Abstract
Purpose
The objective of the present study was to evaluate the efficiency of lorlatinib compared to alectinib and brigatinib for the treatment of adult patients with anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC) previously not treated, in Spain.
Methods
A partitioned survival model comprised progression free, non-intracranial progression, intracranial progression, and death health states was constructed to estimate the total costs, life-years gained (LYG) and quality-adjusted life years (QALYs) accumulated in a lifetime horizon. Overall survival (OS) and progression-free survival (PFS) for lorlatinib were obtained from the CROWN study. For alectinib and brigatinib, a network meta-analysis of randomized controlled trials was conducted to estimate OS and PFS hazard ratios versus crizotinib. Utilities were estimated based on EQ-5D-5L data derived from the CROWN (lorlatinib), ALEX (alectinib) and ALTA-1L (brigatinib) studies. According to the Spanish National Health Service perspective the total costs (expressed in euros using a 2021 cost year) included drug acquisition and the administration's subsequent treatment, ALK+ advanced NSCLC management and adverse-event management, and palliative care. Unitary costs were obtained from local cost databases and literature. Costs, LYGs and QALYs were discounted at 3% annually. Deterministic and probabilistic sensitivity analyses were used to test the model's robustness.
Results
Lorlatinib provided higher health outcomes (+0.70 LYG/patient, +1.42 QALYs/patient) and lower costs (-€9239/patient) than alectinib. Lorlatinib yielded higher LYG (+1.74) and QALYs (+2.30) versus brigatinib but higher costs/patient (+€36,627), resulting in an incremental-cost-effectiveness-ratio of €15,912/QALY gained.
Conclusion
The results of this study suggest that lorlatinib may be a dominant treatment option versus alectinib. Considering a willingness-to-pay threshold of €25,000/QALY, lorlatinib may be an efficient option compared to brigatinib.
Keywords: advanced non-small cell lung cancer, lorlatinib, ALK+, cost-effectiveness analysis, cost-utility analysis
Introduction
Lung cancer represents the third most common cancer among both men and women and the first cause of death among all cancers in Spain, with more than 30,900 new annual cases and 21,900 deaths.1 The most common type of lung cancer is the non-small cell lung cancer (NSCLC), accounting for approximately 85% of cases,1 of which 65% will be diagnosed at advanced disease stage (IIIB-IV) and 12.2% will progress from early to advanced stage.2
The diagnosis of lung cancer is increasingly precise thanks to the application of biomarker techniques that allow the identification of molecular alterations, including the anaplastic lymphoma tyrosine kinase receptor gene (ALK) which presents in approximately 4.3% of NSCLC patients.2 Thanks to identification, new therapies are being developed that act against molecular targets in patients with a specific genomic profile, as is the case with tyrosine kinase inhibitor drugs (TKIs), the first targeted therapy for NSCLC, which has contributed to a decrease in the mortality rate of these patients.3
Crizotinib is a first-generation ALK TKI, which was followed by a second-generation of ALK TKIs (ceritinib, alectinib and brigatinib). Alectinib has shown systematic and central nervous system efficacy in the treatment of ALK+ advanced NSCLC in a randomized, open-label, Phase 3 trial, in which 303 patients underwent randomization (152 in the alectinib arm and 151 in the crizotinib arm).4 Brigatinib, compared to crizotinib, demonstrated, in an open-label, phase 3 trial in which 137 patients were randomized to brigatinib and 138 to crizotinib, robust efficacy in patients with ALK+ NSCLC who had not previously received ALK inhibitors.5 Due to their demonstrated superiority to crizotinib, the second-generation TKIs were adopted as standard first-line treatment.6
However, despite the efficacy of these TKIs, recurrent disease, drug resistance and central nervous system (CNS) progression, a major cause of illness and death, still constitute a major problem in the treatment of ALK+ advanced NSCLC.7–9
Lorlatinib is a third generation ALK TKI indicated for the second- or third-line treatment of adult patients with ALK+ advanced NSCLC,10 which crosses the blood-brain barrier to reach high exposures in the CNS and to inhibit the solvent-front ALK G1202R mutation, one of the most common causes of resistance against first and second generation ALK TKIs.11 Lorlatinib was approved in May 2019 by the European Medicines Agency (EMA) for the first-line treatment of patients with ALK+ advanced or metastatic NSCLC,12 based on the clinical benefits observed in the CROWN clinical trial.13
The CROWN trial was a global, randomized Phase III trial which evaluated the efficacy and safety of lorlatinib compared to crizotinib in patients with previously untreated ALK+ advanced NSCLC. At data cutoff, lorlatinib demonstrated significantly longer progression-free survival (PFS) compared to crizotinib (median not reached with lorlatinib vs 9.3 months with crizotinib), higher percentage of survival patients without disease progression at 12 months (78% vs 39%), and higher frequency of intracranial response than those who were treated with crizotinib.13 After a median follow-up time of 36 months, the median PFS had not yet been reached for lorlatinib, and the percentage of patients with PFS at 36 months was 63.5% with lorlatinib and 18.9% with crizotinib.14
As a result of the increasing number of new therapeutic options against ALK+ advanced NSCLC and the lack of direct clinical trials comparisons, economic evaluations have become an important tool for decision makers. This study was developed with the objective of assessing the cost effectiveness of lorlatinib as a first-line treatment option in Spain when compared with alectinib and brigatinib for ALK+ advanced NSCLC patients.
Materials and Methods
Model Description
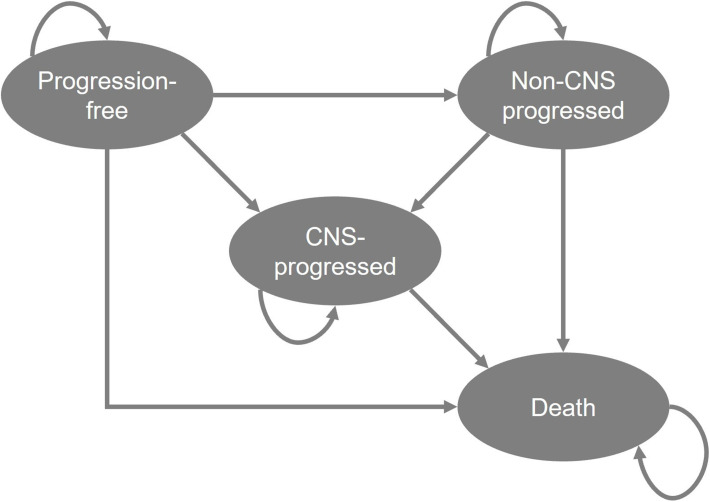
The cost-effectiveness analysis, which was developed in Microsoft Excel, used a previously validated partitioned survival model composed of four health states: progression free, non-CNS progressed disease, CNS-progressed disease and death (Figure 1).15 Partitioned survival analyses are common in oncology, and the chosen model structure is in accordance with technology appraisal guidance previously developed by the National Institute for Health Care Excellence (NICE) in the treatment of ALK+ NSCLC.16,17 In partitioned survival models, the area under the PFS curve determines the proportion of patients who are progression free, the area between the overall survival (OS) and PFS curve defines the proportion of patients that have progressed disease. A CNS-PFS curve was used to determine the proportion of patients with or without CNS progression. Those who are dead are estimated as 1 minus the OS curve. Each health state is related with explicit drug costs and patient’s preferences (utility values), being possible to estimate total drug costs, life years gained (LYG) and quality-adjusted life years (QALYs). In this model, lorlatinib was compared to alectinib and brigatinib. Patients were initiated in the progression-free health state, receiving lorlatinib or comparators treatments, and were able to remain in that state, progress in the disease (progression with or without CNS involvement) or die. The alive health states were divided into on- and off-treatment and patients could discontinue treatment before progression or receive treatment beyond progression depend on the modelled time on treatment (ToT). Subsequent treatments following progression of lorlatinib, alectinib and brigatinib were considered in the analysis at the time point of progression, affecting costs exclusively.
Figure 1.
Structure of cost-effectiveness model.
Abbreviation: CNS, central nervous system.
The analysis was carried out using 30-day cycles over a lifetime horizon of 30 years to estimate LYG, QALYs gained, and the total costs accrued with lorlatinib, alectinib and brigatinib. The incremental cost-effectiveness ratio (ICER) was the determiner and was evaluated according to the acceptable willingness to pay threshold for oncology medicines in Spain.18,19
The perspective adopted on analysis was the Spanish National Healthcare System (NHS) and costs and health outcomes were discounted at 3% per year, according with the Spanish recommendations for the development of economic evaluations.20 A half-cycle correction was applied to all costs (excepting drug and administration cost which were known to occur at the start of the cycle) and outcomes.21
Parameters, assumptions and health resource use considered in the analysis were validated by a medical advisory group composed of two oncologist experts with wide expertise and knowledge in the management of lung cancer.
Patient Population
Adult patients with untreated ALK+ advanced NSCLC were included in the analysis. The clinical characteristics, including average patient age (57 years-old) and the proportion of males (40.9%), were defined based on the intention-to-treat population included in CROWN study.13 Based on the recommendations for the Spanish population, an average body surface of 1.70 m2 was considered.22
Efficacy Data
To extrapolate clinical outcomes beyond the trial and distribute patients among health states over a lifetime horizon, parametric distributions (Weibull, log-normal, log-logistic, exponential, Gompertz, and generalized gamma distributions) were fitted to OS, PFS, CNS-PFS and ToT data.
For lorlatinib, parametric distributions were fitted separately to crizotinib (according with the NICE recommendations)23 to patient-level data from CROWN.13 Based on Akaike and Bayesian information criteria, which provided an indication of the statistical goodness of fit, and the plausibility of the long-term extrapolation, the Weibull parametric curve, which provided the most conservative OS estimation, was selected. For PFS, CNS-PFS and ToT curves, exponential distributions were used, which produced conservative long-term extrapolations.
Because of there are no head-to-head clinical trials comparing the efficacy of lorlatinib, alectinib and brigatinib, an indirect treatment comparison was required to obtain OS and PFS data in the alectinib and brigatinib arms of the model. A network meta-analysis (NMA) of randomized controlled trials was conducted, producing hazard ratios (HRs) versus the control treatment in each of the studies (crizotinib) as the treatment effect estimate for alectinib and brigatinib. Due to differences in treatment practices and healthcare systems, studies conducted only in Asian countries were excluded from NMA. According with the NMA results, alectinib (HR 0.69; 95% Credible Interval [CrI] 0.47–1.01) and brigatinib (HR 0.87; 95% CrI 0.41–1.85) demonstrated higher OS than crizotinib. In the same way, alectinib (HR 0.50; 95% CrI 0.36–0.70) and brigatinib (HR 0.49; 95% CrI 0.35–0.68) reduce the risk of progression compared with crizotinib. As CNS-PFS data were not reported in the alectinib and brigatinib studies, their estimated PFS HR versus crizotinib was assumed to be applicable to CNS-PFS. ToT curves for alectinib and brigatinib were estimated by an exponential distribution using the median treatment duration reported in the ALEX and ALTA-1 studies (28.1 months for alectinib and 24.3 months for brigatinib).4,5 More details about the NMA results and the extrapolation of survival curves were detailed in a previously published study.15
In all treatments, the OS curve was capped based on the expected age- and sex-matched survival of the general population,24 and treatment beyond progression was allowed when ToT was higher than PFS based on the modelled curves.
Safety Data
Grade 3 or higher adverse events (AEs) reported by at least 5% of patients treated with lorlatinib, alectinib or brigatinib were considered in the analysis to estimate their potential management costs and their impact on quality of life. The incidence rates of AEs for each treatment were obtained from CROWN,13 ALEX4 and ALTA-15 clinical trials.
Utility Values
Utility values express the preference that patients gave to each health state and generally range from 0 (death) to 1 (perfect health).
To estimate QALYs, utility values associated with the progression-free and post-progression health states were considered depending on treatment status (on treatment or off treatment) and treatment arm (lorlatinib, alectinib or brigatinib). For lorlatinib, health-state utilities were derived from EQ-5D-5L data collected during CROWN clinical trial and mapped to the equivalent EQ-5D-3L results using a crosswalk algorithm.25
For alectinib and brigatinib, utility values show no difference between treatment status due to lack of data, and health state utilities were sourced from NICE technology appraisals,16,17 based on mixed-models from ALEX4 and ALTA-15 data, respectively.
To reflect the lower quality of life of patients with ALK+ NSCLC progression and CNS metastases, a multiplier of 75.4% was applied to the post-progression utility to estimate the CNS-progressed health state utility value (Table 1).26
Table 1.
Utility Values
| Treatment | Progression-Free | Non-CNS Progressed | CNS-Progressed | |||
|---|---|---|---|---|---|---|
| On Treatment | Off Treatment | On Treatment | Off Treatment | On Treatment | Off Treatment | |
| Lorlatinib | 0.85 | 0.77 | 0.82 | 0.74 | 0.62 | 0.56 |
| Alectinib | 0.81 | 0.81 | 0.73 | 0.73 | 0.55 | 0.55 |
| Brigatinib | 0.79 | 0.79 | 0.62 | 0.62 | 0.47 | 0.47 |
Abbreviation: CNS, central nervous system.
Disutility values associated with the presence of AE were not considered assuming that health state utilities already capture the effect of any AE.
Resource Use and Costs
In concordance with the NHS perspective, direct healthcare costs were considered in the analysis, including lorlatinib, brigatinib and alectinib acquisition costs, subsequent treatment, drug administration and monitoring, AE and ALK+ advanced NSCLC management, and palliative care costs. Drug costs were estimated based on published ex-factory prices,27 with national mandatory deduction of 7.5% applied (Table 2).28 To reflect treatment costs more accurately, relative dose intensity was applied for lorlatinib (94.5%),13 alectinib (95.6%),4,16 and brigatinib (85.5%).5,17
Table 2.
Drug Costs (€, 2021)
| Treatment | Dosage | Model Cycle Costa | ||
| Lorlatinib | 100 mg per day | €4571.67 | ||
| Alectinib | 1200 mg per day | €5319.39 | ||
| Brigatinib | 90 mg/day 7 days followed 180 mg/day | €4757.96 | ||
| Subsequent treatment | Dosage | Treatment Duration | Proportion of Useb | Total Cost |
| After lorlatinib | ||||
| Pemetrexed | 500 mg/m2 | 4.2 months29 | 10% | €10,373.38 |
| Pemetrexed + cisplatin | 500 mg/m2 + 75 mg/m2 | 4 cycles of 21-dayb | 45% | |
| Pemetrexed + carboplatin | 500 mg/m2 + 5 AUCc | 4 cycles of 21-dayb | 45% | |
| After alectinib | ||||
| Lorlatinib | 100 mg per day | 9.60 months30 | 70% | €39,618.64 |
| Ceritinib | 450 mg per day | 8.30 months31 | 10% | |
| Brigatinib | 90 mg/day 7 days followed 180 mg/day | 7.8 months32 | 10% | |
| Pemetrexed + carboplatin | 500 mg/m2 + 5 AUCc | 4 cycles of 21-dayb | 10% | |
| After brigatinib | ||||
| Lorlatinib | 100 mg per day | 9.60 months30 | 70% | €42,034.85 |
| Ceritinib | 450 mg per day | 8.30 months31 | 10% | |
| Alectinib | 1200 mg per day | 6.23 months33 | 10% | |
| Pemetrexed + carboplatin | 500 mg/m2 + 5 AUCc | 4 cycles of 21-dayb | 10% | |
Notes: aDrug cost estimated for 30-day model cycle considering the dosage, ex-factory price applying the Royal Decree-law 8/2010, and the relative dose intensity. bExpert panel assumption. cBased on the practical clinical of expert clinicians 5 AUC was equivalent to 625 mg.
Abbreviation: AUC, area under the curve.
Regarding the subsequent treatment, the proportion of patients receiving subsequent lines after disease progression was proportioned by the advisory board of oncologists based on their current clinical practice. Treatment durations were obtained from second-line studies (Table 2)29–33 and the cost of oncology day hospital (€351,55 per visit) for those treatments which required intravenous administration (chemotherapy) was obtained from the national database.34
ALK+ advanced NSCLC management and monitoring costs depended on whether the patient had a stable disease, progression without CNS involvement, or progression and CNS involvement, and were estimated based on healthcare resource consumption. Disease and monitoring costs were mostly related to general practitioner and specialist visits, computerized tomography scans, x-rays and radiotherapy (Table 3). Palliative care costs were applied as a one-off cost to each of the death events.
Table 3.
Resource Consumption and Costs (€, 2021)
| Resource | Annual Frequencya | Proportion of Usea | Unitary Cost34 | Total Cost |
|---|---|---|---|---|
| Progression-free management | €3417.06 | |||
| Oncology visit | 10 | 100% | €79.28 | €792.80 |
| Oncology unit stay | 7 days | 10% | €479.15 | €335.41 |
| Cranial CT | 3 | 100% | €206.05 | €618.15 |
| Thoraco-abdominal CT | 4 | 100% | €190.63 | €762.50 |
| Spirometry | 1 | 20% | €59.11 | €11.82 |
| Electrocardiogram | 3 | 100% | €24.09 | €72.27 |
| Biochemistry + hemogram | 12 | 100% | €68.68 | €824.11 |
| Non-CNS progression management | €4668.58 | |||
| Oncology visit | 14 | 100% | €79.28 | €1079.62 |
| Emergency visit | 1 | 50% | €221.50 | €110.75 |
| Oncology unit stay | 7 days | 35% | €479.15 | €1173.92 |
| Biopsy | 1 | 10% | €154.37 | €15.44 |
| Cranial CT | 3 | 100% | €206.05 | €618.15 |
| Thoraco-abdominal CT | 4 | 100% | €190.63 | €762.50 |
| Spirometry | 1 | 20% | €59.11 | €11.82 |
| Electrocardiogram | 3 | 100% | €24.09 | €72.27 |
| Biochemistry + hemogram | 12 | 100% | €68.68 | €824.11 |
| CNS progression management | €5320.7867 | |||
| Oncology visit | 14 | 100% | €79.28 | €1079.62 |
| Emergency visit | 2 | 50% | €221.50 | €221.50 |
| Oncology unit stay | 7 days | 45% | €479.15 | €1509.32 |
| Biopsy | 1 | 10% | €154.37 | €15.44 |
| Cranial CT | 3 | 100% | €206.05 | €824.20 |
| Thoraco-abdominal CT | 4 | 100% | €190.63 | €762.50 |
| Spirometry | 1 | 20% | €59.11 | €11.82 |
| Electrocardiogram | 3 | 100% | €24.09 | €72.27 |
| Biochemistry + hemogram | 12 | 100% | €68.68 | €824.11 |
| End-of-life care | €7093.80 | |||
| Home hospitalization unit | 10 days | 100% | €215.48 | €2154.79 |
| Palliative care unit stay | 45.7 days | 100% | €138.56 (first 15 days) €103.93 (between day 16–30) €83.14 (from day 30) |
€4939.01 € |
Note: aHealthcare resource use (annual frequency and proportion of use) were proportioned by oncologists based on the clinical practice.
Abbreviations: CNS, central nervous system; CT, computerized tomography.
AEs-related costs were calculated considering their frequency and the cost per event obtained from literature35–39 or the national database.34 In those AEs in which cost data were not available from the literature, management cost was estimated based on healthcare resource consumption detailed by the advisory board of oncologists (Table 4).
Table 4.
Adverse Events Management Costs (€, 2021)
| Adverse Event | Cost per Event |
|---|---|
| ALT/AST increased | €1508.82a 35,36,38,39 |
| Blood CPK increased | €357.46b |
| GGT increased | €260.7639 |
| Hypercholesterolemia | €1022.4835 |
| Hypertension | €2934.92a 36–38 |
| Hypertriglyceridemia | €593.8435 |
| Lipase increased | €3252.7536 |
| Weight increased | €178.29c |
Notes: aAverage management cost per event estimated based on literature data. bManagement cost per event was estimated based on the oncologist clinical practice: 10% of patients remain in the oncology unit for 3 days, 72% undergo electromyography, electrocardiogram and biochemistry + hemogram, and 81% visit the oncologist. cManagement cost per event was estimated based on the oncologist clinical practice: 10% of patients remain in the oncology unit for 3 days, 90% visit the nurse and 18% undergo a nutritionist consultation.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine phosphokinase; GGT, gamma-glutamyltransferase.
Healthcare resources costs were obtained from a local national database of health costs34 and were expressed in euros, 2021 values. For those costs obtained from the literature, values were inflated to a 2021 year based on the Spanish general consumer price index.40
Sensitivity Analyses
Deterministic one-way sensitivity analyses (OWSA) and probabilistic sensitivity analyses (PSA) were carried out to assess the robustness of the overall results and to determine the uncertainty surrounding the most influential parameters. For the OWSA, the key parameters were varied: time horizon (10 years), discount rate (0% and 5%), alectinib and brigatinib PFS HR versus crizotinib based on the intention to treat NMA data, alectinib and brigatinib OS HR versus crizotinib from the crossover-adjusted NMA, alectinib and brigatinib CNS progressed estimated based on intracranial time to progression (TTP) HR, lorlatinib utility values according to health and treatment status for alectinib and brigatinib, subsequent treatment after lorlatinib considering 50% of patients are treated with alectinib and 50% with chemotherapy, drug costs without applying the mandatory deduction of 7.5% and considering net ex-factory price, ALK+ advanced NSCLC management costs (±20%), palliative care (±20%) and AEs management costs (±20%). The tornado diagrams are used to show which of the parameters have the greatest influence on analysis results. The PSA was performed by Monte Carlo method, running the model 5.000 times. Parameters were varied using randomly sampled values associated with probability distributions: beta distributions were applied for utility values and AEs frequency, log-normal distributions for HRs, multivariate normal distributions for survival curves, and normal distributions for all other parameters.
Results
Base Case
Over a lifetime horizon, lorlatinib yielded 7.40 LYG per patient, which were higher than the LYG obtained in patients treated with alectinib (6.69 LYG/patient) and brigatinib (5.66 LYG/patient). In terms of quality of life, lorlatinib providing higher QALYs per patient (5.89 QALYs/patient) compared with alectinib (4.46 QALYs/patient) and brigatinib (3.59 QALYs/patient) (Table 5).
Table 5.
Base Case results
| Lorlatinib | Alectinib | Lorlatinib vs Alectinib | Brigatinib | Lorlatinib vs Brigatinib | |
|---|---|---|---|---|---|
| Total LYG | 7.40 | 6.69 | 0.70 | 5.66 | 1.74 |
| LYG progression-free disease | 3.99 | 2.31 | 1.69 | 2.35 | 1.64 |
| LYG progression disease | 3.40 | 4.39 | −0.98 | 3.30 | 0.10 |
| Total QALY | 5.89 | 4.46 | 1.42 | 3.59 | 2.30 |
| QALY progression-free disease | 3.37 | 1.88 | 1.50 | 1.87 | 1.51 |
| QALY progression disease | 2.51 | 2.59 | −0.07 | 1.72 | 0.79 |
| Total Costs | €268,827 | €278,066 | -€9239 | €232,200 | €36,627 |
| Treatment costs | €222,545 | €201,587 | €20,958 | €158,171 | €64,374 |
| Subsequent treatment costs | €8125 | €32,561 | -€24,436 | €34,506 | -€26,381 |
| Health-state management costs | €29,539 | €37,831 | -€8292 | €32,195 | -€2656 |
| End-of-life costs | €5400 | €5627 | -€227 | €5870 | -€470 |
| AE costs | €3218 | €459 | €2758 | €1457 | €1760 |
| ICER (€/LYG) | Dominant | €21,040/LYG | |||
| ICUR (€/QALY) | Dominant | €15,912/QALY |
Abbreviations: AE, adverse events; ICER, incremental cost-effectiveness ratio; ICUR, incremental cost-utility ratio; QALY, quality-adjusted life year; LYG, life-year gained.
Regarding the total expenditure, lorlatinib represented a total cost of €268,827 per patient compared with €278,066 with alectinib and €232,200 with brigatinib (Table 5).
The analysis indicated that lorlatinib, as a first-line treatment for ALK+ advanced NSCLC, was a dominant option (more effective and less costly) relative to alectinib. The incremental cost-utility ratio (ICUR) for lorlatinib versus brigatinib was €15,912/QALY gained; as such, lorlatinib could be a cost-effective treatment compared to brigatinib in the management of untreated ALK+ advanced NSCLC, according to the referenced willingness to pay threshold of €25,000/QALY used in Spain.18,19
Sensitivity Analysis
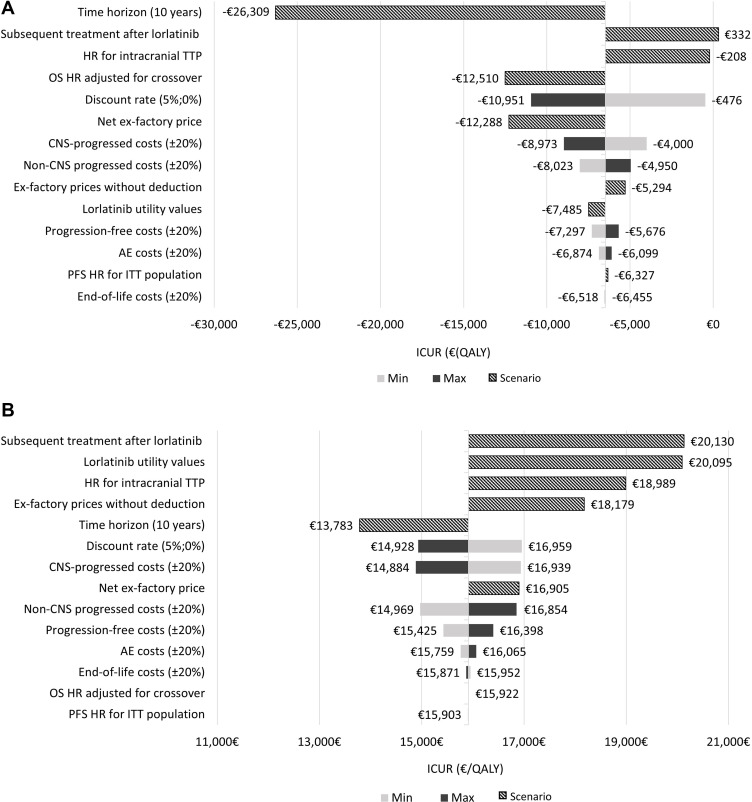
Regarding the OWSA, lorlatinib remained as a dominant option compared to alectinib in 12 of 13 scenarios tested. The scenario with the most influence on results was considering a time horizon of 10 years (Figure 2A). In the analysis of lorlatinib versus brigatinib, the OWSA illustrated that the primary drivers of the model results were the subsequent treatment cost after lorlatinib, followed by the utility values and the acquisition drug costs (Figure 2B).
Figure 2.
(A) Tornado diagram for one-way sensitivity analyses results: lorlatinib vs alectinib. (B) Tornado diagram for one-way sensitivity analyses results: lorlatinib vs brigatinib.
Abbreviations: AE, adverse events; CNS, central nervous system; HR, hazard ratio; ICUR, incremental cost-utility ratio; ITT, intention to treat; QALY, quality-adjusted life year; OS, overall survival; PFS, progression-free survival.
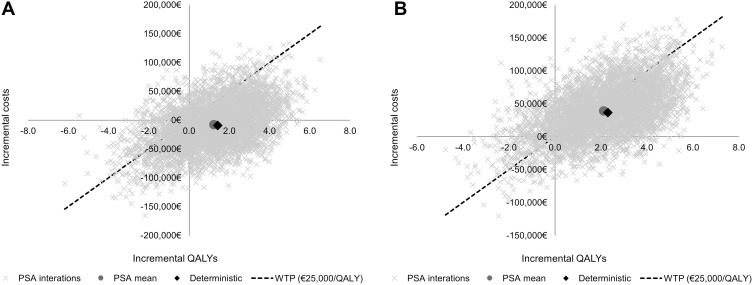
PSA results were consistent with the results from the base case in terms of total costs, LYG and QALYs for each treatment. Like in the deterministic results, alectinib was extendedly dominated by lorlatinib (mean dominant; median €4020/QALY; interquartile range [IQR] dominant - €21,154/QALY). Lorlatinib compared to brigatinib was associated with a mean ICUR of €18,508/QALY (median €16,327/QALY; IQR €6012/QALY-€28,256/QALY). To show PSA results, a cost-effectiveness plane was used (Figure 3A and 3B). At a willingness to pay threshold of €25,000/QALY,18,19 lorlatinib was a cost-effective option in the 81% of 5000 simulations compared to alectinib, and in the 63% of 5000 simulations compared to brigatinib.
Figure 3.
(A) Cost-effectiveness plane comparing lorlatinib vs alectinib. (B) Cost-effectiveness plane comparing lorlatinib vs brigatinib.
Abbreviations: PSA; probabilistic sensitivity analysis; QALY, quality-adjusted life year; WTP, willingness-to-pay threshold.
Discussion
Economic evaluations are an important tool that provide useful information for health decision-makers in the adoption of new therapies. Their inclusion in the reimbursement process would help to promote efficiency and financial sustainability, especially in the area of oncology healthcare technology, which drug expenditure has raised from €10 billion to €32 billion between 2005 and 2018.41
The present study is the first cost-effectiveness analysis of lorlatinib in first-line treatment of patients with ALK+ advanced NSCLC developed from the Spanish NHS perspective. The results obtained in the base case analysis positioned lorlatinib as a dominant alternative versus alectinib and a cost-effective option versus brigatinib. Although our analysis carries some inherent uncertainties associated with an NMA, these ICURs are well below the threshold which establishes what is considered a cost-effective alternative in Spain (below €25,000/QALY).18,19
A previously cost-effectiveness analysis of lorlatinib versus crizotinib in the first-line treatment of ALK+ advanced NSCLC was developed in the United States from the payer perspective.42 Over a lifetime horizon, lorlatinib accrued 0.72 incremental QALY and $293,528 incremental cost compared to crizotinib, resulted in an ICUR of $409,667/QALY gained.42 However, in this study lorlatinib was not compared with alectinib or with brigatinib, which are the alternatives that are most used today to treat ALK+ advanced NSCLC patients. Another study conducted from a Swedish societal perspective, in the second- or third-line treatment of ALK+ advanced NSCLC, showed that lorlatinib compared to chemotherapy was a cost-effective option, resulting in an ICUR of Swedish krona (SEK)603,934/QALY gained (€57,810/QALY), less than the WTP threshold for a high-severity disease treatment in Sweden (SEK 988,000/QALY or €94,574/QALY).43 Recently, has been published the cost-effectiveness analysis of lorlatinib versus crizotinib, alectinib and brigatinib in the first-line treatment of ALK+ advanced NSCLC in Sweden,15 using the same model as in the present analysis. According with the results, brigatinib was dominated by alectinib, and subsequently alectinib was dominated by lorlatinib.15 Lorlatinib compared to crizotinib resulted in a cost-effective option considering the WTP threshold for high-severity diseases in Sweden (SEK 613,032/QALY or €59,555/QALY).15
The strength of our study is the use of data, wherever possible, from clinical trials (although it was necessary to perform an NMA to compare the 3 relevant treatment options in the model), credible and publicly available data sources, or published peer-review studies in accurate medical journals.
Due to the nature of economic models, the study has potential limitations, some of which are inherent to this type of economic evaluation due to its theoretical nature that may not be representative of exact clinical practice. Firstly, in the absence of head-to head clinical trial evidence, an indirect treatment comparison, conducted through an NMA of randomized controlled trials, was used to estimate the alectinib and brigatinib efficacy data. However, this methodology is widely accepted due to provides useful evidence and represents a valuable set of analytical tools to inform clinical evidence in cost-effectiveness analysis.44,45
Secondly, the current cost-effectiveness analysis supposed that the utility data obtained in clinical trials were applicable to the Spanish healthcare setting without any adaptation, however, this assumption was validated by the oncologist experts and considered appropriate. Given the absence of local utility values and to test their influence on the results, this variable was included in the deterministic and probabilistic sensitivity analysis. In the OWSA, to avoid the differences in quality of life between treatments options, a scenario considering the lorlatinib utility values for alectinib and brigatinib were evaluated. Although in the lorlatinib versus brigatinib comparison, the utility values resulted a key input, the ICUR obtained was below the WTP threshold (€25,000/QALY). Thirdly, another limitation lies in the immaturity of the PFS, CNS-PFS and OS data for lorlatinib, which median was not reached at CROWN data cut-off.13 Consequently, survival extrapolation was subject to a high uncertainty and several survival modelling approaches were explored to extrapolate the efficacy data beyond the clinical trial duration. Moreover, because CNS-PFS data were not reported in the alectinib and brigatinib clinical trials, the model assumed that the PFS HR estimated for each treatment versus crizotinib from the NMA was applicable to the CNS-PFS curve, in line with the approach undertaken in the NICE appraisal of brigatinib.17 To analyze the impact of this assumption on the results, an alternative approach was tested in the OWSA, estimating the CNS progression for alectinib and brigatinib based on the intracranial TTP HR obtained from NMA. In this scenario, lorlatinib continued being a cost-effective alternative (ICUR €18.989/QALY) compared to brigatinib and a dominant option compared to alectinib.
Despite the limitations described previously; the results obtained in the OWSA and PSA confirmed the robustness of the model. In OWSA, the results obtained when most parameters were tested did not show a great variation with respect to the base case ICER. In the PSA, lorlatinib dominated alectinib and the mean cost per additional QALY gained with lorlatinib compared with brigatinib remained below the WTP threshold of €25,000/QALY.18,19
The results observed in the present analysis demonstrated that lorlatinib is an efficient option compared to brigatinib and a dominant alternative versus alectinib for the first-line treatment of ALK+ advanced NSCLC. Lorlatinib has shown improved health outcomes over a lifetime horizon, delaying the progression of the disease and increasing the survival compared to brigatinib and alectinib, and reduces the pharmaceutical costs and healthcare resource costs versus alectinib. The efficiency demonstrated would allow clinicians and other stakeholders to make informed decisions regarding the most appropriate treatment, as well as guiding the price and reimbursement process, especially in advanced NSCLC whose management requires long-term treatment.
Despite the robustness of the model, tested by several types of sensitivity analysis, it would be advisable to perform a head-to-head study comparing the second- and third- generation of ALK TKI for the treatment of advanced NSCLC to confirm these results.
Conclusion
Our cost-effectiveness analysis, based on a decision analytic model and an NMA, suggests that lorlatinib could be a dominant treatment option (more effective and less expensive) compared to alectinib, and a cost-effective treatment option versus brigatinib when using the commonly applied WTP in Spain, in the first-line treatment of ALK+ advanced NSCLC.
Funding Statement
This work was supported by Pfizer.
Author Contributions
All authors made a significant contribution to the conception, study design, execution, acquisition of data, analysis, and interpretation; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
MP is an employee of PORIB (PORIB received consultancy fees from Pfizer Spain to adapt the global cost-effectiveness model for lorlatinib into the Spanish environment and NHS). DV reports speaker honoraria from Merck, Sharp & Dohme, Pfizer and AstraZeneca as well as travel/accommodation expenses from Bristol Myers Squibb, Gilead, Roche, Novartis, Merck, Sharp & Dohme and Boehringer-Ingelheim. AC has received honorary/consulting fees from AstraZeneca, Boehringer-Ingelheim, Pfizer, Roche, Eli Lilly company, Novartis, Merck, Sharp & Dohme, Bristol-Myers Squibb, Takeda, Sanofi, Bayer and Janssen. LSO is an employee of PORIB. JN was an employee of BresMed at the time this study was conducted (BresMed received consultancy fees from Pfizer Inc for the development of the global cost-effectiveness model for lorlatinib and supporting statistical analysis). LFG and JS are employees of Pfizer Spain and holds Pfizer stock and stock options. The authors report no other conflicts of interest in this work.
References
- 1.Majem M, Juan O, Insa A., et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018). Clin Transl Oncol. 2019;21(1):3–17. doi: 10.1007/s12094-018-1978-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Provencio M, Carcereny E, Rodríguez-Abreu D, et al. Lung cancer in Spain: information from the Thoracic Tumors Registry (TTR study). Transl Lung Cancer Res. 2019;8(4):461–475. doi: 10.21037/tlcr.2019.08.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–649. doi: 10.1056/NEJMoa1916623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–838. doi: 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- 5.Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379(21):2027–2039. doi: 10.1056/NEJMoa1810171 [DOI] [PubMed] [Google Scholar]
- 6.Selvaggi G, Wakelee HA, Mok T, et al. Phase III randomized study of ensartinib vs crizotinib in anaplastic lymphoma kinase (ALK) positive NSCLC patients: EXALT3. Presented at the International Association for the Study of Lung Cancer World Conference on Lung Cancer, Singapore; 2020. [Google Scholar]
- 7.Bauer TM, Shaw AT, Johnson ML, et al. Brain penetration of lorlatinib: cumulative incidences of CNS and non-CNS progression with lorlatinib in patients with previously treated ALK-positive non-small-cell lung cancer. Target Oncol. 2020;15(1):55–65. doi: 10.1007/s11523-020-00702-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gainor JF, Chi AS, Logan J, et al. Alectinib dose escalation reinduces central nervous system responses in patients with anaplastic lymphoma kinase-positive non-small cell lung cancer relapsing on standard dose alectinib. J Thorac Oncol. 2016;11(2):256–260. doi: 10.1016/j.jtho.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali A, Goffin JR, Arnold A, Ellis PM. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol. 2013;20(4):300–306. doi: 10.3747/co.20.1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Medicines Agency. Summary of product characteristics: Lorviqua [homepage on the Internet]. London: European Medicines Agency; 2021. Available from: https://cima.aemps.es/cima/pdfs/es/ft/1191355002/FT_1191355002.pdf. Accessed November, 2021. [Google Scholar]
- 11.Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57(11):4720–4744. doi: 10.1021/jm500261q [DOI] [PubMed] [Google Scholar]
- 12.European Medicines Agency. Lorviqua® [homepage on the Internet]. London: European Medicines Agency; 2022. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/lorviqua. Accessed March 08, 2022. [Google Scholar]
- 13.Shaw AT, Bauer TM, de Marinis F, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018–2029. doi: 10.1056/NEJMoa2027187 [DOI] [PubMed] [Google Scholar]
- 14.Solomon B, Bauer T, Mok T, et al. Updated efficacy and safety from the phase 3 CROWN study of first-line lorlatinib vs crizotinib in advanced Anaplastic Lymphoma Kinase (ALK)–positive Non-Small Cell Lung Cancer (NSCLC). Poster presented at American Association for Cancer Research (AACR) Congress; April, 2022. New Orleans, USA. [Google Scholar]
- 15.Naik J, Beavers N, Nilsson FOL, et al. Cost‑effectiveness of lorlatinib in first-line treatment of adult patients with Anaplastic Lymphoma Kinase (ALK)‑positive non‑small‑cell lung cancer in Sweden. Appl Health Econ Health Policy. 2023;21(4):661–672. doi: 10.1007/s40258-023-00807-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence. Alectinib for untreated ALK-positive advanced non-small-cell lung cancer (TA536) [homepage on the Internet]. London: National Institute for Health and Care Excellence; 2018. Available from: https://www.nice.org.uk/guidance/ta536. Accessed August 16, 2023. [Google Scholar]
- 17.National Institute for Health and Care Excellence. Brigatinib for ALK-positive advanced non-small-cell lung cancer that has not been previously treated with an ALK inhibitor (TA670) [homepage on the Internet]. London; National Institute for Health and Care Excellence; 2021. Available from: https://www.nice.org.uk/guidance/ta670. Accessed November, 2021. [Google Scholar]
- 18.Sacristán JA, Oliva J, Campillo-Artero C, et al. ¿Qué es una intervención sanitaria eficiente en España en 2020? [What is an efficient health intervention in Spain in 2020?] Gac Sanit. 2020;34(2):189–193. Spanish. doi: 10.1016/j.gaceta.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 19.Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018;27(4):746–761. doi: 10.1002/hec.3633 [DOI] [PubMed] [Google Scholar]
- 20.López Bastida J, Oliva J, Antoñanzas F, et al. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias [Guide proposal for economic evaluation applied to health technologies]. Gac Sanit. 2010;24(2):154–170. Spanish. doi: 10.1016/j.gaceta.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 21.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322–338. doi: 10.1177/0272989X9301300409 [DOI] [PubMed] [Google Scholar]
- 22.Ortega A, Marín R, Fraga MD, López-Briz E, Puigventós F. Guía de evaluación económica e impacto presupuestario en los informes de evaluación de medicamentos. Guía práctica asociada al programa MADRE v 4.0 [Economic evaluation and budgetary impact guide in drug evaluation reports. Practical guide associated with the MADRE v 4.0 program] [homepage on the Internet]. Madrid: Sociedad Española de Farmacia Hospitalaria; 2017. Spanish. Available from: http://gruposdetrabajo.sefh.es/genesis. Accessed February 23, 2021. [Google Scholar]
- 23.Latimer N NICE DSU Technical Support Document 14: undertaking survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data. 2011[homepage on the Internet]. London: National Institute for Health and Care Excellence; 2013. Available from: http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf. Accessed September 16, 2019. [PubMed] [Google Scholar]
- 24.National Statistics Institute. Population mortality tables for Spain by year, sex, age and functions [homepage on the Internet]. Madrid: National Statistics Institute; 2019. Available from: https://www.ine.es/jaxiT3/Tabla.htm?t=27153&L=0. Accessed February 14, 2022. [Google Scholar]
- 25.Van Hout B, Janssen M, Feng Y-S, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–715. doi: 10.1016/j.jval.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 26.Roughley A, Damonte E, Taylor-Stokes G, Rider A, Munk VC. Impact of brain metastases on quality of life and estimated life expectancy in patients with advanced non-small cell lung cancer. Value Health. 2014;17(7):A650. doi: 10.1016/j.jval.2014.08.2364 [DOI] [PubMed] [Google Scholar]
- 27.Botplus web 2.0 [homepage on the Internet]. Madrid: General Council of Pharmacists Official College; 2021. Available from: www.portalfarma.com. Accessed November, 2021. [Google Scholar]
- 28.Real Decreto-ley 8/2010, de 20 de mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público [Royal Decree-law] [homepage on the Internet]. Madrid: BOE de; 2010:126. Available from: www.boe.es/boe/dias/2010/05/24/pdfs/BOE-A-2010-8228.pdf. Accessed November, 2021. [Google Scholar]
- 29.Rodríguez-Abreu D, Powell SF, Hochmair MJ, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32(7):881–895. doi: 10.1016/j.annonc.2021.04.008 [DOI] [PubMed] [Google Scholar]
- 30.Felip E, Shaw AT, Bearz A, et al. Intracranial and extracranial efficacy of lorlatinib in patients with ALK-positive non-small-cell lung cancer previously treated with second-generation ALK TKIs. Ann Oncol. 2021;32(5):620–630. doi: 10.1016/j.annonc.2021.02.012 [DOI] [PubMed] [Google Scholar]
- 31.Gridelli C, Baas P, Barlesi F, et al. Second-line treatment options in non-small-cell lung cancer: report from an international experts panel meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer. 2018;19(4):301–314. doi: 10.1016/j.cllc.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 32.Huber RM, Hansen KH, Paz-Ares Rodríguez L, et al. Brigatinib in crizotinib-refractory ALK+ NSCLC: 2-Year follow-up on systemic and intracranial outcomes in the Phase 2 ALTA trial. J Thorac Oncol. 2020;15(3):404–415. doi: 10.1016/j.jtho.2019.11.004 [DOI] [PubMed] [Google Scholar]
- 33.Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a Phase II Global Study. J Clin Oncol. 2016;34(7):661–668. doi: 10.1200/JCO.2015.63.9443 [DOI] [PubMed] [Google Scholar]
- 34.Oblikue Consulting [homepage on the Internet]. Barcelona: base de datos de costes sanitarios eSalud; 2021. Available from: http://www.oblikue.com/bddcostes/. Accessed January 31, 2022.
- 35.Llibre-Codina JM, Casado-Gómez MÁ, Sánchez-de la Rosa R, et al. Costes de la toxicidad asociada a los análogos de nucleósidos inhibidores de la transcriptasa inversa en pacientes con infección por el VIH-1 [Costs of toxicity associated with nucleoside reverse transcriptase inhibitors in patients with HIV-1 infection]. Enferm Infecc Microbiol Clin. 2007;25(2):98–107. doi: 10.1157/13098570 [DOI] [PubMed] [Google Scholar]
- 36.Martín-Escudero V, García-Muro X, Trigo JM, et al. Uso de recursos y costes relacionados con el manejo de los acontecimientos adversos asociados al uso de terapias dirigidas en el tratamiento del carcinoma de células renales metastático en España [Resources use and costs related to the management of adverse events associated with the use of targeted therapies in the treatment of metastatic renal cell carcinoma in Spain]. Póster presentado en las XXX Jornadas de Economía de la Salud Valencia, España; 2010. Spanish. [Google Scholar]
- 37.Rivera F, Valladares M, Gea S, López-Martínez N. Cost-effectiveness analysis in the Spanish setting of the PEAK trial of panitumumab plus mFOLFOX6 compared with bevacizumab plus mFOLFOX6 for first-line treatment of patients with wild-type RAS metastatic colorectal cancer. J Med Econ. 2017;20(6):574–584. doi: 10.1080/13696998.2017.1285780 [DOI] [PubMed] [Google Scholar]
- 38.Wehler E, Zhao Z, Pinar Bilir S, Munakata J, Barber B. Economic burden of toxicities associated with treating metastatic melanoma in eight countries. Eur J Health Econ. 2017;18(1):49–58. doi: 10.1007/s10198-015-0757-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campone M, Yang H, Faust E, et al. Cost of adverse events during treatment with everolimus plus exemestane or single-agent chemotherapy in patients with advanced breast cancer in Western Europe. J Med Econ. 2014;17(12):837–845. doi: 10.3111/13696998.2014.959589 [DOI] [PubMed] [Google Scholar]
- 40.Instituto Nacional de Estadística. General consumer price index [homepage on the Internet]. Madrid: Instituto Nacional de Estadística; 2022. Spanish. Available from: https://www.ine.es/. Accessed January 31, 2022. [Google Scholar]
- 41.Wilking N, Bradvik G, Lindgren P, Svedman C, Jönsson B, Hofmarcher T. A comparative study on costs of cancer and access to medicines in Europe. Ann Oncol. 2020;31(Suppl 4):S1197. doi: 10.1016/j.annonc.2020.08.2303 [DOI] [Google Scholar]
- 42.Li S, Li J, Peng L, Li Y, Wan X. Cost-effectiveness of lorlatinib as a first-line therapy for untreated advanced anaplastic lymphoma kinase-positive non-small cell lung cancer. Front Oncol. 2021;11:684073. doi: 10.3389/fonc.2021.684073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilsson FOL, Asanin ST, Masters ET, et al. The cost-effectiveness of lorlatinib versus chemotherapy as a second- or third-line treatment in Anaplastic Lymphoma Kinase (ALK)-positive non-small-cell lung cancer in Sweden. Pharmacoeconomics. 2021;39(8):941–952. doi: 10.1007/s40273-021-01015-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14(4):417–428. doi: 10.1016/j.jval.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 45.Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR task force on indirect treatment comparisons good research practices: part 2. Value Health. 2011;14(4):429–437. doi: 10.1016/j.jval.2011.01.011 [DOI] [PubMed] [Google Scholar]