Abstract
Hatchery’s goals include maximizing revenue by achieving high hatchability with day-old birds of excellent quality. The advancement of technology has benefited the poultry sector since breeding and genetics technology have increased the rates of meat maturation in developing birds in a short period of time. Excessive use of in-feed antibiotics has been shown in studies to increase the chance of resistance to human infections. Bacterial resistance and antibiotic residues in animal products raised concerns about using antibiotics as growth promoters, eventually leading to a prohibition on using in-feed antibiotics in most industrialized nations. In ovo technology is a novel method for delivering bioactive chemicals to developing avian embryos. In ovo feeding technologies may provide additional nutrients to the embryos before hatching. The introduction of bioactive compounds has the potential to assist in decreasing and eventually eliminating the problems associated with traditional antibiotic delivery in chicken production. Phytobiotics were advocated as an alternative by researchers and dietitians. So far, several studies have been conducted on the use of phytogenic feed additives in poultry and swine feeding. They have primarily demonstrated that phytobiotics possess antibacterial, antioxidant, anti-inflammatory, and growth-stimulating properties. The antioxidant effect of phytobiotics can improve the stability of animal feed and increase the quality and storage duration of animal products. In general, the existing documentation indicates that phytobiotics improve poultry performance. To effectively and efficiently use the in ovo technique in poultry production and advance research in this area, it is important to have a thorough understanding of its potential as a means of nutrient delivery during the critical stage of incubation, its effects on hatching events and posthatch performance, and the challenges associated with its use. Overall, this review suggests that in ovo feeding of phytobiotics has the potential to improve the antioxidant status and performance of chickens.
Keywords: antioxidant, growth promoter, in ovo, phytobiotics, stress
Some in ovo phytobiotics’ bioactive compounds can influence embryonic and posthatch development
Introduction
The perinatal period from late-term embryo to a few days post-hatch is important for the development of the gastrointestinal tract and the immune system in poultry. In contrast to mammals that influence the growth of the fetus during pregnancy, avian species can only depend on the composition of the egg and egg microenvironment (Uni and Ferket, 2004). This critical period is crucial for the development of the gastrointestinal tract and the immune system of poultry (Uni and Ferket, 2004; Uyanga et al., 2022). This restriction on nutrient reserves may prevent freshly hatched chicks from growing and developing to their full potential (Uni and Ferket, 2004). In ovo feeding technologies may be used to get around this restriction by giving the embryo extra nutrients before it hatches (Uni et al., 2005).
In ovo technology was used for vaccination in poultry hatcheries many years ago (Ricks et al., 1999). Since chicken has a limited amount of nutrients available for the development of embryos, attention has focused on providing additional nutrients to the embryos (Uni et al., 2012). In ovo feeding of substances such as antioxidants during incubation may improve the antioxidant status of the chicken embryo and posthatch growth phases (Yigit et al., 2014; El-Saadany et al., 2019). Moreover, in ovo feeding of chickens with extracts from numerous plant products has enhanced their defenses against the contagious bursal virus, avian influenza virus, and fowlpox virus (Sood et al., 2013; Nyandoro et al., 2014).
The antioxidant level of the chicken embryo may be enhanced by in ovo injection of antioxidants because they have an effective defense against free radicals (Salary et al., 2014). Recently, attention has been shifted to the use of herbal additives as growth promoters and antioxidant components from herbs, spices, and their products due to their benefits (Oke et al., 2016, 2017; Oke, 2018; Voemesse et al., 2019; Tokofai et al., 2020; Kpomasse et al., 2021, 2023; Adjei-Mensah et al., 2022). With the growing interest in the use of in ovo phytobiotics, it is crucial to have a thorough understanding of the potential of in ovo feeding of phytobiotics as a method of nutrient delivery during the crucial stages of incubation, hatching events, and posthatch performance and the challenges associated with it to use the technique in poultry production effectively and efficiently and advance research in this area.
Classification of Phytochemicals
Due to the large number of phytochemicals, accurate categorization has been difficult to obtain. Phytochemicals are classified as secondary or major components according to how they function in plant metabolism (Koche et al., 2016). chlorophylls, pyrimidines, nucleic acid purines, vitamins, amino acids, sugars, and other fundamental components are all found in plants (Harborne, 1984). Secondary components include plant lignans, phenolics, steroids, flavonoids, alkaloids, terpenes, curcumins, saponins, flavonoids, and glucosides (Grashorn, 2010; Dhama et al., 2015; Tiwari et al., 2018). According to a literature review, phenolics are the most abundant and structurally varied plant phyto-components (Huang, 2009).
Phytobiotics or phytogenic feed additives can be classified based on their processing features and source. The four main categories of phytogenic feed additives are plants, spices, essential oils, and oleoresins (Windisch et al., 2008; Yang et al., 2009; Huyghebaert et al., 2011; Gheisar et al., 2015). Plants refer to non-permanent blooming and non-woody plants, such as herbs and shrubs, which are used as a source of phytogenic feed additives. Whole plants, flowers, leaves roots, and can be used to produce phytogenic feed additives (Grashorn, 2010). Spices are plants that have a dense odor or flavor and are commonly used as food additives to enhance flavor. Examples of spices that are commonly used as phytogenic feed additives include garlic, ginger, cinnamon, and oregano. Essential oils are particularly volatile lipophilic components that are extracted from plants. They are often used as natural fragrances, flavorings and for their therapeutic properties. Extraction of essential oil can be made from different plant parts, like roots, flowers, and leaves using different methods, such as steam distillation (Vankar, 2004). Oleoresins are extracted from spices and are a concentrated form of the plant’s active compounds. They are obtained by solvent extraction and contain both the plant’s essential oil and non-volatile components. Oleoresins are commonly used in the food industry as flavorings and colorings (Procopio et al., 2022). Categories of phytogenic feed additives have their own unique set of properties and benefits. The utilization of phytobiotics as feed additives has been found to enhance animal performance and health in the poultry industry (Alghirani et al., 2021).
Beneficial Effects of Phytochemicals on Animal Performance and Health
Phytogenic feed additives are a class of bioactive compounds derived from several sources of herbs like plants, spices, and other botanicals. These compounds have been found to have various beneficial effects on animal performance and health when included in animal diets. They are also known as phytogenics, a term used to describe natural feed additives derived from plant sources (Windisch et al., 2008; Grashorn, 2010; Tiwari et al.,2018). Phytogenic feed additives have been used in animal feed for many years, and their popularity has recently increased as consumers demand natural and organic products (Alagawany et al., 2021). These compounds have been shown to have a wide range of beneficial effects on animal health, such as improving gut health, enhancing immune function, and increasing feed efficiency (Alagawany et al., 2021). They are believed to work by stimulating the natural processes in the animal’s body, leading to improved overall health and performance. Thousands of phytochemicals have been identified to be beneficial due to numerous advantages and have biological activities such as growth-promoting effects such as anticancer, antibacterial, antioxidant, antidiarrheal, analgesic, easy availability, and low cost (Mahima et al., 2012; Rahal et al., 2014; Dhama et al., 2015, 2018; Yadav et al., 2016; Tiwari et al., 2018).
Phytogenic feed additives have been found to have a wide range of beneficial effects on poultry nutrition. One of the most significant benefits of phytogenic feed additives is their ability to stimulate feed intake, which is likely due to the improved palatability of the diet. Additionally, phytogenic feed additives have been shown to improve nutrient digestibility, resulting in enhanced feed conversion ratio (FCR) and weight gain (Grashorn, 2010; Dhama et al., 2015; Gheisar et al., 2015; Yadav et al., 2016).
Phytobiotics also have the potential to improve gut microflora by preventing the development of dangerous bacteria while encouraging the development of beneficial populations. This can lead to improved gut health and reduced risk of disease. Some phytogenic feed additives also have antimicrobial properties, which can further contribute to the reduction of disease incidence in poultry (Grashorn, 2010; Dhama et al., 2015; Gheisar et al., 2015; Yadav et al., 2016). Another beneficial effect of phytogenic feed additives is their coccidiostatic effect, which can help to prevent and control coccidiosis, a common poultry disease caused by a protozoan parasite. Phytogenic feed additives can also have immunostimulating effects, which can improve the overall immune response of poultry and reduce the risk of infectious diseases (Grashorn, 2010; Dhama et al., 2015; Gheisar et al., 2015; Yadav et al., 2016). Furthermore, some phytogenic feed additives have anti helmintic effects, which can help to control intestinal parasites in poultry. This can be particularly important in free-range and organic poultry production systems where outdoor access increases the risk of parasitic infections (Grashorn, 2010; Dhama et al., 2015; Gheisar et al., 2015; Yadav et al., 2016). The antioxidative properties of phytogenic feed additives can improve the stability of animal feed and the quality and storage time of animal products such as meat and eggs (Gheisar et al., 2015). This can lead to a reduction in waste and an increase in the shelf life of these products. Phytogenic feed additives have a wide range of beneficial effects on poultry nutrition and can be a valuable tool for improving the health and performance of poultry (Gheisar et al., 2015).
Mechanism of Action of Phytochemicals
Phytochemicals have been related to several different mechanisms of action. Inhibition of microorganisms, disruption of metabolic processes, and modulation of signal transduction pathways and gene expression are all possibilities (Kris-Etherton et al., 2002; Surh, 2003; Omojate et al., 2014). Plant extracts and essential oils may act against bacterial strains in a variety of ways, including disruption of enzymes involved in cellular energy production and the synthesis of structural components, increased permeability and loss of cellular components, destruction or inactivation of genetic material, and interaction with the phospholipid bilayer of the cell membrane. The mechanism of action is thought to break the cytoplasmic membrane, affecting the proton motive force, electron flow, active transport, and cell coagulation (Kotzekidou et al., 2008).
Incubation of Eggs
There are two types of incubation: natural and artificial (Booth, 2004). Incubation is simply the embryo’s development within the egg under appropriate environmental conditions (Berntsen and Bech, 2016). For natural incubation, the broody hen, preferably with good mothering instincts, once laying ends, begins to sit on the eggs, ruffles her feather, spreads out her wings, and makes a special clucking sound (Head, 2011). The hen tries to maintain the general conditions around the eggs, including temperature and humidity, and turns the egg at intervals to ensure the survival of the embryo, normal formation and development of the embryo. In closed systems, feed and water are often provided close to the mother hen for easy access and to be still close to the eggs to avoid cold shock resulting from the mother hen staying away from the eggs for an extended period of time (Spotila, 2004). This incubation period lasts for an average of 21 d in chickens.
On the other hand, artificial incubation uses an incubator to control the environment around the egg in a way similar to natural incubation to ensure the survival and proper development of the embryo (Ospina et al., 2018). One of the advantages of artificial incubation over natural incubation is that it ensures the hatching of more eggs compared to broody hens, which have limits.
In this artificial incubation system, the eggs are exposed to these plants’ phytochemicals. Research by Widowski et al. (2022) and Iyasere et al., (2022) shows that the mother hen’s environment may affect the egg composition, leading to physical and behavioral effects in the chicks. Maternal exposure to environmental stressors may affect offspring, so if the mother hen is in a good environment, it will affect the embryo positively. While the mother hen may pass on several nutrients to the eggs, eggs may also be injected in ovo with rich phytochemicals, which will affect the physical and behavioral properties of the chicks when they hatch and grow (Shehata et al., 2021).
The quality of day-old chicks is often affected by what they are exposed to during the incubation phase (Bergoug et al., 2013). This adds to why the eggs in incubators must be provided with the right conditions. Research by Hedlund and Jensen (2021) shows that incubation and hatching cause long-term stress in chickens. Hence, it is worth looking at what can be done to reduce this stress effect on chickens. A substance that has been used previously is phytochemicals. Phytochemicals are chemical compounds derived from plants, and they help in providing resistance against microbial infections. One of the outstanding properties of phytochemicals is their role as antioxidants. They have the potential to boost the immune system, prevent the growth of cancerous cells, and regulate hormones.
In Ovo Technology
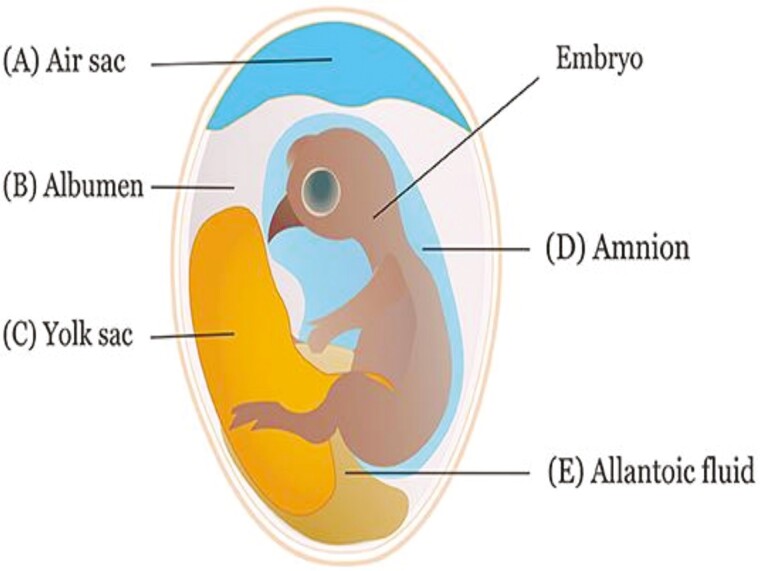
Broilers spend 30% to 40% of their lifespan inside the egg, while the market age continues to decline along with advances in genetic and nutritional knowledge (Hulet et al., 2007). The incubation and neonatal stages of broiler chickens account for almost half of the bird’s productive lifetime (Proszkowiec-Weglarz et al., 2019). The period between embryonic day eighteen and four days posthatch is important for the chick’s survival and growth since it marks the physiological and metabolic shift from egg to feed nutrition (Proszkowiec-Weglarz et al., 2019). As a result, the embryonic life stage now has a lot more significance. In ovo feeding appears to boost the late-term broiler’s energy status by helping the chick reach its potential for late embryonic and early posthatch growth (Uni et al., 2005). The chick will have its first meal around embryonic day 18, when it consumes the amniotic fluid prior to internal pipping (Ferket and Gernat, 2006). In ovo feeding involves injecting liquid feed components into an egg’s amnion many days before hatching. In ovo feeding is thought to be a viable option of administering nutrients to embryos prior to hatching. At a later stage of development (day 18 of incubation), the nutrients supplemented to the developing embryo through the amniotic sac to provide a constant supply of vital nutrients during the first few days (3 to 4 d) of the posthatch phase, promoting metabolism and intestinal development (Kadam et al., 2013b).
The foremost egg injection device was commercially available in 1992, with a capacity of 20,000 to 30,000 eggs per hour (Johnston et al., 1997; Jochemsen and Jeurissen, 2002). It was created to deliver vaccinations but has been tested for other uses, such as in ovo injections.
Figure 1 shows the various in ovo injection methods that have been used to examine the effects of various bioactive substances. The efficacy of in ovo feeding can be influenced by the site of injection (Avakian et al., 2002; Williams, 2007; Williams and Hopkins, 2011; Peebles, 2018). Generally, the timing and type of the biological substance determine the route of administration. When the injection is done in the initial stages of incubation, the air sac is usually the target (Berrocoso et al., 2017). When the embryo in the egg consumes the amniotic fluids later in the incubation process (Ma et al., 2020), the amnion is intended to provide chemicals in the amniotic fluid. The ability of the amnion to aspirate and digest the vaccine for more thorough systemic assimilation in the embryo has made it the best site for administration (Peebles, 2018). However, the findings of Kop Bozbay et al. (2016) revealed that in ovo injection of propolis and injection sites had no effect on hatching traits, including chick survival, chick weight at hatch, or hatchability. A typical injection depth to target the amnion is 2.49 cm from the top of the big end of the broiler hatching egg (Zhai et al., 2011).
Figure 1.
Different Sites of in ovo injection (Das et al., 2021).
Advantage of Early Feeding and In Ovo Applications in Poultry
In modern hatchery practices, the timing of hatching can vary between poults and chicks (Careghi et al., 2005). Typically, they hatch out of the egg at different times, with chicks hatching first, followed by poults around 36 to 48 h later. Once the majority of eggs have hatched, the chicks and poults are removed from the hatcher. After hatching, the chicks undergo various hatchery treatments, such as sexing, vaccination, and beak trimming, to ensure their health and welfare (Glatz, 2000). Depending on the hatchery’s protocols, these treatments can take a few hours to a few days. During this time, the chicks are held in the hatchery before being transported to the production farm. During transportation, the chicks may undergo a further holding time of up to 72 h. This period can be stressful for the chicks, as they are deprived of food and water, leading to a period of starvation. However, the residual yolk that is still present in their bodies after hatching provides them with the energy needed for growth and maintenance during this time. The yolk is a crucial source of nutrition for the chicks, as it contains essential nutrients such as proteins, fats, and minerals (Lesnierowski and Stangierski, 2018). It also provides antibodies that help protect the chick from diseases until its own immune system develops (Lesnierowski and Stangierski, 2018). The yolk is gradually absorbed by the chick’s body over several days, providing a steady supply of energy and nutrients during the early stages of life (Sklan and Noy, 2000). Restriction in feed access does not only impairs the chicks’ growth but also their immune system’s ability to fight off pathological diseases (Willemsen et al. 2010; Bhuiyan and Iji, 2015).
In ovo administration of various compounds such as bacteriophages, electrolyte solutions, glycerol, hormones, organic acids, peptides, silver nanoparticles, trace elements, amino acids, vitamins, carbohydrates, and plant extracts directly into the egg during incubation is well documented in the literature (Roto, Kwon, and Ricke 2016). One of the potential benefits of in ovo technology is that it can stimulate favorable immunological responses in birds, leading to improved disease resistance and overall health (Jha et al., 2019). Additionally, in ovo technology can address perinatal nutritional deficiencies in birds (Jha et al., 2019). During the perinatal period, which encompasses the time before and after hatching, birds may experience nutritional deficiencies due to the transition from embryonic yolk nutrition to exogenous feeding (Proszkowiec-Weglarz et al., 2019). This can be compounded by the long hatchery window of 24 to 36 h and time-consuming hatchery logistics such as sorting, sexing, vaccinations, beak trimming, and chick transport (Noy and Uni, 2010). By administering nutrients and other compounds directly into the egg during incubation, in ovo technology can potentially mitigate these perinatal nutritional deficiencies (Kadam et al., 2013). For example, in ovo administration of amino acids, vitamins, and trace elements can help support the development of the chick’s immune system, muscles, and other tissues (Bakyara et al., 2012). Furthermore, in ovo administration of other compounds such as bacteriophages, organic acids, and plant extracts can also help to promote gut health and reduce the risk of disease (Yadav et al., 2016). This is particularly important in commercial poultry production, where disease outbreaks can have significant economic impacts.
In ovo technology offers several additional benefits; one potential advantage is that it can stimulate the colonization of the embryonic gut with beneficial microbiota (Shehata et al., 2021). The embryonic gut is a sterile environment before hatching, and establishing a healthy gut microbiome is critical for proper immune system development and the bird’s overall health (Shehata et al., 2021). In ovo administration of probiotics or phytobiotics can help promote the growth of beneficial gut bacteria and improve gut health, potentially reducing the risk of disease (Rubio, 2019). In ovo technology can also promote the development of the embryonic gastrointestinal tract (GIT) and gut-associated lymphoid tissue (Siwek et al., 2018). The GIT is responsible for the digestion and absorption of nutrients, while the gut-associated lymphoid tissue plays a critical role in immune function (Ahluwalia et al., 2017). In ovo administration of nutrients and other compounds can help support the development and maturation of these systems, improving the bird’s overall health and disease resistance (Shehata et al., 2021). Recently, in ovo sexing has been proposed as an application of this technology to address an important animal welfare and ethics issue in commercial layer production (Gautron et al., 2021). Male chicks are of limited value in the egg-laying industry and are typically culled by maceration or suffocation shortly after hatching. In ovo sexing can determine the sex of the chick prior to hatching, allowing for the selective culling of male embryos and eliminating the need for posthatch culling (Jennison, 2021). This approach has the potential to significantly reduce the number of male chicks culled, improving animal welfare and reducing the environmental impact of commercial layer production. In ovo technology has the potential to address several significant challenges in poultry production, including disease control, perinatal nutrition, and animal welfare (Ferket, 2009). While further research is needed to understand this technology’s benefits and limitations fully, it represents a promising approach to improving the efficiency and sustainability of the poultry industry.
Effect of in ovo Administration of Phytogenic Substance on Poultry Animals
Nutritional Need of Embryos
Energy is stored in the embryo of fast-growing chicks after incubation for rapid growth and development (Gous, 2010). During the last phase of embryogenesis, the chick depletes the energy stored in the embryo through gluconeogenesis, which occurs mainly in the yolk sac and liver (Fasenko, 1996). The glycogen produced is then stored in the liver, muscle, and yolk sac for hatching purposes and growth and development posthatch (Fasenko, 1996). Over the years, chicken strains have been selectively bred to grow faster and reach market size quickly (Humphrey et al., 2014). This has led to changes in the bird’s genetic makeup, with the liver maturing earlier in heritage lines (Humphrey et al., 2014). The embryonic development phase, therefore, has become a critical period to prepare and equip newly hatched chicks for this fast-growing process (Ravindran and Abdollahi, 2021).
To ensure optimal growth and development of the chick embryo, researchers have focused on supplying various phytogenic nutrient substrates to the developing embryos (Abd El-Hack et al., 2022). Phytogenic substances are derived from plants and have been found to have potential energy for animals (Karásková et al., 2015). In the context of in ovo injection, phytogenic substances can be delivered directly to the developing embryo through the eggshell, which may improve nutrient absorption and utilization.
Studies have shown that the late embryonic phase, specifically between days 14 and 18 of incubation, is crucial for supplying phytogenic nutrients (Oladokun and Adewole, 2020; Oladokun et al., 2022). This period is characterized by a high caloric demand to fuel the hatching operation, and the embryo begins to consume the nutrients in the egg towards the end of incubation.
In Ovo Phytobiotics on Hatchability
Different authors have documented the effects of in ovo phytogenic nutrients on embryo growth and hatchability. This review protrudes different phytogenic substance use as in ovo supplements with a different result. Coskun et al. (2014) found that the injection of sunflower pollen extract into the amnion did not affect hatchability. Therefore, providing phytogenic nutrients during this phase can help increase nutrient reserve to support organs and structural development posthatch. In ovo injection of phytogenic nutrients during embryonic development has emerged as a promising strategy to improve the hatchability and posthatch performance of chickens in the context of fast-growing strains (Oladokun and Adewole, 2020).
Using in ovo injection is a potential strategy for improving chickens’ hatchability and posthatch performance. This technique involves the injection of various substances, such as nutrients, drugs, or vaccines, directly into the egg during the incubation period (Bello et al., 2013; Li et al., 2016). The perinatal period, which encompasses both the pre and posthatch period, is a critical stage in the development of the chick embryo. During this period, the embryo has a high caloric demand to fuel the hatching process (Ferket and Uni, 2006). The availability of nutrients for in ovo injection is essential for embryonic development and hatchability. As the incubation period progresses, the embryo consumes the nutrients in the egg and begins to use its body reserves to prepare for emergence (Ferket, 2006). Therefore, it is crucial to provide the developing embryo with adequate nutrients to prevent weight loss and ensure successful hatching.
Several studies have demonstrated the potential benefits of in ovo phytogenic feed additives injection for improving hatchability and posthatch performance in chickens. For instance, in ovo administration of nutrients, such as amino acids, vitamins, and minerals, has been shown to improve hatchability, chick weight, and FCR (Bello et al., 2013; Li et al., 2016). Additionally, in ovo vaccination against various pathogens, such as Marek’s disease virus, has been shown to enhance immune responses and reduce mortality in chickens (Peebles et al., 2017). The use of in ovo phytogenic feed additives injection is a promising approach for improving the hatchability of chickens. Providing the developing embryo with adequate nutrients and vaccines through in ovo injection can support healthy embryonic development, reduce weight loss during the perinatal period, and improve hatchability.
In contrast, N’nanle et al. (2017) reported that in ovo administration of Moringa oleifera extract improved hatchability and resulted in heavier chickens at hatch. Similarly, Ngueda et al. (2021) found that injection of Manihot esculenta extract solution on the 18th day of incubation improved hatchability and reduced total incubation duration. In other studies, Hajati et al. (2014) reported that in ovo injection of grape seed extract (GSE) increased hatchability, while Heidary et al. (2020) found that in ovo administration of nano curcumin decreased hatchability. Morovate et al. (2016) observed no differences in the hatchability of birds that were administered in ovo Silybum marianum extract. Additionally, Fazli et al. (2015) reported that garlic and tomato extract increased the percentage of male chicks, while Mahjar and Al-Salhie (2022) found that in ovo injection of garlic extract influenced the hatchability of fertilized broiler eggs. Oke et al. (2021) discovered that in ovo inclusion of black cumin at a 2-mg dose increased hatchability when broiler birds were thermally challenged during incubation. Peşmen (2022) also reported no significant difference in hatchability when in ovo injection of black cumin was administered to broiler birds. Akosile et al. (2023b) administered clove and cinnamon in ovo to broiler birds and concluded that hatchability was significantly influenced compared to the control birds. Overall, these results suggest that the effects of in ovo administration of these extracts on hatchability can vary depending on the specific extract and dosage used. However, most studies found that the solutions of phytogenic feed additives injected were safe for the developing embryos, and some extracts showed a dose-dependent effect on hatchability.
In Ovo Feeding and Chick Quality
Through various assessment methods and supplementation strategies of supplementing eggs in the hatchery with an adequate amount of phytogenic feed additives to enhance the growth of the embryo to produce effective chick weight and good chick quality parameters, it is possible to optimize chick quality and promote the healthy growth of broiler chickens. However, there is limited research on the effects of in ovo phytobiotic supplementation on chick weight and quality.
Ngueda et al. (2021) studied the in ovo feeding of Manihot esculenta extract solution at day 18 of embryonic development of broilers and reported improved chick weight and chick quality. As comparison to the saline solution and the negative control groups, the authors reported that the weights of 1-d-old chicks in the Manihot esculenta extract group were higher. Similarly, in ovo pollen extract injection (PEI) into the amniotic fluid of fertile broiler eggs on the 16th day of incubation increased subsequent chick weight from 70.1% to 73.5% among the control and PEI groups, respectively, to improve the genetic potential for late embryonic and early posthatch growth (Cocksun et al., 2014). In contrast, Morovate et al. (2016) and Fazli et al. (2015) also concluded that no differences were observed in increased weight at hatch of birds that were administered in ovo Silybum marianum extract and garlic extract.
In Ovo Phytobiotics and Performance Effect
Modern broiler lines are developed to mature quickly and reach a high body weight at the point of sale (Asche et al., 2018). These strains are chosen for characteristics that improve the FCR and shorten the duration to market weight (Lippens et al., 2000; Tůmová et al., 2002). Consequently, during the incubation phase, the day-old chick acquires a substantial quantity of body weight, and this weight increase persists after hatching (Decuypere et al., 2007). In reality, a broiler chicken’s body weight can grow by up to 5,000%, or 2 kg, in 5 to 6 wk. This rapid development strains the bird’s physiological systems, including its nutrient needs to support this fast growth and development, the bird’s food must provide adequate nutrients (Ravindran and Abdollahi, 2021). Meeting these nutritional needs during embryonic development is critical to ensuring the chick has the necessary reserves to support growth and development after hatching.
The effects of different types and doses of in ovo phytogenic feed additives on hatching and performance of chickens are shown in Table 1. It has been mostly reported that in ovo supplementation of phytogenic substances has been shown to influence chicken performance parameters and has a growth-promoting effect on poultry. Ngueda et al. (2021) proposed that the early feeding of Manihot esculenta extract via an in ovo route on the 18th day of incubation is utilized by chick embryos along with yolk sac reserves and promotes the growth performance than those of the other group. Feed intake was higher in the Manihot esculenta group and FCR showed no significant differences across the treatments. Injection of GSE into broiler eggs at the dose rates of 3 (GSE) and 4 (GSE) into the airspace on the 5th day of incubation Feed intake, body weight, body weight gain (BWG) and FCR appeared with no significant differences (Zeinab et al., 2019). Similarly, Hajati et al. (2014) investigated the effect of (3 mg/egg), (4.5 mg/egg), and (6 mg/egg) GSE on ileal microflora, performance, hatchability, antioxidant activity, and yolk sac weight of broiler chickens. The findings demonstrated that, in comparison to control groups, in ovo treatment of 4.5 mg GSE or vitamin C enhanced the average daily weight gain and daily feed consumption of chickens. In ovo feeding (IOF) of black cumin (2, 4, 6, and 8 mg) into the airspace of the egg at 17.5 d of incubation improved the final weight of the birds injected with black cumin as compared with the control. The feed intake was similar, while the FCR of the in the in ovo-treated birds was lower than that of the control birds (Oke et al., 2021).
Table 1:
Effects of different types and doses of in ovo phytobiotics on hatching and performance of chickens
| Extracts | Dose | Hatch (%) | FI | BWG | FCR | Reference |
|---|---|---|---|---|---|---|
| Nano curcumin | 0.01 | 100a | 15.89 | 13.56 | 1.007 | Heidary et al., 2020 |
| 0.03 | 86.67b | 15.89 | 11.89 | 1.003 | ||
| 0.05 | 86.67b | 17.43 | 12.09 | 1.125 | ||
| Control | 100a | 18.62 | 12.29 | 1.291 | ||
| Grape seed extract | 3 mg | 84.28bc | 29.03bc | 21.46abc | 1.35 | Hajati et al., 2014 |
| 4.5 mg | 92.85a | 28.15b | 21.71ab | 1.35 | ||
| 6 mg | 75.71c | 30.94a | 20.45bcd | 1.37 | ||
| Control | 80.00c | 27.42d | 19.74d | 1.39 | ||
| Black cumin | 2 mg | 86.48cd | 4963.8b | 2087.5bc | 2.38b | Oke et al., 2020 |
| 4 mg | 87.75bc | 5215.3ab | 2175.0b | 2.40b | ||
| 6 mg | 91.93ab | 5321.8ab | 2455.0a | 2.17c | ||
| 8 mg | 87.75d | 4887.0b | 2030.0b | 2.41ab | ||
| Control | 86.07cd | 4835.3b | 1900.00c | 2.55ab | ||
| Silybum marianum extract | 0 mg/kg | 86.14 | 96.99 | 2179.96a | 1.63 | Morovate et al., 2016 |
| 100 mg/kg | 85.88 | 96.18 | 2125.20b | 1.65 | ||
| 200 mg/kg | 84.79 | 97.86 | 2142.54ab | 1.67 | ||
| Tomato and garlic | Tom | 68 | 107 | 2263 | 2.02 | Fazli et al., 2015 |
| Gar | 72 | 111 | 2388 | 1.99 | ||
| Control | 77 | 110 | 2346 | 2.01 | ||
| Plant extracts | Cinnamon | 3390.00 | 2639.10b | 1.29b | El-Kholy et al., 2021 | |
| Thyme | 3525.50 | 2752.60a | 1.29b | |||
| Clove | 3365.14 | 2799.80a | 1.20b | |||
| Control | 3604.08 | 2172.10c | 1.66a | |||
| Clove and cinnamon | Clove 2 mg | 92.0a | Akosile et al., 2023a | |||
| Clove 4 mg | 74.0e | |||||
| Cinnamon 2 mg | 89.0b | |||||
| Cinnamon 4 mg | 81.0d | |||||
| Control | 87.0c | |||||
| Moringa | 0 g | 68.8b | 23.4 ± 3.77 | N’nanle et al., 2017 | ||
| 0.5 g | 93.8a | 26.2 ± 4.39 | ||||
| 5 g | 71.1b | 28.5 ± 4.81 | ||||
| 50 g | 73.9b | 33.3 ± 5.41 | ||||
| Pollen Extract | Pollen extract | 73.1b | Coşkun et al., 2014 | |||
| Control | 82.3a | |||||
| Manihot esculenta extract |
M. esculenta
extract |
91.30 ± 2.00a | 6921.84 ± 36.62 | 1933.42 ± 20.05a | 2.32 ± 0.08 | Ngueda et al., 2021 |
| Control | 82.90 ± 2.00c | 6499.95 ± 34.19 | 1861.69 ± 20.02b | 2.48 ± 0.08 | ||
| Resveratrol | 52 µg RV/egg | 86.81c | EL-Saadany et al., 2019 | |||
| 50 µg RV/egg | 93.89a | |||||
| Control | 87.31c |
In the literature, reports of production parameter values are not always consistent. According to recent findings, several phytogenic compounds have increased feed intake while decreasing the FCR, resulting in unforeseen consequences. Heidary et al. (2020) studied the influence of IOF of nanocurcumin (NC) on growth immune responses, performance, intestinal morphology, and antioxidant status of broiler chickens challenged with thermal stress. The findings demonstrated that the IOI of broiler embryo with 0.01, 0.03, and 0.05 mL/egg of NC during the 17.5th day of incubation had no influence on daily weight gain, feed intake, or FCR from days 1 to 10, nor did it affect body weight on those days. Treatments had a significant impact on feed intake from days 1 through 24, so all groups receiving NC had lower average feed intake than control groups. Additionally, the study Nnanle et al. (2017) revealed influence of in ovo supplementation of 0.5, 5, and 50 μg/mL of Moringa oleifera leaves at 18 d of incubation in the air chamber of broilers’ hatching eggs. The authors revealed that the IOI nutrient of Moringa oleifera leaf extract did not affect the feed intake and BWG up to 7 wk of age. However, the authors observed increased body weights with increased extract concentration. The doses of the various bioactive compounds, environmental factors, and managerial variations may be attributed to these contradictory results.
Recently, El-Kholy et al. (2021) conducted a study on in-ovo injection of herbal extracts clove extract (0.1 mL), (thyme extract (0.1 mL), and cinnamon extract (0.1 mL) on immunological and physiological responses and posthatch performance of broilers on day 10 of incubation. The authors indicated that in-ovo administration of herbal extracts influence the FCR, daily weight gain, hatching weights, final weights, and weight gain during various experimental times. The BWG and FCR of chickens hatched from eggs injected with herbal extracts were superior to those of the control group. While research has shown interesting finding on the use of bioactive substances in in ovo supplementation to improve chicken hatchability and posthatch performance, there are still challenges that must be overcome before these technologies can be adopted on a larger scale by the poultry industry.
One of the difficulties is creating in ovo supplementation standards that can be replicated and implemented reliably across various production methods. This would necessitate taking into account variables such as bioactive substance timing, dose, and transport mechanism, as well as genetic and environmental factors that can impact the reaction of the chicken embryo. To resolve these issues, additional study is required to investigate the impacts of various bioactive substances and supplementation methods on performance parameters, as well as to create standardized procedures and recommendations for in ovo supplementation. This would allow the poultry business to more easily and effectively implement these technologies to enhance the performance and profitability of their operations.
In Ovo Feeding of Phytobiotics and Antioxidant Status
Optimal antioxidant status is crucial during the perinatal development of chicks. According to Oke et al. (2020) and Zhang et al. (2018), heat stress is known to destabilize the redox status and cause oxidative damage in poultry and it is characterized by reduced levels of cellular antioxidant activity. Recent studies also showed that administering a mixture of essential oils via the in ovo route enhanced the blood antioxidant status of broiler chickens without impairing growth performance parameters (Gouda et al., 2020; Oladokun et al., 2021). Using in ovo black cumin extract, Oke et al. (2021) indicated that the use of 6 mg of the extract meliorated the antioxidant parameters of broiler chicken at day 21 of incubation. Similar to this, the study of Akosile et al. (2023a) showed that the antioxidant status of broiler chicks was enhanced at hatch using 2 mg clove. In addition, Farag et al. (2023) demonstrated that the in ovo feeding of polyphenolic compounds of chicoric and rosmarinic improved oxidative stress in chicks, while Abdelghani et al. (2023) also demonstrated that 3 mg/egg of soy isoflavone had a positive effect on the antioxidant status of newly hatched chicks.
Gut Development and In ovo Phytogenic Nutrition
In ovo injection of phytogenic substances involves the injection of phytogenic nutrients directly into the developing embryos, which provides several benefits. One of these benefits is that the nutrients provided are exposed to the tissues of the GIT after the embryo orally consumes the amniotic contents prior to pipping (when the chick begins to break through the eggshell) (Shehata et al., 2021). This early exposure to nutrients helps to accelerate the development of the GIT, resulting in a greater digestive and nutrient-absorptive capacity in the hatchling (Shehata et al., 2021). This means that the chick is better able to digest and utilize the nutrients in its diet after hatching. However, it should be noted that the digestive tract of hatchlings has a limited ability to digest and utilize diets rich in proteins and carbohydrates (Kadam et al., 2013). Therefore, in ovo feeding provides an opportunity to enhance the nutrient profile of the chick before hatching, which can improve the overall health and growth performance of the chick posthatch (Kadam et al., 2013). Additionally, in ovo feeding can reduce the stress on the chick during the immediate posthatch period when feed intake is typically low due to stress from the hatching process (Uni and Ferket, 2004).
The small intestine is a vital organ in the digestion and absorption of nutrients in newly hatched chicks (Ravindran and Abdollahi, 2021). It is responsible for breaking nutrients into their basic components, such as proteins into amino acids, carbohydrates into simple sugars, and fats into fatty acids and glycerol (Ravindran and Abdollahi, 2021). These nutrients are then absorbed into the bloodstream through the intestinal wall and transported to the body tissues for growth and development. Although the small intestine is fully formed and functional at hatch, it undergoes further development and maturation as the chick transitions from dependent on the yolk sac to exogenous nutrient sources, such as feed. This development and maturation include an increase in villi height, crypt depth, and surface area, which provide a larger surface area for nutrient absorption. The intestinal morphology and function can be influenced by various factors such as diet, management, and early nutrition interventions (Lucas, 1998). Therefore, proper nutrition during the embryonic phase is crucial for the development of the small intestine in newly hatched chicks, which will affect their ability to digest and absorb nutrients and impact their overall growth and development (Lucas, 1998).
Due to the genetic selection for fast growth in modern poultry, the intestinal development of chicks needs to occur quickly to reach full functional digestive and absorptive capacities to meet the high nutrient demands of rapid growth (Ravindran and Abdollahi, 2021). The small intestine is crucial for the digestion and absorption of nutrients, and any deficiency in its function could lead to poor growth performance and health problems.
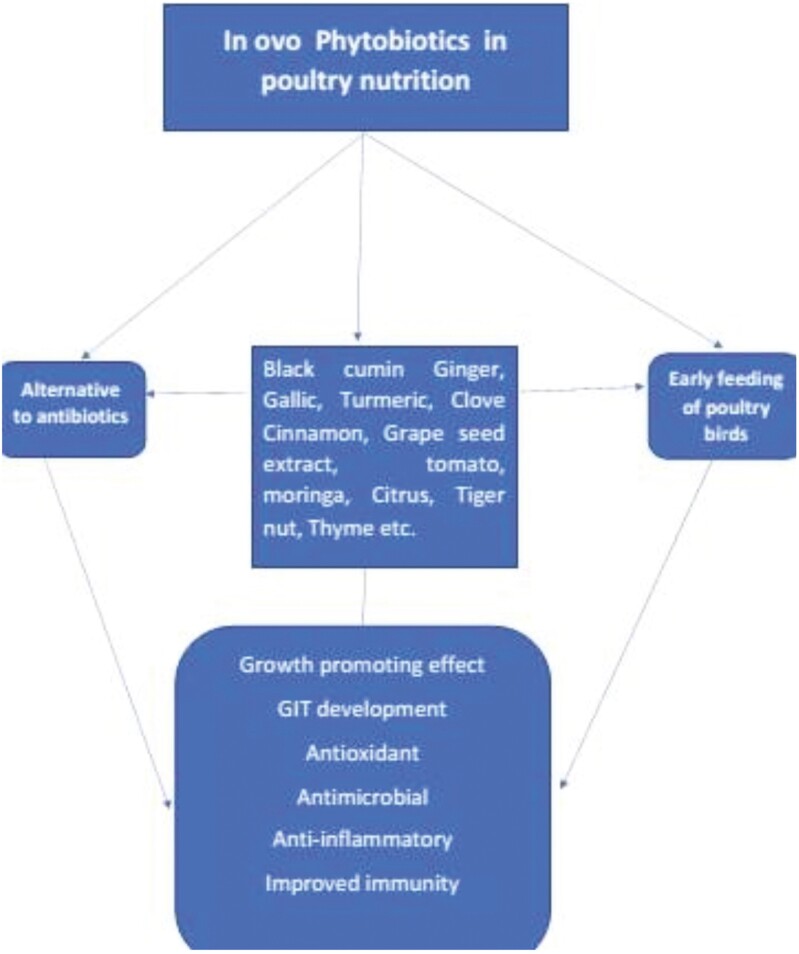
Phytogenic feed additives, such as herbal plants and their extracts, have been reported to have positive effects on the gastrointestinal tract, including spasmolytic and laxative effects (Windisch and Kroismayr, 2006). It has been demonstrated that in ovo feeding with bioactive compounds that boost the activity of digestive enzymes enhances digestion in hatchling chicks (Siwek et al., 2018). These bioactive substances can be delivered directly to the developing embryo through in ovo injection and subsequently be exposed to the tissues of the gastrointestinal tract after the embryo orally consumes the amniotic contents before hatching. This can accelerate enteric development and increase digestive and nutrient absorptive capacity in the hatchling chicks. Oke et al. (2020) showed the effect of in ovo black cumin on the intestinal weight of thermally exposed broiler birds. The result showed that intestinal weight was significantly affected by birds administered in ovo black cumin. Similarly, Akosile et al. (2023a, b) showed positive influence of in ovo administration of clove and cinnamon on the intestinal weight of broiler birds in hot, humid environments. Villus height in the jejunum was significantly affected on day 10 by in ovo injection of nano curcumin, thereby enhancing the intestinal growth and performance ability of the birds (Heidary et al., 2020). The summary of different in ovo phytogenic feed additives (PFA) and their effects are shown in Figure 2.
Figure 2:
Summary of different in ovo phytobiotics and their effects.
Conclusion
This review collates information on the potential of in ovo feeding of phytogenic feed additives as emerging advances in avian sciences to provide insight into the use of the technology. Existing data indicate that in ovo supplementation with phytogenic feed additives is a promising technique and it might positively affect the antioxidant status of embryos, hatching, and posthatch growth performance of chickens. To demonstrate the long-term effects of in ovo supplementation and the financial benefits for poultry industry, more research is required. Although some herbs and their derivatives have been explored, more studies are needed on novel phytogenic feed additives to further increase their scale in poultry production.
Glossary
Abbreviations:
- BWG
Body weight gain
- FCR
Feed conversion ratio
- FI
Feed intake
- g
Gram
- GALT
Gut-Associated Lymphoid Tissue
- GIT
Gastrointestinal tract
- GSE
Grape seed extract
- IOF
In ovo feeding
- IOI
In ovo injection
- Kg
Kilogram
- Mg
Milligram
- NC
Nanocurcumin
- PFA
Phytogenic feed additives
- RV
Resveratrol
Contributor Information
Oluwaseun Ayomide Akosile, Department of Animal Physiology, Federal University of Agriculture, Abeokuta, Nigeria.
Festus Olasehinde Kehinde, Department of Animal and Environmental Biology, Faculty of Natural Science, Kogi State University, Anyigba, Nigeria.
Aderanti Ifeoluwa Oni, Department of Animal Physiology, Federal University of Agriculture, Abeokuta, Nigeria.
Oyegunle Emmanuel Oke, Department of Animal Physiology, Federal University of Agriculture, Abeokuta, Nigeria.
Conflict of interest statementThe authors declare no conflict of interest.
Literature Cited
- Abdelghani, E., Fathi M. A., Zhojian L., Pengyuan D., Li Y., and Li C... 2023. In ovo injection of soy isoflavones on hatching performance and intestinal development of newly hatched chicks. J. Anim. Physiol. Anim. Nutr. (Berl) doi: 10.1111/jpn.13850. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack, M. E., El-Saadony M. T., Salem H. M., El-Tahan A. M., Soliman M. M., Youssef G. B., Taha A. E., Soliman S. M., Ahmed A. E., El-Kott A. F.,. et al. 2022. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird’s health and production. Poult. Sci. 101:101696. doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjei-Mensah, B., Oke E. O., Ali M. M., Hamidu J. A., and Tona K... 2022. Response of layer chicks to the dietary inclusion of allicin-rich extract. J. Appl. Poult. Res. 31:100291. doi: 10.1016/j.japr.2022.100291. [DOI] [Google Scholar]
- Ahluwalia, B., Magnusson M. K., Öhman L.. 2017. Mucosal immune system of the gastrointestinal tract: maintaining balance between the good and the bad. Scandinavian journal of gastroenterology. 52:1185–1193. [DOI] [PubMed] [Google Scholar]
- Akosile, O. A., Majekodunmi B. C., Sogunle O. M., Baloyi J. J., Fushai F., Bhebhe E., and Oke O. E... 2023a. Research note: responses of broiler chickens to in ovo feeding with clove and cinnamon extract under hot-humid environments. Poult. Sci. 102:102391. doi: 10.1016/j.psj.2022.102391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akosile, O. A., Sogunle O. M., Majekodunmi B. C., and Oke O. E... 2023b. In ovo injection of cinnamon or clove alters the physiology and growth of broilers in a hot-tropical environment. Transl Anim Sci. 7:1–8. doi: 10.1093/tas/txad036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany, M., Elnesr S. S., Farag M. R., Abd El-Hack H. E., Barkat R. A., Gabr A. A., Foda M. A., Noreldin A. E., Khafaga A. F., El-Sabrout K.,. et al. 2021. Potential role of important nutraceuticals in poultry performance and health: a comprehensive review. Res. Vet. Sci. 137:9–29. doi: 10.1016/j.rvsc.2021.04.009. [DOI] [PubMed] [Google Scholar]
- Alghirani, M. M., Chung E. L. T., E. L. T.>, Jesse F. F. A., Sazili A. Q., and Loh T. C... 2021. Could phytobiotics replace antibiotics as feed additives to stimulate production performance and health status in poultry? An overview. J. Adv. Vet. Res. 11:254–265. https://advetresearch.com/index.php/AVR/article/view/810#:~:text=In%20summary%2C%20phytobiotics%20can%20be,security%20while%20preventing%20antibiotic%20resistance. [Google Scholar]
- Asche, F., Cojocaru A. L., and Roth B... 2018. The development of large scale aquaculture production: a comparison of the supply chains for chicken and salmon. Aquaculture 493:446–455. doi: 10.1016/j.aquaculture.2016.10.031. [DOI] [Google Scholar]
- Avakian A., Wakenell P. S., Bryan T., Schaeffer J. L., Williams C. J., Whitfill C. E... 2002. In ovo administration of Marek’s disease vaccine: Importance of vaccine deposition site in the fertile egg. Proc. 51st Western Poultry Disease Conf., Puerto Vallarta, Mexico. O’Fallon, IL: Veterinary Software Publishing Inc. [Google Scholar]
- Bakyaraj, S., Bhanja S. K., Majumdar S., and Dash B... 2012. Modulation of post‐hatch growth and immunity through in ovo sulemented nutrients in broiler chickens. J. Sci. Food Agric. 92:313–320. doi: 10.1002/jsfa.4577. [DOI] [PubMed] [Google Scholar]
- Bello, A., Zhai W., Gerard P. D., and Peebles E. D... 2013. Effects of the commercial in ovo injection of 25-hydroxycholecalciferol on the hatchability and hatching chick quality of broilers. Poult. Sci. 92:2551–2559. doi: 10.3382/ps.2013-03086. [DOI] [PubMed] [Google Scholar]
- Bergoug, H., Burel C., Guinebretiere M., Tong Q., Roulston N., Romanini C. E. B., Exadaktylos V., McGonnell I. M., Demmers T. G. M., Verhelst R.,. et al. 2013. Effect of pre-incubation and incubation conditions on hatchability, hatch time and hatch window, and effect of post-hatch handling on chick quality at placement. Worlds Poult. Sci. J. 69:313–334. doi: 10.1017/s0043933913000329. [DOI] [Google Scholar]
- Berntsen, H. H., and Bech C... 2016. Incubation temperature influences survival in a small passerine bird. J. Avian Biol. 47:141–145. doi: 10.1111/jav.00688. [DOI] [Google Scholar]
- Berrocoso, J. D., Kida R., Singh A. K., Kim Y. S., and Jha R... 2017. Effect of in ovo injection of raffinose on growth performance and gut health parameters of broiler chicken. Poult. Sci. 96:1573–1580. doi: 10.3382/ps/pew430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan, M. M., Iji P. A.. 2015. Energy value of cassava products in broiler chicken diets with or without enzyme supplementation. Asian-Australasian J. Anim. Sci. 28:1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth, D.T. 2004. Artificial incubation. Reptilian Incubation: environment, evolution and behaviour. UK: Nottingham University Press; p. 253–263. [Google Scholar]
- Careghi, C., Tona K., Onagbesan O., Buyse J., Decuypere E., and Bruggeman V... 2005. The effects of the spread of hatch and interaction with delayed feed access after hatch on broiler performance until seven days of age. Poult. Sci. 84:1314–1320. doi: 10.1093/ps/84.8.1314. [DOI] [PubMed] [Google Scholar]
- Coşkun, I., Cayan H., Yilmaz O., Taskin A., Tahtabiçen E., and Samli H... 2014. Effects of in ovo pollen extract injection to fertile broiler eggs on hatchability and subsequent chick weight. Türk Tarım ve Doğa Bilimleri Dergisi 1:485–489. https://dergipark.org.tr/en/pub/turkjans/issue/13308/160752. [Google Scholar]
- Das, R., Mishra P., and Jha R... 2021. In ovo feeding as a tool for improving performance and gut health of poultry: a review. Front. Vet. Sci. 8:754246. doi: 10.3389/fvets.2021.754246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuypere E., and Bruggeman V... 2007. The endocrine interface of environmental and egg factors affecting chick quality. Poult. Sci. 86:1037–1042. doi: 10.1093/ps/86.5.1037. [DOI] [PubMed] [Google Scholar]
- Dhama, K., Karthik K., Khandia R., Munjal A., Tiwari R., Rana R., Khurana S. K., Sana U., Khan R. U., and Alagawany M... 2018. Medicinal and therapeutic potential of herbs and plant metabolites/ extracts countering viral pathogens – current knowledge and future prospects. Curr. Drug Metab. 19:236–263. doi: 10.2174/1389200219666180129145252. [DOI] [PubMed] [Google Scholar]
- Dhama, K., Latheef S. K., Saminathan M., Samad H. A., Karthik K., Tiwari R., Khan R. U., Alagawany M., Farag M. R., and Gazi Mahabubul A... 2015. Multiple beneficial applications and modes of action of herbs in poultry health and production – a review. Int. J. Pharmacol. 11:152–176. doi: 10.3923/ijp.2015.152.176. [DOI] [Google Scholar]
- El-Kholy, K. H., Sarhan D. M., El-Said E. A.. 2021. Effect of in-ovo Injection of herbal extracts on post-hatch performance, immunological, and physiological responses of broiler chickens. J. World's Poultry Res. 11:183–192. [Google Scholar]
- El-saadany, A. S. 2019. Effect of in ovo injection with resveratrol on hatching traits and physiological response of mandara chicks. Egypt. Poult. Sci. J. 39:973–991. https://epsj.journals.ekb.eg/article_67517_308d6bbe74bea97e0be623a3f608b1e0.pdf. [Google Scholar]
- Farag, M. R., Zizzadoro C., Alagawany M., Abou-Zeid S. M., Mawed S. A., El Kholy M. S., Di Cerbo A., Azzam M. M., Mahdy E. A., Khedr M. H.,. et al. 2023. In ovo protective effects of chicoric and rosmarinic acids against thiacloprid-induced cytotoxicity, oxidative stress, and growth retardation on newly hatched chicks. Poult. Sci. 102:102487. doi: 10.1016/j.psj.2023.102487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasenko, G.M. 1996. Factors influencing embryo and poult viability and growth during long term storage of turkey eggs. North Carolina State University. [Google Scholar]
- Fazli, N., Hassanabadi A., Mottaghitalab M., and Hajati H... 2015. Manipulation of broiler chickens sex differentiation by in ovo injection of aromatase inhibitors, and garlic and tomato extracts. Poult. Sci. 94:2778–2783. doi: 10.3382/ps/pev236. [DOI] [PubMed] [Google Scholar]
- Ferket, P.R., 2006. Incubation and in ovo nutrition affects neonatal development. In: 33rd Annual Carolina Poultry Nutrition Conference (Vol. 26). p. 18–28. [Google Scholar]
- Ferket, P.R., 2009. Epigenetic adaptions in poultry: a case for in ovo feeding strategies. https://conservancy.umn.edu/bitstream/handle/11299/204171/SF95_M658a-70-2009_magr56189.pdf?sequence=1
- Ferket, P., and Uni Z.. 2006. Early Feeding – in ovo feeding enhances of early gut development and digestive capacity of poultry. XII European Poultry Conference, Verona. Italy. [Google Scholar]
- Gautron, J., Réhault-Godbert S., Van de Braak T. G. H., Dunn I. C... 2021. What are the challenges facing the table egg industry in the next decades and what can be done to address them? Animal 15:100282. doi: 10.1016/j.animal.2021.100282. [DOI] [PubMed] [Google Scholar]
- Gheisar, M. M., Im Y. W., Lee H. H., Choi Y. I., and Kim I. H... 2015. Inclusion of phytogenic blends in different nutrient density diets of meat-type ducks. Poult. Sci. 94:2952–2958. doi: 10.3382/ps/pev301. [DOI] [PubMed] [Google Scholar]
- Glatz, P. C. 2000. Beak trimming methods: review. Asian-Australas. J. Anim. Sci. 13:1619–1637. doi: 10.5713/ajas.2000.1619. [DOI] [Google Scholar]
- Gouda, A., Amer S. A., Gabr S., and Tolba S. A... 2020. Effect of dietary supplemental ascorbic acid and folic acid on the growth performance, redox status, and immune status of broiler chickens under heat stress. Trop. Anim. Health Prod. 52:2987–2996. doi: 10.1007/s11250-020-02316-4. [DOI] [PubMed] [Google Scholar]
- Gous, R. M. 2010. Nutritional limitations on growth and development in poultry. Livestock Sci. 130:25–32. doi: 10.1016/j.livsci.2010.02.007. [DOI] [Google Scholar]
- Grashorn, M. A. 2010. Use of phytobiotics in broiler nutrition: an alternative to infeed antibiotics. J. Anim. Feed Sci. 19:338–347. doi: 10.22358/jafs/66297/2010. [DOI] [Google Scholar]
- Hajati, H., Hassanabadi A., Golian A., Nassiri-Moghaddam H., and Nassiri M. R... 2014. The effect of in ovo injection of grape seed extract and vitamin C on hatchability, antioxidant activity, yolk sac weight, performance and ileal micro flora of broiler chickens. Res. Opin. Anim. Vet. Sci. 4:633–638. https://profdoc.um.ac.ir/paper-abstract-1045139.html. [Google Scholar]
- Harborne, J. 1984. Methods of Plant Analysis, Phytochemical Methods. 1–36. [Google Scholar]
- Head, V. 2011. Keeping chickens and other poultry. Arcturus Publishing. [Google Scholar]
- Hedlund, L., and Jensen P.. 2021. Incubation and hatching conditions of laying hen chicks explain a large part of the stress effects from commercial large-scale hatcheries. Poultry Sci. 100:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidary, M., Hassanabadi A., and Mohebalian H... 2020. Effects of in ovo injection of nanocurcumin and vitamin E on antioxidant status, immune responses, intestinal morphology and growth performance of broiler chickens exposed to heat stress. J. Livestock Sci. Technol. 8:17–27. doi: 10.22103/JLST.2020.15352.1310. [DOI] [Google Scholar]
- Huang, W. Y., Cai Y. Z., and Zhang Y... 2009. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr. Cancer 62:1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- Hulet, R., Gladys G., Hill D., Meijerhof R., and El-Shiekh T... 2007. Influence of egg shell embryonic incubation temperature and broiler breeder flock age on posthatch growth performance and carcass characteristics. Poult. Sci. 86:408–412. doi: 10.1093/ps/86.2.408. [DOI] [PubMed] [Google Scholar]
- Humphrey, S., Chaloner G., Kemmett K., Davidson N., Williams N., Kipar A., Humphrey T., and Wigley P... 2014. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. MBio 5:e01364–e01314. doi: 10.1128/mBio.01364-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghebaert, G., Ducatelle R., and Van Immerseel F... 2011. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Iyasere, O. S., Olajumoke O. P., Durosaro S. O., Oke O. E., Famosaya O. O., Oliyide K. M. and Oyeniran V. J.. 2022. Nigerian indigenous hens show more discomfort-related behavior with visual separation than physical separation from their chicks: An exploratory study. Frontiers in Veterinary Sci. 9:978848. doi: 10.3389/fvets.2022.978848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennison, R. 2021. Hatchery practice. Poultry Health: A Guide for Professionals 72–78. doi: 10.1079/9781789245042.0011. [DOI] [Google Scholar]
- Jha, R., Singh A. K., Yadav S., Berrocoso J. F. D., and Mishra B... 2019. Early nutrition programming (in ovo and post-hatch feeding) as a strategy to modulate gut health of poultry. Front. Vet. Sci. 6:82. doi: 10.3389/fvets.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochemsen, P., and Jeurissen S. H... 2002. The localization and uptake of in ovo injected soluble and particulate substances in the chicken. Poult. Sci. 81:1811–1817. doi: 10.1093/ps/81.12.1811. [DOI] [PubMed] [Google Scholar]
- Johnston, P. A., Liu H., O’Connell T., Phelps P., Bland M., Tyczkowski J., Kemper A., Harding T., Avakian A., Haddad E.,. et al. 1997. Alications in in ovo technology. Poult. Sci. 76:165–178. doi: 10.1093/ps/76.1.165. [DOI] [PubMed] [Google Scholar]
- Kadam, M. M., Barekatain M. R., Bhanja S., and Iji P. A... 2013. Prospects of in ovo feeding and nutrient sulementation for poultry: the science and commercial alications—a review. J. Sci. Food Agric. 93:3654–3661. doi: 10.1002/jsfa.6301. [DOI] [PubMed] [Google Scholar]
- Karásková, K., Suchý P., and Straková E... 2015. Current use of phytogenic feed additives in animal nutrition: a review. Czech J. Anim. Sci. 60:521–530. doi: 10.17221/8594-cjas. [DOI] [Google Scholar]
- Koche, D., Shirsat R., and Kawale M. A. H. E. S. H... 2016. An overerview of major classes of phytochemicals: their types and role in disease prevention. Hislopia J. 9:0976–2124. [Google Scholar]
- Kop Bozbay, C., Konanç K., Ocak N., and Öztürk E... 2016. The effects of in ovo injection of propolis and injection site on hatchability, hatching weight and survival of chicks. Türkiye Tarımsal Araștırmalar Dergisi 3:48–54. http://dergipark.gov.tr/.../262512. [Google Scholar]
- Kotzekidou, P., Giannakidis P., and Boulamatsis A... 2008. Antimicrobial activity of some plant extracts and essential oils against foodborne pathogens in vitro and on the fate of inoculated pathogens in chocolate. LWT Food Sci. Technol. 41:119–127. doi: 10.1016/j.lwt.2007.01.016. [DOI] [Google Scholar]
- Kpomasse, C. C., Oke O. E., Houndonougbo F. M., and Tona K... 2021. Broilers production challenges in the tropics: a review. Vet. Med. Sci. 7:831–842. doi: 10.1002/vms3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kpomasse, C. C., Oso O. M., Lawal K. O., and Oke O. E... 2023. Juvenile growth, thermotolerance and gut histomorphology of broiler chickens fed Curcuma longa under hot-humid environments. Heliyon 9:e13060. doi: 10.1016/j.heliyon.2023.e13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris-Etherton, P. M., and Keen C. L... 2002. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr. Opin Lipidol 13:41–49. doi: 10.1097/00041433-200202000-00007. [DOI] [PubMed] [Google Scholar]
- Lesnierowski, G., and Stangierski J... 2018. What’s new in chicken egg research and technology for human health promotion? A review. Trends Food Sci. Technol. 71:46–51. doi: 10.1016/j.tifs.2017.10.022. [DOI] [Google Scholar]
- Li, S., Zhi L., Liu Y., Shen J., Liu L., Yao J., and Yang X... 2016. Effect of in ovo feeding of folic acid on the folate metabolism, immune function and epigenetic modification of immune effector molecules of broiler. Br. J. Nutr. 115:411–421. doi: 10.1017/S0007114515004511. [DOI] [PubMed] [Google Scholar]
- Lippens, M., Room G., De Groote G., and Decuypere E... 2000. Early and temporary quantitative food restriction of broiler chickens. 1. Effects on performance characteristics, mortality and meat quality. Br. Poult. Sci. 41:343–354. doi: 10.1080/713654926. [DOI] [PubMed] [Google Scholar]
- Lucas, A. 1998. Programming by early nutrition: an experimental approach. J. Nutr. 128:401S–406S. doi: 10.1093/jn/128.2.401S. [DOI] [PubMed] [Google Scholar]
- Ma, Y. B., Zhang F. D., Wang J., Wu S. G., Qi G. H., and Zhang H. J... 2020. Effect of in ovo feeding of β-hydroxy-β-methylbutyrate on hatchability, muscle growth and performance in prenatal and posthatch broilers. J. Sci. Food Agric. 100:755–763. doi: 10.1002/jsfa.10080. [DOI] [PubMed] [Google Scholar]
- Mahima, R. A., Deb R., Latheef S. K., Abdul Samad H., Tiwari R., Verma A. K., Kumar A., and Dhama K... 2012. Immunomodulatory and therapeutic potentials of herbal, traditional/indigenous and ethnoveterinary medicines. Pak. J. Biol. Sci. 15:754–774. doi: 10.3923/pjbs.2012.754.774. [DOI] [PubMed] [Google Scholar]
- Mahjar, N. T., and Al-Salhie K. C... 2022. The effects of in ovo injection of garlic (Allium sativum L.) extract on hatchability, liver enzymes and antioxidant status of broiler chickens. Basrah J. Agric. Sci. 35:61–70. doi: 10.37077/25200860.2022.35.1.05. [DOI] [Google Scholar]
- Morovat, M., Chamani M., A., Zarei, and Sadeghi A. A... 2016. Dietary but not in ovo feeding of Silybum marianum extract resulted in an improvement in performance, immunity and carcass characteristics and decreased the adverse effects of high temperatures in broilers. Br. Poult. Sci. 57:105–113. doi: 10.1080/00071668.2015.1121537. [DOI] [PubMed] [Google Scholar]
- Ngueda, D. O. R., N’nanle O., Voemesse K., Onagbesan O., Decuypere E., and Tona K... 2021. Effects of in ovo injection of Manihot esculenta extract on hatchability and post-hatch performance of Sasso broiler chickens. Eur. Poult. Sci. 85:1–11. doi: 10.1399/eps.2021.335. [DOI] [Google Scholar]
- N’nanle, O., Tété-Bénissan A., Tona K., Teteh A., Voemesse K., Decuypere E., and Gbeassor M... 2017. Effect of in ovo inoculation of Moringa oleifera leaves extract on hatchability and chicken growth performance. Eur. Poult. Sci. 81. doi: 10.1399/eps.2017.213. [DOI] [Google Scholar]
- Noy, Y., Uni Z.. 2010. Early nutritional strategies. World's Poultry Science Journal, 66:639–646. [Google Scholar]
- Nyandoro, S. S., Nkunya M. H., Cosam J. C., and Msoffe P. L... 2014. In ovo antiviral potency of the leaf constituents of Tanzanian Toussaintia species against infectious bursal disease virus and newcastle disease virus. Int. J. Biol. Chem. Sci. 8:1308–1318. doi: 10.4314/ijbcs.v8i3.43. [DOI] [Google Scholar]
- Oke, O. E. 2018. Evaluation of physiological response and performance by supplementation of Curcuma longa in broiler feed under hot humid tropical climate. Trop. Anim. Health Prod. 50:1071–1077. doi: 10.1007/s11250-018-1532-8. [DOI] [PubMed] [Google Scholar]
- Oke, O. E., Alo E. T., Oke F. O., Oyebamiji Y. A., Ijaiya M. A., Odefemi M. A., Kazeem R. Y., Soyode A. A., Aruwajoye O. M., Ojo R. T., Adeosun S. M., Onagbesan O. M.. 2020. Early Age Thermal Manipulation on the Performance and Physiological Response of Broiler Chickens under Hot Humid Tropical Climate. J. Therm. Bio. 88:102517. doi: 10.1016/j.jtherbio.2020.102517. [DOI] [PubMed] [Google Scholar]
- Oke, O. E., Emeshili U. K., Iyasere O. S., Abioja M. O., Daramola J. O., Ladokun A. O., Abiona J. A., Williams T. J., Rahman S. A., Rotimi S. O.,. et al. 2017. Physiological responses and performance of broiler chickens offered olive leaf extract under hot humid tropical climate. J. Appl. Poult. Res. 26:376–382. doi: 10.3382/japr/pfx005. [DOI] [Google Scholar]
- Oke, O. E., Oyelola O. B., Iyasere O. S., Njoku C. P., Oso A. O., Oso O. M., Fatoki S. T., Bankole K. O., K. O., Jimoh I. O., Sybill N. I., et al. 2021. In ovo injection of black cumin (Nigella sativa) extract on hatching and post hatch performance of thermally challenged broiler chickens during incubation. Poult. Sci. 100:100831. doi: 10.1016/j.psj.2020.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke, O. E., Sorungbe F. O., Abioja M. O., Oyetunji O., and Onabajo A. O... 2016. Effect of different levels of honey on physiological, growth and carcass traits of broiler chickens during dry season. Acta Argic. Slov. 108:45–53. doi: 10.14720/aas.2016.108.1.5. [DOI] [Google Scholar]
- Oladokun, S., and Adewole D. I... 2020. In ovo delivery of bioactive substances: an alternative to the use of antibiotic growth promoters in poultry production—a review. J. Appl. Poult. Res. 29:744–763. doi: 10.1016/j.japr.2020.06.002. [DOI] [Google Scholar]
- Oladokun, S., Clark K. F., and Adewole D. I... 2022. Microbiota and transcriptomic effects of an essential oil blend and its delivery route compared to an antibiotic growth promoter in broiler chickens. Microorganisms 10:861. doi: 10.3390/microorganisms10050861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladokun, S., Macisaac J., Rathgeber B., and Adewole D... 2021. Essential oil delivery route: effect on broiler chicken’s growth performance, blood biochemistry, intestinal morphology, immune, and antioxidant status. Animals 11:3386. doi: 10.3390/ani11123386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omojate Godstime, C., Enwa Felix O., Jewo Augustina O., and Eze Christopher O... 2014. Mechanisms of antimicrobial actions of phytochemicals against enteric pathogens–a review. J. Pharm. Chem. Biol. Sci. 2:77–85. http://www.jpcbs.info/. [Google Scholar]
- Ospina, E. A., Merrill L., and Benson T. J... 2018. Incubation temperature impacts nestling growth and survival in an open‐cup nesting passerine. Ecol. Evol. 8:3270–3279. doi: 10.1002/ece3.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles, E. 2018. In ovo applications in poultry: a review. Poult. Sci. 97:2322–2338. doi: 10.3382/ps/pey081. [DOI] [PubMed] [Google Scholar]
- Peebles, E., Barbosa T., Cummings T., Dickson J., Womack S., Gerard P.. 2017. Comparative effects of in ovo versus subcutaneous administration of the Marek's disease vaccine and pre-placement holding time on the processing yield of Ross 708 broilers. Poultry Sci. 96:3944–3948. doi: 10.3382/ps/pex201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peşmen, G. 2022. Effects of in ovo injection of black cumin (Nigella sativa) extract on hatching performance of broiler eggs. Open Chem. 20:1502–1507. doi: 10.1515/chem-2022-0256. [DOI] [Google Scholar]
- Procopio, F. R., Ferraz M. C., Paulino B. N., do Amaral Sobral P. J., and Hubinger M. D... 2022. Spice oleoresins as value-added ingredient for food industry: recent advances and perspectives. Trends Food Sci. Technol. 122:123–139. doi: 10.1016/j.tifs.2022.02.010. [DOI] [Google Scholar]
- Proszkowiec-Weglarz, M., Schreier L. L., Miska K. B., Angel R., Kahl S., and Russell B... 2019. Effect of early neonatal development and delayed feeding post-hatch on jejunal and ileal calcium and phosphorus transporter genes expression in broiler chickens. Poult. Sci. 98:1861–1871. doi: 10.3382/ps/pey546. [DOI] [PubMed] [Google Scholar]
- Rahal, A., Kumar A., Singh V., Yadav B., Tiwari R., Chakraborty S., and Dhama K... 2014. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res. Int. 2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran, V. M. R., and Abdollahi M. R... 2021. Nutrition and digestive physiology of the broiler chick: state of the art and outlook. Animals 11:2795. doi: 10.3390/ani11102795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran, V. M. R. A. 2021. Nutrition and digestive physiology of the broiler chick: state of the art and outlook. Animals 11:2795. doi: 10.3390/ani11102795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricks, C. A., Avakian A., Bryan T., Gildersleeve R., Haddad E., Ilich R., King S., Murray L., Phelps P., Poston R.,. et al. 1999. In ovo vaccination technology. Adv. Vet. Med. 41:495–515. https://pubmed.ncbi.nlm.nih.gov/9890038/. [PubMed] [Google Scholar]
- Roto, S. M., Kwon Y. M., and Ricke S. C... 2016. Alications of in ovo technique for the optimal development of the gastrointestinal tract and the potential influence on the establishment of its microbiome in poultry. Front. Vet. Sci. 3:63. doi: 10.3389/fvets.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio, L. A. 2019. Possibilities of early life programming in broiler chickens via intestinal microbiota modulation. Poult. Sci. 98:695–706. doi: 10.3382/ps/pey416. [DOI] [PubMed] [Google Scholar]
- Salary, J., Sahebi-Ala F., Kalantar M., and Matin H. R... 2014. In ovo injection of vitamin E on post-hatch immunological parameters and broiler chicken performance. Asian Pac. J. Trop. Biomed. 4:S616–S619. doi: 10.12980/APJTB.4.2014APJTB-2014-0088. [DOI] [Google Scholar]
- Shehata, A. M., Paswan V. K., Attia Y. A., Abdel-Moneim A. M. E., Abougabal M. S., Sharaf M., Elmazoudy R., Alghafari W. T., Osman M. A., Farag M. R.,. et al. 2021. Managing gut microbiota through in ovo nutrition influences early-life programming in broiler chickens. Animals 11:3491. doi: 10.3390/ani11123491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwek, M., Slawinska A., Stadnicka K., Bogucka J., Dunislawska A., and Bednarczyk M... 2018. Prebiotics and synbiotics–in ovo delivery for improved lifespan condition in chicken. BMC Vet. Res. 14:1–17. doi: 10.1186/s12917-018-1738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklan, D., and Noy Y... 2000. Hydrolysis and absorption in the small intestines of posthatch chicks. Poult. Sci. 79:1306–1310. doi: 10.1093/ps/79.9.1306. [DOI] [PubMed] [Google Scholar]
- Sood, R., Bhatia S., Bhatnagar H., Gupta V., Kumar M., Dimri U., and Swarup D... 2013. Phytochemical analysis and in vitro screening of selected Indian medicinal plants for antiviral activity against highly pathogenic avian influenza virus. Spatula DD 3:81–88. doi: 10.5455/spatula.20130812030904. [DOI] [Google Scholar]
- Spotila, J.R. 2004. Sea turtles: a complete guide to their biology, behavior, and conservation. JHU Press. [Google Scholar]
- Surh, Y. J. 2003. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Tiwari, R., Latheef S. K., Ahmed I., Iqbal H. M. N., Bule M. H., Dhama K., Samad, H. A.Karthik K., Alagawany M., El-Hack M. E. A., Yatoo M. I., and Farag M. R... 2018. Herbal immunomodulators – a remedial panacea for designing and developing effective drugs and medicines: current scenario and future prospects. Curr. Drug Metab. 19:264–301. doi: 10.2174/1389200219666180129125436. [DOI] [PubMed] [Google Scholar]
- Tokofai, M. B., Idoh K., Oke O. E., and Agbonon A... 2020. Growth performance, haematological and biochemical parameters changes in broilers chickens feed varying levels of Vernonia amygdalina leaf meal. Eur. Poult. Sci. 84:1–12. doi: 10.1399/eps.2020.321. [DOI] [Google Scholar]
- Tůmová, E., Skřivan M., Skřivanová V., Kacerovska L. , 2002. Effect of early feed restriction on growth in broiler chickens, turkeys and rabbits. Czech J. Anim. Sci. 47:418–428. [Google Scholar]
- Uni, Z., and Ferket P. R... 2004. Methods for early nutrition and their potential. Worlds Poult. Sci. J. 60:101–111. doi: 10.1079/WPS20038. [DOI] [Google Scholar]
- Uni, Z., Ferket P. R., Tako E., and Kedar O... 2005. In ovo feeding improves energy status of late-term chicken embryos. Poult. Sci. 84:764–770. doi: 10.1093/ps/84.5.764. [DOI] [PubMed] [Google Scholar]
- Uni, Z., Yadgary L., and Yair R... 2012. Nutritional limitations during poultry embryonic development. J. Appl. Poult. Res. 21:175–184. doi: 10.3382/japr.2011-00478. [DOI] [Google Scholar]
- Uyanga, V. A., Oke O. E., Amevor F. K., Zhao J., Wang X., Jiao H., Onagbesan O. M., and Lin H... 2022. Functional roles of taurine, L-theanine, L-citrulline, and betaine during heat stress in poultry. J. Anim. Sci. Biotechnol. 13:23. doi: 10.1186/s40104-022-00675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankar, P. S. 2004. Essential oils and fragrances from natural sources. Resonance 9:30–41. doi: 10.1007/bf02834854. [DOI] [Google Scholar]
- Voemesse, K., Teteh A., Nideou D., N’nanlé O., Tété-Benissan A., Oke O. E., Gbeassor M., Decuypere E., and Tona K... 2019. Effects of Moringa oleifera leave meal in the diet on layer performance, haematological and serum biochemical values. Eur. Poult. Sci. 83:1–12. doi: 10.1399/eps.2019.263. [DOI] [Google Scholar]
- Widowski, T. M., Cooley L., Hendriksen S., and Peixoto M. R. L. V... 2022. Maternal age and maternal environment affect egg composition, yolk testosterone, offspring growth and behaviour in laying hens. Sci. Rep. 12:1–12. doi: 10.1038/s41598-022-05491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen, H., Debonne M., Swennen Q., Everaert N., Careghi C., Han H., Bruggeman V., Tona K., and Decuypere E... 2010. Delay in feed access and spread of hatch: importance of early nutrition. Worlds Poult. Sci. J. 66:177–188. doi: 10.1017/s0043933910000243. [DOI] [Google Scholar]
- Williams, C. J. 2007. In ovo vaccination for disease prevention. Int. Poult. Prod. 15:7–9. http://www.positiveaction.info/pdfs/articles/pp15.8p7.pdf. [Google Scholar]
- Williams, C. J., and Hopkins B. A... 2011. Field evaluation of the accuracy of vaccine deposition by two different commercially available in ovo injection systems. Poult. Sci. 90:223–226. doi: 10.3382/ps.2010-00759. [DOI] [PubMed] [Google Scholar]
- Windisch, W., Kroismayr A.. 2006. The effects of phytobiotics on performance and gut function in monogastrics. In World nutrition forum: The future of animal nutrition (pp. 85–90). Austria, Vienna: University of Natural Resources and Applied Life Sciences Vienna. [Google Scholar]
- Windisch, W., Schedle K., Plitzner C., and Kroismayr A... 2008. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 86:E140–E148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- Yadav, A. S., Kolluri G., Gopi K., Karthik K., and Singh Y... 2016. Exploring alternatives to antibiotics as health promoting agents in poultry: a review. J. Exp. Biol. 4:368–383. doi: 10.18006/2016.4(3S).368.383. [DOI] [Google Scholar]
- Yang, Y., Iji P. A., and Choct M... 2009. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. Worlds Poult. Sci. J. 65:97–114. doi: 10.1017/s0043933909000087. [DOI] [Google Scholar]
- Yigit, A. A., Panda A. K., and Cherian G... 2014. The avian embryo and its antioxidant defense system. Worlds Poult. Sci. J. 70:563–574. doi: 10.1017/s0043933914000610. [DOI] [Google Scholar]
- Zeinab S., Majid M., Mostafa G.. 2019. Effect of in-ovo injection of nanoparticles of grape seed extract on sex differentiation and gonadal structure in broiler chicks. Iranian J. Anim. Sci. 49:495–504. [Google Scholar]
- Zhai, W., Rowe D. E., and Peebles E. D... 2011. Effects of commercial in ovo injection of carbohydrates on broiler embryogenesis. Poult. Sci. 90:1295–1301. doi: 10.3382/ps.2010-01130. [DOI] [PubMed] [Google Scholar]
- Zhang, C., Chen K., Zhao X., and Geng Z... 2018. Protective effects of resveratrol against high ambient temperature-induced spleen dysplasia in broilers through modulating splenic redox status and apoptosis. J. Sci. Food Agric. 98:5409–5417. doi: 10.1002/jsfa.9084. [DOI] [PubMed] [Google Scholar]