Abstract
The growing concern of consumers with the welfare of production animals searches welfare in a production system extremely important; thus, the study of animal temperament is necessary to select less excitable temperament animals resulting in healthy development and fewer accidents. The objective of this study was to estimate genetic parameters for traits related to animal temperament and growth traits of Nellore cattle. In addition to exploring the genetic pattern of these traits through cluster and principal component analysis (PCA), to reveal possible groups of individuals that express less excitable temperament and greater growth. A total of 2,332 measurements from 1,245 male and female Nellore cattle born between 2008 and 2016 were utilized in the study. The (co)variance components were estimated by Bayesian inference using a two-trait animal model. The heritability for temperament score (TS), flight speed (FS), body condition score (BCS), live weight (LW), and hip height (HH) were 0.08, 0.12, 0.06, 0.13, and 0.48, respectively. The genetic correlation between the temperament indicator traits was strong and positive (0.78 ± 0.24). The TS and FS showed a favorable or null genetic correlation with LW, BCS, and HH. The third cluster included animals with low EBV for TS and FS and with high EBV for BCS, LW, and HH. In the PCA, the PC1 was what best evidenced the aim of this study; thus, our findings suggest that we could explore select animals based on cluster 3 and PC1 in breeding programs to select Nellore cattle with less excitable temperament and greater growth.
Keywords: cluster analysis, genetic correlation, heritability, temperament, zebu
Some scientific studies estimate the genetic parameters of temperament characteristics of Nellore animals. However, not many studies correlate temperament traits with growth traits through multivariate analyses to use them as a tool to assist in selecting animals with high genetic value for growth and low for temperament. Thus, we believe this study may interest its readers to update the scientific literature on this subject.
Introduction
The increasing demand for food derived from animals in the last decades resulted in an intensification of production systems that were often not concerned with the issue of sustainability and especially the welfare of animals. However, in recent years, this issue has become increasingly relevant. According to Alonso et al. (2020), consumers’ concerns about animal welfare can prevent them from consuming that kind of product, influencing the sustainability systems.
Temperament assessments of domestic animals are an essential tool that may be employed to improve production systems, given that animals with more excitable temperaments are significantly more difficult to handle. This represents a risk factor for animal handlers and the animals themselves, generating additional costs due to increased handling time and the incidence of work accidents (Fordyce et al., 1988; Grandin, 1993; Maffei, 2009). However, a less excitable animal or one of calmer temperament is not only associated with ease in handling but also with other economically important traits (Maffei, 2009; Costilla et al., 2020).
Animals with more excitable temperaments or aggressive behavior, both in confinement and/or in extensive systems, may exhibit production problems since they gain less weight (Hoppe et al., 2010; Sant’Anna et al., 2012, 2013; Lucena et al., 2015); display inferior meat quality (Kadel et al., 2006; Cafe et al., 2011; Francisco et al., 2015); retain inferior reproductive efficiency (Barrozo et al., 2012; Rueda et al., 2015; Cooke et al., 2017); and are more susceptible to diseases, due to compromised immune systems based on physiological stress indicators, such as elevated concentrations of basal serum cortisol in more excitable animals (Curley Jr et al., 2008; Burdick et al., 2011; Bates et al., 2014). For these reasons, selecting animals with calmer temperaments should be beneficial for enhancing animal performance, welfare, and safety (Norris et al., 2014).
Some studies suggest that temperament may respond to selection (Prayaga et al., 2009; Sant’Anna et al., 2013; Lucena et al., 2015). The heritability estimates for cattle are generally of low to moderate magnitude and vary according to the method, population, and breed, indicating that this trait can be modified by selection (Fordyce et al., 1982; Hoppe et al., 2010; Burdick et al., 2011).
Several studies in Nellore cattle found heritability estimates for temperament scores that varied between 0.15 and 0.26 (±0.03) (Barrozo et al., 2012; Sant’Anna et al., 2013, 2015; Lucena et al., 2015; Valente et al., 2015). Heritability estimates for flight speed for the Nellore breed varied between 0.21 (±0.03) and 0.35 (Sant’Anna et al., 2012, 2013, 2015; Valente et al., 2015, 2016). Favorable genetic correlations between temperament and production traits were observed in some studies (Hoppe et al., 2010; Sant’Anna et al., 2015), indicating that genetic selection for improving animal temperament will result in an indirect genetic increment in production, such as in the study conducted by Lucena et al. (2015), which found a genetic correlation of −0.33 (±0.01) between the temperament score and weaning weight in Nellore cattle. In other words, the worse the temperament scores, the lower the weaning weight of the animal.
Multivariate cluster analysis, also known as multivariate clustering, is largely used to evaluate temporal and spatial variations and to interpret large and complex data sets. (Hair et al., 2009). Is indeed a powerful statistical tool used to explore large datasets where multiple variables are evaluated simultaneously (Oliveira et al., 2018). This analysis aims to identify and group individuals or objects based on the similarity of their traits or attributes. However, this method is still not widely applied in animal breeding studies to group animals based on estimated breeding values of important economic traits (Oliveira et al., 2018).
The process of multivariate cluster analysis involves several steps. First, a set of variables or attributes is selected based on the research question or problem at hand. These variables should be relevant and meaningful in the context of the analysis. Examples of variables could include demographic information, behavioral traits, or performance measures. Next, a distance or similarity measure is applied to quantify the dissimilarity or similarity between pairs of individuals based on their values for the selected variables (Backhaus et al., 2021).
The resulting clusters are then interpreted and analyzed to gain insights into the underlying patterns or subgroups present in the data. This analysis can help identify distinct segments within a population, uncover relationships between variables, or discover hidden structures within the dataset.
Cruz et al. (2016) used multivariate cluster analysis to identify additive genetic patterns for the persistency of lactation in Guzera cattle. Savegnago et al. (2016) identified additive e genetic patterns of Holstein to select animals that met the selection goals of the breeding program using cluster analysis.
Similar to Multivariate cluster analysis, principal component analysis (PCA) is a widely used statistical technique for dimensionality reduction and data exploration (Johnson and Wichern, 2007). It is particularly useful when dealing with datasets that have a large number of variables or features. The main objective of PCA is to transform the original variables into a new set of uncorrelated variables, known as principal components while retaining most of the information present in the data. Overall, PCA is a powerful tool for exploring and understanding the underlying structure of high-dimensional data. It allows for data reduction while preserving the essential information, facilitating further analysis and interpretation (Vargas et al., 2018).
The objectives of this study were to 1) estimate the genetic parameters for indicator traits for animal temperament and growth traits in Nellore cattle; 2) use the estimated breeding value of the evaluated animals in cluster analyses to assess whether there are groups within the population that can be used as candidates for selection; 3) investigate the feasibility of the PCA to genetically select for a specific breeding objective based on calm temperament animals with high merit to growth trait.
Materials and methods
The experimental procedures of this study were approved by the Ethics Committee of the Animal Science Institute of Sao Paulo State, Brazil (Protocol 192-14). The study was conducted with data from the Beef Cattle Research Center of the Institute for Animal Sciences/APTA/SAA, Sertãozinho, São Paulo, Brazil, from October 2014 to August 2017.
The beef cattle selection program works with three Nellore selection lines. The control (NeC) line is a closed line in which sires from the same center were used, and the animals were selected for average postweaning weight. The selection (NeS) line is another closed line, while the traditional (NeT) line is an open line in which sires from other populations both within and outside the same center were used, particularly during the early years of the breeding program. In the NeS and NeT lines, the animals were selected for the highest differentials to increase postweaning weight (Cardoso et al., 2018; Freitas et al., 2021). The animals were weighed at calving, at 120 d of age, and after weaning at 210 d of age (when the assessment began). After weaning, all of the animals were selected for yearling weight (YW) where they were weighed at the beginning, middle, and end of the performance test. In general, males were sold or slaughtered after the performance test. The females that were selected to remain in the herd were weighed three times per year, at the beginning and end of the mating season (November and February, respectively), and the weaning of the calves in May.
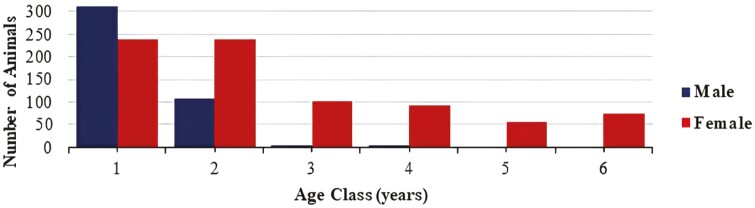
Table 1 summarizes the descriptive data of the genetic analyses. A total of 2,332 records from 1,245 Nellore cattle, of which 440 were male, and 805 were female, were used. The animals came from three selection lines, were born between 2008 and 2016, and were divided into six age classes. class 1: 7 mo to 1 yr; class 2: 1 to 2 yr; class 3: 2 to 3 yr; class 4: 3 to 4 yr; class 5: 4 to 5 yr, and class 6: above 5 yr of age (Figure 1). Of the 1,245 animals analyzed, 596 were recorded only once (in the majority consisted of animals born at the end of 2016 and that were males recorded before the slaughter), 310 animals were recorded twice, and 339 underwent three or more recordings.
Table 1.
Descriptive statistics of the analyzed dataset
| Trait | N | Mean | Minimum | Maximum | SD | CG |
|---|---|---|---|---|---|---|
| TS | 999 | 2.30 | 1.00 | 5.00 | 1.09 | 39 |
| FS (m/s) | 1,040 | 1.98 | 0.07 | 10.71 | 1.12 | 42 |
| BCS | 642 | 5.98 | 2.00 | 9.00 | 1.07 | 31 |
| Live weight (kg) | 1,076 | 348.73 | 96.00 | 901.00 | 127.48 | 37 |
| Hip height (m) | 529 | 1.37 | 1.05 | 1.62 | 0.09 | 31 |
| Age (d) | 1,245 | 657.00 | 210.00 | 2,432.00 | 510.86 | 42 |
TS, temperament score; FS, flight speed; BCS, body condition score; N, number of animals; SD, standard deviation; CG, number of contemporary groups.
Figure 1.
Distribution of the number of animals per age class in years.
The behavioral and growth traits were observed/measured for all animals in the herd. At the same time, they were handled in corrals for routine farm procedures (e.g., weighing, vaccination, and blood collection) to assess the behavior of the animals in their regular environment. After vaccinations or blood sample collections, the handlers moved away from the animal and waited about 30 s before releasing the animal. The animals were evaluated in three different corrals existing in the Center. Therefore, the recording of the traits was conducted at random in the three corrals according to the farm’s study schedule.
The body condition score (BCS) of the animals was determined by a single, adequately trained observer on a scale from 1 to 9, where 1 corresponded to an emaciated animal and 9 to an extremely obese animal (Nicholson and Butterworth, 1986). Hip height (HH, in meters) was measured using a measuring tape fixed to the chute, and the live weight (LW) was determined using a digital scale. These measurements were conducted simultaneously with the temperament assessments.
The behavior of the animals during restraint was evaluated using a temperament score (TS) adapted from Fordyce et al. (1982), which ranges from 1 to 5, classifying the animal as very calm (1), characterized by gentle movements of the head during containment; little agitated (2), defined by the movement of the head and body, taking a maximum of one step; agitated (3), hectic movements of the head and body and attempts to walk in the chute; very agitated (4), defined when the animal shakes the head frequently, moves a lot, and tries to walk in the chute, and struggling (5), characterized by struggling of the animal, with brisk movements, attempting to escape from the handler during restraint.
The flight speed (FS) was measured with specific equipment installed on metal support at the exit of the chute, which analyzed the time spent by the animal to move 1.5 m towards the exit. The recorded times were converted into meters per second (m/s). Many of the exit velocity or time tests that are currently used are adapted from the flight speed test (Burrow et al., 1988), with alterations in distance (Paranhos da Costa et al., 2002; Cafe et al., 2011). In this evaluation, higher flight speeds are associated with greater excitability of the animal.
A preliminary analysis of variance was performed using the GLM procedure of the SAS program (SAS Inst. Inc., Cary, NC, USA) to determine which nongenetic effects should be included in the model. The models for all of the traits included the fixed effects of age class nested in sex; contemporary group, which was composed of the selection lineage, year of birth, year of recording and handling corral; and an interaction between sex and contemporary group. Contemporary groups with at least five observations were kept in the analyses. The genetic effects for all traits included direct additive genetic, permanent environmental, and residual effects as random effects.
The (co)variance components for all of the traits were estimated with the software GIBBS2F90 (Misztal et al., 2002) by Bayesian inferences via the Gibbs sampling, using a two-trait animal model. A single chain of 500,000 cycles was performed, with a conservative burn-in period of 50,000 cycles and a thinning interval of 50 cycles. Thus, 9,000 samples were effectively used to obtain the means, standard deviations, and highest posterior density intervals of the variance components and genetic parameter estimates. Convergence was verified using Geweke’s criterion (Geweke, 1991). The posterior estimates were obtained using the POSTGIBBSF90 software (Misztal et al., 2002).
The model can be written in matrix form, as follows:
where y corresponds to the vector of observations; β is the vector of the fixed effects (contemporary group, interaction between sex and contemporary group and age class nested in sex); α symbolizes the vector of random direct additive genetic effects; pe is the vector of random permanent environmental effects; ε represents the vector of random residual effects; X, Z, and W are incidence matrices that assign observations to fixed effects and random additive genetic, permanent environmental, and residual effects, respectively. Faria et al. (2008) and Pires et al. (2010) did not observe differences in the genetic parameters for visual scores using either a threshold or a linear model for a similar variable in Nellore cattle. Therefore, the following assumptions were made:
where A, G, P, R, and In represent the additive genetic relationship matrix amongst animals (2,791 animals), the covariance matrix between traits for additive genetic, permanent environmental, and residual effects, and an identity matrix of order n, respectively; symbolizes the Kronecker product; Sg and vg, Sp and vp, and Sr and vr correspond to prior values and the degrees of freedom for direct additive genetic, permanent environmental, and residual covariances, respectively.
In the multivariate analysis of the data, the genetic values for all traits were estimated with the BLUPF90 software (Misztal et al., 2002), where the values of (co)variances previously estimated by bi-traits analysis were fixed. Only predicted breeding values (EBVs) from 1,245 animals with information were used in the multivariate analysis.
The hierarchical and nonhierarchical cluster analyses were performed using the EBVs of all traits. As described by Oliveira et al. (2018), the aim was to group animals based on the similarities of their EBVs and then to evaluate the additive genetic pattern of the groups formed within the studied population. Initially, all of the genetic values were standardized with means equal to 0 and a standard deviation of 1.
Hierarchical cluster analysis was used to determine the number of clusters into which the population could be divided. The Euclidean distance was used as a measure of similarity between the animals and the Ward cluster algorithm (Ward Jr., 1963) was used to form the groups (Savegnago et al., 2016).
The analysis of nonhierarchical groupings was conducted using the k-means method (Hartigan, 1975; Hartigan and Wong, 1979) to explore the additive genetic pattern of the clusters, based on the breeding values of the evaluated traits. The cluster analysis was carried out using CLUSTER procedure of the SAS statistical program (SAS Inst. Inc., Cary, NC, USA).
PCA consists of a summary of important information from multivariate data in sets of new variables named principal components (PCs), reducing the number of variables (Johnson and Wichern, 2007). In animal breeding, these new variables can be used to study the associations between multiple traits using the magnitude and direction of the PCA coefficients (in the eigenvectors) for each trait (Vargas et al., 2018). The PC eigenvalue is associated with the variance of all five traits included in the PC. Each eigenvalue has a corresponding unitary vector named eigenvector which represents the strength and direction of the variance of each variable within the PC and eigenvectors with positive or negative loadings above 0.5 are more strongly bound to their components. Thus, in practice, PCs are a combination of traits that potentially have a biological meaning.
There are different criteria to determine how many components should be excluded from the analysis, in this study we used the Kaiser criterion i.e., eigenvalue greater than 1.0 (Kaiser, 1960) to identify the PCs that explained the largest proportion of the total genetic variation of the traits. The first PC explains the largest percentage of the variation of genetic variance of the traits, the second PC explains the second largest percentage, and so on.
The PCA also was performed using the EBVs of the all traits studied, just as in cluster analysis, the EBVs were standardized with means equal to 0 and a standard deviation of 1. The PCA was carried out using PRINCOMP procedure of the SAS statistical program (SAS Inst. Inc., Cary, NC, USA).
Results
Estimation of genetic parameters
The direct additive genetic variance was higher than the permanent environmental variance for TS and HH, indicating that the effect of the permanent environment had a lower contribution to the phenotypic variability of these traits. On the other hand, for FS, LW, and BCS, the permanent environmental variation was the primary cause of phenotypic variation (Table 2).
Table 2.
Posteriori mean, standard deviation, and 95% high posterior density (HPD) for the estimates of genetic parameters for each assessed trait
| Mean | Standard deviation | HPD 95% | |
|---|---|---|---|
| Temperament score (TS) | |||
| σ²a | 0.08 | 0.03 | 0.02 to 0.15 |
| σ²pe | 0.04 | 0.03 | 0.00 to 0.11 |
| σ²e | 0.85 | 0.04 | 0.77 to 0.93 |
| h² | 0.08 | 0.03 | 0.02 to 0.15 |
| r | 0.12 | 0.04 | 0.05 to 0.19 |
| Flight speed (FS) | |||
| σ²a | 0.11 | 0.04 | 0.04 to 0.19 |
| σ²pe | 0.14 | 0.04 | 0.06 to 0.23 |
| σ²e | 0.63 | 0.03 | 0.57 to 0.69 |
| h² | 0.12 | 0.04 | 0.04 to 0.21 |
| r | 0.24 | 0.03 | 0.18 to 0.31 |
| Body condition score (BCS) | |||
| σ²a | 0.03 | 0.02 | 0.01 to 0.08 |
| σ²pe | 0.21 | 0.03 | 0.16 to 0.27 |
| σ²e | 0.27 | 0.02 | 0.24 to 0.31 |
| h² | 0.06 | 0.04 | 0.01 to 0.15 |
| r | 0.48 | 0.03 | 0.41 to 0.15 |
| Live weight (LW) | |||
| σ²a | 229.68 | 88.12 | 84.58 to 481.44 |
| σ²pe | 934.77 | 88.49 | 761.28 to 1,107.13 |
| σ²e | 606.41 | 30.02 | 550.75 to 667.11 |
| h² | 0.13 | 0.05 | 0.05 to 0.23 |
| r | 0.66 | 0.02 | 0.62 to 0.69 |
| Hip height (HH) | |||
| σ²a | 0.0009 | 0.0002 | 0.000 to 0.001 |
| σ²pe | 0.0005 | 0.0001 | 0.000 to 0.001 |
| σ²e | 0.0004 | 0.0003 | 0.0004 to 0.0005 |
| h² | 0.48 | 0.07 | 0.34 to 0.62 |
| r | 0.77 | 0.02 | 0.73 to 0.81 |
σ²a, direct additive genetic variance; σ²pe, permanent environmental variance; σ²e, residual variance; h², heritability; r, repeatability.
The studied behavioral traits, TS and FS, exhibited low heritability, indicating greater environmental effects for these traits. The repeatability estimates for the same traits ranged from low to moderate (Table 2). The heritability for BCS, LW, and HH ranged from low to moderate, and their repeatability ranged from moderate to high.
The genetic correlation between TS and FS was positive and high, indicating that the lower the TS, the lower the flight speed of the animals and, consequently, the calmer their temperament during handling. The correlations between the behavioral and growth traits were mostly negative, with magnitude ranging from low to moderate (Table 3), except for the correlations between TS and HH, and FS and BCS which were positive, but both with high standard deviation. The genetic correlations between TS and BCS, and FS and LW, even with high HPD intervals, may be indicative that calmer temperaments can be related to higher performance. The results of this study highlight the importance of identifying animals with calmer temperaments because docile animals tend to have greater growth potential. All the values of phenotypic correlations between temperament and growth traits were zero or close to zero, except for FS × TS (Table 3).
Table 3.
Genetic and phenotypic correlations (above and below the diagonal, respectively), respective standard deviations, and 95% high posterior density (HPD) between parentheses for the analyzed traits
| TS | FS | BCS | LW | HH | |
|---|---|---|---|---|---|
| TS | 0.78 ± 0.24 (0.20 to 0.99) | −0.37 ± 0.35 (−0.96 to 0.35) | −0.29 ± 0.31 (−0.83 to 0.33) | 0.09 ± 0.29 (−0.42 to 0.86) | |
| FS (m/s) | 0.20 ± 0.04 (0.13 to 0.28) | 0.19 ± 0.44 (−0.71 to 0.97) | −0.47 ± 0.29 (−0.97 to 0.11) | −0.18 ± 0.19 (−0.54 to 0.22) | |
| BCS | −0.06 ± 0.04 (−0.14 to 0.02) | −0.01 ± 0.04 (−0.10 to 0.07) | 0.10 ± 0.42 (−0.84 to 0.74) | 0.16 ± 0.37 (−0.61 to 0.97) | |
| LW (kg) | −0.02 ± 0.04 (−0.10 to 0.05) | 0.001 ± 0.04 (−0.08 to 0.08) | 0.39 ± 0.04 (0.12 to 0.26) | 0.82 ± 0.11 (0.58 to 0.99) | |
| HH (m) | −0.01 ± 0.05 (−0.10 to 0.08) | −0.04 ± 0.05 (−0.14 to 0.06) | −0.06 ± 0.06 (−0.11 to 0.11) | 0.30 ± 0.05 (0.18 to 0.38) |
TS, temperament score; FS, flight speed; LW, live weight; BCS, body condition score; HH, hip height of Nellore cattle.
Clustering analysis
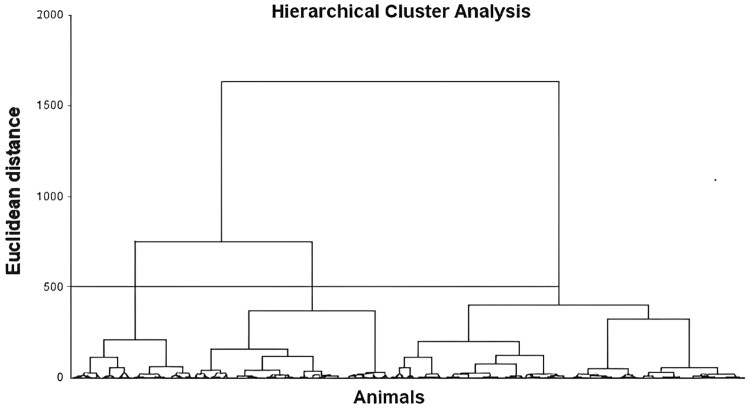
The hierarchical clustering analysis using the EBVs of animals was performed and generated the dendrogram presented in Figure 2, in which it was possible to observe the clustering of the population into three large clusters.
Figure 2.
Dendrogram based on the predicted breeding values (EVBs) regarding the temperament score (TS), flight speed (FS), body condition score (BCS), live weight (LW), and hip height (HH) using Hierarchical cluster analysis with Ward’s method.
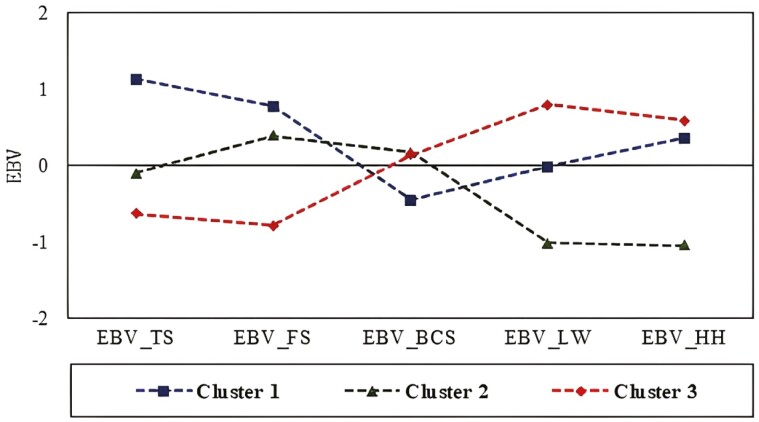
The genetic patterns of each of the three major groups, established using the standardized genetic values of the animals, are shown in Figure 3. Group 1 included animals with EBV for TS, FS, and HH above the mean of the population, EBV for BCS below the mean, and EBV for LW close to the mean of the population (zero). Group 2 was composed of animals with EBV for TS and BCS close to the mean of the population (slightly below and slightly above, respectively), EBV for FS above the mean, and EBV for LW and HH below the mean. Group 3 was composed of animals with EBV for TS and FS below the mean, EBV for BCS slightly above the mean of the population, and EBV for LW and HH above the mean of the population (Figure 3).
Figure 3.
Standardized means of the breeding values (EBVs) for the traits used to divide Nellore cattle into three clusters by the k-means method. EBV_TS, temperament score; EBV_FS, flight speed; EBV_BCS, condition score; EBV_LW, live weight; and EBV_HH, hip height.
PCA
Table 4 presents the eigenvalues, proportion, and cumulative sum of the explained variance over the five components. According to the Kaiser’s criterion (Kaiser, 1960), the first three PCs were chosen explaining 99.3% of the total variance of the EBVs.
Table 4.
Principal components (PC), eigenvalues, difference, proportion, and cumulative sum of the explained variance over the five components studied
| Eigenvalue | Difference | Proportion | Cumulative | |
|---|---|---|---|---|
| PC1 | 2.468 | 1.039 | 0.494 | 0.494 |
| PC2 | 1.429 | 0.360 | 0.286 | 0.779 |
| PC3 | 1.069 | 1.041 | 0.214 | 0.993 |
| PC4 | 0.028 | 0.023 | 0.006 | 0.999 |
| PC5 | 0.006 | 0.001 | 1.000 |
Table 5 shows the eigenvector coefficients for the first three PCs. From the five original dimensions using the eigen-decomposition, PC1 explained 49.4% of the total variation and had contrasting coefficients for temperament. The variables that most contributed to this component were FS and LW, with contrasting coefficients, reflecting those animals with a higher LW and calmer temperament. The PC2 accounted for 28.6% of the total genetic variation and in this component, highlighted the high positive loading for TS and HH, reflecting those animals with a higher HH and more temperamental according to TS. The PC3 accounted for 21.4% of the total genetic variation, where the strongest correlation was with BCS.
Table 5.
Eigenvectors coefficients for the first three principal components (PCs).
| Eigenvectors | |||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| EBV_TS | −0.397 | 0.653 | 0.010 |
| EBV_FS | −0.546 | 0.298 | 0.354 |
| EBV_BCS | 0.049 | −0.290 | 0.904 |
| EBV_LW | 0.578 | 0.326 | 0.087 |
| EBV_HH | 0.456 | 0.543 | 0.224 |
Predicted genetic values of the traits: temperament score (EBV_TS), flight speed (EBV_FS), body condition score (EBV_BCS), live weight (EBV_LW), and hip height (EBV_HH).
Discussion
The estimated heritability regarding the TS was lower than those reported heritabilities in the literature for bovine animals of the Nellore breed ranging between 0.15-0.21 (Barrozo et al., 2012; Sant’Anna et al., 2013, 2015; Valente et al., 2015). The heritability estimate for FS for Nellore cattle was also considerably lower than those in the literature, ranging between 0.21 and 0.35 (Sant’Anna et al., 2012, 2013, 2015; Valente et al., 2015, 2016).
As well as the research conducted by Sant’Anna et al. (2013, 2015) and Valente et al. (2014) with Nellore beef cattle, the FS retained slightly higher heritability than when compared with the TS. The permanent environment effect had a more significant contribution to the phenotypic variability of the FS. Thus, the low heritability estimates described in the present study may have been due to the fact that those traits were measured in animals with a large age difference, different from the age of the animals in the studies by Sant’Anna et al. (2012) and Valente et al. (2015), which were about 495 and 550 d, respectively.
Nevertheless, genetic gain by way of selection regarding FS and TS can still be accomplished, given the impact that these traits cause on traits of economic importance and mainly in the welfare of the animals, facilitating the handling, and avoiding aggression.
Growth traits are essential to establish the economic efficiency of any given production system (Laureano et al., 2011). One measure that is commonly employed as a growth indicator is LW, mainly due to its convenience. The estimated heritability of LW in this study was also lower than values found in other studies with the weight heritability in Nellore animals ranging from 0.22 (±0.03) to 0.44 (±0.05), according to age (Yokoo et al., 2009). However, animals that are reared in extensive systems often exhibit periodic fluctuations in LW, and, therefore, the heritability of this trait may vary according to age, as described by Yokoo et al., (2009).
Another important growth indicator utilized in this study was the BCS, which consists of a subjective measurement obtained by the visual inspection of the animal’s muscle mass and fat cover, enabling monitoring of the energetic balance of the animals. In the present study, the heritability regarding the BCS was lower when compared to what is described in the literature for Nellore cattle: 0.23 (±0.05) for BCS on a scale from 1 to 5 measured in Nellore females at about four years of age (Silveira et al., 2015) and 0.21 (±0.03) in a scale from 1 to 9 (Mercadante et al., 2006), but only from females born at the same Institute, between the years 1961 and 2001.
The difference found in the heritability estimates for BCS and LW may be explained by the significant difference in age because, in the literature, age is usually standardized, as previously mentioned (Barrozo et al., 2012; Sant’Anna et al., 2012; Valente et al., 2014; Lucena et al., 2015). In addition to being a study composed of males and females of different ages, unlike the studies mentioned (Mercadante et al., 2006; Silveira et al., 2015). The heritability of HH was similar to that observed in the study conducted by Yokoo et al, (2009) which estimated heritability of 0.46 (±0.09) to males and females Nellore with ages ranging from 450 to 599 d.
The genetic correlation between the TS and FS (Table 3) suggests the existence of a strong genetic association between both traits and that selection regarding either one may lead to genetic modifications in the other. In other words, the lower the TS, the lower the FS. Moreover, both traits are considered favorable to selection in the animal breeding program that uses weight as a selection criterion, as was described in a study conducted by Sant’Anna et al. (2013) with Nellore animals, in which a high and positive correlation between TS and FS was estimated, of 0.85 ± 0.05.
The genetic correlations between the temperament and growth traits were, in general, of low to moderate magnitude, corroborating with the literature (Barrozo et al., 2012; Lucena et al., 2015; Sant’Anna et al., 2012, 2013, 2015; Valente et al., 2014, 2016). However, except for the positive correlation between TS and HH, and FS and BCS, all other correlations were negative (even with high HPD intervals), being able to indicate that calmer behavior (lower scores) may be genetically associated with higher values of BCS, LW, and HH. The FS was the behavioral trait that retained the strongest genetic association with the LW, although it exhibited a positive correlation with BCS. The TS was shown to be more genetically associated with BCS. Thus, the FS can be considered an important trait to be integrated into animal breeding programs that use weight as a selection criterion. Furthermore, FS is currently one of the most understood and employed behavioral measures in beef cattle worldwide and is validated for bovine breeds in the most distinct handling situations due to its objectivity and convenience in measuring (Cafe et al., 2011; Sant’Anna et al., 2013).
Even with a high standard deviation, it is possible to observe a low, but negative correlation between TS and BCS, indicating a relative association between both traits, in which the greater the BCS, the lower the TS. This result corroborates the findings observed in the study conducted by Sant’Anna et al. (2015), who stated that, in general, the results found in their studies, indicated that both the visual scores conformation, precocity, muscularity, BCS, and frame are weakly associated with temperament.
Unexpectedly, the genetic correlation between FS and the BCS was positive, although with a high standard deviation (Table 3). This result indicates that when carrying out selection for increments in BCS, a response in animal temperament will possibly not occur. Using Spearman correlations, Petherick et al. (2002) reported significant and negative associations that varied from −0.23 to −0.33 between FS and the BCS during the confinement period in crossbred Bos indicus heifers. In another study that aimed at evaluating the impact of temperament in Nellore cows on handling quality and the rates of pregnancy by artificial insemination, significant, negative, and low-magnitude Pearson correlations were reported between the BCS and FS (−0.14) and the BCS and the crushing score (−0.11) (Rueda et al., 2015).
The genetic correlation between the TS and LW was negative, but with a high standard deviation, indicating that animals retaining greater genetic potential for LW may exhibit calm temperament since the genetic correlation between FS and LW was also negative, but with a greater magnitude and by the fact FS and TS are highly correlated. This result is in accordance with what is reported in the assessed literature, in which the genetic correlation was negative (−0.33 ± 0.03) between the TS and the weaning weight in bovine animals of the Nellore breed (Lucena et al., 2015). A similar result was described in another study with Nellore animals, in which the correlation between the TS and the body weight gain at weaning was −0.18 ± 0.07 and the genetic correlation between FS and average daily gain was −0.08 (±0.07) (Sant’Anna et al., 2013). Another study with the same breed reported an association between FS and weaning weight of −0.20 (±0.07) (Sant’Anna et al., 2015). Thus, selection for increments in LW may provide improvements in the temperament of the animals (lower TS and FS).
The antagonistic genetic correlations of the behavioral traits with HH (TS × HH, positive, and FS × HH, negative), and with BCS (TS × BCS, negative, and FS × BCS, positive) indicate that, when selecting for taller animals or with greater body condition, a response in the temperament of the animals will possibly not occur. However, overall, the selection is aimed at increasing not only height but also weight, given that taller animals may be less precocious and less efficient and exhibit inferior carcass quality (Yokoo et al., 2009).
Therefore, selection aimed at weight increments in the animals will also increase their height due to the highly correlated response between LW and HH (Table 3) (Yokoo et al., 2007, 2009) and, consequently, the temperament of these animals will tend to be calmer. The strong genetic correlation between LW and HH observed in the present study agrees with the literature showing estimated genetic correlations that ranged from 0.58 to 0.80 at different ages (Yokoo et al., 2007).
The existence of genetic variation allows the division of genetically similar animals into groups using multivariate cluster analysis based on EBVs for all studied traits. This division will enable a selection of animals with low EBVs for TS and FS and high values for BCS, LW, and, consequently, HH, which comprise the desired traits in a production system composed of Nellore animals. Thus, cluster analysis is a tool that can be used in addition to selection indexes.
Groups 1, 2, and 3 consisted of 324, 401, and 520 animals, respectively. When analyzing the average genetic value of each variable (within its respective group), if selection were performed for the animals in group 1, excitable animals (higher TS and FS) with low or no genetic merit for BCS and LW, respectively, and possibly larger animals would have been chosen. If selection were performed for the animals in group 2, animals with low or no genetic merit for TS, BCS, LW, and HH and with an excitable temperament (higher FS) would have been chosen. In turn, if selection would prioritize animals in group 3, less excitable animals with more significant genetic merit for growth traits (BCS, LW, and HH) would be selected, given that these are the most desired traits in a production system.
In general, the genetic relationship among traits is related to the three PCs that explain the highest proportion (99.3%) of the total additive genetic variance of the 5 traits. Vargas et al. (2018) applied three different approaches of PCA using standardized EBVs for growth and visual score traits at weaning and yearling in Nellore beef cattle and reported that three PCs met the Kaiser criterion (>1) and explained 79.3%, 87.1%, and 87.8% of the total variance in each approach, respectively. Boligon et al. (2016) reported that the first three PCs explained 79.1% of the total variation in EBVs for growth and reproductive traits in Nellore cattle.
The magnitude of the eigenvector (either positive or negative) indicates the importance of the corresponding trait for the PC; thus, a higher coefficient would indicate greater discriminatory power. The first PC showed a high coefficient for the original FS and LW traits, suggesting a greater discriminatory power for these traits. An opposite sign between temperament traits and growth traits indicates a different direction of variation for these variables, as already demonstrated by the genetic correlations. These findings again show that animals with higher genetic merit for growth tend to be less excitable.
The second PC showed a greater discriminatory power for the original TS and HH traits but with the same sign (positive), as also evidenced by the genetic correlations, reflecting those animals with a higher HH and more temperamental according to TS. In contrast, the third PC showed a greater discriminatory power for BCS, with a positive and high coefficient.
Conclusion
The inclusion of temperament indicator traits such as TS and FS in selection indexes, which is aimed at achieving more significant genetic progress in animal temperament and welfare, desirable mainly for Bos indicus, may result in actual benefits for productivity and the well-being of animals since those that exhibit less excitable temperament retain more significant genetic increments in growth traits. However, progress tends to be slower due to low heritabilities.
Multivariate cluster analysis and PCA help selectors (livestock farmers) more easily choose the animals they want to select for breeding, making it easier for the producer to visualize which animals have the traits that should be chosen together. Therefore, the fact that 1,056 of the 1,245 animals evaluated came from herds selected for yearling weight is one of the reasons why cluster 3, a group formed by high-performance animals, is the largest group with 520 animals showing that selection for weight is correlated with temperament improvement as well as the principal component 1, shows us.
Our findings suggest that the inclusion of temperament indicator traits such as TS and FS, as well as cluster and PCA, may be explored in animal breeding programs to select Nellore cattle with less excitable temperament and more significant genetic increment in growth traits, implying directly in animals’ welfare and benefits for productivity.
Acknowledgments
The authors would like to acknowledge the São Paulo Research Foundation (FAPESP, Brazil, grants 2017/50339-5 and 2016/19222-1) for providing financial support to conduct the research and National Council of Technological and Scientific Development (CNPq, Brazil, grant 409485/2018-7) for providing financial support and productivity fellowships. This study was partly financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) – Finance Code 001. A.P. Freitas received a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil, Finance Code 001).
Glossary
Abbreviations:
- TS
temperament score
- FS
flight speed
- LW
live weight
- BCS
body condition score
- HH
hip height
- PC
principal component
- PCA
principal component analysis
- NeC
Nellore Control
- NeS
Nellore Selection
- NeT
Nellore Traditional
- YW
yearling weight
- EBVs
predicted breeding values
Contributor Information
Anielly P Freitas, Beef Cattle Research Center, Animal Science Institute/APTA/SAA, Sertãozinho, São Paulo 14174-000, Brazil; Department of Genetics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, São Paulo, 14049-900, Brazil.
Maria Lúcia P Lima, Beef Cattle Research Center, Animal Science Institute/APTA/SAA, Sertãozinho, São Paulo 14174-000, Brazil.
Flávia F Simili, Beef Cattle Research Center, Animal Science Institute/APTA/SAA, Sertãozinho, São Paulo 14174-000, Brazil.
Flávio S Schenkel, Centre for Genetic Improvement of Livestock, Department of Animal Biosciences, University of Guelph, Guelph, ON, Canada, N1G 2W1.
Lenira E Faro, Beef Cattle Research Center, Animal Science Institute/APTA/SAA, Sertãozinho, São Paulo 14174-000, Brazil.
Mario L Santana, Beef Cattle Research Center, Animal Science Institute/APTA/SAA, Sertãozinho, São Paulo 14174-000, Brazil.
Claudia Cristina P Paz, Beef Cattle Research Center, Animal Science Institute/APTA/SAA, Sertãozinho, São Paulo 14174-000, Brazil; Department of Genetics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, São Paulo, 14049-900, Brazil.
Conflict of interest statement
Authors declare no Conflict of Interests for this article.
Literature Cited
- Alonso, M. E., González-Montaña, J.R., and Lomillos, J. M.. 2020. Consumers' Concerns and Perception of Farm Animal Welfare. Animals, 10 (3), 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus, K., Erichson B., Gensler S., Weiber R., and Weiber T... 2021. Cluster Analysis. In: Multivariate Analysis. Springer Gabler, Wiesbaden. https://link.springer.com/chapter/10.1007/978-3-658-32589-3_8#citeas [Google Scholar]
- Barrozo, D., Buzanskas M. E., Oliveira J. A., Munari D. P., Neves H. H. R., and Queiroz S. A... 2012. Genetic parameters and environmental effects on temperament score and reproductive traits of Nellore cattle. Animals. 6:36–40. doi: 10.1017/S1751731111001169 [DOI] [PubMed] [Google Scholar]
- Bates, K. E., Weaber R. L., Bormann J. M., Moser D. W., Salak-Johnson J. L., Chase C. C. L., Peel R. K., Van Campen H., Loneragan G. H., Wagner J. J.,. et al. 2014. Genetic relationships among temperament immune function and carcass merit in beef cattle. In: Kansas State University editor Cattlemen’s Day Manhattan. Manhattan; 39–44. http://hdl.handle.net/2097/17773 [Google Scholar]
- Boligon, A. A., Vicente I. S., Vaz R. Z., Campos G. S., Souza F. R. P., and Carvalheiro L. G., Albuquerque R... 2016. Principal component analysis of breeding values for growth and reproductive traits and genetic association with adult size in beef cattle. J. Anim. Sci. 94:5014–5022. doi: 10.2527/jas.2016-0737 [DOI] [PubMed] [Google Scholar]
- Burdick, N. C., Randel R. D., Carroll J. A., and Welsh T. H... 2011. Interactions between temperament stress and immune function in cattle. Int. J. Zool. 2011. doi: 10.1155/2011/373197 [DOI] [Google Scholar]
- Burrow, H. M., Seifert, G. W., Corbet, N. J.. 1988. A new technique for measuring temperament in cattle. In: Proceedings of the Australian Society of Animal Production: 18th biennial meeting; May, 1988; Sydney. ASAP; 154-157. procite:7b7e0050-0d9d-487d-ad32-9158fe45240d. http://hdl.handle.net/102.100.100/263651?index=1. [Google Scholar]
- Cafe, L. M., Robinson D. L., Ferguson D. M., Mcintyre B. L., Geesink G. H., and Greenwood P. L... 2011. Cattle temperament: persistence of assessments and associations with productivity efficiency carcass and meat quality traits. J. Anim. Sci. 89:1452–1465. doi: 10.2527/jas.2010-3304 [DOI] [PubMed] [Google Scholar]
- Cardoso, D. F., Albuquerque L. G., Reimer C., Qanbari S., Erbe M., Nascimento A. V., Venturini G. C., Scalez D. C. B., Baldi F., Camargo G. M. F.,. et al. 2018. Genome-wide scan reveals population stratification and footprints of recent selection in Nellore cattle. Genet. Sel. Evol. 50:1–12. doi: 10.1186/s12711-018-0374-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke, R. F., Schubach K. M., Marques R. S., Peres R. F. G., Silva L. G. T., Carvalho R. S., Cipriano R. S., Bohnert D. W., Pires A. V., and Vasconcelos J. L. M... 2017. Effects of temperament on physiological productive and reproductive responses in Bos indicus beef cows. J. Anim. Sci. 95:1–8. doi: 10.2527/jas.2016.1098 [DOI] [PubMed] [Google Scholar]
- Costilla, R., Kemper, K. E., Byrne, E. M., Porto-Neto, L. R., Carvalheiro, R., Purfield, D. C., ... & Hayes, B. J.. 2020. Genetic control of temperament traits across species: association of autism spectrum disorder risk genes with cattle temperament. Genetics Selection Evolution, 52(1), 1-14. doi: 10.1186/s12711-020-00569-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz, D. A. C., Savegnago R. P., Santana A. B. B., Peixoto M. G. C. D., Bruneli F. A. T., and El Faro L... 2016. Cluster analysis of breeding values for milk yield and lactation persistency in Guzerá cattle. Ciência Rural. 46:1281–1288. doi: 10.1590/0103-8478cr20150418 [DOI] [Google Scholar]
- Curley, K. O.Jr, Neuendorff D. A., Lewis A. W., Cleere J. J., Welsh T. H., and Randel R. D... 2008. Functional traits of the bovine hypothalamic-pituitary-adrenal axis vary with temperament. Horm. Behav. 53:20–27. doi: 10.1016/j.yhbeh.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Faria, C. U., Magnabosco C. U., Albuquerque L. G., Reyes A. D. L., Bezerra L. A. F., and Lobo R. B... 2008. Análise genética de escores de avaliação visual de bovinos com modelos bayesianos de limiar e linear. Pesq. Agropec. Bras. 43:835–841. doi: 10.1590/S0100-204X2008000700007 [DOI] [Google Scholar]
- Fordyce, G., Dodt R. M., and Wythes J. R... 1988. Cattle temperaments in extensive beef herds in northern Queensland 1. Factors affecting temperament. Aust. J. Exp. Agric. 28:683–687. doi: 10.1071/ea9880683 [DOI] [Google Scholar]
- Fordyce, G., Goddard M. E., and Seifert G. W... 1982. The measurement of temperament in cattle and the effect of experience and genotype. Aust. Soc. Anim. Prod. 14:329–332. [Google Scholar]
- Francisco, C. L., Resende F. D., Benatti J. M. B., Castilhos A. M., Cooke R. F., and Jorge A. M... 2015. Impacts of temperament on Nellore cattle: physiological responses feedlot performance and carcass traits. J. Anim. Sci. 93:5419–5429. doi: 10.2527/jas.2015-9411 [DOI] [PubMed] [Google Scholar]
- Freitas, A. P., Santana M. L., Schenkel F. S., Mercadante M. E. Z., Cyrillo JNSG, and Paz C. C. P... 2021. Different selection practices affect the environmental sensitivity of beef cattle. PLoS One. 16:e0248186. doi: 10.1371/journal.pone.0248186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geweke, J. 1991. Evaluating the accuracy of sampling-based approaches to the calculation of posterior moments. In Bernardo, J. M., Berger J. O., Dawid A. P., Smith A. F. M., editors. v4 Bayesian statistics. New York USA: Oxford University Press; p. 625–631 [Google Scholar]
- Grandin, T. 1993. Behavioral agitation during handling of cattle is persistent over time. Appl. Anim. Behav. Sci. 36:1-9. doi: 10.116/0168-1591(93)9094-6 [DOI] [Google Scholar]
- Hair, J. F., Black W. C., Black B. J., Anderson R. E., and Tatham R. L... 2009. Multivariate data analysis, 6th ed.Edinburgh Gate: Bookman Editora; p. 428 [Google Scholar]
- Hartigan, J. A. 1975. Clustering algorithms. New York: John Wiley & Sons Inc. [Google Scholar]
- Hartigan, J. A., and Wong M. A... 1979. Algorithm AS 136: a k-means clustering algorithm. J. R. Stat. Soc. C: Appl. Stat. 28:100–108. doi: 10.2307/2346830 [DOI] [Google Scholar]
- Hoppe, S., Brandt H. R., König S., Erhardt G., and Gauly M... 2010. Temperament traits of beef calves measured under field conditions and their relationships to performance. J. Anim. Sci. 88:1982–1989. doi: 10.2527/jas.2008-1557 [DOI] [PubMed] [Google Scholar]
- Johnson, R. A. and Wichern D. W... 2007. Applied multivariate statistical analysis. 6th ed.Upper Saddle River, NJ: Pearson Prentice Hall; p. 773 [Google Scholar]
- Kadel, M. J., Johnston D. J., Burrow H. M., Graser H. U., and Ferguson D. M... 2006. Genetics of flight time and other measures of temperament and their value as selection criteria for improving meat quality traits in tropically adapted breeds of beef cattle. Aust. J. Agric. Res. 57:1029–1035. doi: 10.1071/ar05082 [DOI] [Google Scholar]
- Kaiser, H. F. 1960. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 20:141–151. doi: 10.1177/001316446002000116 [DOI] [Google Scholar]
- Laureano, M. M. M., Boligon A. A., Costa R. B., Forni S., Severo J. L. P., and Albuquerque L. G... 2011. Estimativas de herdabilidade e tendências genéticas para características de crescimento e reprodutivas em bovinos da raça Nellore. Arq. Bras. Med. Vet. Zootec. 63:143–152. doi: 10.1590/S0102-09352011000100022 [DOI] [Google Scholar]
- Lucena, C. R. S., Neves H. H. R., Carvalheiro R., Oliveira J. A., and Queiroz S. A... 2015. Genetic analysis of the temperament of Nellore cattle using linear and threshold models. Animal. 9:388–394. doi: 10.1017/S1751731114002572 [DOI] [PubMed] [Google Scholar]
- Maffei, W. E. 2009. Confinement reactivity. Braz. J. Anim. Sci. 38:81–92. doi: 10.1590/S1516-35982009001300010 [DOI] [Google Scholar]
- Mercadante, M. E. Z., Razook A. G., Silva J. A. D. V., and Figueiredo L. A... 2006. Escore de condição corporal de vacas da raça Nellore e suas relações com características de tamanho e reprodução. ALPA. 14:143–147. https://ojs.alpa.uy/index.php/ojs_files/article/view/494 [Google Scholar]
- Misztal, I., Tsuruta S., Strabel T., Auvray B., Druet T., and Lee D. H... 2002. BLUPF90 and related programs. In: INRA editor. 7th World Congress on Genetics Applied to Livestock Production, Montpellier, France; p. 4 [Google Scholar]
- Nicholson, M. J. and Butterworth M. H... 1986. A guide to condition scoring of zebu cattle. Addis Ababa Ethiopia: International Livestock Centre for Africa; p. 29. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://pdf.usaid.gov/pdf_docs/PNAAV664.pdf [Google Scholar]
- Norris, D., Ngambi J. W., Mabelebele M., Alabi O. J., and Benyi K... 2014. Genetic selection for docility: a review. J. Anim. Plant Sci. 24:374–379. ISSN: 1018-7081http://repository.futminna.edu.ng:8080/jspui/handle/123456789/4164 [Google Scholar]
- Oliveira, E. J., Savegnago R. P., Freitas L. A. D., Freitas A. P., Maia S. R., Simili F. F., Faro E., Costa L. R. D. D., Santana M. L. Júnior, and Paz C. C. P... 2018. Estimates of genetic parameters and cluster analysis for worm resistance and resilience in Santa Inês meat sheep. Pesq. Agropec. Bras. 53:1338–1345. doi: 10.1590/s0100-204x2018001200006 [DOI] [Google Scholar]
- Paranhos da Costa, M. J. R., Costa e Silva E. V., Chiquitelli Neto M., and Rosa M. S... 2002. Contribuição dos estudos de comportamento de bovinos para implementação de programas de qualidade de carne. In: XX Encontro Anual de Etologia Natal: Sociedade Brasileira de Etologia; p. 71–89. http://grupoetco.org.br/arquivos_br/pdf/contriestcomp.pdf
- Petherick, J. C., Holroyd R. G., Doogan V. J., and Venus B. K... 2002. Productivity carcass and meat quality of lot-fed Bos indicus cross steers grouped according to temperament. Aust. J. Exp. Agric. 42:389–398. doi: 10.1071/ea01084 [DOI] [Google Scholar]
- Pires, B. C., Faria C. U., Viu M. A. O., Terra J. P., Lopes D. T., Magnabosco C. U., and Lôbo R. B... 2010. Modelos bayesianos de limiar e linear na estimação de parâmetros genéticos para características morfológicas de bovinos da raça Nellore. Rev. Bras. Saude Prod. Anim. 11:651–661. ISSN: 1519-9940. http://repositorio.bc.ufg.br/handle/ri/18999 [Google Scholar]
- Prayaga, K. C., Corbet N. J., Johnston D. J., Wolcott M. L., Fordyce G., and Burrow H. M... 2009. Genetics of adaptive traits in heifers and their relationship to growth pubertal and carcass traits in two tropical beef cattle genotypes. Anim. Prod. Sci. 49:413–425. doi: 10.1071/ea08247 [DOI] [Google Scholar]
- Rueda, P. M., Sant’Anna A. C., Valente T. S., and Paranhos da Costa M. J. R... 2015. Impact of the temperament of Nellore cows on the quality of handling and pregnancy rates in fixed-time artificial insemination. Livest.Sci. 177:189–195. doi: 10.1016/j.livsci.2015.04.021 [DOI] [Google Scholar]
- Sant’Anna, A. C., Baldi F., Valente T. S., Albuquerque L. G., Menezes L. M., Boligon A. A., and Paranhos da Costa M. J. R... 2015. Genetic associations between temperament and performance traits in Nellore beef cattle. J. Anim. Breed. Genet. 132:42–50. doi: 10.1111/jbg.12117 [DOI] [PubMed] [Google Scholar]
- Sant’Anna, A. C., Paranhos da Costa M. J. R., Baldi F., and Albuquerque L. G... 2013. Genetic variability for temperament indicators of Nellore cattle. J. Anim. Sci. 91:3532–3537. doi: 10.2527/jas.2012-5979 [DOI] [PubMed] [Google Scholar]
- Sant’Anna, A. C., Paranhos da Costa M. J. R., Baldi F., Rueda P. M., and Albuquerque L. G... 2012. Genetic associations between flight speed and growth traits in Nellore cattle. J. Anim. Sci. 90:3427–3432. doi: 10.2527/jas.2011-5044 [DOI] [PubMed] [Google Scholar]
- Savegnago, R. P., Nascimento G. B., Rosa G.J. de M., de Carneiro R.L.R., Sesana R. C., El Faro L., and Munari D. P... 2016. Cluster analyses to explore the genetic curve pattern for milk yield of Holstein. Livest. Sci. 183:28–32. doi: 10.1016/j.livsci.2015.11.010 [DOI] [Google Scholar]
- Silveira, D. D., Souza F. R. P., Brauner C. C., Ayres D. R., Silveira F. A., Dionello N. J. L., and Boligon A. A... 2015. Body condition score of Nellore cows and its relation with mature size and gestation length. Livest. Sci. 175:10–17. doi: 10.1016/j.livsci.2015.02.013 [DOI] [Google Scholar]
- Valente, T. S., Baldi F., Sant’Anna A. C., Albuquerque L. G., and Costa M. J. R. P... 2016. Genome-wide association study between single nucleotide polymorphisms and flight speed in Nellore cattle. PLoS One. 11:1–18. doi: 10.1371/journal.pone.0156956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente, T. S., Sant’Anna A. C., Baldi F., Albuquerque L. G., and Costa M. J. R. P... 2015. Genetic association between temperament and sexual precocity indicator traits in Nellore cattle. J. Appl. Genet. 56:349–354. doi: 10.1007/s13353-014-0259-0 [DOI] [PubMed] [Google Scholar]
- Vargas, G., Schenkel F. S., Brito L. F., Rezende Neves H. H., D. P. Munari, Boligon A. A., and Carvalheiro R... 2018. Unraveling biological biotypes for growth visual score and reproductive traits in Nellore cattle via principal component analysis. Livest. Sci. 217:37–43. doi: 10.1016/j.livsci.2018.09.010 [DOI] [Google Scholar]
- Ward, J. H.Jr. 1963. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 58:236–244. doi: 10.1080/01621459.1963.10500845 [DOI] [Google Scholar]
- Yokoo, M. J. I., Albuquerque L. G., Lôbo R. B., Sainz R. D., Carneiro J. M., Bezerra L. A. F., and Araujo F. R. C... 2007. Estimativas de parâmetros genéticos para altura do posterior peso e circunferência escrotal em bovinos da raça Nellore. Rev. Bras. Zootec. 36:1761–1768. doi: 10.1590/S1516-35982007000800008 [DOI] [Google Scholar]
- Yokoo, M. J., Lobo R. B., Araujo F. R. C., Bezerra L. A. F., Sainz R. D., and Albuquerque L. G... 2009. Genetic associations between carcass traits measured by real-time ultrasound and scrotal circumference and growth traits in Nellore cattle. J. Anim. Sci. 88:52–58. doi: 10.2527/jas.2008-1028 [DOI] [PubMed] [Google Scholar]