Abstract
Traumatic Brain Injury (TBI) is a risk factor for Alzheimer's disease and Alzheimer's disease related dementias (AD/ADRD) and otherwise classified post-traumatic neurodegeneration (PTND). Targeted research is needed to elucidate the circumstances and mechanisms through which TBI contributes to the initiation, development, and progression of AD/ADRD pathologies including multiple etiology dementia (MED). The National Institutes of Health hosts triennial ADRD summits to inform a national research agenda, and TBI was included for a second time in 2022. A multidisciplinary expert panel of TBI and dementia researchers was convened to re-evaluate the 2019 research recommendations for understanding TBI as an AD/ADRD risk factor and to assess current progress and research gaps in understanding post-TBI AD/ADRD. Refined and new recommendations were presented during the MED special topic session at the virtual ADRD Summit in March 2022. Final research recommendations incorporating broad stakeholder input are organized into four priority areas as follows: (1) Promote interdisciplinary collaboration and data harmonization to accelerate progress of rigorous, clinically meaningful research; (2) Characterize clinical and biological phenotypes of PTND associated with varied lifetime TBI histories in diverse populations to validate multimodal biomarkers; (3) Establish and enrich infrastructure to support multimodal longitudinal studies of individuals with varied TBI exposure histories and standardized methods including common data elements (CDEs) for ante-mortem and post-mortem clinical and neuropathological characterization; and (4) Support basic and translational research to elucidate mechanistic pathways, development, progression, and clinical manifestations of post-TBI AD/ADRDs. Recommendations conceptualize TBI as a contributor to MED and emphasize the unique opportunity to study AD/ADRD following known exposure, to inform disease mechanisms and treatment targets for shared common AD/ADRD pathways.
Keywords: adult brain injury; Alzheimer's disease related dementias; head trauma; National Institute of Neurological Disorders and Stroke, post-TBI; TBI
Introduction
Dementia is an increasingly concerning public health challenge as the population ages; it is estimated that 14,000,000 people in the United States,1 and 152,000,000 people worldwide2 will have dementia by 2050.3 There is an urgent need to develop effective treatments, which will require an expanded understanding of individual, genetic, environmental, and lifestyle factors that contribute to the development and progression of pathological processes that contribute and predispose individuals to the clinical expression of dementia syndromes. In 2012, Health and Human Services (HHS; please see list of abbreviations in Supplementary Material) released the National Plan to Address Alzheimer's Disease4 in response to the National Alzheimer's Project Act (NAPA).5 The National Plan strives to coordinate federal, private, and state-level efforts to address Alzheimer's disease (AD)/Alzheimer's disease related dementias (ADRD) through improved clinical care and education, support services, and research. Since 2013, the ADRD summits are hosted and led by the National Institute of Neurological Disorders and Stroke (NINDS), in collaboration with the National Institute of Aging (NIA) to identify research priorities and monitor progress toward achieving the goals of the plan. The National Institutes of Health (NIH) triennial ADRD summits, AD summits, and the National Research Summit on Care, Services and Support for Persons with Dementia and their Caregivers are key components of the NIH response to NAPA.
Traumatic brain injury (TBI) was first identified as a potential contributor to dementia in a small series of case studies from the late 1930s to the 1970s, after which decades of evidence from large-scale epidemiological and smaller cohort studies documented an association between TBI and all-cause dementia, AD, and other neurodegenerative diseases. The 2020 report of the Lancet Commission on Dementia Prevention, Intervention, and Care reiterated the importance of TBI as a potentially modifiable dementia risk factor.6 Approximately 64,000,000–74 000,000 people sustain a TBI each year worldwide,7 and 22% of people in the United States have sustained at least one TBI with loss of consciousness (LOC) during their lifetime.8,9 Despite substantial evidence that TBI is associated with elevated risk for dementia and other neurodegenerative diseases, many studies find no risk for cognitive decline or AD, including large population-based and epidemiological studies,10–13 and studies with gold standard autopsy end-points.14–16 Efforts to synthesize extant literature17-–21 suggest that myriad factors can influence dementia risk following TBI, as is the case in individuals without this exposure history. TBI can range in severity from a bump or blow to the head or neck that results in a brief alteration in mental state,22 to one that can result in prolonged disorders of consciousness or death; some evidence suggests that dementia risk is greater following more severe injury.19 Most published studies of TBI and dementia risk were not designed for this purpose, and analyses have been largely limited by imprecise data on exposure and outcomes and traditional methodological approaches to investigating group-level associations. As an example, evidence suggests that using diagnostic codes to identify dementia in veterans under the age of 65 yields mostly false positive cases,23 and studies using administrative data only include TBIs for which care was sought and received during the time for which records are available. Important questions remain about how TBI, alone or together with other life course exposures, impacts dementia risk. Well-designed studies of individuals with TBI offer an exciting opportunity to investigate AD/ADRD in a population that is enriched for dementia risk by virtue of having sustained a known and potentially quantifiable exposure.
The 2019 ADRD Summit24 hosted by NINDS was the first to include a subcommittee on TBI as a risk factor for AD/ADRD25 as an emerging topic, “TBI and AD/ADRD Risk.” This subcommittee set forth four research priorities based on expert and stakeholder-informed recommendations to advance scientific knowledge of whether and how TBI increases risk for AD/ADRD. Recognizing the opportunities made possible through targeted investments into research studying TBI as a dementia risk factor, the summit planning committee invited inclusion of this working group as part of the multiple etiology dementia (also referred to as mixed etiology dementia) (MED) committee for the 2022 ADRD Summit. Per the 2018 update to NAPA,26 ADRDs include frontotemporal dementias (FTD), Lewy body dementias (LBD), vascular contributions to cognitive impairment and dementia (VCID), and MEDs. The TBI and AD/ADRD subcommittee recognized that evidence suggests elevated risk for clinical expression and pathological hallmarks of other ADRDs (i.e., FTD, LBD, and VCID) among TBI survivors; post-TBI AD/ADRD is inherently a MED wherein TBI is one of many environmental exposures that may have important implications for dementia risk. Because studying TBI as a “risk factor” for ADRD implies a restrictive set of methodological approaches, the subcommittee elected to approach this research topic with a revised session name: “Post-TBI AD/ADRD.” This conceptual shift is motivated by the need to elucidate key distinctions in individual and injury characteristics and their associations with distinct clinical dementia phenotypes and underlying pathological processes.
The Post-TBI AD/ADRD subcommittee was tasked with developing four prioritized recommendations, along with estimated timelines for each to reflect the number of years needed to achieve fully operational status and/or to complete implementation of the proposed work following its initiation. Here, we describe the research priorities outlined at the 2022 ADRD Summit, as recommended and refined by stakeholder input, for the investigation of Post-TBI AD/ADRD.
Methods
The post-TBI ADRD subcommittee followed methods deployed in the 2013 ADRD Conference,27 the 2016 ADRD Summit,28 and the 2019 ADRD Summit,24 as previously described25 and explained in greater detail in the ADRD Summit 2022 Report to the National Advisory Neurological Disorders and Stroke (NANDS) Council.29 A brief summary is provided below in the next section.
Pre-summit activities
The session chair of the post-TBI AD/ADRD special topic subcommittee (K.D.O'C.), together with NIH session leads, formed a subcommittee by selecting from a roster of experts with diverse and relevant expertise in TBI, post-traumatic neurodegeneration, neuropathology of TBI and AD/ADRDs, basic and translational TBI and AD/ADRD science, TBI and dementia epidemiology, and neuroimaging and fluid biomarkers of TBI and dementia. The subcommittee met regularly via teleconference between November 2021 and March 2022 to evaluate progress since the 2019 ADRD Summit, examine enduring gaps in knowledge, and develop and refine scientific recommendations.
A joint NINDS/NIA request for information solicited public input on updating the ADRD research priorities, and NIH staff provided the subcommittee with responses for review. Cross-committee coordination was facilitated by monthly teleconferences of the Summit Organizing Committee which consisted of scientific committee chairs, the Summit Scientific Chair, NIH and other federal officials including the NINDS/NIH Summit lead, and the Steering Committee. The Post-TBI AD/ADRD Subcommittee chair attended MED committee meetings to facilitate coordination and complementarity of recommendations, and other subcommittee members rotated attendance at all AD/ADRD Committee meetings to facilitate interdisciplinary and cross-committee communication. Committees and their participants are detailed in the ADRD Summit 2022 Report to the NANDS Council.30 The ADRD Summit 2022 agenda and draft recommendations were posted online and distributed to ADRD Summit registrants to gather input from stakeholders and the public.
Summit
Because of the global COVID-19 pandemic, the 2022 ADRD Summit was held on a virtual 2-day platform on March 22 and 23, 2022. Engagement of stakeholders was encouraged through interactive question and answer sessions as a proxy for an open microphone; efforts were made to closely approximate the open interactions achieved at prior in-person summits. Participants were invited to speak during the live session by using a virtual hand-raising feature or typing their names into the chat so that they could be called upon to address the speakers and panels directly.
More than 1500 individuals registered for the 2022 ADRD Summit, nearly double the registration numbers from prior summits. Up to 700 registrants attended most sessions, which were also recorded for archival viewing.31,32 Post-TBI AD/ADRD subcommittee chair (K.D.O'C.) provided a brief introduction to the topic area, and subcommittee members presented a summary of scientific rationale and draft recommendations for investigating post-TBI AD/ADRD. As with prior ADRD summits, the primary goal was to seek public input on recommendations from a wide stakeholder audience including researchers; clinicians; representatives from government, industry, and non-profit organizations; and individuals with lived experience, their loved ones, caregivers, and advocacy groups.
Post-summit follow-up
Following the summit proceedings, NINDS led a closed executive session with session chairs, NIH and other federal officials, Steering Committee members, and the scientific chair to review stakeholder input from the summit, consider proposed revisions, and edit the draft recommendations. Over teleconference, the TBI-ADRD Subcommittee met to further refine the content, prioritization, and proposed timelines for research recommendations. The finalized ADRD Summit 2022 Prioritized Research Milestones recommendations were submitted in a report30 to the NANDS Council, approved by them in September 2022, and then submitted together with the ADRD Summit 2022 Success Criteria to the Department of Health and Human Services (DHHS) NAPA Council in November 2022. Upon acceptance by the NAPA Council, the research recommendations became ADRD research implementation milestones in the National Plan.
Results
Evaluation of progress since 2019 Summit.
As an emerging topic, the subcommittee's recommendations in the 2019 Summit were informed by decades of research on TBI and AD/ADRD, and combined studies of TBI and AD/ADRD. In preparation for the 2022 Summit, the subcommittee considered new advancements in research and progress toward recommendations made at the 2019 Summit. Following the 2019 Summit, advancements consistent with the 2019 recommendations include the publication of consensus-based chronic traumatic encephalopathy (CTE)33 and traumatic encephalopathy syndrome (TES)34 research diagnostic criteria, both of which are believed to be unique to repetitive sub-concussive head impact exposures. An NIH/NINDS-supported initiative was launched in 2021 to define CDEs for the post-mortem neuropathological investigation of brain tissue derived from decedents with a history of TBI, as well as CDEs for post-mortem clinical characterization of TBI decedents per informant report; these CDEs were made available to the research community in March 2022.35
Incorporation of stakeholder input
Open discussion at the 2022 Summit yielded valuable feedback from public stakeholders. A stakeholder with lived experience emphasized the need for expanded public education regarding potential implications of repetitive head impacts (RHI) for long-term brain health, to allow individuals to make informed decisions regarding risk tolerance. Attendees opined on the centrality of biomarkers to understand clinical heterogeneity across injury mechanisms. Further discussions emphasized the importance of imaging biomarkers for characterizing pathophysiology, injury, and progression of AD/ADRD, and also to understand heterogeneous recovery processes and individual resilience to injury. There was a comment and ensuing discussion regarding the complexity of CTE and post-TBI dementia, with participants agreeing on the importance of characterizing lifetime TBI and RHI exposure histories. Additional comments addressed the need for focused study of post-TBI ADRD in pre-clinical models, with panelists agreeing that collaboration between clinical and pre-clinical researchers is essential to achieve clinically relevant models across injury exposure patterns and mechanics.
Priorities for the investigation of post-TBI AD/ADRD
Final recommendations (see Table 1) reflect persistent gaps in scientific knowledge and high-yield opportunities to advance the understanding of post-TBI AD/ADRD. Some recommendations build upon gains made since 2019, others elaborate on recommendations made in 2022, and still others are entirely new expansions beyond prior recommendations. Recommendations highlight infrastructure needs and unanswered questions viewed by the subcommittee as being critical to advancing the goals of the National Plan, and research priorities span pre-clinical and clinical methods to investigate underlying mechanisms and their clinical manifestations to define post-traumatic neurodegeneration (PTND) and better understand its relationship to AD/ADRDs.
Table 1.
Recommendations for the Study of Post-Traumatic Brain Injury (TBI) Alzheimer's Disease and Alzheimer's Disease Related Dementias (AD/ADRD)
| Recommendation 1 – Priority 1. Promote collaboration among TBI and dementia researchers through working groups, retrospective and prospective data and measurement harmonization, and interdisciplinary research. (1–3 years) |
| • Convene a working group of stakeholders from the TBI & multiple-etiology dementia communities to evaluate the extent to which current knowledge (e.g., mechanistic pathways, environmental and genetic risk factors, independent and interactive effects of multiple proteinopathies on pathological proliferation and AD/ADRD clinical manifestation) in AD/ADRD can be applied to the study of dementia after TBI, and how TBI (an AD/ADRD risk factor with a “time zero”) contributes to AD/ADRD. • Harmonize existing data across longitudinal TBI outcome studies and TBI-AD/ADRD studies using data harmonization and advanced psychometric methods; improve data annotation in existing studies to facilitate cross-study comparisons. • Maximize measurement harmonization across longitudinal TBI and dementia clinical cohort studies by establishing and prospectively collecting common data elements (CDEs) (clinical, psychometric, neuroimaging, fluid biomarkers) to facilitate comparisons and data sharing. • Encourage collaboration among community stakeholders, clinical researchers, biostatisticians, epidemiologists, data scientists, and implementation scientists to incorporate multidimensional/multimodal data, employ causal inference methodologies, and maximize clinical translatability in the study of TBI-AD/ADRD. |
| Recommendation 2 – Priority 2. Characterize the heterogeneous clinical and biological phenotypes and time course of progressive dementia following varied TBI exposure histories by developing biomarkers and methods to quantify lifetime head trauma exposure and diagnose post-TBI dementias. (1–10 years) |
| • Establish and validate a quantitative index of lifetime head trauma exposure. • Establish and validate a provisional clinical definition of post-TBI dementia(s) that distinguishes chronic static TBI-related symptoms from a progressive neurodegenerative disease, as measured by clinical decline and changes in clinically accessible biofluid and imaging biomarkers. • Conduct longitudinal studies to characterize the clinical phenotype, phenotypic heterogeneity, clinical course, environmental and genetic protective factors, and effect modifiers (e.g., post-traumatic stress disorder, sleep disorders) of post-TBI AD/ADRDs in samples of men and women from diverse backgrounds with distinct and varied lifetime exposure histories, as characterized by age at injury, severity, mechanism, and chronicity. • Develop and validate TBI-AD/ADRD biomarkers (e.g., psychometric, wearable sensors, imaging, and biofluid) to non-invasively identify progressive post-TBI AD/ADRD pathologies, monitor disease progression over time, elucidate pathological substrates of domain-specific clinical decline, and predict resilience to cognitive decline and to behavioral disorders. |
| Recommendation 3 – Priority 3. Establish research infrastructure, including multimodal longitudinal studies with autopsy end-points that employ standardized CDEs and methodologies, to study post-TBI AD/ADRD. (1–3 years) |
| • Enrich the design of longitudinal TBI studies to include multimodal clinical and biological/biochemical end-points relevant to neurodegenerative diseases and incident dementia diagnostics. Similarly, enrich AD/ADRD studies with expanded lifetime TBI ascertainment methods. • Establish clinic- and community-based prospective studies of individuals with diverse head trauma exposure histories (e.g., single TBI, repetitive head trauma in the contexts of contact sports, military service, domestic violence, and intimate partner violence [IPV]) for longitudinal study using multimodal clinical evaluations and neuroimaging and neuropathological end-points to inform clinically actionable diagnostics for post-TBI AD/ADRD. • Expand efforts to build and enhance existing brain biorepositories to include optimally preserved tissues from individuals with diverse head trauma exposure histories (e.g., a history of participation in contact sports or military service, single or repetitive TBI of all severities) and clinical and/or post-mortem neuroimaging, medical records, and CDE structured post-mortem interview data. • Launch nationwide inter-agency efforts to expand and standardize the use of National Institute of Neurological Disorders and Stroke (NINDS) CDEs for Human Neuropathological Studies in TBI for harmonized neuropathological evaluation and post-mortem clinical characterization across tissue banking centers. Promote tissue sharing and develop digital pathology infrastructure to facilitate research across tissue banks. |
| Recommendation 4 – Priority 4. Basic and translational research to elucidate the mechanistic pathways, development, and progression of post-TBI AD/ADRD neuropathologies to better understand clinical symptom expression. (7–10 years) |
| • Accelerate the development, standardization, and validation of clinically relevant experimental models of aspects of TBI that accommodate diversity of injury mechanisms and biomechanics; and accurately reproduce their distinct and interactive acute and chronic neuropathological and behavioral sequelae. Collaborate with clinical researchers to refine models as knowledge of human TBI neuropathology evolves. • Deploy traditional, quantitative, and/or molecular (omics) approaches to deeply characterize post-TBI neuropathologies, identify selectively vulnerable/resilient cells/regions, and determine underlying pathological mechanisms common with, or unique from, other multi-etiology dementias and neurodegenerative disorders. • Determine how the relative extent, distribution, and temporal progression of individual neuropathologies (and their potential interactions) contribute to the clinical manifestation of dementia following TBI. Identify how TBI exposure history (e.g., mechanism and severity of injury, number of exposures) influences the nature and evolution of autonomic and central nervous system pathologies in humans and experimental models. • Identify intrinsic (e.g., genetic, proteomic) and environmental (e.g., socioeconomic, educational, lifestyle) factors that confer resilience to cognitive decline and behavioral disorders after TBI and during aging. |
Each of the four recommendations and their scientific rationale are discussed in turn.
Recommendation 1: Promote collaboration among TBI and dementia researchers through working groups, retrospective and prospective data and measurement harmonization, and interdisciplinary research
High impact, clinically meaningful, and methodologically rigorous research on post-TBI AD/ADRDs is inherently and necessarily interdisciplinary. Decades of research on AD and ADRDs can inform and refine our approach to studying PTND, and studying TBI sequelae in prospective studies offers a unique opportunity to elucidate contributory mechanisms to MEDs. This crosstalk and foundational collaboration may be best facilitated by a formal, face-to-face, meeting process.36
Sharing data across TBI and ADRD studies has historically been hampered by the use of different measures of the same constructs, a lack of detailed head impact exposure ascertainment in AD/ADRD studies, and the absence of AD/ADRD clinical and biological end-points in many TBI studies. Advanced psychometric methods offer an opportunity to fully harness the value of existing data from AD/ADRD and/or TBI studies, and multiple approaches37–39 have shown strong promise for overcoming long-standing obstacles to permit cross-study comparisons, meta-analyses, and even pooled data analyses. Investments in careful data annotation and the use of CDEs relevant to TBI and dementia (including those most recently developed for the study of PTND),35 will be pivotal for facilitating harmonization of data from existing and future studies.
In addition to interdisciplinary TBI and AD/ADRD content expertise, methodological expertise is needed to conduct rigorous post-TBI AD/ADRD research and address existing gaps. Longitudinal methods based in sociology, psychology, and epidemiology are needed to study TBI in the life course context.40 Advanced psychometric methods are needed to optimize outcome precision and detection of change over time, and sophisticated statistical models are to investigate causal inference are needed to examine the associations of longitudinal head trauma exposures with post-TBI AD/ADRD in observational data.41 Finally, there is an urgent need to expand translation of research findings into clinical and community practice; this will require investment in research that is responsive to the needs of end users, engagement and crosstalk between researcher and stakeholders/end-users through community-based participatory research, and use of implementation science methods to inform best methods to communicate findings across diverse environments and audiences.42
Recommendation 2: Characterize the heterogeneous clinical and biological phenotypes and time course of progressive dementia following varied TBI exposure histories by developing biomarkers and methods to quantify lifetime head trauma exposure and diagnose post-TBI dementias
A foundational challenge in studying post-TBI AD/ADRD is quantifying lifetime head trauma exposure. Reliance on administrative claims data results in the exclusion of injuries sustained outside the period of time for which records are available, as well as of injuries for which care was not sought. Well-validated self-report TBI exposure measures exist,43,44 and methods for characterizing repetitive head impacts are in development; however, methods for quantifying lifetime exposure to head trauma are lacking. Prospective studies employing granular clinical injury exposure metrics and validation of these metrics with data from wearable devices and clinical biomarkers may facilitate more objective and quantifiable head trauma exposure data. A multimodal approach to lifetime head trauma exposure ascertainment will address growing concerns about the utility and relevance of long-standing methods for TBI severity characterization.45
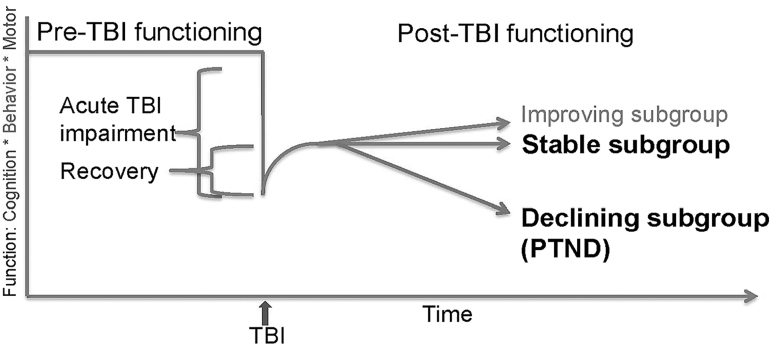
A unified definition of PTND is required to distinguish the acute effects of injury from chronic injury outcomes, and to distinguish chronic stable effects of TBI from a progressive degenerative process. When considering TBI in a life course context, PTND is best conceptualized as a MED that may include clinical features and biological signatures of a wide range of ADRDs. Identification of in-vivo clinical and biological markers of PTND during life is necessary for in-vivo diagnosis, monitoring disease progression, and evaluating intervention efficacy. Understanding the underlying pathology of PTND will inform the development of disease-modifying interventions (Fig. 1).
FIG. 1.
Traumatic brain injury (TBI) of varying severities can result in acute impairments in multiple domains of function. The degree of impairment can range from mild transient symptoms to prolonged disordered consciousness and death. The degree and rate of recovery is similarly variable. Some survivors may continue to improve for years following injury. Most reach a recovery plateau, after which some remain stable while others experience a progressive decline in one or more area of function (post-traumatic neurodegeneration [PTND]).
A barrier to in-vivo biomarker discovery is the lack of large-scale longitudinal studies following TBI patients from the acute phase of injury for years or even decades to characterize the chronic sequelae that may take many years to manifest. Acute TBI studies typically follow patients for 3–6 months or at most 1 year, and many chronic TBI studies are cross-sectional. Recent multi-center acute TBI studies have documented large disparities in symptomatic, cognitive, behavioral, and functional outcomes during the 1st year after injury;46,47 however, the further progression of impairments resulting in PTND is not well understood because of the lack of long-term longitudinal data in most acute studies. The largest prospective TBI outcome study in the world, the National Institute on Disability Independent Living and Rehabilitation Research (NIDILRR)-funded TBI Model Systems,48 includes rich patient-reported outcomes data but lacks biological data and AD/ADRD end-points. Well-designed prospective longitudinal investigations incorporating genetic, proteomic, and neuroimaging biomarker collection can elucidate the biological mechanisms of PTND.
Fluid biomarker discovery efforts in TBI to date have focused on acute diagnostics, and opportunities to investigate biomarkers of chronic TBI and/or PTND have been limited. As has been well documented in AD/ADRD research, the clinical manifestations of PTND may be preceded by pathological changes that are detectable in biofluids. As such, investigation of acute markers of TBI in longitudinal studies that permit discovery of clinically accessible biomarkers of chronic post-recovery decline will pave the way for early detection of PTND, in-vivo diagnostics, and elucidation of the underlying pathobiology of post-TBI AD/ADRDs. A recent systematic review found that blood-based biomarker concentrations after TBI correlate with burden of intracranial disease as characterized by neuroimaging, but associations with imaging phenotypes (e.g., diffuse axonal injury, intracranial hemorrhage) were inconsistent, further illustrating the heterogeneous nature of TBI.49 A wide range of neuropathological processes are found in the brain tissue of decedents who survived many years following TBI;50–56 these present potential mechanisms through which TBI may elevate the risk for subsequent development of AD/ADRD. Some of these processes begin at the acute stage of injury and evolve chronically, whereas others may develop and progress years after TBI. Progress in acute TBI biomarkers and recent expansions of TBI brain banking efforts are laudable, but there remains a major gap in knowledge such that little is known about biological signatures of TBI in the years between acute TBI and death.
Neuroimaging similarly provides a clinically accessible window into the brain as it evolves in the years following TBI. TBI has traditionally been studied using imaging biomarkers of brain macrostructure from high-resolution three-dimensional (3D) structural magnetic resonance imaging (sMRI), brain microstructure and white matter connectivity from diffusion MRI (dMRI) brain activation, and gray matter connectivity from functional MRI (fMRI), as well as brain blood flow from perfusion MRI.57–67 Improvements to standard processing pipelines to address large TBI-related lesions68–71 are needed to ensure that those with greatest intracranial burden are not excluded from research. Meanwhile, imaging studies of neurodegeneration often instead focus on molecular biomarkers from positron emission tomography (PET), including radiotracers that map fluorodeoxyglucose (FDG), amyloid, and tau for Alzheimer's disease, the latter also for frontotemporal dementia, as well as single-photon emission computed tomography (SPECT) radiotracers such as ioflupane for Parkinson's disease and LBD.72–76 There is a paucity of longitudinal investigations combining MR-based and PET/SPECT-based biomarkers for characterizing the development of the different biological phenotypes of PTND, the pathogenesis of which remains poorly understood. Combining these multimodal imaging biomarkers with specific blood-based biomarkers of pathogenesis as well as longitudinal clinical, cognitive, and behavioral data can address this vital explanatory gap regarding initiation and progression of PTND.
Recommendation 3: Establish research infrastructure, including multimodal longitudinal studies with autopsy endpoints that employ standardized CDEs and methodologies, to study post-TBI AD/ADRD
Research on post-traumatic AD/ADRD can greatly benefit from well-developed and long-standing AD/ADRD research infrastructure. For over three decades, NIH/NIA has supported the Alzheimer's Disease Research Center Network,77 which has developed effective structured research pipelines and robust national data and biospecimen repositories78,79 that support AD/ADRD research. Funded infrastructure makes biospecimen and data resources widely accessible, as each aging and disability resource center (ADRC) hosts data, biomarker, biospecimen, and other funded ADRC cores and other core consortia78–80 support data and biospecimen collection, characterization, and sharing with AD/ADRD researchers. Post-TBI AD/ADRD research can benefit from this existing infrastructure and expertise, with early successes including expansion of the NIH-funded Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) brain bank81 and CONNECT-TBI Center Without Walls82 which formed a network of brain banks dedicated to post-TBI AD/ADRD neuropathology research. Further progress is needed, perhaps to leverage existing AD/ADRD NIH working groups, including the ADRC-NeuroBiobank Working Group, whose goal is to identify opportunities between the brain bank networks to promote and support AD/ADRD brain donation; enhance communication and collaboration; as well as biospecimen, tissue, and data sharing across the networks; and the Digital Pathology Working Group, whose focus is to identify opportunities for generation and sharing of whole slide imaging data across the AD/ADRD research community. In addition to biospecimen resources, digital pathology represents a tremendous opportunity for broad sharing and access to neuropathology resources, and represents one aspect of the ways that post-TBI AD/ADRD research could be enhanced through collaboration and harmonization with existing AD/ADRD data and biospecimen resources.
This infrastructure can be leveraged and/or replicated to enhance post-TBI AD/ADRD research that includes dementia end-points linked with enhanced TBI-exposure ascertainment data combined with cognitive, imaging, and biofluid biomarker measurements and robust biospecimen banking. Prospective, longitudinal study designs in representative cohorts are critical to understanding the broader impact of post-TBI AD/ADRD. Studies should include research participants from diverse backgrounds and a broad range of TBI exposures, including unexposed controls, who are assessed with multimodal evaluations and biospecimen collection and banking that will enable connection of cognitive trajectories with underlying structural injury and biological progression. In this design, community-based recruitment is essential to mitigate risks associated with clinic-based and convenience samples that may over- or underestimate risk factor/exposure/outcome relationships and may poorly generalize to a larger population. This is particularly true in TBI, wherein multiply marginalized individuals are at disproportionate risk for worst outcomes.
At the time of the 2019 ADRD Summit, there were very few TBI brain tissue archives, a paucity of detailed clinical data available, and no standardization of protocols for neuropathological examination or clinical data harmonization. Creation and early implementation of neuropathological CDEs, which were developed in consensus by harmonizing with, and building upon, existing AD/ADRD neuropathology criteria,83–85 now provides a framework for research standardization, cross-communication, and collaborative and integrative research on TBI-related AD/ADRD across TBI and AD/ADRD researchers. Most existing TBI studies do not include in-vivo biological markers of PTND or post-mortem neuropathology. Few TBI cohort studies span more than 1 year post-injury, and even fewer include control comparisons or ADRD consensus diagnostics. There is a critical need for well-designed prospective studies including dementia outcomes and autopsy.
Finally, technological advancements permit unique opportunities to study the human brain at the cellular and molecular level, to correlate rich neuroimaging and highly sensitive biofluid assays with cognitive outcomes and subtle alterations in brain structure and connectivity. NIH has invested in efforts to understand the neurotypical human brain through the BRAIN Initiative Cell Atlas Network, and the Human Connectome Project, and AD/ADRD research is already leveraging these efforts to understand the cellular and molecular underpinnings of age-related neurodegeneration.86–89 Post-TBI AD/ADRD studies can benefit from forward-looking applications of state-of-the-art neuroimaging and biospecimen collection techniques to permit advanced molecular and analytical approaches, including deep learning and artificial intelligence, to understand the structural and functional changes associated with, and potentially specific for, post-TBI AD/ADRD. This approach will inform next generation experimental models in silico, in culture, or in vivo to develop and test advanced therapeutics.
Recommendation 4: Conduct basic and translational research to elucidate the mechanistic pathways, development, and progression of post-TBI AD/ADRD neuropathologies to better understand clinical symptom expression
Pre-clinical models provide a valuable platform to examine how acute biomechanical injury might precipitate progressive neurodegenerative processes. Multiple pre-clinical TBI studies over several decades have been examined for various degenerative pathologies, including neurodegeneration-associated proteinopathies. Employing mostly rodent models, including transgenic models, these studies report a wide range of occasionally conflicting outcomes.90–95 Therefore, there is a pressing need to carefully consider the utility, development, and standardization of models for the study of neurodegeneration after TBI. Critically, validation of pre-clinical models will depend on bidirectional translational research informed by ongoing human clinical and neuropathology studies. Anatomic structure and biomechanical forces across the spectrum of injury severity are other important factors to consider in biofidelic modeling. For example, the pathognomonic lesion of CTE is defined, in part, by its location at the depths of cortical sulci,33 which is proposed as a region of biomechanical vulnerability.96 Therefore, gyrencephalic structure may be an important consideration in modeling aspects of this pathology. Recapitulating the biomechanics of mild TBI is also a notable challenge, particularly in rodents, and the careful application of input biomechanics informed by known real-world data will be essential. Ultimately, characterizing reproducible models that reflect the diversity of injury biomechanics, evolution of pathologies, life stage, and complex clinical manifestations of neurodegeneration will generate novel opportunities to explore mechanisms of disease. The Department of Veterans Affairs (VA) is leading the development of an interagency (Department of Defense [DoD], NINDS, and NIA) pre-clinical TBI resource center, Pre-Clinical Interagency Research Resource-TBI (PRECISE TBI),97 which will catalog pre-clinical TBI models (based on relevancy to clinical TBI), further develop standardized methods, and current preclinical common data elements.98,99 The goal of this initiative is to make all information available to those involved in TBI pre-clinical research, to reduce variability and improve reproducibility among laboratories, especially regarding chronic models investigating TBI-related neurodegeneration.
To date, there have been considerable efforts to characterize CTE, defined by a stereotypical distribution of hyperphosphorylated (p-tau) pathology at the depths of cortical sulci as its pathognomonic lesion.33 Now, there is increasing recognition that TBI may lead to far more diverse and varied evolving pathologies, including white matter degeneration, neuroinflammation, multiple proteinopathies, and blood–brain barrier breakdown,17,19,50,55 with this heterogeneity of neuropathological changes potentially reflected in similarly diverse clinical outcomes. However, the relationship between the nature, severity, and number of TBI exposures and specific europathological and clinical outcomes remains poorly defined. Detailed correlation of clinicopathological outcomes with robust measures of lifetime TBI exposure will be critical to addressing this knowledge gap. Further, examination of individuals with good outcomes, despite significant TBI exposure, may provide valuable information regarding possible intrinsic and environmental factors conferring resilience.
An additional and important avenue of examination will be to elucidate both the similarities and differences between trauma-related neurodegeneration and other established neurodegenerative disorders. Notably, although immunohistochemical examinations indicate that tau in CTE is indistinguishable from that of AD or other age-related tauopathies,100 recent cryogenic electron microscopy (cryo-EM) studies indicate that tau in CTE may have a structure distinct from that of AD.101 Using state-of-the-art quantitative and molecular approaches, deep phenotyping of pathologies following diverse trauma exposures will be integral not only to advancing understanding disease pathogenesis, but also to identifying possible distinguishing features of TBI- related neurodegeneration that might inform the development of targeted diagnostic and therapeutic interventions.
Conclusion
Investigation of post-TBI AD/ADRD in well-designed multimodal longitudinal studies with autopsy end-points offers a unique opportunity for uncovering mechanisms of AD/ADRD. Unlike other causes of neurodegeneration and dementias, “time zero” – the moment of the initiating TBI event or events – is usually known, permitting a temporal examination of the evolving clinical changes and pathological processes triggered by TBI.
Approval of the 2022 Prioritized Recommendations for the study of Post-TBI AD/ADRD by the National Advisory Neurological Disorders and Stroke Council and the NAPA Council as research milestones in the National Plan to Address Alzheimer's Disease will inform the Department of Health and Human Services, the NIH, and the entire national and international research community on high priority steps to advance TBI research related to AD/ADRD outcomes.
Supplementary Material
Authors' Contributions
K.D.O'C. was responsible for conceptualization and writing (original draft, review and editing). H.O.A. was responsible for conceptualization and writing (review and editing). S.H. was responsible for conceptualization and writing (review and editing). M.J.P. was responsible for conceptualization and writing (original draft, review and editing). V.E.J. was responsible for conceptualization and writing (original draft, review and editing). C.D.K. was responsible for conceptualization and writing (original draft, review and editing). L.M. was responsible for writing (review and editing). P.M. was responsible for conceptualization and writing (original draft, review and editing). L.O. was responsible for writing (review and editing). N.U. was responsible for conceptualization and writing (review and editing). G.S. was responsible for conceptualization and writing (review). H.Z. was responsible for conceptualization and writing (original draft, review and editing).
Funding Information
The conference was supported by NINDS, organized in collaboration with NIA, with assistance from the NIH Office of Disease Prevention, and the Foundation for the National Institutes of Health with support from their contributors: Alzheimer's Association; GHR Foundation; Biogen; Accelerate Cure/Treatments for Alzheimer's Disease (ACT-AD) Coalition; Alzheimer's Drug Discovery Foundation; American Stroke Association, a division of the American Heart Association; CurePSP; EIP Pharma; The John A. Hartford Foundation; and the WellMed Charitable Foundation. M.J.P.'s time was supported by VA Health Services Research and Development Service Research Career Scientist Award (RCS 17-297, Award No IK6HX002608). K.D.O'C.'s time was supported by the NIH through U54 NS115322 (CONNECT-TBI), U54NS115266, R01 AG061028 (LEARN-TBI-AD), RF1 NS115268 and RF1 NS128961 (LETBI), and Department of Defense W81XWH2210999 (ENRICH). V.E.J.'s time was supported by the NIH through U54 NS115322 (CONNECT-TBI). C.D.K.'s time was supported by the NIH through P30 AG066509 (UW ADRC), U54 NS115322 (CONNECT-TBI), R01 AG061028 (LEARN-TBI-AD), RF1 NS115268 (LETBI), and the Nancy and Buster Alvord endowment.
Author Disclosure Statement
H.Z. has served on scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Passage Bio, Pinteon Therapeutics, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. The other authors have nothing to disclose.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute; the National Institute of Neurological Disorders and Stroke; the National Institute on Aging; or the National Institutes of Health and the U.S. Department of Health and Human Services.
Supplementary Material
References
- 1. Lopez OL, Kuller LH. Epidemiology of aging and associated cognitive disorders: Prevalence and incidence of Alzheimer's disease and other dementias. Handb Clin Neurol 2019;167:139–148; doi: 10.1016/b978-0-12-804766-8.00009-1 [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Dementia. 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia [Last accessed February 16, 2023].
- 3. GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022;7(2):e105–e125; doi: 10.1016/s2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Assistant Secretary for Planning and Evaluation. National Plan to Address Alzheimer's Disease. Available from: https://aspe.hhs.gov/collaborations-committees-advisory-groups/napa/napa-documents/napa-national-plan [Last accessed February 16, 2023].
- 5. United States Congress. National Alzheimer's Project Act. 2010. [Google Scholar]
- 6. Livingston G, Huntley J, Sommerlad A, et al. . Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396(10248):413–446; doi: 10.1016/s0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dewan MC, Rattani A, Gupta S, et al. . Estimating the global incidence of traumatic brain injury. J Neurosurg 2018; doi: 10.3171/2017.10.Jns17352 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8. Bogner J, Corrigan JD, Yi H, et al. . Lifetime history of traumatic brain injury and behavioral health problems in a population-based sample. J Head Trauma Rehabil 2020;35(1):E43–e50; doi: 10.1097/htr.0000000000000488 [DOI] [PubMed] [Google Scholar]
- 9. Corrigan JD, Hammond FM. Traumatic brain injury as a chronic health condition. Arch Phys Med Rehabil 2013;94(6):1199–1201; doi: 10.1016/j.apmr.2013.01.023;10.1016/j.apmr.2013.01.023 [DOI] [PubMed] [Google Scholar]
- 10. Launer L, Andersen K, Dewey M, et al. . Rates and risk factors for dementia and Alzheimer's disease: results from EURODEM pooled analyses. Neurology 1999;52(1):78; doi: 10.1212/wnl.52.1.78 [DOI] [PubMed] [Google Scholar]
- 11. Grasset L, Glymour MM, Yaffe K, et al. . Association of traumatic brain injury with dementia and memory decline in older adults in the United States. Alzheimers Dement 2020;16(6):853–861; doi: 10.1002/alz.12080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tripodis Y, Alosco ML, Zirogiannis N, et al. . The effect of traumatic brain injury history with loss of consciousness on rate of cognitive decline among older adults with normal cognition and Alzheimer's disease dementia. J Alzheimers Dis 2017;59(1):251–263; doi: 10.3233/jad-160585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dams-O'Connor K, Gibbons LE, Bowen JD, et al. . Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J Neurol Neurosurg Psychiatry 2013;84(2):177–182; doi: 10.1136/jnnp-2012-303938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chosy EJ, Gross N, Meyer M, et al. . Brain Injury and Later-Life Cognitive Impairment and Neuropathology: The Honolulu–Asia Aging Study. J Alzheimers Dis 2020;73(1):317–325; doi: 10.3233/jad-190053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crane PK, Gibbons LE, Dams-O'Connor K, et al. . Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol 2016;73(9):1062–1069; doi: 10.1001/jamaneurol.2016.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sugarman MA, McKee AC, Stein TD, et al. . Failure to detect an association between self-reported traumatic brain injury and Alzheimer's disease neuropathology and dementia. Alzheimers Dement 2019;15(5):686–698; doi: 10.1016/j.jalz.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson L, Stewart W, Dams-O'Connor K, et al. . The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol 2017;16(10):813–825; doi: 10.1016/s1474-4422(17)30279-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dams-O'Connor K, Guetta G, Hahn-Ketter AE, et al. . Traumatic brain injury as a risk factor for Alzheimer's disease: current knowledge and future directions. Neurodegener Dis Manag 2016;6(5):417–429; doi: 10.2217/nmt-2016-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brett BL, Gardner RC, Godbout J, et al. . Traumatic brain injury and risk of neurodegenerative disorder. Biol Psychiatry 2022;91(5):498–507; doi: 10.1016/j.biopsych.2021.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weiner MW, Crane PK, Montine TJ, et al. . Traumatic brain injury may not increase the risk of Alzheimer disease. Neurology 2017;89(18):1923–1925; doi: 10.1212/wnl.0000000000004608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. LoBue C, Cullum CM. POINT/COUNTER-POINT-Beyond the headlines: the actual evidence that traumatic brain injury is a risk factor for later-in-life dementia. Arch Clin Neuropsychol 2020;35(2):123–127; doi: 10.1093/arclin/acz074 [DOI] [PubMed] [Google Scholar]
- 22. Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J Head Trauma Rehabil 1993;8(3):86–87; doi: 10.1097/000001199-199309000-00010 [DOI] [Google Scholar]
- 23. Marceaux JC, Soble JR, O'Rourke JJF, et al. . Validity of early-onset dementia diagnoses in VA electronic medical record administrative data. Clin Neuropsychol 2020;34(6):1175–1189; doi: 10.1080/13854046.2019.1679889 [DOI] [PubMed] [Google Scholar]
- 24. National Institute of Neurological Disorders and Stroke, Natcher Auditorium Bethesda, Maryland, United States, 14–15 March 2019. [Google Scholar]
- 25. Dams-O'Connor K, Bellgowan PSF, Corriveau R, et al. . Alzheimer's Disease-Related Dementias Summit 2019: National Research Priorities for the Investigation of Traumatic Brain Injury as a Risk Factor for Alzheimer's Disease and Related Dementias. J Neurotrauma 2021;38(23):3186–3194; doi: 10.1089/neu.2021.0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. U.S. Department of Health and Human Services. National Plan to Address Alzheimer's Disease: 2018 Update. Bethesda, MD; 2018. [Google Scholar]
- 27. Montine TJ, Koroshetz WJ, Babcock D, et al. . Recommendations of the Alzheimer's disease–related dementias conference. Neurology 2014;83(9):851–860; doi: 10.1212/WNL.0000000000000733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Corriveau RA, Koroshetz WJ, Gladman JT, et al. . Alzheimer's Disease-Related Dementias Summit 2016: National Research Priorities. Neurology 2017;89(23):2381–2391; doi: 10.1212/wnl.0000000000004717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. NIH National Institute of Neurological Disorders and Stroke. Alzheimer's Disease-Related Dementias Summit 2022. 2022. Available from: https://www.ninds.nih.gov/news-events/events/adrd-summit-2022 [Last accessed April 19, 2023].
- 30. Rost NS. ADRD Summit 2022 Report. 2022; Available from: https://www.ninds.nih.gov/sites/default/files/documents/ADRD%20Summit%202022%20Report%20to%20NINDS%20Council%20FINAL.pdf [Last accessed February 16, 2023].
- 31. ADRD Summit 2022: Day 1. 2022. Available from: https://videocast.nih.gov/watch=45149 [Last accessed February 16, 2023].
- 32. ADRD Summit 2022: Day 2. 2022. Available from: https://videocast.nih.gov/watch=45150 [Last accessed February 16, 2023].
- 33. Bieniek KF, Cairns NJ, Crary JF, et al. . The Second NINDS/NIBIB Consensus Meeting to Define Neuropathological Criteria for the Diagnosis of Chronic Traumatic Encephalopathy. J Neuropathol Exp Neurol 2021;80(3):210–219; doi: 10.1093/jnen/nlab001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Katz DI, Bernick C, Dodick DW, et al. . National Institute of Neurological Disorders and Stroke Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome. Neurology 2021;96(18):848–863; doi: 10.1212/wnl.0000000000011850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Research FITBI. Chronic TBI-Related Neurodgeneration Common Data Elements. Available from: https://fitbir.nih.gov/chronic-tbi-related-neurodegeneration-cdes [Last accessed February 16, 2023].
- 36. Higgins LE, Smith JM. Documenting development of interdisciplinary collaboration among researchers by visualizing connections. Research Evaluation 2022;31(1):159–172; doi.org/ 10.1093/reseval/rvab039 [DOI] [Google Scholar]
- 37. Kennedy E, Dennis EL, Lindsey HM, et al. . Harmonizing PTSD severity scales across instruments and sites. Neuropsychology 2022; doi: 10.1037/neu0000823 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mukherjee S, Choi SE, Lee ML, et al. . Cognitive domain harmonization and cocalibration in studies of older adults. Neuropsychology 2022; doi: 10.1037/neu0000835 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kobayashi LC, Gross AL, Gibbons LE, et al. . You say tomato, I say radish: can brief cognitive assessments in the U.S. Health Retirement Study be harmonized with its international partner studies? J Gerontol B Psychol Sci Soc Sci 2021;76(9):1767–1776; doi: 10.1093/geronb/gbaa205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hanson MA, Cooper C, Aihie Sayer A, et al. . Developmental aspects of a life course approach to healthy ageing. J Physiol 2016;594(8):2147–2160; doi: 10.1113/jp270579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaplan D. Causal inference for observational studies. J Infect Dis 2019;219(1):1–2; doi: 10.1093/infdis/jiy392 [DOI] [PubMed] [Google Scholar]
- 42. Woolf SH, Purnell JQ, Simon SM, et al. . Translating evidence into population health improvement: strategies and barriers. Annual review of public health 2015;36:463; doi: 10.1146/annurev-publhealth-082214-110901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Corrigan JD, Bogner J. Initial reliability and validity of the Ohio State University TBI Identification Method. J Head Trauma Rehabil 2007;22(6):318–329; doi: 10.1097/01.Htr.0000300227.67748.77 [DOI] [PubMed] [Google Scholar]
- 44. Dams-O'Connor K, Cantor JB, Brown M, et al. . Screening for traumatic brain injury: findings and public health implications. J Head Trauma Rehabil 2014;29(6):479–489; doi: 10.1097/HTR.0000000000000099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tenovuo O, Diaz-Arrastia R, Goldstein LE, et al. . Assessing the severity of traumatic brain injury–time for a change? J Clin Med 2021;10:1; doi: 10.3390/jcm10010148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nelson LD, Temkin NR, Dikmen S, et al. . Recovery after mild traumatic brain injury in patients presenting to US level I trauma centers: a transforming research and clinical knowledge in traumatic brain injury (TRACK-TBI) study. JAMA Neurol 2019;76(9):1049–1059; doi. 10.1001/jamaneurol.2019.1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van der Naalt J, Timmerman ME, de Koning ME, et al. . Early predictors of outcome after mild traumatic brain injury (UPFRONT): an observational cohort study. Lancet Neurol 2017;16(7):532–540; doi: 10.1016/S1474-4422(17)30117-5 [DOI] [PubMed] [Google Scholar]
- 48. Dijkers MP, Marwitz JH, Harrison-Felix C. Thirty years of national institute on disability, independent living, and rehabilitation research traumatic brain injury model systems center research–an update. J Head Trauma Rehabil 2018;33(6):363–374; doi: 10.1097/htr.0000000000000454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Whitehouse DP, Vile AR, Adatia K, et al. . Blood biomarkers and structural imaging correlations post-traumatic brain injury: a systematic review. Neurosurgery 2022;90(2):170–179; doi: 10.1227/neu.0000000000001776 [DOI] [PubMed] [Google Scholar]
- 50. Johnson VE, Stewart JE, Begbie FD, et al. . Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013;136(Pt 1):28–42; doi: 10.1093/brain/aws322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Agrawal S, Leurgans SE, James BD, et al. . Association of traumatic brain injury with and without loss of consciousness with neuropathologic outcomes in community-dwelling older persons. JAMA Netw Open 2022;5(4):e229311; doi: 10.1001/jamanetworkopen.2022.9311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kenney K, Iacono D, Edlow BL, et al. . Dementia after moderate-severe traumatic brain injury: coexistence of multiple proteinopathies. J Neuropathol Exp Neurol 2018;77(1):50–63; doi: 10.1093/jnen/nlx101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Postupna N, Rose SE, Gibbons LE, et al. . The delayed neuropathological consequences of traumatic brain injury in a community-based sample. Front Neurol 2021;12:624696; doi: 10.3389/fneur.2021.624696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McKee AC, Gavett BE, Stern RA, et al. . TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 2010;69(9):918–929; doi: 10.1097/NEN.0b013e3181ee7d85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 2013;9(4):211; doi: 10.1038/nrneurol.2013.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smith DH, Johnson VE, Trojanowski JQ, et al. . Chronic traumatic encephalopathy – confusion and controversies. Nat Rev Neurol 2019;15(3):179–183; doi: 10.1038/s41582-018-0114-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Amyot F, Arciniegas DB, Brazaitis MP, et al. . A review of the effectiveness of neuroimaging modalities for the detection of traumatic brain injury. J Neurotrauma 2015;32(22):1693–1721; doi: 10.1089/neu.2013.3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bigler ED. Systems biology, neuroimaging, neuropsychology, neuroconnectivity and traumatic brain injury. Front Syst Neurosci 2016;10:55; doi: 10.3389/fnsys.2016.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hayes JP, Bigler ED, Verfaellie M. Traumatic brain injury as a disorder of brain connectivity. J Int Neuropsychol Soc 2016;22(2):120–137; doi: 10.1017/S1355617715000740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iraji A, Chen H, Wiseman N, et al. . Connectome-scale assessment of structural and functional connectivity in mild traumatic brain injury at the acute stage. Neuroimage Clin 2016;12:100–115; doi: 10.1016/j.nicl.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Narayana PA, Yu X, Hasan KM, et al. . Multi-modal MRI of mild traumatic brain injury. Neuroimage Clin 2015;7(87–97); doi: 10.1016/j.nicl.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Palacios EM, Owen JP, Yuh EL, et al. . The evolution of white matter microstructural changes after mild traumatic brain injury: a longitudinal DTI and NODDI study. Sci Adv 2020;6(32):eaaz6892; doi: 10.1126/sciadv.aaz6892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Palacios EM, Yuh EL, Mac Donald CL, et al. . Diffusion tensor imaging reveals elevated diffusivity of white matter microstructure that is independently associated with long-term outcome after mild traumatic brain injury: a TRACK-TBI study. J Neurotrauma 2022;39(19–20):1318–1328; doi: 10.1089/neu.2021.0408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sharp DJ, Scott G, Leech R. Network dysfunction after traumatic brain injury. Nat Rev Neurol 2014;10(3):156–166; doi: 10.1038/nrneurol.2014.15 [DOI] [PubMed] [Google Scholar]
- 65. Wiegand TL, Sollmann N, Bonke EM, et al. . Translational neuroimaging in mild traumatic brain injury. J Neurosci Res 2022;100(5):1201–1217; doi: 10.1002/jnr.24840 [DOI] [PubMed] [Google Scholar]
- 66. Hannawi Y, Abers MS, Geocadin RG, et al. . Abnormal movements in critical care patients with brain injury: a diagnostic approach. Crit Care 2016;20(1):1–10; doi: 10.1186/s13054-016-1236-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wu J, Aguilera T, Shultz D, et al. . Early-stage non–small cell lung cancer: quantitative imaging characteristics of 18F fluorodeoxyglucose PET/CT allow prediction of distant metastasis. Radiology 2016;281(1):270; doi: 10.1148/radiol.2016151829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Diamond BR, Donald CLM, Frau-Pascual A, et al. . Optimizing the accuracy of cortical volumetric analysis in traumatic brain injury. MethodsX 2020;7:100994; doi: 10.1016/j.mex.2020.100994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Maffei C, Gilmore N, Snider SB, et al. . Automated detection of axonal damage along white matter tracts in acute severe traumatic brain injury. Neuroimage Clin 2022;37:103294; doi: 10.1016/j.nicl.2022.103294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. King DJ, Novak J, Shephard AJ, et al. . Lesion induced error on automated measures of brain volume: data from a pediatric traumatic brain injury cohort. Front Neurosci 2020;14:4914780; doi: 10.3389/fnins.2020.491478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Radwan AM, Emsell L, Blommaert J, et al. . Virtual brain grafting: enabling whole brain parcellation in the presence of large lesions. Neuroimage 2021;229:117731; doi: 10.1016/j.neuroimage.2021.117731 [DOI] [PubMed] [Google Scholar]
- 72. Brooks DJ, Pavese N. Imaging biomarkers in Parkinson's disease. Prog Neurobiol 2011;95(4):614–628; doi: 10.1016/j.pneurobio.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 73. Burkett BJ, Babcock JC, Lowe VJ, et al. . PET Imaging of Dementia: Update 2022. Clin Nucl Med 2022;47(9):763–773; doi: 10.1097/RLU.0000000000004251 [DOI] [PubMed] [Google Scholar]
- 74. Chételat G, Arbizu J, Barthel H, et al. . Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer's disease and other dementias. Lancet Neurol 2020;19(11):951–962; doi: 10.1016/S1474-4422(20)30314-8 [DOI] [PubMed] [Google Scholar]
- 75. Leuzy A, Chiotis K, Lemoine L, et al. . Tau PET imaging in neurodegenerative tauopathies—still a challenge. Mol Psychiatry 2019;24(8):1112–1134; doi: 10.1038/s41380-018-0342-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tagai K, Ono M, Kubota M, et al. . High-contrast in vivo imaging of tau pathologies in Alzheimer's and non-Alzheimer's disease tauopathies. Neuron 2021;109(1):42–58; doi: 10.1016/j.neuron.2020.09.042 [DOI] [PubMed] [Google Scholar]
- 77. NIH National Institute on Aging. Alzheimer's Disease Research Centers. Available from: https://www.nia.nih.gov/health/alzheimers-disease-research-centers [Last accessed February 16, 2023].
- 78. The NIA Alzheimer's Disease Research Centers. National Alzheimer's Coordinating Center. Available from: https://naccdata.org [Last accessed February 16, 2023].
- 79. National Centralized Repository for Alzheimer's Disease and Related Dementias. NCRAD. Available from: https://ncrad.iu.edu/ [Last accessed February 16, 2023].
- 80. The National Institute on Aging Genetics of Alzheimer's Disease Data Storage Site. NIAGADS. Available from: https://www.niagads.org/ [Last accessed February 16, 2023].
- 81. McKee AC, Stein TD, Huber BR, et al. . Chronic traumatic encephalopathy (CTE): criteria for neuropathological diagnosis and relationship to repetitive head impacts. Acta Neuropathol 2023;145(4):371–394; doi: 10.1007/s00401-023-02540-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Smith DH, Dollé JP, Ameen-Ali KE, et al. . COllaborative Neuropathology NEtwork Characterizing ouTcomes of TBI (CONNECT-TBI). Acta Neuropathol Commun 2021;9(1):32; doi: 10.1186/s40478-021-01122-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hyman BT, Phelps CH, Beach TG, et al. . National Institute on Aging-Alzheimer's Association Guidelines for the Neuropathologic Assessment of Alzheimer's Disease. Alzheimers Dement 2012;8(1):1–13; doi: 10.1016/j.jalz.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Montine TJ, Phelps CH, Beach TG, et al. . National Institute on Aging–Alzheimer's Association Guidelines for the Neuropathologic Assessment of Alzheimer's Disease: a practical approach. Acta Neuropathol 2012;123(1):1–11; doi: 10.1007/s00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. National Alzheimer's Coordinating CenterNeuropathology (NP) v.11. Available from: https://naccdata.org/data-collection/forms-documentation/np-11 [Last accessed February 16, 2023].
- 86. National Institutes of Health. The BRAIN Initiative. Available from: https://braininitiative.nih.gov/ [Last accessed February 16, 2023].
- 87. National Institutes of Health. Cell Census Network (BICCN). Available from: https://braininitiative.nih.gov/brain-programs/cell-census-network-biccn [Last accessed February 16, 2023].
- 88. Alliance BI. BRAIN Initiative Cell Atlas Network (BICAN): Comprehensive Center on Human and Non-human Primate Brain Cell Atlases. Available from: https://www.braininitiative.org/funding-opportunity/brain-initiative-cell-atlas-network-bican-comprehensive-center-on-human-and-non-human-primate-brain-cell-atlases-um1-clinical-trial-not-allowed/ [Last accessed February 16, 2023].
- 89. Connectome Coordination Facility. What is the Connectome Coordination Facility? Available from: https://www.humanconnectome.org/ [Last accessed February 16, 2023].
- 90. Smith DH, Kochanek PM, Rosi S, et al. . Roadmap for advancing pre-clinical science in traumatic brain injury. J Neurotrauma 2021;38(23):3204–3221; doi: 10.1089/neu.2021.0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ojo JO, Mouzon BC, Crawford F. Repetitive head trauma, chronic traumatic encephalopathy and tau: challenges in translating from mice to men. Exp Neurol 2016;275(Pt 3):389–404; doi: 10.1016/j.expneurol.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 92. Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-β pathology: a link to Alzheimer's disease? Nat Rev Neurosci 2010;11(5):361–370; doi: 10.1038/nrn2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zanier ER, Bertani I, Sammali E, et al. . Induction of a transmissible tau pathology by traumatic brain injury. Brain 2018;141(9):2685–2699; doi: 10.1093/brain/awy193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mouzon B, Bachmeier C, Ojo J, et al. . Chronic white matter degeneration, but no tau pathology at one-year post-repetitive mild traumatic brain injury in a tau transgenic model. J Neurotrauma 2019;36(4):576–588; doi: 10.1089/neu.2018.5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nakagawa Y, Reed L, Nakamura M, et al. . Brain trauma in aged transgenic mice induces regression of established abeta deposits. Exp Neurol 2000;163(1):244–252; doi: 10.1006/exnr.2000.7375 [DOI] [PubMed] [Google Scholar]
- 96. Ghajari M, Hellyer PJ, Sharp DJ. Computational modelling of traumatic brain injury predicts the location of chronic traumatic encephalopathy pathology. Brain 2017;140(2):333–343; doi: 10.1093/brain/aww317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. PRECISE-TBI. PRE Clinical Interagency reSearch resourcE-TBI. Available from: https://www.precise-tbi.org/ [Last accessed February 16, 2023].
- 98. LaPlaca MC, Huie JR, Alam HB, et al. . Pre-clinical common data elements for traumatic brain injury research: Progress and use cases. J Neurotrauma 2021;38:1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Smith DH, Hicks RR, Johnson VE, et al. . Pre-clinical traumatic brain injury common data elements: Toward a common language across laboratories. J Neurotrauma 2015;32:1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Arena JD, Smith DH, Lee EB, et al. . Tau immunophenotypes in chronic traumatic encephalopathy recapitulate those of ageing and Alzheimer's disease. Brain 2020;143(5):1572–1587; doi: 10.1093/brain/awaa071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Falcon B, Zivanov J, Zhang W, et al. . Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 2019;568(7752):420–423; doi: 10.1038/s41586-019-1026-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.