Eckert GJ, Zyromski NJ, Leonardi MJ, Lillemoe KD, Howard TJ. Natural history of pancreatitis-induced splenic vein thrombosis: a systematic review and meta-analysis of its incidence and rate of gastrointestinal bleeding. HPB (Oxford) 2011;13:839–845. doi: 10.1111/j.1477-2574.2011.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley EL 3rd. The natural history of splenic vein thrombosis due to chronic pancreatitis: indications for surgery. Int J Pancreatol. 1987;2:87–92. doi: 10.1007/BF03015001. [DOI] [PubMed] [Google Scholar]
- 28.Heider TR, Azeem S, Galanko JA, Behrns KE. The natural history of pancreatitis-induced splenic vein thrombosis. Ann Surg. 2004;239:876–80; discussion 880. doi: 10.1097/01.sla.0000128685.74686.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakorafas GH, Sarr MG, Farley DR, Farnell MB. The significance of sinistral portal hypertension complicating chronic pancreatitis. Am J Surg. 2000;179:129–133. doi: 10.1016/s0002-9610(00)00250-6. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Wang Q, Ding X, Liu Q, Huang W, Gu J, Wang Z, Wu W, Wu Z. The clinical applicability of percutaneous splenic vein stent implantation for pancreatic portal hypertension. BMC Gastroenterol. 2022;22:136. doi: 10.1186/s12876-022-02214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber SM, Rikkers LF. Splenic vein thrombosis and gastrointestinal bleeding in chronic pancreatitis. World J Surg. 2003;27:1271–1274. doi: 10.1007/s00268-003-7247-6. [DOI] [PubMed] [Google Scholar]
- 32.Khuroo MS, Rather AA, Khuroo NS, Khuroo MS. Portal biliopathy. World J Gastroenterol. 2016;22:7973–7982. doi: 10.3748/wjg.v22.i35.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valeriani E, Riva N, Di Nisio M, Ageno W. Splanchnic Vein Thrombosis: Current Perspectives. Vasc Health Risk Manag. 2019;15:449–461. doi: 10.2147/VHRM.S197732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajesh S, Mukund A, Arora A. Imaging Diagnosis of Splanchnic Venous Thrombosis. Gastroenterol Res Pract. 2015;2015:101029. doi: 10.1155/2015/101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uy PPD, Francisco DM, Trivedi A, O'Loughlin M, Wu GY. Vascular Diseases of the Spleen: A Review. J Clin Transl Hepatol. 2017;5:152–164. doi: 10.14218/JCTH.2016.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tessler FN, Gehring BJ, Gomes AS, Perrella RR, Ragavendra N, Busuttil RW, Grant EG. Diagnosis of portal vein thrombosis: value of color Doppler imaging. AJR Am J Roentgenol. 1991;157:293–296. doi: 10.2214/ajr.157.2.1853809. [DOI] [PubMed] [Google Scholar]
- 37.Lai L, Brugge WR. Endoscopic ultrasound is a sensitive and specific test to diagnose portal venous system thrombosis (PVST) Am J Gastroenterol. 2004;99:40–44. doi: 10.1046/j.1572-0241.2003.04020.x. [DOI] [PubMed] [Google Scholar]
- 38.Gupta P, Madhusudhan KS, Padmanabhan A, Khera PS. Indian College of Radiology and Imaging Consensus Guidelines on Interventions in Pancreatitis. Indian J Radiol Imaging. 2022;32:339–354. doi: 10.1055/s-0042-1754313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bach AM, Hann LE, Brown KT, Getrajdman GI, Herman SK, Fong Y, Blumgart LH. Portal vein evaluation with US: comparison to angiography combined with CT arterial portography. Radiology. 1996;201:149–154. doi: 10.1148/radiology.201.1.8816536. [DOI] [PubMed] [Google Scholar]
- 40.Riva N, Ageno W. Clinical manifestations and imaging tools in the diagnosis of splanchnic and cerebral vein thromboses. Thromb Res. 2018;163:252–259. doi: 10.1016/j.thromres.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 41.Kreft B, Strunk H, Flacke S, Wolff M, Conrad R, Gieseke J, Pauleit D, Bachmann R, Hirner A, Schild HH. Detection of thrombosis in the portal venous system: comparison of contrast-enhanced MR angiography with intraarterial digital subtraction angiography. Radiology. 2000;216:86–92. doi: 10.1148/radiology.216.1.r00jl2386. [DOI] [PubMed] [Google Scholar]
- 42.Gralnek IM, Camus Duboc M, Garcia-Pagan JC, Fuccio L, Karstensen JG, Hucl T, Jovanovic I, Awadie H, Hernandez-Gea V, Tantau M, Ebigbo A, Ibrahim M, Vlachogiannakos J, Burgmans MC, Rosasco R, Triantafyllou K. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:1094–1120. doi: 10.1055/a-1939-4887. [DOI] [PubMed] [Google Scholar]
- 43.Rana SS, Bush N, Sharma R, Dhalaria L, Gupta R. Forward-viewing EUS-guided combined coil and glue injection in bleeding gastric varices secondary to splenic vein thrombosis in chronic pancreatitis. Endosc Ultrasound. 2022;11:246–247. doi: 10.4103/EUS-D-21-00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Besselink MG. Splanchnic vein thrombosis complicating severe acute pancreatitis. HPB (Oxford) 2011;13:831–832. doi: 10.1111/j.1477-2574.2011.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Liu GJ, Chen YX, Dong HP, Wang LX. Sinistral portal hypertension: clinical features and surgical treatment of chronic splenic vein occlusion. Med Princ Pract. 2012;21:20–23. doi: 10.1159/000329888. [DOI] [PubMed] [Google Scholar]
- 46.Liu Q, Song Y, Xu X, Jin Z, Duan W, Zhou N. Management of bleeding gastric varices in patients with sinistral portal hypertension. Dig Dis Sci. 2014;59:1625–1629. doi: 10.1007/s10620-014-3048-z. [DOI] [PubMed] [Google Scholar]
- 47.Gandini R, Merolla S, Chegai F, Abrignani S, Lenci I, Milana M, Angelico M. Trans-splenic Embolization Plus Partial Splenic Embolization for Management of Variceal Bleeding Due to Left-Sided Portal Hypertension. Dig Dis Sci. 2018;63:264–267. doi: 10.1007/s10620-017-4863-9. [DOI] [PubMed] [Google Scholar]
- 48.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959–974. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: literature review. J Comput Assist Tomogr. 2015;39:7–12. doi: 10.1097/RCT.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 50.Evans RP, Mourad MM, Pall G, Fisher SG, Bramhall SR. Pancreatitis: Preventing catastrophic haemorrhage. World J Gastroenterol. 2017;23:5460–5468. doi: 10.3748/wjg.v23.i30.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi K, Futagawa S, Ochi M, Sakamoto I, Hayashi K. Pancreatic pseudoaneurysm converted from pseudocyst: transcatheter embolization and serial CT assessment. Radiat Med. 2000;18:147–150. [PubMed] [Google Scholar]
- 52.Chiang KC, Chen TH, Hsu JT. Management of chronic pancreatitis complicated with a bleeding pseudoaneurysm. World J Gastroenterol. 2014;20:16132–16137. doi: 10.3748/wjg.v20.i43.16132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rana SS, Chhabra P, Ahuja C, Gunjan D, Bhasin DK. Gastroduodenal artery pseudoaneurysm resulting from a plastic stent after pseudocyst drainage. Endoscopy. 2015;47 Suppl 1:E631–E632. doi: 10.1055/s-0034-1393589. [DOI] [PubMed] [Google Scholar]
- 54.Mendelson RM, Anderson J, Marshall M, Ramsay D. Vascular complications of pancreatitis. ANZ J Surg. 2005;75:1073–1079. doi: 10.1111/j.1445-2197.2005.03607.x. [DOI] [PubMed] [Google Scholar]
- 55.Zabicki B, Limphaibool N, Holstad MJV, Juszkat R. Endovascular management of pancreatitis-related pseudoaneurysms: A review of techniques. PLoS One. 2018;13:e0191998. doi: 10.1371/journal.pone.0191998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gamanagatti S, Thingujam U, Garg P, Nongthombam S, Dash NR. Endoscopic ultrasound guided thrombin injection of angiographically occult pancreatitis associated visceral artery pseudoaneurysms: Case series. World J Gastrointest Endosc. 2015;7:1107–1113. doi: 10.4253/wjge.v7.i13.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huizinga WK, Baker LW. Surgical intervention for regional complications of chronic pancreatitis. Int Surg. 1993;78:315–319. [PubMed] [Google Scholar]
- 58.Gambiez LP, Ernst OJ, Merlier OA, Porte HL, Chambon JP, Quandalle PA. Arterial embolization for bleeding pseudocysts complicating chronic pancreatitis. Arch Surg. 1997;132:1016–1021. doi: 10.1001/archsurg.1997.01430330082014. [DOI] [PubMed] [Google Scholar]
- 59.Cui HY, Jiang CH, Dong J, Wen Y, Chen YW. Hemosuccus pancreaticus caused by gastroduodenal artery pseudoaneurysm associated with chronic pancreatitis: A case report and review of literature. World J Clin Cases. 2021;9:236–244. doi: 10.12998/wjcc.v9.i1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pang TC, Maher R, Gananadha S, Hugh TJ, Samra JS. Peripancreatic pseudoaneurysms: a management-based classification system. Surg Endosc. 2014;28:2027–2038. doi: 10.1007/s00464-014-3434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zyromski NJ, Vieira C, Stecker M, Nakeeb A, Pitt HA, Lillemoe KD, Howard TJ. Improved outcomes in postoperative and pancreatitis-related visceral pseudoaneurysms. J Gastrointest Surg. 2007;11:50–55. doi: 10.1007/s11605-006-0038-2. [DOI] [PubMed] [Google Scholar]
- 62.Kalva SP, Yeddula K, Wicky S, Fernandez del Castillo C, Warshaw AL. Angiographic intervention in patients with a suspected visceral artery pseudoaneurysm complicating pancreatitis and pancreatic surgery. Arch Surg. 2011;146:647–652. doi: 10.1001/archsurg.2011.11. [DOI] [PubMed] [Google Scholar]
- 63.Saad NE, Saad WE, Davies MG, Waldman DL, Fultz PJ, Rubens DJ. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005;25 Suppl 1:S173–S189. doi: 10.1148/rg.25si055503. [DOI] [PubMed] [Google Scholar]
- 64.Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol. 2008;31:957–970. doi: 10.1007/s00270-007-9138-y. [DOI] [PubMed] [Google Scholar]
- 65.Sharma M, Somani P, Prasad R, Jindal S. EUS imaging of splenic artery pseudoaneurysm. VideoGIE. 2017;2:219–220. doi: 10.1016/j.vgie.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soto JA, Múnera F, Morales C, Lopera JE, Holguín D, Guarín O, Castrillón G, Sanabria A, García G. Focal arterial injuries of the proximal extremities: helical CT arteriography as the initial method of diagnosis. Radiology. 2001;218:188–194. doi: 10.1148/radiology.218.1.r01ja13188. [DOI] [PubMed] [Google Scholar]
- 67.Múnera F, Soto JA, Palacio D, Velez SM, Medina E. Diagnosis of arterial injuries caused by penetrating trauma to the neck: comparison of helical CT angiography and conventional angiography. Radiology. 2000;216:356–362. doi: 10.1148/radiology.216.2.r00jl25356. [DOI] [PubMed] [Google Scholar]
- 68.Madhusudhan KS, Gopi S, Singh AN, Agarwal L, Gunjan D, Srivastava DN, Garg PK. Immediate and Long-Term Outcomes of Percutaneous Radiological Interventions for Hemorrhagic Complications in Acute and Chronic Pancreatitis. J Vasc Interv Radiol. 2021;32:1591–1600.e1. doi: 10.1016/j.jvir.2021.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Leyon JJ, Littlehales T, Rangarajan B, Hoey ET, Ganeshan A. Endovascular embolization: review of currently available embolization agents. Curr Probl Diagn Radiol. 2014;43:35–53. doi: 10.1067/j.cpradiol.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Madhusudhan KS, Gamanagatti S, Garg P, Shalimar, Dash NR, Pal S, Peush S, Gupta AK. Endovascular Embolization of Visceral Artery Pseudoaneurysms Using Modified Injection Technique with N-Butyl Cyanoacrylate Glue. J Vasc Interv Radiol. 2015;26:1718–1725. doi: 10.1016/j.jvir.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 71.Jesinger RA, Thoreson AA, Lamba R. Abdominal and pelvic aneurysms and pseudoaneurysms: imaging review with clinical, radiologic, and treatment correlation. Radiographics. 2013;33:E71–E96. doi: 10.1148/rg.333115036. [DOI] [PubMed] [Google Scholar]
- 72.Loffroy R, Rao P, Ota S, De Lin M, Kwak BK, Krause D, Geschwind JF. Packing technique for endovascular coil embolisation of peripheral arterial pseudo-aneurysms with preservation of the parent artery: safety, efficacy and outcomes. Eur J Vasc Endovasc Surg. 2010;40:209–215. doi: 10.1016/j.ejvs.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 73.Spiliopoulos S, Sabharwal T, Karnabatidis D, Brountzos E, Katsanos K, Krokidis M, Gkoutzios P, Siablis D, Adam A. Endovascular treatment of visceral aneurysms and pseudoaneurysms: long-term outcomes from a multicenter European study. Cardiovasc Intervent Radiol. 2012;35:1315–1325. doi: 10.1007/s00270-011-0312-x. [DOI] [PubMed] [Google Scholar]
- 74.Madhusudhan KS, Venkatesh HA, Gamanagatti S, Garg P, Srivastava DN. Interventional Radiology in the Management of Visceral Artery Pseudoaneurysms: A Review of Techniques and Embolic Materials. Korean J Radiol. 2016;17:351–363. doi: 10.3348/kjr.2016.17.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarioglu O, Capar AE, Belet U. Interventional treatment options in pseudoaneurysms: different techniques in different localizations. Pol J Radiol. 2019;84:e319–e327. doi: 10.5114/pjr.2019.88021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Künzle S, Glenck M, Puippe G, Schadde E, Mayer D, Pfammatter T. Stent-graft repairs of visceral and renal artery aneurysms are effective and result in long-term patency. J Vasc Interv Radiol. 2013;24:989–996. doi: 10.1016/j.jvir.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 77.Dzijan-Horn M, Langwieser N, Groha P, Bradaric C, Linhardt M, Böttiger C, Byrne RA, Steppich B, Koppara T, Gödel J, Hadamitzky M, Ott I, von Beckerath N, Kastrati A, Laugwitz KL, Ibrahim T. Safety and efficacy of a potential treatment algorithm by using manual compression repair and ultrasound-guided thrombin injection for the management of iatrogenic femoral artery pseudoaneurysm in a large patient cohort. Circ Cardiovasc Interv. 2014;7:207–215. doi: 10.1161/CIRCINTERVENTIONS.113.000836. [DOI] [PubMed] [Google Scholar]
- 78.Weinmann EE, Chayen D, Kobzantzev ZV, Zaretsky M, Bass A. Treatment of postcatheterisation false aneurysms: ultrasound-guided compression vs ultrasound-guided thrombin injection. Eur J Vasc Endovasc Surg. 2002;23:68–72. doi: 10.1053/ejvs.2001.1530. [DOI] [PubMed] [Google Scholar]
- 79.Rai P, Kc H, Goel A, Aggarwal R, Sharma M. Endoscopic ultrasound-guided coil and glue for treatment of splenic artery pseudo-aneurysm: new kid on the block! Endosc Int Open. 2018;6:E821–E825. doi: 10.1055/a-0608-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gunjan D, Gamanagatti S, Garg P. Endoscopic ultrasonography-guided obliteration of a left inferior phrenic artery pseudoaneurysm in a patient with alcoholic chronic pancreatitis. Endoscopy. 2018;50:449–450. doi: 10.1055/s-0043-124867. [DOI] [PubMed] [Google Scholar]
- 81.Udd M, Leppäniemi AK, Bidel S, Keto P, Roth WD, Haapiainen RK. Treatment of bleeding pseudoaneurysms in patients with chronic pancreatitis. World J Surg. 2007;31:504–510. doi: 10.1007/s00268-006-0209-z. [DOI] [PubMed] [Google Scholar]
- 82.Agarwal AK, Kalayarasan R, Javed A. Vascular Complications in Chronic Pancreatitis. In: Domínguez-Muñoz JE. Clinical Pancreatology for Practising Gastroenterologists and Surgeons. United States: John Wiley & Sons Ltd, 2021: 322-332. [Google Scholar]
- 83.Jiang TA, Xie LT. Algorithm for the multidisciplinary management of hemorrhage in EUS-guided drainage for pancreatic fluid collections. World J Clin Cases. 2018;6:308–321. doi: 10.12998/wjcc.v6.i10.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strand DS, Law RJ, Yang D, Elmunzer BJ. AGA Clinical Practice Update on the Endoscopic Approach to Recurrent Acute and Chronic Pancreatitis: Expert Review. Gastroenterology. 2022;163:1107–1114. doi: 10.1053/j.gastro.2022.07.079. [DOI] [PubMed] [Google Scholar]
- 85.Bernades P, Baetz A, Lévy P, Belghiti J, Menu Y, Fékété F. Splenic and portal venous obstruction in chronic pancreatitis. A prospective longitudinal study of a medical-surgical series of 266 patients. Dig Dis Sci. 1992;37:340–346. doi: 10.1007/BF01307725. [DOI] [PubMed] [Google Scholar]
- 86.Bergert H, Dobrowolski F, Caffier S, Bloomenthal A, Hinterseher I, Saeger HD. Prevalence and treatment of bleeding complications in chronic pancreatitis. Langenbecks Arch Surg. 2004;389:504–510. doi: 10.1007/s00423-004-0478-7. [DOI] [PubMed] [Google Scholar]
- 87.Sethi H, Peddu P, Prachalias A, Kane P, Karani J, Rela M, Heaton N. Selective embolization for bleeding visceral artery pseudoaneurysms in patients with pancreatitis. Hepatobiliary Pancreat Dis Int. 2010;9:634–638. [PubMed] [Google Scholar]
- 88.Mallick B, Malik S, Gupta P, Gorsi U, Kochhar S, Gupta V, Yadav TD, Dhaka N, Sinha SK, Kochhar R. Arterial pseudoaneurysms in acute and chronic pancreatitis: Clinical profile and outcome. JGH Open. 2019;3:126–132. doi: 10.1002/jgh3.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Balachandra S, Siriwardena AK. Systematic appraisal of the management of the major vascular complications of pancreatitis. Am J Surg. 2005;190:489–495. doi: 10.1016/j.amjsurg.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 90.Hsu JT, Yeh CN, Hung CF, Chen HM, Hwang TL, Jan YY, Chen MF. Management and outcome of bleeding pseudoaneurysm associated with chronic pancreatitis. BMC Gastroenterol. 2006;6:3. doi: 10.1186/1471-230X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tulsyan N, Kashyap VS, Greenberg RK, Sarac TP, Clair DG, Pierce G, Ouriel K. The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg. 2007;45:276–83; discussion 283. doi: 10.1016/j.jvs.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 92.Kim J, Shin JH, Yoon HK, Ko GY, Gwon DI, Kim EY, Sung KB. Endovascular intervention for management of pancreatitis-related bleeding: a retrospective analysis of thirty-seven patients at a single institution. Diagn Interv Radiol. 2015;21:140–147. doi: 10.5152/dir.2014.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dhali A, Ray S, Sarkar A, Khamrui S, Das S, Mandal TS, Biswas DN, Dhali GK. Peripancreatic arterial pseudoaneurysm in the background of chronic pancreatitis: clinical profile, management, and outcome. Updates Surg. 2022;74:1367–1373. doi: 10.1007/s13304-021-01208-y. [DOI] [PubMed] [Google Scholar]