Abstract
Background:
Little is known about pulmonary hypertension (PH) in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV).
Objectives:
The aims of this retrospective study, in which echocardiography was used for detection of PH, were to identify the potential causes of PH in AAV and to analyze the risk factors for mortality.
Methods:
We performed a retrospective descriptive review of 97 patients who had AAV with PH at our institution from January 1, 1997, through December 31, 2015. These patients with PH were compared with 558 patients who had AAV without PH. Demographic and clinical data were abstracted from electronic health records.
Results:
Among the patients who had PH, 61% were men; mean (SD) age was 70.5 (14.1) years at the time of PH diagnosis. The majority of patients with PH (73.2%) had more than 1 potential cause of PH, with left heart disease and chronic lung disease being the most common causes. Older age, male sex, smoking history, and kidney involvement were associated with the presence of PH. PH was associated with an increased risk of death (hazard ratio, 3.15; 95% CI, 2.37-4.18). On multivariate analysis, PH, age, smoking status, and kidney involvement were independent risk factors for death. Median survival after the diagnosis of PH was 25.9 months (95% CI, 12.2-49.9).
Conclusions:
PH in AAV is often multifactorial, is commonly associated with left heart disease, and is associated with a poor prognosis.
Keywords: antineutrophil cytoplasmic antibody, pulmonary hypertension, vasculitis
Introduction
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a rare multisystem small-vessel vasculitis with 3 major variants: granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA). The 2 major targeted antigens that combine with ANCA are proteinase 3 (PR3) and myeloperoxidase (MPO). ANCA-negative AAV can occur as well. MPA primarily manifests as necrotizing glomerulonephritis or pulmonary capillaritis (or both). In contrast, GPA produces granulomatous inflammation of the upper and lower respiratory tracts in addition to glomerulonephritis.
Pulmonary hypertension (PH), defined as mean pulmonary arterial pressure greater than 20 mm Hg at rest (1), has rarely been described in patients with AAV. Right heart catheterization is the gold standard for diagnosis of PH, but echocardiography, which is typically the initial test of choice when PH is suspected, can be used to assess probability of PH (2). According to some studies, echocardiographic findings for detection of PH correlate well with those from right heart catheterization (3-5). The World Health Organization has classified PH into 5 clinical groups (1, 6): pulmonary arterial hypertension (PAH) (group 1), PH due to left heart disease (group 2), PH due to chronic lung disease (group 3), chronic thromboembolic PH (group 4), and PH with unclear or multifactorial mechanisms (group 5).
PAH is commonly associated with connective tissue disease and autoimmune conditions. It is particularly described as occurring with systemic sclerosis and systemic lupus erythematosus but also with other kinds of vasculitis, such as Takayasu arteritis (7, 8) and polyarteritis nodosa (9), raising the possibility that PAH may be the consequence of autoimmune vascular inflammation. Distinct from PAH, PH in AAV may also be due to abnormalities elsewhere (eg, lungs or left heart) that may predispose to PH. These abnormalities may or may not be a direct result of vasculitis itself.
Little is known about PH in AAV. Although some case reports and case series (10, 11) have described PH among patients with AAV, no large cohort studies have been published.
The aims of this retrospective study, in which echocardiography was used for detection of PH, were 1) to identify potential causes of PH in AAV, 2) to assess the impact of PH on the mortality of patients who have AAV, and 3) to analyze the risk factors for mortality of patients who have AAV and PH.
Methods
The Mayo Clinic Institutional Review Board approved this study (No. 20-009407). Patients were excluded if they did not authorize the research use of their medical records.
A computer-assisted search of the electronic health record was conducted to identify all adults with AAV at Mayo Clinic in Rochester, Minnesota, from January 1, 1997, through December 31, 2015. We searched clinical notes in the electronic health record for the following terms: ANCA associated vasculitis, granulomatosis polyangiitis, PR3 associated vasculitis, c-ANCA associated vasculitis, microscopic polyangiitis, MPO associated vasculitis, and p-ANCA associated vasculitis. The initial search yielded 2,785 patients. A clinician with expertise in AAV (M.B.) performed chart reviews to verify the diagnoses. All patients were seen and evaluated by experts in our specialty vasculitis clinic. The diagnosis of AAV was made according to the definitions for vasculitides adopted by the 2012 International Chapel Hill Consensus Conference on the Nomenclature of Vasculitides (12).
A total of 1,260 patients were excluded, predominantly because of a diagnosis of EGPA. Other reasons for exclusion were that they did not have vasculitis or they did not specifically have AAV. Of the remaining 1,525 patients, 655 had an echocardiogram at our institution and were included in the final analysis.
Patients were included in the study 1) if they were adults 18 years or older at the time of echocardiography, 2) if they received a diagnosis of AAV (not including EGPA), and 3) if they had echocardiography at our institution. Patients were excluded from the study 1) if their disease overlapped with other connective tissue diseases (eg, myositis or lupus) or 2) if the diagnosis of AAV was not clear.
PH probability was assessed with guidelines established by the European Society of Cardiology, European Respiratory Society, and British Society of Echocardiography (2, 13). If multiple echocardiograms were available for 1 patient, the date of the first echocardiogram that showed PH was abstracted. In accordance with current guidelines, we used the following echocardiographic criteria to define PH: 1) right ventricular systolic pressure (RVSP) greater than 50 mm Hg or 2) peak tricuspid regurgitation velocity greater than 3.4 m/s (indicating a high probability of PH). When risk was indeterminate (ie, if RVSP was 35-50 mm Hg or tricuspid regurgitation velocity was 2.9-3.4 m/s), we used the presence of other echocardiographic signs of PH (RV or right atrial dilatation, RV dysfunction, moderate to severe tricuspid regurgitation, or interventricular septal flattening) as assessed by an experienced echocardiographer (N.S.V.S.) to determine the presence of PH.
Heart failure with preserved ejection fraction (HFpEF) was diagnosed according to recommendations from the American Society of Echocardiography guidelines for the evaluation of left ventricular diastolic function with echocardiography (14). Heart failure with reduced ejection fraction (HFrEF) was defined as left ventricular ejection fraction (LVEF) less than 40%, and heart failure with midrange ejection fraction (HFmrEF) was defined as ejection fraction of 41% to 49%.
Clinical notes and diagnoses were carefully examined for any history of coronary artery disease, valvular heart disease, cardiomyopathy, congenital heart disease, or coronary artery intervention. We reviewed medical records, chest images, and test results to assess specific risk factors for different groups of patients with PH. To capture data on chronic thromboembolic PH, we collected data on its diagnosis, history of pulmonary embolism or deep vein thrombosis, use of anticoagulation, ventilation-perfusion ( /Q) scan results, and computed tomography (CT) of the chest with a contrast agent. For a diagnosis of chronic thromboembolic pulmonary hypertension, we considered the results of a
/Q) scan results, and computed tomography (CT) of the chest with a contrast agent. For a diagnosis of chronic thromboembolic pulmonary hypertension, we considered the results of a  /Q scan and CT angiography only when the procedures had been performed within 1 year of the date of diagnosis of PH. Reports from CT scans were abstracted to determine the presence or absence of interstitial lung disease, emphysema, and other lung diseases such as bronchiectasis and bronchiolitis.
/Q scan and CT angiography only when the procedures had been performed within 1 year of the date of diagnosis of PH. Reports from CT scans were abstracted to determine the presence or absence of interstitial lung disease, emphysema, and other lung diseases such as bronchiectasis and bronchiolitis.
We also reviewed the records to document any history of liver disease or cirrhosis, obstructive sleep apnea (OSA), and use of supplemental oxygen. We collected demographic and other clinical data such as patient age, sex, smoking history, body mass index, vital status, date of death, and date of last clinical encounter. Variables related to AAV included date of AAV diagnosis, organs involved with vasculitis, ANCA type (MPO, PR3, or negative), and activity of vasculitis at the time of PH diagnosis. Activity was assessed with the Birmingham Vasculitis Activity Score (BVAS) for GPA (15, 16). Active AAV was defined as an increase in the BVAS of more than 1, and remission was defined as a BVAS of 0 with a prednisone dose less than 10 mg daily.
Statistical analysis
Descriptive data were expressed as mean (SD) for continuous variables and number of patients (percentage of sample) for categorical variables. Data were compared between patients with PH and patients without PH with the use of a paired t test, χ2 test, or Fisher exact test, as appropriate. A Cox proportional hazards model was used to determine the effect of PH and other prognostic factors on mortality among patients with AAV. Statistically significant variables on univariate analysis (P<.05) were included in a multivariate model if they remained significant (P<.10) after adjustment. We limited our model to no more than 6 predictors to avoid overfitting. Kaplan-Meier survival curves were generated with log-rank tests for comparison of patients with PH and patients without PH. Statistical analysis was performed with SAS version 9.4 (SAS Institute Inc).
Results
A total of 655 patients with AAV and echocardiograms were included in the study. Of those patients, 14.8% (n=97) had echocardiographic PH. Among those 97 patients, 79 (81.4%) had RVSP greater than 50 mm Hg, 17 (17.5%) had RVSP between 40 and 50 mm Hg, and 1 patient (1.0%) had RVSP less than 40 mm Hg; tricuspid regurgitant jet velocity was greater than 3.4 m/s in 54 of the 97 patients (55.7%) and was 2.9 to 3.4 m/s in 43 of the 97 patients (44.3%).
PH Cohort (n=97)
Secondary causes of PH
Demographic and clinical data from patients with AAV and PH (n=97) are summarized in Table 1. Most patients had multiple possible causes or risk factors for PH; the cause of PH was not identified for only 3 patients. The most common possible contributors to PH were left heart disease, including valvular heart disease (41.2%) (moderate to severe mitral regurgitation in 18 patients, moderate to severe aortic stenosis in 14, moderate to severe aortic regurgitation in 5, moderate to severe mitral stenosis in 2, and severe tricuspid stenosis in 1); HFpEF (40.2%); and systolic heart failure (28.9%), including HFrEF and HFmrEF. Eight patients (8.2%) had a history of valvular repair or replacement, and 22 (22.7%) had a history of coronary artery bypass graft surgery or coronary stents. Chronic lung disease was the second most common risk factor for PH: OSA (28.9%), interstitial lung disease (24.7%), and chronic obstructive pulmonary disease (COPD) (22.7%). Twenty-four patients (24.7%) either had a history of pulmonary embolism or deep vein thrombosis (n=18) or had thromboembolism at the time of PH diagnosis (n=6). Chronic thromboembolic disease was not identified but could not be definitively ruled out in 49 patients (50.5%) since no CT angiography, Doppler venous ultrasonography, or  /Q scan was performed. Thirty-four patients underwent imaging with at least 1 modality, and the results were negative for thromboembolism.
/Q scan was performed. Thirty-four patients underwent imaging with at least 1 modality, and the results were negative for thromboembolism.
Table 1.
Clinical Characteristics of Patients With AAV and PH (n=97)
| Feature | Value |
|---|---|
| Age at PH diagnosis, mean (SD), y | 70.5 (14.1) |
| Sex, No. (%) | |
| Male | 61 (63) |
| Female | 36 (37) |
| RVSP, mean (SD), mm Hg | 58.7 (12.5) |
| Tricuspid regurgitant jet velocity, mean (SD), m/s | 3.4 (0.4) |
| Patients according to No. of identifiable possible causes of PH, No. (%) 0 1 2 3 4 >4 |
3 (3.1) 23 (23.7) 34 (35.1) 23 (23.7) 11 (11.3) 3 (3.1) |
| Patients according to conditions that were possible causes of PH, No. (%) | |
| Valvular heart diseasea | 40 (41.2) |
| Heart failure with preserved LVEF | 39 (40.2) |
| Systolic heart failure | 28 (28.9) |
| With reduced EF | 17 (17.6) |
| With midrange EF | 11 (11.3) |
| Obstructive sleep apnea | 28 (28.9) |
| Interstitial lung disease | 24 (24.7) |
| Chronic thromboembolic disease | 24 (24.7) |
| Chronic obstructive pulmonary disease | 22 (22.7) |
| Other lung disease causing hypoxia | 13 (13.4) |
| Other cardiac causesb | 9 (9.3) |
| Other pulmonary artery obstructions (pulmonary embolism or tumor emboli) | 1 (1.0) |
| Hematologic disorders (myeloproliferative disorder) | 1 (1.0) |
| Systemic and metabolic disorders (Gaucher disease) | 1 (1.0) |
| Use of supplemental oxygen at the time of PH diagnosis, No. (%) | 56 (57.7) |
| Interval between AAV diagnosis and PH diagnosis, mean (SD), y | 6.6 (6.9) |
| Patients according to No. of systems or organs involved with AAV, No. (%) (n=96)c 1 2 3 4 5 6 |
21 (21.9) 35 (36.5) 30 (31.3) 6 (6.3) 3 (3.1) 1 (1.0) |
| ANCA, No. (%) (n=93)d PR3 MPO Negative |
55 (59.1) 34 (36.6) 4 (4.3) |
| Active vasculitis at the time of diagnosis of PH, No. (%) | 21 (21.6) |
| Patient deaths, No. (%) | 71 (73.2) |
| Survival after PH diagnosis, median (95% CI), mo | 25.9 (12.2-49.9) |
Abbreviations: AAV, antineutrophil cytoplasmic antibody–associated vasculitis; ANCA, antineutrophil cytoplasmic antibody; EF, ejection fraction; LVEF, left ventricular ejection fraction; MPO, myeloperoxidase; PH, pulmonary hypertension; PR3, proteinase 3; RVSP, right ventricular systolic pressure. a Moderate to severe mitral regurgitation (n=18); moderate to severe aortic stenosis (n=14); moderate to severe aortic regurgitation (n=5); moderate to severe mitral stenosis (n=2); and severe tricuspid stenosis (n=1). b Constrictive pericarditis (n=2); infective endocarditis (n=2); congenital heart diseases (n=2); atrial mass (n=1); cardiac transthyretin amyloidosis (n=1); dilated cardiomyopathy (n=1). c Information about system or organ involvement was missing for 1 patient. d ANCA status was unknown for 4 patients.
Other lung diseases that may have contributed to hypoxia and resultant PH were bronchiectasis (n=8), bronchiolitis (n=2), lung adenocarcinoma (n=1), and asbestosis (n=1). Other less common cardiac diseases were present in 9.3% of patients and may have contributed to PH: constrictive pericarditis (n=2); infective endocarditis (n=2); congenital heart diseases (n=2); atrial mass due to GPA (involving the anterior mitral leaflet and causing noncompliant left atrium and left ventricle and severe mitral regurgitation) (n=1); cardiac transthyretin amyloidosis (n=1); and dilated cardiomyopathy (n=1).
Patients without an identifiable cause of PH
Three patients did not have an identifiable cause of PH other than perhaps vasculitis itself. One patient was a nonsmoker without known heart or lung disease; CT angiography was negative for pulmonary embolism, and right heart catheterization showed precapillary PH, which supported a diagnosis of PAH. A second patient with PH also did not have an underlying risk factor for PH, but right heart catheterization was not performed. The third patient had biopsy-proven giant cell myocarditis and associated restrictive physiology that may have led to PH, but PAH could not be ruled out.
Timing of AAV and PH diagnoses
For most patients (82.5%), the diagnosis of AAV preceded the diagnosis of PH. Eleven patients (11.3%) received a diagnosis of AAV and PH concurrently; 4 (4.1%) received a diagnosis of PH before a diagnosis of AAV; and the timing of diagnosis of either AAV or PH was not clear for 2 patients. The mean (SD) interval between AAV diagnosis and PH diagnosis was 6.6 (6.9) years.
Organ involvement With AAV and treatment of AAV
The majority of patients with a diagnosis of AAV (77.3%) had more than 1 organ or system involved. The most common vasculitis antigen was PR3 (56.7%). Treatment of AAV was with immunosuppressants for 92.8% of patients. At the time of diagnosis of PH, 21.6% had active vasculitis.
Follow-up and outcome
After the diagnosis of PH, the median follow-up was 18.6 months (IQR, 2.5-58.5). During that period, 71 patients (73.2%) died (Figure 1). Median survival after the diagnosis of PH was 25.9 months (95% CI, 12.2-49.9). Among the patients with identifiable causes of PH, survival was not different for patients with one cause of PH compared to others.
Fig. 1.
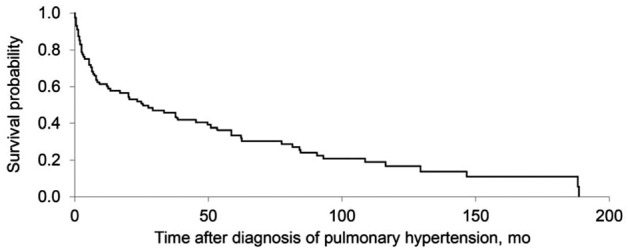
Kaplan-Meier estimates of survival after diagnosis of pulmonary hypertension for patients who had antineutrophil cytoplasmic antibody–associated vasculitis (n=97).
Comparison of patients With PH (n=97) and without PH (n=558)
Compared with patients who did not have PH, those with PH were older (mean age, 63 years vs 54 years) at the time of the vasculitis diagnosis, were predominantly male (62.9% vs 49.5%), and were more likely to be current or former smokers (60.9% vs 47.9%) (Table 2). Differences between the 2 groups were not significant for body mass index (P=.91), ANCA antigen type (PR3, MPO, or negative) (P=.13), or number of organs or systems involved with AAV (P=.52).
Table 2.
Comparison of Patients With PH and Without PH
| Feature | With PH (n=97) | Without PH (n=558) | P value |
|---|---|---|---|
| Age at vasculitis diagnosis, mean (SD), y | 63 (14.4) | 54 (18.1) | <.001 |
| Sex, No. (%) | |||
| Male | 61 (62.9) | 276 (49.5) | .02 |
| Female | 36 (37.1) | 282 (50.5) | |
| BMI, mean (SD)a | 28.3 (7.2) | 29.4 (7.1) | .91 |
| Smoking statusb | |||
| Smoker, No. (%) | 56 (60.9) | 259 (47.9) | .03 |
| Pack-years, mean (SD) | 38.81 (39.8) | 28.66 (21.6) | .09 |
| No. of organs or systems involved with AAV, mean (SD) | 2.4 (1.1) | 2.7 (1.2) | .52 |
| Organs or systems involved with AAV, No. (%) | |||
| General | 14 (14.4) | 292 (52.3) | <.001 |
| Cutaneous | 6 (6.2) | 84 (15.1) | .02 |
| Mucous membranes, eyes | 13 (13.4) | 82 (14.7) | .73 |
| Ear, nose, throat | 35 (36.1) | 310 (55.6) | <.001 |
| Chest | 66 (68.0) | 338 (60.6) | .17 |
| Cardiovascular | 4 (4.1) | 73 (13.1) | .01 |
| Abdominal | 0 (0) | 8 (1.4) | .23 |
| Kidney | 69 (71.1) | 268 (48.0) | <.001 |
| Nervous | 17 (17.5) | 75 (13.4) | .29 |
| ANCA status, No. (%)c | .13 | ||
| PR3 | 55 (59.1) | 353 (65.0) | |
| MPO | 34 (36.6) | 148 (27.2) | |
| Negative | 4 (4.3) | 43 (7.9) |
Abbreviations: AAV, antineutrophil cytoplasmic antibody–associated vasculitis; ANCA, antineutrophil cytoplasmic antibody; BMI, body mass index; MPO, myeloperoxidase; PH, pulmonary hypertension; PR3, proteinase 3. a BMI was not available for 40 patients in the group without PH. b Smoking status was not known for 5 patients in the group with PH and for 17 patients in the group without PH. c ANCA status was not known for 4 patients in the group with PH and for 15 patients in the group without PH.
Organ involvement with AAV
Patients with PH were less likely to have general organ or system involvement (myalgias, arthralgias or arthritis, fever ≥38 °, or weight loss ≥2 kg); cutaneous involvement (infarct, purpura, ulcer, gangrene, or other skin vasculitis); ear, nose, or throat manifestations (nasal discharge, crusts, ulcers, granulomata, paranasal sinus involvement, subglottic stenosis, conductive hearing loss, or sensorineural hearing loss); or cardiovascular involvement (loss of pulses, valvular heart disease, pericarditis, ischemic cardiac pain, cardiomyopathy, or congestive heart failure). However, kidney involvement (hypertension, proteinuria, hematuria, or increase in serum creatinine) was significantly more common in the group with PH compared to the group without PH (71.1% vs 48.0%, P<.001). Additionally, 17 of 69 patients (25%) with kidney involvement in the PH group were undergoing hemodialysis, and 13 of the 17 (76.5%) had atriovenous fistula at the time of PH diagnosis.
Survival
After the diagnosis of AAV, mean (SD) follow-up for the group with PH was 9.35 (7.50) years, and median follow-up was 7.0 (range, 0-30) years; for the group without PH, mean follow-up was 11.20 (8.11) years and median follow-up was 10.0 (range, 0-43) years. Kaplan-Meier survival curves for the 2 groups are shown in Figure 2. Compared with patients without PH, patients with PH had a significantly higher risk of death (hazard ratio, 3.15; 95% CI, 2.37-4.18; P<.001).
Fig. 2.

Kaplan-Meier estimates of survival for patients who had antineutrophil cytoplasmic antibody–associated vasculitis with pulmonary hypertension (PH) (n=97) and without PH (n=558).
Risk factors for mortality
Among patients with AAV, the presence of PH, age at AAV diagnosis, smoking status (current or former), MPO positivity, and kidney involvement with AAV were associated with an increased risk of death according to univariate analysis (Table 3). On multivariate analysis, the presence of PH, age at AAV diagnosis, smoking status, and kidney involvement with AAV were independent risk factors for death.
Table 3.
Risk Factors Associated With Mortality Among Patients With AAV According to Logistic Regression Models
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Pulmonary hypertension | <.001 | <.001 | ||
| Absent | 1.00 (ref) | 1.00 (ref) | ||
| Present | 3.15 (2.37-4.18) | 1.91 (1.41-2.59) | ||
| Age (at AAV diagnosis) | 1.06 (1.05-1.08) | <.001 | 1.07 (1.05-1.08) | <.001 |
| Smoker | 1.78 (1.36-2.35) | <.001 | 1.48 (1.12-1.96) | .006 |
| ANCA status | ||||
| PR3 | 1.00 (ref) | |||
| MPO | 1.48 (1.11-1.98) | .007 | ||
| Negative | 1.28 (0.76-2.15) | .86 | ||
| Kidney involvement with AAV | 1.52 (1.16-1.98) | .002 | 1.31 (0.99-1.75) | .06 |
Abbreviations: AAV, antineutrophil cytoplasmic antibody–associated vasculitis; ANCA, antineutrophil cytoplasmic antibody; MPO, myeloperoxidase; PR3, proteinase 3; ref, reference.
Discussion
In this single-center retrospective cohort study, we found that PH is relatively common in patients with AAV, is often associated with left heart or lung disease, and is associated with an increased risk of death. In addition to identifying PH as an independent prognostic factor associated with a 3-fold higher risk of death among patients with AAV, we also identified other prognostic factors, including age, male sex, smoking history, and kidney involvement with AAV. Our study provides important insight into the prognostic importance of PH in AAV, regardless of the cause or classification.
Other studies have identified prognostic risk factors in AAV and explored the relationship between AAV and smoking, age at the time of diagnosis of AAV, and kidney involvement with AAV and MPO. The role of smoking in the development of AAV is not completely understood, but a similar phenomenon is apparent in other autoimmune diseases such as rheumatoid arthritis.
In a case-control study, the risk of AAV was 1.7 times higher for smokers compared with controls (17). In the same study when patients were stratified by ANCA type, smoking was strongly associated with MPO vasculitis.
Among patients with AAV, those with MPO-positive disease tend to be older, have more kidney involvement, and have a greater likelihood of progressing to end-stage kidney disease (18-22). In multiple studies the major determinant of prognosis in AAV was kidney involvement with the disease (23, 24). In animal models when MPO was injected into mice, more kidney damage occurred (25). Older age, severity of kidney disease, and MPO status together contribute to the increased mortality among patients with AAV (22, 26-30).
In a study of patients with AAV, older patients (>65 years) compared with younger patients (<65 years) had a higher vasculitis damage index score, an increased likelihood of developing malignancies, a decreased chance of recovery of kidney function, and an increased risk of infections (31). With increasing age, immune system function seems to decrease and the likelihood of adverse effects from disease and from treatment seems to increase (22, 32, 33).
In our study, MPO was not an independent risk factor for mortality in multivariate analysis. Possibly the effects of age, MPO status, and kidney involvement with AAV overlap, and all 3 factors influence the overall prognosis for patients with AAV.
As mentioned above, age, smoking, MPO status, and kidney involvement have previously been associated with a greater risk of death among patients with AAV, but previous studies have not identified the association between PH and death. All the factors mentioned above can be risk factors for development of PH, which may have been an unmeasured mediator of effect in prior studies. The chances of collinearity between these other factors and PH exist, but our study found that PH was an independent predictor of mortality. After PH was detected in patients in our study, the prognosis was poor, which is similar to other studies where PH is described as “deadly” (34). The role of smoking is well established in conditions associated with PH, such as COPD and left heart disease. Smoking can directly cause PAH, which may explain “out of proportion” PH with mild airflow limitation in patients with COPD (35). The major mechanisms by which smoking causes PH could be vasoconstriction, inflammation, thrombosis, endothelial dysfunction, and smooth muscle proliferation (36-39). Even in the absence of hypoxia, infection and inflammation can cause vascular remodeling in patients with a genetic predisposition. In animal studies smoking upregulates the genes involved in vasoconstriction and vascular proliferation (40). It has been shown that smoking causes an increase in vascular endothelial growth factor, a decrease in endothelial nitric oxide synthase, and an increase in endothelin, a pulmonary vasoconstrictor (41, 42). The prevalence of PH increases with age not only because of increased comorbidities, such as cardiac conditions, but also because of increased arterial stiffness causing increased pulmonary resistance (43).
PH associated with chronic kidney disease (CKD) is currently classified in PH group 5 (1). The prevalence of PH among patients with nonhemodialysis CKD stage 5 is 9% to 39%, which is 2 to 8 times higher than the prevalence in the general population (44-47). The prevalence is even higher among patients who undergo hemodialysis (48). Regardless of whether a patient is undergoing hemodialysis, CKD itself is associated with left ventricular dysfunction resulting in volume overload and increased venous return, which results in increased pulmonary venous pressure (49). Patients with CKD have an imbalance between vasodilators and vasoconstrictors; for example, decreased nitric oxide (NO) levels have been identified in patients with CKD and PH (6). Another mechanism contributing to PH in patients with CKD could be the copresence of OSA, which causes sympathetic activation leading to increased activity of asymmetric dimethylarginine, an endogenous inhibitor of NO synthase, causing left ventricular hypertrophy (50, 51).
Similar to other epidemiologic studies in PH, our study found that left heart disease was the most common potential cause of PH (34, 52). Among the cardiac causes, valvular heart diseases, especially mitral and aortic valvular dysfunction, were most common. In AAV these valvular problems may be secondary to dilatation of the aortic root or the left ventricle, but primary valvulitis could also be the reason (53, 54).
PAH in AAV has been previously described as in other autoimmune diseases. In our study we could not definitively classify PAH because of lack of right heart catheterization data and comprehensive PH clinical assessments, but the majority of patients had risk factors for other PH groups (eg, groups 2 and 3). However, 3 patients with PH in our study did not have another identifiable cause other than perhaps vasculitis itself.
Our study has several practical clinical implications. First, PH should be considered in all patients with AAV, particularly those with signs or symptoms of PH or right heart failure, such as dyspnea or lower-extremity edema. The presence of risk factors for PH, such as smoking history, older age, MPO positivity, and kidney involvement with AAV, should prompt early consideration of PH, and right heart catheterization should be considered for accurate diagnosis. When PH is detected, all the potential causes of PH, such as left heart disease (especially valvular disease), chronic lung disease, thromboembolism, and liver disease, should be explored and addressed. An early referral to PH experts should be made for definitive diagnostic evaluation and consideration of treatment if appropriate. PH should be recognized as an independent risk factor for mortality in AAV.
Our study is the largest one describing the causes and impact of PH in AAV. This study, however, is not without limitations. The biggest limitation is that assessment of PH was based on echocardiography rather than right heart catheterization and thus limits accurate classification of PH. As a noninvasive and readily available screening test for PH, however, the use of echocardiogram to define PH increases generalizability. Lack of other testing, such as  /Q scan, also limited complete assessment of other PH groups. In addition, we included only patients who had an echocardiogram because this was necessary to define the presence or absence of PH. We recognize that the patients may have been a biased cohort with signs or symptoms that prompted the echocardiogram, and thus our results may not be generalizable to all patients with AAV. We cannot comment on the frequency of PH in AAV because this study was conducted with patients who underwent echocardiography.
/Q scan, also limited complete assessment of other PH groups. In addition, we included only patients who had an echocardiogram because this was necessary to define the presence or absence of PH. We recognize that the patients may have been a biased cohort with signs or symptoms that prompted the echocardiogram, and thus our results may not be generalizable to all patients with AAV. We cannot comment on the frequency of PH in AAV because this study was conducted with patients who underwent echocardiography.
In conclusion, PH in AAV is often multifactorial, is commonly associated with left heart disease or chronic lung disease, and is associated with a poor prognosis. PH, age, smoking history, and kidney involvement with AAV are risk factors for increased mortality in AAV. Future prospective studies are warranted to determine the pathogenesis and accurate classification of PH in AAV.
Acknowledgments:
Randall J. Fritz, DVM, Mayo Clinic, substantively edited the manuscript. The Scientific Publications staff at Mayo Clinic provided proofreading, administrative, and clerical support.
Abbreviations:
AAV, antineutrophil cytoplasmic antibody–associated vasculitis; ANCA, antineutrophil cytoplasmic antibody; BVAS, Birmingham Vasculitis Activity Score; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CT, computed tomography; EGPA, eosinophilic granulomatosis with polyangiitis; GPA, granulomatosis with polyangiitis; HFmrEF, heart failure with midrange ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; MPA, microscopic polyangiitis; MPO, myeloperoxidase; NO, nitric oxide; OSA, obstructive sleep apnea; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; PR3, proteinase 3; RVSP, right ventricular systolic pressure;  /Q, ventilation-perfusion
/Q, ventilation-perfusion
Data Statement:
All relevant data supporting the findings of this study are reported within the article.
Conflict of Interest:
None of the authors have any conflicts of interest to disclose.
Role of the Funding Source:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1) doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- Denton CP, Cailes JB, Phillips GD, Wells AU, Black CM, Bois RM. Comparison of Doppler echocardiography and right heart catheterization to assess pulmonary hypertension in systemic sclerosis. Br J Rheumatol. 1997;36(2):239–43. doi: 10.1093/rheumatology/36.2.239. [DOI] [PubMed] [Google Scholar]
- Gabbay E, Reed A, Williams TJ. Assessment and treatment of pulmonary arterial hypertension: an Australian perspective in 2006. Intern Med J. 2007;37(1):38–48. doi: 10.1111/j.1445-5994.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- Mukerjee D, St George D, Knight C, et al. Echocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosis. Rheumatology (Oxford) 2004;43(4):461–6. doi: 10.1093/rheumatology/keh067. [DOI] [PubMed] [Google Scholar]
- Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333(4):214–21. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhu YJ, Zhou YP, et al. Clinical features and survival in Takayasu's arteritis-associated pulmonary hypertension: a nationwide study. Eur Heart J. 2021;42(42):4298–305. doi: 10.1093/eurheartj/ehab599. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y. Takayasu arteritis-associated pulmonary hypertension. Eur Heart J. 2021;42(42):4306–8. doi: 10.1093/eurheartj/ehab688. [DOI] [PubMed] [Google Scholar]
- Marette P, Molle B, Mas JL, et al. Angina without involvement of large coronary trunks and precapillary pulmonary hypertension in a patient with periarteritis nodosa. Ann Cardiol Angeiol (Paris) 1989;38(6):305–8. [PubMed] [Google Scholar]
- Launay D, Souza R, Guillevin L, et al. Pulmonary arterial hypertension in ANCA-associated vasculitis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23(3):223–8. [PubMed] [Google Scholar]
- Li Y, Yi Q. Pulmonary arterial hypertension associated with rare cause of ANCA-associated vasculitis misdiagnosed as idiopathic one. Int J Clin Exp Med. 2015;8(9):16850–3. [PMC free article] [PubMed] [Google Scholar]
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- Augustine DX, Coates-Bradshaw LD, Willis J, et al. Echocardiographic assessment of pulmonary hypertension: a guideline protocol from the British Society of Echocardiography. Echo Res Pract. 2018;5(3):G11–G24. doi: 10.1530/ERP-17-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87(11):671–8. [PubMed] [Google Scholar]
- Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener's granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS) Arthritis Rheum. 2001;44(4):912–20. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- McDermott G, Fu X, Stone JH, et al. Association of Cigarette Smoking With Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. JAMA Intern Med. 2020;180(6):870–6. doi: 10.1001/jamainternmed.2020.0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad AJ, Segelmark M. A population-based study showing better renal prognosis for proteinase 3 antineutrophil cytoplasmic antibody (ANCA)-associated nephritis versus myeloperoxidase ANCA-associated nephritis. J Rheumatol. 2014;41(7):1366–73. doi: 10.3899/jrheum.131038. [DOI] [PubMed] [Google Scholar]
- Berti A, Cornec-Le Gall E, Cornec D, et al. Incidence, prevalence, mortality and chronic renal damage of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis in a 20-year population-based cohort. Nephrol Dial Transplant. 2019;34(9):1508–17. doi: 10.1093/ndt/gfy250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai YH, Li ZY, Chang DY, Chen M, Kallenberg CG, Zhao MH. The BVAS is an independent predictor of cardiovascular events and cardiovascular disease-related mortality in patients with ANCA-associated vasculitis: A study of 504 cases in a single Chinese center. Semin. Arthritis Rheum. 2018;47(4):524–9. doi: 10.1016/j.semarthrit.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Mohammad AJ, Jacobsson LT, Westman KW, Sturfelt G, Segelmark M. Incidence and survival rates in Wegener's granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome and polyarteritis nodosa. Rheumatology (Oxford) 2009;48(12):1560–5. doi: 10.1093/rheumatology/kep304. [DOI] [PubMed] [Google Scholar]
- Harper L, Savage CO. ANCA-associated renal vasculitis at the end of the twentieth century--a disease of older patients. Rheumatology (Oxford) 2005;44(4):495–501. doi: 10.1093/rheumatology/keh522. [DOI] [PubMed] [Google Scholar]
- Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. 2003;41(4):776–84. doi: 10.1016/s0272-6386(03)00025-8. [DOI] [PubMed] [Google Scholar]
- Mahr A, Girard T, Agher R, Guillevin L. Analysis of factors predictive of survival based on 49 patients with systemic Wegener's granulomatosis and prospective follow-up. Rheumatology (Oxford) 2001;40(5):492–8. doi: 10.1093/rheumatology/40.5.492. [DOI] [PubMed] [Google Scholar]
- Wang Q, van Timmeren MM, Petersen AH, et al. Age-determined severity of anti-myeloperoxidase autoantibody-mediated glomerulonephritis in mice. Nephrol Dial Transplant. 2017;32(2):254–64. doi: 10.1093/ndt/gfw202. [DOI] [PubMed] [Google Scholar]
- Chen M, Yu F, Zhang Y, Zhao MH. Antineutrophil cytoplasmic autoantibody-associated vasculitis in older patients. Medicine (Baltimore) 2008;87(4):203–9. doi: 10.1097/MD.0b013e31817c744b. [DOI] [PubMed] [Google Scholar]
- Weiner M, Goh SM, Mohammad AJ, et al. Outcome and treatment of elderly patients with ANCA-associated vasculitis. Clin J Am Soc Nephrol. 2015;10(7):1128–35. doi: 10.2215/CJN.00480115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoganson DD, From AM, Michet CJ. ANCA vasculitis in the elderly. J Clin Rheumatol. 2008;14(2):78–81. doi: 10.1097/RHU.0b013e31816b2fbd. [DOI] [PubMed] [Google Scholar]
- Jayne D. Current attitudes to the therapy of vasculitis. Kidney Blood Press Res. 2003;26(4):231–9. doi: 10.1159/000072990. [DOI] [PubMed] [Google Scholar]
- Wallace ZS, Fu X, Harkness T, Stone JH, Zhang Y, Choi H. All-cause and cause-specific mortality in ANCA-associated vasculitis: overall and according to ANCA type. Rheumatology (Oxford) 2020;59(9):2308–15. doi: 10.1093/rheumatology/kez589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti A, Felicetti M, Monti S, et al. Disease and treatment-related morbidity in young and elderly patients with granulomatosis with polyangiitis and microscopic polyangiitis. Semin Arthritis Rheum. 2020;50(6):1441–8. doi: 10.1016/j.semarthrit.2020.02.008. [DOI] [PubMed] [Google Scholar]
- Berti A, Caporali R, Montecucco C, Paolazzi G, Monti S. Aging in Primary Systemic Vasculitis: Implications for Diagnosis, Clinical Manifestations, and Management. Drugs Aging. 2019;36(1):53–63. doi: 10.1007/s40266-018-0617-4. [DOI] [PubMed] [Google Scholar]
- Higgins RM, Goldsmith DJ, Connolly J, et al. Vasculitis and rapidly progressive glomerulonephritis in the elderly. Postgrad Med J. 1996;72(843):41–4. doi: 10.1136/pgmj.72.843.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange G, Playford D, Stewart S, et al. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart. 2012;98(24):1805–11. doi: 10.1136/heartjnl-2012-301992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiess R, Senn O, Fischler M, et al. Tobacco smoke: a risk factor for pulmonary arterial hypertension? A case-control study. Chest. 2010;138(5):1086–92. doi: 10.1378/chest.09-2962. [DOI] [PubMed] [Google Scholar]
- Wright JL, Dai J, Zay K, Price K, Gilks CB, Churg A. Effects of cigarette smoke on nitric oxide synthase expression in the rat lung. Lab Invest. 1999;79(8):975–83. [PubMed] [Google Scholar]
- Wright JL, Farmer SG, Churg A. A neutrophil elastase inhibitor reduces cigarette smoke-induced remodelling of lung vessels. Eur Respir J. 2003;22(1):77–81. doi: 10.1183/09031936.03.00095703. [DOI] [PubMed] [Google Scholar]
- Santos S, Peinado VI, Ramirez J, et al. Enhanced expression of vascular endothelial growth factor in pulmonary arteries of smokers and patients with moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167(9):1250–6. doi: 10.1164/rccm.200210-1233OC. [DOI] [PubMed] [Google Scholar]
- Barbera JA, Peinado VI, Santos S, Ramirez J, Roca J, Rodriguez-Roisin R. Reduced expression of endothelial nitric oxide synthase in pulmonary arteries of smokers. Am J Respir Crit Care Med. 2001;164(4):709–13. doi: 10.1164/ajrccm.164.4.2101023. [DOI] [PubMed] [Google Scholar]
- Wright JL, Tai H, Churg A. Vasoactive mediators and pulmonary hypertension after cigarette smoke exposure in the guinea pig. J Appl Physiol (1985) 2006;100(2):672–8. doi: 10.1152/japplphysiol.00274.2005. [DOI] [PubMed] [Google Scholar]
- Goerre S, Staehli C, Shaw S, Luscher TF. Effect of cigarette smoking and nicotine on plasma endothelin-1 levels. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S236–8. [PubMed] [Google Scholar]
- Santos S, Peinado VI, Ramirez J, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19(4):632–8. doi: 10.1183/09031936.02.00245902. [DOI] [PubMed] [Google Scholar]
- Akmal M, Barndt RR, Ansari AN, Mohler JG, Massry SG. Excess PTH in CRF induces pulmonary calcification, pulmonary hypertension and right ventricular hypertrophy. Kidney Int. 1995;47(1):158–63. doi: 10.1038/ki.1995.18. [DOI] [PubMed] [Google Scholar]
- Yigla M, Fruchter O, Aharonson D, et al. Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int. 2009;75(9):969–75. doi: 10.1038/ki.2009.10. [DOI] [PubMed] [Google Scholar]
- Havlucu Y, Kursat S, Ekmekci C, et al. Pulmonary hypertension in patients with chronic renal failure. Respiration. 2007;74(5):503–10. doi: 10.1159/000102953. [DOI] [PubMed] [Google Scholar]
- Issa N, Krowka MJ, Griffin MD, Hickson LJ, Stegall MD, Cosio FG. Pulmonary hypertension is associated with reduced patient survival after kidney transplantation. Transplantation. 2008;86(10):1384–8. doi: 10.1097/TP.0b013e318188d640. [DOI] [PubMed] [Google Scholar]
- Abdelwhab S, Elshinnawy S. Pulmonary hypertension in chronic renal failure patients. Am J Nephrol. 2008;28(6):990–7. doi: 10.1159/000146076. [DOI] [PubMed] [Google Scholar]
- Pabst S, Hammerstingl C, Hundt F, et al. Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: results of the PEPPER-study. PLoS One. 2012;7(4):e35310. doi: 10.1371/journal.pone.0035310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul F, Yigla M, Gilman R, Reisner SA, Abassi Z. The pathogenesis of pulmonary hypertension in haemodialysis patients via arterio-venous access. Nephrol Dial Transplant. 2005;20(8):1686–92. doi: 10.1093/ndt/gfh840. [DOI] [PubMed] [Google Scholar]
- Ressl J, Urbanova D, Widimsky J, Ostadal B, Pelouch V, Prochazka J. Reversibility of pulmonary hypertension and right ventricular hypertrophy induced by intermittent high altitude hypoxia in rats. Respiration. 1974;31(1):38–46. doi: 10.1159/000193097. [DOI] [PubMed] [Google Scholar]
- Sica AL, Greenberg HE, Ruggiero DA, Scharf SM. Chronic-intermittent hypoxia: a model of sympathetic activation in the rat. Respir Physiol. 2000;121(2-3):173–84. doi: 10.1016/s0034-5687(00)00126-2. [DOI] [PubMed] [Google Scholar]
- Wijeratne DT, Lajkosz K, Brogly SB, et al. Increasing Incidence and Prevalence of World Health Organization Groups 1 to 4 Pulmonary Hypertension: A Population-Based Cohort Study in Ontario, Canada. Circ Cardiovasc Qual Outcomes. 2018;11(2):e003973. doi: 10.1161/CIRCOUTCOMES.117.003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanda RJ, Guis MS, Rabkin JM. Aortic valvulitis in a patient with Wegener's granulomatosis. West J Med. 1989;151(5):555–6. [PMC free article] [PubMed] [Google Scholar]
- Gerbracht DD, Savage RW, Scharff N. Reversible valvulitis in Wegener's granulomatosis. Chest. 1987;92(1):182–3. doi: 10.1378/chest.92.1.182. [DOI] [PubMed] [Google Scholar]


