Abstract
Background:
Individuals with self-declared sarcoidosis are at increased risk of COVID-19 related morbidity and mortality for which vaccination can be lifesaving. Despite this, vaccine hesitancy remains a large barrier to global acceptance of vaccination against COVID-19. We aimed to identify individuals with sarcoidosis who had and had not been vaccinated against COVID-19 vaccine to 1) establish a safety profile of COVID-19 vaccination in those with sarcoidosis and 2) to elucidate factors that contribute to COVID-19 vaccine hesitancy.
Methods:
A questionnaire inquiring about COVID-19 vaccination status, vaccination side effects, and willingness for future vaccination was distributed from December 2020 to May 2021 to individuals with sarcoidosis living in the US and European countries. Details regarding sarcoidosis manifestations and treatment were solicited. Vaccine attitudes were classified as pro or anti-COVID-19 vaccination for subgroup analysis.
Results:
At the time of questionnaire administration, 42% of respondents had already received a COVID-19 vaccination, most of whom either denied side effects or reported a local reaction only. Those not on sarcoidosis therapy were more likely to report systemic side effects. Among subjects who had not yet received a COVID-19 vaccine, 27% of individuals reported they would not receive one once available. Reasons against vaccination were overwhelmingly related to the lack of confidence in vaccine safety and/or efficacy and less related to concerns associated with convenience or complacency. Black individuals, women, and younger adults were more likely to decline vaccination.
Conclusions:
Among individuals with sarcoidosis, COVID-19 vaccination is well-accepted and well-tolerated. Subjects on sarcoidosis therapy reported significantly less vaccination side effects, and thus the correlation between side effects, vaccine type, and vaccine efficacy requires further investigation. Strategies to improve vaccination should focus on improving knowledge and education regarding vaccine safety and efficacy, as well as targeting sources of misinformation, particularly in young, black, and female subpopulations.
Keywords: Sarcoidosis, COVID-19, Vaccination
Supplementary file


Introduction
Vaccines have proven to be an effective strategy to combat infection-related morbidity and mortality. For example, global vaccination programs against smallpox and polio effectively resulted in near-eradication of these diseases (1-3). In addition, while influenza remains a yearly seasonal threat, there have been substantial mortality benefits to vaccination of at-risk-groups (4, 5). Current vaccine recommendations are therefore intended to provide direct individual protection as well as to achieve herd immunity, both of which extend benefit to the most vulnerable populations. By the time the first COVID-19 vaccine was introduced in December 2020, there were more than 71 million confirmed cases of COVID-19 infection with more than 1.7 million deaths globally (6). High risk individuals include those of older age, male sex, non-white race, lower socioeconomic status, and those with various comorbidities such as obesity, hypertension, immunodeficiency, and chronic lung disease (7). National and international governing bodies subsequently emphasized the need for vaccination in these higher-risk populations to mitigate disease severity, hospitalization, and death (8, 9).
Sarcoidosis is an immune-mediated inflammatory disease that results in granuloma formation which subsequently risks multiorgan dysfunction. While the etiology of sarcoidosis is largely unknown, mechanisms driving the disease highlight defects in innate, cellular, and adaptive immunity (10-16). Immune dysfunction is compounded by the impact of advanced structural lung disease (17) as well as frequent need for immunosuppressive medications (18), both of which may diminish inherent immune defenses. Numerous studies support the increased risk of infection in this population though the overall risk of infection with COVID-19 specifically remains unclear (19-21). However, if infected with COVID-19, sarcoidosis patients with moderate-severe pulmonary dysfunction are at increased risk of infection-related morbidity and mortality (22). In addition, individuals on chronic immunosuppressants have higher mortality rates from COVID-19 infection (23). Taken together, primary infection prevention with vaccination is of great importance to protect this population.
Unfortunately, despite the promise of protection from a potentially deadly disease, attitudes towards vaccinations vary. Vaccine hesitancy describes the uncertainty or ambivalence that an individual may harbor regarding the need for vaccination. Reasons for hesitancy are complex and have been modeled into domains by the WHO Vaccine Hesitancy Working Group. These domains include 1) confidence in the effectiveness, safety, and delivery of the vaccine, as well as perceived motivation of those recommending the vaccine; 2) complacency, referring to the balance of perceived risk of infection and need for vaccination to prevent the disease; and 3) convenience, or the affordability, availability, and accessibility of the vaccine (24). Overall, understanding the reasons for vaccine hesitancy is crucial to develop strategies to improve vaccine acceptance.
While the concept of vaccine hesitancy is well recognized and extends to many vaccine types, the study of the novel COVID-19 vaccines offers unique insight into the raw attitudes towards vaccination, particularly after their emergency-use introductions. While phase three studies provided much needed data regarding the safety profiles and efficacy of the COVID-19 vaccines, questions regarding use in specific populations were left unanswered. The objective of this study was therefore two-fold; to assess the global behaviors regarding COVID-19 vaccination in individuals with sarcoidosis to 1) identify self-reported safety profiles in those who received a vaccine to better inform the sarcoidosis community and 2) identify the characteristics and rationale of those who are hesitant to receive the vaccination to better target interventions to improve acceptance.
Methods
A questionnaire (Supplement S1) to assess the COVID-19 vaccination statuses and attitudes among sarcoidosis subjects was developed jointly by the University of Cincinnati (UC) and Albany Medical Center (AMC) (authors MAJ, EEL, and RPB) and appended to an existing questionnaire (19). The amendment was approved by the University of Cincinnati Institutional Review Board.
The UC/AMC questionnaire was distributed via Redcap to subjects with sarcoidosis followed at UC and posted on the Foundation for Sarcoidosis Research (FSR) website. While the FSR has international reach, the UC/AMC questionnaire was only available in English. An Italian questionnaire was adapted from the UC/AMC questionnaire, with minimal variation owing to translation and regional relevance, and distributed to individuals of the Italian Association for Sarcoidosis (ACSI, Amici Contro La Sarcoidosi Italia). Both questionnaires were distributed in the initial months of vaccine introduction between December 2020 and May 2021. Questionnaire responses were collected in an anonymous manner without patient-identifiable information and a question confirming consent was included. The brand, type, or dose of COVID-19 vaccine was not elicited in the questionnaire. Individuals without sarcoidosis were excluded.
The questionnaire asked participants whether they had already received a COVID-19 vaccine and, if so, to identify any vaccine-related reactions experienced. As individuals may have responded to the questionnaire before a vaccine was available to them, those who had not received a vaccine were asked if they would receive one once available. If the individual was against receiving a COVID-19 vaccine, they were asked to select a reason. Options included safety concerns, concern for side-effects, perception of lack of vaccine efficacy, prior COVID-19 infection with subsequent perception that the vaccine is not needed, cost, and desire to review data regarding the experience of vaccinated individuals. The UC/AMC questionnaire further inquired about patient demographics, country of residence, comorbidities, COVID-19 infection history, and data involving sarcoidosis organ manifestations and treatment. Survey participants were also asked if they had received the influenza vaccine and answers were correlated to COVID-19 vaccine attitudes.
Statistical analysis
All responses to the UC/AMC questionnaire and Italian questionnaires were pooled. The primary outcomes assessed included rates of vaccine administration at time of the questionnaire, as well as rates of intended vaccination once the vaccine was available. Secondary outcomes included adverse reactions among those who had already received COVID-19 vaccination and reasons against COVID-19 vaccination among those who declined the vaccine. Vaccination reaction answer options varied among questionnaires, so these results are reported separately. Subgroups analysis was performed on the UC/AMC questionnaire data to determine if reactions experienced varied by use of sarcoidosis specific therapy (i.e. no treatment versus any treatment). In addition, reactions were categorized into ‘no reaction”, ‘local reaction only’ (i.e. sore arm), and ‘systemic reaction’ (i.e. headache, fever, nausea, worsened sarcoidosis) to evaluate any variation in age, sex, or race among these subgroups. All subjects were reallocated into a US and non-US based cohort for location specific analysis. Subjects were then categorized into those who were pro-COVID-19 vaccine, as defined by those who had already received or planned to receive a COVID-19 vaccine, and anti-COVID-19 vaccine which included subjects who indicated they would not receive a vaccine. Reasons cited to not receive a COVID-19 vaccine were assessed among the anti-COVID-19 vaccine group and compared among US and non-US based subjects. Subgroup analyses were then performed on the US-cohort by age, race, sex, the presence of comorbidities, and receipt of the influenza vaccine in the previous year. Age, race, and sex were also assessed among individuals based on their combined influenza and COVID-19 vaccine attitudes.
Differences in categorical data were assessed for significance using either the Chi-square test of independence or the Fisher’s exact test as appropriate. Continuous data were assessed using the Wilcoxon rank sum test for non-parametric data or students t-test for parametric data as appropriate. Analyses were performed in R version 4.1.2 (https://www.R-project.org/) using the stats package (25). Odds ratios were calculated for numerous variables utilizing the epitools package (26).
Results
A total of 1653 individuals with sarcoidosis completed the vaccination questionnaires between December 2020 and May 2021, of whom 1155 (70%) completed the UC/AMC questionnaire and 498 (30%) completed the Italian questionnaire (Table 1). Of all respondents, 1063 (64%) lived in the United States (US) while 587 (36%) lived in other countries, including 500 from Italy, 42 from Germany, 16 from the United Kingdom, 5 from Canada, with the remaining 23 from other European, African, and Asian countries. Most respondents were women (65%). Race, which was only consistently reported for respondents of the UC/AMC questionnaire, was predominantly white (66%). Details regarding sarcoidosis organ involvement and treatment are listed in Table 1.
Table 1.
Demographics of questionnaire respondents.
| n | |
|---|---|
|
Total respondents
UC/AMC questionnaire Italian questionnaire |
1653 1155 498 |
|
Country of Residence
US Non-US |
1063 587 |
|
Sex
Women Men |
1072 578 |
| Already Received COVID-19 Vaccine | 689 |
|
Race *
White Black Other |
752 (66%) 377 (33%) 19 (1%) |
| History of COVID Infection | 145 (14%) |
|
Sarcoidosis Organ Involvement
†
Lung Heart Brain Other |
742 (71%) 107 (10%) 132 (13%) 430 (41%) |
|
Sarcoidosis Treatment
‡
None Prednisone/Prednisolone MTX/AZA/Mycophenolate/Leflunomide Infliximab/Adalimumab HCQ or Chloroquine Rituximab Other |
304 (29%) 424 (40%) 352 (33%) 156 (15%) 139 (13%) 29 (3%) 57 (5%) |
*Race data available for 1147 subjects (UC/AMC questionnaire)
†Organ involvement data available for 1052 subjects (UC/AMC questionnaire); ‡Treatment data available for 1055 subjects (UC/AMC questionnaire); NA: not available.
At the time of questionnaire administration, 689 (42%) respondents had already received a COVID-19 vaccination. This included 31% of UC/AMC questionnaire respondents and 65% of Italian questionnaire respondents, which likely reflected the geographic variability in COVID-19 vaccine introduction during the time period that the questionnaire was administered. Of those who answered the Italian questionnaire, 21% reported no side effects, while others reported sore arm (55%), fatigue (38%), muscle pain (25%), headache (24%), arthralgia (19%), GI symptoms (16%), fever (11%), respiratory symptoms (8%), and other (12%). Of those who answered the UC/AMC questionnaire, a majority reported no side effects (74%), while others reported ‘local reaction only’ (i.e. sore arm, 10%) and ‘systemic reactions’ (16%) which included fever (11%), worsening sarcoid-related symptoms (7%), headache (3%), nausea (2%), and other (4%). In addition, 96% of UC/AMC respondents stated they would receive a repeat dose of the COVID-19 vaccine in the future which alludes to the overall tolerability of vaccine side effects in this group. Subgroup analysis was performed to determine which factors were associated with reporting local or systemic side effects (Figure 1).
Figure 1.
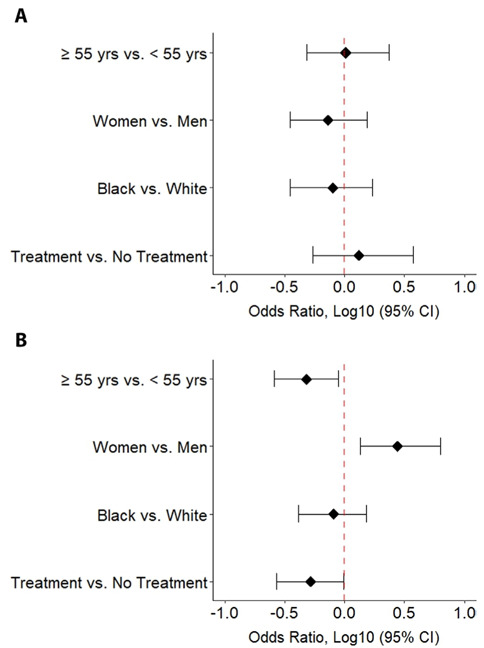
Odds of developing A) only local reaction and B) any systemic reaction after receiving the COVID-19 vaccination for various subgroups. ‘Other” race was excluded from statistical analysis given low number of subjects. ‘Treatment’ refers to any sarcoidosis specific therapy. The second variable listed in the y-axis is considered the baseline variable.
Overall, of those who reported ‘local reaction only’, there was no difference in age, sex, race, or use of sarcoidosis specific treatment. In contrast, individuals younger than 55 years, women, and those off sarcoidosis therapy had a significantly greater odds of reporting ‘systemic reactions’ (age <55: OR 2.07, 95% CI 1.12-3.80, p-val 0.0165; women: OR 2.78, 95% CI 1.35-6.26, p-val 0.0059; no treatment: OR 1.94, 95% CI 1.01-3.65, p-val 0.0390). Of the 964 individuals who had not yet received a COVID-19 vaccine at the time of the questionnaire, 18 subjects did not answer the follow up question asking if they would receive the vaccine once available and were excluded from further analyses. Of the remaining 946 subjects, 73% reported they would receive a COVID-19 vaccine while 27% reported they would not (Table 2).
Table 2.
Intent to receive the COVID-19 vaccine and reasons against stratified by location.
| Total | Non-US Respondents | US Respondents | p value | |
|---|---|---|---|---|
| Have Not Received | 964 (58.3%) | 253 (43.1%) | 709 (66.7%) | |
| Will Receive Vaccine | 687 / 946 (73%) | 190 / 244 (78%) | 495 / 700 (71%) | 0.0310 |
| Will Not Receive Vaccine | 259 / 946 (27%) | 54 / 244 (22%) | 205 / 700 (29%) | - |
| Reasons Cited to Not Receive Vaccine * | 268 | 65 | 203 | |
|
Confidence Safety Concerns Reaction to Vaccine Does Not Think it will Help |
205 (76%) 45 (17%) 10 (4%) |
46 (71%) 15 (23%) 6 (9%) |
159 (78%) 30 (15%) 4 (2%) |
0.2112 0.1192 0.0152 |
|
Complacency Prior COVID Infection / Won’t Help |
10 (4%) | 5 (8%) | 5 (2%) | 0.0529 |
|
Convenience Cost |
- | NA | 0 (0%) | - |
| Waiting to observe vaccination outcomes | 109 (41%) | 8 (12%) | 101 (50%) | <0.0001 |
| Other | 12 (4%) | 4 (6%) | 8 (3.9%) | 0.4927 |
*Multiple answers allowed; NA: not applicable.
Subjects were sub-grouped by country of residence into a US and non-US based cohort for subsequent analyses. Significantly more subjects from the US indicated they would not receive a COVID-19 vaccine than those from non-US countries (29% versus 22%, p value 0.0310). Concerns for safety and fear of reaction were the most cited reasons against a vaccine and indicated a general lack of confidence in the COVID-19 vaccines. Concern regarding a lack of efficacy was less commonly cited, but more likely to be reasoned by non-US respondents (9% versus 2% in US respondents, p val 0.0074). Complacency was also rarely cited, as only 3.7% of subjects felt that a personal history of COVID-19 infection would negate the need for COVID-19 vaccination. Convenience of vaccine as assessed by concern for cost, was not cited by any US participants and was not reported in the non-US survey, likely due to widespread availability in surveyed countries, governmental funding, and/or nationalized health care. Finally, significantly more US respondents stated they were waiting to observe COVID-19 vaccination outcomes before making a final decision on receiving a COVID-19 vaccine (50% versus 12% non-US respondents, p val <0.0001).
To better target interventions to improve vaccine acceptance, subgroup analyses were performed on the US participants based on various demographic factors (Figure 2).
Figure 2.
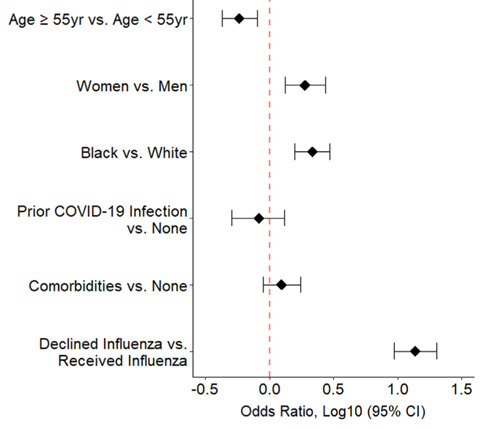
Odds of expressing anti-COVID-19 vaccination attitudes for various subgroups within the US-residing cohort are shown. Prior Infection refers to self-reported history of COVID-19 infection. Comorbidities includes COPD/asthma, cancer, diabetes, heart disease, and/or hypertension. The second variable listed in the y-axis is considered the baseline variable.
While most surveyed subjects were pro-COVID-19 vaccine, those who were anti-COVID-19 vaccine were significantly younger (median age 53.9 years versus median age 58.1 years, p-val <0.0001; <55 year OR 1.72, 95% CI 1.25-2.33, p-val 0.0006) and consisted of more women (OR 1.89, 95% CI 1.33-2.73, p-val 0.0004) and black individuals (OR 2.16, 95% CI 1.58-2.95, p-val <0.0001). In addition, those who had declined the influenza vaccine had a significantly higher odds of being anti-COVID (OR 13.73, 95% CI 9.43-20.19, p val <0.0001).
Lastly, to assess generalizability of COVID-19 vaccination attitudes, decision to have received the influenza vaccine was assessed among age, sex, and race subgroups (Figure 3).
Figure 3.
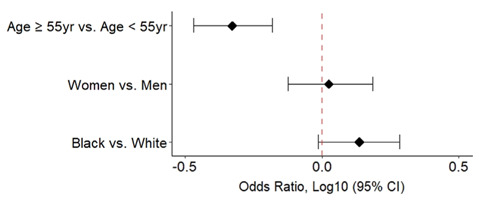
Odds of having declined the influenza vaccine among various subgroups within the US-residing cohort are shown. The second variable listed in the y-axis is considered the baseline variable.
Younger individuals (age <55 years) had a significantly higher odds of declining the influenza vaccine than older individuals (OR 2.13, 95% CI 1.52-2.94, p-val <0.0001). In contrast to anti-COVID-19 vaccine attitudes, black individuals had a non-significant trend towards increased influenza vaccine refusal (OR 1.37, 95% CI 0.97-1.92, p-val 0.0725) whereas influenza refusal was not significantly associated with sex.
Discussion
In summary, our study is an international evaluation of COVID-19 vaccine behaviors and attitudes among individuals with sarcoidosis. Our findings indicate the COVID-19 vaccines were well accepted at time of introduction as most respondents had already received a vaccine or indicated willingness to receive a COVID-19 vaccine once available to them. Concern for safety remains the largest barrier to receiving a COVID-19 vaccine, to which the safety profile elucidated by our study speaks directly. While younger age was more likely to be both anti-COVID-19 vaccine and anti-influenza vaccine, black race and female sex indicated vaccine hesitancy that was COVID-19 specific.
The COVID-19 pandemic had profound impact on everyday life which led governments and pharmaceutical companies to spend billions of dollars on vaccine development to decrease the severity and the risk of the spread of COVID-19. Emergency use authorization of various COVID-19 vaccines, with an efficacy of as high as 95% and mild yet tolerable safety profiles, was granted by February 2022 (27-30). Unfortunately, the proportion of people questioning vaccines has long been a growing threat (31). Concerns are generally related to lack of confidence of the vaccine, complacency surrounding the perceived risk of disease, and convenience of vaccine accessibility, which is further compounded by the influence of the media, the internet, health care policy, and health care professionals (24, 31). In regards to the COVID-19 vaccine, the rapidity of the vaccine development and roll-out process with subsequent concern that approval will occur before safety and effectiveness are fully understood contributes to vaccination hesitancy (32). Moreover, not only does COVID-19 vaccine acceptance depend on the type of vaccine offered, but it also varies based on the individuals most trusted source of information(33). As such, misinformation is a plausible contributor to fear of vaccination, and when spread by social media is more likely to be endorsed by younger individuals (34, 35). In addition, mistrust of health care, lower levels of knowledge and awareness, and sociopolitical factors further complicate overall attitudes(36, 37). Despite the evidence underlying vaccine mechanism, efficacy, and safety, individuals opt out of vaccination for complex reasons ultimately risking the ability to achieve herd immunity and protect our most vulnerable populations.
While moderate and severe impairment in pulmonary function is associated with increased covid mortality in sarcoidosis, our prior research in a small cohort of sarcoidosis subjects who underwent BNT162b2 vaccination indicates that they have comparable immunity post-COVID-19 vaccination when compared to the general population(17, 22, 31, 38), arguing for a need to overcome vaccine hesitancy this group. In our study, sarcoidosis patients in the US were more likely to decline the COVID-19 vaccine than non-US participants. When the specific reasons to decline COVID-19 vaccination were investigated, safety was the largest concern for both cohorts. It is unclear if these concerns extend to general safety or sarcoidosis-related safety, as the potential for triggering sarcoidosis-like symptoms has been reported with COVID-19 vaccination (39, 40). While the survey did not point to an explanation for this geographic difference, considerations include variations in the type of vaccine offered regionally, variation in local health care provider beliefs and management, and/or differences in health care policy, as all have been found to influence COVID-19 vaccine hesitancy (33, 36, 41). Notably, a majority of UC/AMC respondents indicated preference to wait for more outcome data prior to making a final decision on COVID-19 vaccination, which may suggest a desire to learn more about safety profiles.
Among respondents in the US cohort, younger individuals, female sex, and black race were the most likely to be anti-COVID-19 vaccine, which largely echoes findings of existing studies (36, 41-43). Overall, older individuals are more likely to be concerned about COVID-19 and its detrimental health consequences compared to younger individuals(44) and therefore more likely to accept COVID-19 vaccine. In contrast, younger individuals are more subject to misinformation regarding COVID-19 vaccination and therefore may be less trusting of receiving it (34). Interesting, anti-vaccine views of younger individuals extended beyond the COVID-19 vaccine as younger respondents in our cohort had higher rates of declining the influenza vaccine as well. Black individuals and women are also significantly more likely to be anti-COVID-19 vaccine, which is particularly interesting as both demographics are more likely to perceive COVID-19 as a major health threat (44). In general, black individuals have a higher level of distrust in medical research (37) which potentially extends to attitudes regarding receipt of a newly developed vaccine. Interventions to improve vaccination rates should therefore focus on improving overall confidence and trust in the COVID-19 vaccine in these populations. Efforts should be made to increase general knowledge and education of the COVID-19 vaccines, of which this manuscript may contribute, as well as to decrease misinformation that may reach these vulnerable populations. In addition, as minorities may be less likely to participate in research (37) efforts should be made to increase participation of these minority groups to improve generalization of findings to these populations.
Despite limited data on the safety of COVID-19 vaccination in sarcoidosis subjects, which is the major contributor to vaccine hesitancy in our population, vaccination is strongly recommended based on expert opinion(40). Our findings, though retrospective and subject to recall bias, demonstrate that vaccination against COVID-19 was generally well tolerated in subjects with sarcoidosis with mild side effects in a minority of patients comparable to what is experienced by the general population (27-30). Interestingly, those on sarcoidosis treatment were significantly less likely to experience systemic side effects. This suggests that side effects are limited by anti-inflammatory therapy use directly, or alternatively, limited by the underlying sarcoidosis activity that requires treatment. Additionally, despite comparable immunity observed in those on and off sarcoidosis specific treatment suggested by our prior observations of BNT162b2 vaccination in sarcoidosis, the lack of reported side effects in this study raises concerns since decreased vaccine efficacy has been observed in other immunosuppressed populations (45, 46).There was noticeable difference in the rates of various reactions reported by the UC/AMC and Italian questionnaires. It is unclear if these differences reflect demographics, sarcoidosis disease severity or need for treatment as suggested by analyses of the UC/AMC questionnaire, as this specific data was unavailable for the Italian questionnaire. Alternatively, a history of COVID infection or variation in vaccine brand administered may be relevant. Thus, further investigation is warranted to elucidate the association between side effects and vaccine brand, type, and efficacy in subjects with sarcoidosis to inform vaccination strategies in this population.
Limitations of our study include an overwhelming representation of the US and European populations; therefore, conclusions should be cautiously generalized to underrepresented geographical regions. Given minimal variation in questionnaires, to include discrepancy in the provided responses to vaccine side effects, some answers may be under reported. Questionnaires also did not consistently inquire about vaccine type, so conclusions as to hesitancy towards or side effects of specific vaccines cannot be gleaned. Finally, we report on attitudes and behaviors towards COVID-19 vaccination from the early period of vaccine introduction. While this information is still very relevant in providing information to target interventions, the evolution of vaccine willingness has likely evolved throughout the pandemic because of changes in the perceived risk of COVID-19 infection (47, 48), emotional effects of lockdowns and their resolution (49), the emergence of new variants, and the development of novel vaccines with improved safety profiles.
Despite these limitations, we report the first study evaluating vaccine behaviors and attitudes in subjects with sarcoidosis, explore COVID-19 side effects, and evaluate reasons associated with hesitancy to receive the COVID-19 vaccine. Overall, vaccination against COVID-19 is considered safe in subjects with sarcoidosis, regardless of immunosuppressive medications, with mild tolerable side effects comparable to that of the general population. Subjects most at risk to decline COVID-19 vaccine were identified as young, black, and/or female and cited lack of confidence in vaccine safety and efficacy as the largest barrier to vaccine acceptance. Our study not only informs strategies to improve education regarding side effects, but also identifies subpopulations towards whom interventions should be targeted, with overall intent to increase the rate of vaccination against COVID-19.
Conclusion
Vaccination against COVID-19 is generally accepted and well tolerated among subjects with sarcoidosis. Of those who exhibit hesitancy towards vaccination, confidence in vaccine safety is the largest barrier. Interventions to improve vaccination rates should focus on increasing knowledge of vaccine safety, work to limit misinformation, and improve minority participation in safety studies to help generalize results to all populations.
Acknowledgements:
The authors acknowledge the contributions of Amelia Carlucci, the secretary of Sarcoidosis Patients Association, who assisted with questionnaire collection and data elaboration.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Authors Contribution:
Conception, questionnaire development, and study design: A. Wells, M. Judson, N.J. Sweiss, E. Lower, and R.P. Baughman. Questionnaire translation: P. Rotolli, F. Martone. Subject recruitment, questionnaire distribution, data collection: P. Rottoli, F. Martone, M. Judson, N.J. Sweiss, E. Lower, and R.P. Baughman. Analysis and interpretation: C.L. Vagts, C. Ascoli, N. J. Sweiss, R. P. Baughman Drafting of manuscript for important intellectual content: C.L. Vagts, J. Sweis, N. Sweis, C. Ascoli, N. J. Sweiss, A. Wells, M. Judson , E. Lower, R. P. Baughman
References
- Berche P. Life and death of smallpox. Presse Med. 2022;51(3):104117. doi: 10.1016/j.lpm.2022.104117. [DOI] [PubMed] [Google Scholar]
- Meyer H, Ehmann R, Smith GL. Smallpox in the Post-Eradication Era. Viruses. 2020;12(2) doi: 10.3390/v12020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward B, Tangermann R. The global polio eradication initiative: lessons learned and prospects for success. Vaccine. 2011;29(Suppl 4):D80–5. doi: 10.1016/j.vaccine.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Barberis I, Myles P, Ault SK, Bragazzi NL, Martini M. History and evolution of influenza control through vaccination: from the first monovalent vaccine to universal vaccines. J Prev Med Hyg. 2016;57(3):E115–E20. [PMC free article] [PubMed] [Google Scholar]
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007;7(10):658–66. doi: 10.1016/S1473-3099(07)70236-0. [DOI] [PubMed] [Google Scholar]
- Organization WH. WHO Coronavirus (COVID-19) Dashboard Geneva, Switzerland. 2020 Available from: https://covid19.who.int/ [Google Scholar]
- Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. 2021;76(2):428–55. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- Callaghan T, Moghtaderi A, Lueck JA, et al. Correlates and disparities of intention to vaccinate against COVID-19. Soc Sci Med. 2021;272:113638. doi: 10.1016/j.socscimed.2020.113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH. Coronavirus disease (COVID-19): Vaccines Geneva, Switzerland. 2022 [Google Scholar]
- Ascoli C, Huang Y, Schott C, et al. A Circulating MicroRNA Signature Serves as a Diagnostic and Prognostic Indicator in Sarcoidosis. Am J Respir Cell Mol Biol. 2018;58(1):40–54. doi: 10.1165/rcmb.2017-0207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman L, Pelikan RC, Rasmussen A, et al. Single Cell Transcriptomics Implicate Novel Monocyte and T Cell Immune Dysregulation in Sarcoidosis. Front Immunol. 2020;11:567342. doi: 10.3389/fimmu.2020.567342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemzadeh K, Fatemipour M, Zahra Mirfeizi S, et al. Serum B cell activating factor (BAFF) and sarcoidosis activity. Arch Rheumatol. 2021;36(1):72–9. doi: 10.46497/ArchRheumatol.2021.8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryavtsev I, Serebriakova M, Starshinova A, et al. Imbalance in B cell and T Follicular Helper Cell Subsets in Pulmonary Sarcoidosis. Sci Rep. 2020;10(1):1059. doi: 10.1038/s41598-020-57741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saussine A, Tazi A, Feuillet S, Rybojad M, et al. Active chronic sarcoidosis is characterized by increased transitional blood B cells, increased IL-10-producing regulatory B cells and high BAFF levels. PLoS One. 2012;7(8):e43588. doi: 10.1371/journal.pone.0043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweiss NJ, Salloum R, Gandhi S, et al. Significant CD4, CD8, and CD19 lymphopenia in peripheral blood of sarcoidosis patients correlates with severe disease manifestations. PLoS One. 2010;5(2):e9088. doi: 10.1371/journal.pone.0009088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli C, Schott CA, Huang Y, et al. Altered transcription factor targeting is associated with differential peripheral blood mononuclear cell proportions in sarcoidosis. Front Immunol. 2022;13:848759. doi: 10.3389/fimmu.2022.848759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaritopoulos GA, Antoniou KM, Wells AU. Comorbidities in interstitial lung diseases. Eur Respir Rev. 2017;26(143) doi: 10.1183/16000617.0027-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis Based on Health Care Use. Ann Am Thorac Soc. 2016;13(8):1244–52. doi: 10.1513/AnnalsATS.201511-760OC. [DOI] [PubMed] [Google Scholar]
- Baughman RP, Lower EE, Buchanan M, et al. Risk and outcome of COVID-19 infection in sarcoidosis patients: results of a self-reporting questionnaire. Sarcoidosis Vasc Diffuse Lung Dis. 2020;37(4):e2020009. doi: 10.36141/svdld.v37i4.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dureault A, Chapelon C, Biard L, et al. Severe infections in sarcoidosis: Incidence, predictors and long-term outcome in a cohort of 585 patients. Medicine (Baltimore) 2017;96(49):e8846. doi: 10.1097/MD.0000000000008846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungprasert P, Crowson CS, Matteson EL. Sarcoidosis Increases Risk of Hospitalized Infection. A Population-based Study, 1976-2013. Ann Am Thorac Soc. 2017;14(5):676–81. doi: 10.1513/AnnalsATS.201610-750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthau AS, Levin MA, Freeman R, Reich DL, Klang E. Moderate or Severe Impairment in Pulmonary Function is Associated with Mortality in Sarcoidosis Patients Infected with SARSCoV2. Lung. 2020;198(5):771–5. doi: 10.1007/s00408-020-00392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek MS, Lee MT, Kim WY, Choi JC, Jung SY. COVID-19-related outcomes in immunocompromised patients: A nationwide study in Korea. PLoS One. 2021;16(10):e0257641. doi: 10.1371/journal.pone.0257641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald NE, Hesitancy SWGoV. Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015;33(34):4161–4. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- Team. RC. R: A language and environment or statistical computing. R Foundation for Statistical Computing, Vienna, Australia. 2020 [Google Scholar]
- Aragon TJ. epitools: Epidemiology Tools. R package version 05-101. 2020 [Google Scholar]
- Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N Engl J Med. 2021;385(25):2348–60. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath PT, Galiza EP, Baxter DN, et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N Engl J Med. 2021;385(13):1172–83. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J, Gray G, Vandebosch A, et al. Final Analysis of Efficacy and Safety of Single-Dose Ad26.COV2.S. N Engl J Med. 2022;386(9):847–60. doi: 10.1056/NEJMoa2117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Hum Vaccin Immunother. 2013;9(8):1763–73. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson AJC, Funk CUS. Public Now Divided Over Whether To Get COVID-19 Vaccine. Pew Resarch Center. Sept 17, 2020 [Google Scholar]
- Kutasi K, Koltai J, Szabo-Morvai A, et al. Understanding hesitancy with revealed preferences across COVID-19 vaccine types. Sci Rep. 2022;12(1):13293. doi: 10.1038/s41598-022-15633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy B. Coronavirus: vaccine misinformation and the role of social media. The Policy Institute at the King's College London; 2020 Dec 14, 2020. [Google Scholar]
- Islam MS, Kamal AM, Kabir A, et al. COVID-19 vaccine rumors and conspiracy theories: The need for cognitive inoculation against misinformation to improve vaccine adherence. PLoS One. 2021;16(5):e0251605. doi: 10.1371/journal.pone.0251605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khubchandani J, Sharma S, Price JH, Wiblishauser MJ, Sharma M, Webb FJ. COVID-19 Vaccination Hesitancy in the United States: A Rapid National Assessment. J Community Health. 2021;46(2):270–7. doi: 10.1007/s10900-020-00958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb FJ, Khubchandani J, Striley CW, Cottler LB. Correction to: Black-White Differences in Willingness to Participate and Perceptions About Health Research: Results from the Population-Based HealthStreet Study. J Immigr Minor Health. 2019;21(2):306. doi: 10.1007/s10903-018-0747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagts CCY, Ascoli C, Lee JM, et al. Trimer IgG and Neutralising Antibody Response to COVID-19 mRNA Vaccination in Individuals with Sarcoidosis. ERJ Open Res. 2022;8(4) doi: 10.1183/23120541.00025-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher JG, Tampe B, Korsten P. First Report of Two Cases of Lofgren's Syndrome after SARS-CoV-2 Vaccination-Coincidence or Causality? Vaccines (Basel) 2021;9(11) doi: 10.3390/vaccines9111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manansala M, Chopra A, Baughman RP, et al. COVID-19 and Sarcoidosis, Readiness for Vaccination: Challenges and Opportunities. Front Med (Lausanne) 2021;8:672028. doi: 10.3389/fmed.2021.672028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. 2021;194:245–51. doi: 10.1016/j.puhe.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndugga NHL, Artiga S, Parker N. Latest Data on COVID-19 Vaccinations by Race/Ethnicity: Kaiser Family Foundation. 2021 Available from: https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-on-covid-19-vaccinations-by-race-ethnicity/ [Google Scholar]
- Viswanath K, Bekalu M, Dhawan D, Pinnamaneni R, Lang J, McLoud R. Individual and social determinants of COVID-19 vaccine uptake. BMC Public Health. 2021;21(1):818. doi: 10.1186/s12889-021-10862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nino M, Harris C, Drawve G, Fitzpatrick KM. Race and ethnicity, gender, and age on perceived threats and fear of COVID-19: Evidence from two national data sources. SSM Popul Health. 2021;13:100717. doi: 10.1016/j.ssmph.2020.100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021;325(21):2204–6. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall VG, Ferreira VH, Ku T, et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N Engl J Med. 2021;385(13):1244–6. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley SJ, Stanton R, Browne M, et al. As the Pandemic Progresses, How Does Willingness to Vaccinate against COVID-19 Evolve? Int J Environ Res Public Health. 2021;18(2) doi: 10.3390/ijerph18020797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padamsee TJ, Bond RM, Dixon GN, et al. Changes in COVID-19 Vaccine Hesitancy Among Black and White Individuals in the US. JAMA Netw Open. 2022;5(1):e2144470. doi: 10.1001/jamanetworkopen.2021.44470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WS, Budenz A. Considering Emotion in COVID-19 Vaccine Communication: Addressing Vaccine Hesitancy and Fostering Vaccine Confidence. Health Commun. 2020;35(14):1718–22. doi: 10.1080/10410236.2020.1838096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file





