Abstract
Background
Aging, influenced by intrinsic and extrinsic factors, leads to visible skin changes such as dryness, surface roughness, and loss of luminosity. Proper skin care can mitigate some of these effects, with topical combination products offering support through complementary mechanisms.
Objectives
To test efficacy and safety of GSYBS-7 (Goop Beauty Youth-Boost Peptide Serum; Goop Inc., Santa Monica, CA), a topical postbiotic and botanical combination serum, used twice daily on facial skin for 6 weeks.
Methods
In this 19-patient pilot study, the primary efficacy measure was improvement on the Global Ranking Scale (GRS) at 42 days. A 12-point patient satisfaction survey and the serum's effect on healing after ablative laser treatment served as secondary and exploratory measures, respectively.
Results
Statistically significant improvements in all GRS domains were observed as early as day 7 with ongoing improvement up to day 42. A >1-point improvement was observed for dehydration (1.8; 95% CI, 1.5-2.2), visible pores (1.6; 95% CI, 1.3-2.0), surface roughness (1.6; 95% CI, 1.3-1.9), imbalance (1.3; 95% CI, .9-1.7), static wrinkles (1.3; 95% CI, .9-1.6), pigmentation (1.3; 95% CI, 1.0-1.5), and vasculature (1.2; 95% CI, .8-1.5). Independent photographic review and patient satisfaction surveys corroborated these findings. At day 42, 94.4% of patients were very satisfied with the results, and 88.9% would recommend GSYBS-7 to family and friends. No adverse events were reported, and 100% of patients indicated that GSYBS-7 was gentle enough for everyday use.
Conclusions
GSYBS-7 appears to be an effective and well-tolerated combination topical for the management of age-related and environment-induced skin changes.
Level of Evidence: 4

Intrinsic and extrinsic processes, as well as the complex interactions between them, are known to contribute to aging. Extrinsic processes include factors such as diet, ultraviolet radiation, environmental and fluctuating climactic conditions, pollutants, and chemical exposure, while intrinsic processes include genetic predisposition, hormonal changes, and cellular senescence.1 These types of changes comprise the physiologic process of aging and, over time, lead to alterations in the structure of the extracellular matrix, the biology of each skin layer, and the biology of underlying structures and tissues.2 Both behavior (ie, sun exposure, smoking) and genetics are known to drive the rate at which the manifestations of aging occur.3 While some of these contributors are established and relatively well defined, others, like the impact of the gut and skin microbiome on the skin, are emerging and continuing to evolve. For example, changes in the microbiome are associated with several aspects of skin aging, including barrier function as well as susceptibility to photodamage4; however, the exact mechanism remains undefined.
Clinically, patients experience skin changes such as dryness, surface roughness, and loss of luminosity (ie, “dull-looking skin”), which can occur, in part, as a result of transepidermal water loss. Visible pores, dyspigmentation (redness/telangiectasia or the appearance of melasma or lentigines), and the emergence of static and dynamic wrinkles can also diminish the appearance of the skin.4 Skin quality is an indicator of overall health, and adequate at-home care is an important first step in any aesthetic approach.5,6 Importantly, it is not uncommon for patients to present for treatment of age-related skin changes with minimally invasive aesthetic devices but still not be utilizing optimal skin care at home. In the authors' experience, common weaknesses in skin care include inadequate sun protection; the use of too-aggressive products that cause irritation, dryness, or excess sebum production; the use of too many products that do not adequately or specifically support skin health; and the use of products that contain irritants, perfumes, or colorants that could worsen the patient's skin and may have negative impacts on overall health.
Topical combination products are an important way for patients to mitigate the effects of skin aging by supporting skin health via a variety of complementary mechanisms. In the current prospective study, a novel postbiotic and botanical combination topical serum, Goop Beauty Youth-Boost Peptide Serum (GSYBS-7; Goop Inc., Santa Monica, CA), was tested for efficacy and safety on facial skin. The formulation was designed to target apparent signs of skin aging, including barrier function, tonality, radiance, surface roughness, and texture, with a blend of dermatologic ingredients, naturally derived botanicals, and postbiotic extracts.
METHODS
Study Design
This single-center pilot study assessed the clinical effects of GSYBS-7 topical serum when applied twice daily to facial skin. The serum contains a proprietary blend of a postbiotic (bifida ferment lysate), heptapeptide-7, and botanical agents for skin firming (paracress [Acmella oleracea extract] and astaxanthin [Haematococcus pluvialis extract]), moisturization (Daemonorops draco extract), and exfoliation (black tea ferment [Saccharomyces/Xylinum] and salicylic acid), along with ceramide-NP and niacinamide, to address a range of cosmetic skin quality concerns, including tonality, healthy skin barrier function, and overall surface radiance. The serum is designed to be appropriate for sensitive skin types and is free of synthetic colorants and fragrances.
A total of 19 patients were enrolled; demographics are shown in Table. Patients were selected who presented to the clinic voicing concerns around skin quality related to aging. Patients who had received botulinum toxin A, filler, or energy-based treatments within 3 months of study enrollment or who had undergone rejuvenation procedures to the face or neck (eg, microneedling, microdermabrasion, chemical peels) within 30 days were excluded. Those who were current smokers, had a history of heavy smoking, had uncontrolled systemic inflammatory conditions, or had active dermatologic conditions in the area to be treated were also excluded. For the duration of the study, patients did not receive any aesthetic treatments on their face or neck, and limited their use of topical products to the product under study, a gentle facial cleanser, and routine physical mineralized zinc oxide–based sunscreen application daily as recommended by the American Academy of Dermatology.
Table.
Study Demographics
| Subject characteristic (n = 19) | Value |
|---|---|
| Median age, years (range) | 32.0 (21-60) |
| Gender, n (%) | |
| Male | 3 (15.8) |
| Female | 16 (84.2) |
| Fitzpatrick skin type, n (%) | |
| I | 0 (0) |
| II | 3 (15.8) |
| III | 5 (26.3) |
| IV | 1 (5.3) |
| V | 10 (52.6) |
| VI | 0 (0) |
In this pilot study, there was no placebo group for the facial skin assessment; rather, patients served as their own control. Following baseline assessment (day 1), each patient was provided with a bottle of GSYBS-7 topical serum in an unbranded, white container. Patients were instructed to apply a thin layer of serum over the surface of their entire face following use of a mild facial cleanser in the morning and evening. In the mornings, patients were instructed to apply a thin layer of nonmedicated physical sunscreen. This regimen was continued for 42 days (6 weeks), with follow-up visits at days 7, 21, and 42.
Given the antioxidant and reported wound-healing attributes of several GSYBS-7 ingredients, the effect on healing following spot treatment with ablative laser (HALO; Sciton, Palo Alto, CA) was also assessed as an exploratory endpoint. Patients, blinded, were provided with 2 bottles of serum to apply to either their left or right arm in the morning and evening for 7 days: One bottle, labeled “Serum for Left Arm,” contained GSYBS-7, and the second, labeled “Serum for Right Arm,” was a placebo, containing only the GSYBS-7 carrier without active ingredients. Recovery was evaluated based on patient photographs and the cutaneous changes present at day 7.
Compliance was assured by weighing the container at the end of the study. Patients also filled out a daily diary in which they recorded any changes to their skin appearance and any side effects. Patients were not reimbursed for their time, but the cost of parking was covered.
Efficacy and Safety Measures
Primary Endpoint
At baseline and each follow-up visit, standardized photographs of the treatment areas were captured. Patients were assessed live (co-assessment included both the patient and the treating clinician) using the Global Ranking Scale (GRS; Appendix A) with Comprehensive Skin Analysis.7 The primary endpoint in this pilot study was improvement on the GRS on day 42 compared to baseline; however, GRS assessments were completed on days 7, 21, and 42 to better understand time to onset. For each of the 13 domains included in the GRS (loss of elasticity, surface roughness, dehydration, static wrinkles, dynamic wrinkles, volume loss, sagging, asymmetry, imbalance, scar presence, visible pores, pigmentation, and vasculature), grading ranges from 0 to 3 (0, none; 1, mild; 2, moderate; 3, severe). The patient and investigator were blinded to baseline scores and any scores from prior follow-up at all evaluations.
With the use of 2-tailed paired t tests unadjusted for multiple comparisons, each of the GRS domains showed a statistically significant improvement from baseline to day 42. To better understand the magnitude of the treatment effect, paired mean differences and lower and upper 95% confidence intervals for each GRS domain were calculated.
Independent Photographic Review
Because the GRS is intended to be used jointly by the physician and patient, independent review of before-and-after images was carried out by a blinded board–certified plastic surgeon working in a clinic remote to the study site. The independent reviewer, who was blinded to patient GRS scores, was provided with patient images at baseline and 42 days and asked to rate the after image as “improved,” “not improved,” or “not assessed” for each of the GRS domains.
Domains that were difficult to evaluate based on photographs were marked as “not assessed.” The percentage of patients considered improved in each domain was then calculated. Because patients served as their own control, there was no comparison to a placebo group or alternative treatment arm in this study.
Secondary Endpoint
A 12-question subject satisfaction questionnaire was completed by patients independently, at home, prior to their office visit and completion of the GRS. Satisfaction questionaries were completed on days 7, 21, and 42. These questions include those dealing with functional outcomes and satisfaction, as well as several queries that overlap with GRS skin quality domains (Appendix B).
Exploratory Measures
To assess the effect of GSYBS-7 ingredients, on healing, a 2 cm2 area on each patient's left and right volar forearm was treated with subablative settings, which were the most appropriate given patient skin types within the study (1470 nm setting, 200 microns over 10%; 2940 nm setting, 20 microns over 10%). Patients recorded each application of GSYBS-7/placebo in their diary along with any notes on irritation, side effects, and/or aesthetic effects, and photographed the areas with their smartphone once daily. On day 7, recovery was evaluated based on patient photographs and cutaneous changes. All subjects were evaluated for redness, color, texture, recovery, healing, and reepithelialization to determine which side displayed better improvement. The reviewer and the patients were blinded to active vs placebo sides. Note that all subjects had a history of sunscreen use prior to the study.
Patients also underwent a video evaluation at baseline and at 42 days. This video assessment permitted identification of any changes apparent during motion and dynamic expression that may not have been appreciable in static images. Patients were asked to share their age, favorite color, and ideal vacation, and they were then asked to repeat this same information at the final follow-up evaluation. This endpoint was intended to further develop 4-dimensional patient assessment as a tool for evaluating treatment outcomes.8
Safety
Safety was monitored at a virtual visit on day 3 and at each follow-up visit (days 7, 21, and 42) through assessment of the facial skin by the investigator and by review of patient diaries. This study was approved by an Institutional Review Board (Advarra IRB, Columbia, MD) and adhered to the standards set forth in the World Medical Association's Declaration of Helsinki. Written consent was provided, by which the patients agreed to the use and analysis of their data and publication of their photographs and videos.
RESULTS
The study took place from January to March 2023 and enrolled 19 patients (16 females [84.2%] and 3 males [15.8%]), with a median age of 32 years (range, 21-60). Of the 19 patients enrolled, 19 completed all GRS evaluations, and 18 completed the satisfaction survey at day 42. Patients with a wide range of skin types were included in the study (Table 1). Mean baseline scores (SE) were reflective of mild-to-moderate severity, with the most severe average scores observed for surface roughness (2.2 [0.6]), dynamic wrinkles (2.1 [0.8]), visible pores (2.1 [0.5]), dehydration (1.8 [0.7]), and imbalance (1.8 [0.7]) as well as pigmentation (1.8 [0.7]). Mean GRS scores with standard errors are shown in Figure 1 for baseline and each follow-up visit. For each domain, any observed improvement was apparent by day 7 and continued to improve at weeks 3 and 6.
Figure 1.
Mean Global Ranking Scale (GRS) scores with error bars for each time point within each of the GRS domains.
While an improvement was observed for each GRS domain, including those for which one would not expect a topical treatment to have an effect (ie, volume loss), assessment of the degree of improvement in each domain reveals that the greatest changes (from day 0 to day 42) were present in those domains consistent with topical treatments (Figure 2). Domains in which a >1-point improvement was observed included dehydration (1.8; 95% CI, 1.5-2.2), visible pores (1.6; 95% CI, 1.3-2.0), surface roughness (1.6; 95% CI, 1.3-1.9), imbalance (1.3; 95% CI, 0.9-1.7), static wrinkles (1.3; 95% CI, 0.9-1.6), pigmentation (1.3; 95% CI, 1.0-1.5), and vasculature (1.2; 95% CI, 0.8-1.5). While “imbalance” is considered a morphologic domain, and refers to the profile (“asymmetry” refers to the frontal view of the face), this term may be somewhat unclear to patients, and the degree of change observed in this domain, as well as the larger confidence interval, may be reflective of some patients' assessing balanced skin tone rather than balance of facial anatomic structures.
Figure 2.
Mean change in Global Ranking Scale (GRS) scores from baseline to day 42. The mean change in each GRS domain (listed on y-axis) is shown (closed circles) along with 95% confidence intervals (shaded bars). The dashed line indicates a 1-point mean change.
When photographs were independently reviewed by a board-certified plastic surgeon, the domains in which the greatest degree of improvement were apparent and similar included dehydration (94.7% of patients were improved), vasculature (94.7% were improved), surface roughness (89.5% were improved), pigmentation (84.2% were improved), and visible pores (78.9% were improved; Figure 3). Of note, not all patients had each of the aesthetic issues measured by the GRS at baseline. For example, while only 31.6% of patients reported improvement in scars, 21.1% did not have scars present at baseline (baseline score of 0), suggesting the potential for a greater degree of improvement in those who have this aesthetic issue than the total percentage of improvement for all patients would suggest. For reference, the percentages of patients with a baseline score of 0 are shown in Figure 3 as dashed white lines with percentages indicated, and the percentages of patients with a baseline score of 1 are indicated by green dashed lines.
Figure 3.
Independent review of patient baseline and 42-day images. The percentages of patients whom the independent reviewer deemed “improved” are shown as green bars. Gray bars indicate “no improvement” or “not assessed” (NA). Because not all patients had severe issues in all categories measured, the percentages of patients who had a score of 0 at baseline (white dashed lines) or a score of 1 at baseline (green dashed lines) are indicated.
In Figures 4 through 7, patient before-and-after images are shown. Exploratory dynamic assessments are shown in the Video.
Figure 4.
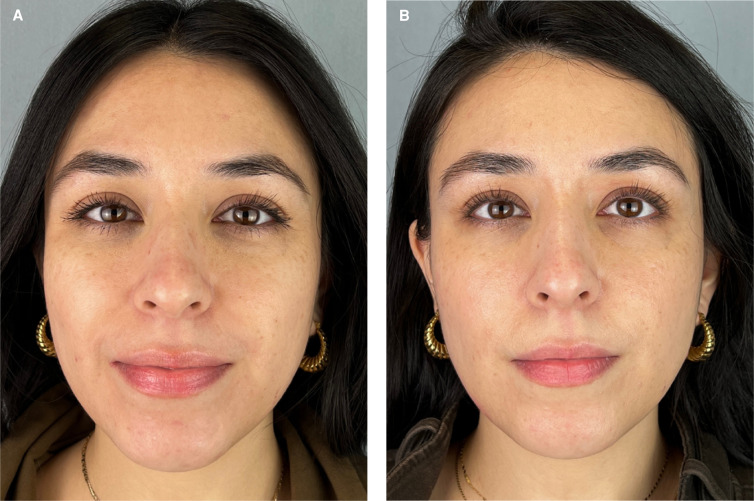
A 36-year-old female at (A) baseline and (B) 42 days after twice-daily application of GSYBS-7 (Goop Beauty Youth-Boost Peptide Serum; Goop Inc.). Decreased pore visibility, improved hydration, and more even texture were identified. On the Global Ranking Scale, this patient showed a 2-point improvement in hydration and 1-point improvements in static wrinkles, visible pores, and pigmentation.
Figure 7.
A 40-year-old female at (A) baseline and (B) 42 days after twice-daily application of GSYBS-7 (Goop Beauty Youth-Boost Peptide Serum; Goop Inc.). Note the decrease in redness, improved evenness in tone, and improved luminosity. On the Global Ranking Scale, this patient had 1-point improvements in static wrinkles, dynamic wrinkles, volume loss, sagging, surface roughness, scars, visible pores, pigmentation, and vasculature, as well as a 3-point improvement in dehydration.
Figure 5.
A 32-year-old female at (A) baseline and (B) 42 days after twice-daily application of GSYBS-7 (Goop Beauty Youth-Boost Peptide Serum; Goop Inc.). A decrease in redness and improved skin texture throughout the facial skin were noted, especially on the forehead, as well as improvement in skin roughness. On the Global Ranking Scale, this patient had 2-point improvements in static wrinkles, imbalance, and dehydration as well as 1-point improvements in dynamic wrinkles, asymmetry, surface roughness, visible pores, pigmentation, and vasculature.
Figure 6.
A 31-year-old female at (A) baseline and (B) 42 days after twice-daily application of GSYBS-7 (Goop Beauty Youth-Boost Peptide Serum; Goop Inc.). A decrease in redness and improved hydration of the skin in the midface and forehead were observed. On the Global Ranking Scale, this patient experienced 2-point improvements in imbalance, surface roughness, dehydration, and visible pores, as well as 1-point improvements in static and dynamic wrinkles, volume loss, asymmetry, loss of elasticity, scars, pigmentation, and vasculature.
The subject questionnaire completed on days 7, 21, and 42 revealed that at day 42, 94.5% of subjects either strongly agreed (66.7%) or agreed (27.8%) that there was a significant improvement in the radiance/luminosity of their skin, and 88.9% either strongly agreed (61.1%) or agreed (27.8%) that there was a significant improvement in the texture/smoothness of their skin (Figure 8). Consistent with the improvement in GRS scores observed before the final study endpoint, these improvements in radiance/luminosity and skin texture were apparent at 21 days. Even as early as 21 days, 77.8% either strongly agreed (66.7%) or agreed (11.1%) that they observed a significant increase in radiance/luminosity and a total of 83.3% either strongly agreed (50%) or agreed (33%) that they observed an improvement in texture. Overall, at day 42, 94.4% of patients were very satisfied with the results of the treatment, and 88.9% would recommend GSYBS-7 to family and friends. A total of 83.3% of patients felt that GSYBS-7 gave them more confidence in their appearance. Responses to all survey questions are detailed in Figure 8.
Figure 8.
Subject satisfaction and functional outcomes measured using the subject survey after using GSYBS-7 (Goop Beauty Youth-Boost Peptide Serum; Goop Inc.) for 6 weeks. The percentages of patients who agreed (navy) and strongly agreed (green) with the survey statement are indicated. The total percentage of patients who at least agreed with the statement is shown at the outside end of the respective bar.
At day 42, 100% of subjects felt that GSYBS-7 was gentle enough for everyday use (77.8% strongly agreed and 22.2% agreed), and 94.5% felt that it was comfortable and soothing on the skin. Throughout the study, there were no reports of adverse events or skin reactions in patient diaries, and none were reported to the treating clinician upon questioning.
For the exploratory endpoint of accelerated healing following treatment with an ablative laser, 17 of the 19 patients (89.5%) showed some accelerated healing on the arm treated with GSYBS-7 based on the defined parameters. Select images for comparison may be found in Appendix C.
DISCUSSION
The GSYBS-7 combination topical is a unique and novel formulation that includes ingredients known to be supportive of several aspects of skin health. To our knowledge, this represents the first study of its kind to evaluate such a combination of botanical ingredients for cosmetic skin enhancement and the promotion of wound healing after laser resurfacing. This study provides evidence that this combination is effective for improving surface roughness, luminosity, skin texture and smoothness, dehydration, appearance of pores, and pigmentation. These improvements are consistently captured across 3 different measures: the GRS scale, patient satisfaction surveys, and independent review. Importantly, on the GRS, patients also reported an effect on dynamic wrinkles. This finding is consistent with the GSYBS-7 formulation, which was designed with peptides, niacinamide, and other botanicals to improve the appearance of dynamic lines and wrinkles. The observed product efficacy and associated aesthetic improvements are accompanied by a high degree of patient satisfaction with supporting results: 94.4% of patients were satisfied, and 88.9% would recommend the product to family and friends. As is the case with any skin care product intended for everyday use, tolerability is of central importance. GSYBS-7 was well tolerated not only by clinical standards but also by patient standards: 100% of patients felt it was gentle enough for everyday use, and there were no adverse events or tolerability issues reported. This combination of aesthetic improvement and tolerability suggests that the GSYBS-7 combination topical is well suited for everyday patient skin care. Accelerated healing following treatment with ablative laser was observed in most patients, which presents an opportunity for future, more in-depth study of healing, in particular in patients with skin color, a group for whom healing following procedures is a concern and for which there are very few evidence-based practices.
The effects of natural extracts on the skin can be difficult to predict, especially when used in combination. Thus, clinical investigation is critical for defining both efficacy and tolerability. While a full review of the body of evidence for each ingredient in GSYBS-7 is beyond the scope of this paper, a brief summary of the product ingredients and rationale for inclusion in the formulation is given below.
Bifida ferment lysate, thought to have postbiotic effects, has been shown to reduce the molecular hallmarks of inflammation while decreasing skin sensitivity and dryness and increasing skin barrier resistance to disruption relative to controls.9 Theoretically, it may support the native skin microbiome, which has the potential to affect age-related changes in this complex system. Acmella oleracea extract has been shown in preclinical models to improve collagen content and organization following tendon injury10 and also has both anti-inflammatory and antioxidant activities.11 Astaxanthin (H. pluvialis extract) is a potent antioxidant with a unique capacity to quench reactive oxygen species in the cell membrane through inhibition of lipid peroxidation.12 With regard to skin health, it is thought to protect against collagen reduction resulting from ultraviolet skin damage.12 Of note, as an oral supplement, astaxanthin has been shown to be effective for sebum control and for reducing dynamic lines as well as transepidermal water loss.13 Daemonorops draco extract has anti-inflammatory and antimicrobial properties and has also been used to support wound healing and skin repair in a variety of settings.14,15 While there are limited data for black tea ferment (Saccharomyces/Xylinum) specifically, Saccharomyces cerevisiae extract is emerging as a popular ingredient in many cosmetics due to its amino acid, protein, and peptide content, and it is hypothesized to support wound healing and the function of the skin barrier.16 Heptapeptide-7,17 ceramide-NP,18,19 niacinamide,20,21 and salicylic acid also have established roles in dermatologic care.
Strengths of this study include excellent retention as well as the inclusion of a wide range of patient skin types, which makes for more informative study results and exploratory findings. Limitations include a small cohort of patients and lack of a placebo control for the primary endpoint. While the inclusion of a blinded photographic review and a patient satisfaction survey with questions overlapping with the domains within the GRS assessment increase the rigor of the study beyond what would be achievable with the GRS alone, the authors recognize that these assessments are subjective, and like all subjective measures, may be prone to individual interpretation and bias. Inclusion of an objective scale or outcome measure would strengthen the study’s findings. In addition, the safety and tolerability described here are short-term and do not necessarily reflect long-term safety.
GSYBS-7 was formulated to address a range of aging concerns via the supportive effects of each individual ingredient. This study supports a role for the formulation in effective at-home care for a wide range of skin types with an array of aesthetic concerns.
CONCLUSIONS
Based on the evidence gathered for this pilot study, GSYBS-7 is a promising and well-tolerated combination topical for an array of skin quality issues. In particular for skin hydration, luminosity, surface texture, and visibility of pores, the formulation is associated with improvement and a high degree of patient satisfaction. In addition, observed improvements in pigmentation and in both static and dynamic wrinkles suggest a possible effect on skin quality. Taken together, these data support additional study of GSYBS-7 topical for facial skin rejuvenation as well as accelerated healing following energy-based treatments.
Supplementary Material
Acknowledgments
Medical writing assistance was provided by Ginny Vachon, PhD, Principal Medvantage, LLC (Atlanta, GA), under the direction of the authors. Funding for this support was provided by Aforé (Chicago, IL). The authors thank Karen Copeland of Boulder Statistics for her assistance with data analysis and Iulia Imbrogno for her support in the coordination and execution of this study.
Supplemental Material
This article contains supplemental material located online at www.asjopenforum.com.
Disclosures
Dr Few is a consultant and investigator for Merz (Raleigh, NC), 3-D Matrix (Tokyo, Japan), and Allergan (Irvine, CA); a consultant for Revance (Nashville, TN); a part owner of Aforé, LLC (Chicago, IL); and holds stock in Venus Concept (Toronto, Canada), Sciton (Palo Alto, CA), and Revance. Mr Semersky is a part owner of Aforé, LLC. Dr Talati is an employee of Goop Inc. (Santa Monica, CA). The remaining authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
Funding for this research was provided by Goop Inc. (Santa Monica, CA) and Aforé, LLC (Chicago, IL).
REFERENCES
- 1.Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27(5):729–738. doi: 10.1177/0963689717725755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC. Skin anti-aging strategies. Dermatoendocrinol. 2012;4(3):308–319. doi: 10.4161/derm.22804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guinot C, Malvy DJ, Ambroisine L, et al. Relative contribution of intrinsic vs extrinsic factors to skin aging as determined by a validated skin age score. Arch Dermatol. 2002;138(11):1454–1460. doi: 10.1001/archderm.138.11.1454 [DOI] [PubMed] [Google Scholar]
- 4.Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5(1):a015370. doi: 10.1101/cshperspect.a015370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphrey S, Manson Brown S, Cross SJ, Mehta R. Defining skin quality: clinical relevance, terminology, and assessment. Dermatol Surg. 2021;47(7):974–981. doi: 10.1097/DSS.0000000000003079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etcoff NL. Survival of the Prettiest: The Science of Beauty. Anchor Books; 2000. [Google Scholar]
- 7.Jain R, Huang P, Ferraz RM. A new tool to improve delivery of patient-engaged care and satisfaction in facial treatments: the Aesthetic Global Ranking Scale. J Cosmet Dermatol. 2017;16(1):132–143. doi: 10.1111/jocd.12297 [DOI] [PubMed] [Google Scholar]
- 8.Few J, Lee MJ, Semersky A, Mariscal E, Vachon G. A single-center study evaluating the effects of a novel retinol and cannabidiol combination topical on facial skin. Aesthet Surg J Open Forum. 2022;4:ojac002. doi: 10.1093/asjof/ojac002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guéniche A, Bastien P, Ovigne JM, et al. Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp Dermatol. 2010;19(8):e1–e8. doi: 10.1111/j.1600-0625.2009.00932.x [DOI] [PubMed] [Google Scholar]
- 10.Moro SDS, de Oliveira Fujii L, Teodoro LFR, et al. Acmella oleracea extract increases collagen content and organization in partially transected tendons. Microsc Res Tech. 2021;84(11):2588–2597. doi: 10.1002/jemt.23809 [DOI] [PubMed] [Google Scholar]
- 11.Rondanelli M, Fossari F, Vecchio V, et al. Acmella oleracea for pain management. Fitoterapia. 2020;140:104419. doi: 10.1016/j.fitote.2019.104419 [DOI] [PubMed] [Google Scholar]
- 12.Ambati RR, Phang SM, Ravi S, Aswathanarayana RG. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Mar Drugs. 2014;12(1):128–152. doi: 10.3390/md12010128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tominaga K, Hongo N, Karato M, Yamashita E. Cosmetic benefits of astaxanthin on human subjects. Acta Biochim Pol. 2012;59(1):43–47. doi: 10.18388/abp.2012_2168 [DOI] [PubMed] [Google Scholar]
- 14.Fan JY, Yi T, Sze-To CM, et al. A systematic review of the botanical, phytochemical and pharmacological profile of Dracaena cochinchinensis, a plant source of the ethnomedicine “dragon's Blood”. Molecules. 2014;19(7):10650–10669. doi: 10.3390/molecules190710650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Namjoyan F, Kiashi F, Moosavi ZB, Saffari F, Makhmalzadeh BS. Efficacy of dragon's blood cream on wound healing: a randomized, double-blind, placebo-controlled clinical trial. J Tradit Complement Med. 2015;6(1):37–40. doi: 10.1016/j.jtcme.2014.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaspar LR, Camargo FB Jr, Gianeti MD, Maia Campos PM. Evaluation of dermatological effects of cosmetic formulations containing Saccharomyces cerevisiae extract and vitamins. Food Chem Toxicol. 2008; 46(11):3493–3500. doi: 10.1016/j.fct.2008.08.028 [DOI] [PubMed] [Google Scholar]
- 17.Falla TJ, Zhang L. Efficacy of hexapeptide-7 on menopausal skin. J Drugs Dermatol. 2010;9(1):49–54. [PubMed] [Google Scholar]
- 18.Uchida Y, Park K. Ceramides in skin health and disease: an update. Am J Clin Dermatol. 2021;22(6):853–866. doi: 10.1007/s40257-021-00619-2 [DOI] [PubMed] [Google Scholar]
- 19.Coderch L, López O, de la Maza A, Parra JL. Ceramides and skin function. Am J Clin Dermatol. 2003;4(2):107–129. doi: 10.2165/00128071-200304020-00004 [DOI] [PubMed] [Google Scholar]
- 20.Bissett DL, Oblong JE, Berge CA. Niacinamide: a B vitamin that improves aging facial skin appearance. Dermatol Surg. 2005;31(7pt 2):860–865. doi: 10.1111/j.1524-4725.2005.31732 [DOI] [PubMed] [Google Scholar]
- 21.Wohlrab J, Kreft D. Niacinamide—mechanisms of action and its topical use in dermatology. Skin Pharmacol Physiol. 2014;27(6):311–315. doi: 10.1159/000359974 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.