Abstract
Background and Purpose
Over-sedation may confound neurologic assessment in critically ill neurologic patients and prolong duration of mechanical ventilation (MV). Decreased sedative use may facilitate early functional independence when combined with early mobility. The objective of this study was to evaluate the impact of a stepwise, multidisciplinary analgesia-first sedation pathway and early mobility protocol on medication use and mobility in the neuroscience intensive care unit (ICU).
Methods
We performed a single-center prospective cohort study with adult patients admitted to a neuroscience ICU between March and June 2016-2018 who required MV for greater than 48 hours. Patients were included from three separate phases of the study: Phase I – historical controls (2016); Phase II – analgesia-first pathway (2017); Phase III – early mobility protocol (2018). Primary outcomes included propofol requirements during MV, total rehabilitation therapy provided, and functional mobility during ICU admission.
Results
156 patients were included in the analysis. Decreasing propofol exposure was observed during Phase I, II, and III (median 2243.7 mg/day vs 2065.6 mg/day vs 1360.8 mg/day, respectively; P = .04 between Phase I and III). Early mobility was provided in 59.7%, 40%, and 81.6% of patients while admitted to the ICU in Phase I, II, and III, respectively (P < .01). An increased proportion of patients in Phase III were walking or ambulating at ICU discharge (26.7%; 8/30) compared to Phase I (7.9%, 3/38, P = .05).
Conclusions
An interdisciplinary approach with an analgesia-first sedation pathway with early mobility protocol was associated with less sedative use, increased rehabilitation therapy, and improved functional mobility status at ICU discharge.
Keywords: sedation, analgesia, analgosedation, early mobility, neurocritical care
Introduction
Critically ill neurologic patients may require prolonged analgesia and sedation for endotracheal intubation and mechanical ventilation (MV), management of elevated intracranial pressure (ICP), and control of pain, agitation, and delirium. 1 Over-sedation may confound neurological examination, increase need for neuroimaging, delay emergence of consciousness, contribute to muscle atrophy, cause hemodynamic instability, and prolong duration of MV.1,2 In contrast, under-sedation can contribute to agitation, distress, elevated ICP, MV dyssynchrony, and accidental removal of intravenous lines, drains, or endotracheal tubes. 1 A multicenter survey of neuroscience intensive care units (ICUs) found titration of sedatives to a sedation scale goal was rarely performed and increased need for pain and delirium assessments. 3 Additionally, early mobility in critically ill stroke patients may facilitate early functional independence.4,5 Balancing sedation needs for critically ill neurologic patients with early mobility is critical to optimizing patient outcomes.
The Society of Critical Care Medicine recommends a protocolized, assessment-driven approach to pain and sedation management in critically ill patients. 6 An analgosedation approach may lead to reduced sedative requirements and pain intensity along with decreased duration of MV and ICU length of stay, though significant differences in outcomes is with mixed results.6–8 While patients admitted to medical and surgical ICUs have been shown to derive benefit in an analgosedation approach, critically ill neurologic patients have traditionally been underrepresented. Furthermore, implementation of early mobility in medical and surgical ICUs has been associated with decreased delirium and duration of MV and improved muscle strength at surgical and medical ICU discharge.9–11
A quality improvement task force was established in 2016 to optimize sedation practices in the Brigham and Women’s Hospital (BWH) neuroscience ICU (Boston, MA, USA), which provides primary critical care of neurology and neurosurgery patients. The task force implemented an analgesia-first sedation pathway with a stepwise approach to pain, agitation, and delirium management and provided enhanced provider education to optimize pharmacotherapy management of critically ill neurologic patients requiring MV. Subsequently, early ICU mobility in conjunction with physical therapy (PT) was added as a component of multidisciplinary care for patients. The objective of this study was to evaluate the impact of this stepwise, multidisciplinary implementation of an education-driven analgesia-first sedation pathway and early mobility protocol in neuroscience ICU patients on medication use and functional outcomes.
Methods
A single-center, prospective cohort study with retrospective controls was performed using patients admitted to the neuroscience ICU at BWH, a tertiary academic medical center, designated Level I Trauma Center, and Comprehensive Stroke Center. Patients were included in the study if they underwent MV for greater than 48 hours and received intensive care exclusively in the neuroscience ICU. Patients were excluded if they were admitted to another ICU for greater than 24 hours prior to neuroscience ICU admission to reduce confounding of unit-specific sedation practices. The Phase I cohort represented retrospective controls prior to implementation of an analgesia-first pathway and included patients admitted between March 15, 2016 and June 15, 2016. Phase I cohort was retrospectively evaluated. The phase II cohort represented patients treated between March 15, 2017 and June 15, 2017, after the analgesia-first pathway was approved and unit-based clinician education was provided. Phase II cohort was prospectively followed. The phase III cohort represented patients admitted between March 12, 2018 and June 12, 2018, after an early mobility protocol was implemented and utilized by PT and nursing staff in routine patient care. Phase III cohort was prospectively followed. Three identical 90-day periods were selected across all phases to minimize confounding effects of rotating trainees, additional new staff training, and major holidays. The BWH institutional review board approved this protocol prior to initiation as a quality improvement initiative with waiver of patient consent.
An analgesia-first pathway (eFigure1) was implemented in August 2016 and included assessment of pain, level of sedation, and development of delirium. Presence of severe pain was evaluated using the critical care pain observation tool (CPOT), level of sedation was evaluated using the Richmond Agitation-Sedation Scale (RASS) score, and delirium was assessed using the Confusion Assessment Method for the ICU (CAM-ICU), each of which were documented in the electronic medical record. 6 Eligibility for a spontaneous awakening trial was evaluated daily by the patient’s provider and Registered Nurse (RN). In-person education of the provider team was conducted prior to initiation of the analgesia-first sedation pathway and routinely thereafter by the neurocritical care attending, fellow, or pharmacist. Education was provided through formal educational conferences, informal teaching while performing daily bedside rounding, and as needed to support individual patient care plans.
An early mobility protocol was developed to compliment the initial analgesia-first pathway after resource allocation allowed for increased PT services for patients with decreased sedation use (eFigure2). The early mobility protocol was initiated for patients intubated for at least 48 hours, with a target goal for patients to perform early mobility twice daily. Early mobility was led by a physical therapist, often with assistance from the RN, to the highest level of functional mobility as possible. To facilitate active patient participation, sedation was decreased to the lowest doses tolerated while maintaining patient safety. During early mobility, patients were progressed through functional positions based on the Johns Hopkins Highest Level of Mobility (JH-HLM) scale according to a formalized protocol, see eFigure2. 12 Patients requiring medical paralysis with continuous intravenous neuromuscular blocking agents, undergoing burst suppression therapy, or sustained ICPs > 25 mmHg were deferred early mobility services until resolved. Potential strategies to address barriers to performing early mobility, such as moderate or deep sedation during analgosedation therapy or patient safety, were discussed during multidisciplinary team rounds.
Outcome Measures
The primary outcomes included propofol use per day of MV, the amount of early mobility provided, and functional mobility outcomes during admission to the neuroscience ICU. Early mobility provided was expressed as number of patients included, time to initiation of early mobility, number of patients seen, number of sessions, and amount of time. Functional outcomes were evaluated in survivors to hospital discharge and expressed as change in JH-HLM from initial early mobility session to ICU and hospital discharge and total number of patients standing/walking on ICU discharge. 12 Data was collected from the electronic medical record with sedative and opioid exposure captured by medication administration record documentation and mobility information captured in daily PT documentation.
Secondary outcome measures included opioid medication utilization, ICU and hospital length of stay, duration of MV, reintubation rates, and adverse events related to early mobility. We additionally evaluated medication utilization of adjunct sedative agents, non-opioid analgesics, and antipsychotic medications during MV. For adverse effects related to propofol infusion, we evaluated the incidence of patients with a creatinine kinase elevation > 500 U/L, serum triglyceride > 400 mg/dL, amylase and lipase > three times upper limit normal (>330 U/L and >240 U/L, respectively). Presence of severe pain (CPOT >2) and persistent pain (two consecutive assessments reporting CPOT >2) after initiation of early mobility was evaluated in Phase III. Adverse events were captured through electronic medical record documentation and related to early mobility including falls, unplanned extubation or airway compromise, sustained deterioration to respiratory status, loss of critical lines (including external ventricular drains, central lines, percutaneous endoscopic gastrostomy tubes, and urinary catheters), cardiopulmonary arrest or seizures.
Data Analysis
Statistical analyses was performed using Wilcoxon rank sum test to compare nonparametric continuous outcomes between Phase I, Phase II, and Phase III study periods. Chi-square and Fisher exact test were used to compare categorical data between Phase I, Phase II, and Phase III study periods as appropriate. A two-sided P-value <.05 was considered statistically significant. Multivariate stepwise forward logistic regression analysis was performed to assess whether implementation of an analgesia-first and early mobility protocol was associated with improved outcomes after adjustment for baseline covariates (including sex, race, acute physiology and chronic health evaluation II [APACHE II] score, admission diagnosis, and baseline level of function). The APACHE II score is calculated based on patient age, physiologic measurements (temperature, heart rate, respiratory rate, blood, mean arterial pressure, pH, sodium, potassium, creatinine, hematocrit, which blood cells, glasgow coma score, FiO2), and presence of baseline severe organ dysfunction, immunocompromised state, or acute renal failure. All statistical analyses were perform using JMP®, Version 13.0. SAS Institute, Inc., Cary, NC, 1989-1921.
Results
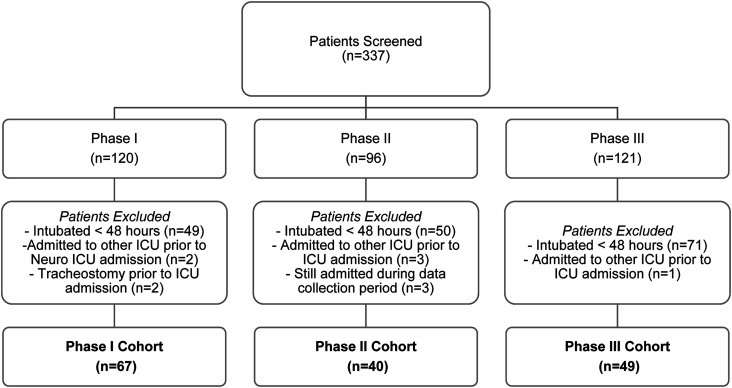
Of the 337 patients evaluated, 156 patients met study inclusion criteria for Phase I (n = 67), Phase II (n = 40), or Phase III (n = 49) cohorts, Figure 1. Median age was 63.4 years [52.2-74.0] and 42.3% (66/156) of patients were female, Table 1. Ninety-one (58.3%) patients were admitted to the neurology primary service and 65 (42.7%) admitted to the neurosurgical primary service. Patient age, gender, race, were similar between Phase I, II, and III cohorts. Most patients (81.4%; 127/156) were independent at baseline prior to admission to the neuroscience ICU. Baseline acute physiology and chronic health evaluation (APACHE) score was a median of 17 [interquartile range (IQR) 13, 21], 15 [13, 20], and 17 [14, 21] in Phase I, II, and III, respectively, P = .75.
Figure 1.
Methods.
Table 1.
Baseline Characteristics.
| Characteristic a | Phase I (n = 67) | Phase II (n = 40) | Phase III (n = 49) | P-value |
|---|---|---|---|---|
| Age, years | 61.1 [50.8, 73.8] | 64.4 [53.7, 73.5] | 63.4 [52.4, 74.4] | .91 |
| Female sex | 34 (50.7) | 16 (40.0) | 16 (32.7) | .14 |
| Race | .53 | |||
| Caucasian | 53 (79.1) | 30 (75.0) | 39 (79.6) | |
| African American | 10 (14.9) | 3 (7.5) | 5 (10.2) | |
| Asian | 1 (1.5) | 3 (7.5) | 1 (2.0) | |
| Other | 1 (1.5) | 3 (7.5) | 3 (6.1) | |
| Unknown | 2 (3.0) | 1 (2.5) | 1 (2.0) | |
| Diagnosis | .05 | |||
| Seizures/status epilepticus | 16 (23.8) | 2 (5.0) | 7 (14.3) | |
| Ischemic stroke | 14 (20.9) | 10 (25.0) | 6 (12.2) | |
| Intracerebral hemorrhage | 9 (13.4) | 11 (27.5) | 12 (24.5) | |
| Intracranial mass | 8 (11.9) | 3 (7.5) | 8 (16.3) | |
| Subarachnoid hemorrhage | 8 (11.9) | 1 (25.0) | 3 (6.1) | |
| Traumatic brain injury/trauma | 3 (4.5) | 4 (10.0) | 8 (16.3) | |
| Infectious/inflammatory disorder | 3 (4.5) | 2 (5.0) | 2 (4.1) | |
| Subdural hemorrhage | 2 (3.0) | 4 (10.0) | 0 (0) | |
| Neuromuscular disease | 1 (1.5) | 0 (0) | 2 (4.1) | |
| Other | 3 (4.5) | 3 (7.5) | 0 (0) | |
| APACHE II score | 17 [13, 21] | 15 [13, 20] | 17 [14, 21] | .75 |
| Baseline level of function | .57 | |||
| Independent | 49 (83.1) | 34 (85.0) | 44 (89.8) | |
| Household mobility without assist | 3 (5.1) | 1 (2.5) | 2 (4.1) | |
| Limited household mobility with assist | 3 (5.1) | 3 (7.5) | 0 (0) | |
| Primarily wheelchair, transfer with assist | 3 (5.1) | 1 (2.5) | 2 (4.1) | |
| Limited household mobility without assist | 1 (1.7) | 0 (0) | 0 (0) | |
| Primarily wheelchair, transfer without assist | 0 (0) | 0 (0) | 0 (0) | |
| Dependent at baseline | 0 (0) | 1 (2.5) | 0 (0) | |
| Unknown | 8 (11.9) | 0 (0) | 1 (2.0) |
aData presented as number (%) or median [interquartile range].
Abbreviations: APACHE-acute physiology and chronic health evaluation.
Implementation of an analgesia-first sedation pathway guided by educational in-service trainings alone did not result in a significant reduction in propofol or opioid exposure during MV between Phase I and Phase II but was associated with a higher proportion of patients administered adjunct acetaminophen, continuous opioid infusions, and antipsychotic therapy, Table 2. These differences were no longer evident after implementation of an early mobility protocol in Phase III. A significant decrease in propofol dose per day of MV was observed following early mobility protocol implementation. An increase in dexmedetomidine use was also observed in Phase III when compared to Phase II and Phase I (42.9% vs 10% vs 3%, respectively; P < .01). A 1.5 to 1.8-fold increase in patient sedation assessments per day of MV was observed in Phase III compared to Phase II and Phase I, respectively. While no difference in analgesic exposure was observed after early mobility protocol implementation, the early mobility protocol was associated with a 30% increase in patient pain assessments per day of MV.
Table 2.
Pain, Agitation, and Delirium Management.
| Characteristic a | Phase I (n = 67) | Phase II (n = 40) | Phase III (n = 49) | P-value All cohorts | Phase I vs II | Phase II vs III | Phase I vs III |
|---|---|---|---|---|---|---|---|
| Sedation Management | |||||||
| Number of RASS assessments | 2088 | 1233 | 2582 | - | - | - | - |
| RASS Score | -3 [-4, -1] | -3 [-4, -2] | -3 [-4, -1] | .50 | .33 | .99 | .32 |
| RASS assessment/days of MV | 4.8 [3.7, 4.8] | 4.1 [3.6, 5.5] | 7.4 [5.3, 8.9] | <.01 | .53 | <.01 | <.01 |
| Sedative administration | |||||||
| Propofol utilization | 67 (100) | 40 (100) | 47 (96.0) | .16 | 1 | .50 | .18 |
| Midazolam CIVI | 12 (17.9) | 5 (20) | 6 (12.2) | .63 | .46 | 1 | .41 |
| Dexmedetomidine | 2 (3.0) | 4 (10.0) | 21 (42.9) | <.01 | .19 | <.01 | <.01 |
| Sedative exposure | |||||||
| Propofol dose per days of MV, mg | 2243.7 [1285.1, 3417.7] | 2065.6 [1197.7, 3824.6] | 1360.8 [740.7, 3013.0] | .07 | .93 | .05 | .04 |
| Midazolam dose per days of MV, mg | 5.6 [.4, 86.7] | 8.1 [.9, 90.8] | 1.2 [.3, 12.2] | .38 | .70 | .31 | .21 |
| Duration of sedation | |||||||
| Propofol, days | 3.0 [1.7, 6.5] | 2.4 [1.7, 4.1] | 2.5 [1.6, 5.8] | .56 | .32 | .40 | .92 |
| Midazolam CIVI, hours | 57.3 [8.4, 117.6] | 78 [22.8, 229.3] | .9 [.05, 3.3] | <.01 | .64 | <.01 | <.01 |
| Dexmedetomidine, hours | 8.1 [7.1, 9] | 40.9 [8.7, 67.6] | 2.6 [1.2, 5.6] | .008 | .49 | <.01 | .07 |
| Pain Management | |||||||
| Number of CPOT assessments | 3015 | 1762 | 2860 | - | - | - | - |
| CPOT assessments per days of MV | 6.7 [4.7, 9.2] | 6.4 [5.5, 7.7] | 8.3 [7.4, 9.4] | <.01 | .95 | <.01 | <.01 |
| CPOT score > 2 | 290 (9.6) | 177 (10.0) | 415 (14.5) | <.01 | .63 | <.01 | <.01 |
| Patients with CPOT score > 2 | 49 (73.1) | 35 (87.5) | 44 (89.8) | .04 | .08 | .75 | .03 |
| Analgesic administration | |||||||
| Opioid | 52 (77.6) | 36 (90) | 43 (87.8) | .16 | .12 | 1 | .22 |
| Opioid CIVI | 22 (32.8) | 22 (55) | 16 (32.7) | .04 | .04 | .06 | .86 |
| Acetaminophen | 57 (85.1) | 40 (100) | 47 (96.0) | <.01 | .01 | .50 | .07 |
| NSAID | 1 (1.5) | 0 (0) | 2 (4.1) | .47 | 1 | .50 | .57 |
| Gabapentin | 5 (7.5) | 2 (5) | 0 (0) | .13 | .71 | .20 | .07 |
| Analgesic exposure | |||||||
| Opioid oral MME per days of MV, mg | 11.5 [2.1, 54.3] | 21.1 [4.2, 153.04] | 17.7 [7.4, 41.6] | .19 | .10 | .13 | .60 |
| Acetaminophen, mg | 4550 [1950, 10 400] | 5200 [1950, 7800] | 5850 [3250, 13 325] | .45 | .97 | .27 | .27 |
| NSAID, mg | 2000 | 0 | 4000 [400, 7600] | 1 | - | - | 1 |
| Gabapentin, mg | 4800 [900, 12 000] | 4250 [3725, 4775] | 0 | 1 | 1 | - | - |
| Delirium Management | |||||||
| Number of CAM-ICU assessments | 949 | 516 | 808 | - | - | - | - |
| CAM-ICU assessments per days of MV | 2.2 [1.4, 2.9] | 1.9 [1.1, 2.9] | 1.0 [1.8, 5] | .50 | .18 | .52 | .93 |
| Patients with CAM-ICU positive | 20 (29.9) | 8 (20.0) | 12 (24.5) | .52 | .26 | .62 | .52 |
| Antipsychotic administration | 21 (31.3) | 19 (47.5) | 15 (30.6) | .17 | .09 | .13 | 1 |
| Duration of antipsychotic use, days | 6.5 [0-16.2] | 4.5 [1.4-9.7] | 7.3 [1.8-12.3] | .73 | .71 | .47 | .60 |
| Quetiapine | 13 (19.4) | 16 (40) | 12 (24.5) | .06 | .02 | .12 | .51 |
| Haloperidol | 12 (17.9) | 8 (20) | 8 (16.3) | .90 | .79 | .65 | .82 |
| Olanzapine | 3 (4.5) | 0 (0) | 4 (8.3) | .20 | .29 | .12 | .45 |
| Other | 3 (4.5) | 0 (0) | 0 (0) | .26 | .29 | 1 | .26 |
aData presented as number (%) or median [interquartile range].
Abbreviations: CIVI – continuous intravenous infusion; MME – milligram morphine equivalents; MV – mechanical ventilation; NSAID – non-steroidal anti-inflammatory drugs; RASS – richmond agitation sedation scale.
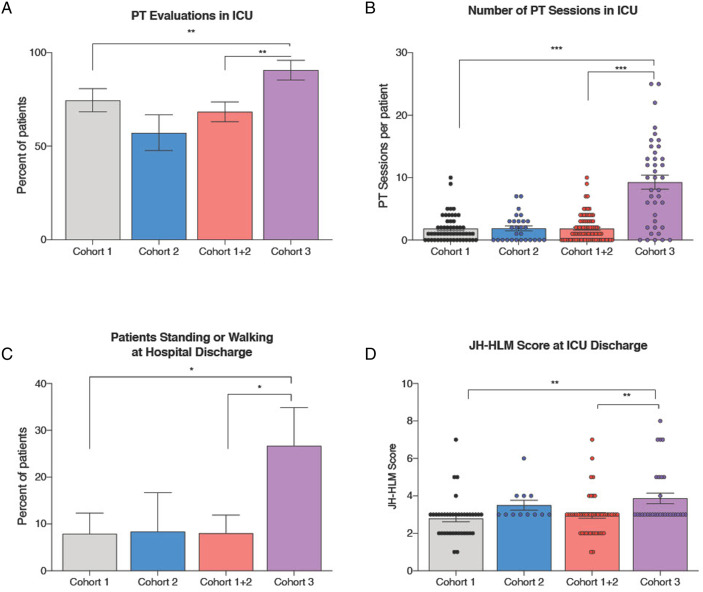
Implementation of the early mobility protocol resulted in an increase in the proportion of patients who received early mobility during ICU admission in Phase III (81.6%; 40/49) compared to Phase II (40%; 16/40) and Phase I (59.7%; 40/67), P < .01 (Table 3, Figure 2). Median days from neuroscience ICU admission to early mobility was reduced by 52% from 7.3 days in Phase I to 3.5 days in Phase III. The median number of times a patient received early mobility intervention in the ICU per patient increased to 8 [2-14] in Phase III compared to 0 [0-3] in Phase II and 1 [0-2] in Phase I, P < .01. An increase in duration of mobility sessions performed during ICU admission was observed between Phase I (median 39 minutes), Phase II (median 60 minutes) and Phase III (median 210 minutes).
Table 3.
Clinical Outcome Measures.
| Characteristic a | Phase I (n = 67) | Phase II (n = 40) | Phase III (n = 49) | P-value All cohorts | Phase I vs II | Phase II vs III | Phase I vs III |
|---|---|---|---|---|---|---|---|
| ICU length of stay, days | 9.5 [6.5, 17.5] | 10.0 [5.3, 13.1] | 12.0 [5.4, 20.1] | .46 | .59 | .22 | .42 |
| Hospital length of stay, days | 15.1 [9.7, 23.1] | 17.1 [8.3, 23.8] | 18.1 [10.8, 25.8] | .53 | .81 | .47 | .27 |
| In-hospital mortality | 12 (17.9) | 12 (30.0) | 10 (20.4) | .34 | .16 | .30 | .74 |
| Duration of MV, days | 5.3 [3.3, 8.5] | 5.1 [3.8, 7.4] | 6.0 [3.3, 10.6] | .91 | .96 | .70 | .74 |
| Re-intubation | 18 (26.9) | 5 (12.5) | 14 (28.6) | .15 | .08 | .07 | .84 |
| Vasopressor use | 36 (53.7) | 22 (55) | 25 (51) | .93 | .89 | .71 | .78 |
| Laboratory Outcomes | |||||||
| Amylase > 330 U/L | 0 (0) | 0 (0) | 0 (0) | - | - | - | - |
| Lipase > 240 U/L | 2 (3.0) | 1 (2.5) | 1 (2.0) | 1 | 1 | 1 | 1 |
| Serum triglyceride > 400 mg/dL | 4 (6.0) | 1 (2.5) | 0 (0) | .17 | .64 | .45 | .14 |
| Creatinine kinase > 500 U/L | 7 (10.5) | 2 (5.0) | 4 (8.2) | .66 | .48 | .69 | .76 |
| Physical Therapy while Admitted to the Intensive Care Unit | |||||||
| Number of PT sessions performed | 100 | 52 | 362 | <.01 | .08 | <.01 | .02 |
| Patients who received PT evaluations | 42 (62.7) | 16 (40) | 40 (81.6) | <.01 | .02 | <.01 | .03 |
| Time from ICU admission to PT evaluation, days | 7.3 [2.7, 13.7] | 7.7 [6.0, 12.5] | 3.5 [2.3, 5.6] | <.01 | .28 | <.01 | <.01 |
| PT sessions performed/patient | 1 [0, 2] | 0 [0, 3] | 8 [2, 14] | <.01 | .21 | <.01 | <.01 |
| Percentage of goal PT sessions met/patient | 3.8 [0, 8] | 0 [ 0, 10.6] | 32.1 [9.5, 41.4] | <.01 | .35 | <.01 | <.01 |
| Duration of PT therapy, minutes | 39 [20, 70] | 60 [50, 84] | 210 [120, 315] | <.01 | .01 | <.01 | <.01 |
| Early termination of Early Mobility Program (Phase III Only) | |||||||
| Elevated ICP | - | - | 5 (10.2) | - | - | - | - |
| Hemodynamic instability | - | - | 1 (2.0) | - | - | - | - |
| Difficulty with positioning while sitting | - | - | 2 (4.1) | - | - | - | - |
aData presented as number (%) or median [interquartile range].
Abbreviations: ICU- intensive care unit; MV – mechanical ventilation; PT - Physical Therapy.
Figure 2.
Significant Increase in Mobility at ICU Discharge in Patients who Survived to Hospital Discharge. After implementation of an early mobility protocol in the neuroscience intensive care unit (ICU), a significant increase in physical therapy evaluations performed while patient was admitted in the ICU (Figure 2a), number of physical therapy sessions during ICU admission (Figure 2b) was observed. This translated into increased in the proportion of patients standing or walking at hospital discharge (Figure 2c) and JH-HLM Scores are ICU discharge (Figure 2d). * denotes P-value <.05; ** denotes P-value <.01; *** denotes P-value <.001.
A higher proportion of patients in Phase III achieved JH-HLM score > 5, indicating ability to ambulate or walk, on ICU discharge (26.7%; 8/30) compared to Phase II (8.3%; 1/12, P = .25) and Phase I (7.9%; 3/38, P = .05). In patients who survived to hospital discharge and were evaluated for JH-HLM at both initial early mobility session and ICU discharge, 10.8% (4/37) of patients improved by > 2 points on the scale in Phase I, compared to 16.7% (2/12) in Phase II, and 26.7% (8/30) in Phase III. On multivariate analysis adjusting for baseline covariates and severity, admission after implementation of both an analgesia-first sedation pathway and early mobility protocol (Phase III) remained a significant predictor of propofol and opioid exposure during MV (P = .0392 and .0005, respectively) along with admission diagnosis but not JH-HLM score > 5.
Implementation of an analgesia-first sedation pathway and early mobility protocol was not associated with decreased ICU or hospital length of stay, duration of MV, or incidence of delirium in patients who were mechanically ventilated for > 48 hours. In Phase III, after implementation of an early mobility protocol, presence of severe pain was recorded in 11 patients the day prior to first PT evaluation and 16 patients the day of first PT evaluation. Persistent pain was reported in 5 patients the day prior to first PT evaluation and 5 patients on the day of first PT evaluation. There was no difference in the incidence of laboratory abnormalities associated with propofol exposure amongst cohorts. No adverse events related to early mobility were observed.
Discussion
Only the combined multidisciplinary approach to goal-directed therapy targeting pain management, decreased sedation, and participation in an early mobility protocol was associated with decreased propofol exposure during MV, increased early mobility, and increased mobility at ICU discharge. The combined analgosedation pathway and early mobility protocol was safe across a wide range of critically ill neurologic patients. Implementing an analgosedation pathway with multidisciplinary education alone was ineffective at decreasing sedation during the Phase II study period. However, the addition of PT for early mobility interventions necessitating decreased sedation was associated with an increased effect of the analgosedation approach during the Phase III study period.
Reduced sedation exposure has been associated with improved outcomes, including decreased duration of MV, hospital length of stay, and early mobility, in the general ICU patient population. 13 Patients with acute brain injury have traditionally been excluded from studies evaluating lightened sedation protocols, due to concern for exacerbation of intracranial hypertension and presumed need for deep sedation. 13 This has led to a significant gap in literature surrounding the safety and efficacy of analgosedation protocols in the broad critically ill neurologic patient population. Sedative utilization must be carefully balanced in patients with acute brain injury as excess sedation may be neurotoxic, exacerbate hemodynamic instability inducing secondary brain injury and confound the neurologic examination.14,15 In patients with impaired autoregulation, significant hypotension induced by sedative agents may result in a decrease in cerebral perfusion pressure and oxygen delivery compounding brain tissue ischemia and hypoxia. 13 The hemodynamic effects of sedative agents are often dose-dependent; therefore, a reduction in sedative exposure may result in improved hemodynamic stability during acute brain injury. Propofol has been observed in pre-clinical studies to induce impairment of neuronal mitochondrial function resulting in neurotoxicity and neuronal dysfunction while midazolam has been associated with increased risk of delirium and prolonged duration of MV.16–18 We observed increased utilization of dexmedetomidine, which has been shown in clinical trials to facilitate improved communication of pain compared to midazolam and propofol. 19 This may in turn translate to improved ability for clinicians to perform neurologic examinations and accurately assess neurologic deficits. Our multidisciplinary approach was able to significantly decrease median daily propofol exposure during MV without increasing midazolam or dexmedetomidine exposure during the study period.
While select critically ill neurologic patients may benefit from deep sedation (eg status epilepticus, refractory intracranial hypertension), this strategy in patients otherwise eligible for lightened sedation may mask untreated pain that is commonly present in patients with neurologic injury. Adequate analgesia has been associated with reduced agitation, duration of MV, nosocomial infections, and pulmonary complications. 20 During the study period, an increase in pain assessments was observed and increased identification of patients reporting severe pain during MV. We suspect that reduced sedation enhanced recognition of the presence of pain, allowing appropriate non-pharmacological and pharmacologic interventions with opioid and non-opioid therapies.
The multidisciplinary approach to early mobility in the neuroscience ICU facilitated an increase in early mobility interventions provided. A significant increase was observed in early mobility interventions provided, length of time from hospital admission to onset of early mobility, and number of minutes of early mobility intervention. Early mobility in critically ill neurologic patients has been associated with increased home discharge, decreased mortality, and improved functional mobility.21–23 Improvements in perceived functional status, or functional independence using the Barthel Index have also been observed.9,11 Other benefits of early mobility in the ICU include decreased length stay, decreased incidence of ICU delirium, shortened time of MV, and decreased need for post-acute care services.32–37 Previous studies have excluded patients who were non-verbal, unable to follow commands, suffered baseline cognitive impairment, or had inadequate baseline functional status from early mobility protocols.8,24–31,33,38,39 Early mobility studies in the critical care setting often exclude patients with neurologic injuries such as stroke, seizures and/or status epilepticus, spinal cord injury, and baseline neuromuscular diseases.8,9,11,33,35,38–40 The AVERT trial has provided needed insight into the effects of early mobility within 24-hours, highlighting risks of very early mobilization following stroke, but demonstrating benefits of shorter more frequent mobilization on favorable outcome at 12 months. 38 In our study, functional outcomes were improved in patients after implementation of an early mobility protocol with analgesia-first sedation compared to retrospective controls on ICU discharge, however JH-HLM outcomes on hospital discharge were not observed. One possible explanation why functional improvement in the ICU did not translate to improved functional status at hospital discharge is that the early mobility protocol may have been discontinued once transferring to the step-down floor. If the dosage of therapy consistently remained higher, its possible the functional outcomes at hospital discharge would have also improved.
Our early mobility protocol contained less exclusion criteria than many published protocols. Our protocol relied on communication within a team of multidisciplinary experts in their discipline in neurology and critical care to assess medical stability for mobility. We did not have a step in our protocol consisting of only bed level activities. Even with a more aggressive protocol, we were able to successfully perform 44% of intended PT sessions with the lowest level of early mobility involving sitting at the edge of the bed. No adverse events related to early mobility were observed. Occasionally, early mobility was stopped before goal due to changes in ICP or hemodynamics, to maintain patient safety. All patients were returned to pre-session baseline neurologic function shortly after termination of early mobility.
Limitations
Due to the heterogenous nature of patients admitted to our neuroscience ICU and specified inclusion criteria, we were unable to adjust for baseline diagnoses or comorbidities and sedation exposure. Nevertheless, we believe that this study highlights the generalizability of unit-based protocols to guide sedation, analgesia, and early mobility management across a broad range of neurologic illnesses. Data analysis was reliant on documentation of assessments and activities within the electronic health record, therefore subtle improvements in neurologic examination or pain management were not captured within the RASS and CPOT scoring tools. Commonly used assessment scales in critically ill patients with neurologic injury are limited in their ability to detect pain, agitation, and delirium compared to the general ICU patient population. Due to the nature of the study, assessments and outcome variables were not blinded to authors, and a component of performance bias may be present when sedation weaned in Phase III. Daily sedative exposures may have been confounded by increased therapy sessions but this highlights the influence supplemental adjunct therapy may have on ensuring pharmacotherapy targets are met. Increased use of dexmedetomidine during Phase III may have partly been due to shifting practices over time, with increased available safety data of dexmedetomidine in critically ill neurologic patients during the study period. Implementation of early mobility protocols required increased PT service utilization and nursing workload of increased patient assessments. Institutions considering addition of early mobility protocols into clinical care should ensure infrastructure and personnel are adequate to support this initiative.
Conclusions
Implementation of an analgesia-first sedation pathway guided by educational in-service trainings alone did not result in a significant reduction in propofol or opioid exposure during MV between Phase I and Phase II, but was associated with a higher proportion of patients administered adjunct pharmacotherapy. An interdisciplinary approach with an analgesia-first sedation pathway and early mobility may be associated with less sedative use, and facilitate successful provision of rehabilitation therapy and optimize functional mobility status at neuroscience ICU discharge. Further research is warranted in the neurocritical care patient population on optimal analgosedation and physical therapy treatment strategies to support patient recovery.
Supplemental Material
Supplemental Material for Multidisciplinary Approach to Sedation and Early Mobility of Intubated Critically Ill Neurologic Patients Improves Mobility at Discharge by Megan E. Barra, PharmD, Christine Iracheta, PT, Joseph Tolland, PT, Johnathan Jehle, MSN, AGNP, Ljubica Minova, PharmD, Karen Li, BS, Mary Amatangelo, DNP, Patricia Krause, PharmD, Ayush Batra, MD, and Henrikas Vaitkevicius, MD in The Neurohospitalist
Supplemental Material for Multidisciplinary Approach to Sedation and Early Mobility of Intubated Critically Ill Neurologic Patients Improves Mobility at Discharge by Megan E. Barra, PharmD, Christine Iracheta, PT, Joseph Tolland, PT, Johnathan Jehle, MSN, AGNP, Ljubica Minova, PharmD, Karen Li, BS, Mary Amatangelo, DNP, Patricia Krause, PharmD, Ayush Batra, MD, and Henrikas Vaitkevicius, MD in The Neurohospitalist
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: H.V. and M.B. have no conflicts relevant to this article, this work was conceived and completed during employment at Brigham and Women’s Hospital (H.V. and M.B.) and Massachusetts General Hospital (M.B.), since then, H.V. and M.B. have been employed by Marinus Pharmaceutical Inc. All other authors (C.I., J.T., J.J., L.M., K.L., A.M., P.K., A.B.,) have no conflicts of interest to disclose.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Megan E. Barra https://orcid.org/0000-0002-7696-1540
Ayush Batra https://orcid.org/0000-0002-4783-5801
References
- 1.Lele A, Souter MJ. Sedation practices in the Neurocritical Care Unit. J Neuroanaesth Crit Care. 2016;03:81-87. [Google Scholar]
- 2.Mahmoud L, Zullo AR, Thompson BB, Wendell LC. Outcomes of protocolised analgesia and sedation in a neurocritical care unit. Brain Inj. 2018;32(7):941-947. doi: 10.1080/02699052.2018.1469167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu A, Teitelbaum J, Scott J, et al. Evaluating pain, sedation, and delirium in the neurologically critically ill-feasibility and reliability of standardized tools: A multi-institutional study. Crit Care Med. 2013;41(8):2002-2007. doi: 10.1097/CCM.0b013e31828e96c0 [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Zhang X, Wang K, Wen J. Effects of early mobilization after acute stroke: A meta-analysis of randomized control trials. J Stroke Cerebrovasc Dis. 2018;27(5):1326-1337. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.021 [DOI] [PubMed] [Google Scholar]
- 5.Cumming TB, Churilov L, Collier J, et al. Early mobilization and quality of life after stroke: Findings from AVERT. Neurology. 2019;93(7):e717-e728. doi: 10.1212/WNL.0000000000007937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873. doi: 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 7.Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238-2243. [DOI] [PubMed] [Google Scholar]
- 8.Morris PE, Berry MJ, Files DC, et al. Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: A randomized clinical trial. JAMA. 2016;315(24):2694-2702. doi: 10.1001/jama.2016.7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet. 2009;373(9678):1874-1882. doi: 10.1016/S0140-6736(09)60658-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dantas CM, Silva PFDS, Siqueira FHT de, et al. Influence of early mobilization on respiratory and peripheral muscle strength in critically ill patients. Rev Bras Ter Intensiva. 2012;24(2):173-178. [PubMed] [Google Scholar]
- 11.Burtin C, Clerckx B, Robbeets C, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37(9):2499-2505. doi: 10.1097/CCM.0b013e3181a38937 [DOI] [PubMed] [Google Scholar]
- 12.Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-347. doi: 10.1002/jhm.2546 [DOI] [PubMed] [Google Scholar]
- 13.Oddo M, Crippa IA, Mehta S, et al. Optimizing sedation in patients with acute brain injury. Crit Care. 2016;20(1):128. doi: 10.1186/s13054-016-1294-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts DJ, Hall RI, Kramer AH, Robertson HL, Gallagher CN, Zygun DA. Sedation for critically ill adults with severe traumatic brain injury: A systematic review of randomized controlled trials. Crit Care Med. 2011;39(12):2743-2751. doi: 10.1097/CCM.0b013e318228236f [DOI] [PubMed] [Google Scholar]
- 15.Kvolik S, Koruga N, Skiljic S. Analgesia in the neurosurgical intensive care unit. Front Neurol. 2021;12:819613. doi: 10.3389/fneur.2021.819613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berndt N, Rösner J, Haq RU, et al. Possible neurotoxicity of the anesthetic propofol: evidence for the inhibition of complex II of the respiratory chain in area CA3 of rat hippocampal slices. Arch Toxicol. 2018;92(10):3191-3205. doi: 10.1007/s00204-018-2295-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sebastiani A, Granold M, Ditter A, et al. Posttraumatic Propofol Neurotoxicity Is Mediated via the Pro-Brain-Derived Neurotrophic Factor-p75 Neurotrophin Receptor Pathway in Adult Mice. Crit Care Med. 2016;44(2):e70-82. doi: 10.1097/CCM.0000000000001284 [DOI] [PubMed] [Google Scholar]
- 18.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial. JAMA. 2009;301(5):489-499. doi: 10.1001/jama.2009.56 [DOI] [PubMed] [Google Scholar]
- 19.Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA. 2012;307(11):1151-1160. doi: 10.1001/jama.2012.304 [DOI] [PubMed] [Google Scholar]
- 20.Petzold A, Girbes A. Pain management in neurocritical care. Neurocrit Care. 2013;19(2):232-256. doi: 10.1007/s12028-013-9851-0 [DOI] [PubMed] [Google Scholar]
- 21.Olkowski BF, Shah SO. Early mobilization in the neuro-ICU: How far can we go? Neurocrit Care. 2017;27(1):141-150. doi: 10.1007/s12028-016-0338-7 [DOI] [PubMed] [Google Scholar]
- 22.Mulkey M, Bena JF, Albert NM. Clinical outcomes of patient mobility in a neuroscience intensive care unit. J Neurosci Nurs. 2014;46(3):153-161; quiz E1-2. doi: 10.1097/JNN.0000000000000053 [DOI] [PubMed] [Google Scholar]
- 23.Klein K, Mulkey M, Bena JF, Albert NM. Clinical and psychological effects of early mobilization in patients treated in a neurologic ICU: A comparative study. Crit Care Med. 2015;43(4):865-873. doi: 10.1097/CCM.0000000000000787 [DOI] [PubMed] [Google Scholar]
- 24.Hashem MD, Parker AM, Needham DM. Early mobilization and rehabilitation of patients who are critically ill. Chest. 2016;150(3):722-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: A quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536-542. doi: 10.1016/j.apmr.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 26.Schaller SJ, Anstey M, Blobner M, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet. 2016;388:1377-1388. [DOI] [PubMed] [Google Scholar]
- 27.Stiller K. Physiotherapy in Intensive Care: An Updated Systematic Review. Chest. 2013;144(3):825-847. doi: 10.1378/chest.12-2930 [DOI] [PubMed] [Google Scholar]
- 28.Lai CC, Chou W, Chan KS, et al. Early mobilization reduces duration of mechanical ventilation and intensive care unit stay in patients with acute respiratory failure. Arch Phys Med Rehabil. 2017;98:931-939. [DOI] [PubMed] [Google Scholar]
- 29.Corcoran JR, Herbsman JM, Bushnik T, et al. Early rehabilitation in the medical and surgical intensive care units for patients with and without mechanical ventilation: An interprofessional performance improvement project. PM R. 2017;9:113-119. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt UH, Knecht L, MacIntyre NR. Should early mobilization be routine in mechanically ventilated patients? Respir Care. 2016;61(6):867-875. [DOI] [PubMed] [Google Scholar]
- 31.McWilliams D, Weblin J, Atkins G, et al. Enhancing rehabilitation of mechanically ventilated patients in the intensive care unit: A quality improvement project. J Crit Care. 2015;30:13-18. [DOI] [PubMed] [Google Scholar]
- 32.Hashem MD, Nelliot A, Needham DM. Early mobilization and rehabilitation in the ICU: Moving back to the future. Respir Care. 2016;61(7):971-979. [DOI] [PubMed] [Google Scholar]
- 33.Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238-2243. doi: 10.1097/CCM.0b013e318180b90e [DOI] [PubMed] [Google Scholar]
- 34.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536-542. doi: 10.1016/j.apmr.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 35.Schaller SJ, Anstey M, Blobner M, et al. Early, goal-directed mobilisation in the surgical intensive care unit: A randomised controlled trial. Lancet. 2016;388(10052):1377-1388. doi: 10.1016/S0140-6736(16)31637-3 [DOI] [PubMed] [Google Scholar]
- 36.Stiller K. Physiotherapy in intensive care: An updated systematic review. Chest. 2013;144(3):825-847. doi: 10.1378/chest.12-2930 [DOI] [PubMed] [Google Scholar]
- 37.Schmidt UH, Knecht L, MacIntyre NR. Should early mobilization be routine in mechanically ventilated patients? Respir Care. 2016;61(6):867-875. doi: 10.4187/respcare.04566 [DOI] [PubMed] [Google Scholar]
- 38.AVERT Trial Collaboration group . Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): A randomised controlled trial. Lancet. 2015;386(9988):46-55. doi: 10.1016/S0140-6736(15)60690-0 [DOI] [PubMed] [Google Scholar]
- 39.Hodgson CL, Bailey M, Bellomo R, et al. A binational multicenter pilot feasibility randomized controlled trial of early goal-directed mobilization in the ICU. Crit Care Med. 2016;44(6):1145-1152. doi: 10.1097/CCM.0000000000001643 [DOI] [PubMed] [Google Scholar]
- 40.Thomas K, Wright SE, Watson G, et al. Extra physiotherapy in critical care (EPICC) trial protocol: A randomised controlled trial of intensive versus standard physical rehabilitation therapy in the critically ill. BMJ Open. 2015;5(5):e008035. doi: 10.1136/bmjopen-2015-008035 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Multidisciplinary Approach to Sedation and Early Mobility of Intubated Critically Ill Neurologic Patients Improves Mobility at Discharge by Megan E. Barra, PharmD, Christine Iracheta, PT, Joseph Tolland, PT, Johnathan Jehle, MSN, AGNP, Ljubica Minova, PharmD, Karen Li, BS, Mary Amatangelo, DNP, Patricia Krause, PharmD, Ayush Batra, MD, and Henrikas Vaitkevicius, MD in The Neurohospitalist
Supplemental Material for Multidisciplinary Approach to Sedation and Early Mobility of Intubated Critically Ill Neurologic Patients Improves Mobility at Discharge by Megan E. Barra, PharmD, Christine Iracheta, PT, Joseph Tolland, PT, Johnathan Jehle, MSN, AGNP, Ljubica Minova, PharmD, Karen Li, BS, Mary Amatangelo, DNP, Patricia Krause, PharmD, Ayush Batra, MD, and Henrikas Vaitkevicius, MD in The Neurohospitalist