Abstract
Clobazam is a 1,5-benzodiazepine frequently used as an adjunctive agent for refractory seizures and status epilepticus. Clobazam undergoes metabolism to an active metabolite norclobazam which is subsequently hydroxylated by CYP2C19, a cytochrome with several pharmacogenetic variants. Patients with poor metabolizer phenotypes may have elevated norclobazam levels and subsequent adverse effects. We present a case of an Asian American male receiving clobazam at a standard therapeutic dose for seizure disorder who became comatose secondary to significantly elevated norclobazam concentrations. Genetic testing revealed the patient was a poor CYP2C19 metabolizer, accounting for the impaired clearance. Clinicians should be aware of the patient populations at risk for these genetic polymorphisms and adjust initial doses based on package labeling or consider therapeutic drug monitoring to avoid adverse effects.
Keywords: clobazam, norclobazam, pharmacogenomics, CYP2C19 polymorphism, case report
Introduction
Clobazam is a unique 1,5-benzodiazepine which potentiates GABAergic neurotransmission with increased affinity for the alpha2 subunit of the GABAA receptor. 1 While its only labeled indication is Lennox-Gastaut syndrome, it is frequently used as an adjunctive agent in refractory seizures and status epilepticus given its broad spectrum of action and tolerability. Like other benzodiazepines, clobazam’s primary adverse reaction is somnolence which is primarily driven by its active metabolite, norclobazam. 2 Norclobazam is the product of clobazam’s demethylation by CYP3A4 and is subsequently hydroxylated by CYP2C19, a cytochrome with several pharmacogenomic variants which can substantially alter the pharmacokinetics of norclobazam.3,4 Norclobazam concentrations are typically 3-5 times higher than the parent compound and with a relative potency of 1/5 to approximately equal potency of clobazam, but norclobazam concentrations can be as much as 30-times higher in the setting of poor CYP2C19 metabolism. 5
Clobazam and norclobazam are notable for long half-lives at 36-42 hours and 71-82 hours, respectively. In patients who are poor CYP2C19 metabolizers or who are taking strong CYP2C19 inhibitors, the half-life of norclobazam is further prolonged with an estimated 85% reduction in clearance. 6 Poor metabolizers of CYP2C19 have been noted to have norclobazam levels over seven times higher than extensive metabolizers. 4 While elevated norclobazam levels have been associated with improved anti-seizure activity, 5 they are also associated with excessive sedation, weight gain, and hypersalivation. 7 Because of the prolonged clearance, steady state concentrations may not be achieved for weeks and adverse effects may appear late into therapy. Here we report a case of severe toxic-metabolic encephalopathy caused by reduced clobazam clearance due to a CYP2C19 genetic polymorphism. While coma secondary to clobazam toxicity has been reported, this has been previously in the context of clobazam overdose; our patient was taking a therapeutic dose of clobazam and had a slow decline in mental status over time.8,9
Case Description
We present a 56-year-old male who self-identified as Asian American with a past medical history significant for schizophrenia, hypertension, diabetes, and hypothyroidism. At baseline, the patient lived in a group home and was independent with activities of daily living (ADL). The patient’s schizophrenia had been well-controlled for 10 years with clozapine 600 mg daily; other psychiatric medications included lorazepam 1 mg twice daily, venlafaxine XR 150 mg daily, and trazodone 50 mg at night. Informed consent from the Health Care Proxy was obtained to describe the details of this case report.
Three months prior, the patient was hospitalized for suicidal ideation. The hospital course was complicated by first-time seizure and all antipsychotics were held. Antiseizure medications (ASMs) were initiated, and the patient was discharged to a skilled nursing facility (SNF) on lacosamide 200 mg twice daily, clobazam 10 mg twice daily, and olanzapine 7.5 mg nightly. After admission to the SNF, the patient’s family was concerned about his functional status, noting severe decline in strength and inability to perform basic ADL. They also reported the patient to be significantly below his psychiatric baseline, with flat affect, worsening wakefulness, and monosyllabic responses despite the significantly reduced antipsychotic regimen.
After two months at the SNF, EMS was called after the patient was found down unresponsive. He was noted to be hypotensive (68/40 mmHg) with a GCS of 3 and evidence of tongue biting. On arrival to the emergency department the patient was febrile (38.6°C) and had pinpoint pupils that were non-reactive bilaterally. His GCS remained 3 and the patient was intubated for the inability to protect his airway and admitted to the Medical Intensive Care Unit. The patient’s ASM regimen of clobazam 10 mg twice daily and lacosamide 200 mg twice daily was continued. He was found to have Pseudomonas aeruginosa and Escherichia coli bacteremia and was treated with piperacillin-tazobactam with improvement in his septic shock.
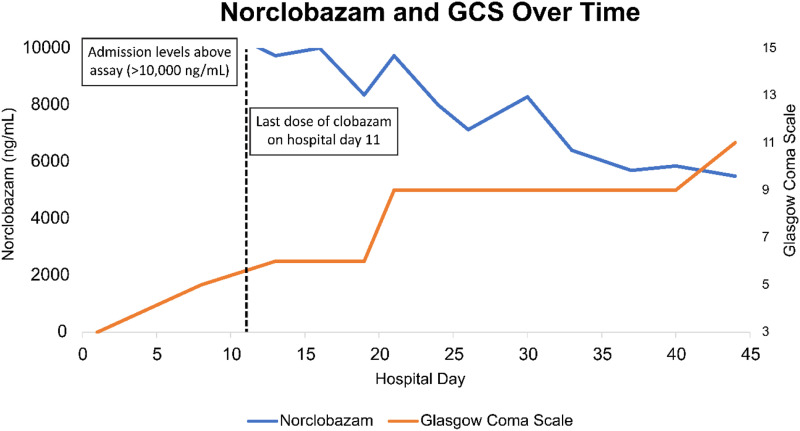
Initial labs (Table 1) were significant for a clobazam concentration of 250 ng/mL (reference range 30-300 ng/mL) with a norclobazam concentration of >10000 ng/mL (reference range 300-3000 ng/mL). Serum levels of clobazam and norclobazam were analyzed by liquid chromatography-tandem mass spectrometry at a reference laboratory and were not available until day 5 of admission. Once elevated norclobazam concentrations were identified, clobazam was reduced to 5 mg twice daily and labs were re-sent on day 8. Despite dose reduction and adequate management of the patient’s bacteremia, his mental status failed to improve and investigation into his persistent encephalopathy was initiated. Magnetic resonance imaging (MRI) demonstrated mild symmetric globus pallidus diffusion restriction suggestive of hypoxic or toxic metabolic derangements and possible vascular inflammatory changes but no acute hemorrhage, mass, or mass effect was noted. On day 11 of admission, repeat clobazam concentrations resulted at 191 ng/mL with norclobazam concentrations remaining greater than assay. Clobazam was discontinued and levels were serially monitored (Figure 1). During this time, continuous electroencephalography (cEEG) revealed disorganized and diffuse delta slowing consistent with toxic metabolic encephalopathy. There was also a background of excess beta activity consistent with benzodiazepine exposure which persisted for weeks despite clobazam discontinuation.
Table 1.
Admission Laboratory Values.
| Laboratory Test | Measured Value |
|---|---|
| Sodium | 145 mEq/L |
| Potassium | 3.7 mEq/L |
| Chloride | 107 mEq/L |
| Carbon dioxide | 15 mmol/L |
| BUN | 25 mg/dL |
| Creatine | 2.52 mg/dL |
| AST | 44 U/L |
| ALT | 34 U/L |
| Alk phos | 184 U/L |
| Total bilirubin | 1.5 mg/dL |
| Direct bilirubin | .9 mg/dL |
| Albumin | 3.5 g/dL |
| White blood cells | 10.10 K/μL |
| Hemoglobin | 15.3 g/dL |
| Hematocrit | 49.1% |
| Platelets | 188 K/μL |
| Lacosamide | 15.5 mcg/mL |
| Clobazam | 250.0 ng/mL |
| Norclobazam | >10 000 ng/mL |
Figure 1.
Norclobazam concentration and GCS over time.
Based on discordant clobazam and norclobazam concentrations, CYP2C19 genotyping using a PCR-based 5′-nuclease assay was sent and revealed a CYP2C19 polymorphism of genotype *2/*3 (poor metabolizer phenotype). The patient’s mental status gradually improved over the course of the hospitalization as the norclobazam concentration declined. At the time of hospital discharge (day 47 of admission), the norclobazam concentration remained elevated at 5490 ng/mL. The calculated terminal norclobazam half-life was approximately 770 hours. At hospital discharge, the patient was awake, oriented to person and place, and able to follow commands intermittently. He was discharged to a rehabilitation facility to further promote neurorecovery on lacosamide monotherapy and no antipsychotics.
Discussion
We present a case of a patient with progressively declining mental status attributable to impaired clobazam metabolism from poor CYP2C19 metabolic polymorphism. CYP2C19 is responsible for the metabolism of various drugs including antidepressants, clopidogrel, some proton pump inhibitors, and certain ASMs such as phenytoin, phenobarbital, brivaracetam, clobazam, and diazepam. The CYP2C19 gene is prone to genetic polymorphisms, with 39 identified variant alleles to date. Of particular interest include the identified gene variants that lead to altered enzymatic activity, including the common variants of CYP2C19*2 and CYP2C19*3 which cause reduced or absent enzyme activity or, in contrast, the CYP2C19*17 allele which leads to increased CYP2C19 expression and subsequent activity. 10
Patients of East Asian descent are at high risk of CYP2C19 polymorphism, with 45.9% being intermediate metabolizers (IM) and 12.9% being poor metabolizers (PM). 11 Despite this, guidelines for selecting appropriate patients for CYP2C19 function screening and managing the clobazam-CYP2C19 interaction are not available. Pharmacogenomic testing is not routinely performed when initiating clobazam which may lead to increased risk of adverse events in populations with higher likelihood of expressing polymorphisms. In addition to genetic influence on CYP2C19 metabolism, prescribers should be aware of drug-drug interactions with agents such as diltiazem, ketoconazole, oxcarbazepine, cannabidiol, ritonavir, stiripentol, cenobamate and verapamil which may also increase norclobazam concentrations by a similar mechanism of reduced CYP2C19 activity.
To date, only one case report highlights a patient receiving therapeutic doses of clobazam who experienced hypersomnolence and was found to have a poor metabolizer phenotype. In this case, genetic testing was pursued after seeking a second opinion, after clobazam had been discontinued, and thus clobazam and norclobazam concentration assessment was unavailable. 12 The currently presented case highlights the only report to date of a patient taking therapeutic doses of clobazam with notable hypersomnolence and confirmed CYP2C19 polymorphism with subsequent norclobazam monitoring to confirm clearance over time. Current package labeling recommends initiating clobazam at 5mg daily in patients who are known to be PM of CYP2C19, however, recommendations for whom genotype testing should be pursued are not provided. Routine CYP2C19 genotype testing for assessment of clopidogrel effectiveness has been shown to be cost-effective with potential efficacy benefits for certain indications including high-risk transient ischemic attacks, minor strokes, and acute coronary syndromes,13,14 but the role of routine testing of CYP2C19 function for clobazam dosing has not been evaluated. It may be reasonable to pursue routine phenotype testing in patients in or descendant of biogeographic groups who have a high prevalence of poor CYP2C19 function, including patients of Oceanic (57% PM, 23% IM), East Asian (13% PM, 45% IM), and South Asian (8% PM, 40% IM) descent to inform clobazam dosing and titration. 10 Additionally, in patients who experience adverse reactions out of proportion to their prescribed dose, clinicians could evaluate clobazam: norclobazam ratios; with a ratio exceeding 1:5 to 1:10 indicative to consider genotype testing. In one study of Japanese patients, a norclobazam to clobazam dose ratio in mcg/mL:mg/kg/day of greater than 10 had a 94.4% specificity and 95.7% sensitivity for poor metabolizer status. 15 Our patient’s admission ratio exceeded 41.
Clobazam is a potent, broad-spectrum ASM with efficacy in a range of seizure disorders, however, our case report demonstrates that caution is required in patients at risk for CYP2C19 polymorphism. While our patient had a complex past medical history which contributed to a multifactorial toxic metabolic encephalopathy, after an extensive diagnostic workup, delayed clearance of norclobazam was a significant contributor to this patient’s altered mental status and declining functional status. Drug-gene interactions are increasingly recognized as contributors to excess morbidity and clinicians should be aware of these potential interactions when initiating new therapies.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Kristy M. Phillips https://orcid.org/0009-0005-5013-3578
References
- 1.Sankar R. GABAA receptor physiology and Its relationship to the mechanism of action of the 1,5-benzodiazepine clobazam. CNS Drugs. 2012;26(3):229-244. doi: 10.2165/11599020-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 2.Bardy AH, Seppälä T, Salokorpi T, Granström ML, Santavuori P. Monitoring of concentrations of clobazam and norclobazam in serum and saliva of children with epilepsy. Brain Dev. 1991;13(3):174-179. doi: 10.1016/S0387-7604(12)80025-8 [DOI] [PubMed] [Google Scholar]
- 3.Rupp W, Badian M, Christ O, et al. Pharmacokinetics of single and multiple doses of clobazam in humans. Br J Clin Pharmacol. 1979;7(Suppl 1):51S-57S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosaki K, Tamura K, Sato R, Samejima H, Tanigawara Y, Takahashi T. A major influence of CYP2C19 genotype on the steady-state concentration of N-desmethylclobazam. Brain Dev. 2004;26(8):530-534. doi: 10.1016/j.braindev.2004.02.010 [DOI] [PubMed] [Google Scholar]
- 5.Seo T, Nagata R, Ishitsu T, et al. Impact of CYP2C19 polymorphisms on the efficacy of clobazam therapy. Pharmacogenomics. 2008;9(5):527-537. doi: 10.2217/14622416.9.5.527 [DOI] [PubMed] [Google Scholar]
- 6.Saruwatari J, Ogusu N, Shimomasuda M, et al. Effects of CYP2C19 and P450 oxidoreductase polymorphisms on the population pharmacokinetics of clobazam and N-desmethylclobazam in Japanese patients with epilepsy. Ther Drug Monit. 2014;36(3):302-309. doi: 10.1097/FTD.0000000000000015 [DOI] [PubMed] [Google Scholar]
- 7.de Leon J, Spina E, Diaz FJ. Clobazam therapeutic drug monitoring: A comprehensive review of the literature with proposals to improve future studies. Ther Drug Monit. 2013;35(1):30-47. doi: 10.1097/FTD.0b013e31827ada88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pok PRP, Mauras M, De Saint Léger MN, et al. Blood concentrations of clobazam and norclobazam in a lethal case involving clobazam, meprobamate and clorazepate. Leg Med. 2010;12(6):300-304. doi: 10.1016/j.legalmed.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 9.Proença P, Teixeira H, Pinheiro J, Marques EP, Vieira DN. Forensic intoxication with clobazam: HPLC/DAD/MSD analysis. Forensic Sci Int. 2004;143(2):205-209. doi: 10.1016/j.forsciint.2004.03.029 [DOI] [PubMed] [Google Scholar]
- 10.Scott SA, Sangkuhl K, Shuldiner AR, et al. PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenet Genomics. 2012;22(2):159-165. doi: 10.1097/FPC.0b013e32834d4962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorji PW, Tshering G, Na-Bangchang K. CYP2C9, CYP2C19, CYP2D6 and CYP3A5 polymorphisms in South-East and East Asian populations: a systematic review. J Clin Pharm Ther. 2019;44(4):508-524. doi: 10.1111/jcpt.12835 [DOI] [PubMed] [Google Scholar]
- 12.Hamilton KE, Shelton CM, Wheless J, Phelps SJ. Persistent hypersomnolence following clobazam in a child with epilepsy and undiagnosed CYP2C19 polymorphism. J Pediatr Pharmacol Ther. 2020;25(4):320-327. doi: 10.5863/1551-6776-25.4.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Limdi NA, Cavallari LH, Lee CR, et al. Cost-effectiveness of CYP2C19-guided antiplatelet therapy in patients with acute coronary syndrome and percutaneous coronary intervention informed by real-world data. Pharmacogenomics J. 2020;20(5):724-735. doi: 10.1038/s41397-020-0162-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Z, Cai D, Wang R, et al. Cost-effectiveness of CYP2C19 genotyping to guide antiplatelet therapy for acute minor stroke and high-risk transient ischemic attack. Sci Rep. 2021;11(1):7383. doi: 10.1038/s41598-021-86824-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto Y, Takahashi Y, Imai K, et al. Influence of CYP2C19 polymorphism and concomitant antiepileptic drugs on serum clobazam and N-desmethyl clobazam concentrations in Patients with epilepsy. Ther Drug Monit. 2013;35(3):305-312. doi: 10.1097/FTD.0b013e318283b49a [DOI] [PubMed] [Google Scholar]