Abstract
Venous Ulcers (VU) represent 60–80% of all leg ulcers and are the final stage of the disease secondary to venous hypertension or valve insufficiency. Conventional treatment that focuses on its etiological factors continues to be the gold standard; however, 30% of ulcers do not heal with this treatment; thus, it has been seen that the use of growth factor can be used as an adjuvant for this pathology. A literature review was carried out to evaluate the evidence from systematic reviews, meta-analyses, case studies, and quantitative studies that respond to the objective of this analysis review in the different databases with specific inclusion criteria with publications between 2002 and 2022, initially finding the topical application of the factor and later, more recently, the intralesional and perilesional application, the latter being an alternative treatment for this type of pathology and generating some recommendations for using the Factor.
Keywords: chronic venous fibroblast dysfunctions, recombinant human epidermal growth factor, rhEGF, intralesional, perilesional, fibroblast dysfunctions
Introduction
Chronic venous insufficiency (CVI) is one of the main pathologies treated by vascular surgeons. Its most serious presentation is venous ulcer (VU), being the most common ulcer in the lower extremities and feet.1 It accounts for 70–90% of all ulcers in the lower extremities; its overall prevalence ranges between 25% and 40%,2 and its onset rate is 1 out 300 adults who may suffer this condition at some point in their life. It affects 1% of the adult world population in industrialized countries, and the incidence is expected to grow due to the increase in sedentism and obesity rates.3,4 Twenty-two per cent of people present their first episode in the fourth decade of life, while this percentage rises to 70% in the sixth decade.1 Its onset is more common in women, with a male–female ratio of 1/3. Incidence increases at ≥65 years, with a percentage that affects 5.6% of the population.5 Other known risk factors for VU include trauma, family history, phlebitis, and deep vein thrombosis (DVT).6–8 Leg venous ulcers (VU) are opened lesions between the knee and ankle joint secondary to venous hypertension, valve insufficiency, or post-thrombotic syndrome.9,10
Health system is also weighed down by the cost of VU, with estimated costs of about USD 1.9–2.5 billion. In addition, patients experience social isolation due to LVU and certain degrees of disabilities.11 The current VU treatment focuses on the management of reflux and the management of the local ulcer, with conservative compression therapy being the gold standard.12
To provide an objective evaluation, the CEAP (Clinical-Etiology-Anatomy-Pathophysiology) classification system was created. This tool is the international standard for describing patients with chronic venous disorders.13 In 2020, the latest CEAP update by the CEAP Working Group for the clinical classification (C) reported VU as the final stage of CVI (C6), which creates a physical and financial burden for patients. VU recurrence rates after treatment are around 50–70% in 6 months, and up to 5% VU will not heal after proper treatment.14,15
Vein wall damage and valve damage are the main events leading to this disease. Evidence has shown that they are pathologically altered, with microcirculation being a critical component in pathophysiology through the endothelium, which is a key regulator that releases vasoactive agents, inflammatory molecules, cytokines, and prothrombotic precursors, which in turn activate leukocyte recruitment, thus initiating a cascade of inflammatory processes. An important element within the pathophysiology is the activity of matrix metalloproteinases (MMPs), specific elements in the inflammatory process, with deleterious effects on the vein wall, the venous valve, and the endothelium,16 MMPs are a family of zinc-dependent endopeptidases that degrade the components of the extracellular matrix and the basement membrane. Its activity is hampered by protease inhibitors, including tissue inhibitors of metalloproteinases (TIMP) and α-2-macroglobulin,17 and its regulation is carried out by different actors including among others the epidermal growth factor, which have immunohistochemistry degradation, and their imbalance causes an uncontrolled proteolysis characteristic of a chronic inflammatory state.17–19
Furthermore, in chronic ulcers, fibroblast dysfunctions such as increased apoptosis, premature senescence, senescence-like phenotype, or poor growth response in the absence of senescence markers have been reported. Some of these differential dysfunctions may be secondary to differences in the age or sex of the patient, the size, or the duration of the ulcer. Some specific studies for venous ulcers have shown that fibroblasts taken from the edge of these ulcers behave differently from fibroblasts from unaffected tissue. These fibroblasts are differentiated or senescent and have a larger cell size and macromolecular mass with enclosed cells that cannot replicate. This suggests that the fibroblast population at the venous ulcer site has changed due to venous hypertension, the chronic wound environment, or some other unknown factor; however, the full spectrum of fibroblast dysfunction may exist and be secondary to a response to varying degrees of oxidative stress, experiencing excessive inflammation.20,21
As the normal healing process is impaired and does not progress normally, the addition of growth factors can stimulate the formation of granulation tissue. In the past two decades, growth factors have been increasingly used for the treatment of chronic wounds such as platelet-derived growth factor (PDGF), keratinocyte growth factor-2 (KGF-2), epidermal growth factor (EGF), transforming growth factor-β2 (TGF-β2), granulocyte-macrophage colony–stimulating factor (GM-CSF), which have similar operating mechanisms of granulation, tissue regeneration, chemotaxis, fibroblast proliferation and collagenase production in venous ulcers, positively interfering in the healing process, with epidermal growth factor being the most studied to date.15
The therapy for chronic venous ulcers has not changed significantly in decades, which is focused on the control of the causal factors; bandages, grafts, and sequential compression devices are the current standard of care for this disease along with resting therapy for venous stasis and compression therapy to decrease chronic venous hypertension. It is important to combine all treatment with an appropriate topical therapy to maintain a moist environment for the prevention of recurrences. However, 30% of ulcers do not heal with this type of therapy, so the addition of growth factors in this percentage of ulcers may be an alternative treatment.22
Epidermal growth factor (EGF) is a 53-amino acid polypeptide that Cohen first isolated from the submandibular glands of mice. It stimulates the growth of fibroblasts, keratinocytes, and vascular endothelial cells, which contribute to the formation of scar tissue.23
Its action is triggered by the interaction with specific receptors located in the fibroblast cell membrane, where success has been reported in the regeneration of diabetic ulcers, raising the potential benefits of its use as adjuvant therapy for other types of ulcers such as ULV.24,25 The epidermal growth factor to be administered is the human recombinant type (hrEGF), which is the one that shows efficacy, tolerability and safety.26 In 2009, Montequin et al evidenced the beneficial effect of topical application of hrEGF in low-grade neuropathic ulcers; however, the effect of topical hrEGF formulation may be reduced, particularly in high-grade wounds, since an increase in protease activity has been identified. Direct intralesional administration of an EGF-based formulation may overcome this limitation as reported in previous studies in diabetic foot ulcer (DFU),27 pathology that also mediates an inflammatory process and oxidative stress as in venous ulcer.16 Intralesional infiltration of hrEGF has been associated with a significant recovery of markers of oxidative stress and antioxidant reserve, contributing to restoring the systemic redox balance, with intralesional and perilesional application.28
This document aims to make some recommendations by a panel of experts based on the literature found on the use of epidermal growth factor for the indication of treatment of venous ulcers locally, which can be useful for medical professionals devoted to VU treatment, being aware of the impact of the quality of life that VU can cause, with the possibility of having a management alternative that can reduce treatment time. Therefore, this literature review of the effectors of hrEGF in VU was carried out, and updated management guidelines based on this panel of experts were provided.
Methods
In order to identify available literature on the use of intralesional and perilesional rhEGF in the local treatment of venous ulcers, a literature review was carried out to identify systematic reviews and quantitative studies that respond to the objective of this analysis. Consultation databases were PubMed (Medline), Cochrane Library and Google Scholar using the following terms: EGF, h-EGF, epidermal growth factor, venous insufficiency ulcer and venous leg ulcer.
The inclusion criteria considered were Spanish and English articles published between 2002 and 2022: The typology of the selected articles was systematic reviews, meta-analyses, clinical trials, case studies, cohort studies, cases, prospective and retrospective controls. The indications currently registered in the rhEGF marketing authorization in Colombia were also evaluated as well as its current indications for wound healing (adjuvant in epidermal regeneration processes in skin ulcers and ulcers of vascular origin). As an exclusion criterion, the articles that did not consider venous ulcers were determined.
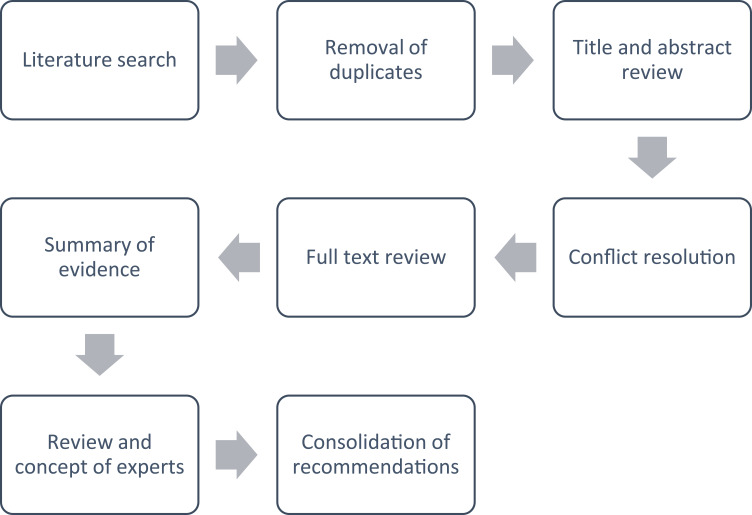
Reference screening was carried out by two reviewers independently, and differences were resolved by consensus. SANRA’s guidelines were followed to guarantee the quality of the review; however, the current evidence is heterogeneous from a methodological point of view, with respect to growth factors in the indication of VU. This, in a way, prevents a rigorous methodological analysis typical of a meta-analysis study or a systematic review. Therefore, this publication is narrative in nature, which aims to provide a broad perspective on the use of the rhEGF in the indication for Venous Ulcers in relation to the progress found, experiences and guides in this indication (Figure 1).29
Figure 1.
Flowchart for the literature review process and expert recommendations.
The screening of the studies and the extraction of information were carried out using a Microsoft Excel matrix, where the study title, authors, year of publication, place of publication, type of study and relevant findings for this review were mentioned.
For the preparation of the expert recommendations based on the review results, it was shared with 10 experts specialized in peripheral vascular surgery from different countries, with the aim of analyzing the available evidence and based on this, determining and recommending how rhEGF can be used in the treatment of venous ulcers.
Results
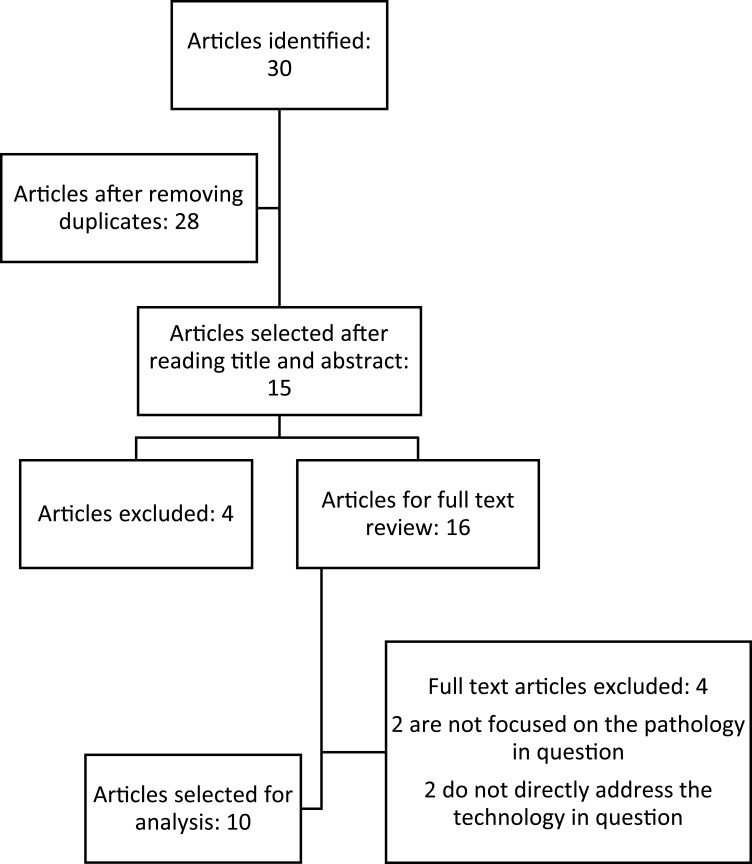
After applying the inclusion and exclusion criteria, relevant articles were identified that reported comparative retrospective results of case report studies, RCTs and systematic reviews. Thirty studies were identified, by title and abstract selection. Ten studies were selected for full-text review (Figure 2 and Table 1), and then the analysis of the findings is presented.
Figure 2.
Perspective of literature review.
Table 1.
Characteristics of the Studies Included in the Analysis
| N | Study Title | Year | Method | Results |
|---|---|---|---|---|
| 1 | Epidermal growth factor therapy and wound healing—past, present, and future perspectives30 | 2008 | Review in Medline | The FCE showed positive results in the wound healing process, but due to its topical application it has been criticized due to the limited effect on the healing of chronic wounds and the association with invasive malignancies at local level |
| 2 | An approach to treating ulcers of vascular origin: review and role of epidermal growth factor26 | 2016 | Evidence Review | The review of scientific evidence of (rhEGF) for vascular ulcers shows that it has efficacy, and tolerability, considered as an adjunctive or emerging treatment that can be considered for the management of vascular ulcers. |
| 3 | Comparative study of Four Layer Compression Bandaging and Topical Human Epidermal Growth Factor in Chronic Venous Leg Ulcer31 | 2018 | A prospective, open-label, randomized, comparative, single-center study | Topical h-EGF along with the four-layer bandage speeds the healing time in chronic leg venous ulcer |
| 4 | Human recombinant epidermal growth factor in skin lesions: 77 cases in EPithelized project32 | 2018 | Multicenter case series | Topical rhEGF presents results in effectiveness and tolerance. It is not compared with other technologies or drug presentations. |
| 5 | A new alternative in the management of complex vascular ulcer with recombinant epidermic growth factor, EPIPROT® (Nepidermine)33 | 2019 | Follow-up to three cases | rhEGF contributes to granulation and stimulates short term healing, which can reduce complications. |
| 6 | Treatment of venous ulcers with growth factors: systematic review and meta-analysis34 | 2019 | Systematic Review | The effect of the application of growth factors for complete healing in venous ulcers is not clear, clinical trials with methodological quality are necessary for more precise recommendations. |
| 7 | Experience with the use of perilesional and intralesional recombinant human epidermal growth factor (nepidermin) in the treatment of patients with chronic venous ulcers25 | 2019 | Retrospective multicenter case series study | rhEGF achieved an important level of improvement in the severity index of the injury, which allowed epithelialization and wound healing in most cases in a brief period of time |
| 8 | Clinical guideline on topical growth factors for skin wounds35 | 2020 | GPC for the application of growth factors | Topical application of growth factors is promising in the treatment of several types of skin wounds |
| 9 | Efficacy of Human Recombinant Epidermal Growth Factors vs Conventional Therapy for the Treatment of Chronic Venous Ulcers: A Retrospective Case Series36 | 2021 | Case Series Retrospective Study | rhEGF presents a higher probability of epithelization of venous ulcers compared to hydrocolloids |
| 10 | Growth factors for treating chronic venous leg ulcers: A systematic review and meta-analysis15 | 2022 | Systematic Review | EGFs can increase the percentage of reduced ulcer area during treatment |
In 2008, in a review carried out by Hardwicke et al, they showed that the use of the EGF may be promising to improve ulcer healing but that its topical application where chronic ulcers possibly degrade the factor properties alters its statistical significance, due to the hostile environment of this type of ulcers, but specifies that there is a recent research focus on new administration systems that are capable of protecting and stabilizing the EGF.30
Esquirol et al, in 2016, evidence very similar results demonstrating efficacy, tolerability and safety, considering adjuvant or emerging treatment in evidence-based clinical practice guidelines 28 and in another review of this same author in 2017, which includes 25 articles that concludes that although evidence on the use of hrEGF in skin lesions is not very large compared to other treatments, it shows positive or at least very promising results without adverse effects with excellent tolerability.37
Balasubrahmanya KS et al, 2018, compared the healing rate and healing area in chronic venous ulcer with topical use of human epidermal growth factor (hEGF) along with four-layer compression bandages versus four-layer compression bandages alone in 90 patients with venous ulcers. The results of this study show that the hEGF promotes faster healing by stimulating granulation tissue and accelerating the rate of epithelialization. No side effects caused by hEGF were observed.31
Esquirol et al, 2019, evaluated EGF in 77 patients with different vascular type ulcers (venous, arterial, and diabetic foot type), other surgical wounds, trauma, burns, and scars. The most frequent pathologies were type 2 diabetes mellitus and chronic venous insufficiency. The average decrease in wound area was 66.7%, about 43.3% of the included venous ulcers had a healing rate greater than 40% in 4 weeks.32
Daza et al, 2019, made a case report with 3 patients, of which 2 patients had a diabetic foot ulcer and a third case a venous ulcer. The case of a 35-year-old chronic venous ulcer occurred in a 66-year-old woman to whom intralesional and perilesional hrEGF was applied, resulting in 95% granulation tissue, 90% epithelialization, in a subsequent exam with full closure.33
Carvalho et al, 2019, conducted a systematic review, in which they found that 802 participants were recruited by the 10 studies, including 472 in the intervention group (growth factors) and 330 as controls. Participants who received platelet-rich plasma and epidermal growth factor had a slight tendency to complete cure, but without statistical significance (p < 0.05). In conclusion, the effect of the application of growth factors for the complete healing of venous ulcers is not clear.34
In Colombia, Cacua et al, 2019, made a case report of the intralesional and perilesional application of hrEGF in 28 patients with 35 venous ulcers. On average, the patients were 60 years old. After treatment, 69% of the patients achieved complete epithelialization and 86% achieved granulation tissue, with an application time of 8 weeks.25
Several researchers in conjunction with the Chinese Burn Association developed a clinical guide to the topical use of growth factors in different pathologies. The guide mentioned that the use of EGF in venous ulcers, as it exerts chemotactic and mitogenic effects, helps regulate cellular metabolism, differentiation, and other biological activities. It is suggested that EGF promotes the healing of venous ulcers and issued an opinion with an already moderate level of evidence, although the recommendation level is still low.35
In another study, Cacua et al conducted a retrospective study in the city of Bogotá, where 48 patients were examined. All patients were treated with compression therapy, but 24 patients were treated with hydrocolloid, and the other 24 patients were treated with hrEGF applied perilesionally and intralesionally. The results showed that patients whose wounds were treated with hydrocolloids closed in an average of 29.5 weeks, while patients with hrEGF closed in 8 weeks (71%) and the rest between 9 and 12 weeks.36
A systematic review and meta-analysis of randomized trials were performed, and 13 trials (n = 991) were included. There was a significant difference between any growth factor and placebo in complete wound healing (P = 0.04). Any growth factor compared to placebo significantly increased the probability of percent wound reduction by 48.80% (P = <0.00001). This meta-analysis suggests that growth factors have a beneficial effect on the complete healing of VLU wounds, although the application of EGF was also topical.15
Discussion and Recommendations
Considering that the epidermal growth factor is an endogenously produced polypeptide, we can determine that its function within the organism is to generate the proliferative action of the tissues, giving the organism the opportunity to regenerate spontaneously and naturally,37 However, we know that in a complex ulcer, which can have multiple etiologies, the inflammatory environment secondary to constant stress generates a vicious circle that makes spontaneous healing difficult with a degradation of all the pro-healing actors, including EGF. The specific treatment of an ulcer with these characteristics is based on managing its underlying pathology, but the treatment at local level is based on maintaining an adequate environment for the ulcer at local level,38 thus there has been an increasing use of different types of growth factors, of which we know that there are different classes and families, looking for alternatives that make local treatment more efficient,39 and it has been seen that its topical treatment has a certain tendency to improve ulcers characterized by a decrease in size or sometimes epithelialization, depending on its complexity, as evidenced in the present review. However, this trend is not statistically significant, so we can infer that the topical application mode is not conclusive in its results. However, in the present review, studies with a different application could be found, such as intralesional and perilesional application, which can cross that inflammatory barrier and be directly available to the fibroblast,27 to fulfill a function that acts directly on the imbalance produced in a complex ulcer, such as UVP, being a very good alternative for local treatment together with elastocompression for this pathology. It is clearly necessary to expand the current evidence with studies such as clinical trials to validate its use in this indication, but with intralesional and perilesional application.
Therefore, all experts give the following recommendations for its application and the design of future studies in the indication for venous ulcers:
The perilesional and intralesional application of the human recombinant epidermal growth factor (hrEGF) in patients with venous ulcer should be used to reduce the time to healing.23,25
Growth factors, particularly human recombinant type, help in wound healing and wound size reduction.26
hrEGF plus compression therapy seems to promote chronic VU epithelialization within 12 weeks of treatment or less.25,33,36
The appropriate epidermal growth factor to be applied must be the human recombinant type (hrEGF).26
The application of hrEGF should not be topically, nor by means of a dressing. Its application should be intralesionally and perilesionally.25,33,36
The hrEGF is an alternative treatment that can be used to decrease the inflammatory burden of chronic venous ulcer and its oxidative stress burden.28
When added to standard conservative therapy, growth factors, particularly of human recombinant type, can provide additional benefits for wound healing.25,33,36
Its application should be performed as an adjuvant to the standard venous ulcer therapy, which will continue to be elastic compression therapy; therefore, it is essential to perform the local treatment with hrEGF together with this therapy.25,33,36
The use of hrEGF is a local-type treatment alternative for the treatment of chronic venous ulcers; therefore, the background therapy of venous disease must remain the same which will be focused on causal factors with adequate systemic support, compression therapy and surgical management when indicated.25,33,36
According to the etiology of venous ulcer and its risk factors, it is essential to make the appropriate etiological diagnosis of the origin of this ulcer to determine the appropriate treatment and therefore the necessary alternative for the appropriate treatment. For this, taking a venous and arterial Doppler ultrasound is required before starting hrEGF treatment.5,8
The use of hrEGF applied perilesionally and intralesionally is safe for the patient. No adverse events or harmful effects have been reported with its use in patients with complex ulcers.34
Conclusion
Although the evidence suggests that hrEGF treatment in the venous ulcer indication is limited, many authors have conducted research on this indication with a topical application modality or through dressings, where good results have been reported but these are not statistically significant. However, there are authors who have applied hrEGF using another route of application such as the perilesional and intralesional route with a needle, which, unlike topical application or by dressing, show completely different results, generating a higher percentage of granulation and epithelialization in a short time, making it a suitable application alternative to overcome the hostile inflammatory barrier and the biofilm of a complex ulcer. Concluding that to face a complex ulcer, hrEGF cannot be used topically, but rather applied intralesionally and perilesionally, which also applies to future clinical studies. However, it is always mandatory to support local ulcer treatment with hrEGF treatment along with conventional supportive therapy for venous ulceration based on its causative factors with adequate systemic support, compression therapy, and surgical management when indicated.
Disclosure
Jhon Jairo Berrio Caicedo reports personal fees from Praxis Pharmaceutical Colombia, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Blumtritt DG. Leg venous ulcer in elder people: A problem transcending health. Cicatriz Ar. 2015;01:1. [Google Scholar]
- 2.Al Shammeri O, Alhamdan N, Al-Hothaly B, Hussain M, Al-Mohaimeed A, Al-Mohaimeed A. Chronic venous insufficiency: prevalence and effect of compression stockings. Int J Health Sci. 2014;8(3):231–236. doi: 10.12816/0023975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franks PJ, Barker J, Collier M, et al. Management of patients with venous leg ulcers: challenges and current best practice. J Wound Care. 2016;25(Sup6):S1–67. doi: 10.12968/jowc.2016.25.Sup6.S1 [DOI] [PubMed] [Google Scholar]
- 4.Finlayson K, Edwards H, Courtney M. Relationships between preventive activities, psychosocial factors and recurrence of venous leg ulcers: a prospective study. J Adv Nurs. 2011;67(10):2180–2190. doi: 10.1111/j.1365-2648.2011.05653.x [DOI] [PubMed] [Google Scholar]
- 5.Gómez Ayala AE. Vascular ulcers; risk factors, clinic and prevention. Farm Profes. 2008;22:6. [Google Scholar]
- 6.Abbade LPF, Lastória S. Venous ulcer: epidemiology, physiopathology, diagnosis and treatment. Int J Dermatol. 2005;44(6):449–456. doi: 10.1111/j.1365-4632.2004.02456.x [DOI] [PubMed] [Google Scholar]
- 7.Scott TE, Lamorte WW, Gorin DR, Menzoian JO. Risk factors for chronic venous A dud case, control study insufficiency. J Vasc Surg. 1995;22(5):622–628. doi: 10.1016/S0741-5214(95)70050-1 [DOI] [PubMed] [Google Scholar]
- 8.Etufugh CN, Phillips TJ. Venous ulcers. Clin Dermatol. 2007;25(1):121–130. doi: 10.1016/j.clindermatol.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 9.O’Meara S, Al-Kurdi D, Ologun Y, Ovington LG, Martyn-St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev. 2014;2014:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alavi A, Sibbald RG, Phillips TJ, et al. What’s new: management of venous leg ulcers: treating venous leg ulcers. J Am Acad Dermatol. 2016;74(4):643–664. doi: 10.1016/j.jaad.2015.03.059 [DOI] [PubMed] [Google Scholar]
- 11.Rice JB, Desai U, Cummings AKG, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. J Med Econ. 2014;17(5):347–356. doi: 10.3111/13696998.2014.903258 [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell TF, Passman MA, Marston WA, et al. Management of venous leg ulcers: clinical practice guidelines of the society for vascular surgery® and the American venous forum. J Vasc Surg. 2014;60(2):3S–59S. doi: 10.1016/j.jvs.2014.04.049 [DOI] [PubMed] [Google Scholar]
- 13.Lurie F, Passman M, Meisner M, et al. CEAP classification system and reporting standard, revision 2020. J Vasc Surg Venous Lymphat Disord. 2020;8(3):342–352. doi: 10.1016/j.jvsv.2019.12.075 [DOI] [PubMed] [Google Scholar]
- 14.Nicolaides AN. The most severe stage of chronic venous disease: an update on the management of patients with venous leg ulcers. Adv Ther. 2020;37:19–24. doi: 10.1007/s12325-020-01219-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y, Lee MH, Phillips SA, Stacey MC. Growth factors for treating chronic venous leg ulcers: a systematic review and meta-analysis. Wound Repair Regen. 2022;30(1):117–125. doi: 10.1111/wrr.12982 [DOI] [PubMed] [Google Scholar]
- 16.Chi YW, Raffetto JD. Venous leg ulceration pathophysiology and evidence based treatment. Vasc Med. 2015;20(2):168–181. doi: 10.1177/1358863X14568677 [DOI] [PubMed] [Google Scholar]
- 17.Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound Repair Regen. 2008;16(5):642–648. doi: 10.1111/j.1524-475X.2008.00415.x [DOI] [PubMed] [Google Scholar]
- 18.Pérez-García LJ. Metalloproteinases and the skin. Actas Dermosifiliogr. 2004;95(7):413–423. doi: 10.1016/S0001-7310(04)76850-7 [DOI] [Google Scholar]
- 19.Raffetto JD. Inflammation in chronic venous ulcers. Phlebology. 2013;28(1_suppl):61–67. doi: 10.1177/0268355513476844 [DOI] [PubMed] [Google Scholar]
- 20.Clark RAF. Oxidative stress and “senescent” fibroblasts in non-healing wounds as potential therapeutic targets. J Invest Dermatol. 2008;128:2361–2364. doi: 10.1038/jid.2008.257 [DOI] [PubMed] [Google Scholar]
- 21.Stanley AC, Park H, Phillips TJ, Russakovsky V, Menzoian JO. Reduced growth of dermal fibroblasts from chronic venous ulcers can be stimulated with growth factors. J Vasc Surg. 1997;6:6. [DOI] [PubMed] [Google Scholar]
- 22.Broszczak DA, Sydes ER, Wallace D, Parker TJ. Molecular aspects of wound healing and the rise of venous leg ulceration: omics approaches to enhance knowledge and aid diagnostic discovery. Tony J Parker Clin Biochem Rev. 2017;38(1):2017. [PMC free article] [PubMed] [Google Scholar]
- 23.López-Saura PA, Berlanga-Acosta J, Fernández-Montequín JI, et al. Intralesional Human Recombinant Epidermal Growth Factor for the Treatment of Advanced Diabetic Foot Ulcer: from Proof of Concept to Confirmation of the Efficacy and Safety of the Procedure. In: Dinh T, editor. Global Perspective on Diabetic Foot Ulcerations. Rijeka: IntechOpen; 2011:12. [Google Scholar]
- 24.Dumantepe M, Fazliogullari O, Seren M, Uyar I, Basar F. Efficacy of intralesional recombinant human epidermal growth factor in chronic diabetic foot ulcers. Growth Factors. 2015;33(2):128–132. doi: 10.3109/08977194.2015.1031898 [DOI] [PubMed] [Google Scholar]
- 25.Cacua Sánchez MT, Giraldo LF. Experience with the use of perilesional and intralesional recombinant human epidermal growth factor (nepidermin) in the treatment of patients with chronic venous ulcers. Vasc Dis Manag. 2019;16:1. [Google Scholar]
- 26.Esquirol Caussa J, Herrero Vila E. One approach to treating vascular ulcers: review and role of epidermal growth factor. Angiologia. 2016;68:322–330. [Google Scholar]
- 27.Fernández-Montequín JI, Betancourt BY, Leyva-Gonzalez G, et al. Intralesional administration of epidermal growth factor-based formulation (Heberprot-P) in chronic diabetic foot ulcer: treatment up to complete wound closure. Int Wound J. 2009;6(1):67–72. doi: 10.1111/j.1742-481X.2008.00561.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ojalvo AG, Acosta JB, Marí YM, et al. Healing enhancement of diabetic wounds by locally infiltrated epidermal growth factor is associated with systemic oxidative stress reduction. Int Wound J. 2017;14(1):214–225. doi: 10.1111/iwj.12592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidal EI, Fukushima FB. The art and science of writing a scientific review article. Cad Saude Publica. 2021;37:e00063121. doi: 10.1590/0102-311x00063121 [DOI] [PubMed] [Google Scholar]
- 30.Hardwicke J, Schmaljohann D, Boyce D, Thomas D. Epidermal growth factor therapy and wound healing—past, present and future perspectives. Surgeon. 2008;6(3):172–177. doi: 10.1016/S1479-666X(08)80114-X [DOI] [PubMed] [Google Scholar]
- 31.Balasubrahmanya KS, Vinay G, Srinidhi M, Sunil Kumar APV. Comparative study of four layer compression bandaging and topical human epidermal growth factor in chronic venous leg ulcer. Madridge J Surg. 2018;1(1):24–28. doi: 10.18689/mjs-1000106 [DOI] [Google Scholar]
- 32.Esquirol-Caussa J, Herrero-Vila E. Human recombinant epidermal growth factor in skin lesions: 77 cases in epitelizando project. J Dermatolog Treat. 2019;30(1):96–101. doi: 10.1080/09546634.2018.1468546 [DOI] [PubMed] [Google Scholar]
- 33.Daza J, Garcia R, Lozano E, Tolstano A. A new alternative in the management of complex vascular ulcer with recombinant epidermic growth factor, EPIPROT® (Nepidermine). Rev Latinoam Cir Vascular Angiol. 2019;2:1. [Google Scholar]
- 34.Carvalho MR, Silveira IA, Oliveira BG. Treatment of venous ulcers with growth factors: systematic review and metaanalysis. Rev Bras Enferm. 2019;72(1):200–210. doi: 10.1590/00347167-2017-0865 [DOI] [PubMed] [Google Scholar]
- 35.Han C-M, Cheng B, Wu P. Biao Cheng, Pan Wu, Clinical guideline on topical growth factors for skin wounds. Burns Trauma. 2020;8:35. doi: 10.1093/burnst/tkaa035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cacua M, Giraldo L, Diaz J. Efficacy of human recombinant epidermal growth factors vs conventional therapy for the treatment of chronic venous ulcers: a retrospective case series. Wound. 2021;33(2):41–49. [PubMed] [Google Scholar]
- 37.Esquirol-Caussa J, Herrero-Vila E. Epidermal Growth Factor (EGF) and silicone gels in wounds, burns and scars management: literature review. Cir Plast Ibero-Latinoam. 2017;43(4):387–394. [Google Scholar]
- 38.Gibson D, Cullen B, Legerstee R, Harding KG, Schultz G. MMP easy. Wound Int. 2009;1:1. [Google Scholar]
- 39.Barbeito C, Laube A. GROWTH FACTORS. BASIC CONSIDERATIONS AND POTENTIAL THERAPEUTICS. Analecta Vet. 2005;25(1):8–27. [Google Scholar]