Abstract
A challenging step in human risk assessment of chemicals is the derivation of safe thresholds. The Threshold of Toxicological Concern (TTC) concept is one option which can be used for the safety evaluation of substances with a limited toxicity dataset, but for which exposure is sufficiently low. The application of the TTC is generally accepted for orally or dermally exposed cosmetic ingredients; however, these values cannot directly be applied to the inhalation route because of differences in exposure route versus oral and dermal. Various approaches of an inhalation TTC concept have been developed over recent years to address this. A virtual workshop organized by Cosmetics Europe, held in November 2020, shared the current state of the science regarding the applicability of existing inhalation TTC approaches to cosmetic ingredients. Key discussion points included the need for an inhalation TTC for local respiratory tract effects in addition to a systemic inhalation TTC, dose metrics, database building and quality of studies, definition of the chemical space and applicability domain, and classification of chemicals with different potencies. The progress made to date in deriving inhalation TTCs was highlighted, as well as the next steps envisaged to develop them further for regulatory acceptance and use.
Keywords: Risk assessment, Inhalation, Threshold of toxicological concern, Workshop, Cosmetic ingredients
1. Introduction
The main route of exposure from most cosmetic products is dermal, but oral (e.g. lipstick, toothpaste) and inhalation exposures (e.g. sprays, loose powders) also occur. One of the most challenging steps in human risk assessment is the derivation of safe thresholds. In recent years, due to a combination of concerns over the ethics of animal tests and regulatory bans on animal testing of cosmetic ingredients, there has been a lot of effort to find new ways to assure the safety of cosmetics ingredients in the absence of animal testing data. The Threshold of Toxicological Concern (TTC) concept is one option which can be used for cosmetics ingredients with a limited dataset and sufficiently low consumer exposure (SCCS, 2021), and is a common feature of tiered approaches to non-animal safety assessment (Berggren et al., 2017; Dent et al., 2018). TTC values were originally devised for the exposure to chemicals with known structures but unknown toxicity via the oral route. Kroes et al. (2004) presented a tiered TTC approach that established several human exposure thresholds over several orders of magnitude ranging from 0.0025 to 30 μg/kg-day. The lowest tier was set for substances that posed a concern based on structural alerts for genotoxicity. Substances which could act as neurotoxicants, possessing alerts for carbamates and organophosphates, were assigned to a separate category, whereas substances without structural alerts were assigned to one of three structural classes using the Cramer et al. (1978) decision tree. Cramer Class 1 substances comprised simple chemical structures and of low potential toxicity (TTC of 30 μg/kg-day). Cramer Class 2 contained substances that were intermediate (TTC of 9 μg/kg-day), whereas Cramer Class 3 contained structural features indicative of significant toxicity (TTC of 1.5 μg/kg-day). According to the analysis of the route-dependent differences in bioavailability by Kroes et al. (2007), established oral TTC values have also been shown to be valid for dermal exposures. However, they cannot be applied directly to the inhalation route because of relevant differences in exposure routes, including specific portal of entry effects on the respiratory tract. In recent years, the inhalation TTC concept has been developed to support the consumer safety assessment of cosmetic ingredients, to provide threshold values to address unintentional inhalation exposure of workers, and for screening and prioritization of chemicals for registration. If by using inhalation TTC an acceptable level of risk is achieved in a cosmetic ingredient safety assessment, then additional data would not need to be generated.
A virtual workshop organized by Cosmetics Europe was held on 3rd and 4th November 2020 to share the current state of the science regarding (a) the existing inhalation TTC approaches and (b) propose modifications for a more robust approach to gain scientific and regulatory acceptance. The workshop included the following presentations by experts in the field of inhalation exposure and toxicity:
Inhalation exposure assessment and hazard plus workshop objectives (Anthony Bowden, Unilever)
Introduction to the TTC values derived from the RepDose databases and outlook to other relevant aspects to address inhalation toxicity (Sylvia Escher, Fraunhofer ITEM)
Development of inhalation TTC for local respiratory effects - Building upon the Carthew et al. approach (Jane Rose, Procter and Gamble and Nikaeta Sadekar, RIFM)
Derivation of new TTC values for exposure via inhalation for environmentally relevant chemicals (Grace Patlewicz, US EPA)
The second part of the workshop was to discuss several key questions and draw conclusions where possible:
Defining the chemical space and domain of applicability: What other data sources are available to grow the inhalation TTC dataset and which chemicals should be included/excluded?
How is the study quality defined for inclusion into the database?
Do we need to include an applicability domain to know when the inhalation TTC concept can be applied, and do we need better tools to predict the applicability domain?
What is the appropriate approach to discriminate chemicals based on toxicological potency?
What are the appropriate dose metric values for local and systemic effects?
How are local versus systemic effects addressed?
The robustness of the various approaches was debated, vulnerabilities were highlighted, along with what new work was needed to be undertaken to manage any gaps. To this end, in addition to a systemic TTC, the application of a TTC for local effects was a key discussion point, alongside main topics that would build consensus and enable future application. The discussions to the extent to which consensus was reached are summarized in this report.
2. Derivation of inhalation TTC values from different databases
Compared to the database relating to toxicity data derived from oral repeat dose studies in animals where there are several thousand studies (Patel et al., 2020), the number of available high quality repeated dose inhalation exposure studies is limited (e.g. there are only ~400 rodent inhalation studies in the Research Institute for Fragrance Materials (RIFM) database https://rifm.org/rifm-database/). Thus, inhalation TTC values have been typically developed using smaller databases of different origin and containing different chemical spaces. This section describes some of the key work and considerations involved in deriving inhalation TTCs for low throughput (e.g. cosmetics ingredients) verses high throughput (environmental chemicals) purposes.
2.1. RepDose derived inhalation TTCs
In 2010, Escher et al. (2010) published inhalation TTC values derived from the RepDose database (Bitsch et al., 2006). They collected inhalation toxicity data for 203 chemicals and expanded the dataset to 296 chemicals (608 studies) later on (Tluczkiewicz et al., 2016). The dataset comprised only organic compounds (no metals, inorganic compounds, or polymers) with a defined chemical structure and smiles code. Several quality criteria were applied such as chemical purity >90% and repeated dose toxicity (RDT) rodent studies via the inhalation route with an examination scope comparable to guideline studies. When several high-quality studies per compound were available, the “no observed effect concentration” (NOEC) was derived from the longer-term study, prioritizing chronic (>1 year) studies above sub-chronic and sub-acute studies.
The doses in RDT studies are normally reported as nominal concentrations using the units of ppm and/or mg/m3. The former was considered the best descriptor in this study because it is independent of the molecular weight (MW). The inhalation TTC values were derived based on the 5th percentile distribution of NOEC values. A safety factor of 25 was applied, as recommended by the European Chemicals Agency (ECHA) (ECHA, 2012), to consider the remaining species toxicodynamic differences (2.5-fold) and intra-individual variability (10-fold). To compare the obtained inhalation TTC values to oral TTC values, the 5th percentile given in ppm was converted to body dose (μg/kg bw/day). This calculation of body dose was corrected for the daily exposure period and used default values to account for a standard human respiration rate, absorption rate (set to 100%) and an average body weight. The Cramer classification scheme was applied, and values were derived for Cramer Class 1 and 3 chemicals. An insufficient number of substances were assigned to Cramer Class 2 to permit derivation of a TTC value (see Table 1).
Table 1.
TTC values – results using 203 organic compounds. Published in Escher et al. (2010).
| Cramer Class | No. Chemicals in class | TTC (ppm/day) | TTC (μg/person/day) |
|---|---|---|---|
|
| |||
| 1 | 58 | 1.5 × 10−3 | 71 |
| 3 | 138 | 2.2 × 10−5 | 4 |
A more in-depth analysis investigated showed that there were no marked differences between local and systemic effects observed between chemicals in Cramer Class 1 and 3 (Escher et al., 2010). After oral dosing, the most frequent effect is on body weight, as well as other effects on liver, clinical symptoms, clinical chemistry, and hematology. After inhalation dosing, the nose was most commonly affected in rodents, particularly after nose-only exposure since they are obligate nose breathers. Approximately 50% of the chemicals showed local effects in the RDT studies. Of 102 of the chemicals, there was a difference between local and systemic inhalation TTC values, mainly for the less toxic Cramer Class 1 chemicals. However, in terms of practical application, the most sensitive effect in the studies shows only a very few chemicals have exclusively local effects.
As Cramer classification failed to discriminate toxic potency in this dataset, a new classification was developed to improve the definition of potency classes (Tluczkiewicz et al., 2016). The approach grouped 296 compounds according to their observed toxicological potency (log NOEC value) from in vivo animal studies with chronic exposure. Three statistical parameters were used to identify several structural classes being predominately toxic or low toxic: concordance, sensitivity and predictivity (Schüürmann et al., 2016). These were closely examined to look for structural homogeneity, e.g. Kow, differences in absorption and toxicity profile. The review resulted in 28 classes, which exhibited a shared Mode of Action (MoA). The new classification described by Tluczkiewicz et al. (2016) (shown in Table 2) derived two potency classes: “L” for low toxic compounds (TTC of 5 × 10−2 ppm) and “T” for toxic compounds (TTC of 2.0 × 10−5 ppm). Sixty compounds could not be classified as they had unique structural features.
Table 2.
TTC values – results using 296 organic compounds. Published in Tluczkiewicz et al. (2016), NA = not applicable.
| Potency class | No. Chemicals in class | TTC (ppm/day) | TTC (μg/person/day) |
|---|---|---|---|
|
| |||
| L | 81 | 5 × 10−2 | 4260 |
| T | 155 | 2.0 × 10−5 | 2 |
| Not classified | 60 | NA | NA |
Both publications investigated the impact of DNA reactive (genotoxic) chemicals on inhalation TTC values. Normally, genotoxic chemicals are allocated an oral TTC of 0.15 μg/person/day (Kroes et al., 2004). There were some predicted genotoxic chemicals (based on QSAR tools, thus an approximation and potentially over-predictive) in the databases. When genotoxic chemicals were excluded from the Escher et al. (2010) dataset, the inhalation TTC for Cramer Class 1 increased by 2-fold (from 71 to 180 μg/person/day) while for Cramer Class 3, the inhalation TTC stayed approximately the same (4 μg/person/day). In the larger data set, the inhalation TTC values for the L- and T-classes also stayed the same with genotoxic chemicals removed. The conclusion from this work was that genotoxic chemicals do not have a high impact on the 5th percentiles and the resulting inhalation TTC values.
2.2. Development of inhalation TTC by building upon the Carthew et al. approach
Carthew et al. (2009) used publicly available sources of data, specifically from RDT inhalation studies including chronic, sub-chronic and sub-acute (>28 days) studies. The original dataset was small, with 92 industrial chemicals (commonly found in consumer products) tested in rodents for which “no observed adverse effect concentration” (NOAEC) values for specific chemical classes were identified. Carthew et al. differentiated between local and systemic effects and when a local effect was reported, it was assumed that it was in the respiratory tract. They used various safety adjustment factors from the ECHA 2008 guidance (REACH Technical Guidance Document, 2008). Inhalation TTCs were derived for local (1400 and 470 μg/person/day for Cramer Class 1 and 3, respectively) and systemic (980 and 170 μg/person/day for Cramer Class 1 and 3, respectively) effects. Cramer Class 2 contained only 4 chemicals and therefore was defaulted to Cramer Class 3 inhalation TTC limits. Carthew et al. calculated local respiratory TTC levels by taking the 5th percentile NOAEC for chronic local effects normalized to rat lung weight. This was then converted to human exposure by using the human lung weight. This raises the question as to the type of correction needed for local effects and whether they should be derived based on lung weight. Dosimetry within the lung is complex and converting from an air concentration to a deposited dose in the rat and human lung is complex. An exposure duration correction was used to express the exposure per day to show inhaled dose. A conservative yet practical dataset was built with Cramer Class 1, 2 and 3 chemicals. The range of NOAEC values is relatively wide, but the 5th percentile inhalation TTC limits included some quite toxic chemicals in the low end of the distribution.
A joint Procter & Gamble (P&G) and RIFM project aimed to develop an inhalation TTC using the dataset of Carthew et al. (2009) as a starting point. Studies from different sources (RIFM, P&G, ECHA, Carthew et al. (2009), Escher et al. (2010)) were combined to increase the inventory to 246 chemicals (excluding genotoxic carcinogens, heavy metals, nerve agents and organophosphates) for which rodent RDT inhalation studies were publicly available. NOAEC exposure values represented in mg/m3 were used, together with safety factors, like those used by Carthew et al. (2009) (i.e. based on the ECHA 2008 guidance (REACH Technical Guidance Document, 2008) and ECETOC). There was a clear distinction between values for local and systemic effects for Cramer Class 1 and 3 chemicals; however, the suitability of Cramer Class to distinguish between toxic potency following inhalation was still unresolved (since Cramer Classification system is based on systemic effects derived from oral studies). Also, since the chemical domain within Carthew’s original dataset was limited to industrial chemicals commonly found in consumer products, this expanded dataset needs to be analyzed to ensure relevant chemical spaces are captured. Correlation of physicochemical properties with site-specific effects (i.e. site of contact effects in different parts of the respiratory tract) to identify those properties that drive such effects was considered for evaluating the dataset. However, this was not possible because in most studies observations were reported for general respiratory tract effects (not specifically for upper or lower respiratory effects). A combination of chemical features and toxicological potency was evaluated to determine whether these were more suitable for defining potency groups for inhalation TTC thresholds. This evaluation required advanced machine learning approaches using both, supervised (predicting the cluster labels of chemicals) and unsupervised (for clustering of chemicals) approaches to identify key descriptors defining potency groups for inhalation TTC. The clustering was analyzed using different methods, e.g. KMeans, MiniBatchKMeans, Gaussian Mixture Model, Agglomerative Clustering and Birch Clustering, which were visualized using hierarchical clustering. The performance of the clusters was assessed and compared according to the accuracy and cross-validation scores, thus providing a measure of confidence. The machine learning features were narrowed from over 900 to 10 to gain confidence. These included the number of atoms, bonds, and carbons; molecule radius; shape and size of a molecule; electronegativity; and ionization potential – all of which are correlated. They identified the minimum features required to reproduce the optimal cross-validation score, which was >80%. This means that at least 80% of the dataset is represented by these minimum features identified in the machine learning analysis. The next steps identified included analysis of the range of local and systemic points of departure across the clusters, with the aim to move away from Cramer classifications, identification of the 5th percentile threshold values, and a detailed scrutiny of the studies driving the 5th percentile.
2.3. Derivation of inhalation TTCs for environmentally relevant chemicals
The Office of Research & Development of the US Environmental Protection Agency (EPA) were looking to evaluate how the TTC approach could facilitate risk-based prioritization for thousands of chemicals. To that end, Patlewicz et al. (2018) proposed an approach of using TTC (based on the Kroes et al. (2004) workflow) in conjunction with high throughput exposure predictions (Wambaugh et al., 2014) to facilitate risk-based prioritization of several thousand chemicals. The approach showed promise, but two questions remained: 1) whether the existing oral TTC values were appropriate for the environmentally-relevant substances, and 2) how to extend the approach for inhalation exposures. For the first question, Nelms et al. (2019), used the EPA’s Toxicity Values Database (ToxValDB) to derive new oral TTC values and compared them to the established values from Munro et al. (1996). The oral TTC values derived from ToxValDB were similar, but not identical to the Munro oral TTC values. Cramer Class 3 oral TTC values were found to be statistically different, but these differences could be rationalized based on the chemical features represented. For question 2, the feasibility of deriving new inhalation TTC values was also investigated using ToxValDB using a stepwise approach (Fig. 1). Briefly, repeated toxicity studies conducted in rats, mice or rabbits where the portal of entry was inhalation were extracted from ToxValDB version 7. Chemicals were profiled into different TTC categories in accordance with the workflow by Kroes et al. (2004) to differentiate those chemicals possessing genotoxicity alerts from those that were assigned into one of the Cramer structural classes. For substances with multiple NOAEL/NOAEC values, the minimum point of departure was calculated after removal of statistical outliers (>1.5 Interquartile range). NOAEL/NOAEC values were found not to be normally distributed (unlike oral NOAELs) and there was no clear separation between the cumulative distributions of the NOAEL/NOEC values for Cramer Class 1 and 3 substances. Furthermore, there was an insufficient number of chemicals in Cramer Class 2 to evaluate further. The resulting ToxValDB inhalation TTC for Cramer Class 3 was similar to that reported by Escher et al. (2010) (Table 3).
Fig. 1.
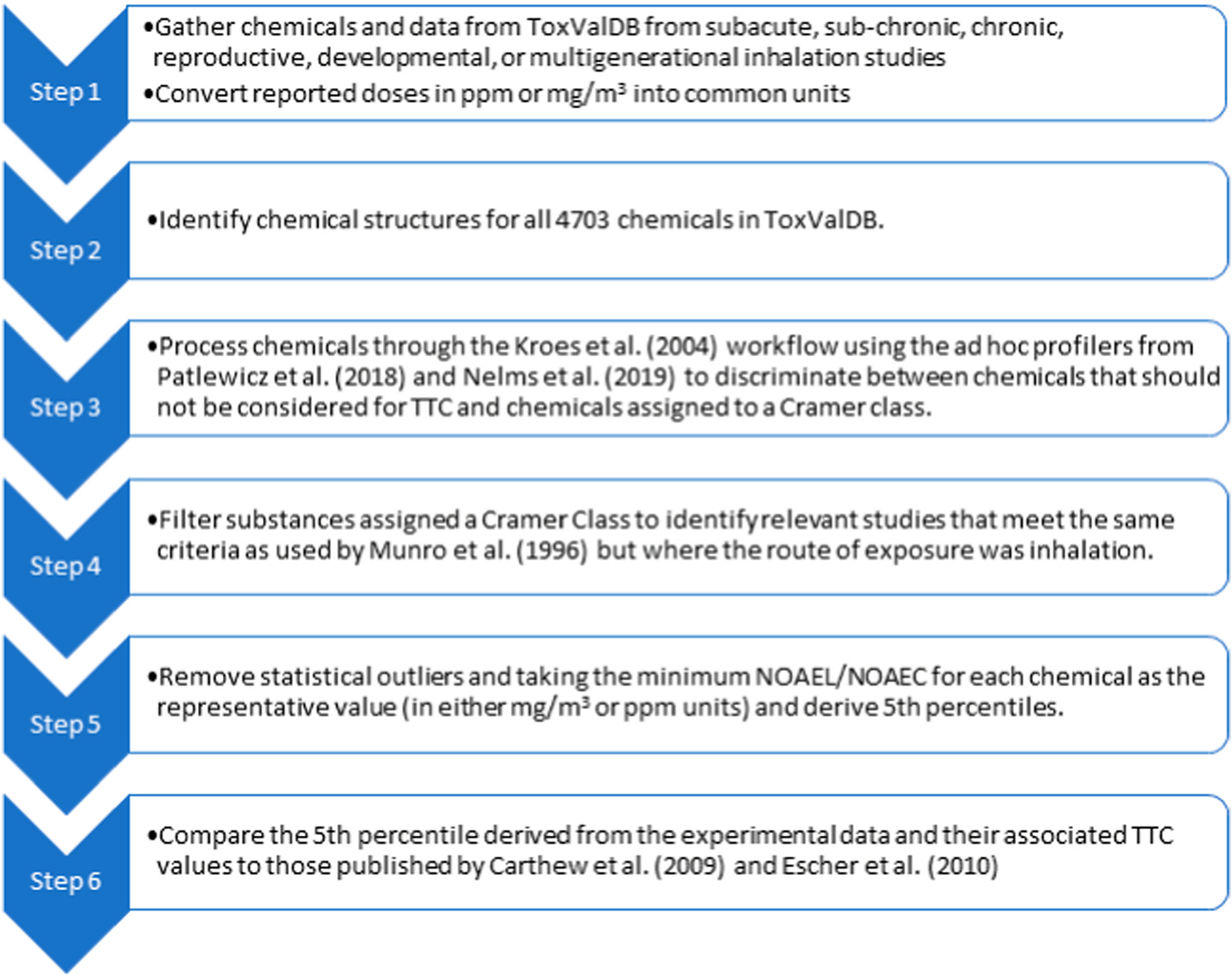
Step-wise process approach taken to derive TTCs for inhalation.
Table 3.
Comparison of inhalation TTC values derived from the ToxValDB evaluation with those from Escher et al. (2010) and Carthew et al. (2009).
| Cramer Class 1 |
Cramer Class 2 |
Cramer Class 3 |
||||
|---|---|---|---|---|---|---|
| TTC mg/m3 (No. of chemicals) | TTC μg/person/day (No. of chemicals) | TTC mg/m3 (No. of chemicals) | TTC μg/person/day (No. of chemicals) | TTC mg/m3 (No. of chemicals) | TTC μg/person/day (No. of chemicals) | |
|
| ||||||
| ToxValDB | 4.14 × 10−4 (244) | 8.27 (244) | 2.975 × 10−3 (14) | 59.5 (14) | 2.14 × 10−4 (261) | 4.28 (261 |
| Escher et al. | 3.6 × 10−3 (58) | 71 (58) | 4.8 × 10−4 (7) | 10 (7) | 1.8 × 10−4 (138) | 4 (138) |
| Carthew et al. | 1.4 (38) | 1400 (38) | 0.47 (50) | 470 (50) | ||
The reason for the different inhalation TTC values was investigated – in terms of the chemistry coverage and the differences in the underlying toxicity data (which mostly overlapped, with some outliers) – with the main difference found to be due to the very conservative PoD assigned based on the minimum value. An attempt was made to reproduce the inhalation TTC values using the data reported in Escher et al. (2010), similar inhalation TTC values were derived (Table 4).
Table 4.
Comparison of inhalation TTC values using the ToxValDB approach and the Escher et al. (2010) dataset.
| Structural category | No. of chemicals in Escher database | Reported TTC (μg/m3) | Reported TTC (μg/person/day) | No. reproduced values | TTC (μg/m3) | TTC (μg/person/day) |
|---|---|---|---|---|---|---|
|
| ||||||
| Cramer 1 | 58 | 3.6 × 10−3 | 71 | 58 | 4.57 × 10−3 | 91.44 |
| Cramer 3 | 138 | 1.8 × 10−4 | 4 | 137a | 2.78 × 10−4 | 5.56 |
One of the NOEC values reported was 0 which was dropped from consideration.
In a final step, other means to categorize the substances beyond Cramer designations were explored and revised inhalation TTC values were derived (Table 5 (Nelms and Patlewicz, 2020)). Substances were profiled according to an aquatic Mode of Action (MoA) based on a proposal made by Veith et al. (2009). Veith et al. (2009) investigated the feasibility of applying the same principles used to develop QSAR models for acute fish toxicity to acute rodent inhalation. He determined that although fish and mammalian baseline was not directly compatible due to the different external media, the thermodynamic activity was the same. Motivated by this work, the substances were profiled into their aquatic MoA to explore whether there was any discrimination in potency, indeed a good separation was found between the baseline and reactive MoA classes from which new inhalation TTC values were proposed. A number of next steps were outlined including: re-considering exclusion criteria and how these are implemented in the Kroes et al. (2004) module within Toxtree, since better specificity is needed (this has since been explored and is described in Patlewicz et al. (2022)); harmonization of the genotoxicity alerts used as part of the Kroes et al. (2004) implementation within Toxtree (if another genotoxicity rule base is used, there could be some differences in the output); the utility of acute oral/inhalation data within TTC could be investigated and an reexamination of the study data used beyond the arbitrary minimum point of departure used.
Table 5.
Inhalation TTC values for substances profiled according to an aquatic MoA (Nelms and Patlewicz, 2020).
| MoA class | No. of chemicals | 5th percentile median bootstrapped | TTC (μg/m3) | TTC (μg/ person/day) |
|---|---|---|---|---|
|
| ||||
| Baseline | 190 | 0.1567 | 1.11 × 10−3 | 22.39 |
| Reactive | 118 | 0.0299 | 2.14 × 10−4 | 4.286 |
3. Key questions discussed in the workshop
3.1. Defining the chemical space and domain of applicability: what other data sources are available to grow the inhalation TTC dataset and which chemicals should be included/excluded?
The group decided that the database should be expanded as much as possible, not limiting the contents to cosmetic products, thus allowing the approach to be used for all types of sprayable consumer products. It was mentioned that substances on the Cosmetics Regulation Annexes, as well as environmental and other organic chemicals should be considered for inclusion into the database. The group pointed out that a vastly expanded database allows better searches for compound classes of special concern, e.g. those that accumulate at the low/high end of the compiled NOEC distribution. Such analyses might help to identify compound classes that share a specific MoA and
must be excluded because of comparatively high toxicity due to high pharmacological activity, e.g. as done for steroids, the Cohort of Concern in the oral TTC concept;
or contain enough members to justify the derivation of a group specific value, e.g. as proposed for organophosphates.
The group acknowledged that the definition of these MoAs and exclusion categories (e.g. DNA reactive compounds, sensitisers or organophosphates) would need some consideration (most likely based on structural properties). Thus only chemicals with a defined structure would have a place in the database (rather than unknowns, or UVCBs (unknown variable composition or biological substances)).
Although nanoparticles are currently not included in the oral TTC databases, it was suggested to include solid particles in the inhalation database to assess the extent to which (nano)particle-specific limits can be derived. It was highlighted that there are fundamental differences in the toxicokinetics and dynamics of chemicals in solution compared to solid (nano)particles. Therefore, the creation of a distribution and a point of departure (PoD) using a dose metric to compare particles with soluble chemicals is not easy. It was suggested that a solid particle inhalation TTC could be based on lung surface area rather than body weight. To include solid particles, it was acknowledged that they would have different descriptors (potentially sub-categorized into micro/macro, soluble/insoluble) and dosimetry (deposition having a dependency on particle size and the amount of dose being delivered to the cells, and systemic exposure having a dependency on solubility and clearance).
3.2. How is the study quality defined for inclusion into the database?
In order to define study quality for inclusion into the database, a starting point for evaluation of a study could be to apply similar inclusion criteria as employed in the oral TTC dataset, and although some of the criteria for inhalation studies have evolved as the inhalation TTC databases have been built, a systematic and transparent way of including studies is needed. One possibility that resolved from the group discussion was the option to consider adopting the ToxRTool (an evolution of Klimisch (Schneider et al., 2009)) for a systematic evaluation of studies. Furthermore, the group decided in the case of studies with less-than-ideal design/scope of examination, but which still provided useful supporting information, that the addition of uncertainty annotation would prevent the exclusion of useful data.
3.3. Do we need to include an applicability domain to know when the inhalation TTC concept can be applied, and do we need better tools to predict the applicability domain?
It was discussed that applicability domain should be included to know when the inhalation TTC concept can be applied. However, the database would need to identify any unique structures/chemicals that would have a relevant impact on the derivation of inhalation TTC values, e.g. proteins. As for the oral TTC, these should be excluded from the inhalation TTC database and handled separately. While the exclusion of proteins was accepted by the group, the inclusion of chemical respiratory sensitizers was discussed. Since it is mainly epidemiological human data that identifies respiratory sensitisers, with only a small set of chemicals (less than 10) having compelling clinical evidence (Sadekar et al., 2021), and with the inhalation RDT study databases not containing the appropriate data on respiratory sensitization, the available data are not suitable for the definition of TTC values for chemical respiratory sensitization. Thus, these chemicals would also need to be excluded from inhalation TTC. The group identified that where chemicals are excluded, this would need to be included in the decision tree, e. g. ask if there is any concern for protein content or structural similarity to known respiratory sensitizers, and then exclude from further consideration within the approach.
3.4. What is the appropriate approach to discriminate chemicals based on toxicological potency?
The group discussions acknowledged that the work to date has shown that there was not good discrimination between the toxicological potencies of chemicals after inhalation based on the Cramer decision tree. It was also clear from the workshop proceedings that there were different ways of classifying chemicals based on toxicological potency. While the group did not decide on an appropriate approach to discriminate chemicals, this provides the opportunity to develop a new classification scheme as industry. Thus, the focus of any future work should be to investigate whether a different scheme can be defined that better discriminates the potencies, for example by considering the most relevant applicability domains for the datasets.
3.5. What are the appropriate dose metric values for local and systemic effects?
The group reflected on how doses could be expressed in a uniform way for solids, liquids and gases/vapours (and on a per day basis), and whether there was one single dose metric suitable for all. Arguments for using ppm and the reasoning for converting this value to μg/person/day when the exposure is expressed in ppm were discussed. While it was agreed that a metric of ppm is probably the best dose metric for a gas or vapor, this may not be the best metric for a solid, liquid or an aerosol (as a conversion is needed with regards to droplet/particle size), and therefore, mg/m3 was considered by some as the more appropriate dose metric. However, using mg/m3 would relate the value to MW which was shown to vary by a factor of 10 in the analyses of Escher et al. (2010), and so the group agreed this required further consideration. Nonetheless, all the factors that were identified in the machine learning processing were based on key chemical structure and were thus inclusive of solids, liquids and gases and consequently it was agreed at the end of this discussion that both ppm and mg/m3 should be used as dose metrics (acknowledging that ppm can be converted to mg/m3 by using the MW).
The group recommended the consideration of the duration of toxicity studies and consumer exposure in relation to dose metrics. Rodent RDT studies mostly test 6-h exposures and while the aim is to extrapolate these exposures for cosmetics and apply an inhalation TTC, cosmetics generally have a very brief inhalation exposure of 5–12 min (considered in exposure models e.g. Creme model (Comiskey et al., 2017)), if any. However, as the inhalation TTC approach should be extended to all consumer products, the group was clear in the fact that the approach needed to be developed to encompass longer durations and chronic use throughout a lifetime, and consequently, the 6-h RDT data becomes important. The group mentioned Haber’s law in the description of dose – this law states that toxic effects from inhalation exposure are dependent both on the concentration and duration of exposure (C × t) (Rozman and Doull, 2001)). With inhalation, since the blood vessels are near to the tissue surface, the chemical can enter the blood quickly, resulting in a high initial concentration, which could trigger the effect. Mostly, it is the rate of intake in a specific time that is important (a bolus or a dose spread over a long time). However, there are cases, e.g. coumarin, where systemic toxicity only occurs when a certain concentration in the respective tissue is exceeded (Cmax) although the toxic dose in the tissue (AUC) is reached. Overall, it might be beneficial to derive an inhalation TTC based on exposure durations/scenarios. In this case, the MoA is needed as well as the dose-dependency. The group also observed that a 12-min exposure per day (i.e. repeated acute exposure) is also a repeat exposure over a long period of time and that rodent assays show a decrease in the NOEC over time, as they become more sensitive to the toxicity over time. Therefore, the group agreed that inhalation TTC values should reflect repeated exposure, even if it is a short duration each day.
3.6. How are local versus systemic effects addressed?
The group discussed the assessment of both local and systemic effects of chemicals in situations where a compound only shows local effects at the concentrations used in the study (i.e. systemic effects are not observed and the systemic effects NOAEC is not identified, e.g. isobutylene (NTP, 1998)). Several questions were raised during this discussion:
Can a difference be distinguished between local and systemic doses?
When should a local dose be applied?
Would a prediction of the type of effect, in this case local versus systemic, be possible based on the structure of an unknown compound?
Is the “local” threshold applicable or should the most sensitive endpoint for a study be chosen, whether it is local or systemic?
The group was reminded that in the analyses of Escher et al. (2010) a local effect was “any effect in the respiratory tract” linked with a dose (e. g. ranging from inflammatory changes to fibrosis and tumor formation), and that the difference between the associated inhalation TTCs was not high in this analysis. The group was also reminded that Tluczkiewicz et al. (2016) classified chemicals based on effect and potency and looked at different classes with respect to their MoAs. A conclusion here, based on the that there were only a few classes of chemicals that specifically caused local effects, was that it may be more appropriate to base the effect on MoA (if one can be identified) rather than an artificial general local effect.
As a result of the discussion and the questions raised there was some agreement that there didn’t seem to be a benefit of developing separate local and systemic inhalation TTCs. However, it was felt by others that since local effects would depend on surface area and are point-of-entry effects it would still be preferable to investigate a local effect inhalation TTC value. On the other hand, if absorbed into the systemic circulation, the effect could be covered by general repeated dose studies in the absence of inhalation systemic effects.
4. Conclusions
In recent years, the inhalation TTC concept (versions published since 2009 are summarized in Table 6) has been developed in part to support the safety assessment of substances with a limited toxicity dataset, but for which exposure via the inhalation route is sufficiently low. There are numerous reasons for differences between values including: the number of chemicals and related inhalation RDT studies; chemical spaces covered; study types; exposure duration; differences in study design; derivation of LOEC/NOEC; variability of underlying toxicity data; adjustment/safety factors used; chemical grouping; data curation; overlapping chemicals between categories and limited discrimination of local versus systemic effects. This workshop highlighted the progress made so far in deriving inhalation TTCs, as well as the next steps envisaged to develop them further for regulatory acceptance. It also paved the way for a larger collaboration between the US-EPA, Fraunhofer ITEM, P&G and RIFM to 1) harmonize the datasets and outline the criteria for evaluating data quality, 2) evaluating observed local and systemic effects and their respective PoDs, and 3) determining a relevant classification system for the database and derivation of inhalation TTC values. This effort is also supported in part by Cosmetics Europe. In addition to the aim of using the inhalation TTC for exposure-based waiving for regulatory purposes, this concept may already be used as a screening/prioritization tool earlier on in a risk assessment framework when the material is of sufficient low-level exposure.
Table 6.
Summary of the TTC models published since 2009.
| Reference | Data input | No. of chemicals | Grouping- discriminate chemicals based on toxicity potency | Discriminate local/systemic | TTC | |
|---|---|---|---|---|---|---|
|
| ||||||
| Carthew et al., 2009 | Local NOAEC Systemic NOAEL (EPA, BfR, TNO, ECETOC) | 92 | Cramer Classes (CC) | Yes | Local: | CC1: 1400 μg/d CC3: 470 μg/d |
| Systemic: | CC1: 980 μg/d CC3: 170 μg/d |
|||||
| Escher et al., 2010 | NOEC RepDose, local/sys, organic compounds | 203 | Cramer Classes | CC1 only | CC1: 71 μg/d CC3: 4 μg/d |
|
| Tluczkiewicz et al., 2016 | NOEC RepDose | 296 | Structure factors identified for high vs low NOEC; machine learning; 28 groups: 19 high, 9 low | No | Low toxic: 4260 μg/d High toxic: 2 μg/d |
|
| Schüürmann et al., 2016 | NOEC RepDose | 296 | Structural alerts identified for high vs low NOEC, machine learning. Physicochemical properties, bioavailability, metabolism, MoA, to explore | No | Low Tox NOEC >12 ppm High Tox NOEC <0.75 ppm |
|
| Hoersch et al., 2018 | IFA GESTIS DNEL list | 1876 | Statistical DNEL distribution (99th percentile, 8 h occupational exposure) | Yes | 50 μg/m3 corresponding to 500 μg/worker/d | |
| Nelms and Patlewicz, 2020 | ToxVal database (subacute, subchronic, chronic, reproductive, developmental, multigeneration toxicity studies) | 4703 (of which 613 used in TTC) | Identified chemical structure, process through Kroes and Patlewicz profilers (OECD Toolbox, ToxTree). Filter for relevant studies (species, duration), remove statistical outliers, taking minimum NOAEL/NOAEC MoA sub-categories, Bootstrapping to explain uncertainty of 95thpercentile CC3, MoA profiling scheme for aquatic toxicity → reactive and baseline | No (inconclusive) | Comparable to Escher et al.: CC3 CC1: 8.23 μg/d CC3: 4.28 μg/d Baseline: 22.4 μg/d Reactive: 4.3 μg/d |
|
| RIFM/P&G | Carthew, RepDos, P&G, RIFM, ECHA | 246 | Hierarchical clustering with machine learning 5 clusters, 4 features | Yes | ||
Funding body information
This work was funded through the Long Range Science Strategy (LRSS) programme of Cosmetics Europe (available at https://www.lrsscosmeticseurope.eu).
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Anthony M. Bowden: Conceptualization, Writing – review & editing. Sylvia E. Escher: Conceptualization, Writing – review & editing. Jane Rose: Conceptualization, Writing – review & editing. Nikaeta Sadekar: Conceptualization, Writing – review & editing. Grace Patlewicz: Conceptualization, Writing – review & editing. Lara O’Keeffe: Conceptualization, Writing – review & editing. Dagmar Bury: Conceptualization, Writing – review & editing. Nicola J. Hewitt: Writing – original draft, Writing – review & editing. Arianna Giusti: Project administration. Helga Rothe: Conceptualization, Writing – review & editing.
Data availability
No data was used for the research described in the article.
References
- Berggren E, White A, Ouedraogo G, Paini A, Richarz A-N, Bois FY, Exner T, Leite S, van Grunsven LA, Worth A, Mahony C, 2017. Ab initio chemical safety assessment: a workflow based on exposure considerations and non-animal methods. Comput. Toxicol. 4, 31–44. 10.1016/j.comtox.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsch A, Jacobi S, Melber C, Wahnschaffe U, Simetska N, Mangelsdorf I, 2006. REPDOSE: a database on repeated dose toxicity studies of commercial chemicals - a multifunctional tool. Regul. Toxicol. Pharmacol. 46, 202–210. [DOI] [PubMed] [Google Scholar]
- Carthew P, Clapp C, Gutsell S, 2009. Exposure based waiving: the application of the toxicological threshold of concern (TTC) to inhalation exposure for aerosol ingredients in consumer products. Food Chem. Toxicol. 47, 1287–1295. 10.1016/j.fct.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Comiskey D, Api AM, Barrett C, Ellis G, McNamara C, O’Mahony C, Robison SH, Rose J, Safford B, Smith B, Tozer S, 2017. Integrating habits and practices data for soaps, cosmetics and air care products into an existing aggregate exposure model. Regul. Toxicol. Pharmacol. 88, 144–156. 10.1016/j.yrtph.2017.05.017. Epub 2017 May 27. [DOI] [PubMed] [Google Scholar]
- Cramer GM, Ford RA, Hall RL, 1978. Estimation of toxic hazard - a decision tree approach. J. Cosmet. Toxicol. 16, 255–276. [DOI] [PubMed] [Google Scholar]
- Dent M, Amaral RT, Da Silva PA, Ansell J, Boisleve F, Hatao M, Hirose A, Kasai Y, Kern P, Kreiling R, Milstein S, Montemayor B, Oliveira J, Richarz A, Taalman R, Vaillancourt E, Verma R, Posada NVO’RC, Weiss C, Kojima H, 2018. Principles underpinning the use of new methodologies in the risk assessment of cosmetic ingredients. Comput. Toxicol. 7, 20–26. 10.1016/j.comtox.2018.06.001. [DOI] [Google Scholar]
- ECHA, 2012. Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.15: Consumer Exposure Estimation. [Google Scholar]
- Escher SE, Tluczkiewicz I, Batke M, Bitsch A, Melber C, Kroese ED, Buist HE, Mangelsdorf I, 2010. Evaluation of inhalation TTC values with the database RepDose. Regul. Toxicol. Pharmacol. 58, 259–274. 10.1016/j.yrtph.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Hoersch J, Hoffmann-Doerr S, Keller D, 2018. Derivation of an inhalation TTC for the workplace based on DNEL values reported under REACH. Toxicol. Lett. 290, 110–115. 10.1016/j.toxlet.2018.03.030. [DOI] [PubMed] [Google Scholar]
- Kroes R, Renwick AG, Cheeseman M, Kleiner J, Mangelsdorf I, Piersma A, Schilter B, Schlatter J, van Schothorst F, Vos JG, Würtzen G, 2004. European branch of the International Life Sciences Institute. Structure-based thresholds of toxicological concern (TTC): guidance for application to substances present at low levels in the diet. Food Chemical Toxicology, Jan 42 (1), 65–83. [DOI] [PubMed] [Google Scholar]
- Kroes R, Renwick AG, Feron V, Galli CL, Gibney M, Greim H, Guy RH, Lhuguenot JC, van de Sandt JJ, 2007. Application of the threshold of toxicological concern (TTC) to the safety evaluation of cosmetic ingredients. Food Chem. Toxicol. 45, 2533–2562. 10.1016/j.fct.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Munro IC, Ford RA, Kennepohl E, Sprenger JG, 1996. Correlation of structural class with no-observed-effect levels: a proposal for establishing a threshold of concern. Food Chemical Toxicology, Sep 34 (9), 829–867. 10.1016/s0278-6915(96)00049-x. [DOI] [PubMed] [Google Scholar]
- Nelms MD, Pradeep P, Patlewicz G, 2019. Evaluating potential refinements to existing threshold of toxicological concern (TTC) values for environmentally-relevant compounds. Dec Regul. Toxicol. Pharmacol. 109, 104505. 10.1016/j.yrtph.2019.104505. Epub 2019 Oct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelms MD, Patlewicz G, 2020. Derivation of new threshold of toxicological concern values for exposure via inhalation for environmentally-relevant chemicals. Frontiers in Toxicology 2, 580347. 10.3389/ftox.2020.580347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP, 1998. Toxicology and Carcinogenesis Studies of Isobutene in F344/N Rats and B6C3F1 Mice (Inhalation Studies). Report number TR-487. Retrieved from [website]: https://ntp.niehs.nih.gov/publications/reports/tr/400s/tr487/index.html. [PubMed] [Google Scholar]
- Patel A, Joshi K, Rose J, Laufersweiler M, Felter SP, Api AM, 2020. Bolstering the existing database supporting the non-cancer Threshold of Toxicological Concern values with toxicity data on fragrance-related materials. Regul. Toxicol. Pharmacol. 116, 104718 10.1016/j.yrtph.2020.104718. Epub 2020 Jun 27. [DOI] [PubMed] [Google Scholar]
- Patlewicz G, Nelms M, Rua D, 2022. Evaluating the utility of the Threshold of Toxicological Concern (TTC) and its exclusions in the biocompatibility assessment of extractable chemical substances from medical devices, 2022 Nov Comput Toxicol 24, 1–11. 10.1016/j.comtox.2022.100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlewicz G, Wambaugh JF, Felter SP, Simon TW, Becker RA, 2018. Utilizing threshold of toxicological concern (TTC) with high throughput exposure predictions (HTE) as a risk-based prioritization approach for thousands of chemicals. Comput. Toxicol. 7, 58–67. 10.1016/j.comtox.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REACH Technical Guidance Document. European Chemicals Agency. http://echa.europa.eu. [Google Scholar]
- Rozman KK, Doull J, 2001. The role of time as a quantifiable variable of toxicity and the experimental conditions when Haber’s c x t product can be observed: implications for therapeutics. Journal of Pharmacology and Experimental Therapeutics, Mar 296 (3), 663–668. [PubMed] [Google Scholar]
- Sadekar N, Boisleve F, Dekant W, Fryer AD, Gerberick GF, Griem P, Hickey C, Krutz NL, Lemke O, Mignatelli C, Panettieri R, Pinkerton KE, Renskers KJ, Sterchele P, Switalla S, Wolter M, Api AM, 2021. Identifying a reference list of respiratory sensitizers for the evaluation of novel approaches to study respiratory sensitization. Crit. Rev. Toxicol. 51 (10), 792–804. 10.1080/10408444.2021.2024142. [DOI] [PubMed] [Google Scholar]
- SCCS (Scientific Committee on Consumer Safety), 2021. SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation, 11th revision, 30–31 March 2021, SCCS/1628/21. [DOI] [PubMed]
- Schneider K, Schwarz M, Burkholder I, Kopp-Schneider A, Edler L, Kinsner-Ovaskainen A, Hartung T, Hoffmann S, 2009. ToxRTool, a new tool to assess the reliability of toxicological data. Toxicol. Lett. 189 (2), 138–144. [DOI] [PubMed] [Google Scholar]
- Schüürmann G, Ebert R-U, Tluczkiewicz I, Escher SE, Kühne R, 2016. Inhalation threshold of toxicological concern (TTC) - structural alerts discriminate high from low repeated-dose inhalation toxicity. Environ. Int. 88, 123–132. 10.1016/j.envint.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Tluczkiewicz I, Kühne R, Ebert R-U, Batke M, Schüürmann G, Mangelsdorf I, Escher SE, 2016. Inhalation TTC values: a new integrative grouping approach considering structural, toxicological and mechanistic features. Regul. Toxicol. Pharmacol. 78, 8–23. 10.1016/j.yrtph.2016.03.022. [DOI] [PubMed] [Google Scholar]
- Veith GD, Petkova EP, Wallace KB, 2009. A baseline inhalation toxicity model for narcosis in mammals. SAR and QSAR in Environmental Research, Jul 20 (5–6), 567–578. 10.1080/10629360903278669. [DOI] [PubMed] [Google Scholar]
- Wambaugh JF, Wang A, Dionisio KL, Frame A, Egeghy P, Judson R, Setzer RW, 2014. High throughput heuristics for prioritizing human exposure to environmental chemicals. Environ. Sci. Technol. 48 (21), 12760–12767. 10.1021/es503583j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


